Abstract
The susceptibilities to telithromycin of 203 Streptococcus pneumoniae isolates prospectively collected during 1999 and 2000 from 14 different geographical areas in Spain were tested and compared with those to erythromycin A, clindamycin, quinupristin-dalfopristin, penicillin G, cefotaxime, and levofloxacin. Telithromycin was active against 98.9% of isolates (MICs, ≤0.5 μg/ml), with MICs at which 90% of isolates are inhibited being 0.06 μg/ml, irrespective of the resistance genotype. The corresponding values for erythromycin were 61.0% (MICs, ≤0.25 μg/ml) and >64 μg/ml. The erm(B) gene (macrolide-lincosamide-streptogramin B resistance phenotype) was detected in 36.4% (n = 74) of the isolates, which corresponded to 93.6% of erythromycin-intermediate and -resistant isolates, whereas the mef(A) gene (M phenotype [resistance to erythromycin and susceptibility to clindamycin and spiramycin without blunting]) was present in only 2.4% (n = 5) of the isolates. One of the latter isolates also carried erm(B). Interestingly, in one isolate for which the erythromycin MIC was 2 μg/ml, none of these resistance genes could be detected. Erythromycin MICs for S. pneumoniae erm(B)-positive isolates were higher (range, 0.5 to >64 μg/ml) than those for erm(B)- and mef(A)-negative isolates (range, 0.008 to 2 μg/ml). The corresponding values for telithromycin were lower for both groups, with ranges of 0.004 to 1 and 0.002 to 0.06 μg/ml, respectively. The erythromycin MIC was high for a large number of erm(B)-positive isolates, but the telithromycin MIC was low for these isolates. These results indicate the potential usefulness of telithromycin for the treatment of infections caused by erythromycin-susceptible and -resistant S. pneumoniae isolates when macrolides are indicated.
Macrolide resistance among Streptococcus pneumoniae isolates has risen to prominence during the last decade (1, 11, 12). This situation is of particular concern as, in most cases, it is coupled with resistance to other first-line antibiotics (11). According to recent surveys, almost 40% of pneumococci in Spain are penicillin resistant. This resistance is frequently associated with resistance to macrolides, tetracyclines, and chloramphenicol (1, 12). Ketolides, a novel class of antibiotics (5), appear to be an alternative to macrolides for the treatment of pneumococcal infections in which multidrug-resistant strains are involved. A high intrinsic in vitro activity coupled with a favorable pharmacokinetic profile and the lack of inducibility properties make these compounds promising alternatives for the treatment of infections caused by respiratory pathogens (3, 4, 9).
In the present study, the in vitro activity of telithromycin was evaluated against clinical isolates of S. pneumoniae prospectively collected in different geographical areas of Spain and was compared with those of erythromycin A, clindamycin, penicillin G, quinupristin-dalfopristin, cefotaxime, and levofloxacin. The resistance mechanisms involved in erythromycin-intermediate and -resistant strains were phenotypically and genotypically characterized. The lack of inductive activity of telithromycin was also assessed.
(This work was presented in part at the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Ontario, Canada, 2000.)
MATERIALS AND METHODS
Antibiotics.
The following antibiotics were supplied as powders of known potency by the indicated manufacturers: telithromycin (HMR 3647), erythromycin A, penicillin G, cefotaxime, and levofloxacin, Aventis Pharma, Romainville, France; clindamycin, The Upjohn Co., Kalamazoo, Mich.; and quinupristin-dalfopristin, Rhône Poulenc Rorer, Paris, France. Telithromycin (15 μg), erythromycin (15 μg), clindamycin (2 μg), spiramycin (100 μg), chloramphenicol (30 μg), and tetracycline (30 μg) disks were purchased from Oxoid Ltd. (Basingstoke, United Kingdom).
Bacterial strains.
A total of 203 clinical isolates of S. pneumoniae prospectively collected during 1999 and 2000 from 14 Spanish hospitals representing 14 different geographical areas were studied. Isolates obtained from clinical samples were distributed as follows: sputum (n = 69) and other samples from the lower respiratory tract (n = 48), blood (n = 43), eye (n = 16), nasopharynx (n = 12), middle ear (n = 7), catheter (n = 5), and organic fluids (n = 3).
Susceptibility testing.
The MICs for the S. pneumoniae isolates were determined by an adaptation of the standard agar dilution test recommended for other organisms by the National Committee for Clinical Laboratory Standards (NCCLS) (18). Mueller-Hinton agar (Oxoid Ltd.) supplemented with 5% sheep blood was used, and the plates were incubated overnight in ambient air at 35°C. S. pneumoniae ATCC 49619 was included in each run as a control strain to ensure that the results were within the acceptable quality control limits of the NCCLS microdilution method for pneumococci (18). The MIC breakpoints recommended by NCCLS were considered for all antibiotics (18). In the case of telithromycin, the MIC breakpoint proposed by the manufacturer (Aventis Pharma) was applied (susceptible, ≤0.5 μg/ml; resistant, ≥4 μg/ml). This breakpoint has been validated by two committees in Europe, including MENSURA (Mesa Española de Normalización de las Sensibilidad y Resistancia a los Antimicrobianos) and CA-SFM (Comité de l'Antibiogramme de la Société Française de Microbiologie) (26).
Agar disk diffusion assays.
All strains were screened for macrolide-lincosamide-streptogramin B (MLSB) resistance by the disk diffusion method on Mueller-Hinton agar supplemented with 5% sheep blood. The plates were inoculated by using a swab with a cell suspension with a turbidity equivalent to that of a 0.5 McFarland standard; and commercial erythromycin, clindamycin, and spiramycin disks were placed on the plates approximately 15 to 20 mm apart (triple-disk induction test) (13). The plates were incubated overnight in ambient air at 35°C. Blunting of the clindamycin and spiramycin inhibition zones proximal to the erythromycin disk was interpreted as the inducible type of MLSB resistance. Resistance to all three antibiotics (erythromycin, clindamycin, and spiramycin) was considered the constitutive type of MLSB resistance. Isolates resistant to erythromycin and susceptible to clindamycin and spiramycin without blunting were assigned the M phenotype (efflux). The inductive activity of telithromycin was tested by replacing the erythromycin disk by the ketolide disk (3). Susceptibilities to chloramphenicol and tetracycline were also determined by the standard agar diffusion test with the commercial disks cited above (19).
Detection of erythromycin resistance genes.
Total DNA was obtained from all erythromycin-intermediate and -resistant streptococcal strains and from five susceptible isolates (negative controls) by using the InstaGene Matrix (Bio-Rad Laboratories, Hercules, Calif.). The 25-μl PCR mixture contained 15 mM Tris-HCl, 50 mM KCl (pH 8.0), 2 mM MgCl, 100 μM (each) deoxynucleotide, 2 pmol of each primer, 2 U of AmpliTaq Gold DNA polymerase (Perkin-Elmer Applied Biosystems, Foster City, Calif.), and 10 μl of the DNA preparation. Amplifications were performed on a PTC-100 (MJ Research Inc., Watertown, Mass.) thermocycler; and the program was as follows: 94°C for 12 min and 35 cycles of denaturation at 94°C for 1 min, annealing at 50°C for 45 s, and elongation at 72°C for 45 s. A final elongation step of 72°C for 10 min was included. Electrophoresis was carried out on 2% agarose gels stained with ethidium bromide, and the sizes of the PCR products were estimated with DNA molecular weight markers (DNA Markers III and V; Roche Diagnostic GmbH, Mannheim, Germany). Detection of the erm(B) and mef(A) (formerly mefE) (23) genes was performed with the primers reported previously (27) (Amersham Pharmacia Biotech, Uppsala, Sweden): for erm(B), 5′-GAA AAG GTA CTC AAC CAA ATA-3′ and 5′-AGT AAC GGT ACT TAA ATT GTT TAC-3′ (PCR product of ca. 640 bp); for mef(A), 5′-AGT ATC ATT AAT CAC TAG TGC-3′ and 5′-TTC TTC TGG TAC TAA AAG TGG-3′ (PCR product of ca. 350 bp). Genomic DNAs from Streptococcus pyogenes AC1 and S. pyogenes 02C1064 were used as positive controls in the PCRs for the erm(B) and mef(A) genes, respectively. A negative control in which DNA was omitted was also included in each run.
RESULTS
Overall susceptibility and resistance rates.
The results obtained for S. pneumoniae ATCC 49619 were within acceptable quality control limits of the NCCLS microdilution method for pneumococci (18). The MICs, ranges of MICs, and percent susceptibilities to each antibiotic for all S. pneumoniae isolates tested are shown in Table 1.
TABLE 1.
Comparative in vitro activities of telithromycin against 203 S. pneumoniae isolates
| Antimicrobial agent | MIC (μg/ml)a
|
% of fully susceptible isolatesb | ||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| Penicillin | ≤0.008–4 | 0.06 | 2 | 51.7 |
| Cefotaxime | ≤0.004–1 | 0.01 | 0.5 | 94.0 |
| Telithromycin | ≤0.002–1 | 0.01 | 0.06 | NAc |
| Erythromycin | ≤0.008–>64 | 0.03 | >64 | 61.0 |
| Clindamycin | ≤0.008–>64 | 0.06 | >64 | 68.0 |
| Quinupristin-dalfopristin | 0.06–8 | 1 | 2 | 69.9 |
| Levofloxacin | 0.12–8 | 0.5 | 1 | 99.6 |
50% and 90%, MICs at which 50 and 90% of isolates are inhibited, respectively.
NCCLS breakpoints (18).
NA, not applicable.
Telithromycin and levofloxacin were the most active agents among the antibiotics tested, with 98.9 and 99.6% of the strains being susceptible to these two antibiotics, respectively. The overall rate of penicillin susceptibility among the 203 S. pneumoniae isolates was 51.7%; 34.5% of the strains showed intermediate resistance and 13.8% were fully resistant to this antibiotic. The corresponding values for cefotaxime were 94, 6.0, and 0%, respectively. The overall rate of resistance (intermediate plus resistant isolates) to erythromycin was 39.0%. As has been noted in worldwide studies of the susceptibilities of S. pneumoniae isolates (11), erythromycin resistance was more prevalent among penicillin-resistant (75.0%) and penicillin-intermediate (62.8%) isolates than among penicillin-susceptible isolates (13.3%). The rates of clindamycin and quinupristin-dalfopristin resistance were 32 and 30.1%, respectively.
According to the results of the disk diffusion tests, the rate of resistance to tetracycline was 38.4% (n = 78), and in the case of chloramphenicol, the rate of resistance was 21.7% (n = 44). Tetracycline and chloramphenicol resistance was more frequently found among penicillin- and erythromycin-intermediate and -resistant strains, confirming previous findings (11).
Identification of erythromycin resistance determinants: implication in antibiotic susceptibility findings.
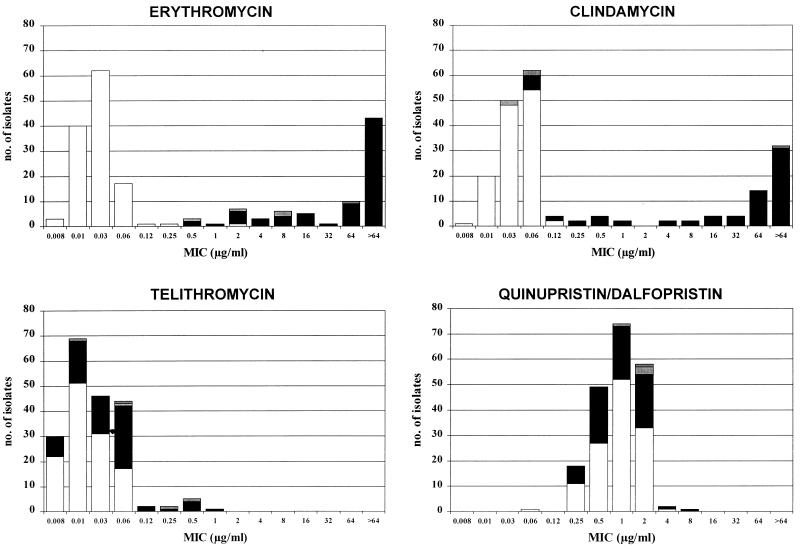
The erm(B) and/or the mef(A) gene was found to be present in 78 erythromycin-intermediate and -resistant S. pneumoniae isolates. Antimicrobial susceptibility patterns based on the presence or the absence of the erythromycin erm(B) and mef(A) resistance determinants are shown in Table 2. The distributions of the MICs of each antimicrobial agent tested according to the PCR results are also presented in Fig. 1.
TABLE 2.
Susceptibilities of S. pneumoniae isolates according to the presence or absence of erm(B) and mef(A) erythromycin resistance genes
| MLS resistance genotype (no. of isolates) and antibiotic | MIC (μg/ml)a
|
|||
|---|---|---|---|---|
| Range | 50% | 90% | Geometric mean | |
| erm(B) and mef(A) negative (125) | ||||
| Penicillin | 0.008–4 | 0.03 | 1 | 0.06 |
| Cefotaxime | 0.004–1 | 0.01 | 0.25 | 0.02 |
| Telithromycin | 0.002–0.06 | 0.01 | 0.06 | 0.01 |
| Erythromycin | 0.008–2 | 0.03 | 0.06 | 0.02 |
| Clindamycin | 0.008–0.12 | 0.03 | 0.06 | 0.03 |
| Quinupristin-dalfopristin | 0.06–4 | 1 | 2 | 0.9 |
| Levofloxacin | 0.12–1 | 1 | 1 | 0.65 |
| erm(B) positive and mef(A) negative (73) | ||||
| Penicillin | 0.01–4 | 0.5 | 2 | 0.45 |
| Cefotaxime | 0.008–1 | 0.25 | 1 | 0.15 |
| Telithromycin | 0.004–1 | 0.03 | 0.12 | 0.03 |
| Erythromycin | 0.5–>64 | >64 | >64 | 45.0 |
| Clindamycin | 0.06–>64 | 64 | >64 | 18.7 |
| Quinupristin-dalfopristin | 0.25–8 | 1 | 2 | 0.90 |
| Levofloxacin | 0.25–8 | 0.5 | 1 | 0.57 |
| erm(B) negative and mef(A) positive (4) | ||||
| Penicillin | 0.03–2 | 0.17 | ||
| Cefotaxime | 0.008–0.25 | 0.06 | ||
| Telithromycin | 0.01–0.5 | 0.09 | ||
| Erythromycin | 0.5–8 | 2.82 | ||
| Clindamycin | 0.03–0.06 | 0.04 | ||
| Quinupristin-dalfopristin | 1–2 | 1.68 | ||
| Levofloxacin | 0.25–2 | 0.84 | ||
| erm(B) and mef(A) positive (1) | ||||
| Penicillin | 0.5 | |||
| Cefotaxime | 0.12 | |||
| Telithromycin | 0.06 | |||
| Erythromycin | 64 | |||
| Clindamycin | >64 | |||
| Quinupristin-dalfopristin | 2 | |||
| Levofloxacin | 1 | |||
50% and 90%, MICs at which 50 and 90% of isolates are inhibited, respectively.
FIG. 1.
Correlation of MICs of erythromycin, telithromycin, clindamycin, and quinupristin-dalfopristin with presence of macrolide resistance determinants [□, no determinants; ▪, erm(B) alone; ▥, mef(A) alone; ▨, erm(B) and mef(A)] for 203 S. pneumoniae isolates.
With the exception of one strain (see below), all isolates for which the erythromycin MICs were greater than or equal to 0.5 μg/ml had at least one known erythromycin resistance determinant. The erm(B) gene, being the most prevalent, was detected in 74 isolates (94.9% of all erythromycin-intermediate and -resistant isolates and 36.4% of all isolates tested). In contrast, mef(A) was detected in only five isolates (2.4% of all isolates tested); in one of them it was detected simultaneously with erm(B). Isolates for which erythromycin MICs were equal to or less than 0.25 μg/ml, which were chosen as negative controls (five isolates), did not have any of these resistance genes. It is worth noting that in one isolate for which the erythromycin MIC was 2 μg/ml, which indicates resistance according to standard NCCLS guidelines (18), neither the mef(A) determinant nor the erm(B) determinant was detected.
Erythromycin MICs for S. pneumoniae erm(B)-positive isolates were higher (range, 0.5 to >64 μg/ml; geometric mean, 45 μg/ml) than those for erm(B)- and mef(A)-negative isolates (range, 0.008 to 2 μg/ml; geometric mean, 0.02 μg/ml). The corresponding MICs of telithromycin were lower for both groups, with ranges of 0.004 to 1 μg/ml (geometric mean, 0.03 μg/ml) and 0.002 to 0.06 μg/ml (geometric mean, 0.01 μg/ml), respectively. Erythromycin MICs for erm(B)-positive S. pneumoniae isolates have a heterogeneous distribution (Fig. 1). An important number of erm(B)-positive isolates had a relatively low level of resistance, with MICs ranging from 0.5 to 16 μg/ml, the same MIC range found for mef(A)-positive isolates. The rest of the strains in the erm(B)-positive population exhibited a higher level of resistance to erythromycin, with MICs being greater than 32 μg/ml. Although the telithromycin MICs for isolates for which the erythromycin MICs were higher were also more likely to be higher, these two groups of strains could not be easily differentiated according to their telithromycin MICs, as all strains were inhibited by telithromycin at concentrations equal to or less than 1 μg/ml (Fig. 1), a value achieved in serum after administration of a normal dosage (5). These results are consistent with the fact that the therapeutic activity of telithromycin is slightly affected by either the erm(B)- or the mef(A)-related erythromycin resistance mechanisms.
The classical inducible MLSB phenotype (erythromycin resistance and clindamycin susceptibility) and constitutive MLSB phenotype (high-level cross-resistance), first distinguished in Staphylococcus aureus, are difficult to apply to S. pneumoniae, as many strains with the constitutive phenotype are even inducible (24). Indeed, we found a wide range of erythromycin concentrations inhibitory for erm(B)-positive S. pneumoniae isolates (0.5 to >64 μg/ml), particularly among those isolates for which erythromycin MICs are lower (0.5 to 16 μg/ml), which may correspond to strains with different levels of inducibility. Because telithromycin is a weak inducer, most of these strains retain their susceptibilities to this drug. Moreover, the erythromycin, clindamycin, and spiramycin triple-disk induction test was not always positive for S. pneumoniae isolates with the classical inducible MLSB phenotype according to the MICs for the isolates. In fact, only 11 of 74 erm(B)-positive isolates (14.8%) displayed an inducible phenotype by the disk induction test. Nevertheless, disk diffusion tests also confirmed that telithromycin has no inducing activity against erythromycin, clindamycin, and spiramycin for those strains for which, on the contrary, this profile was clearly detected for erythromycin. As expected, no inducing activity of erythromycin or telithromycin was observed in mef(A)-positive isolates.
Quinupristin-dalfopristin, which combines the activities of streptogramins A and B, which are synergistic, was active against 70% of the strains tested; and significant levels of susceptibility to quinupristin-dalfopristin were retained even in the presence of the erm(B) determinant. For 67.5% of the erm(B)-positive isolates, the quinupristin-dalfopristin MIC was ≤1 μg/ml. The last group included isolates with the inducible phenotype (11 isolates) as well as isolates with the constitutive MLSB phenotype by the disk induction test (39 isolates).
DISCUSSION
Multidrug resistance in S. pneumoniae is well documented all over the world. As a consequence, current oral antibiotics are losing their efficacies for the treatment of infections caused by this organism (2, 15). Although the frequency of penicillin-intermediate and -resistant S. pneumoniae isolates varies among countries, it appears to be increasing worldwide. Moreover, macrolide resistance is increasing among both penicillin-resistant and -susceptible isolates (1, 11, 12). In this scenario, new antimicrobials, such as ketolides, have emerged. These drugs have specifically been designed to overcome MLS resistance mechanisms (5). Different studies have previously assayed the activities of telithromycin, a novel ketolide, against S. pneumoniae isolates (8, 14, 16, 17, 21, 22). However, the available information on the in vitro activities of telithromycin against a collection of S. pneumoniae isolates with well-characterized erythromycin resistance mechanisms remains scarce.
In our study, telithromycin displayed significant in vitro activity, with 98.9% of S. pneumoniae isolates tested being susceptible, regardless of the presence of macrolide resistance determinants. This result confirmed previous findings about the good in vitro activity of this ketolide against pneumococci (14, 16), even among resistant isolates. In our pneumococcal population, erm(B)-mediated methylation of the ribosomal target was the most prevalent resistance mechanism in erythromycin-intermediate and -resistant isolates (94.9%), with 36.4% isolates in the collection studied exhibiting the erm(B) determinant. In contrast, mef(A)-positive isolates accounted for only 2.4% of strains, with only one isolate carrying both mechanisms simultaneously (Table 2). Although the erm(B) determinant is always found among erythromycin-resistant isolates from different countries (16, 17, 20), our study revealed a higher incidence of this determinant in Spain than in other areas of the world, including Mediterranean countries and North America (25). This situation could be related to antibiotic consumption and differential selective pressures or to the clonal spread of both penicillin- and erythromycin-resistant strains (6, 10). Notably, erm(B) was detected more among penicillin-resistant (71.4%) and -intermediate (61.4%) isolates than among isolates that were part of the susceptible population (10.5%).
Descheemaeker et al. (8) recently found that 3 of 33 S. pneumoniae isolates displayed an M phenotype, with a range of telithromycin MICs of 0.125 to 0.5 μg/ml. A similar range was found for our four strains with a confirmed mef(A) resistance determinant, for which the telithromycin MICs placed them in the susceptible population (MIC range, 0.01 to 0.5 μg/ml). These results confirm that telithromycin is less affected than erythromycin by efflux-based mechanisms in S. pneumoniae, as erythromycin MICs were 0.5 to 8 μg/ml for isolates with the M phenotype or the mef(A) determinant.
As stated earlier, one isolate for which the erythromycin MIC was 2 μg/ml was negative for the erm(B) and mef(A) genes. Although it has been established that macrolide-resistant pneumococci that are isolated from clinical specimens and that do not carry either the erm or the mef gene occur infrequently, ribosome and/or ribosomal protein mutations that are the same as or similar to those observed in recent in vitro mutants may be present in this isolate (28, 29; A. Canu, B. Malbruny, M. Coquemont, T. A. Davies, P. C. Appelbaum, and R. Leclercq, Abstr., 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1927, 2000). Interestingly, the telithromycin MIC for this isolate was 0.03 μg/ml, which is in the susceptible range. Moreover, this value was lower than that obtained for laboratory mutants selected from serial passages with different MLS antibiotics (7).
In summary, the erm(B) resistance determinant was responsible for most of the erythromycin resistance detected in S. pneumoniae. Telithromycin had a higher level of intrinsic activity than erythromycin against susceptible strains and was slightly affected by the erm(B)-mediated erythromycin resistance mechanism. Despite the small number of strains with the mef(A) resistance mechanism, telithromycin displayed higher levels of in vitro activity than erythromycin. This excellent in vitro activity, together with a lesser capacity to select resistant mutants compared to the capacities of other MLS agents (28) and the lack of inductive activity (3), merits further clinical studies on the efficacy of telithromycin against infections caused by S. pneumoniae when macrolides are indicated.
ACKNOWLEDGMENTS
We thank Milagro Reig for providing reference strains.
This study was supported, in part, by a grant from Aventis Pharma. J.-C. Galán is the recipient of a fellowship (BEFI 98/9060) from the Fondo de Investigaciones Sanitarias de la Seguridad Social, Spain.
S. pneumoniae isolates were provided in part by the Spanish Collaborative Group, which consists of the Hospital Gregorio Marañón, Madrid (Bouza); Hospital de Bellvitge, Barcelona (Martín); Hospital Juan Canalejo, La Coruña (Guerrero); Hospital Clínico de Valencia (García de Lomas); Hospital Clínico de Zaragoza (Gómez Lus); Hospital de Basurto, Bilbao (Cisterna); Hospital Clínico de Murcia (Segovia); Hospital Clínico de Salamanca (García Rodríguez); Hospital Clínico de Sevilla (Perea); Hospital Son Dureta, Palma de Mallorca (Alomar); Hospital Insular, Las Palmas (Martín Sánchez); Hospital Central de Asturias (Méndez); and Hospital Virgen de las Nieves, Granada (de la Rosa).
REFERENCES
- 1.Baquero F, García-Rodríguez J A, García de Lomas J, Aguilar L the Spanish Surveillance Group for Respiratory Tract Pathogens. Antimicrobial resistance of 1,113 Streptococcus pneumoniae isolates from patients with respiratory tract infections in Spain: results of a 1-year (1996–1997) multicenter surveillance study. Antimicrob Agents Chemother. 1999;43:357–359. doi: 10.1128/aac.43.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barlett J G, Breiman R F, Mandell L A, File T M. Community-acquired pneumonia in adults: guidelines for management. Clin Infect Dis. 1998;26:811–838. doi: 10.1086/513953. [DOI] [PubMed] [Google Scholar]
- 3.Bonnefoy A, Girard A M, Agouridas C, Chantot J F. Ketolides lack inducibility properties of MLSB resistance phenotype. J Antimicrob Chemother. 1997;40:85–90. doi: 10.1093/jac/40.1.85. [DOI] [PubMed] [Google Scholar]
- 4.Boswell F J, Andrews J M, Ashby J P, Fogarty C, Brenwald N P, Wise R. The in-vitro activity of HMR 3647, a new ketolide antimicrobial agent. J Antimicrob Chemother. 1998;42:703–709. doi: 10.1093/jac/42.6.703. [DOI] [PubMed] [Google Scholar]
- 5.Bryskier A. Kétolides. In: Bryskier A, editor. Antibiotiques agents antibactériens et antifongiques. Paris, France: Ellipses Édition Marketing S.A.; 1999. pp. 563–594. [Google Scholar]
- 6.Corso A, Severina E P, Petruk V F, Mauriz Y R, Tomasz A. Molecular characterization of penicillin-resistant Streptococcus pneumoniae isolates causing respiratory disease in the United States. Microb Drug Resist. 1998;4:325–337. doi: 10.1089/mdr.1998.4.325. [DOI] [PubMed] [Google Scholar]
- 7.Davies T A, Dewasse B E, Jacobs M R, Appelbaum P C. In vitro development of resistance to telithromycin (HMR 3647), four macrolides, clindamycin, and pristinamycin in Streptococcus pneumoniae. Antimicrob Agents Chemother. 2000;44:414–417. doi: 10.1128/aac.44.2.414-417.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Descheemaeker P, Chalpelle S, Lammens C, Hauchecorne M, Wijdooghe M, Vandamme P, Ieven M, Goossens H. Macrolide resistance and erythromycin resistance determinants among Belgian Streptococcus pyogenes and Streptococcus pneumoniae isolates. J Antimicrob Chemother. 2000;45:167–173. doi: 10.1093/jac/45.2.167. [DOI] [PubMed] [Google Scholar]
- 9.Douthwaite S, Hansen L H, Mauvais P. Macrolide-ketolide inhibition of MLS-resistant ribosomes is improved by alternative drug interaction with domain II of 23S rRNA. Mol Microbiol. 2000;36:183–192. doi: 10.1046/j.1365-2958.2000.01841.x. [DOI] [PubMed] [Google Scholar]
- 10.Felmingham D, Gruneberg R N. The Alexander Project 1996–1997: latest susceptibility data from this international study of bacterial pathogens from community-acquired lower respiratory tract infections. J Antimicrob Chemother. 2000;45:191–203. doi: 10.1093/jac/45.2.191. [DOI] [PubMed] [Google Scholar]
- 11.Fenoll A, Jado I, Vicioso D, Pérez A, Casal J. Evolution of Streptococcus pneumoniae serotypes and antibiotic resistance in Spain: update (1990 to 1996) J Clin Microbiol. 1998;36:3447–3454. doi: 10.1128/jcm.36.12.3447-3454.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giovanetti E, Montanari M P, Mingoia M, Varaldo P E. Phenotypes and genotypes of erythromycin-resistant Streptococcus pyogenes strains in Italy and heterogeneity of inducibly resistant strains. Antimicrob Agents Chemother. 1999;43:1935–1940. doi: 10.1128/aac.43.8.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granizo J J, Aguilar L, Casal J, García-Rey C, Dal-Re R, Baquero F. Streptococcus pneumoniae resistance to erythromycin and penicillin in relation to macrolide and β-lactam consumption in Spain (1979–1997) J Antimicrob Chemother. 2000;46:767–773. doi: 10.1093/jac/46.5.767. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton-Miller J M, Shah S. Comparative in-vitro activity of ketolide HMR 3647 and four macrolides against gram-positive cocci of known erythromycin susceptibility status. J Antimicrob Chemother. 1998;41:649–653. doi: 10.1093/jac/41.6.649. [DOI] [PubMed] [Google Scholar]
- 15.Heffelfinger J D, Dowell S H, Jorgensen J H, Klugman K P, Mabry L R, Musher D M, Plouffe J F, Rakowsky A, Schuchat A, Whitney C G. Management of community-acquired pneumonia in the era of pneumococcal resistance: a report from the Drug-Resistant Streptococcus pneumoniae Therapeutic Working Group. Arch Intern Med. 2000;160:1399–1408. doi: 10.1001/archinte.160.10.1399. [DOI] [PubMed] [Google Scholar]
- 16.Malathum K, Coque T M, Singh K V, Murray B E. In vitro activities of two ketolides, HMR 3647 and HMR 3004, against gram-positive bacteria. Antimicrob Agents Chemother. 1999;43:930–936. doi: 10.1128/aac.43.4.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marchese A, Tonoli E, Debbia E A, Schito G C. Macrolide resistance mechanisms and expression of phenotypes among Streptococcus pneumoniae circulating in Italy. J Antimicrob Chemother. 1999;44:461–464. doi: 10.1093/jac/44.4.461. [DOI] [PubMed] [Google Scholar]
- 18.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard M7–A5. Wayne, Pa: National Committee for Clinical Laboratory Standards; 2000. [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. National Committee for Clinical Laboratory Standards, Wayne, Pa. 2000. Performance standards for antimicrobial disk susceptibility tests, 6th ed. Approved standard M2–A7. [Google Scholar]
- 20.Nishijima T, Saito Y, Aoki A, Toriya M, Toyonaga Y, Fujii R. Distribution of mefE and ermB genes in macrolide-resistant strains of Streptococcus pneumoniae and their variable susceptibility to various antibiotics. J Antimicrob Chemother. 1999;43:637–643. doi: 10.1093/jac/43.5.637. [DOI] [PubMed] [Google Scholar]
- 21.Pankuch G A, Visalli M A, Jacobs M R, Appelbaum P A. Susceptibility of penicillin- and erythromycin-susceptible and -resistant pneumococci to HMR 3647 (RU 66647), a new ketolide, compared with susceptibilities to 17 other agents. Antimicrob Agents Chemother. 1998;42:624–630. doi: 10.1128/aac.42.3.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reinert R R, Bryskier A, Lütticken R. In vitro activities of the new ketolide antibiotics HMR 3004 and HMR 3647 against Streptococcus pneumoniae in Germany. Antimicrob Agents Chemother. 1998;42:1509–1511. doi: 10.1128/aac.42.6.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts M C, Sutcliffe J, Courvalin P, Jensen L B, Rood J, Seppälä H. Nomenclature for macrolide and macrolide-lincosamide-streptogramin B resistance determinants. Antimicrob Agents Chemother. 1999;43:2823–2830. doi: 10.1128/aac.43.12.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosato A, Vicarini H, Leclercq R. Inducible or constitutive expression of resistance in clinical isolates of streptococci and enterococci cross-resistant to erythromycin and lincomycin. J Antimicrob Chemother. 1999;43:559–562. doi: 10.1093/jac/43.4.559. [DOI] [PubMed] [Google Scholar]
- 25.Shortridge V D, Doern G V, Brueggemann A B, Beyer J M, Flamm R K. Prevalence of macrolide resistance mechanisms in Streptococcus pneumoniae isolates from a multicenter antibiotic resistance surveillance study conducted in the United States in 1994–1995. Clin Infect Dis. 1999;29:1186–1188. doi: 10.1086/313452. [DOI] [PubMed] [Google Scholar]
- 26.Soussy C J, Carret G, Cavallo J D, Chardon H, Chidiac C, Choutet P, Courvalin P, Dabernat H, Drugeon H, Dubreuil L, Goldstein F, Jarlier V, Leclercq R, Nicolas-Chanoine M H, Philippon A, Quentin C, Rouveix B, Sirot J. Antibiogram Committee of the French Microbiology Society. Report 2000–2001. Pathol Biol (Paris) 2000;48:832–871. [PubMed] [Google Scholar]
- 27.Sutcliffe J, Grebe T, Tait-Kamradt A, Wondrack I. Detection of eythromycin-resistant determinants by PCR. Antimicrob Agents Chemother. 1996;40:2562–2566. doi: 10.1128/aac.40.11.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tait-Kamradt A, Davies T, Cronan M, Jacobs M R, Appelbaum P C, Sutcliffe J. Mutations in 23S rRNA and ribosomal protein L4 account for resistance in pneumococcal strains selected in vitro by macrolide passage. Antimicrob Agents Chemother. 2000;44:2118–2125. doi: 10.1128/aac.44.8.2118-2125.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tait-Kamradt A, Davies T, Appelbaum P C, Depardieu F, Courvalin P, Petitpas J, Wondrack L, Walker A, Jacobs M R, Sutcliffe J. Two new mechanisms of macrolide resistance in clinical strains of Streptococcus pneumoniae from Eastern Europe and North America. Antimicrob Agents Chemother. 2000;44:3395–3401. doi: 10.1128/aac.44.12.3395-3401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]