Abstract
The in vitro and in vivo activities of four azole compounds belonging to a new series of 2(2,4-difluorophenyl)-3-(4-substituted piperazin-1-yl)-1-(1,2,4-triazol-1-yl) butanol antifungal agents is described. The compounds were selected from a library of azole compounds synthesized by our group. The in vitro activities of Syn2869, Syn2836, Syn2903, and Syn2921 against a panel of over 240 recently collected clinical isolates of yeast and molds were determined, and the results were compared with those obtained with fluconazole (FLC), itraconazole (ITC), and amphotericin B (AMB). The MICs at which 90% of the isolates were inhibited (MIC90s) for the four test compounds for strains of Candida spp. ranged from <0.048 to 0.78 μg/ml. All compounds were also active against FLC-resistant Candida albicans and other Candida sp. strains. Moreover, MIC90s for strains of Cryptococcus neoformans, Aspergillus spp., Trichophyton spp., and Microsporum spp. were also low and ranged from <0.048 to 0.39 μg/ml. The test compounds produced a fungistatic pattern during the time-kill kinetic studies. In vivo studies indicated that all four test compounds have good efficacies against C. albicans in a murine systemic infection model and significantly improved the survival rates of the infected mice. The results for Syn2903 were similar to those for FLC, while the other compounds were slightly less effective but had ranges of activities similar to the range of activity of ITC. The compounds were also evaluated against an Aspergillus fumigatus systemic infection. Syn2903 was also superior to ITC, whereas the efficacy data for the other compounds were similar to those for ITC. It was concluded from the data generated for this new series of azole compounds in the studies described above that further pharmacokinetic and toxicologic evaluations are warranted prior to selection of a candidate compound for preclinical testing.
The past two decades have seen an increase in the incidence of life-threatening fungal infections. Factors contributing to this increase are the growing numbers of patients on immunosuppressive therapy as a result of transplantation of major organs, the chemotherapeutic treatments administered to cancer patients, as well as the increased numbers of patients with AIDS (1). Disseminated candidiasis caused by Candida albicans comprises the most commonly detected infection (2). However, non-C. albicans candidiasis, pulmonary aspergillosis, and other mold infections, which are occurring at increasing frequencies, also pose serious threats to these patients (4, 7, 8, 11, 12).
Amphotericin B (AMB) is considered the “gold standard” for treatment of these infections. AMB is, however, associated with a number of severe and sometimes life-threatening side effects including fever, chills, and nephrotoxicity. Recently, various new formulations of AMB have been introduced into clinical use. The ability of these formulations to decrease these symptoms at the therapeutic doses used is still under investigation. Furthermore, the cost of treatment with such formulations is a limiting factor (13). Other treatment regimens include azole antifungal drugs, of which fluconazole (FLC) and itraconazole (ITC) are the most widely used (3). The emergence of resistance to FLC (10) and the lack of efficacy of FLC and the limited efficacy of ITC against pulmonary aspergillosis have highlighted the need for a new broad-spectrum antifungal agent.
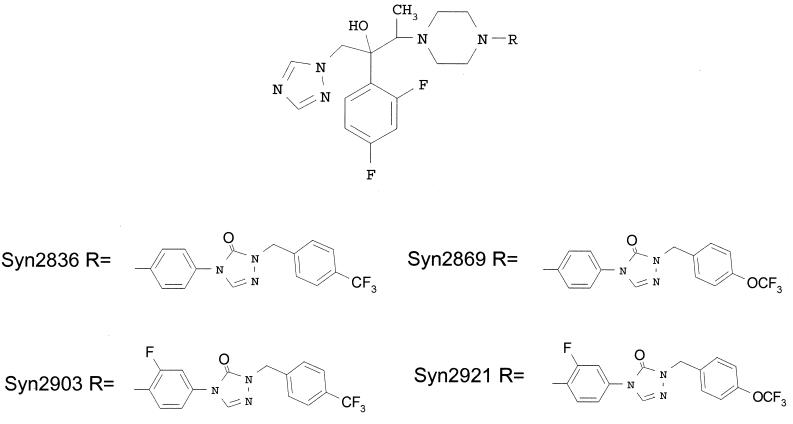
Syn2836, Syn2869, Syn2903, and Syn2921 belong to novel series of 2(2,4-difluorophenyl)-3-(4-substituted piperazin-1-yl)-1-(1,2,4-triazol-1-yl) butanols (M. D. Able, Y. Bathini, C. Ha, T. Furukawa, G. Kasitu, J. Khan, R. G. Micetich, D. Q. Nguyen, S. M. Salama, G. Samari, I. Sidhu, P. Spevak, N. Unemi, and M. Daneshtalab, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., p. 270, 1998) (Fig. 1). These compounds were discovered as a result of our search for a potent, broad-spectrum antifungal agent. The data presented here describe the results of evaluations of the in vitro and in vivo efficacies of these four antifungal compounds.
FIG. 1.
Chemical structures of Syn2836, Syn2869, Syn2903, and Syn2921.
MATERIALS AND METHODS
Antifungal agents.
Syn2836, Syn2869, Syn2903, and Syn2921 (SynPhar Laboratories, Inc., Edmonton, Alberta, Canada), FLC (Pfizer, Sandwich, England), ITC (Janssen, Beerse, Belgium), and AMB (Bristol Meyers Squib, Princeton, N.J.) were stored in a desiccator at 4°C until use. Stock solutions for in vitro testing were prepared by first dissolving the powder in dimethyl sulfoxide (Sigma, St. Louis, Mo.) and then diluting the solution to a working solution of 0.4 mg/ml in deionized water. Solutions for in vivo studies were prepared by suspending Syn2836, Syn2869, Syn2903, and Syn2921 in 0.5% hydroxymethylcellulose solution, FLC in saline, and ITC in 0.05 N HCl containing 20% hydroxypropyl-β-cyclodextrin (Nihon Shokuhin Kako Co. Ltd., Tokyo, Japan) and 2.5% polyethylene glycol 200 (Wako Pure Chemical Industries, Osaka, Japan).
Fungal strains.
The clinical isolates of C. albicans, Candida tropicalis, Candida kefyr, Candida guilliermondii, Candida krusei, Candida glabrata, Cryptococcus neoformans, Aspergillus fumigatus, Aspergillus flavus, Aspergillus niger, Trichophyton rubrum, Trichophyton mentagrophytes, and Microsporum canis which were used during the in vitro susceptibility study were obtained from the National Mycology Reference Laboratory, University of Alberta Hospital, Edmonton, Alberta, Canada. These strains were collected from clinical mycology laboratories throughout Canada between 1996 and 1997. Upon arrival, all strains were harvested into RPMI 1640 medium supplemented with 20% glycerol and were stored at −80°C until needed. Strain C. albicans Y01-09 is a clinical isolate donated by Taiho Pharmaceutical Co., Tokyo, Japan. Strain A. fumigatus TIMM1776 was obtained from the Teikyo University Institute for Medical Mycology, Tokyo, Japan. The MICs for the latter two strains are listed in Table 2.
TABLE 2.
Comparative in vitro activities of Syn2836, Syn2869, Syn2903, and Syn2921 and other antifungal agents against C. albicans Y01–09 and A. fumigatus TIMM1776
| Antifungal agent | MIC (μg/ml)
|
|
|---|---|---|
| C. albicans Y01–09 | A. fumigatus TIMM1776 | |
| Syn2836 | <0.048 | 0.19 |
| Syn2869 | <0.048 | 0.39 |
| Syn2903 | <0.048 | 0.19 |
| Syn2921 | <0.048 | 0.19 |
| ITC | 0.09 | 0.19 |
| FLC | 0.39 | >100 |
| AMB | 0.09 | 0.19 |
In vitro antifungal susceptibility testing.
The broth microdilution method was used to test the in vitro activities of the compounds. The test procedures applied were in accordance with the M27-A broth dilution method recommended by NCCLS (9). Briefly, twofold serial dilutions of the test compounds were prepared in 96-well plates by using RPMI 1640 broth buffered with morpholinepropanesulfonic acid (MOPS; pH 7.0). The plates were then inoculated with the fungal suspensions to give a final inoculum size of 0.5 × 103 CFU/ml. The plates were then incubated for 48 h at 35°C for all strains except C. neoformans, which required 72 h of incubation. The MIC was defined as the lowest concentration that resulted in an 80% reduction of growth compared with the growth on a drug-free control plate for the azoles and no visible growth compared with the growth on a drug-free control plate for AMB. The MICs at which 50% of strains are inhibited (MIC50s), MIC80s, and MIC90s were obtained.
Time-kill kinetics.
To determine the time-kill kinetic activities of the test compounds, the MIC of each compound was first determined, as described above. Test tubes containing 10-ml volumes of RPMI 1640 medium were supplemented with the test or reference compounds at concentrations equal to 2, 4, 8, and 16 times the MIC. Test tubes were also supplemented with chloramphenicol at 12 μg/ml to suppress bacterial contamination. C. albicans Y01-09 at a final inoculum size of 5 × 102 CFU/ml was added to the test tubes. Test tubes were incubated in a shaking incubator at 35°C. Growth control tubes did not contain the antifungal agents. Ten samples, each of 10 μl in volume, were obtained from each tube at times 0, 3, 5, 8, 16, 28, 33, and 50 h postinoculation and were inoculated onto plates of Sabouraud dextrose agar (SDA; Difco Laboratories, Detroit, Mich.) to determine the total number of viable cells (number of CFU per milliliter).
In vivo systemic candidiasis.
An inoculum equal to 2.9 × 106 CFU/mouse was prepared from an overnight culture of C. albicans Y01-09 grown on SDA plates. Four-week-old mice (ddY mice; weight, 18 to 22 g; SLC, Osaka, Japan) were challenged intravenously with the inoculum described above. At 1 h postinoculation, control vehicle or the test compounds were administered orally. Ten mice were used in each group, and the test doses ranged from 0.78 to 50 mg/kg of body weight. Survival of the mice was observed for 5 days after drug administration. The survival curves were plotted against the doses. Survival on day 5 was used to define the end points.
In vivo systemic aspergillosis.
An inoculum equal to 3.26 × 106 CFU/mouse was prepared from a 48-h culture of A. fumigatus TIMM1776 grown onto slants of SDA. Four-week-old ddY mice were challenged intravenously with the inoculum described above. Control vehicle or the test compounds were administered orally daily on days 0 through 5. Ten mice were used in each group, and the test doses ranged from 25 to 100 mg/kg/day. Survival of the mice was observed for 8 days after inoculation. The survival curves were plotted against the doses. Survival on day 8 was used to define end points.
RESULTS
In vitro antifungal susceptibility testing.
As shown in Table 1, Syn2836, Syn2869, Syn2903, and Syn2921 possess broad-spectrum activities against almost all clinical isolates tested including FLC-resistant C. albicans, C. guilliermondii, Aspergillus spp., and dermatophytes. Syn2921 did not inhibit the growth of one strain of C. glabrata. This strain was also resistant to FLC but not the other azoles used in the present study. The MIC90s of the rest of the compounds for Candida spp. ranged from <0.048 to 0.78 μg/ml. Similar results were obtained with ITC and AMB. FLC was the least active against these strains. Trailing-end results were recorded for all azoles tested against C. albicans and were more prominent against C. glabrata. The MIC90s of the test compounds for C. neoformans, Aspergillus spp., and dermatophytes were 0.19, 0.19 to 0.39, and <0.048 to 0.39 μg/ml, respectively.
TABLE 1.
Comparative in vitro activities of Syn2836, Syn2869, Syn2903, and Syn2921 and reference compounds against clinical isolates
| Organism (no. of strains) and antifungal agenta | MIC (μg/ml)
|
|||
|---|---|---|---|---|
| Range | 50% | 80% | 90% | |
| C. albicans Flus (51) | ||||
| Syn2836 | <0.048–0.39 | <0.048 | <0.048 | 0.09 |
| Syn2869 | <0.048–0.19 | <0.048 | <0.048 | 0.09 |
| Syn2903 | <0.048–0.19 | <0.048 | <0.048 | 0.09 |
| Syn2921 | <0.048–0.19 | <0.048 | <0.048 | 0.09 |
| ITC | <0.048–0.19 | <0.048 | <0.048 | 0.09 |
| FLC | <0.048–0.39 | 0.19 | 0.39 | 0.39 |
| AMB | <0.048–0.78 | 0.19 | 0.39 | 0.39 |
| C. albicans Flur (6) | ||||
| Syn2836 | <0.09–0.19 | <0.09 | <0.19 | 0.19 |
| Syn2869 | <0.09–0.19 | <0.09 | <0.09 | 0.19 |
| Syn2903 | <0.09–0.19 | <0.09 | <0.19 | 0.19 |
| Syn2921 | <0.09–0.19 | <0.09 | <0.19 | 0.19 |
| ITC | 0.09–0.19 | 0.09 | 0.19 | 0.19 |
| FLC | 6.25–100 | 12.5 | 25 | 100 |
| AMB | <0.048–0.78 | 0.19 | 0.39 | 0.39 |
| C. tropicalis (20) | ||||
| Syn2836 | <0.048–0.19 | <0.048 | <0.048 | 0.19 |
| Syn2869 | <0.048–0.19 | <0.048 | <0.048 | 0.19 |
| Syn2903 | <0.048–0.19 | <0.048 | <0.048 | 0.19 |
| Syn2921 | <0.048–0.19 | <0.048 | <0.048 | 0.19 |
| ITC | <0.048–0.19 | <0.048 | <0.048 | <0.048 |
| FLC | <0.048–6.25 | 0.19 | 0.39 | 3.12 |
| AMB | <0.048–0.39 | 0.19 | 0.19 | 0.19 |
| C. kefyr (5) | ||||
| Syn2836 | <0.048–0.19 | <0.048 | 0.09 | 0.19 |
| Syn2869 | <0.048–0.19 | <0.048 | 0.09 | 0.19 |
| Syn2903 | <0.048–0.19 | <0.048 | 0.09 | 0.19 |
| Syn2921 | <0.048–0.19 | <0.048 | 0.09 | 0.19 |
| ITC | <0.048–0.09 | <0.048 | 0.09 | 0.09 |
| FLC | 0.09–1.56 | 0.39 | 0.78 | 1.56 |
| AMB | 0.09–0.39 | 0.19 | 0.19 | 0.39 |
| C. guilliermondii (6) | ||||
| Syn2836 | 0.09–0.39 | 0.19 | 0.19 | 0.39 |
| Syn2869 | 0.09–0.39 | 0.19 | 0.19 | 0.39 |
| Syn2903 | 0.09–0.78 | 0.19 | 0.19 | 0.78 |
| Syn2921 | 0.09–0.78 | 0.19 | 0.19 | 0.78 |
| ITC | 0.09–1.56 | 0.19 | 0.19 | 1.56 |
| FLC | 3.12–>100 | 6.25 | 25 | >100 |
| AMB | <0.048–0.19 | 0.19 | 0.19 | 0.19 |
| C. krusei (20) | ||||
| Syn2836 | 0.09–0.19 | 0.19 | 0.19 | 0.19 |
| Syn2869 | 0.09–0.19 | 0.09 | 0.19 | 0.19 |
| Syn2903 | 0.09–0.19 | 0.09 | 0.19 | 0.19 |
| Syn2921 | 0.09–0.19 | 0.09 | 0.19 | 0.19 |
| ITC | 0.09–0.19 | 0.09 | 0.19 | 0.19 |
| FLC | 1.56–100 | 50 | 50 | 50 |
| AMB | 0.09–0.39 | 0.19 | 0.19 | 0.39 |
| C. glabrata (20) | ||||
| Syn2836 | <0.048–>100 | 0.39 | 0.39 | 0.78 |
| Syn2869 | <0.048–>100 | 0.39 | 0.39 | 0.78 |
| Syn2903 | <0.048–>100 | 0.39 | 0.39 | 0.78 |
| Syn2921 | 0.09–>100 | 0.39 | 1.56 | >100 |
| ITC | <0.048–>100 | 0.19 | 0.39 | 0.78 |
| FLC | 1.56–>100 | 6.25 | 25 | 25 |
| AMB | 0.09–0.19 | 0.19 | 0.19 | 0.19 |
| C. neoformans (8) | ||||
| Syn2836 | <0.048–0.19 | <0.048 | <0.048 | 0.19 |
| Syn2869 | <0.048–0.19 | <0.048 | <0.048 | 0.19 |
| Syn2903 | <0.048–0.19 | <0.048 | <0.048 | 0.19 |
| Syn2921 | <0.048–0.19 | <0.048 | <0.048 | 0.19 |
| ITC | <0.048–1.56 | <0.048 | <0.048 | 0.19 |
| FLC | 1.56–6.25 | 3.12 | 3.12 | 6.25 |
| AMB | 0.09–0.19 | 0.09 | 0.19 | 0.19 |
| A. fumigatus (54) | ||||
| Syn2836 | <0.048–0.19 | 0.09 | 0.19 | 0.19 |
| Syn2869 | <0.048–0.19 | 0.09 | 0.09 | 0.19 |
| Syn2903 | <0.048–0.19 | 0.09 | 0.09 | 0.09 |
| Syn2921 | <0.048–0.19 | 0.09 | 0.09 | 0.09 |
| ITC | <0.048–0.19 | 0.09 | 0.09 | 0.09 |
| FLC | >100 | >100 | >100 | >100 |
| AMB | <0.048–0.78 | 0.09 | 0.19 | 0.19 |
| A. flavus (8) | ||||
| Syn2836 | <0.048–0.39 | 0.09 | 0.09 | 0.39 |
| Syn2869 | <0.048–0.39 | 0.09 | 0.09 | 0.39 |
| Syn2903 | <0.048–0.39 | <0.048 | 0.09 | 0.19 |
| Syn2921 | <0.048–0.39 | 0.09 | 0.09 | 0.19 |
| ITC | <0.048–0.39 | <0.048 | <0.048 | 0.19 |
| FLC | 50–>100 | 100 | 100 | >100 |
| AMB | 0.39–3.12 | 0.39 | 0.39 | 0.39 |
| A. niger (27) | ||||
| Syn2836 | <0.048–0.39 | 0.19 | 0.19 | 0.39 |
| Syn2869 | 0.09–0.39 | 0.19 | 0.19 | 0.19 |
| Syn2903 | 0.09–0.39 | 0.19 | 0.19 | 0.39 |
| Syn2921 | 0.09–0.39 | 0.19 | 0.19 | 0.39 |
| ITC | <0.048–0.39 | 0.19 | 0.19 | 0.19 |
| FLC | >100 | >100 | >100 | >100 |
| AMB | <0.048–0.19 | 0.09 | 0.09 | 0.09 |
| T. rubrum (5) | ||||
| Syn2836 | <0.048 | <0.048 | <0.048 | <0.048 |
| Syn2869 | <0.048 | <0.048 | <0.048 | <0.048 |
| Syn2903 | <0.048 | <0.048 | <0.048 | <0.048 |
| Syn2921 | <0.048–0.39 | <0.048 | <0.048 | 0.39 |
| ITC | <0.048 | <0.048 | <0.048 | <0.048 |
| FLC | 12.5–>100 | >100 | >100 | >100 |
| AMB | <0.048 | <0.048 | <0.048 | <0.048 |
| T. mentagrophytes (10) | ||||
| Syn2836 | <0.048–0.19 | <0.048 | 0.09 | 0.19 |
| Syn2869 | <0.048–0.78 | <0.048 | <0.048 | <0.048 |
| Syn2903 | <0.048–0.39 | <0.048 | <0.048 | 0.39 |
| Syn2921 | <0.048–0.19 | <0.048 | <0.048 | <0.048 |
| ITC | <0.048 | <0.048 | <0.048 | <0.048 |
| FLC | 3.12–>100 | 50 | >100 | >100 |
| AMB | <0.048–0.19 | <0.048 | 0.09 | 0.19 |
| M. canis (4) | ||||
| Syn2836 | <0.048 | <0.048 | <0.048 | <0.048 |
| Syn2869 | <0.048 | <0.048 | <0.048 | <0.048 |
| Syn2903 | <0.048 | <0.048 | <0.048 | <0.048 |
| Syn2921 | <0.048–0.09 | <0.048 | <0.048 | 0.09 |
| ITC | <0.048 | <0.048 | <0.048 | <0.048 |
| FLC | 50–>100 | 100 | >100 | >100 |
| AMB | <0.048 | <0.048 | <0.048 | <0.048 |
Flus and Flur, FLC susceptible and FLC resistant, respectively.
Time-kill kinetics.
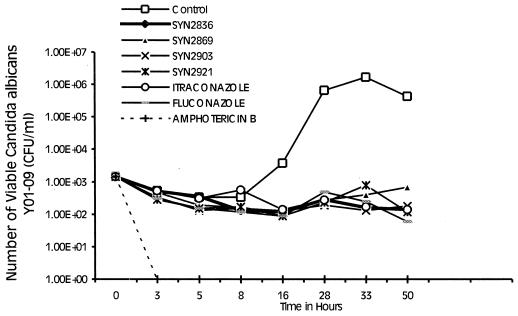
The time-kill kinetics of Syn2836, Syn2869, Syn2903, and Syn2921 were compared to those of FLC, ITC, and AMB. The MICs of the test and reference compounds for C. albicans Y01-09 are shown in Table 2. The time-kill kinetics were determined at 2, 4, 8, and 16 times the MIC of each compound. It was found that all azoles tested behaved in an identical manner; i.e., all compounds produced a stationary phase that was stable throughout the 50-h study. This occurred even at the highest concentration, 16 times the MIC. AMB, on the other hand, inhibited the growth of the organisms within 3 h of incubation. This remarkable activity of AMB occurred at the lowest concentration tested (two times the MIC) (Fig. 2).
FIG. 2.
Time-kill kinetics of Syn2836, Syn2869, Syn2903, and Syn2921 compared with those of FLC, ITC, and AMB against C. albicans Y01-09. Compounds were tested at 16 times the MIC. The results obtained at lower concentrations were similar to those obtained at 16 times the MIC.
Systemic candidiasis.
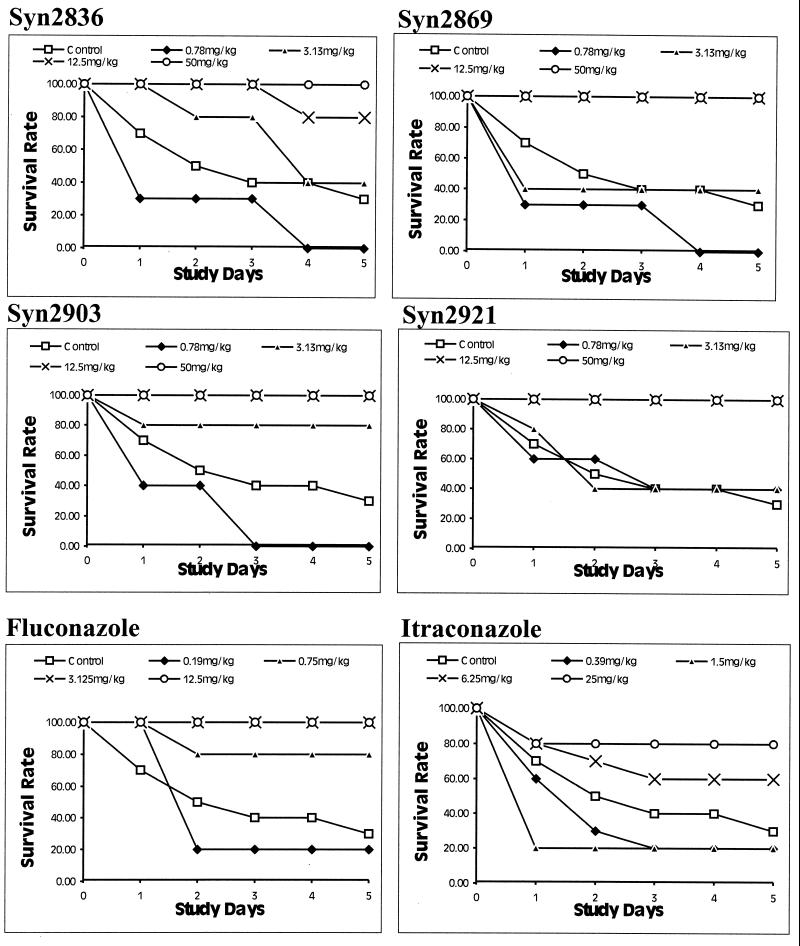
Results of tests of the in vivo efficacies of the four test compounds as well as those of FLC and ITC are shown in Fig. 3. At doses of 12.5 mg/kg or higher, survival rates of 90% or more were achieved with all compounds tested. Syn2903 at a dose of 3.13 mg/kg increased the survival rate to 80%. No efficacy was noted with Syn2836, Syn2869, Syn2921, Syn2903, or ITC at dosages less than 1 mg/kg. FLC, on the other hand, improved the survival rates for infected mice when it was used at a concentration of 0.75 mg/kg or more.
FIG. 3.
In vivo efficacies of Syn2836, Syn2869, Syn2903, and Syn2921 against C. albicans Y01-09 in a mouse model of systemic infection. The results are compared with those obtained with FLC and ITC. Test compounds were administered orally 1 to 3 h postinoculation as a single bolus dose. Experimental compounds were administered at doses ranging from 0.78 to 50 mg/kg, whereas reference compounds FLC and ITC were given at doses ranging from 0.19 to 12.5 and 0.39 to 25 mg/kg, respectively. Animals were observed for survival over a 5-day period.
Systemic aspergillosis.
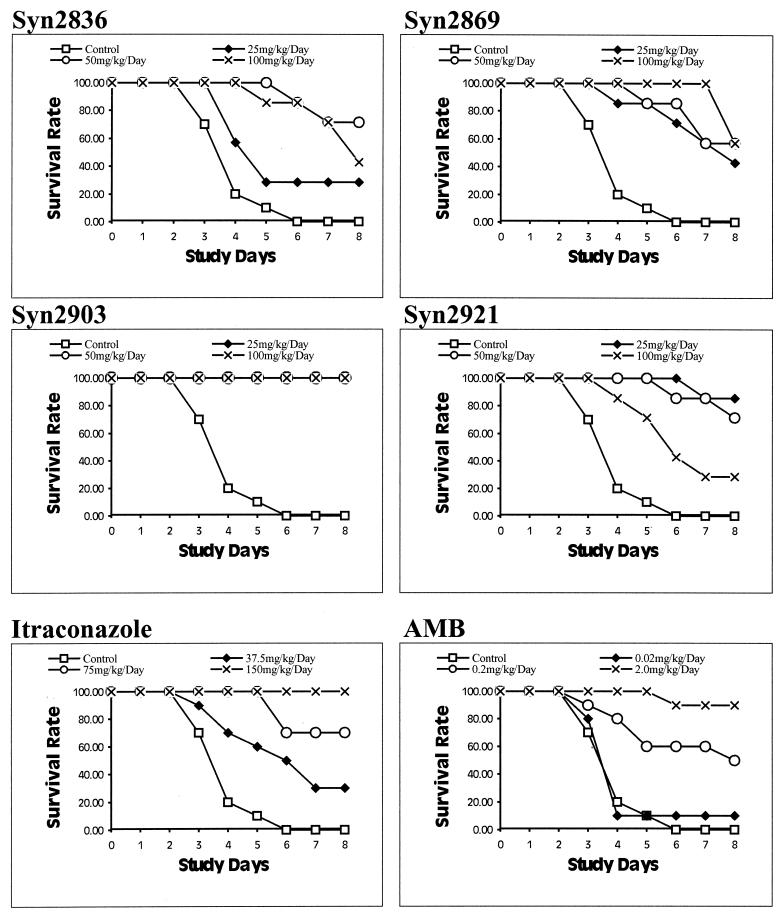
Results of studies of the in vivo efficacies of the four test compounds as well as those of ITC and AMB are shown in Fig. 4. None of the untreated control mice survived to the end of the test period. Syn2903 at all doses used (25, 50, and 100 mg/kg/day) improved the survival rates by 100%. Syn2836, Syn2869, and Syn2921 also significantly improved the survival rates for the test mice. However, the survival rate obtained with Syn2921 at 100 mg/kg was only 30%. ITC at 150, 75, and 37.5 mg/kg/day achieved 100, 70, and 30% improvements in survival rates, respectively. AMB was effective at 2 mg/kg/day. With AMB at 0.2 mg/kg/day the survival rate was only 50%, and with AMB at 0.02 mg/kg/day the survival rate was 10%.
FIG. 4.
In vivo efficacies of Syn2836, Syn2869, Syn2903, and Syn2921 against A. fumigatus TIMM1776 in a mouse model of systemic infection. The results are compared with those obtained with ITC and AMB. Test and reference compounds were administered as single oral daily doses for 5 days starting 3 h after intravenous inoculation. Experimental compounds were administered at doses ranging from 25 to 100 mg/kg/day, whereas ITC was given at doses ranging from 37.5 to 150 mg/kg/day and AMB was given at doses ranging from 0.02 to 2 mg/kg/day. Animals were observed for survival over an 8-day period.
DISCUSSION
A large library of azole compounds was synthesized by our group as part of an effort to identify a broad-spectrum antifungal compound with demonstrated activity, safety, and pharmacokinetic properties. The present report presents the data on the in vitro and in vivo activities of four lead compounds belonging to a new series of 2(2,4-difluorophenyl)-3-(4-substituted piperazin-1-yl)-1-(1,2,4-triazol-1-yl) butanols, namely, Syn2836, Syn2869, Syn2903, and Syn2921.
Data on the MICs of the four test compounds for C. albicans strains have shown that they possess potent in vitro activities against both FLC-susceptible and FLC-resistant strains. The MIC90s ranged from 0.09 to 0.19 μg/ml. The compounds were also active against non-C. albicans Candida sp. strains including C. tropicalis (MIC90, 0.19 μg/ml), C. kefyr (MIC90, 0.19 μg/ml), and the FLC-resistant organisms C. guilliermondii (MIC90, 0.78 μg/ml), C. krusei (MIC90, 0.19 μg/ml), and C. glabrata (MIC90, 0.78 μg/ml). Moreover, the compounds were active against strains of C. neoformans, Aspergillus spp., Trichophyton spp. and Microsporum spp. The in vitro activities were comparable to those of ITC and AMB but were far superior to the in vitro activity of FLC. Other investigators (A. W. Fothergill, S. M. Salama, and M. G. Rinaldi, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., p. 271, 1998; A. P. Gibb and H. Van Den Elzen, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., p. 270, 1998) also presented these findings previously. In addition, the compounds were shown to be active against less common molds including Absidia spp., Cladophialophora spp., Exophiala spp., Fonsecaea spp., Scedosporium spp., and Scopulariopsis spp. (E. M. Johnson, A. Sezekly, and D. W. Warnock, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., p. 271, 1998).
Studies of the time-kill kinetics of the test compounds against a strain of C. albicans revealed a static rather than a cidal nature, as is seen with AMB. This pattern was similar to that found with other ergosterol synthesis inhibitors such FLC and ITC.
We have shown previously that Syn2869 is active against systemic infections caused by C. glabrata and C. neoformans (T. Furukawa, H. Saito, T. Uji, K. Nishida, F. Higashitani, N. Unemi, and H. Yamaguchi, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., p. 270, 1998; S. M. Salama, H. Atwal, A. Gandhi, J. Khan, H. Montaseri, M. Poglod, R. G. Micetich, and M. Daneshtalab, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., p. 271, 1998). In vivo efficacy testing in mouse models of systemic candidiasis as well as systemic aspergillosis was also conducted in the present study. The data generated during the study of systemic candidiasis showed that at doses of 12.5 mg/kg or higher all compounds significantly increased the rates of survival of the infected animals (survival rate, >80%). Syn2903 was particularly active and provided protection at doses of 3.13 mg/kg. The latter result was similar to the results obtained with FLC but unlike those obtained with ITC, which provided protection at 25 mg/kg. FLC is a metabolically stable compound that remains mostly unchanged (70%) upon excretion (5). It is speculated that this stability combined with the high level of tissue exposure and lack of toxicity all play a major role in the excellent efficacy observed for FLC.
The in vivo efficacy in the mouse A. fumigatus systemic infection model has shown that Syn2903 provided the most protection of all the azoles tested. The activities of the other three test compounds were similar to the activity of ITC, and the activities of all compounds tested except Syn2921 were also dose dependent. Syn2921 is thought to be toxic at higher doses. In a separate study performed to determine the safety profiles of the test compounds, it was observed that administration of Syn2921 at doses of 100 mg/kg/day reduced the rates of survival of test mice by 40% by day 5 (data not shown). We are not certain about the exact mechanism of toxicity of this compound. This observed toxicity might have contributed to the lack of a dose-response during the A. fumigatus systemic infection study.
On the basis of the results of the in vitro and in vivo studies described here, it has been concluded that the compounds selected from this new series are active and warrant further pharmacokinetic and toxicologic evaluations prior to selection of a candidate compound for preclinical testing. These studies have been completed (6; J. K. Khan, H. Montaseri, M. Poglod, H.-Z. Bu, S. Salama, R. G. Micetich, and M. Daneshtalab, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., p. 271, 1998), and as a result of these studies and the in vivo efficacy data from studies with the lung aspergillosis model (Furukawa et al., 38th ICAAC), Syn2869 was declared a lead candidate for further development.
REFERENCES
- 1.Denning D W, Lee J Y, Hostetler J S, Pappas P, Kauffman C A, Dewsnup D H, Glagiani J N, Graybill J R, Sugar A M, Catanzaro A, Gallis H, Perfect J R, Dockery B, Dismukes W E, Stevens D A. NIAID Mycoses Study Group multicentre trial of oral itraconazole therapy for invasive aspergillosis. Am J Med. 1994;97:135–144. doi: 10.1016/0002-9343(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 2.Fisher-Hoch S P, Hutwanger L. Opportunistic candidiasis: an epidemic of the 1980's. Clin Infect Dis. 1995;21:897–904. doi: 10.1093/clinids/21.4.897. [DOI] [PubMed] [Google Scholar]
- 3.Graybill J R. New antifungal agents. Eur J Clin Microbiol Infect Dis. 1989;5:402–412. doi: 10.1007/BF01964056. [DOI] [PubMed] [Google Scholar]
- 4.Hofflin J M, Potasman I, Baldwin J C, Oyer P E, Stinson E B, Remignton J S. Infectious complications in heart transplant recipients receiving cyclosporine and corticosteroids. Ann Intern Med. 1987;106:209–216. doi: 10.7326/0003-4819-106-2-209. [DOI] [PubMed] [Google Scholar]
- 5.Humphrey M J, Jevons S, Tarbit M H. Pharmacokinetic evaluation of UK-49,858, a metabolically stable triazole antifungal drug, in animals and humans. Antimicrob Agents Chemother. 1985;28:648–653. doi: 10.1128/aac.28.5.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan J K, Montaseri H, Poglod M, Bu H-Z, Zuo Z, Salama S M, Daneshtalab M, Micetich R G. Interspecies comparison of pharmacokinetics of novel triazole antifungal agent Syn2869 and its derivatives. Antimicrob Agents Chemother. 2000;44:910–915. doi: 10.1128/aac.44.4.910-915.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linder J. Infection as complication of heart transplantation. J Heart Transplant. 1988;7:390–394. [PubMed] [Google Scholar]
- 8.Moran G P, Sullivan D J, Nenman M C, McCarthy C E, Harrington B J, Shanley D B, Coleman D C. Antifungal susceptibilities of oral Candida dubliniensis isolates from human immunodeficiency virus (HIV)-infected and non-HIV-infected subjects and generation of stable fluconazole-resistant derivatives in vitro. Antimicrob Agents Chemother. 1997;41:617–623. doi: 10.1128/aac.41.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing for yeasts. Proposed standard. Document M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 2000. [Google Scholar]
- 10.Nolte F S, Parkinson T, Falconer D J, Dix S, Williams J, Gilmore C, Geller R, Wingard J R. Isolation and characterization of fluconazole and amphotericin B-resistant Candida albicans from blood of two patients with leukemia. Antimicrob Agents Chemother. 1997;44:196–199. doi: 10.1128/aac.41.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parkinson T, Falconer D J, Hitchcock C A. Fluconazole resistance due to energy-dependent drug efflux in Candida glabrata. Antimicrob Agents Chemother. 1995;39:1696–1699. doi: 10.1128/aac.39.8.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfaller M, Wenzel R. Impact of the changing epidemiology of fungal infections in the 1990's. Eur J Clin Microbiol Infect Dis. 1992;11:287–291. doi: 10.1007/BF01962067. [DOI] [PubMed] [Google Scholar]
- 13.Tang C M, Bowler I C J W. Do the new lipid formulations of amphotericin B really work. Clin Microbiol Infect. 1997;3:283–288. [PubMed] [Google Scholar]