Abstract
Lactic acid (LA) is an effective sanitizer for disinfection of fresh produce. Tap water is generally used to wash disinfected fresh produce because sanitizer residues negatively affect the quality and organoleptic properties of the produce. However, tap water is ineffective for secondary disinfection compared with sanitizers. Thus, we propose a disinfection method using LA plus aqueous ozone (AO), an oxidizing sanitizer that does not lead to secondary residue. We compared the proposed method of 1% LA (90 s) plus 1 mg L−1 AO (30 s) or 2 mg L−1 AO (30 s) with the traditional method of 100 ppm chlorine (120 s) or 1% LA (120 s) plus tap water (30 s) and 2 mg L−1 AO (150 s). Microbial analysis showed that LA plus AO led to the greatest reductions in microbes (Escherichia coli O157:H7, aerobic mesophilic counts, aerobic psychrophilic counts, moulds, and yeasts) during storage (0–5 days at 5 °C). Quality analysis (colour, sensory qualities, electrolyte leakage, polyphenolic content, and weight loss) showed that LA + AO did not cause additional quality loss compared with tap water treatment. These results indicate that the hurdle technology proposed (LA plus AO) has a good potential for use in fresh produce disinfection.
Lactic acid plus aqueous ozone is an effective hurdle technology for fresh produce disinfection.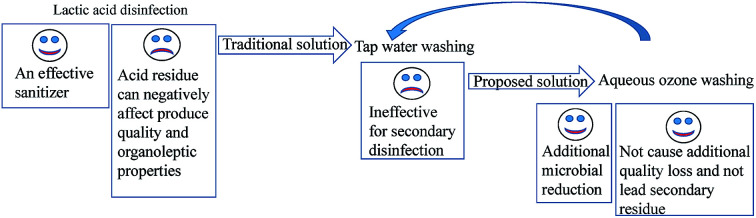
Introduction
Fresh produce is an important source of nutrition. Fresh-cut vegetables are convenient foods for today's fast-paced lifestyle and have expanded the vegetable industry.1 A large portion of fresh produce is consumed raw, and the number of foodborne outbreaks has increased correspondingly.2 In 2011 alone, foodborne pathogens caused 9.4 million cases of infection, resulting in 55 961 hospitalizations and 1351 deaths in the United States of America (USA).3 In the USA and European Union (EU), 39.5% and 42.6% of foodborne disease outbreaks from fresh produce consumption, respectively, were caused by pathogenic bacteria.4 In developing countries, an investigation in Brazil showed that Escherichia coli is present in 53.1% of ready-to-eat (RTE) vegetables, whereas Listeria spp. and Salmonella spp. accounted for 3.7% and 1.2%, respectively.5 In Rwanda, 15% of raw vegetables are contaminated by pathogenic microorganisms, with E. coli accounting for the largest portion (6.1%).6 Therefore, disinfection is a key postharvest operation before fresh produce is packaged for sale.
In recent years, physical (e.g., pulsed light, cold plasma, and ultraviolet light) and biopreservation (e.g., bacteriophages and bacteriocins) disinfection strategies have been used for fresh produce.7 However, the low-cost and easy-to-use characteristics of chemical sanitizers have not been challenged.8–10 Chemical sanitizers are generally divided into two categories, i.e., oxidizing agents and organic acids. As typical oxidizing agents, chlorine-based sanitizers have been widely used for fresh produce disinfection at free chlorine concentrations ranging from 50 to 200 ppm and for a maximum disinfection time of 5 min.11 However, during the washing process, chlorine in disinfectants can react with organic matter to form carcinogenic and mutagenic compounds, such as chloroform, trihalomethanes, chloramines, and haloacetic acids.9,12,13 The EU has therefore significantly restricted the use of chlorine for fresh produce disinfection, and in several EU countries, including Germany, the Netherlands, Switzerland, and Belgium, disinfection of fresh produce using chlorine is prohibited.12 Most organic acids are approved as generally recognized as safe (GRAS) by the US Food and Drug Administration and are used as a pH regulators and flavouring agents in the food industry. Organic acids are superior to chlorine because they do not generate toxic or carcinogenic compounds.9 When comparing disinfection efficacy, organic acids are more effective than chlorine-based sanitizers. For example, lactic acid (LA) is more effective than sodium hypochlorite (SH) in reducing Listeria innocua on broccoli sprouts.14 LA causes a more significant reduction in E. coli and Salmonella counts on spinach than gaseous chlorine dioxide;15 the commercially available sanitizer Purac (containing 90% LA, adjusted to 2% for use) is superior to chlorine for controlling the growth of mould and yeast during storage.16 Citric acid (CA) is better than SH to control E. coli and L. innocua on spinach during storage.17 LA, CA, and acetic acid (AA) are commonly used for minimal processing.10 Other GRAS organic acids (i.e., succinic acid [SA], tartaric acid [TA], propionic acid [PA], and malic acid [MA]) are also used to disinfect fresh produce.9,18,19 Among these seven GRAS acids, we previously showed that LA reduced the aerobic mesophilic count (AMC) the most on lettuce.20 Huang and Chen18 compared the disinfection effects between LA, CA, MA, TA, and AA and found that LA caused the greatest reduction in E. coli O157:H7 on spinach. Moreover, Akbas and Olmez found that the reduction in L. monocytogenes caused by LA was significantly higher than those by AA and CA.21
Although organic acids have many advantages, acid residues often remain on produce after use, giving the produce poor organoleptic characteristics and affecting the visual quality of the produce.10,22 Thus, tap water is generally used to wash fresh produce after acid disinfection. However, this method is ineffective for secondary disinfection and could lead to crosscontamination of the disinfected produce under circulation.23–25 Thus, there is a need for a sanitizer with additional microbial reduction capacity to be used following acid disinfection for removal of acid residues without leaving secondary residues.
Ozone has been deemed as GRAS and is widely used as a sanitizer owing to its low cost (produced from air), efficacy, and lack of residue deposition (because it is unstable and will decompose to oxygen). Aqueous ozone (AO, 1–3 ppm) shows microbial reduction capacity comparable to that of 100 ppm chlorine on lettuce.10 Moreover, disinfection efficacy is enhanced by the combined use of AO and organic acids. For example, the combination of 3 ppm AO and 1% CA reduces E. coli counts on lettuce to a greater extent than either one did alone.26 A similar effect was observed with E. coli and L. monocytogenes on mushrooms treated with both 3 ppm AO and 1% CA.27 On durum wheat, AO plus AA is more effective against native microbiota than AO, AA, or chlorinated water alone.28 Additionally, AO plus MA not only caused the greatest reduction in Shigella spp. counts but also stimulated radical scavenging activity in radishes and mung bean sprouts.29 Moreover, the low-pH environment caused by organic acid can shorten AO preparation time and prolong the half-life of AO.28 However, the combined use of AO and organic acids can leave acid residues on fresh produce.
Accordingly, in this study, we used AO instead of tap water to wash fresh-cut lettuce disinfected with LA and evaluated the effects of this sequential washing method on the quality of lettuce and on its microbial counts (naturally present and inoculated E. coli O157:H7).
Materials and methods
Sample preparation
Green leaf lettuce (Lactuca sativa var. Crispa L.) was purchased from a local market on the day of the experiment. Two outer leaves, inner baby leaves, broken leaves, and stems were removed. The remaining parts were rinsed with tap water to remove the soil. A circle knife was used to cut the lettuce into pieces with a diameter of approximately 5.8 cm.20 Excess water was removed using a manual salad spinner.
Inoculation of lettuce leaves
Nontoxic E. coli O157:H7 (ATCC700728) was kindly provided by Dr Yeting Sun, Vegetable Research Center, Beijing Academy of Agriculture and Forestry Sciences. Obtained cultures were stored in 50% glycerol solution at a ratio of 1 : 1.30 Before every use, the stock culture was purified using sorbitol MacConkey agar (SMAC; Hopebio, Qingdao, China). Then, single colonies were inoculated into tryptic soy broth medium (Hopebio) to prepare the working culture. The inoculation was carried out as previously reported by Huang and Chen,18 with slight modifications. Briefly, the working culture was adjusted to approximately 109 CFU mL−1 (optical density at 600 nm). Then, 5 mL of the adjusted cell suspension was mixed with 200 mL 0.85% sodium chloride in a sterilized plastic bag, and 10 g of the lettuce leaves was submerged in the cell suspension and gently massaged for 5 min. The drained leaves (106 to 107 CFU g−1) were placed in a sterilized plastic box for 24 h at 4 °C to facilitate the attachment of bacteria.
AO preparation
Ozone was prepared using the corona discharge method. Oxygen and ozone generators were mounted on a machine (20 g h−1; Ruifeng Technology, Zhuhai, China), and dried oxygen was introduced into the corona discharge pipe to obtain highly pure gaseous ozone. Tap water was used to prepare AO; 30 L water was introduced into the tank and circulated using a gas–liquid mixing pump (1000 kg h−1; CNP, Hangzhou, China). Gaseous ozone was absorbed by the pump and circulated. The AO concentration gradually increased and was measured in real-time using an ozone electrochemical probe (0–20 mg L−1; Nobo Science, Qingdao, China); data were corrected using the indigo method.31 The principle of electrochemical detection of ozone concentration was the same, regardless of the manufacturer chosen. The correction process was as follows.
(1) To prepare indigo stock solution, 50 mL distilled water, 0.1 mL concentrated hydrochloric acid (analytical grade), and 77 mg potassium indigotrisulfonate (Sigma-Aldrich, St. Louis, MO, USA) were thoroughly mixed and the final volume was adjusted to 100 mL in a volumetric flask. The solution was allowed to stand for 4 months in the dark.
(2) To prepare indigo working solution, 50 mL of distilled water, 10 mL indigo stock solution, 1 g sodium dihydrogen phosphate (analytical grade), and 0.7 mL concentrated phosphoric acid (analytical grade) were thoroughly mixed and the final volume was adjusted to 100 mL in a volumetric flask. The solution was used within one week.
(3) The electrode was placed in tap water and the concentration was adjusted to zero.
(4) When the electrode was thoroughly oxidized by the AO (at least 5 min), electrical signals on the screen were recorded and 5 mL of AO flowing through the electrode were transferred to volumetric flask B (100 mL; containing 10 mL indigo working solution, 1 mL of 5% malonic acid, and 50 mL of distilled water). The final volume was adjusted to 100 mL. Tap water in flask A was used as a blank. The absorbance at 600 nm was measured. The concentration of dissolved ozone was calculated using the following formula:Dissolved ozone concentration (mg L−1) = (ΔA × 100)/(f × b × v)where ΔA is the absorbance difference between flasks A and B, f is 0.42, b is the cuvette width (usually 1 cm), and v is the added sample volume (5 mL in this experiment).
(5) The AO was re-prepared and the concentration was adjusted to the calculated value when the same electrical signal was recorded.
Sequential washes
Lettuce samples and AO were prepared as described above. LA (90% purity; Macklin, Shanghai, China) was prepared as a 1% solution. Chlorinated water was prepared using SH (Sinopharm, Beijing, China) and adjusted to the desired concentration using a free chlorine test kit (Lohand, Hangzhou, China; 0.05–1 mg L−1). To select the appropriate contact time for LA plus AO, a screening experiment was designed (Table 1). We examined whether LA plus AO could shorten the disinfection time while having similar or better disinfection effects. Thus, the processing time of LA plus tap water was set at 120 s plus 30 s. Based on the screening results, the selected experimental groups, disinfection times, and sanitizer concentrations are shown in Table 2. Lettuce samples (for naturally presented microbial analysis) and inoculated samples (for E. coli O157:H7 analysis) were dipped in sanitizers at a ratio of 1 : 20 (w/v) and shaken at 150 rpm. After washing, samples were dewatered using an alcohol-sterilized salad spinner. Then, samples were transferred to a polyethylene terephthalate box (18 × 13 × 4 cm), and packaged using a polyvinyl chloride cling film (Nan Ya, Tai Wan, China) under air.16 Considering the short shelf-life of minimally processed products and consumer demand for fresh products, the samples were stored for 5 days at 5 °C. Samples without disinfection were selected as the control group. Each treatment was independently performed three times.
Effects of contact times and concentrations of LA and AO on E. coli O157:H7 reductiona.
| Treatment | E. coli O157:H7 reduction (log CFU g−1) | |
|---|---|---|
| First stage | Second stage | |
| Tap water 120 s | 0.38 ± 0.15a | |
| 1% LA 120 s | Tap water 30 s | 1.41 ± 0.18c |
| 1% LA 60 s | 0.5 mg L−1 AO 60 s | 1.12 ± 0.16b |
| 1% LA 60 s | 1.0 mg L−1 AO 60 s | 1.28 ± 0.15bc |
| 1% LA 60 s | 2.0 mg L−1 AO 60 s | 1.38 ± 0.03c |
| 1% LA 90 s | 0.5 mg L−1 AO 30 s | 1.45 ± 0.10c |
| 1% LA 90 s | 1.0 mg L−1 AO 30 s | 1.70 ± 0.08d |
| 1% LA 90 s | 2.0 mg L−1 AO 30 s | 1.72 ± 0.16d |
AO, aqueous ozone; LA, lactic acid. Different letters in a column indicate a significant difference (P < 0.05). Values are expressed as means ± standard deviations.
Experimental settingsa.
| Disinfection stage | Sanitizer residue removal stage |
|---|---|
| Tap water (control) 150 s | |
| 2 mg L−1 AO 150 s | |
| 100 mg L−1 free chlorine 120 s | Tap water 30 s |
| 1% LA 120 s | Tap water 30 s |
| 1% LA 90 s | 1 mg L−1 AO 30 s |
| 1% LA 90 s | 2 mg L−1 AO 30 s |
LA, lactic acid; AO, aqueous ozone.
Microbiological analysis
Microbes were counted at 0, 3, and 5 days. To guarantee complete contact between the lettuce sample and the dilution, after opening the package, each piece was divided into four parts on sterilized gauze and manually mixed. A 5 g sample was immersed in 70 mL sterile 0.85% sodium chloride solution and shaken at 260 rpm for 3 min to prepare a 15-fold dilution. A 10-fold dilution series (minimum dilution series of 100-fold) was prepared in 0.85% sodium chloride solution as needed. For AMC and aerobic psychrophilic counts (APCs), a 1 mL dilution was pour-plated in plate count agar (Hopebio) and incubated for 2 days at 37 °C or 10 days at 7 °C, respectively. For M&Y, a 1 mL dilution was pour-plated in Bengal rose agar (Hopebio) and incubated for 5 days at 28 °C. For E. coli O157:H7, a 0.1 mL dilution was surface-plated on SMAC agar and incubated for 1 day at 37 °C. Three replicates were analysed in duplicate, and the results were expressed as log CFU g−1.
Colour and sensory quality analyses
Colour determination was performed as described by Zhang and Yang,32 with some modifications. At the end of the storage period (5 days), L*, a*, and b* values were determined using a colorimeter (CR400; Konica Minolta, Osaka, Japan), which was calibrated using a white standard plate (Y = 82.80, x = 0.3194, y = 0.3264) before use. Ten pieces of lettuce were randomly selected from each group, and two sites per piece were analysed for a total of 20 readings per treatment.
Sensory analysis was performed as described by Allende et al.,33 with some modifications. Briefly, quality characteristics, including sensory colour, crispness, and odour, were evaluated on day 5. Eight PhD students from the College of Food Science, Shenyang Agricultural University were invited to score using the following scale: 0, very bad, not characteristic of the product; 5, acceptability threshold; and 10, very good product characteristics. The samples were placed on trays with marks at the bottom, and the trays were randomly reorganized to minimize subjectivity and to ensure test accuracy. During evaluation, only one person was allowed to enter the room (30 m2, 2.8 m height; illuminated by a 96 W white light lamp) and was not allowed to communicate with others after evaluation.
Electrolyte leakage, weight loss, and polyphenolic content evaluations
The extent of damage to the lettuce after washing was estimated by measuring electrolyte leakage as previously described,34 with some modifications. Briefly, 5 g of washed sample was immersed in 250 mL distilled water (1–3 μs cm−1) for 20 s to remove acid residue, which can interfere with conductivity measurements. Then, the samples were immersed in 150 mL distilled water, and the conductivity was measured after 30 s and 30 min. After incubation for 24 h at −20 °C, the sample was allowed to stand overnight at room temperature, and the conductivity was measured again. Electrolyte leakage was calculated using the following formula:Electrolyte leakage (%) = (conductivity30 min − conductivity0.5 min)/(conductivity24 h − conductivity0.5 min)
Weight loss was analysed on day 5 and calculated using the following formula:Weight loss (%) = 1 − (weightd5/weightd0)
Polyphenolic content was analysed on day 5. Briefly, 5 g fresh sample was extracted using 75 mL of 80% methanol in a blender for 2 min. After allowing the mixture to stand for 2 h at 4 °C, the slurry was filtered and centrifuged at 12 000 × g for 10 min. Polyphenolic content was determined according to the Folin–Ciocalteu method,35 with some modifications. Briefly, 50 μL of the suspension was added to 3 mL distilled water and oxidized with 250 μL Folin reagent. After allowing the mixture to stand for 6 min, the reaction was neutralised by adding 750 μL of 20% sodium carbonate and then incubated for 90 min in the dark. The absorbance was read at 765 nm, and the results are expressed as gallic acid equivalent (GAE, mg kg−1) expressed on a fresh weight basis.
Acid removal capacity
The acid removal capacity of AO and tap water was evaluated based on changes in conductivity. Following treatment with 1% LA for 90 s, samples were transferred to 150 mL tap water or 1 or 2 mg L−1 AO solutions without dewatering, and conductivity was measured within 150 s.
Statistical analysis
Differences between group means were evaluated using analysis of variance with the Statistical Package for the Social Sciences (SPSS) v.20 software (SPSS, Chicago, IL, USA), and differences (P < 0.05) in mean values were analysed using Duncan's multiple range test.
Results and discussion
Effects of sequential washing on lettuce colour and sensory quality
The screening results showed that E. coli O157:H7 counts in the control group were 6.57 ± 0.15 log CFU g−1. After disinfection, 90 s LA plus 30 s 1 mg L−1 AO and 90 s LA plus 30 s 2 mg L−1 AO reduced the levels by 1.70 and 1.72 log CFU g−1, respectively, which were significantly higher than those of the other combination groups (Table 1). In addition, this was important considering that a short contact time is important for AO disinfection.36 Similarly, organic acid disinfection is unfeasible if the contact time exceeds 5 min, even if it yields a significant reduction in microbial counts.10 Thus, this dual combination (i.e., 90 s LA plus 30 s AO) was selected.
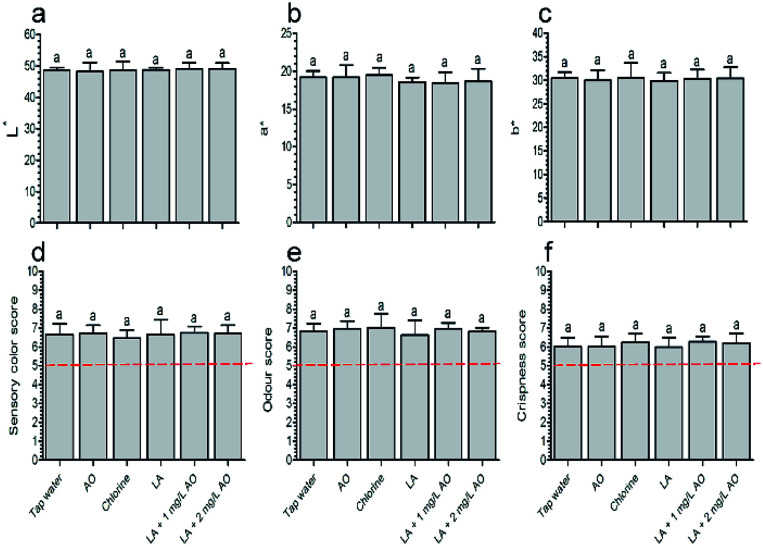
Colour analysis showed that all disinfection treatments had no effect on L*, a*, and b* values relative to samples treated with tap water (Fig. 1a–c). There were also no differences in colour between samples washed with LA (90 s) plus AO (30 s) and LA (120 s). However, it has been reported that oxidizing sanitizers affect lettuce colour to a greater extent than organic acid; one group reported that the L* value was increased relative to the control following treatment with 5 ppm ozone for 15 min, whereas 0.5–1.5% CA did not alter colour quality.37 After treatment with 2% LA and vinegar (6% acetic acid) followed by storage for 7 days, the b* values of lettuce were 21.0 and 18.2 respectively, which were comparable to that of the untreated sample (18.0); whereas the b* value of samples treated with 300 ppm SH was 37.1.38 A previous report showed that AO concentrations higher than 5 mg L−1 can cause physiological injury to the produce.10 Thus, our inconsistent observations in this study may be related to the low AO (1 and 2 mg L−1) and chlorine (100 ppm) concentrations and the short AO contact time (30 s).
Fig. 1. Effects of various treatments on colour (a–c) and sensory quality (d–f) at the end of storage (5 days). The red dotted line indicates the acceptability threshold value (i.e., 5). The columns are means ± standard deviations, and the same letters above the columns indicate insignificant differences (P > 0.05). LA, lactic acid; AO, aqueous ozone.
Sensory analysis showed that the colour was consistent with the colour observed using the colorimeter, indicating that the various treatments did not negatively affect the colour quality (Fig. 1d). The sensory odour and crispness scores were consistent with those of the control group on day 5 and exceeded the acceptability threshold (5 points, Fig. 1e and f), similar to the results described by Martínez-Sánchez et al.16
Effects of sequential washing on lettuce electrolyte leakage, weight loss, and polyphenolic content
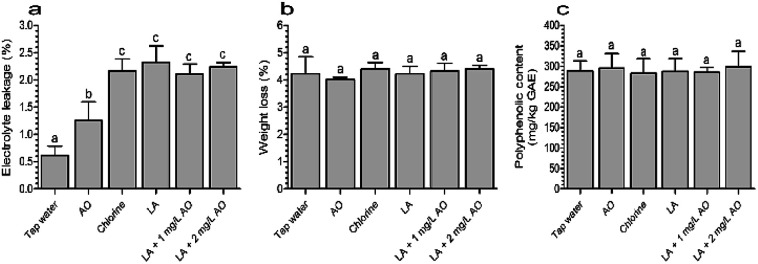
The extent of damage to lettuce samples after washing with various treatments was estimated based on electrolyte leakage. As shown in Fig. 2a, all disinfection treatments caused significant electrolyte leakage compared with tap water. After washing with 2 mg L−1 AO for 120 s, the electrolyte leakage was 1.26%, which was significantly lower than the values of the other disinfection treatments. Compared with LA (120 s), the combination treatment with LA (90 s) plus AO (30 s) did not lead to additional electrolyte leakage. These results indicated that AO had no effect on electrolyte leakage, which was mainly caused by LA.
Fig. 2. Effects of various treatments on electrolyte leakage on day 0 (a), weight loss on day 5 (b), and polyphenolic content on day 5 (c). The columns indicate means ± standard deviations, and the same letters above the columns indicate insignificant differences (P > 0.05). GAE, gallic acid equivalent; LA, lactic acid; AO, aqueous ozone.
Similarly, another study demonstrated that electrolyte leakage rates from lettuce were 1.43% and 1.41% following treatment with 1% hydrogen peroxide and 1% hydrogen peroxide plus electrolyzed water, respectively, which were lower than the rate (3.11%) of samples treated with 1% hydrogen peroxide plus 0.6% CA.32 In contrast, electrolyte leakage in fresh-cut cilantro was found to be comparable between samples washed with AO for 5 min and the control (14.13 vs. 15.78 μs cm−1).39 Moreover, our results showed that the electrolyte leakage of samples treated with LA plus AO was similar to that of chlorine.
During subsequent storage for up to 5 days, nonsignificant weight loss was observed in the disinfection groups (Fig. 2b). Similarly, the polyphenolic content of the disinfection treatment groups ranged from 282.84 to 298.91 mg kg−1 GAE, which was similar to that in the tap water group (289.24 mg kg−1 GAE, Fig. 2c). These results were inconsistent with our initial speculation because the different electrolytes could lead to differences in quality loss.32,40 This phenomenon may result from the slight damage caused by sanitizers and was not sufficient to cause visual quality loss, such as browning. During subsequent storage, the cell self-repair prevented additional losses in weight and polyphenolic contents.40,41
Reduction in microbial counts
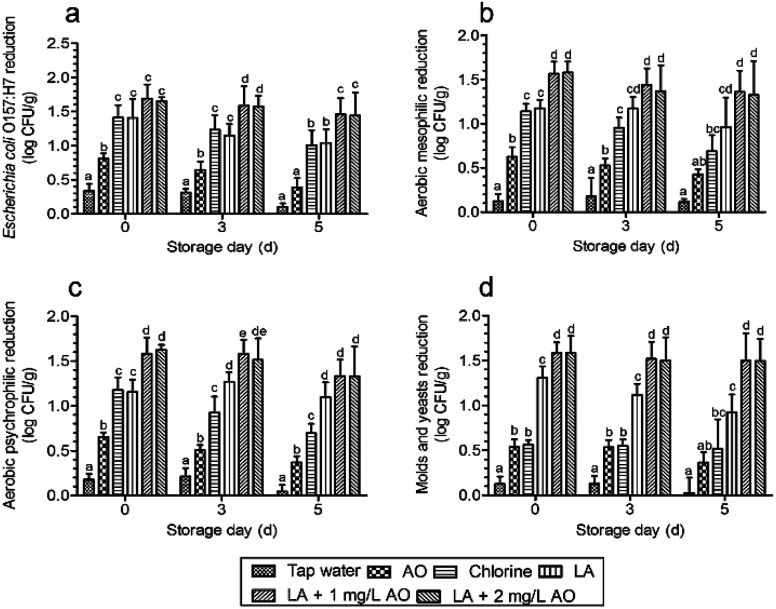
The results showed that the microbial counts of E. coli O157:H7 in the control group were 6.54 ± 0.20 log CFU g−1 on day 0, 7.05 ± 0.26 log CFU g−1 on day 3, and 7.41 ± 0.19 log CFU g−1 on day 5. AMCs were 5.77 ± 0.27 log CFU g−1 on day 0, 6.48 ± 0.27 log CFU g−1 on day 3, and 7.13 ± 0.30 log CFU g−1 on day 5. APCs were 5.73 ± 0.20 log CFU g−1 on day 0, 6.44 ± 0.24 log CFU g−1 on day 3, and 7.13 ± 0.32 log CFU g−1 on day 5. M&Y were 4.86 ± 0.17 log CFU g−1 on day 0, 5.81 ± 0.15 log CFU g−1 on day 3, and 6.57 ± 0.14 log CFU g−1 on day 5. After disinfection with various treatments, the counts of E. coli O157:H7 were all significantly reduced compared with those of tap water (Fig. 3a). Among these treatments, independent AO disinfection led to the lowest E. coli O157:H7 counts, with a 0.8 log reduction, which was significantly lower than that of the other treatments. These results were similar to those of the study by Neal et al.15 The authors found that LA treatment caused 2.7 and 2.3 log reductions in E. coli O157:H7 and Salmonella counts, respectively, whereas AO only reduced the counts by 0.6–1.1 and 0.9–1.0 log, respectively. The E. coli O157:H7 reduction by LA was consistent with that of chlorine, similar to the results described by Akbas and Olmez.21
Fig. 3. Effects of various treatments on Escherichia coli O157:H7 (a) and naturally present microbes (b–d) during storage (0–5 days at 5 °C). The columns show means ± standard deviations, and the different letters above the columns indicate significant differences (P < 0.05) among the presented timepoint groups. Microbial reduction indicates the difference in microbial counts between the control and treatment groups at the same time point. LA, lactic acid; AO, aqueous ozone.
The largest reduction was caused by 90 s LA plus 30 s AO, with a log reduction of 1.65–1.69, which was not significantly higher than that of the independent 120 s LA disinfection plus 30 s tap water, inconsistent with the observations described in Table 1. However, the trends (Fig. 3) were similar to those shown in Table 1. According to previous reports, after washing with organic acid sanitizer, LA and AA are more effective than chlorine in reducing AMCs and E. coli O157:H7 counts on fresh-cut lettuce and result in effective control of microbial growth during storage.38 In contrast, Samara and Koutsoumanis19 found that acid disinfection stimulates the growth of L. monocytogenes during storage. In this work, we found that E. coli O157:H7 reduction by LA plus AO was significantly greater than that of LA, chlorine, and AO disinfection alone during storage (Fig. 3a).
For naturally present microbes, we found that AO plus LA led to the largest AMC reduction after washing, which was significantly larger than that of the other treatments (Fig. 3b). During subsequent storage, LA plus AO also led to the largest AMC reduction, which was significantly larger than those of AO and chlorine alone. APC reductions after washing and during storage were similar to AMC reduction (Fig. 3c). For M&Y, the count reduction induced by chlorine was significantly smaller than that induced by LA (Fig. 3d), consistent with the results described by Allende et al.33 Compared with other disinfection treatments, LA plus AO significantly reduced M&Y counts after disinfection and during storage.
Overall, the largest microbial reduction was achieved after LA plus AO treatment (Fig. 3a–d), which may result from the different disinfection mechanisms of LA and AO. As an oxidizing agent, ozone kills bacteria by reacting with components of the cell envelope, spore coat, or viral capsid.42 The antibacterial activity of organic acids is traditionally attributed to cellular anion accumulation, which is determined by the proportion of undissociated molecules.43 Compared with dissociated anions, undissociated acidic molecules have greater lipophilicity, allowing them to more easily penetrate the microbial cell membrane.43 After penetration, the higher intracellular pH promotes the dissociation of acidic molecules, and the anions accumulate in the cell and exert toxic effects on the cell membrane, acid-sensitive proteins, DNA, and RNA.20,43 Thus, protein denaturation, DNA replication suppression, and membrane disruption are common antibacterial mechanisms.20,43 In this study, we speculate that the mechanism of action of the sequential disinfection (LA plus AO) was as follows: lactate anions act on DNA, proteins, and membranes after penetrating bacterial cells, and after AO washing, the cell membrane is oxidized to accelerate membrane disruption. In contrast, sequential washing with the combined use of sanitizers of the same type (i.e., one with a similar antimicrobial mechanism of action) will not significantly reduce microbial counts compared with samples subjected to independent treatments. For example, application of acidic electrolyzed water alone or followed by washing with AO reduces AMC on cilantro by 0.66 and 0.62 log, respectively,44 and AMCs after treatment with both ozone and chlorine show a similar log reduction to that of samples treated with chlorine only.45
Interestingly, we found that the microbial counts were not additively reduced by increasing the AO concentration from 1 to 2 mg L−1 (Fig. 3a–d and Table 1). For AO, additional microbial reductions were achieved by dramatically increasing the concentration, such as from 3 to 10 ppm, causing a significant decrease in the AMC.46 Moreover, 4 mg L−1 AO yielded a 1.7 log reduction in the AMC,47 which is comparable to the decrease observed with 2 mg L−1 AO (1.5 log).48 Different results were also obtained for pathogen disinfection. For example, L. monocytogenes counts were reduced by 5 log after a 5 min exposure to 5 ppm AO,49 although other investigators reported a reduction of only 0.94 log under the same conditions.26 For practical applications, it is important to maintain ozone concentrations as low as possible to protect the health of workers and reduce corrosion.36,50 In addition, when preparing large-scale AO for a processing line, it is difficult to obtain concentrations exceeding 3 mg L−1.
Acid removal ability of AO and water quality improvement potential of LA residue
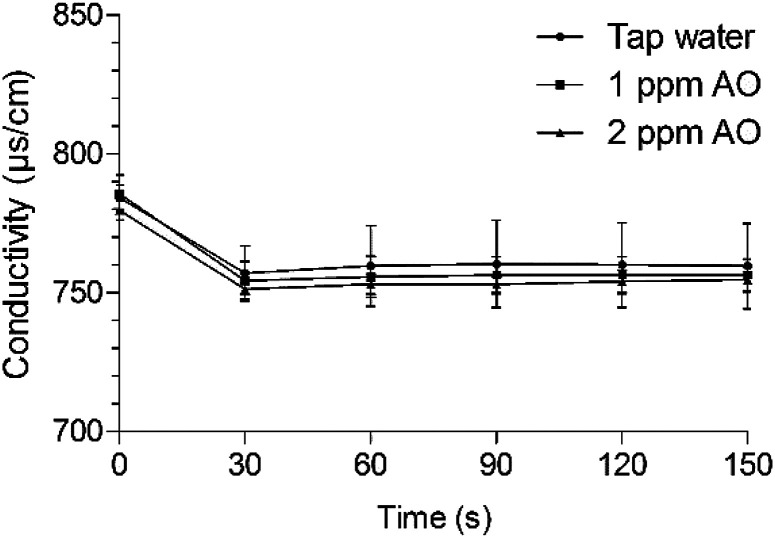
Conductivity is an indicator of the concentration of minerals and ionic compounds dissolved in solution. We investigated whether AO can be used instead to remove residual acid. After immersion in LA for 90 s, lettuce samples were washed with tap water or 1 or 2 ppm AO, which yielded conductivity values of 784.33, 785.67, and 779.67 μs cm−1, respectively (Fig. 4). Organic acids react with minerals and elements dissolved in water because of their acidity and complexing ability.51 The conductivity of drinking water is 50–1500 μs cm−1 and should be maintained as low as possible in water used to wash fresh produce. After immersion in tap water and 1 and 2 mg L−1 AO for 30 s, the conductivity decreased by 27.33, 28, and 28.33 μs cm−1, respectively. Thus, tap water and 1 and 2 mg L−1 AO did not differ in acid-removal ability, and the acid residue was easily removed within 30 s.
Fig. 4. Changes in conductivity after immersing the sample washed with 1% LA for 90 s. Values are expressed as means ± standard deviations. AO, aqueous ozone.
Moreover, crosscontamination during washing is a major concern for researchers in the field of minimal processing. The technology using ozone to improve water quality has advanced. Thus, the preparation of AO in this proposed hurdle technology was important not only for sequential disinfection but also to improve the water quality during AO preparation, consequently reducing crosscontamination risks and water consumption. However, the AO concentration was greatly affected by water chemical oxygen demand (COD), which increased as the washing time was prolonged. Thus, in a subsequent work, we will determine the relationships between fresh wash water COD and AO concentrations and design a corresponding appropriate processing line.
Conclusions
Hurdle technology is attracting increasing interest because it can provide additional microbial reductions compared with traditional independent disinfection strategies. In this study, we designed a hurdle technology using LA disinfection followed by AO disinfection (LA plus AO) and compared its disinfection efficacy and resulting lettuce quality with those of traditional disinfection strategies (LA or chlorine disinfection plus tap water washing and independent AO disinfection). The results indicated that this hurdle technology led to the largest reductions in AMCs, APCs, M&Y, and E. coli O157:H7 counts during storage. Moreover, conductivity analysis showed that the acid removal ability of this hurdle technology was consistent with those of the other disinfection methods. Quality analysis indicated that LA plus AO disinfection did not increase phenolic contents or colour loss compared with other traditional disinfection methods. Interestingly, we also found that the disinfection efficacy of chlorine was similar to that of LA when disinfecting bacteria, whereas the disinfection of fungi by chlorine was significantly less efficient than by LA. Taken together, these results provide a reference for the application of LA plus AO disinfection to control microbial contamination on fresh produce.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
References
- Putnik P. Kovačević D. B. Herceg K. Roohinejad S. Greiner R. Bekhit E. D. A. Levaj B. Food Control. 2017;81:55–64. doi: 10.1016/j.foodcont.2017.05.026. [DOI] [Google Scholar]
- Olaimat A. N. Holley R. A. Food Microbiol. 2012;32:1–19. doi: 10.1016/j.fm.2012.04.016. [DOI] [PubMed] [Google Scholar]
- Scallan E. Hoekstra R. M. Angulo F. J. Tauxe R. V. Widdowson M.-A. Roy S. L. Jones J. L. Griffin P. M. Emerging Infect. Dis. 2011;17:7. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callejón R. M. Rodríguez-Naranjo M. I. Ubeda C. Hornedo-Ortega R. Garcia-Parrilla M. C. Troncoso A. M. Foodborne Pathog. Dis. 2015;12:32–38. doi: 10.1089/fpd.2014.1821. [DOI] [PubMed] [Google Scholar]
- De Oliveira M. A. De Souza V. M. Bergamini A. M. M. De Martinis E. C. P. Food Control. 2011;22:1400–1403. doi: 10.1016/j.foodcont.2011.02.020. [DOI] [Google Scholar]
- Ssemanda J. N. Reij M. W. van Middendorp G. Bouw E. van der Plaats R. Franz E. Muvunyi C. M. Bagabe M. C. Zwietering M. H. Joosten H. Food Control. 2018;89:86–96. doi: 10.1016/j.foodcont.2017.12.034. [DOI] [Google Scholar]
- Ma L. Zhang M. Bhandari B. Gao Z. Trends Food Sci. Technol. 2017;64:23–38. doi: 10.1016/j.tifs.2017.03.005. [DOI] [Google Scholar]
- Gombas D. Luo Y. Brennan J. Shergill G. Petran R. Walsh R. Hau H. Khurana K. Zomorodi B. Rosen J. Varley R. Deng K. J. Food Prot. 2017;80:312–330. doi: 10.4315/0362-028X.JFP-16-258. [DOI] [PubMed] [Google Scholar]
- Meireles A. Giaouris E. Simões M. Food Res. Int. 2016;82:71–85. doi: 10.1016/j.foodres.2016.01.021. [DOI] [Google Scholar]
- Ölmez H. Kretzschmar U. LWT--Food Sci. Technol. 2009;42:686–693. doi: 10.1016/j.lwt.2008.08.001. [DOI] [Google Scholar]
- Goodburn C. Wallace C. A. Food Control. 2013;32:418–427. doi: 10.1016/j.foodcont.2012.12.012. [DOI] [Google Scholar]
- De Corato U. Crit. Rev. Food Sci. Nutr. 2019:1–36. doi: 10.1080/10408398.2018.1553025. [DOI] [PubMed] [Google Scholar]
- Bull R. J. Reckhow D. A. Li X. Humpage A. R. Joll C. Hrudey S. E. Toxicology. 2011;286:1–19. doi: 10.1016/j.tox.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Chen L. Zhang H. Liu Q. Pang X. Zhao X. Yang H. Int. J. Food Microbiol. 2019;295:41–48. doi: 10.1016/j.ijfoodmicro.2019.02.014. [DOI] [PubMed] [Google Scholar]
- Neal J. A. Marquez-Gonzalez M. Cabrera-Diaz E. Lucia L. M. O'Bryan C. A. Crandall P. G. Ricke S. C. Castillo A. Food Res. Int. 2012;45:1123–1128. doi: 10.1016/j.foodres.2011.04.011. [DOI] [Google Scholar]
- Martínez-Sánchez A. Allende A. Bennett R. N. Ferreres F. Gil M. I. Postharvest Biol. Technol. 2006;42:86–97. doi: 10.1016/j.postharvbio.2006.05.010. [DOI] [Google Scholar]
- Finten G. Agüero M. V. Jagus R. J. LWT--Food Sci. Technol. 2017;82:318–325. doi: 10.1016/j.lwt.2017.04.047. [DOI] [Google Scholar]
- Huang Y. Chen H. Food Control. 2011;22:1178–1183. doi: 10.1016/j.foodcont.2011.01.012. [DOI] [Google Scholar]
- Samara A. Koutsoumanis K. P. Int. J. Food Microbiol. 2009;129:1–7. doi: 10.1016/j.ijfoodmicro.2008.10.023. [DOI] [PubMed] [Google Scholar]
- Wang J. Tao D. Wang S. Li C. Li Y. Zheng F. Wu Z. RSC Adv. 2019;9:17514–17520. doi: 10.1039/C9RA03290H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbas M. Y. Ölmez H. Lett. Appl. Microbiol. 2007;44:619–624. doi: 10.1111/j.1472-765X.2007.02127.x. [DOI] [PubMed] [Google Scholar]
- Van Haute S. Uyttendaele M. Sampers I. Int. J. Food Microbiol. 2013;167:161–169. doi: 10.1016/j.ijfoodmicro.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Gil M. I. Selma M. V. Lopez-Galvez F. Allende A. Int. J. Food Microbiol. 2009;134:37–45. doi: 10.1016/j.ijfoodmicro.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Van Haute S. Tryland I. Escudero C. Vanneste M. Sampers I. LWT--Food Sci. Technol. 2017;75:301–304. doi: 10.1016/j.lwt.2016.09.002. [DOI] [Google Scholar]
- Van Haute S. Tryland I. Veys A. Sampers I. Food Control. 2015;50:173–183. doi: 10.1016/j.foodcont.2014.08.028. [DOI] [Google Scholar]
- Yuk H. G. Yoo M. Y. Yoon J. W. Moon K. D. Marshall D. L. Oh D. H. J. Food Sci. 2006;71:83–87. doi: 10.1111/j.1365-2621.2006.tb15636.x. [DOI] [Google Scholar]
- Yuk H.-G. Yoo M.-Y. Yoon J.-W. Marshall D. L. Oh D.-H. Food Control. 2007;18:548–553. doi: 10.1016/j.foodcont.2006.01.004. [DOI] [Google Scholar]
- Dhillon B. Wiesenborn D. Wolf-Hall C. Manthey F. J. Food Sci. 2009;74:E396–E403. doi: 10.1111/j.1750-3841.2009.01275.x. [DOI] [PubMed] [Google Scholar]
- Singla R. Ganguli A. Ghosh M. Food Control. 2011;22:1032–1039. doi: 10.1016/j.foodcont.2010.12.012. [DOI] [Google Scholar]
- Wang C. Chang T. Yang H. Cui M. Food Control. 2015;47:231–236. doi: 10.1016/j.foodcont.2014.06.034. [DOI] [Google Scholar]
- Bader H. Ozone: Sci. Eng. 2008;4:169–176. doi: 10.1080/01919518208550955. [DOI] [Google Scholar]
- Zhang J. Yang H. Food Control. 2017;72:20–26. doi: 10.1016/j.foodcont.2016.07.030. [DOI] [Google Scholar]
- Allende A. Selma M. V. López-Gálvez F. Villaescusa R. Gil M. I. Postharvest Biol. Technol. 2008;49:155–163. doi: 10.1016/j.postharvbio.2007.12.010. [DOI] [Google Scholar]
- Gómez-López V. M. Marín A. Medina-Martínez M. S. Gil M. I. Allende A. Postharvest Biol. Technol. 2013;85:210–217. doi: 10.1016/j.postharvbio.2013.05.012. [DOI] [Google Scholar]
- Singleton V. L. Orthofer R. Lamuela-Raventós R. M. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- Glowacz M. Rees D. J. Sci. Food Agric. 2016;96:4637–4643. doi: 10.1002/jsfa.7763. [DOI] [PubMed] [Google Scholar]
- Bermúdez-Aguirre D. Barbosa-Cánovas G. V. Food Control. 2013;29:82–90. doi: 10.1016/j.foodcont.2012.05.073. [DOI] [Google Scholar]
- Poimenidou S. V. Bikouli V. C. Gardeli C. Mitsi C. Tarantilis P. A. Nychas G. J. Skandamis P. N. Int. J. Food Microbiol. 2016;220:6–18. doi: 10.1016/j.ijfoodmicro.2015.12.013. [DOI] [PubMed] [Google Scholar]
- Wang H. Feng H. Luo Y. Food Res. Int. 2004;37:949–956. doi: 10.1016/j.foodres.2004.06.004. [DOI] [Google Scholar]
- Salgado S. P. Pearlstein A. J. Luo Y. Feng H. LWT--Food Sci. Technol. 2014;56:261–268. doi: 10.1016/j.lwt.2013.11.038. [DOI] [Google Scholar]
- Fan X. Sokorai K. J. Niemira B. A. Mills R. S. Zhen M. Y. HortScience. 2012;47:1108–1112. [Google Scholar]
- Alexopoulos A. Plessas S. Ceciu S. Lazar V. Mantzourani I. Voidarou C. Stavropoulou E. Bezirtzoglou E. Food Control. 2013;30:491–496. doi: 10.1016/j.foodcont.2012.09.018. [DOI] [Google Scholar]
- Lianou A., Koutsoumanis K. P. and Sofos J. N., in Microbial Decontamination in the Food Industry, Woodhead Publishing Inc., Oxford, 2012, pp. 592–664 [Google Scholar]
- Singh N. Singh R. K. Bhunia A. K. Stroshine R. L. LWT--Food Sci. Technol. 2002;35:720–729. doi: 10.1006/fstl.2002.0933. [DOI] [Google Scholar]
- Garcia A. Mount J. R. Davidson P. M. J. Food Sci. 2003;68:2747–2751. doi: 10.1111/j.1365-2621.2003.tb05799.x. [DOI] [Google Scholar]
- Koseki S. Isobe S. J. Food Prot. 2006;69:154–160. doi: 10.4315/0362-028X-69.1.154. [DOI] [PubMed] [Google Scholar]
- Akbas M. Y. Ölmez H. J. Sci. Food Agric. 2007;87:2609–2616. doi: 10.1002/jsfa.3016. [DOI] [PubMed] [Google Scholar]
- Ölmez H. Akbas M. Y. J. Food Eng. 2009;90:487–494. doi: 10.1016/j.jfoodeng.2008.07.026. [DOI] [Google Scholar]
- Rodgers S. L. Cash J. N. Siddiq M. Ryser E. T. J. Food Prot. 2004;67:721–731. doi: 10.4315/0362-028X-67.4.721. [DOI] [PubMed] [Google Scholar]
- Tzortzakis N. Chrysargyris A. Food Rev. Int. 2016;33:270–315. doi: 10.1080/87559129.2016.1175015. [DOI] [Google Scholar]
- Karlsson S. Wolrath H. Dahlén J. Water Res. 1999;33:2569–2578. doi: 10.1016/S0043-1354(98)00485-0. [DOI] [Google Scholar]