PURPOSE
Despite the advances in the approach to non–small-cell lung cancer (NSCLC) with CNS metastasis, access to timely diagnosis and treatment may not be optimal in many instances. Our main objective was to describe a cohort of patients with NSCLC with brain metastases from public and private cancer centers, and the differences between patients' presentation, treatment, and outcomes.
METHODS
GBOT-LACOG 0417 is a multi-institutional retrospective cohort study of patients diagnosed with NSCLC and CNS metastasis in Brazil. All patients had confirmed diagnosis of NSCLC between January 2010 and December 2015. CNS metastases were identified by imaging.
RESULTS
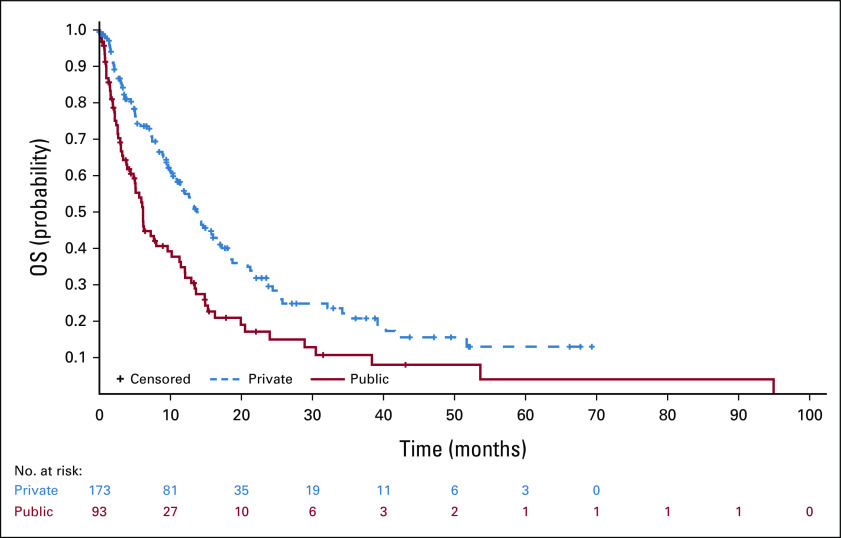
A total of 273 patients were included. Patients treated at public institutions were more often Black or Brown (38.8% v 15.4%), current or former smoker (88.6% v 60.0%), of squamous cell histology (25.0% v 9.1%), EGFR- and ALK-negative (95.9% v 74.9%), and were less frequently assessed by using brain magnetic resonance imaging (38.8% v 83.6%). At public institutions, patients were more often symptomatic (78.1% v 44.6%) and had worse performance status (Eastern Cooperative Oncology Group 2 or higher 61.5% v 10.3%). CNS metastases were larger (median size 25 v 15 mm) and more often surrounded by edema (67.7% v 55.2%) at public institutions. Patients at public institutions were more frequently treated with whole-brain radiation therapy (72.9% v 45.4%) and less frequently with radiosurgery (6.3% v 24.1%). Among patients from private care, median overall survival was 24.2 months (95% CI, 20.0 to 30.6), significantly higher than in public care (median 12.1 months; 95% CI, 6.7 to 13.6; P < .001).
CONCLUSION
Our results demonstrate the discrepancy between public and private health care system in the critical setting of patients with CNS metastasis from NSCLC.
INTRODUCTION
In the past decade, new therapies have revolutionized the approach for non–small-cell lung cancer (NSCLC) and, thus, new patterns of metastasis have been recognized. Although there is a lack of comprehensive studies that estimate the true incidence of CNS metastasis in patients diagnosed with NSCLC, it is believed that up to 50% will develop this complication over the course of disease1-3 and up to 10% have CNS metastasis at diagnosis.4 Young female patients, adenocarcinoma or high-grade histology, tumor larger than 3 cm and lymph node involvement are features related to a higher risk for the development of these metastases.5
CONTEXT
Key Objective
Is proper and timely access to cancer care associated with brain metastasis presentation and survival in patients with lung cancer?
Knowledge Generated
Patients with lung cancer treated in the private health care system present with smaller and less symptomatic brain metastasis in comparison to public health care in Brazil. In the private setting, radiation therapy is often on the basis of modern techniques that more precisely target the tumor, and survival is significantly longer.
Relevance
Our results may reflect the reality of low- and middle-income countries and may serve as a basis for encouraging improvements in clinical practice. Programs to increase quality of lung cancer care and shorten the gap of access are crucial in the upcoming years.
Prognostic factors and indices have been described in an attempt to find a subgroup with a more favorable prognosis, and thus, candidates for more intensive treatment.6-8 The Graded Prognosis Index uses four variables (age, performance status using the Karnofsky scale, number of metastases in the CNS, and presence of extracranial disease) to divide patients into prognostic groups. Although patients with all favorable variables achieve median survival of up to 11 months after the diagnosis of CNS metastasis, patients with all unfavorable variables do not survive more than 3 months.8 This index has recently been updated to incorporate tumor molecular information (presence of EGFR mutations or ALK rearrangements), which increases its prognostic value.9,10
Given its aggressive nature and low activity of classic cytotoxic agents in the CNS because of limited capacity to cross the blood-brain barrier, CNS metastases have been commonly treated with radiation therapy. Whole-brain radiotherapy (WBRT) is the most popular choice, achieving results in reducing tumor size and symptoms related to CNS disease (headache and motor change), but the disease control is of short duration, often < 90 days.5,11,12 Actually, a randomized phase III study demonstrated that WBRT did not increase overall survival (OS) when compared with best supportive care.13 Although several therapeutic dose and fractionation schemes have been studied, none of them have been able to demonstrate benefit over the classic regimen of 30 Gy in 10 fractions.14,15
Oligometastatic patients may benefit from more aggressive treatment for CNS disease. Surgical resection and stereotactic radiotherapy are viable options, including reports of patients who have achieved long survival. Patients with NSCLC and isolated CNS metastasis at diagnosis or those who progress exclusively in the CNS—with the primary tumor remaining under control—are the main candidates for these treatment strategies. In recent years, therapy has moved to more conformed radiation fields, and novel systemic therapies such as small molecule inhibitors and immune checkpoint blockade.16-18
Despite the advances in the approach to NSCLC with CNS metastasis, access to timely diagnosis and treatment may not be optimal in many instances. In low- and middle-income countries, a striking disparity in access exists between public and private care. In this article, we present a comprehensive overview of NSCLC with CNS metastases in Brazil, highlighting prognostic factors and the discrepancies in patients' presentation, treatment, and outcomes according to public or private care setting. This knowledge could be extremely important to better define public health strategies.
METHODS
Study Design
GBOT-LACOG 0417 is a multi-institutional retrospective cohort study that collected data from medical records of patients diagnosed with NSCLC and CNS metastasis. Participating institutions were Instituto COI (state of Rio de Janeiro), Núcleo de Oncologia da Bahia (Bahia)—both private, Instituto Nacional de Câncer (Rio de Janeiro), Instituto do Câncer do Ceará (Ceará), and Hospital de Clínicas de Porto Alegre (Rio Grande do Sul)—public institutions. All patients had confirmed diagnosis of NSCLC by pathologic examination (histology or cytology) between January 2010 and December 2015. CNS metastases were identified by imaging without a requirement of histologic examination. Patients with prior malignancies in the 5 years preceding the diagnosis of NSCLC were excluded.
Approximately 1,000 new patients with lung cancer are seen each year in the five participating institutions. A similar workflow was applied in each institution to identify eligible patient cases. Patient search was done using the International Classification of Diseases (ICD-10) lung cancer code (ICD 34). Subsequently, pathology reports were reviewed to exclude SCLC histology, and medical records were searched for CNS imaging demonstrating the presence of metastasis. Patient cases that did not cover these characteristics or lacked relevant information were excluded. For data collection, an electronic clinical research form was created, including characteristics such as age, histologic subtypes, smoking status, prevalence of molecular alterations, symptoms, evaluation, and treatment of CNS metastasis. Size of brain lesions was determined from medical records. Staging was defined according to the seventh edition of the UICC/IASLC/AJCC (Union for International Cancer Control/International Association for the Study of Lung Cancer/American Joint Committee on Cancer). The main objective was to analyze OS of patients stratified by private or public institution, and timing of CNS metastasis development (upfront or after NSCLC diagnosis). This study was approved by the local research ethics committee at each institution, and the nonuse of an informed consent form was properly justified.
Statistical Analyses
Statistical analyses were performed with information from a cohort of patients with NSCLC with brain metastases. Categorical variables were presented as absolute and relative frequencies. For the purpose of analysis, patient cases lacking EGFR and ALK testing were counted as driver-negative. Quantitative variables were described by median and range. Chi-square test was applied to compare discrete variables between public and private institutions, whereas Wilcoxon rank test was used for continuous variables. OS was estimated using Kaplan-Meier methodology and summarized using the median and the corresponding 95% CI. OS was defined as the time interval between NSCLC diagnosis and death. For the survival analysis after diagnosis of CNS metastasis, OS was defined as the time interval between diagnosis of CNS metastasis and death. Median follow-up was calculated using reverse Kaplan-Meier method. Comparisons of OS distribution between subgroups were done using two-sided log-rank test. Cox proportional hazard models were used to estimate the hazard ratio (HR) and 95% CI. Statistical significance was indicated by P value ≤ .05. All analyses were performed using SAS statistical software (version 9.4; SAS Institute, Inc, Cary, NC).
RESULTS
A total of 273 patients were included, with a median age of 61 years (range, 17 to 86 years). The majority were males (52.8%), White (33.0%), and 70.1% were current or former smokers. Adenocarcinoma was the most common histologic subtype (76.0%), followed by squamous cell carcinoma (SCC; 14.8%). Two-hundred fifteen patients (79.3%) were diagnosed at stage IV, 29 (10.7%) at stage III, nine (3.3%) at stage II, and four (1.5%) at stage I. EGFR mutations were identified in 42 (15.4%) patients and ALK rearrangements in six (2.2%). At initial evaluation, 197 (72.4%) patients had CNS imaging, with magnetic resonance imaging (MRI) in 128 (65.3%) and computed tomography (CT) in 68 (34.7%). Of note, relevant differences were observed among patients from public and private care institutions. Patients treated at public institutions were more often Black or Brown (38.8% v 15.4%), current or former smoker (88.6% v 60.0%), of SCC histology (25.0% v 9.1%), EGFR- and ALK-negative (95.9% v 74.9%), and were less frequently assessed by using brain MRI (38.8% v 83.6%). All patient characteristics are summarized in Table 1.
TABLE 1.
Patient and Tumor Characteristics
At the moment of the CNS metastasis diagnosis, 153 (56.4%) patients had neurologic symptoms and MRI was performed in 177 (66.5%). Ninety-eight (36.3%) patients had only one lesion, whereas 49 (18.2%) had two lesions and 66 (24.4%) had three or more. Median size of the largest lesion was 16 mm (1.2-70), and perilesional edema was detected in 161 (59.6%) cases. Ninety-four (34.7%) patients were classified with performance status of 1, and 77 (28.4%) with 2 or higher; systemic disease was not controlled in 222 (81.9%) patients when CNS metastasis was detected. At public institutions, patients were more often symptomatic (78.1% v 44.6%), had worse performance status (Eastern Cooperative Oncology Group 2 or higher 61.5% v 10.3%), and were less frequently assessed by MRI (40.4% v 80.8%). CNS metastases were larger (median size 25 v 15 mm) and more often surrounded by edema (67.7% v 55.2%) at public institutions. CNS metastasis data are described in Table 2.
TABLE 2.
CNS Metastasis Characteristics
WBRT was applied as first treatment of CNS metastasis in 149 (55.2%) patients, stereotactic radiosurgery in 48 (17.8%), and surgery followed by WBRT in 19 (7.0%). Patients at public institutions were more frequently treated with WBRT (72.9% v 45.4%) and less frequently with radiosurgery (6.3% v 24.1%). One hundred and nine patients had CNS disease progression documented after first diagnosis and treatment. Among these, 24 (22.0%) were submitted to WBRT, 14 (12.8%) to stereotactic radiosurgery, and 47 (43.1%) to exclusive systemic chemotherapy as salvage options. Exclusive systemic treatment was more often performed in public than in private institutions (87.2% v 9.7%). Treatment data are presented in Table 3.
TABLE 3.
Treatment Characteristics

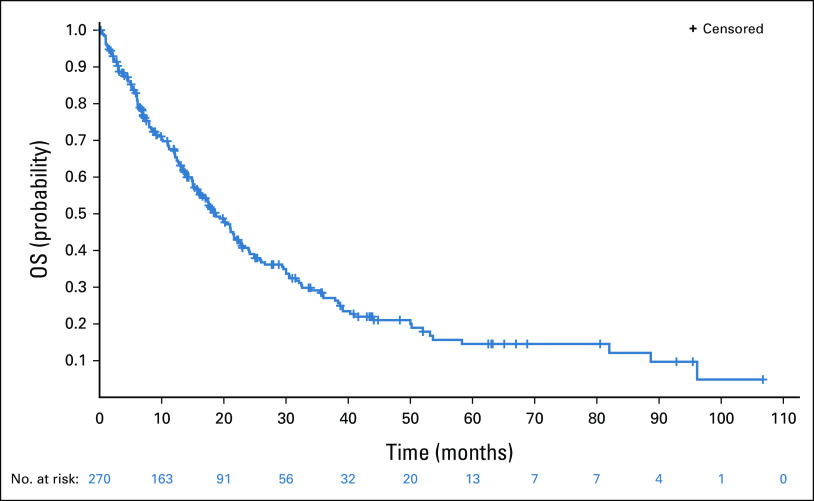
After a median follow-up of 40.9 months (95% CI, 31.0 to 44.1), median OS was 18.7 months (95% CI, 15.8 to 21.6). Among patients from private care, median OS was 24.2 months (95% CI, 20.0 to 30.6), significantly higher than in public care (median 12.1 months; 95% CI, 6.7 to 13.6; P < .001). At multivariate analysis, private institution was independently associated with better OS (HR, 0.49; 95% CI, 0.33 to 0.73; P = .0004), whereas patients age older than 65 years had worse OS (HR, 1.79; 95% CI, 1.22 to 2.62; P = .0030). Patients with CNS metastasis at diagnosis had median OS of 12.1 months (95% CI, 9.0 to 14.9) compared with 26.0 months (95% CI, 21.1 to 32.5) in patients who developed CNS metastasis after NSCLC diagnosis.
After diagnosis of CNS metastasis, median OS was 11.5 months (95% CI, 9.2 to 13.4). Again, OS was greater among patients treated in private (median 13.7 months; 95% CI, 10.9 to 17.0) than in public institutions (median 6.1 months; 95% CI, 4.4 to 9.6; P = .0003). OS after CNS metastasis was also higher in driver-positive than in driver-negative NSCLC (median 17.0 [95% CI, 11.7 to 25.3] v 9.5 months [95% CI, 6.2 to 12.9]; P = .0487). OS data are described in Table 4 and illustrated in Figures 1–5.
TABLE 4.
Survival Analysis
FIG 1.
OS. OS, overall survival.
FIG 5.
OS after metastasis diagnosis stratified by health care system. OS, overall survival.
FIG 2.
OS stratified by time of metastasis diagnosis. OS, overall survival.
FIG 3.
OS stratified by health care system. OS, overall survival.
FIG 4.
OS after metastasis diagnosis. OS, overall survival.
DISCUSSION
This study was carried out to evaluate the patterns of diagnosis, treatment, and outcomes of patients with NSCLC with CNS metastasis in a real-world setting. To the authors' knowledge, this is the first study of this kind in Latin America. Of interest, major disparities were described in all these aspects according to the level of insurance coverage. Given the poor prognosis underlying CNS involvement, outcomes in this critical scenario may be strongly affected by differences in proper and timely access to cancer care. In Brazil, the majority of the population (around 75%) relies on the public health care system, where limited resources are allocated to provide the best care possible. By contrast, private insurance coverage may provide access to examinations and treatment, in a standard that often parallels that of high-income countries.19-21
The social and economic disparities between public and private health care systems are crucial to cause a late diagnosis, and therefore are determinant of poor survival rates. Herein, relevant baseline characteristics distinguished patients in these diverse scenarios. Patients from public institutions were more often smokers and had tumors of SCC histology. Adenocarcinoma rates increased in the past decade, whereas SCC and SCLC rates declined after the 1990s. A major review of lung cancer in Brazil indicated that SCC histology was more prevalent in public health care services, whereas adenocarcinoma predominated in private institutions. In this retrospective study, adenocarcinoma overcame SCC histology in both scenarios, but the latter still has a relevant proportion in public health setting.22-25
Lung carcinomas are a genetically heterogeneous disease. The amount of somatic mutation is notably high in NSCLC, and distinct molecular profiles may occur in tumor from smokers and never smokers. The frequency of EGFR mutation and ALK rearrangements found in the private setting is similar to national data (25% of EGFR mutations, and 3%-4% of ALK rearrangements). However, the frequency of driver mutations in the public care was minimal, and < 10% was submitted to molecular testing in the public setting. It might be justified by the limited access to molecular testing and targeted therapy in this scenario. Unfortunately, access, affordability, and incorporation strategies remain relevant challenges in the public health care.26,27
Because of the relatively high prevalence of occult CNS metastasis in patients with stage III or IV NSCLC, it is suggested that brain MRI should be routinely performed in most cases. In symptomatic patients, brain MRI is the standard test, whereas CT scan is an alternative when MRI is not available. In our analysis, the majority of patients (72.4%) had undergone CNS imaging at presentation. However, a larger number of patients were symptomatic at the public setting (78.1% v 44.6%), and brain MRI was less often used (40.4% v 80.8%), in detriment to brain CT (59.6% v 19.2% in the public and private care, respectively). These data further illustrate the distinct presentation and access to adequate imaging in the clinical practice.28
WBRT was the front-line treatment choice in more than half of the cases, mostly at public setting (72.9% v 45.4%). Furthermore, exclusive systemic chemotherapy was a predominant salvage option for progressing disease in the CNS at public centers. These findings highlight the lack of access to modern radiation techniques in patients who lack private insurance as well as the poor condition in which these patients present when they finally have a chance to be seen in a specialized cancer institution. In low- and middle-income countries, the gap of access to modern therapeutic approaches such as conformed radiation therapy remains considerable.29
The survival difference between patients treated in public and private centers is striking. As presented throughout this article, several factors may concur to determine such contrast. These include distinct characteristics of presentations—worse biology and more advanced disease—but also lack of access to contemporary methods of diagnosis and treatment. Even in patients with CNS metastasis, which per se underlies such an unfavorable prognosis, a huge survival difference could be detected. Altogether, these data uncover the need to define and implement better strategies to guarantee lung cancer prevention, early diagnosis, and workflows for adequate and timely treatment.
Among the potential limitations are the long inclusion period (6 years) and the relatively small sample size. During this period, it is possible that changes in clinical practice may have affected the results described, including increased use of molecular testing and targeted therapy. Also, although a similar workflow was applied in each institution for patient selection, these were not requested to provide the number of patients screened. For this reason, it was not possible to present the patient selection process in a flow diagram. Given the retrospective nature of the study, this issue could represent a significant source of bias. Quality-of-life data are warranted in future prospective assessments. By contrast, the current cohort was representative of private and public health care institutions geographically distributed in the country. Indeed, three of five Brazilian regions were contemplated, increasing the confidence and reproducibility of the results.
Although a large part of the Brazilian population is exclusively covered by the public health system, this subgroup had fewer patients included compared with private practice. It should be noted that large and representative private institutions were selected to participate. These centers tend to have better resources to maintain a dedicated research infrastructure and personnel that enabled adequate patient accrual. Another point of discussion is that public centers usually present a more integrated patient care that includes both outpatient and inpatient services. This characteristic is distinct from private care, where a more fragmented system is the norm. It means that patients selected in the public health could present with greater disease burden and worse prognosis because of its nature of centralized cancer care institution. In the private care, patients may have been selected almost exclusively in outpatient clinics, where prognosis per se is supposably better.
In summary, our results demonstrate the discrepancy between public and private health care system in the critical setting of patients with CNS metastasis from NSCLC. Our results may reflect the reality in other low- and middle-income countries and may serve as a basis for encouraging improvements in clinical practice. Programs to increase quality of lung cancer care and shorten the gap of access are crucial in the upcoming years. As novel and costly approaches are incorporated, continuous effort should be exercised to guarantee value in patient care, especially in settings marked by limited resources.
Fabio Chaves
Consulting or Advisory Role: AstraZeneca/Brazil
Speakers' Bureau: AstraZeneca/Brazil, Servier
Pedro de Marchi
Honoraria: Sanofi
Consulting or Advisory Role: Merck Sharp & Dohme, AstraZeneca, Bristol Myers Squibb, Roche, Bayer
Speakers' Bureau: Merck Sharp & Dohme, Bristol Myers Squibb, Roche, AstraZeneca, Novartis, Merck, Boehringer Ingelheim, Janssen, Takeda
Research Funding: AstraZeneca, Amgen, Merck Sharp & Dohme, Roche
Travel, Accommodations, Expenses: Merck Sharp & Dohme, AstraZeneca, Roche, Bristol Myers Squibb
Gilberto de Castro Jr
Honoraria: AstraZeneca, Pfizer, Merck Sharp & Dohme, Bristol Myers Squibb, Novartis, Roche, Amgen, Janssen
Consulting or Advisory Role: Boehringer Ingelheim, Pfizer, Bayer, Roche, Merck Sharp & Dohme, Bristol Myers Squibb, AstraZeneca, Yuhan, Merck Serono, Janssen, Libbs, Sanofi, Novartis
Speakers' Bureau: AstraZeneca, Bayer, Novartis, Roche, Merck Serono, Bristol Myers Squibb, Merck Sharp & Dohme, Boehringer Ingelheim, Pfizer, Janssen, Amgen
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Novartis, Pfizer, Roche, AstraZeneca, Boehringer Ingelheim, Bayer, Bristol Myers Squibb, Merck Serono
Clarissa Baldotto
Honoraria:AstraZeneca, MSD Oncology Brazil, Roche Oncology Brazil, Takeda Oncology Brazil, BMS Brazil, Janssen Oncology Brazil, Pfizer Oncology Brazil, MD Health
Consulting or Advisory Role: MSD, AstraZeneca, Janssen Oncology Brazil, Novartis, Novartis, Takeda Brazil, Roche Oncology Brazil
Speakers' Bureau: Roche Oncology Brazil, BMS Brazil, Janssen Oncology Brazil, MSD, MSD, Instituto D'Or de Pesquisa e Ensino
Research Funding: AstraZeneca, Roche/Genentech, Janssen Oncology
Travel, Accommodations, Expenses: Janssen Oncology
Eldsamira Mascarenhas
Consulting or Advisory Role: AstraZeneca, Janssen, Pfizer
Gustavo Werutsky
Honoraria: Pfizer, AstraZeneca/MedImmune, Libbs, Merck
Consulting or Advisory Role: Organon
Research Funding: Novartis, Lilly (Inst), AstraZeneca/MedImmune (Inst), Roche/Genentech, GlaxoSmithKline (Inst), Novartis (Inst), Pfizer (Inst), Bristol Myers Squibb Brazil (Inst), Roche (Inst)
Luiz H. Araujo
Consulting or Advisory Role: Merck Sharp & Dohme, Bristol Myers Squibb, AstraZeneca, Pfizer, Boehringer Ingelheim, Merck, Roche
Speakers' Bureau: AstraZeneca, Pfizer, Merck, Roche, Merck Sharp & Dohme, Bristol Myers Squibb, Boehringer Ingelheim
Research Funding: Merck Sharp & Dohme (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Pfizer (Inst), Merck (Inst), Roche (Inst), Boehringer Ingelheim (Inst)
No other potential conflicts of interest were reported.
AUTHOR CONTRIBUTIONS
Conception and design: Juliano Cé Coelho, Gilberto de Castro Jr, Clarissa Baldotto, Gustavo Werutsky, Luiz H. Araujo
Provision of study materials or patients: Fabio Chaves, Gustavo Werutsky
Collection and assembly of data: Juliano Cé Coelho, Fabio Chaves, Clarissa Baldotto, Eldsamira Mascarenhas, Gustavo Werutsky, Luiz H. Araujo
Data analysis and interpretation: Juliano Cé Coelho, Giselle de Souza Carvalho, Pedro de Marchi, Gilberto de Castro Jr, Clarissa Baldotto, Eldsamira Mascarenhas, Patricia Pacheco, Rafaela Gomes, Gustavo Werutsky, Luiz H. Araujo
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution.Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Fabio Chaves
Consulting or Advisory Role: AstraZeneca/Brazil
Speakers' Bureau: AstraZeneca/Brazil, Servier
Pedro de Marchi
Honoraria: Sanofi
Consulting or Advisory Role: Merck Sharp & Dohme, AstraZeneca, Bristol Myers Squibb, Roche, Bayer
Speakers' Bureau: Merck Sharp & Dohme, Bristol Myers Squibb, Roche, AstraZeneca, Novartis, Merck, Boehringer Ingelheim, Janssen, Takeda
Research Funding: AstraZeneca, Amgen, Merck Sharp & Dohme, Roche
Travel, Accommodations, Expenses: Merck Sharp & Dohme, AstraZeneca, Roche, Bristol Myers Squibb
Gilberto de Castro Jr
Honoraria: AstraZeneca, Pfizer, Merck Sharp & Dohme, Bristol Myers Squibb, Novartis, Roche, Amgen, Janssen
Consulting or Advisory Role: Boehringer Ingelheim, Pfizer, Bayer, Roche, Merck Sharp & Dohme, Bristol Myers Squibb, AstraZeneca, Yuhan, Merck Serono, Janssen, Libbs, Sanofi, Novartis
Speakers' Bureau: AstraZeneca, Bayer, Novartis, Roche, Merck Serono, Bristol Myers Squibb, Merck Sharp & Dohme, Boehringer Ingelheim, Pfizer, Janssen, Amgen
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Novartis, Pfizer, Roche, AstraZeneca, Boehringer Ingelheim, Bayer, Bristol Myers Squibb, Merck Serono
Clarissa Baldotto
Honoraria:AstraZeneca, MSD Oncology Brazil, Roche Oncology Brazil, Takeda Oncology Brazil, BMS Brazil, Janssen Oncology Brazil, Pfizer Oncology Brazil, MD Health
Consulting or Advisory Role: MSD, AstraZeneca, Janssen Oncology Brazil, Novartis, Novartis, Takeda Brazil, Roche Oncology Brazil
Speakers' Bureau: Roche Oncology Brazil, BMS Brazil, Janssen Oncology Brazil, MSD, MSD, Instituto D'Or de Pesquisa e Ensino
Research Funding: AstraZeneca, Roche/Genentech, Janssen Oncology
Travel, Accommodations, Expenses: Janssen Oncology
Eldsamira Mascarenhas
Consulting or Advisory Role: AstraZeneca, Janssen, Pfizer
Gustavo Werutsky
Honoraria: Pfizer, AstraZeneca/MedImmune, Libbs, Merck
Consulting or Advisory Role: Organon
Research Funding: Novartis, Lilly (Inst), AstraZeneca/MedImmune (Inst), Roche/Genentech, GlaxoSmithKline (Inst), Novartis (Inst), Pfizer (Inst), Bristol Myers Squibb Brazil (Inst), Roche (Inst)
Luiz H. Araujo
Consulting or Advisory Role: Merck Sharp & Dohme, Bristol Myers Squibb, AstraZeneca, Pfizer, Boehringer Ingelheim, Merck, Roche
Speakers' Bureau: AstraZeneca, Pfizer, Merck, Roche, Merck Sharp & Dohme, Bristol Myers Squibb, Boehringer Ingelheim
Research Funding: Merck Sharp & Dohme (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Pfizer (Inst), Merck (Inst), Roche (Inst), Boehringer Ingelheim (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Delattre JY, Krol G, Thaler HT, et al. : Distribution of brain metastases. Arch Neurol 45:741-744, 1988 [DOI] [PubMed] [Google Scholar]
- 2.Gavrilovic IT, Posner JB: Brain metastases: Epidemiology and pathophysiology. J Neurooncol 75:5-14, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Morgensztern D, Ng SH, Gao F, et al. : Trends in stage distribution for patients with non-small cell lung cancer: A National Cancer Database Survey. J Thorac Oncol 5:29-33, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Waqar SN, Waqar SH, Trinkaus K, et al. : Brain metastases at presentation in patients with non-small cell lung cancer. Am J Clin Oncol 41:36-40, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waqar SN, Morgensztern D, Govindan R: Systemic treatment of brain metastases. Hematol Oncol Clin North Am 31:157-176, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Gaspar L, Scott C, Rotman M, et al. : Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 37:745-751, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Sperduto PW, Kased N, Roberge D, et al. : Summary report on the graded prognostic assessment: An accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 30:419-425, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sperduto PW, Berkey B, Gaspar LE, et al. : A new prognostic index and comparison to three other indices for patients with brain metastases: An analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys 70:510-514, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Sperduto PW, Yang TJ, Beal K, et al. : Estimating survival in patients with lung cancer and brain metastases: An update of the graded prognostic assessment for lung cancer using molecular markers (Lung-molGPA). JAMA Oncol 3:827-831, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sperduto PW, Yang TJ, Beal K, et al. : The effect of gene alterations and tyrosine kinase inhibition on survival and cause of death in patients with adenocarcinoma of the lung and brain metastases. Int J Radiat Oncol Biol Phys 96:406-413, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borgelt B, Gelber R, Kramer S, et al. : The palliation of brain metastases: Final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 6:1-9, 1980 [DOI] [PubMed] [Google Scholar]
- 12.El Gantery MM, Abd El Baky HM, El Hossieny HA, et al. : Management of brain metastases with stereotactic radiosurgery alone versus whole brain irradiation alone versus both. Radiat Oncol 9:116, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mulvenna P, Nankivell M, Barton R, et al. : Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): Results from a phase 3, non-inferiority, randomised trial. Lancet 388:2004-2014, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurtz JM, Gelber R, Brady LW, et al. : The palliation of brain metastases in a favorable patient population: A randomized clinical trial by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 7:891-895, 1981 [DOI] [PubMed] [Google Scholar]
- 15.Murray KJ, Scott C, Greenberg HM, et al. : A randomized phase III study of accelerated hyperfractionation versus standard in patients with unresected brain metastases: A report of the Radiation Therapy Oncology Group (RTOG) 9104. Int J Radiat Oncol Biol Phys 39:571-574, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Weltman E, Salvajoli JV, Brandt RA, et al. : Radiosurgery for brain metastases: A score index for predicting prognosis. Int J Radiat Oncol Biol Phys 46:1155-1161, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Billing PS, Miller DL, Allen MS, et al. : Surgical treatment of primary lung cancer with synchronous brain metastases. J Thorac Cardiovasc Surg 122:548-553, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Chidel MA, Suh JH, Greskovich JF, et al. : Treatment outcome for patients with primary nonsmall-cell lung cancer and synchronous brain metastasis. Radiat Oncol Invest 7:313-319, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Araujo LH, Baldotto C, Castro G Jr, et al. : Lung cancer in Brazil. J Bras Pneumol 44:55-64, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Instituto Brasileiro de Geografia e Estatística (IBGE) : Pesquisa Nacional por Amostra de Domicílios 2008. IBGE. https://biblioteca.ibge.gov.br/visualizacao/monografias/GEBIS%20-%20RJ/panorama.pdf [Google Scholar]
- 21.da Silva MJS, O'Dwyer G, Osorio-de-Castro CGS: Cancer care in Brazil: Structure and geographical distribution. BMC Cancer 19:987, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finke I, Behrens G, Weisser L, et al. : Socioeconomic differences and lung cancer Survival—Systematic review and meta-analysis. Front Oncol 8:536, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ebner PJ, Ding L, Kim AW, et al. : The effect of socioeconomic status on treatment and mortality in non-small cell lung cancer patients. Ann Thorac Surg 109:225-232, 2020 [DOI] [PubMed] [Google Scholar]
- 24.Houston KA, Mitchell KA, King J, et al. : Histologic lung cancer incidence rates and trends vary by race/ethnicity and residential county. J Thorac Oncol 13:497-509, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsukazan MTR, Vigo Á, Silva VDD, et al. : Lung cancer: Changes in histology, gender, and age over the last 30 years in Brazil. J Bras Pneumol 43:363-367, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun S, Schiller JH, Gazdar AF: Lung cancer in never smokers—A different disease. Nat Rev Cancer 7:778-790, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Freitas HC, Torrezan GT, da Cunha IW, et al. : Mutational portrait of lung adenocarcinoma in Brazilian patients: Past, present, and future of molecular profiling in the clinic. Front Oncol 10:1068, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silvestri GA, Gonzalez AV, Jantz MA, et al. : Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 143:e211S-e250S, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Faroni LD, Rosa AA, Aran V, et al. : Access of patients with lung cancer to high technology radiation therapy in Brazil. JCO Glob Oncol 7:726-733, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]