KEY POINTS
The most common form of sickle cell disease occurs in people who carry 2 alleles for hemoglobin S; however, people also have sickle cell disease if they carry 1 allele for hemoglobin S and 1 allele for hemoglobin C.
The hemoglobin SC type of sickle cell disease is less common than the hemoglobin SS type, and is usually more indolent; however, complications may emerge in middle age.
The most serious complications of hemoglobin SC disease include thrombosis, avascular necrosis and sepsis; early treatment with intravenous antibiotics is important in patients with fever.
A 56-year-old, Jamaica-born woman of Afro-Caribbean ethnicity presented to our emergency department with a 3-day history of abdominal pain, jaundice, subjective fever, dark urine and persistent dysuria after nitrofurantoin treatment for possible urinary tract infection. She reported that she had the sickle cell trait, and had had unprovoked pulmonary emboli in 2002 and 2010 and pneumococcal meningitis in her twenties.
In the emergency department, the patient’s temperature was 36.7°C, her heart rate was 107 beats/min, blood pressure was 128/87 mm Hg and oxygen saturation on room air was 100%. She was jaundiced with epigastric tenderness. She had conjugated hyperbilirubinemia, elevated serum creatinine, leukocytosis, normocytic anemia and thrombocytopenia (Table 1). The emergency department team ordered abdominal ultrasonography to investigate the hyperbilirubinemia; there was no biliary duct dilatation or thickening. They drew blood and urine samples for bacterial culture and started broad spectrum antibiotics.
Table 1:
Laboratory results on days 1–3 of presentation
| Laboratory investigation | Value | Reference range | ||
|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | ||
| White blood cell count, × 109/L | 28.6 | 36.7 | 32.9 | 4.5–11.0 |
| Hemoglobin, g/L | 92 | 70 | 71 | 120–150 |
| Platelet count, × 109/L | 92 | 87 | 115 | 150–500 |
| Creatinine, μmol/L | 898 | 961 | 975 | 60–110 |
| Haptoglobin, g/L | 2.23 | 1.90 | – | > 0.4 |
| Lactate dehydrogenase, U/L | 247 | 354 | – | 140–280 |
| Total bilirubin, μmol/L | 273 | 401 | 531 | 5–22 |
| Direct bilirubin, μmol/L | 210 | 220 | 469 | 2–5 |
| Alkaline phosphatase, U/L | 248 | 456 | 993 | 44–147 |
| γ-glutamyl transpeptidase, U/L | 1060 | NA | NA | 0–30 |
| Alanine aminotransferase, U/L | 25 | 31 | 44 | 7–55 |
| Aspartate aminotransferase, U/L | 28 | 73 | 248 | 8–48 |
| INR | 1.75 | 2.15 | 2.34 | < 1.1 |
| PTT, s | 44.9 | 52.3 | – | 25–35 |
| D-dimer, μ/mL | > 4 | > 4 | – | < 0.5 |
| Fibrinogen, g/L | 9.62 | 8.93 | – | 2–4 |
Note: INR = international normalized ratio, NA = not applicable, PTT = partial thromboplastin time.
On day 2, the patient became hemodynamically unstable and was transferred to the intensive care unit (ICU), where she received vasopressors and renal replacement therapy. Nine units of packed red blood cells were administered over 5 days for persistent anemia. Escherichia coli grew in urine and blood samples. The nephrology consultant identified bile casts on urine microscopy and ascribed the renal failure to sepsis and bile cast nephropathy. To investigate the conjugated hyperbilirubinemia, the ICU team ordered autoimmune, viral and toxicological screens, which were all normal. Doppler studies showed no portal vein thrombosis. Computed tomography (CT) and magnetic resonance cholangio-pancreatography showed no intrahepatic or biliary tract obstruction but suggested bilateral pyelonephritis and splenectomy, although the patient had no surgical scar. The working differential diagnosis was nitrofurantoin-induced liver injury, intrahepatic cholestasis of sepsis and ischemic hepatopathy.
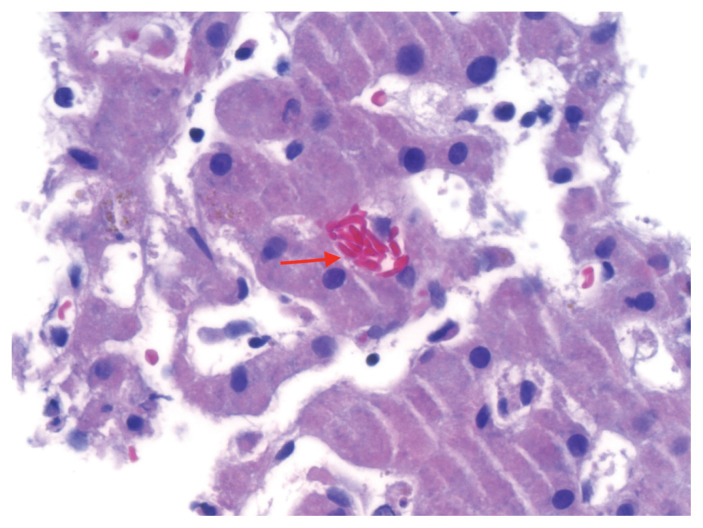
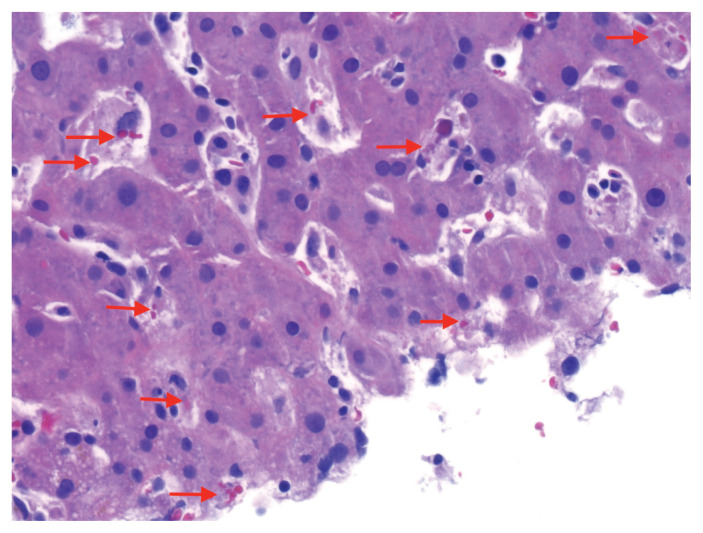
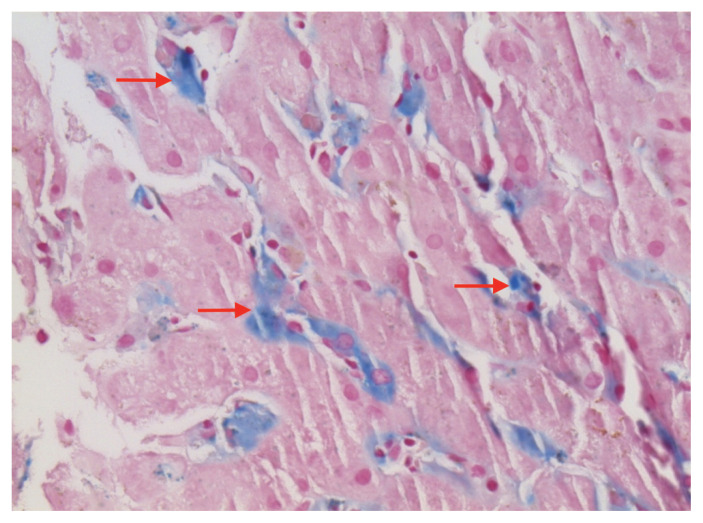
A liver biopsy showed sickle-shaped red blood cells within the hepatic sinusoids (Figure 1), sinusoidal Kupffer cell hyperplasia with erythrophagocytosis (Figure 2) and mild-to-moderate iron deposition (Figure 3). Because of the patient’s self-reported sickle cell trait, the emergency department team had ordered a blood smear and hemoglobin (Hb) fractionation. The automated smear showed both target cells and sickle cells. A normal fractionation consists primarily of HbA with small proportions of fetal HbF (< 1%) and HbA2 (normal variant of HbA, < 3.5%). Our patient’s sample had 2 Hb variants in similar amounts (HbS 46.6%, HbC 47.3%; Figure 4), some HbA2 (3.9%) and small amounts of other Hb types (HbF 1.2%, HbA 1.2%). The laboratory report stated that these results were compatible with hemoglobin SC disease (HbSC), a form of sickle cell disease. Repeat fractionation, ordered in the ICU for our patient after she had received 3 units of packed red blood cells, showed HbS (24.6%), HbC (24.8%), HbF (1.1%), HbA (40%) and HbA2 (3.3%).
Figure 1:
Liver biopsy specimen from a 56-year-old woman with hemoglobin SC sickle cell disease (hematoxylin and eosin at 60× magnification), showing an aggregate of sickled red blood cells within the sinusoidal spaces (arrow).
Figure 2:
Liver biopsy specimen from a 56-year-old woman with hemoglobin SC sickle cell disease (hematoxylin and eosin at 40× magnification). Sinusoidal spaces are expanded by hyperplasia of Kupffer cells, a type of histiocyte. Erythrophagocytosis within some of the histiocytes is shown by arrows.
Figure 3:
Liver biopsy specimen from a 56-year-old woman with hemoglobin SC sickle cell disease (iron stain at 40× magnification), showing mild-to-moderate iron deposition within the Kupffer cells (arrows).
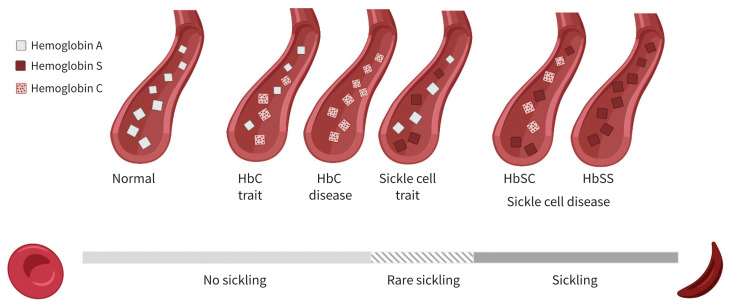
Figure 4:
Hemoglobin alleles and degree of sickling. Most people have 2 hemoglobin (Hb) A alleles. Patients with sickle cell trait have 1 allele for either HbS (sickle cell trait) or HbC (HbC trait) and generally have a low level of sickling. The most common form of sickle cell disease involves 2 HbS alleles (HbSS). Patients with HbSC disease, like our patient, have 1 HbS allele and 1 HbC allele (HbSC). Hemoglobin C disease (2 HbC alleles) does not lead to sickling, but is characterized by hemolysis with associated anemia.1
The patient improved and was transferred to the medical floor to our care. We reviewed the fractionation and liver biopsy results with pathology and hematology consultants. We agreed that the patient’s clinical course was likely related to previously unrecognized HbSC disease. This placed her at risk for urosepsis, which triggered intrahepatic sickling and microscopic biliary obstruction. Together, these caused renal injury. The history of pulmonary emboli and the absence of a spleen on imaging (likely owing to autosplenectomy) were consistent with sickle cell disease. Functional asplenia contributed to our patient’s previous meningitis and overwhelming infection on this admission.
We prescribed folic acid as a substrate for synthesis of red blood cells. The patient had persistent leukocytosis and hip pain, so we ordered blood cultures and a positron emission tomography (PET) scan to rule out infection. Cultures were negative, but the scan was suggestive of pelvic osteomyelitis. The patient declined a bone biopsy and was treated empirically with ceftriaxone. She later developed shoulder pain; CT imaging showed bilateral humeral head avascular necrosis, a known complication of sickle cell disease. She underwent débridement and acromioplasty. She was later found to have avascular necrosis in both shoulders, knees and femoral heads on CT imaging. What was originally considered to show osteomyelitis on the PET scan was likely avascular necrosis. Five months after admission, we discharged the patient to a rehabilitation centre and outpatient follow-up with hematology and internal medicine. She is now at home.
Discussion
There are many types of sickle cell disease. The most common, HbSS, results from homozygosity for the HbS allele at the β-globin gene and presents early in life, often causing painful vaso-occlusive sickling episodes. In contrast, HbSC results from heterozygous inheritance of HbS and HbC alleles (Figure 4). Compared with HbSS, people with HbSC have fewer acute painful episodes and a 2-decade longer life expectancy,2 but, as in our patient, they can experience important clinical consequences.2 Our patient is of Afro-Carribean origin, placing her at higher risk of Hb variants like S and C.
Compared with HbSS, HbSC typically results in less hemolysis, with mild or no anemia. In both HbSC and HbSS, the peripheral blood film may reveal sickle and target cells, which are not present with sickle cell trait.3 Fractionation profiles differ for HbSS and HbSC. Patients with HbSC have high levels of HbS and HbC (as with our patient), and patients with HbSS have higher levels of HbS (70%–90%) and HbF (< 30%). Our patient had S and C bands on initial fractionation, but a subsequent study showed an A band (normal adult hemoglobin), which we believe was owing to the transfusions she received. This may have led some care providers to think that she had sickle cell trait.
Complications in patients with HbSC can be similar to those in patients with HbSS, including vaso-occlusive pain episodes, acute chest pain syndrome, avascular necrosis, functional asplenia, autosplenectomy and hearing loss. However, in patients with HbSC, these manifestations may be delayed to middle age.2,3 Sometimes the diagnosis may be forgotten or misunderstood to be sickle cell trait rather than sickle cell disease. Thromboembolic complications, proliferative retinopathy and avascular necrosis are commonly observed with HbSC.4
Hepatic complications affect about 10% of patients with sickle cell disease who are admitted to hospital, across types. These are usually caused by hepatitis B, hepatitis C or iron overload from repeated transfusions.5 Intrahepatic cholestasis, as seen in our patient’s liver biopsy, is uncommon in patients with sickle cell disease (we identified no previous reports of intrahepatic cholestasis in patients with HbSC)6 and is provoked by occlusion of hepatic arterioles with sickled red blood cells, leading to infarction and obstruction of sinusoidal flow and sequestration of red blood cells in the liver. Intrahepatic cholestasis in HbSS disease has been reported to be triggered by infection, cocaine use and blood transfusions.5,6 In our patient, the trigger was infection. Exchange transfusion is frequently recommended for patients with intrahepatic cholestasis. 7 The 2020 American Society of Hematology guideline8 also recommends exchange transfusion in acute coronary syndrome, cerebrovascular accident or multiorgan failure. The aim is to drive HbS levels below 30%. Our patient had transfusions, but no exchange transfusion.
Sickle cell disease confers a 30-fold increase in the risk of septicemia because of functional asplenia or autosplenectomy, as a result of splenic ischemia from sickling. Patients are particularly susceptible to encapsulated organisms, the leading cause of death in patients with sickle cell disease.9 Common infections include respiratory infections from Streptococcus pneumoniae, skin infections from Staphylococcus aureus and urinary tract infections from Salmonella spp., Escherichia coli or Klebsiella spp.10 Most Canadian provinces include sickle cell disease as a condition warranting publicly funded vaccination against pneumococcus, meningococcus and hepatitis B. The guideline from the Canadian Haemoglobinopathy Association on management of sickle cell disease recommends that patients who are febrile, irrespective of genotype, seek urgent care for parenteral antibiotics. 11 The guideline also provides indications for disease-modifying therapy, such as hydroxyurea and transfusion, and highlights the importance of family screening.
Worldwide, 55 000 children are born with HbSC disease each year.2 In the United States and United Kingdom, HbSC disease accounts for 25%–30% of sickling disorders. Between 1993 and 2013, the overall incidence of hemoglobinopathies in Ontario, including HbSC disease, rose because of immigration from countries where these conditions are more prevalent.12 Several provinces now have routine newborn screening programs that include testing for sickle cell disease, but an American study reported that, among patients who were given diagnoses of sickle cell trait through newborn screening, only 16% self-reported this condition in early-to-middle adulthood.13 Given the more indolent course of HbSC disease, compared with HbSS, diagnosis is delayed until a critical event occurs in adulthood in one-third of cases.2,3
Before discharge, we emphasized to the patient and her family that she had sickle cell disease, not sickle cell trait, and discussed related recommendations, such as intravenous antibiotics in the event of fever. Even after our documentation of her case in the hospital chart, we noted instances in the medical record where she continued to be labelled as having “sickle cell trait.” This highlights the importance of education for clinicians and patients about sickle cell disease in general and HbSC disease in particular.
Acknowledgements
The authors thank Dr. Victoria Marcus and Dr. Catherine Weber of the McGill University Health Centre for their review and comments. They also thank Salma Rakani for her assistance with Figure 4.
Footnotes
For a first-person account of this condition, see www.cmaj.ca/lookup/doi/10.1503/cmaj.220573
Competing interests: None declared.
This article has been peer reviewed.
The authors have obtained patient consent.
Contributors: Thomas McFarland drafted the manuscript, which was revised by David Spillane and Kaberi Dasgupta. Elizaveta Chernetsova drafted the sections reporting the pathology findings and prepared the images. All authors gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
References
- 1.Sheehan VA, Gordeuk VR, Kutlar A. Disorders of hemoglobin structure: sickle cell anemia and related abnormalities. In: Kaushansky K, Prchal JT, Burns LJ, et al. Editors. Williams Hematology, 10th edition. New York: McGraw-Hill Education; 2021. [Google Scholar]
- 2.Pecker LH, Schaefer BA, Luchtman-Jones L. Knowledge insufficient: the management of haemoglobin SC disease. Br J Haematol 2017;176:515–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lionnet F, Hammoudi N, Stojanovic KS, et al. Hemoglobin sickle cell disease complications: a clinical study of 179 cases. Haematologica 2012;97:1136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballas SK, Lewis CN, Noone AM, et al. Clinical, hematological, and biochemical features of Hb SC disease. Am J Hematol 1982;13:37–51. [DOI] [PubMed] [Google Scholar]
- 5.Norris WE. Acute hepatic sequestration in sickle cell disease. J Natl Med Assoc 2004; 96:1235–9. [PMC free article] [PubMed] [Google Scholar]
- 6.Mwirigi AN, Muzah C, Odeh L, et al. A five year experience of acute intrahepatic cholestasis in patients with sickle cell disease at a large teaching hospital in London. Blood 2015;126:3416. [Google Scholar]
- 7.Martí-Carvajal AJ, Martí-Amarista CE. Interventions for treating intrahepatic cholestasis in people with sickle cell disease. Cochrane Database Syst Rev 2017;7:CD010985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chou ST, Alsawas M, Fasano RM, et al. American Society of Hematology 2020 guidelines for sickle cell disease: transfusion support. Blood Adv 2020;4:327–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Booth C, Inusa B, Obaro SK. Infection in sickle cell disease: a review. Int J Infect Dis 2010;14:e2–12. [DOI] [PubMed] [Google Scholar]
- 10.Ochocinski D, Dalal M, Black LV, et al. Life-threatening infectious complications in sickle cell disease: a concise narrative review. Front Pediatr 2020;8:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Consensus statement on the care of patients with sickle cell disease in Canada. Ottawa: The Canadian Haemoglobinopathy Association; 2018. [Google Scholar]
- 12.Simpson E, Klaassen RJ, Chakraborty P, et al. Increasing incidence and prevalence of pathologic hemoglobinopathies among children in Ontario, Canada from 1991–2013. Blood 2018;132(Suppl 1):4698. [Google Scholar]
- 13.Ashorobi D, Ramsey A, Yarrarapu SNS, et al. Sickle cell trait. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022. [Google Scholar]