Abstract
Self-tracking can help personalize self-management interventions for chronic conditions like type 2 diabetes (T2D), but reflecting on personal data requires motivation and literacy. Machine learning (ML) methods can identify patterns, but a key challenge is making actionable suggestions based on personal health data. We introduce GlucoGoalie, which combines ML with an expert system to translate ML output into personalized nutrition goal suggestions for individuals with T2D. In a controlled experiment, participants with T2D found that goal suggestions were understandable and actionable. A 4-week in-the-wild deployment study showed that receiving goal suggestions augmented participants’ self-discovery, choosing goals highlighted the multifaceted nature of personal preferences, and the experience of following goals demonstrated the importance of feedback and context. However, we identified tensions between abstract goals and concrete eating experiences and found static text too ambiguous for complex concepts. We discuss implications for ML-based interventions and the need for systems that offer more interactivity, feedback, and negotiation.
Keywords: Personal Informatics, Machine learning, Goal setting, Diabetes self-management
1. INTRODUCTION
Self-managing chronic conditions like type 2 diabetes (T2D) presents continual burden because it impacts countless choices individuals make in their daily lives [11]. Making healthy choices requires literacy and sustained motivation [11], and self-management is further complicated by the need for reflection and self-discovery due to high individual differences: the same choices can have profoundly different health impacts for different individuals [2, 70, 103]. These challenges contribute to growing health disparities; low income and minority communities have higher prevalence and worse outcomes from chronic diseases and lower access to resources like diabetes education [15, 46, 80].
Mobile health (mHealth) technologies can support self-management by leveraging behavior change techniques to help scaffold and support daily choices [55, 60]. Mobile and wearable devices also enable the collection of personal health data—such as diet and physical activity—which can lead to insights about the relationship between an individual’s behavior and their health state [23, 35, 71]. The field of personal informatics examines the use of personal data for reflection and increased self-knowledge, which could lead to improved health [64].
Despite this potential, many data-driven health interventions suffer from high user burden and low adoption [21, 61, 64]. The majority of interventions that incorporate self-tracking focus on viewing, visualizing, or reflecting on personal data. These approaches place the burden on individuals to derive insights from their data and determine how to change their behavior [47, 66]. As a consequence, individuals with low technology and health literacy, who are most impacted by chronic diseases, are least equipped to reap the benefits [98, 99].
One approach to help individuals more easily derive insights from their data is to apply machine learning (ML) to find patterns and make predictions. Recent research initiatives have demonstrated high accuracy in broadly health-related tasks [44, 85]. ML methods can be applied to personal health data to find patterns of association between multiple streams of self-tracking data [6] or forecast changes in blood sugar levels [27].
However, incorporating ML into personal health applications has its own challenges. Interpreting the output of an ML algorithm also requires knowledge and skills, and can be just as challenging as exploring self-tracking data. What’s more, even if ML can identify insightful patterns, those patterns may not be sufficient to help an individual understand how to change their behaviors: they may not be actionable if there is no information about what an individual can do to change or mitigate the unwanted outcomes [6, 47]. For example, an identified correlation between weather and physical activity may be less actionable without specific suggestions for how to stay active on rainy days [6]. Similarly, a prediction of high blood sugar may be less actionable without explaining what contributed to the forecast or how to mitigate it [27]. Generating suggestions that inform individual action is the heart of the field of recommender systems (RecSys) [87, 95]. However, even for health-aware RecSys, ML is used to infer preferences, rather than the health impact of different choices; the health constraints for recommendations are assumed, not learned with ML [32, 83]. Other recent work has sought to incorporate recommendations based on ML-derived insights from self-tracking data, but were limited to an individual’s own past meals and therefore lacked variability [103], or relied on user’s self-perceptions of what behaviors impact health and were therefore unsurprising and less useful to users [47]. Thus, there a need for new approaches to translating inferences achieved with ML into recommendations that can guide individuals’ action.
To address these research gaps, we developed an approach to couple ML inferences with an expert system in order to generate actionable recommendations; we used this approach to design a system called GlucoGoalie that makes personalized suggestions for nutritional goals for individuals with T2D. GlucoGoalie uses ML to identify patterns in self-tracking data—meals and BG levels captured with the GlucoGoalie smartphone app—regarding the relationship between nutrition and change in BG after meals. Furthermore, GlucoGoalie relies on a rule-based expert system to translate ML output into actionable support by generating natural language recommendations for nutrition goals in order to improve BG levels. These goals reflect both individual patterns identified with ML and expert knowledge regarding ways to improve BG management. Goal setting is a common approach to behavior change interventions, with demonstrated efficacy [30, 74, 75]. Each personalized goal is a suggestion to increase or decrease the amount of a macronutrient in meals, or to replace one macronutrient with another. Finally, GlucoGoalie helps individuals work towards achieving their goals by asking them to self-assess prospective meals on their consistency with selected goals during logging and to review a summary of goal achievement.
We evaluated GlucoGoalie in two complementary studies conducted with individuals with T2D recruited from economically disadvantaged communities. First, in a controlled experiment, we assessed whether participants understood goal recommendations generated by GlucoGoalie, and whether they could act on them to compose meals that were consistent with the recommendations using paper food cutouts. Second, we sought to understand the experience of using ML-derived health goals in-the-wild with a 4-week deployment study.
The controlled experiment showed that participants understood goals and chose the correct meal for a given goal with 89% accuracy when meals were accompanied by nutritional labels (only 49% accuracy with meal images that did not include labels). When composing meals using paper food cutouts, participants chose meals that were consistent with the direction of their selected goal 67% of the time (68 of 102; p < 0.001). This suggested feasibility of using computationally generated goal recommendations to inform individuals’ meal choices.
In the deployment study, participants found GlucoGoalie easy to use, fun, and engaging. Participants’ self-discovery, which is typical for self-tracking apps, was further augmented and informed by the experience of receiving suggestions for personalized goals. How they chose which goal to follow highlighted the importance and multifaceted nature of personal preferences in nutrition recommendations. For example, participants looked for goals that were consistent with both their food preferences and their desire for variety in their diets. When working towards achieving their selected goals, participants described a desire for additional feedback on their progress. Moreover, they alluded to multiple contextual factors, such as their level of physical activity, previous meals, or even the season, that rendered certain goals as more or less useful in their daily lives. In addition, participants wished for a greater connection between goals that focused on their meals and their broader goals in life and health, such as reducing the need for medication. At the same time, some participants described difficulty understanding or acting on goal recommendations. Static text was sometimes ambiguous in communicating the goal’s meaning, and because goals were based on abstract concepts like macronutrients, some participants had difficulty connecting goals to their concrete meal decisions.
This work contributes to a newly emerging understanding of how ML-based methods can be used to make actionable suggestion to support self-management of chronic conditions. Specifically, it outlines a particular approach to translating ML inferences into actionable recommendation by coupling ML with a rule-based expert system. We discuss ways in which systems intended to support action can also support reflection, describe how our approach to personalizing based on health constraints is complementary to existing approaches to ML-based personalization, and argue for the need for systems that offer more interactivity, feedback, and negotiation.
2. RELATED WORK
2.1. Diabetes Self-Management and Personalization
In chronic conditions like type 2 diabetes (T2D), daily lifestyle behaviors, including food choices and physical activity, impact short- and long-term health status. A key goal in T2D self-management is keeping blood glucose (BG) levels within target ranges. Dietary decisions have a direct impact on BG after each meal, but there is a high degree of variability between and within individuals. The same meal can have wildly different glycemic impacts across individuals [103], and the American Diabetes Association (ADA) recommends 1-on-1 counseling with diabetes educators to determine personalized nutrition goals and macronutrient targets [2]. Notably, it can be difficult for individuals to anticipate the impact of a particular meal on BG, even for experts [66].
2.2. Personal Informatics
Technologies for health and wellness have been a vibrant research area within HCI for several decades [55] with research focusing on persuasive behavior change interventions [81], health-focused games [41], and online health communities [68], among many other areas.
In addition to these more general interventions, many previous investigations have specifically focused on approaches to leveraging data collected with self-tracking. Personal informatics refers to a class of interactive technologies that help users to collect data about their behaviors and health, explore those data for patterns and trends, and engage in self-discovery, which is important for conditions with high individual variability like T2D [18, 64]. Li and colleagues described five stages of the self-discovery process with personal informatics: preparation, collection, integration, reflection, and action [64]. While the first few stages focus on self-tracking of personal data, the latter stages—reflection and action—are about how individuals’ beliefs and behaviors change in light of those data.
When it comes to reflection and action, the lion’s share of previous research has focused on supporting reflection as a means towards greater self-knowledge [5, 33, 57, 65, 67]. Typical personal informatics systems rely on users to actively explore their data, which may require considerable literacy and effort [37, 66]. This approach, while appropriate for highly engaged users with available time and appropriate skills and knowledge [18, 64, 65], may present considerable challenges to the larger population with chronic disease, including individuals with low literacy and numeracy [14, 59]. Attempts to reduce the burden of reflection have focused on lightweight logging approaches that may still spur reflection, like photo or audio journals [25, 33, 40]. Other work has endeavored to support reflection on personal data through collaboration with peers or experts [19, 53, 79, 82], or creates more scaffolding for self-discovery by structuring self-experimentation [26, 50, 51]. Yet others have applied ML to identify patterns in multiple streams of self-tracking data and present them to users [6], or to make predictions about future BG levels based on a meal [27], but these approaches still fall short of direct support for action [47]. Notably, EmotiCal makes predictions about an individual’s future mood, accompanied by recommendations for activities that might improve mood, but those recommendations are based on user-perceptions of what will improve mood, not direct inference from self-tracking data, and were therefore unsurprising and less useful to users [47]. In our research, we aim to build upon these previous efforts and use ML not only for arriving at inferences but adapt ML with an expert system to provide direct support for action by generating nutritional goal recommendations.
2.3. Data-Driven Goal Setting For Behavior Change
Goal setting is a common element in behavior change interventions [72] with demonstrated efficacy in mHealth applications [30]. Research in HCI has identified a number of important characteristics for goal setting applications including the need for autonomy in allowing users’ input in choosing their own health goals, as well as the importance of helping individuals track progress in goal attainment with a visual summary [24]; this prior work informed design decisions for GlucoGoalie.
Many goal setting interventions help users articulate their health goals or choose from a set of options. Some approaches have sought to automate aspects of the goal-setting process, for example through interactive dialogs [9], or with adaptive goals [58]; however, these approaches tend to focus on a single dimension of tracking, like step counts or calories, and may not be applicable for more complex nutritional choices [83]. Recent work by Schroeder [92] highlighted an interconnection between an individual’s goals and the types of self-tracking they engage in. In line with goal-directed tracking, GlucoGoalie uses self-tracking of meals and BG to inform personalized nutrition goals, and to assess progress in achieving selected goals over time through tracking.
2.4. Recommender Systems
Another set of methods and interventions for personalized support based on individual user data are recommender systems (RecSys) [87]. RecSys are especially common in the consumer space, for example recommending items for purchase on an e-commerce site, or videos on a streaming platform. In the case of health, however, applying RecSys approaches is more challenging [90]. RecSys aim to personalize recommendations based on user preferences but can have unintended consequences because what individual prefers and what is healthy may be at odds. An emerging research area aims to incorporate health constraints into RecSys methods [31, 32, 89, 102] based on user-specified diet preferences [102], weight loss goals [31], or population-level guidelines [83, 89]. While these approaches make important strides towards healthy recommendations, it is important to note that even these systems use ML primarily to infer user preferences, and impose assumed a priori, rather than learned, constrains on health [88]. Little work has explored how to learn personalized diet constraints or goals from self-tracking data.
3. GLUCOGOALIE
We designed GlucoGoalie through a user-centered design process building on our prior research with individuals with T2D from a predominantly Black and Latino economically disadvantaged community [86]. Figure 1 presents an overview of the pipeline for generating personalized goals. GlucoGoalie’s goal-generating engine includes two main components: a machine learning algorithm for detecting patterns of association between nutrition in meals and changes in BG levels, and an expert system that uses expert knowledge to generate recommendations for nutritional goals in order to improve BG levels. We describe these in more detail below.
Figure 1:
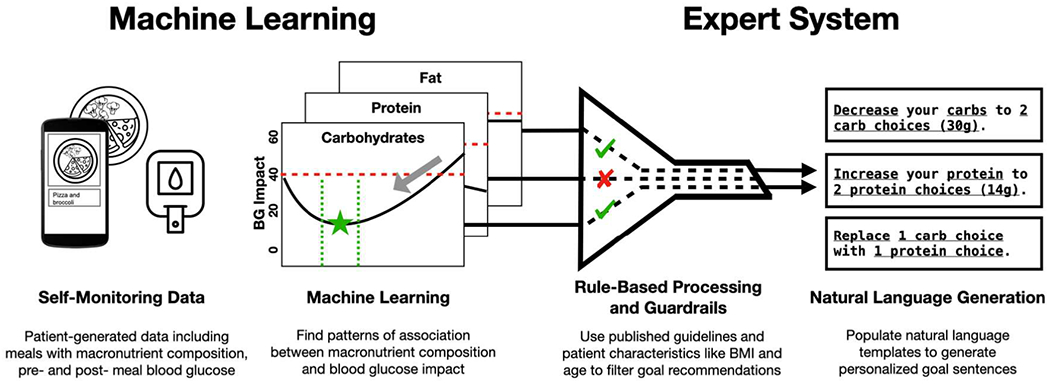
An overview of the pipeline for generating personalized goal recommendations in GlucoGoalie.
3.1. Approach to Goal Setting
Our aim was to generate personalized recommendations for nutritional goals that can be actionable and easily understood by individuals with mixed levels of literacy. One of the key decisions in the design of GlucoGoalie was regarding the level of specificity in nutritional goals. We worked with a group of Certified Diabetes Educators (CDEs, n=3) to formulate goals that are consistent with the ones used in typical diabetes education and that focus on changes to macronutrient composition of meals. We made this choice for three main reasons. First, the macronutrient composition of a meal is directly related to its impact on BG, but the specifics of the relationship vary between individuals [36]. Second, nutrition education in diabetes emphasizes macronutrients to help individuals think flexibly about the nutritional composition of similar foods [101]. Third, using macronutrients as features has advantages for machine learning, offering a denser, low-dimensional feature representation compared to other representations like the specific food items in a meal. We worked with CDEs to create templates for goals that could be populated by an ML algorithm and identified three types of changes to meal composition that could impact post-meal BG: increase the amount of macronutrient, decrease the amount, or replace one macronutrient with another. Goals are meal-level because the balance of each meal has its own impact on BG, making day-level goals (e.g., daily calories) less appropriate. See Table 1 for a selection of goals.
Table 1:
A selection of nutritional goals available in GlucoGoalie. Generic goals are available for all users from when they first use the app. Personalized goals are recommended for an individual based on ML-based analysis of recorded meals and blood glucose readings. Underlined words are personalized for each user based on their data. Note: A food “choice” is a unit similar to a serving size that identifies servings of different foods with similar macronutrient compositions.
| Type | Title | Description |
|---|---|---|
| Personalized | Decrease your carbs to 2½ carb choices | For high carb lunches, decrease your carbs to be about 2½ carb choices (38g). An example of 1 carb choice is 1 slice of whole wheat toast, cup of plantains, or cup of brown rice. |
| Personalized | Increase your protein to 3 protein choices | For low protein dinners, increase your protein to be about 3 lean protein choices (21g). An example of 1 lean protein choice is 1 ounce of lean ground beef, ½ cup of tofu, or 1 ounce of chicken breast. |
| Personalized | Replace 2 carb choices with 2 protein choices | For high carb dinners, replace 2 carb choices with 2 lean protein choices. For example, replace cup of rice with 2 ounces of ground turkey or 2 ounces of tilapia. |
| Generic | Choose whole fruits | Choose whole fruits instead of fruit juices. For example, have a whole orange, an apple, or a cup of berries with your meals. |
| Generic | Choose plant proteins | Include proteins that come from plants, such as beans, nuts and seeds, and legumes. For example, choose a cup of beans, a handful of peanuts, or a cup of lentils to add protein to your meal. |
3.2. Machine Learning
The high-level aim of the ML problem was to infer the relationship between an individual’s nutrition choices and changes in their BG levels after meals. The features in the ML problem are the meals a user has logged, specifically the grams of carbohydrates, protein, and fat. The outcome of interest—change in BG after a meal—is the difference between self-reported BG before the meal, compared with 2 hours after, which is the clinical standard [4]. The ML method to find patterns of association between nutrition and BG was based on Attributable Components Analysis (ACA), a non-parametric method for estimating the conditional expectation of a quantity of interest based on a set of covariates [96]. Because self-monitoring data are manually entered by users, there are often a small number of data points that are prone to include errors and outliers. These characteristics pose challenges for ML, and ACA has advantages over other methods like linear regression because it is able to capture non-linear relationships, is less sensitive to erroneous data points, and more effectively estimates uncertainty [73].
3.3. Expert System Interpretation and Guardrails
While ML can identify patterns in the relationship between meals and BG, these patterns alone are not sufficient to inform behavior. In a series of 10 sessions, we worked with CDEs to establish rules for interpreting the ML output and translating it into goal recommendations. For example, GlucoGoalie suggests goals only if ML infers patterns with an expected increase in BG above a clinically significant threshold (40 mg/dl). In addition, CDEs pointed out that some automatically generated recommendations might be inappropriate irrespective of their impact on BG, for example a goal to eat 100g of fat in a single meal. To mitigate this concern, we added a set of guardrails to filter out extreme recommendations based on population-level nutrition guidelines.
In co-designing the goal templates with CDEs, we also sought to formulate goals such that they could be understood and acted upon by individuals, even those with low nutrition literacy. Because we could not assume nutrition knowledge, we embedded necessary information within the goals themselves. First, each goal includes three examples of concrete foods rich in a target macronutrient. Examples are drawn from a knowledge base created using an ADA resource [101]. To increase their relevance, examples were selected from meal logs captured by participants of a prior self-tracking study; these participants were recruited from a similar population and captured their regular meals for 2-5 weeks, thus creating a rich collection of meals. Second, we considered multiple approaches to describing target macronutrient amounts, including standard units like grams, heuristics like fists and thumbs, or even the proportions of a plate covered with different types of foods, an approach consistent with ChooseMyPlate [97]. However, we dismissed visualizing proportions on a plate due to their lack of precision (15g of rice could be gathered together in a ball or spread thinly over the entire plate). Instead, we opted for a the ADA-endorsed language of food “choices,” a system meant to simplify nutrition education [101]. A food “choice” is a unit similar to a serving size; it identifies servings of different foods with similar macronutrient compositions. For example, 1 carbohydrate choice is 15 grams, which could be 1 slice of toast or cup of rice. In addition, because “choices” are based on grams, the standard unit on food labels, each goal also includes the target amount in grams.
3.4. The GlucoGoalie App
To explore individuals’ perceptions and experience receiving personalized goal suggestions in-the-wild, we included them in a custom smartphone application with logging and goal-setting functionality.
GlucoGoalie helps individuals set goals for improving their diet and work towards achieving these goals. Users begin by choosing one or more nutritional goals from a list in the app (see Table 1 for a selection of goals). To promote engagement with the application before users have tracked enough meals to receive personalized goal recommendations, all users choose from the same set of “generic” goals at the outset. Each generic goal describes a generally healthy behavior, and was developed by experts in nutrition and diabetes [22]. Twice per week, GlucoGoalie analyzes the data of each user with at least 8 meals to generate personalized nutrition goals, described above. If new goals are available, GlucoGoalie sends a push notification, and users can view the new, personalized recommendations and choose any they wish to follow (Figure 2d).
Figure 2:
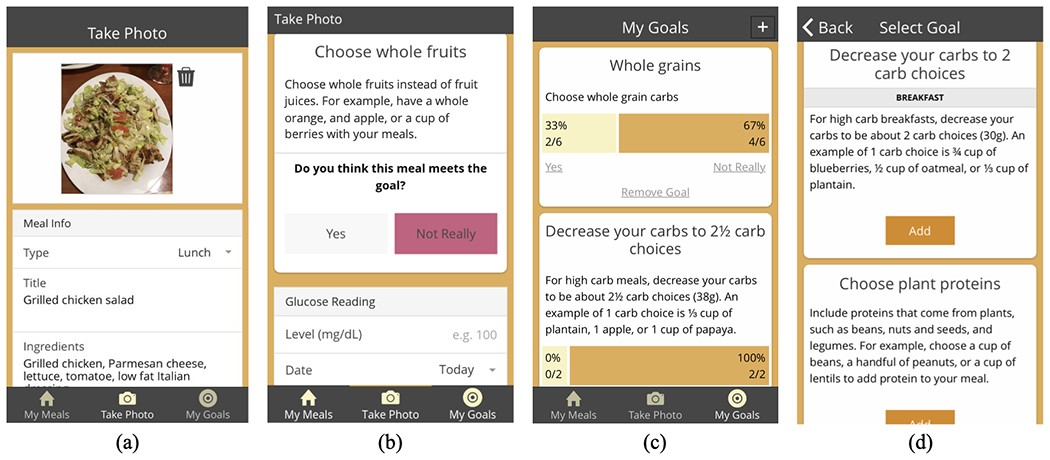
The GlucoGoalie mobile application. (a) Logging a meal with a photo and free text description. (b) Users self-assess whether they met their chosen goals. (c) A summary of goal achievement. (d) Reviewing and choosing new personalized goals to work on after receiving a push notification.
Within the app, user can log their meals and enter their pre-meal BG. Two hours after the meal, GlucoGoalie sends a push notification reminder to enter a post-meal reading. To simplify the logging process, users log meals by taking a picture of the meal and typing a free text description (Figure 2a). Macronutrient data are entered by a team of Registered Dietitians (RDs) who assessed each meal following a standard protocol based on the USDA nutrition database [52], but similar results could be attained via crowdsourcing [78]. To keep goals as a central part of the experience and promote accountability, GlucoGoalie prompts users to assess whether their meal fits with each of their chosen goals while logging with either “Yes” or “Not Really” (Figure 2b); “Not Really” was identified as a preferred and less judgmental option than “No” during user-centered design. Users can view their current goals, remove or choose new goals, and review a summary of goal attainment in the My Goals section of the app (Figure 2c).
4. STUDY 1: CONTROLLED EVALUATION
In the first evaluation study, we sought to assess whether the types of personalized goals generated by the system would be understandable and actionable for individuals with T2D.
4.1. Controlled Evaluation Methods
4.1.1. Participants and Procedure.
Participants were recruited from two types of health centers in a major US city: 1) a Federally Qualified Health Center (FQHC) in Brownsville Brooklyn, and 2) clinics affiliated with Columbia University Irving Medical Center. To be included, participants needed to be between 18- and 65-years-old with a self-reported diagnosis of type 2 diabetes (T2D) and proficient in English. After collecting consent, participants received a 10-minute, in-person nutrition training introducing the concept of food “choices” and reviewing macronutrients. Participants then completed the following three tasks:
Task 1: Goal Comprehension.
To assess whether participants understood the content of the goals output by GlucoGoalie, participants were presented with an example goal “for a friend with diabetes,” and asked to choose which of two meals were a better fit with the goal. Meals were presented as a free text description with a nutrition label in the style of Facts Up Front [105] (see Supplementary Figure A); nutritional labels were included to ensure that this task was testing comprehension of goals, rather than individuals’ ability to assess nutritional composition of meals. This task was repeated twice.
Task 2: Goal/Image Matching.
Because many meals are cooked at home, we included a second task to test comprehension of goal sentences using example meals without nutritional labels. Participants were again asked to choose which of two meals was a better fit with a presented, example goal, but could see only the meal image and description, with no macronutrient information (see Supplementary Figure B). Meal images were selected from a data set collected in ongoing research with individuals from a similar population. Meal pairs were chosen to include similar ingredients but vary in macronutrient content; the incorrect answer was at least 1 macronutrient “choice” different from the correct answer, and the difficulty varied across scenarios. To account for higher variability in meal images, this task was repeated for eight goal/meal-pair combinations.
Task 3. Meal Choice: The Virtual Buffet.
To assess whether goals were actionable and participants could follow them, we simulated the process of choosing meals with a “virtual buffet” made up of food image cutouts (see Supplementary Figure C). Participants then received a tailored goal and were asked to choose additional meals with that goal in mind. Working
1-on-1, researchers asked participants to use the food cutouts to assemble a baseline meal for each type of meal (breakfast, lunch, and dinner) that was “closest to what you would normally eat.” Importantly, there were multiple copies of each food item so participants could vary the amount of each food. Images were labeled with an amount in cups, tablespoons, or ounces, but never choices or grams (the units included in the goals themselves). We used images from a web-based resource [76] and our ongoing research and sought to include common staples like bacon and eggs as well as culturally relevant foods like fried plantains.
The baseline meals were used to identify goals that would require participants to vary from their typical macronutrient behavior by 1 to 1.5 macronutrient choices. For example, if a participant’s baseline meal had 3.5 carb choices and 1 protein choice, they would receive two goals: one to decrease carbs to 2 choices and another to increase protein to 2 choices. Participants chose one of the two goals, and then assembled “breakfast,” “lunch,” and “dinner” for three days in a row, with the chosen goal in mind (9 total meals). Researchers tallied the chosen food items to calculate nutrient compositions. During the 1-on-1 activity, researchers made note of participant comments and feedback, for example, questions about missing or inappropriate food items.
4.1.2. Data Analysis.
For the goal comprehension and goal/image matching tasks, we calculated binary accuracy as a percentage (#correct/ [#correct + #incorrect]). For the “virtual buffet” experiment, we analyzed the data in two dimensions: direction and accuracy. First, we examined whether participants’ meals were consistent with the direction of their chosen goal. For example, if the goal was to increase protein to 2 choices at lunch, we assessed whether subsequent lunches had more protein than baseline. A binomial test was used to determine whether performance was better than chance. Second, accuracy in meeting the goal target was measured with mean absolute error between participant choices and the goal target. For example, if the target was 2 choices, we assessed how close participant’s meals were to the target, on average.
To synthesize participants impressions from their comments during the study, research met to debrief and aggregate notes in a series of meetings to summarize key themes.
4.2. Controlled Evaluation Results
4.2.1. Participants.
We recruited and enrolled a total of 19 participants, including 10 from a Federally Qualified Health Center, and 9 from university-affiliated clinics. Four participants were excluded because of a data collection error for a total of 15 participants included in the analysis. As seen in Table 2, participants were predominantly female, and Black or Hispanic. Most were overweight or obese (body mass index ≥ 25).
Table 2:
Participant demographics for both studies
| Demographics | Study 1: Controlled Experiment | Study 2: Deployment Study |
|---|---|---|
| N Enrolled (Incl. in Analysis) | 19 (15) | 10 (8) |
| Sex | 80% Female | 71% Female |
| Ethnicity | 47% Hispanic | 86% Hispanic |
| Race | 17% White | 43% White |
| 42% Black | 29% Black | |
| 41% Other/Refused | 29% Other/Refused | |
| Age | 54 ± 9 years | 55.7 ± 9.5 years |
| Body Mass Index (BMI) | 37.4 ± 13.9 | 41.8 ± 14.4 |
| Median Household Income | $20,000-$39,999 | $40,000-$59,999 |
| Median Education Level | Some College | Some College |
4.2.2. Results.
For the goal comprehension task, when choosing which of the two nutrition labels met a given goal participants were correct 89% (SD = 21%) of the time. When choosing which of two meal images was a better match with a goal, participants chose the correct meal 49% (SD = 25%) of the time. When composing meals at the virtual buffet, meals were consistent with the direction of chosen goals 67% (68 of 102) of the time, significantly more than chance per a binomial test (p < 0.001). There was no difference in the percentage of meals consistent with the direction of chosen goals by meal type, macronutrient, or direction of goal. At the same time, there was a high degree of variability in precisely meeting the goal target. Meals were an average of 0.83 (SD = 0.56) “choices” away from the goal target. For example, given the goal “reduce carbs to 2 choices (30g),” participants were an average of carb choices (12g), from the target.
We identified two key themes in the comments made by participants during and after the virtual buffet activity. First, most participants commented on the limited selection of food items to choose from. Many recounted what they would normally eat, which was sometimes missing, for example oatmeal at breakfast. Usually, participants were able to select items they do eat from the available choices. Second, when choosing which goal to follow, participants often stated that they understood the goals. However, use of “choices” as a unit led to confusion, and some participants expressed uncertainty about how much food to take. Participants interpreted “2 choices” to mean two different food items, regardless of the amount (e.g., rice and bread) as opposed to a measurable quantity (e.g., cup of rice), as intended.
5. STUDY 2: DEPLOYMENT STUDY
In the second study, we sought to better understand participants’ experience receiving and following personalized goal recommendations from an app, based on their own self-tracking data, and conducted a 4-week deployment study with GlucoGoalie. Due to the timeline of this externally funded project, we did not update the design between the two studies.
5.1. Deployment Study Methods
5.1.1. Participants and Procedure.
Participants were recruited from Columbia University Irving Medical Center, with the same inclusion criteria as study 1. In an initial visit, participants received a one-hour nutrition training introducing relevant concepts like food “choices” and macronutrients. An investigator introduced participants to the GlucoGoalie application, helped them set an initial goal of their choice, and asked them to log meals at home with BG readings before and two-hours after meals, for 4 weeks. During training, we told participants that GlucoGoalie would recommend goals based on their own records, that these goals were made by a computer, not a human expert, and that available goals would change over time. In addition to push notifications, the study coordinator also contacted participants the very first time they received personalized goals. After the 4-week period, participants returned for 1-hour semi-structured interviews. To minimize barriers to participation, individuals without smartphone received an Android phone and could keep it after completing the study (participants who had their own smartphones received its monetary equivalent, $150). All participants received $20 for the initial visit and debrief interview.
5.1.2. Data Analysis.
We downloaded usage log data from the application server and calculated descriptive usage statistics including the numbers of meals logged, goals selected, and goals used. Debrief interviews were audio recorded and transcribed verbatim. We analyzed interview transcripts and usage logs with inductive thematic analysis [12]. The lead author coded 2 transcripts (25%) collaboratively with a second author to create an initial codebook. Then the first and senior author independently coded an additional 2 transcripts (25%), and met in person to discuss coding schemes and resolve all discrepancies through discussion. The remaining interviews were coded independently by the first author with periodic discussion with the research team, followed by an affinity mapping session to group codes into primary themes and subthemes. Participant meal logs, usage, and goal attainment were considered throughout the coding process to contextualize user statements. After coding was complete, we examined data saturation and theme comprehensiveness across participants [39, 43].
5.2. Deployment Study Results
Below, we briefly describe participants’ demographics and usage of the GlucoGoalie app, and then continue to describe the four main themes from the thematic analysis: 1) receiving goal suggestion informs self-discovery, 2) choosing goals highlights individual preferences, 3) following goals demonstrates the importance of feedback and context, and 4) challenges understanding and following goals in practice. As shown in Table 3, data saturation was reached and themes were prevalent across participants.
Table 3:
Prevalence of themes across participant interviews. Each purple-shaded cell indicates that a theme was present for a participant. Theme 1 was prevalent in 100% of interviews, while Theme 4 was prevalent in 50% of interviews.
| P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | |
|---|---|---|---|---|---|---|---|---|
| Theme 1 - Receiving goal suggestion informs self-discovery | ||||||||
| Theme 2 - Choosing goals highlights individual preferences | ||||||||
| A checkpoint or a challenge | ||||||||
| Importance of personal food preferences | ||||||||
| Theme 3 - Following goals demonstrates the importance of feedback and context | ||||||||
| Fitting goals with the context of daily life | ||||||||
| Importance of feedback and seeing progress | ||||||||
| Theme 4 - Challenges understanding and following goals in practice | ||||||||
| Balancing abstract and concrete in nutrition goals | ||||||||
| Imprecision of text for delivery of goal suggestions |
5.2.1. Participant Background and Demographics.
A total of 10 participants were enrolled in the deployment study. Two participants were lost to follow-up or withdrew for personal reasons, for a total of 8 participants who completed the deployment study and were included in the analysis. There was some overlap between the controlled experiment and the deployment study, with 2 participants completing both studies. As shown in Table 2, the demographic breakdown was comparable across the two studies. While median income was higher in the deployment study, the distributions were similar, and the deployment study clinics were in an area with a higher cost of living (Manhattan vs. Brooklyn).
Participants had mixed and often poor experiences with self-management prior to enrolling in the study. Many reported poor eating habits and being indiscriminate about their meals:
“… before that I eat whatever. Yeah, whatever. Dinner time, I eat whatever.” P2
Others often skipped meals, which led to overeating later in the day:
“… I only skip breakfast. I wasn’t always very good about lunch. So, then I’ll be famished. I would eat crap because I was hungry.” P5
Along with challenges with nutrition, participants described challenges keeping their BG within target ranges:
“Sometimes [my blood sugar] goes very high or goes very low.” P4
“Yeah, a mess, my sugar level was high everyday 300, and the doctor was upset to me.” P1
Some participants had tried prior bouts of focused self-management, with mixed success in the long run. Three participants had previously tracked their meals on paper, but none had tracked with an app.
5.2.2. Impressions and Usage.
Overall, participants reported that they enjoyed the experience of using GlucoGoalie, and found it fun, easy, and direct.
“It was fun. They laugh about me because every time I was going to eat — no, wait a minute. I can’t start eating. I’ve got to take a picture of it… It was fun to play.” P2
They also actively engaged with the main part of the app: setting and following goals.
“I try to follow the goals and instructions if I’m trying to improve my intake. That’s what I’m trying to do most of the time. Because every day I try to follow a better diet and try to have more greens.” P4
Usage statistics over the 4-week study are presented in Table 4. Participants showed high engagement with logging features: on average they recorded more than 3 meals per day, and all participants set at least one goal. However, only about 40% (3 of 8) of participants actively engaged with different features of the app, such as setting new goals and viewing progress towards goal achievement; these were savvy users of smartphones with previous experience using apps. In contrast, most of the participants in the study (5 of 8; about 60%) were more accustomed to using their smartphones exclusively to make phone calls and rarely used any apps. These individuals often took a minimalist approach to engaging with GlucoGoalie: they tracked their meals and assessed these meals on fit with chosen goals, but did not engage with any other features without prompts from investigators.
Table 4:
GlucoGoalie usage statistics over the 4-week study (N=8)
| Usage Variable | Value |
|---|---|
| # meals logged | Median: 93.5 |
| Range: 14 to 158 | |
| # BG readings logged | Median: 173 |
| Range: 21 to 314 | |
| % received personalized goals | 88% (7 of 8) |
| % used personalized goals | 63% (5 of 8) |
| # unique personalized goals used during study period | Median: 3 goals |
| Range: 0 to 8 | |
| Pre-meal BG (mean ± SD) | 135.0 ± 36.7 mg/dL |
| Post-meal BG (mean ± SD) | 156.1 ± 47.7 mg/dL |
Regarding personalized goals, 88% of participants (7 of 8) received personalized goals while in the study; one participant did not receive any personalized goals because their BG levels were well-controlled. Of those who received a goal recommendation, 71% (5 of 7) selected at least one of these goals in the app. However, 3 participants did not notice a push notification informing them of a new goal suggestion, and only selected one after a call from the study coordinator.
As a result of following the goals they had chosen, many participants developed new habits, and internalized personalized goal suggestions to the point that they became integrated with their daily practice:
“Even anything longer than two weeks will probably just make it into more of a habit for me. I’ll probably eat two weeks to get comfortable with how much fat I’m taking, let’s say the goal was on fat, so then after that it would just be more of a habit.” P8
At the end of the study, many participants described seeing changes not just in their behaviors, but also in their actual blood glucose levels.
“I did notice because sometimes it was 200. When I see that it was 200, it was after I eat. Oh yeah. After I—but before, 250, 270—because I was eating a lot of food. Five or six in the night.” P2
“And the sugar went down. … Today, I tested, it was 121.” P6
5.3. Theme 1—Receiving Goal Suggestion Informs Self-Discovery
To personalize goals, GlucoGoalie included features for tracking meals and BG levels. The study showed that even these requisite tracking features often led to discoveries and new insights. Furthermore, the experience of viewing both generic and personal goal suggestions helped individuals critically reflect on their behaviors, thus serving as an additional catalyst for learning.
Through tracking and reflecting on their meals, participants described some of the patterns and insights they observed between the foods they were eating and their BG levels.
“I did it for two days and I tested my sugar, oh, this is the rice… So, I stopped eating rice for two days, and then when I stopped eating rice, it got lower.” P6
Beyond tracking, the goal setting features in GlucoGoalie scaffolded the self-discovery process. For example, P2 learned from their goal to “eat whole fruits instead of juice”
“When I drink the juice, I see that sugar is what was high. And I learned that that was the problem. … Now, when I eat, I don’t drink juice.” P2
In many cases, participants used the personalized goal recommendations they received to reflect on their behaviors and sought to reconcile these recommendations with what they had already knew or suspected about themselves.
“And I know that, my carbs like I said, are usually high. I think that, my first, what I gravitate to first in any meal is the carb and that’s what I want more of… So, I’m not like surprised that it recommends reducing the carbs and trying to replace it with something else.” P3
Participants sometimes noted that the goal they received was something they were already trying to work on. For example, P4 described their reaction to receiving a suggestion to reduce the amount of fat in their meals:
“I’m trying to decrease the amount of food and so that’s why, I think it’s important to decrease the amount of fat and that is one of the problems that I have with the fat.” P4
Receiving personalized goal suggestions provided a reference point for participant’s own views of their self-management pitfalls and needs, as well as a jumping off point to guide reflection on their behaviors.
5.4. Theme 2—Choosing Goals Highlights Individual Preferences
5.4.1. A Checkpoint or a Challenge.
Most participants commented that some goals in the GlucoGoalie app seemed harder to achieve than others. However, when choosing which goals to follow, participants took a variety of different approaches. Some participants chose goals that seemed highly achievable, or were the sorts of behaviors they were already doing regularly; these participants viewed goals as a checkpoints or reminders to be more consistent.
“I like that it was a goal that it was more feasible to me. So, it was just a good like a checkpoint for me not sort of a reminder but kind of like, oh it’s going with what I’m doing. So, it’s just reminding me.” P8
In contrast, other participants were interested in choosing goals that were more challenging as self-motivation to change their current habits.
“Yes, I go to the notification and started looking at the new one. That’s why, when I first took the other substitute of water for over sodas. I realized, well that’s not really a goal because I’ve been doing that already. So, I need to change to something more difficult because I was done with the other one.” P4
5.4.2. Importance of personal food preferences.
In addition to the perceived degree of challenge in a given goal, personal likes and dislikes regarding different foods factored in prominently to participants’ decisions of which goal to choose. To illustrate personalized goals, GlucoGoalie included three examples with different foods at the end of each goal (see Table 1). For many participants, these examples were critical factors to deciding whether to try a goal or not. When asked to explain why they selected a particular personalized goal, participants often referenced the examples as their justification for selecting or eliminating a goal from consideration.
“That one is okay, because I used to eat the oatmeal, one slice of toast, yeah that one is okay.” P1
Along with expressing their interest or distaste for certain foods, participants also mentioned the importance of variety, and opted for suggestions that incorporated new ideas to break what they perceived as the monotony of healthy eating. For example, P2 was looking for examples of vegetables they could eat other than broccoli:
“I don’t know, like, if I want to eat like broccoli, I will be tired. And I’m not going to eat it every day.” P2
5.5. Theme 3—Following Goals Demonstrates the Importance of Feedback and Context
5.5.1. Fitting Goals with the Context of Daily Life.
The need for greater personalization extended beyond choosing which goal to pursue and impacted participants’ ability to successfully incorporate new goals within their daily lives. In some cases, participants had established patterns that they did not want to change, for example eating the same thing for breakfast every day because it worked for them, or skipping breakfast entirely because their morning routine did not allow for it. Furthermore, balancing meals within a day or week was just as important. What made sense for an upcoming meal depended in part on what happened earlier in the day.
“Since I’m a busy woman… it kind of just has to go back to like how my day is. So, I know that if I didn’t meet it for one of my meals, I’ll have to meet it for the next meal.” P8
This balance extended to seasonal patterns as well, where different kinds of meals were appealing during different parts of the year.
“I don’t want to have a hearty breakfast compared to like in the winter.” P8
Many participants touted that it was easier to follow goals when preparing their own meals at home, but much harder when eating outside, at a restaurant or other gathering. Goals in GlucoGoalie lent themselves particularly to the home context, but different goals may be useful in other contexts.
“…well at least for me… it was very hard for me to manage using the app when I went out to eat.” P3
When goals felt appropriate also depended on the context of other self-management and health goals, for example exercise. P5 noted that they often include more carbs in their meals after exercising, but less if they have not exercised that day:
“So, I know, if I have exercise, walking or an exercise routine after a meal that’s going to be a little bit more high carbs. That has made an impact.” P5
5.5.2. Importance of Feedback and Seeing Progress.
Participants were eager for feedback on their progress. This included whether they were successfully meeting the goals they had set in GlucoGoalie, for example, whether the amounts of specific macronutrients in their meals were more consistent with their chosen goals. Most participants found this challenging and had to come up with strategies. Some started measuring their foods to get a better sense for portion sizes and proportions:
“When I got after I started, I look for a [measuring] cup and I started to follow the instructions.” P4
In general, participants were eager for feedback on their progress:
“Everybody would like to know how they’re doing… Because if I’m eating less and it’s not doing no good, what’s the point of me doing it?” P2
In particular, many participants described not only the goals they had set with GlucoGoalie, but also their higher-level goals, motivations, and aspirations. These goals were not at the specific and achievable level of “drink more water,” but reflected general desires for leading a healthy life. Importantly, different participants expressed different motivations. Some participants expressed a desire to lose weight, or to see that their blood glucose levels were lowering.
“Definitely in terms of weight loss but like also my actual numbers in terms of my blood sugar.” P3
Other participants were also interested in improving their diabetes management, and had the goal of improving control of blood glucose levels, so that they could reduce their dosage of oral medications like metformin.
“Because I want to keep it as level as possible to try to stay off medications.” P7
5.6. Theme 4—Challenges Understanding and Following Goals in Practice
5.6.1. Balancing Abstract and Concrete in Nutritional Goals.
Nutritional goals in GlucoGoalie included references to both specific foods and food groups, such as “Drink more water” and also macronutrients, such as “replace 1 carb choice with 1 protein choice at lunch”. Many participants’ comments related to the interplay between abstract and concrete when thinking about nutrition.
In general, participants enjoyed goals that were concrete and easy to implement without additional knowledge. This was particularly the case for generic goals that typically targeted familiar foods or food groups.
“Those were right. Those were easy and I’ve been, I have been intentional to drink a bottle of water at every main meal and then have a bottle or two in between.” P5
However, personalized goals were more abstract with a focus on macronutrients rather than specific foods. These goals were typically described as harder to understand and meet.
“The replacement, it was, you know was dropping, half a carb replacing, half carb. That was a little harder to figure out. So, it will require a little more thinking.” P5
Furthermore, participants’ attitudes towards more abstract, macronutrient-oriented goals were influenced by their apparent knowledge of nutrition. About half of participants were comfortable identifying macronutrients, estimating portion size, and discussing steps they could take to meet these goals with their meal choices.
“So, I still go by the basics even from when I went to the nutritionist of like using like my palms, like the two fingers, index fingers. Actually, do work well for like teaspoons and tablespoons.” P8
The other half of participants described themselves as not being familiar with macronutrients and estimating portion sizes. For these participants, goals formulated using macronutrients and “choices” as units presented an impassable barrier and were often dismissed. These participants often referred to using visual proportions of different types of foods on their plate to gauge how healthy their meals were:
“I use my plate, but I try to go as they show me in the program, you see the plate then half it’s a vegetable or fruit, this is a protein and that one is a carbohydrate.” P1
5.6.2. Imprecision of Text for Delivery of Goal Suggestions.
Even for those with higher nutrition literacy, participants were not always consistent in how they interpreted personalized goals, and there were a number of misunderstandings. For example, some terminology, like “choices” as a unit of measure, was often interpreted as an option to choose two different food items, regardless of the amount. P2 described their effort to achieve a goal of eating 2 fat choice (10g) at breakfast by stating that they ate two high fat food, but not the amounts of either:
“Sometimes I put it together, the mozzarella on top of the egg which means I’m taking two fats.” P2 While this meal may have been consistent with P2’s goal, they are saying they believe they achieved their goal because they chose two fat-based ingredients, not because the amount of total fat in the meal is consistent with the goal.
In addition, participants sometimes struggled with the numerical content in goals, for example the combination of both “choices” and “grams” as units.
“ ‘Decrease your fat to about four fat choices.’ That part is pretty clear. The only part that I say, kind of gets tricky where I guess you’re adding numbers with words would be the ‘20 grams’.” P8
In general, static text alone was limited in its ability to convey the more abstract nutrition goals. During the interviews, participants asked a number of clarifying questions, for example asking which foods count as which macronutrients. Some participants suggested that visual aids for portion size estimation would be a welcome addition.
6. DISCUSSION
The goal of this research was to examine individuals’ experiences with receiving, selecting, and following computationally generated nutritional goals for T2D. In designing GlucoGoalie, we took the approach of combining ML analysis of individuals’ self-tracking data with an expert system to computationally generate recommendations for nutritional goals that are likely to lead to improvement in BG levels.
This approach has several important distinctions compared to previously proposed systems. First, the ML inference in GlucoGoalie directly examines the relationship between behavior and a health marker (BG) to inform recommendations; not by assuming which behaviors are healthy [83], or relying on user’s self-perceptions of what behaviors impact health [47]. GlucoGoalie makes recommendations in the multidimensional space of nutritional composition, versus the unidimensional space of steps [58] or calories [83], which makes it more complex. Furthermore, unlike other recommendation approaches (e.g., MyBehavior [83]), integration of expert knowledge within the expert system enables GlucoGoalie to make suggestions that extend beyond individuals’ past behaviors (previously captured meals).
We evaluated this approach in two studies that examined whether individuals can understand and follow nutritional goals generated by the expert system using ML inferences, and what experiences would result from receiving and following such goals in the context of individuals’ daily lives.
In the controlled experiment, we found that participants were largely able to understand and act on computationally derived goals in a controlled setting. Participants correctly chose meals that met a goal 89% of the time when these meals were accompanied by corresponding nutritional labels. When composing meals to meet a chosen goal at a “virtual buffet,” participants assembled meals in the correct direction of the goal 67% of the time. This suggests that individuals were able to understand the personalized goals, and were moderately successful when composing meals to meet goals. At the same time, additional findings highlight the complexity of nutrition decisions. When choosing which of two meal images met a given goal without nutrition labels, participants were correct only 49% of the time. This aligns with prior research suggesting that individuals have difficulty comparing macronutrient quantities from photographs alone [13]. In addition, participant comments during the study indicated confusion about some of the nutrition terminology in goals, and there was considerable variability in meeting the exact goal target. This suggests that participants formed a general idea of how to achieve goals, but had difficulty precisely implementing the recommendations.
The deployment study found similar successes and challenges for participants in understanding and acting on goal recommendations. Specifically, it showed that participants were generally able to understand goals, and at least attempted to follow them. The results also reiterated challenges related to specific design choices, like the use of the word “choice” to describe macronutrient quantities.
In addition, the deployment study revealed a number of insights related to the experience of receiving and following goal suggestions in everyday life. Specifically, it highlighted the relationship between supporting reflection and direct support for action, the alignment between goals with individuals eating practices and larger aspirations, and the need for interactive approaches that enable feedback and negotiation. Below we discuss these themes and relate them to our design choices.
6.1. Balancing Support for Reflection and Action
Personal informatics aims to increase self-knowledge and, ultimately, inform future action through collection of and reflection on self-tracking data [64]. However, reflecting on data can be burdensome, and not everyone has the necessary time, mental energy, and literacy. In contrast, there is a long tradition of research in behavior change interventions that focus less on reflection and provide more direct support for action through a variety of behavior change techniques [72]. One limitation of traditional behavior change interventions is that they rely on predetermined behavior goals to nudge users towards, but in the case of chronic conditions like T2D, different goals may be appropriate for different individuals based on their physiology and response to diet. While a more direct approach may mitigate the burden of reflection, a potential concern is that it could lead to individuals following the system’s recommendations without attaining the benefits of learning and self-discovery, which could have a negative impact on autonomy [49].
Our study suggested that it is possible to reach a middle ground between these extremes. Because GlucoGoalie used an expert system to generate concrete goal recommendations, it was able to provide direct support for action. At the same time, because goals were informed by ML analysis of self-tracking data, the participants often engaged in reflection similar to the one enabled by personal informatics solutions. The participants appreciated the more direct support for action through goal recommendations: those who selected personalized goals in the app described making changes and choosing meals that would be consistent with goals, for example taking increased care to measure the components of their meal. At the same time, the study showed that participants found tracking meals and BG levels to be informative, an experience similar to most personal informatics solutions [47, 65]. Furthermore, we found that participants actively engaged with the recommendations they received and took them as an additional prompt and opportunity for reflection, beyond that provided by the personal data itself. Participants compared goal suggestions to their own self-perceptions of their eating habits and used them as a mirror to re-examine their past choices. In this way, we found a synergy between offering direct support for action as a part of an application that enables reflection via self-tracking.
These findings highlight the potential for solutions that balance support for both reflection and action. First, future work could more directly explore the relationship between actionable recommendations and reflection in self-tracking, for example comparing engagement in self-tracking with and without the addition of actionable recommendations. Second, in this work, the connection between one’s behaviors and the recommendations they received were not explained or made explicit by the application, but relied on users to fill in those gaps. Future work could endeavor to make the connections between personal data and recommendations more salient for users, which may further support engagement and reflection. For example, actionable recommendations could be enhanced by presenting visual summaries of the self-tracking data that informed the specific goal recommendations [34, 91]. This additional information can serve as a form of explanation for the recommendations, and prior work has demonstrated the importance of explanations in facilitating nutritional learning [13]. A growing body of research in explainable ML may offer potential avenues to make recommendations in support of action and ground them with an explanation to support reflection [100]. Future work could further incorporate advances in explainable ML to personal informatics applications.
6.2. Aligning Goals with Eating Experiences and Personal Aspirations
Because GlucoGoalie relied on an expert system to generate recommendations as natural language sentences, one of our challenges was to find the right form to formulate these recommendations. Through the design process, we took the approach of formulating goals in terms of macronutrient amounts [36], which has the advantage of allowing individuals to flexibly apply their goal to different types of foods and meals, with the ability to freely incorporate their food preferences. However, this study demonstrated some limitations of this approach. While participants who expressed comfort with nutrition terminology were able to adopt goals, those with lower nutrition literacy and less comfort measuring or weighing their food had trouble understanding and following goals. Making meal choices ultimately comes down to what’s on one’s plate, and participants sometimes found it difficult to connect somewhat abstract goals to concrete meal choices.
An alternative and common form of nutrition suggestions are recipe or meal plan recommendations, which are much more concrete and consistent with how participants think about their meals and diet. However, as recommendations become more concrete, they need to take into account individual’s food preferences, and there are more opportunities to miss the mark. We found this with the “examples” included with each personalized goal: idiosyncratic preferences for a single food item in the list was a major factor in whether a participant would choose a goal or not. Recommender systems (RecSys) excel at making concrete suggestions based on personal preferences, learned from users’ past behavior or characteristics [87], and can incorporate additional constraints like food allergies [48]. While GlucoGoalie focused on personalizing recommendations based on health constraints, this approach could be complimentary with growing research in health-aware RecSys [32]. Meal logs and macronutrient-centered goals from GlucoGoalie could be used as inputs to a preference-based RecSys to generate concrete suggestions that would help individuals connect their goals to what’s on their plate.
In addition to food preferences, participants highlighted the importance of context in determining when a goal was appropriate, for example the time of year, how active one has been, and what other meals have been eaten recently. Making contextually-appropriate recommendations adds another dimension of complexity [84]. Mobile phones and sensors can offer clues to the user’s current state, and there is a long history of research in context-aware computing within HCI and Ubiquitous Computing [28]. In health, location-based prompts have been used to help prevent relapse triggers [17], and step counts can inform adaptive fitness goals based on recent activity levels [58], but have not been widely used in nutrition [83].
A final tension was participants’ desire for a greater connection between specific nutritional goals and their larger aspirations in life and health. Participants did not always see the connection between concrete, quantifiable self-tracking-related goals and larger, more abstract, qualitative motivations. Niess and Woźniak observed the relationship between tracking goals and qualitative health goals in the context of individuals setting goals with fitness trackers [77]. For example, a quantitative, self-tracking goal of walking 12k steps a day might be connected to a qualitative goal of losing weight, and a higher-level goal of feeling well. In the case of GlucoGoalie, because the algorithm suggests quantitative goals, it’s even more important to draw a connection back to an individual’s qualitative goals, like improving BG levels. Researchers have explored methods to elicit these values and motivations [7] and future work could explore how to connect them to quantitative tracking goal [77].
6.3. Interactivity, Negotiation, and Feedback
By taking the approach of using an expert system to interpret ML output, GlucoGoalie produced static, text-based recommendations. One of the limitations of this approach was that we were unable to resolve the misinterpretations and misunderstandings that are likely to arise in a complex domain like nutrition. In some cases, participants did not understand the nutrition terminology, and in other cases they understood the vocabulary, but misinterpreted the intended meaning. One approach to make nutrition goals more understandable is to incorporate illustrations. In health risk communication, illustrations and infographics have been used successfully to improve comprehension of complex information [3, 42, 104]. A similar visual approach has been applied to assist low literacy adults with portion size estimation [16], and could be used here to better convey numerical content in personalized goals.
A second approach is to offer the opportunity for questions and answers in a back-and-forth exchange. This more interactive approach could introduce concepts, answer users’ questions, and more fully explain goal recommendations. Along these lines, conversational agents have been used to support interactive goal setting, health coaching, and motivational interviewing [10, 62]. Generally, these approaches are based on a set list of goals, not personalized based on user self-tracking data. Combining conversational agents with computationally personalized goal setting is a potential direction for future work. A more interactive and conversational interaction style would also offer another approach to address the challenges of context, discussed above, to allow participants to have input on their goals and negotiate [56]. Finally, this approach might also address the lack of proactive engagement from some users, particularly those with less technology comfort, who did not explore app features and sometimes did not notice updates to their available goals. While many smartphone features rely on users accessing features to pull information or support, conversational approaches can proactively initiate interactions, which may lead to a higher level of engagement with these features [8, 38, 93]. Enabling negotiation within the space of possible goals expands on the complexity of recommendations, and may require more sophisticated and flexible methods than the rule-based expert system used in GlucoGoalie. Machine learning approaches like mechanistic, controller, or reinforcement learning models are a potential vein of future exploration [1, 63, 69].
In another opportunity for increased interactivity, participants expressed resounding interest in more feedback about their progress in achieving their goals, and the impact of this progress on their overall health. Feedback is an important component of learning in goal-setting [29], and while participants were able to self-assess each meal against their goal and view a summary of their goal attainment, they were interested in additional feedback from GlucoGoalie. One approach to providing feedback is to engage dietitians and other healthcare professionals. However, this increases reliance on human experts, thus limiting the scalability of the approach. Previous research in coaching interventions explored offering automated feedback, especially for physical activity [20, 45, 83]. Similar techniques could be applied to nutrition in future research.
6.4. Limitations
As an initial step towards exploring actionable health recommendations, this work has a number of limitations. Both studies had small sample sizes, and while the sample was recruited from economically disadvantaged communities, it was not representative: participants were skewed female, and predominantly black or Latino. This cohort from a single United States metro area, may not account for important cultural differences nationally or globally [94]. In addition, the study ran for 4-weeks, and usage patterns and engagement may change with extended use. Finally, while we report qualitative perceptions, we did not quantitatively examine changes in BG or other health outcomes because of the small sample and short timeframe [54].
6.5. Conclusion
While self-tracking data hold potential to inform action, deriving actionable insights is challenging and burdensome for individuals. In this work, we explored an approach that combines ML with an expert system to generate goals that are personalized based on an individual’s health data. We found that support for action can also support, augment, and inform reflection. We connected our findings with both prior research and open questions in personal informatics, goal-setting, and health-based recommender systems, and argue that future interventions could incorporate more interactive, dialog-based components with conversational agents.
CCS CONCEPTS.
• Applied computing → Life and medical sciences; Consumer health; • Human-centered computing → Human computer interaction (HCI); Empirical studies in HCI; Ubiquitous and mobile computing.
ACKNOWLEDGMENTS
This research was funded by the National Institute of Diabetes and Digestive and Kidney Diseases award number R56DK113189 and the National Library of Medicine award number T15LM007079. Thank you to the fellow students of Columbia’s Department of Biomedical Informatics, who earned the undying gratitude of the corresponding author by arts-and-crafting food images for the virtual buffet.
A. APPENDICES
A. CONTROLLED EXPERIMENT MATERIALS
This section contains supplementary figures with example materials used in the controlled experiment.
Supplementary Figure A.

An example item from the goal comprehension task
Supplementary Figure B.

An example item from the goal/image matching task
Supplementary Figure C.

The “virtual bufet” for breakfast meals.
Contributor Information
Elliot G. Mitchell, Department of Biomedical Informatics, Columbia University.
Elizabeth M. Heitkemper, School of Nursing, The University of Texas at Austin
Marissa Burgermaster, Department of Population Health, Dell Medical School, and Department of Nutritional Sciences, The University of Texas at Austin.
Matthew E. Levine, Department of Computing and Mathematical Sciences, California Institute of Technology
Yishen Miao, Department of Molecular, Cellular, and Developmental Biology, University of California Santa Barbara.
Maria L. Hwang, Science and Math, Fashion Institute of Technology
Pooja M. Desai, Department of Biomedical Informatics, Columbia University
Andrea Cassells, Clinical Directors Network (CDN).
Jonathan N. Tobin, Clinical Directors Network (CDN) and The Rockefeller University
Esteban G. Tabak, Courant Institute of Mathematical Sciences
David J. Albers, University of Colorado, Anschutz Medical Campus, Section of Informatics and Data Science, Departments of Pediatrics, Biomedical Engineering, and Biostatistics and Informatics, and Department of Biomedical Informatics, Columbia University
Arlene M. Smaldone, School of Nursing, Columbia University
Lena Mamykina, Department of Biomedical Informatics, Columbia University.
REFERENCES
- [1].Albers David J, Levine Matthew E, Stuart Andrew, Mamykina Lena, Gluckman Bruce, and Hripcsak George. 2018. Mechanistic machine learning: how data assimilation leverages physiologic knowledge using Bayesian inference to forecast the future, infer the present, and phenotype. Journal of the American Medical Informatics Association 25, 10: 1392–1401. 10.1093/jamia/ocy106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].American Diabetes Association. 2018. 4. Lifestyle Management:Standards of Medical Care in Diabetes-2018. Diabetes care 41, Suppl 1: S38–S50. 10.2337/dc18-S004 [DOI] [PubMed] [Google Scholar]
- [3].Arcia Adriana, Suero-Tejeda Niurka, Bales Michael E., Merrill Jacqueline A., Yoon Sun-moo, Woollen Janet, and Bakken Suzanne. 2016. Sometimes more is more: Iterative participatory design of infographics for engagement of community members with varying levels of health literacy. Journal of the American Medical Informatics Association 23, 1: 174–183. 10.1093/jamia/ocv079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Aschner Pablo. 2017. New IDF clinical practice recommendations for managing type 2 diabetes in primary care. Diabetes Research and Clinical Practice 132: 169–170. 10.1016/J.DIABRES.2017.09.002 [DOI] [PubMed] [Google Scholar]
- [5].Baumer Eric P.S., Khovanskaya Vera, Matthews Mark, Reynolds Lindsay, Sosik Victoria Schwanda, and Gay Geri. 2014. Reviewing refection. In Proceedings of the 2014 conference on Designing interactive systems - DIS ’14, 93–102. 10.1145/2598510.2598598 [DOI] [Google Scholar]
- [6].Bentley Frank, Tollmar Konrad, Stephenson Peter, Levy Laura, Jones Brian, Robertson Scott, Price Ed, Catrambone Richard, and Wilson Jeff. 2013. Health Mashups: Presenting Statistical Patterns betweenWellbeing Data and Context in Natural Language to Promote Behavior Change. ACM Transactions on Computer-Human Interaction 20, 5: 1–27. 10.1145/2503823 [DOI] [Google Scholar]
- [7].Berry Andrew B.L., Lim Catherine, Hartzler Andrea L., Hirsch Tad, Ludman Evette, Wagner Edward H., and Ralston James D.. 2017. Eliciting Values of Patients with Multiple Chronic Conditions: Evaluation of a Patient-centered Framework. AMIA … Annual Symposium proceedings. AMIA Symposium 2017: 430–439. Retrieved September 15, 2020 from /pmc/articles/PMC5977727/?report=abstract [PMC free article] [PubMed] [Google Scholar]
- [8].Bickmore Timothy, Gruber Amanda, and Picard Rosalind. 2005. Establishing the computer–patient working alliance in automated health behavior change interventions. Patient Education and Counseling 59, 1: 21–30. 10.1016/J.PEC.2004.09.008 [DOI] [PubMed] [Google Scholar]
- [9].Bickmore Timothy W., Silliman Rebecca A., Nelson Kerrie, Cheng Debbie M., Winter Michael, Henault Lori, and Paasche-Orlow Michael K.. 2013. A Randomized Controlled Trial of an Automated Exercise Coach for Older Adults. Journal of the American Geriatrics Society 61, 10: 1676–1683. 10.1111/jgs.12449 [DOI] [PubMed] [Google Scholar]
- [10].Bickmore Timothy W, Schulman Daniel, and Sidner Candace L. 2011. A reusable framework for health counseling dialogue systems based on a behavioral medicine ontology. Journal of Biomedical Informatics 44, 2: 183–197. 10.1016/j.jbi.2010.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bodenheimer Thomas, Lorig Kate, Holman Halsted, and Grumbach Kevin. 2002. Patient Self-management of Chronic Disease in Primary Care. JAMA 288, 19: 2469. 10.1001/jama.288.19.2469 [DOI] [PubMed] [Google Scholar]
- [12].Braun Virginia and Clarke Victoria. 2006. Using thematic analysis in psychology. Qualitative Research in Psychology 3, 2: 77–101. 10.1191/1478088706qp063oa [DOI] [Google Scholar]
- [13].Burgermaster Marissa, Gajos Krzysztof Z., Davidson Patricia, and Mamykina Lena. 2017. The Role of Explanations in Casual Observational Learning about Nutrition. In Proceedings of the 2017 CHI Conference on Human Factors in Computing Systems - CHI ’17, 4097–4145. 10.1145/3025453.3025874 [DOI] [Google Scholar]
- [14].Cavanaugh Kerri L. 2011. Health literacy in diabetes care: explanation, evidence and equipment. Diabetes management (London, England) 1, 2: 191–199. 10.2217/dmt.11.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Centers for Disease Control and Prevention. 2018. Racial and Ethnic Approaches to Community Health | DNPAO. Retrieved January 3, 2019 from https://www.cdc.gov/nccdphp/dnpao/state-local-programs/reach/
- [16].Chaudhry Beenish M., Schaefbauer Christopher, Jelen Ben, Siek Katie A., and Connelly Kay. 2016. Evaluation of a Food Portion Size Estimation Interface for a Varying Literacy Population. In Proceedings of the 2016 CHI Conference on Human Factors in Computing Systems - CHI ’16, 5645–5657. 10.1145/2858036.2858554 [DOI] [Google Scholar]
- [17].Chih Ming-Yuan, Patton Timothy, McTavish Fiona M., Isham Andrew J., Judkins-Fisher Chris L., Atwood Amy K., and Gustafson David H.. 2014. Predictive modeling of addiction lapses in a mobile health application. Journal of Substance Abuse Treatment 46, 1: 29–35. 10.1016/J.JSAT.2013.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Eun Kyoung Choe Nicole B. Lee, Lee Bongshin, Pratt Wanda, and Kientz Julie A.. 2014. Understanding Quantified-Selfers’ Practices in Collecting and Exploring Personal Data. Proceedings of the 32nd Annual ACM Conference on Human Factors in Computing Systems: 1143–1152. 10.1145/2556288.2557372 [DOI] [Google Scholar]
- [19].Chung Chia-Fang, Wang Qiaosi, Schroeder Jessica, Cole Allison, Zia Jasmine, Fogarty James, and Munson Sean A.. 2019. Identifying and Planning for Individualized Change. Proceedings of the ACM on Interactive, Mobile, Wearable and Ubiquitous Technologies 3, 1: 1–27. 10.1145/3314394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Clavel Céline, Whittaker Steve, Blacodon Anaïs Anais, and Martin Jean-Claude. 2018. WEnner: A Theoretically Motivated Approach for Tailored Coaching About Physical Activity. In Proceedings of the 2018 ACM International Joint Conference and 2018 International Symposium on Pervasive and Ubiquitous Computing and Wearable Computers - ubiComp ’18 (UbiComp ’18), 1669–1675. 10.1145/3267305.3274190 [DOI] [Google Scholar]
- [21].Clawson James, Pater Jessica A., Miller Andrew D., Mynatt Elizabeth D., and Mamykina Lena. 2015. No longer wearing: investigating the abandonment of personal health-tracking technologies on craigslist. In Proceedings of the 2015 ACM International Joint Conference on Pervasive and Ubiquitous Computing - UbiComp ’15, 647–658. 10.1145/2750858.2807554 [DOI] [Google Scholar]
- [22].Cole-Lewis Heather J., Smaldone Arlene M., Davidson Patricia R., Kukafka Rita, Tobin Jonathan N., Cassells Andrea, Mynatt Elizabeth D., Hripcsak George, and Mamykina Lena. 2016. Participatory approach to the development of a knowledge base for problem-solving in diabetes self-management. International Journal of Medical Informatics 85, 1: 96–103. 10.1016/J.IJMEDINF.2015.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Collins Francis S. and Varmus Harold. 2015. A New Initiative on Precision Medicine. New England Journal of Medicine 372, 9: 793–795. 10.1056/NEJMp1500523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Consolvo Sunny, Klasnja Predrag, McDonald David W., and Landay James A.. 2009. Goal-setting considerations for persuasive technologies that encourage physical activity. In ACM International Conference Proceeding Series, 1. 10.1145/1541948.1541960 [DOI] [Google Scholar]
- [25].Cordeiro Felicia, Bales Elizabeth, Cherry Erin, and Fogarty James. 2015. Rethinking the Mobile Food Journal: Exploring Opportunities for Lightweight Photo-Based Capture. In Proceedings of the 33rd Annual ACM Conference on Human Factors in Computing Systems - CHI ’15, 3207–3216. 10.1145/2702123.2702154 [DOI] [Google Scholar]
- [26].Daskalova Nediyana, Yoon Jina, Wang Yibing, Araujo Cintia, Beltran Guillermo, Nugent Nicole, McGeary John, Williams Joseph Jay, and Huang Jeff. 2020. Sleep-Bandits: Guided Flexible Self-Experiments for Sleep. In Conference on Human Factors in Computing Systems - Proceedings. 10.1145/3313831.3376584 [DOI] [Google Scholar]
- [27].Desai Pooja M., Mitchell Elliot G., Hwang Maria L., Levine Matthew E., Albers David J., and Mamykina Lena. 2019. Personal health oracle: Explorations of personalized predictions in diabetes self-management. In Conference on Human Factors in Computing Systems - Proceedings, 1–13. 10.1145/3290605.3300600 [DOI] [Google Scholar]
- [28].Dey Anind K.. 2001. Understanding and using context. Personal and Ubiquitous Computing 5, 1: 4–7. 10.1007/s007790170019 [DOI] [Google Scholar]
- [29].The Diabetes Prevention Program (DPP) Research Diabetes Prevention Program (DPP) Research Group. 2002. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes care 25, 12: 2165–71. 10.2337/diacare.25.12.2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Donevant Sara Belle, Estrada Robin Dawson, Culley Joan Marie, Habing Brian, and Adams Swann Arp. 2018. Exploring app features with outcomes in mHealth studies involving chronic respiratory diseases, diabetes, and hypertension: a targeted exploration of the literature. Journal of the American Medical Informatics Association. 10.1093/jamia/ocy104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Elsweiler David and Harvey Morgan. 2015. Towards Automatic Meal Plan Recommendations for Balanced Nutrition. Proceedings of the 9th ACM Conference on Recommender Systems: 313–316. 10.1145/2792838.2799665 [DOI] [Google Scholar]
- [32].Elsweiler David, Ludwig Bernd, Said Alan, Schaefer Hanna, and Trattner Christoph. 2016. Engendering Health with Recommender Systems. In Proceedings of the 10th ACM Conference on Recommender Systems - RecSys ’16, 409–410. 10.1145/2959100.2959203 [DOI] [Google Scholar]
- [33].Epstein Daniel A., Cordeiro Felicia, Fogarty James, Hsieh Gary, and Munson Sean A.. 2016. Crumbs: Lightweight Daily Food Challenges to Promote Engagement and Mindfulness. Proceedings of the 2016 CHI Conference on Human Factors in Computing Systems: 5632–5644. 10.1145/2858036.2858044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Epstein Daniel, Cordeiro Felicia, Bales Elizabeth, Fogarty James, and Munson Sean. 2014. Taming Data Complexity in Lifelogs: Exploring Visual Cuts of Personal Informatics Data. DIS ’14 Proceedings of the 2014 conference on Designing interactive systems: 667–676. 10.1145/2598510.2598558 [DOI] [Google Scholar]
- [35].Estrin Deborah. 2014. Small data, where n = me. Communications of the ACM 57, 4: 32–34. 10.1145/2580944 [DOI] [Google Scholar]
- [36].Evert Alison B., Dennison Michelle, Gardner Christopher D., Garvey W. Timothy, Karen Lau Ka Hei, MacLeod Janice, Mitri Joanna, Pereira Raquel F., Rawlings Kelly, Robinson Shamera, Saslow Laura, Uelmen Sacha, Urbanski Patricia B., and Yancy William S.. 2019. Nutrition therapy for adults with diabetes or prediabetes: A consensus report. 10.2337/dci19-0014 [DOI] [PMC free article] [PubMed]
- [37].Feller Daniel J, Burgermaster Marissa, Levine Matthew E, Smaldone Arlene, Davidson Patricia G, Albers David J, and Mamykina Lena. 2018. A visual analytics approach for pattern-recognition in patient-generated data. Journal of the American Medical Informatics Association. 10.1093/jamia/ocy054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Fitzpatrick Kathleen Kara, Darcy Alison, and Vierhile Molly. 2017. Delivering Cognitive Behavior Therapy to Young Adults With Symptoms of Depression and Anxiety Using a Fully Automated Conversational Agent (Woebot): A Randomized Controlled Trial. JMIR mental health 4, 2: e19. 10.2196/mental.7785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Fusch Patricia and Ness Lawrence. 2015. Are We There Yet? Data Saturation in Qualitative Research. The Qualitative Report 20, 9. Retrieved September 15, 2020 from https://nsuworks.nova.edu/tqr/vol20/iss9/3 [Google Scholar]
- [40].Grimes Andrea, Bednar Martin, Bolter Jay David, and Grinter Rebecca E.. 2008. EatWell: Sharing nutrition-related memories in a low-income community. In Proceedings of the ACM Conference on Computer Supported Cooperative Work, CSCW, 87–96. 10.1145/1460563.1460579 [DOI] [Google Scholar]
- [41].Grimes Andrea, Kantroo Vasudhara, and Grinter Rebecca E.. 2010. Let’s play! Mobile health games for adults. In UbiComp’10 - Proceedings of the 2010 ACM Conference on Ubiquitous Computing, 241–250. 10.1145/1864349.1864370 [DOI] [Google Scholar]
- [42].Grossman Lisa, Feiner Steven, Mitchell Elliot, and Creber Ruth Masterson. 2018. Leveraging Patient-Reported Outcomes Using Data Visualization. Applied Clinical Informatics 09, 03: 565–575. 10.1055/s-0038-1667041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Guest Greg, Bunce Arwen, and Johnson Laura. 2006. How Many Interviews Are Enough? Field Methods 18, 1: 59–82. 10.1177/1525822X05279903 [DOI] [Google Scholar]
- [44].Gulshan Varun, Peng Lily, Coram Marc, Stumpe Martin C., Wu Derek, Narayanaswamy Arunachalam, Venugopalan Subhashini, Widner Kasumi, Madams Tom, Cuadros Jorge, Kim Ramasamy, Raman Rajiv, Nelson Philip C., Mega Jessica L., and Webster Dale R.. 2016. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA - Journal of the American Medical Association 316, 22: 2402–2410. 10.1001/jama.2016.17216 [DOI] [PubMed] [Google Scholar]
- [45].Gupta Ankit, Heng Tim, Shaw Chris, Li Linda, and Feehan Lynne. 2018. Towards developing an e-coach to support arthritis patients in maintaining a physically active lifestyle. In Proceedings of the 12th EAI International Conference on Pervasive Computing Technologies for Healthcare - PervasiveHealth ’18, 392–395. 10.1145/3240925.3240954 [DOI] [Google Scholar]
- [46].Hayward Mark D., Miles Toni P., Crimmins Eileen M., and Yang Yu. 2000. The Significance of Socioeconomic Status in Explaining the Racial Gap in Chronic Health Conditions. American Sociological Review 65, 6: 910. 10.2307/2657519 [DOI] [Google Scholar]
- [47].Hollis Victoria, Konrad Artie, Springer Aaron, Antoun Matthew, Antoun Christopher, Martin Rob, and Whittaker Steve. 2017. What Does All This Data Mean for My Future Mood? Actionable Analytics and Targeted Refection for Emotional Well-Being. Human-Computer Interaction 32, 5-6: 208–267. 10.1080/07370024.2016.1277724 [DOI] [Google Scholar]
- [48].Hsu Paris, Zhao Jingshu, Liao Kehan, Liu Tianyi, and Wang Chen. 2017. AllergyBot: A Chatbot technology intervention for young adults with food allergies Dining out. In Conference on Human Factors in Computing Systems - Proceedings, 74–79. 10.1145/3027063.3049270 [DOI] [Google Scholar]
- [49].Kamphorst Bart A.. 2017. E-coaching systems: What they are, and what they aren’t. Personal and Ubiquitous Computing 21, 4: 625–632. 10.1007/s00779-017-1020-6 [DOI] [Google Scholar]
- [50].Karkar Ravi, Zia Jasmine, Schroeder Jessica, Epstein Daniel A., Pina Laura R., Scofield Jeffrey, Fogarty James, Kientz Julie A., Munson Sean A., and Vilardaga Roger. 2017. TummyTrials: A Feasibility Study of Using Self-Experimentation to Detect Individualized Food Triggers. In Proceedings of the 2017 CHI Conference on Human Factors in Computing Systems - CHI ’17, 6850–6863. 10.1145/3025453.3025480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Karkar Ravi, Zia Jasmine, Vilardaga Roger, Sonali R Mishra James Fogarty, Munson Sean A, and Kientz Julie A. 2016. A framework for self-experimentation in personalized health. Journal of the American Medical Informatics Association 23, 3: 440–448. 10.1093/jamia/ocv150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kato Shigeko, Waki Kayo, Nakamura Sadako, Osada Sanae, Kobayashi Haruka, Fujita Hideo, Kadowaki Takashi, and Ohe Kazuhiko. 2016. Validating the use of photos to measure dietary intake: the method used by DialBetics, a smartphone-based self-management system for diabetes patients. Diabetology International 7, 3: 244–251. 10.1007/s13340-015-0240-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kim Yoojung, Ji Sookyoung, Lee Hyunjeong, Kim Jeong-Whun, Yoo Sooyoung, and Lee Joongseek. 2016. “My Doctor is Keeping an Eye on Me!”: Exploring the Clinical Applicability of a Mobile Food Logger. In Proceedings of the 2016 CHI Conference on Human Factors in Computing Systems - CHI ’16, 5620–5631. 10.1145/2858036.2858145 [DOI] [Google Scholar]
- [54].Klasnja Predrag, Consolvo Sunny, and Pratt Wanda. 2011. How to evaluate technologies for health behavior change in HCI research. In Conference on Human Factors in Computing Systems - Proceedings, 3063–3072. 10.1145/1978942.1979396 [DOI] [Google Scholar]
- [55].Klasnja Predrag and Pratt Wanda. 2012. Healthcare in the pocket: Mapping the space of mobile-phone health interventions. Journal of Biomedical Informatics 45, 1: 184–198. 10.1016/J.JBI.2011.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kocielnik Rafal and Hsieh Gary. 2017. New Opportunities for Dialogue-based Interaction in Behavior Change Domain. In CSCW 2017 workshop on Talking with Conversational Agents in Collaborative Action. Retrieved October 30, 2019 from https://talkingwithagents.files.wordpress.com/2017/02/7-kocielnik1.pdf [Google Scholar]
- [57].Kocielnik Rafal, Xiao Lillian, Avrahami Daniel, and Hsieh Gary. 2018. Refection Companion: A Conversational System for Engaging Users in Refection on Physical Activity. Proceedings of the ACM on Interactive, Mobile, Wearable and Ubiquitous Technologies 2, 2: 1–26. 10.1145/3214273 [DOI] [Google Scholar]
- [58].Korinek Elizabeth V., Phatak Sayali S., Martin Cesar A., Freigoun Mohammad T., Rivera Daniel E., Adams Marc A., Klasnja Pedja, Buman Matthew P., and Hekler Eric B.. 2018. Adaptive step goals and rewards: a longitudinal growth model of daily steps for a smartphone-based walking intervention. Journal of Behavioral Medicine 41, 1: 74–86. 10.1007/s10865-017-9878-3 [DOI] [PubMed] [Google Scholar]
- [59].Kutner Mark, Greenberg Elizabeth, and Baer Justin. 2006. A First Look at the Literacy of America’s Adults in the 21st Century. NCES 2006-470. National Center for Education Statistics. Retrieved August 23, 2018 from https://eric.ed.gov/?id=ED489066 [Google Scholar]
- [60].Laranjo Liliana, Lau Annie, and Coiera Enrico. 2017. Design and Implementation of Behavioral Informatics Interventions. Springer, Cham, 13–42. 10.1007/978-3-319-51732-2_2 [DOI] [Google Scholar]
- [61].Lazar Amanda, Koehler Christian, Tanenbaum Joshua, and Nguyen David H.. 2015. Why we use and abandon smart devices. In Proceedings of the 2015 ACM International Joint Conference on Pervasive and Ubiquitous Computing - UbiComp ’15, 635–646. 10.1145/2750858.2804288 [DOI] [Google Scholar]
- [62].Lee Jisoo, Hekler Eric B., Chiauzzi Emil, Towner Auriell, and Fitz-Randolph Marcy. 2016. Helping Users Set Rules for Defining Short-Term Activity Goals. In Proceedings of the 2016 CHI Conference Extended Abstracts on Human Factors in Computing Systems - CHIEA ’16, 2178–2184. 10.1145/2851581.2892488 [DOI] [Google Scholar]
- [63].Lei Huitian, Tewari Ambuj, and Murphy Susan A.. 2017. An Actor-Critic Contextual Bandit Algorithm for Personalized Mobile Health Interventions. Retrieved January 31, 2019 from http://arxiv.org/abs/1706.09090
- [64].Li Ian, Dey Anind, and Forlizzi Jodi. 2010. A stage-based model of personal informatics systems. Proceedings of the 28th international conference on Human factors in computing systems CHI 10: 557. 10.1145/1753326.1753409 [DOI] [Google Scholar]
- [65].Li Ian, Dey Anind K., and Forlizzi Jodi. 2011. Understanding my data, myself: supporting self-refection with ubicomp technologies. In Proceedings of the 13th international conference on Ubiquitous computing - UbiComp ’11, 405. 10.1145/2030112.2030166 [DOI] [Google Scholar]
- [66].Mamykina Lena, Levine Matthew E, Davidson Patricia G, Smaldone Arlene M, Elhadad Noemie, and Albers David J. 2016. Data-driven health management: reasoning about personally generated data in diabetes with information technologies. Journal of the American Medical Informatics Association 23, 3: 526–531. 10.1093/jamia/ocv187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Mamykina Lena, Mynatt Elizabeth, Davidson Patricia, and Greenblatt David. 2008. MAHI: Investigation of social scaffolding for reflective thinking in diabetes management. In Proceedings of the SIGCHI Conference on Human Factors in Computing Systems (CHI ‘08), 477–486. 10.1145/1357054.1357131 [DOI] [Google Scholar]
- [68].Mamykina Lena, Nakikj Drashko, and Elhadad Noemie. 2015. Collective sensemaking in online health forums. In Conference on Human Factors in Computing Systems - Proceedings, 3217–3226. 10.1145/2702123.2702566 [DOI] [Google Scholar]
- [69].Martín César A., Rivera Daniel E., Hekler Eric B., Riley William T., Buman Matthew P., Adams Marc A., and Magann Alicia B.. 2020. Development of a Control-Oriented Model of Social Cognitive Theory for Optimized mHealth Behavioral Interventions. IEEE Transactions on Control Systems Technology 28, 2: 331–346. 10.1109/TCST.2018.2873538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Matthan Nirupa R, Ausman Lynne M, Meng Huicui, Tighiouart Hocine, and Lichtenstein Alice H. 2016. Estimating the reliability of glycemic index values and potential sources of methodological and biological variability. The American journal of clinical nutrition 104, 4: 1004–1013. 10.3945/ajcn.116.137208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].McKillop Mollie, Mamykina Lena, and Elhadad Noemie. 2018. Designing in the Dark. In Proceedings of the 2018 CHI Conference on Human Factors in Computing Systems - CHI ’18, 1–15. 10.1145/3173574.3174139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Michie Susan, Richardson Michelle, Johnston Marie, Abraham Charles, Francis Jill, Hardeman Wendy, Eccles Martin P., Cane James, and Wood Caroline E.. 2013. The Behavior Change Technique Taxonomy (v1) of 93 Hierarchically Clustered Techniques: Building an International Consensus for the Reporting of Behavior Change Interventions. Annals of Behavioral Medicine 46, 1: 81–95. 10.1007/s12160-013-9486-6 [DOI] [PubMed] [Google Scholar]
- [73].Mitchell Elliot G, Tabak Esteban G, Levine Matthew E, Mamykina Lena, and Albers David J. 2021. Enabling personalized decision support with patient-generated data and attributable components. Journal of Biomedical Informatics 113, 103639: 103639. 10.1016/j.jbi.2020.103639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Munson Sean and Consolvo Sunny. 2012. Exploring Goal-setting, Rewards, Self-monitoring, and Sharing to Motivate Physical Activity. In Proceedings of the 6th International Conference on Pervasive Computing Technologies for Healthcare. 10.4108/icst.pervasivehealth.2012.248691 [DOI] [Google Scholar]
- [75].Naik Aanand D., Palmer Nynikka, Petersen Nancy J., Street Richard L., Rao Radha, Suarez-Almazor Maria, and Haidet Paul. 2011. Comparative Effectiveness of Goal Setting in Diabetes Mellitus Group Clinics. Archives of Internal Medicine 171, 5: 453–459. 10.1001/archinternmed.2011.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Nazario Brunilda. 2013. Portion Size Plate | Recommended Serving Sizes for Portion Control. Retrieved April 15, 2018 from https://www.webmd.com/diet/healthtool-portion-size-plate [Google Scholar]
- [77].Niess Jasmin and Wozniak Pawei W.. 2018. Supporting Meaningful Personal Fitness: the Tracker Goal Evolution Model. In Proceedings of the 2018 CHI Conference on Human Factors in Computing Systems - CHI ’18, 1–12. 10.1145/3173574.3173745 [DOI] [Google Scholar]
- [78].Noronha Jon, Hysen Eric, Zhang Haoqi, and Gajos Krzysztof Z.. 2011. Platemate: Crowdsourcing Nutritional Analysis from Food Photographs. In Proceedings of the 24th Annual ACM Symposium on User Interface Software and Technology, 1–12. 10.1145/2047196.2047198 [DOI] [Google Scholar]
- [79].O’Kane Aisling Ann, Park Sun Young, Mentis Helena, Blandford Ann, and Chen Yunan. 2016. Turning to Peers: Integrating Understanding of the Self, the Condition, and Others’ Experiences in Making Sense of Complex Chronic Conditions. Computer Supported Cooperative Work: CSCW: An International Journal 25, 6: 477–501. 10.1007/s10606-016-9260-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Peek Monica E, Cargill Algernon, and Huang Elbert S. 2007. Diabetes health disparities: a systematic review of health care interventions. Medical care research and review: MCRR 64, 5 Suppl: 101S–56S. 10.1177/1077558707305409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Purpura Stephen, Schwanda Victoria, Williams Kaiton, Stubler William, and Sengers Phoebe. 2011. Fit4Life: The design of a persuasive technology promoting healthy behavior and ideal weight. In Conference on Human Factors in Computing Systems - Proceedings, 423–432. 10.1145/1978942.1979003 [DOI] [Google Scholar]
- [82].Puussaar Aare, Clear Adrian K., and Wright Peter. 2017. Enhancing personal informatics through social sensemaking. In Conference on Human Factors in Computing Systems - Proceedings, 6936–6942. 10.1145/3025453.3025804 [DOI] [Google Scholar]
- [83].Rabbi Mashfiqui, Aung Min Hane, Zhang Mi, and Choudhury Tanzeem. 2015. MyBehavior: Automatic personalized health feedback from user behaviors and preferences using smartphones. In UbiComp 2015 - Proceedings of the 2015 ACM International Joint Conference on Pervasive and Ubiquitous Computing, 707–718. 10.1145/2750858.2805840 [DOI] [Google Scholar]
- [84].Raj Shriti, Toporski Kelsey, Garrity Ashley, Lee Joyce M., and Newman Mark W.. 2019. “My blood sugar is higher on the weekends”: Finding a role for context and context-awareness in the design of health self-management technology. In Conference on Human Factors in Computing Systems - Proceedings, 1–13. 10.1145/3290605.3300349 [DOI] [Google Scholar]
- [85].Rajkomar Alvin, Oren Eyal, Chen Kai, Dai Andrew M., Hajaj Nissan, Hardt Michaela, Liu Peter J., Liu Xiaobing, Marcus Jake, Sun Mimi, Sundberg Patrik, Yee Hector, Zhang Kun, Zhang Yi, Flores Gerardo, Duggan Gavin E., Irvine Jamie, Le Quoc, Litsch Kurt, Mossin Alexander, Tansuwan Justin, Wang De, Wexler James, Wilson Jimbo, Ludwig Dana, Volchenboum Samuel L., Chou Katherine, Pearson Michael, Madabushi Srinivasan, Shah Nigam H., Butte Atul J., Howell Michael D., Cui Claire, Corrado Greg S., and Dean Jeffrey. 2018. Scalable and accurate deep learning with electronic health records. npj Digital Medicine 1, 1: 18. 10.1038/s41746-018-0029-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Turchioe Meghan Reading, Burgermaster Marissa, Mitchell Elliot G., Desai Pooja M., and Mamykina Lena. 2020. Adapting the stage-based model of personal informatics for low-resource communities in the context of type 2 diabetes. Journal of Biomedical Informatics 110, 103572. 10.1016/j.jbi.2020.103572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Ricci Francesco, Rokach Lior, and Shapira Bracha. 2015. Recommender Systems: Introduction and Challenges. In Recommender Systems Handbook. Springer US, Boston, MA, 1–34. 10.1007/978-1-4899-7637-6_1 [DOI] [Google Scholar]
- [88].Rohani Darius A., Lopategui Andrea Quemada, Tuxen Nanna, Faurholt-Jepsen Maria, Kessing Lars V., and Bardram Jakob E.. 2020. MUBS: A Personalized Recommender System for Behavioral Activation in Mental Health. In Proceedings of the 2020 CHI Conference on Human Factors in Computing Systems, 1–13. 10.1145/3313831.3376879 [DOI] [Google Scholar]
- [89].Schäfer Hanna. 2016. Personalized Support for Healthy Nutrition Decisions. In Proceedings of the 10th ACM Conference on Recommender Systems - RecSys ’16, 455–458. 10.1145/2959100.2959105 [DOI] [Google Scholar]
- [90].Schäfer Hanna, Hors-Fraile Santiago, Karumur Raghav Pavan, Valdez André Calero, Said Alan, Torkamaan Helma, Ulmer Tom, and Trattner Christoph. 2017. Towards Health (Aware) Recommender Systems. In Proceedings of the 2017 International Conference on Digital Health - DH ’17, 157–161. 10.1145/3079452.3079499 [DOI] [Google Scholar]
- [91].Schroeder Jessica, Karkar Ravi, Fogarty James, Kientz Julie A., Munson Sean A., and Kay Matthew. 2018. A Patient-Centered Proposal for Bayesian Analysis of Self-Experiments for Health. Journal of Healthcare Informatics Research: 1–32. 10.1007/s41666-018-0033-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Schroeder Jessica, Karkar Ravi, Murinova Natalia, Fogarty James, and Munson Sean A.. 2019. Examining Opportunities for Goal-Directed Self-Tracking to Support Chronic Condition Management. Proceedings of the ACM on Interactive, Mobile, Wearable and Ubiquitous Technologies 3, 4: 1–26. 10.1145/3369809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Schroeder Jessica, Wilks Chelsey, Rowan Kael, Toledo Arturo, Paradiso Ann, Czerwinski Mary, Mark Gloria, and Linehan Marsha M.. 2018. Pocket Skills: A Conversational Mobile Web App To Support Dialectical Behavioral Therapy. Proceedings of the SIGCHI Conference on Human Factors in Computing Systems (CHI 2018): 1–15. 10.1145/3173574.3173972 [DOI] [Google Scholar]
- [94].Stowell Elizabeth, Lyson Mercedes C., Saksono Herman, Wurth Reneé C., Jimison Holly, Pavel Misha, and Parker Andrea G.. 2018. Designing and Evaluating mHealth Interventions for Vulnerable Populations. In Proceedings of the 2018 CHI Conference on Human Factors in Computing Systems - CHI ’18, 1–17. 10.1145/3173574.3173589 [DOI] [Google Scholar]
- [95].Swearingen Kirsten and Sinha R. 2001. Beyond Algorithms: An HCI Perspective on Recommender Systems. ACM SIGIR 2001 Workshop on Recommender Systems (2001): 1–11. https://doi.org/10.1.1.23.9764 [Google Scholar]
- [96].Tabak Esteban G. and Trigila Giulio. 2018. Explanation of Variability and Removal of Confounding Factors from Data through Optimal Transport. Communications on Pure and Applied Mathematics 71, 1: 163–199. 10.1002/cpa.21706 [DOI] [Google Scholar]
- [97].United States Department of Agriculture (USDA). ChooseMyPlate. Retrieved September 16, 2020 from https://www.choosemyplate.gov/
- [98].Veinot Tiffany C., Ancker Jessica S., Cole-Lewis Heather, Mynatt Elizabeth D., Parker Andrea G., Siek Katie A., and Mamykina Lena. 2019. Leveling Up. Medical Care 57: S108–S114. 10.1097/MLR.0000000000001032 [DOI] [PubMed] [Google Scholar]
- [99].Veinot Tiffany C, Mitchell Hannah, and Ancker Jessica S. 2018. Good intentions are not enough: how informatics interventions can worsen inequality. Journal of the American Medical Informatics Association. 10.1093/jamia/ocy052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Wang Danding, Yang Qian, Abdul Ashraf, and Lim Brian Y.. 2019. Designing theory-driven user-centric explainable AI. In Conference on Human Factors in Computing Systems - Proceedings, 1–15. 10.1145/3290605.3300831 [DOI] [Google Scholar]
- [101].Wheeler ML, Daly A, Evert A, and others. 2014. Choose Your Foods, Food Lists for Diabetes. Chicago, IL: Academy of Nutrition and Dietetics/American Diabetes Association. [Google Scholar]
- [102].Yang Longqi, Hsieh Cheng-Kang, Yang Hongjian, Dell Nicola, Belongie Serge, Cole Curtis, and Estrin Deborah. 2016. Yum-me: A Personalized Nutrient-based Meal Recommender System. ACM Transactions on Information Systems 36, 1: 7. 10.1145/3072614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Zeevi David, Korem Tal, Zmora Niv, Israeli David, Rothschild Daphna, Weinberger Adina, Ben-Yacov Orly, Lador Dar, Avnit-Sagi Tali, Lotan-Pompan Maya, Suez Jotham, Mahdi Jemal Ali, Matot Elad, Malka Gal, Kosower Noa, Rein Michal, Zilberman-Schapira Gili, Dohnalova Lenka, Pevsner-Fischer Meirav, Bikovsky Rony, Halpern Zamir, Elinav Eran, and Segal Eran. 2015. Personalized Nutrition by Prediction of Glycemic Responses. Cell 163, 5: 1079–1095. 10.1016/j.cell.2015.11.001 [DOI] [PubMed] [Google Scholar]
- [104].Zikmund-Fisher Brian J, Scherer Aaron M, Witteman Holly O, Solomon Jacob B, Exe Nicole L, Tarini Beth A, and Fagerlin Angela. 2016. Graphics help patients distinguish between urgent and non-urgent deviations in laboratory test results. Journal of the American Medical Informatics Association 24, 3: ocw169. 10.1093/jamia/ocw169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Facts Up Front. Retrieved September 15, 2018 from http://www.factsupfront.org/


