Nonalcoholic fatty liver disease is the leading cause of liver disease worldwide and can progress to nonalcoholic steatohepatitis (NASH) through physical inactivity and gut dysbiosis.1 Exercise training reverses gut dysbiosis in non-NASH persons with obesity and in NASH animal models.2,3 Consequently, we conducted a proof-of-concept study investigating the effect of exercise training on gut dysbiosis in NASH patients.
Methods
This study analyzed samples from subjects with biopsy-proven NASH participating in the NASHFit (NASH Fitness Intervention in Thrombosis) trial.4,5 Subjects completed 20 weeks of supervised 30-minute sessions of moderate-intensity aerobic training 5 times a week. Exercise difficulty increased as subjects’ conditioning improved. Subjects received weekly nutritional counseling to control for dietary influences on the microbiome. Home kits (Omnigene Gut OMR 200 kit; Genotek, Ottawa, ON, Canada) were used for stool sample collection for 16S ribosomal RNA (rRNA) analysis and metatranscriptomics.
Results
Six subjects (83% men) with mean age 50 ± 13 years and mean body mass index 34.2 ± 4.0 kg/m2 were included. No subject was prescribed antibiotics in the 6 months preceding enrollment or during the study. Mean Nonalcoholic Fatty Liver Disease Activity Score was 5.7 ± 0.8, mean hepatic fat was 20.4 ± 5.7%, and mean fibrosis stage was 1.5 ± 0.8. Following exercise training, subjects experienced clinically significant improvements in body weight (4% weight loss; P = .167), liver enzymes (alanine aminotransferase = −16 ± 11 IU/L, P = .301; aspartate aminotransferase = −15 ± 18 IU/L, P = .268), and magnetic resonance imaging proton density fat fraction (20% relative reduction; P = .194). No changes were observed in macronutrients or fiber intake.
16S rRNA Analysis
Exercise training led to a minor increase in α-diversity (64.4 amplicon sequence variants pre-exercise vs 70.8 amplicon sequence variants postexercise; P = .630). A defined and consistent shift in β-diversity was observed in a principal coordinate analyses plot based on the Weighted UniFrac Distance metric, as postexercise samples shifted toward the center of the plot. Parabacteroides distasonis was identified as the most differential bacteria (pre-exercise = 0.4%, postexercise = 1.9%; P = .101). However, linear discriminant analysis (LDA) effect size (LEfSe) analysis found no enriched taxa LDA >2.0; P < .05). Co-network analysis demonstrated that P distasonis had the second greatest number of connections in the postexercise network, and positive co-occurring relationships were observed with beneficial commensal gut organisms Lachnospiraceae and Ruminococcaceae.
Metatranscriptomics
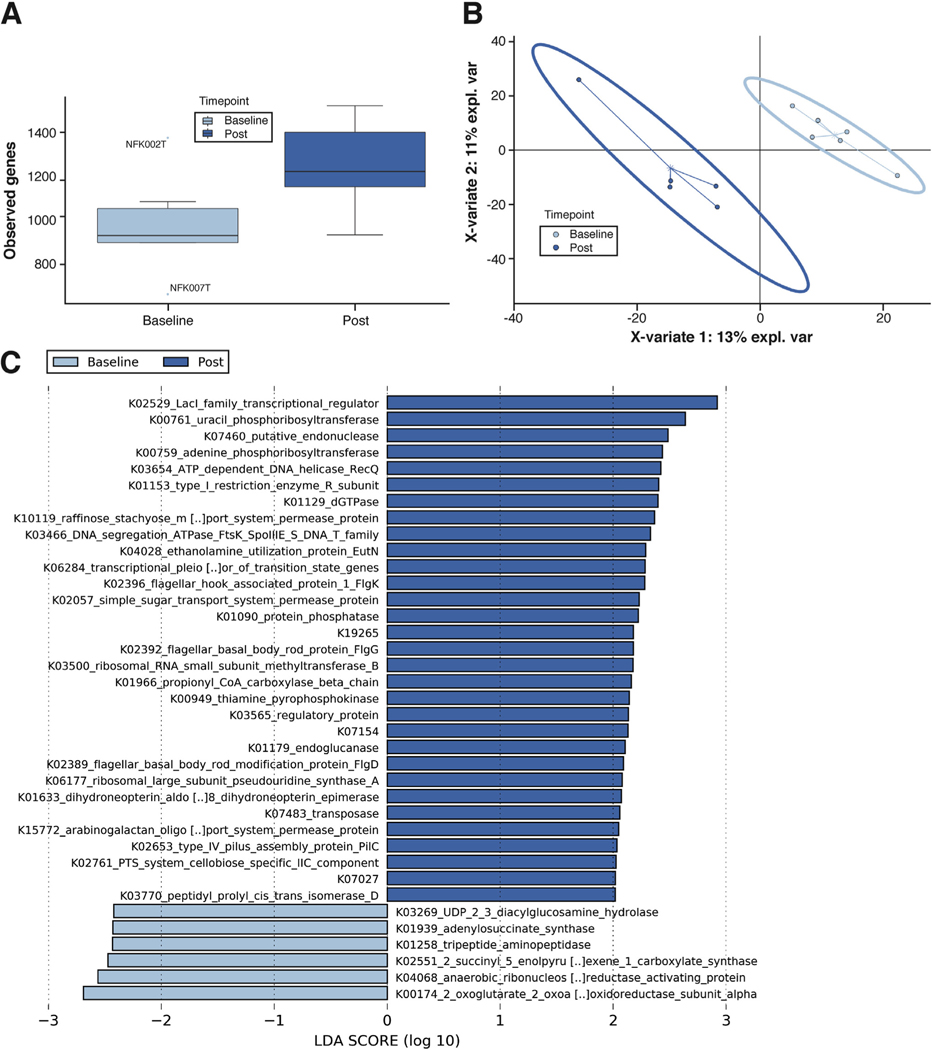
Metatranscriptomics demonstrated functional α-diversity increased much more after exercise training than composition did per 16S rRNA analysis (median 930 vs 1223 unique genes; P = .068) (Figure 1A). Community function shifted after exercise training, as evidenced by the separation among the 95% confidence ellipses shown in the partial least-squares discriminant analysis model (Figure 1B). LEfSe identified 5 taxa as significantly differential after exercise; P distasonis was the most enriched (755 counts per million pre-exercise vs 4781 counts per million postexercise; P = .018).
Figure 1.
Metatranscriptomics demonstrates that aerobic exercise training improves gut dysbiosis. (A) α-Diversity values when considering the observed features metric. The postexercise samples had a noticeably higher number of observed genes. Values were calculated using Qiime2 and represent the average of each sample’s 20 iterations at a depth of 12,000 sequences. (B) β-Diversity assessed by multidimensional plots using partial least-squares discriminant analysis, as implemented in the mixOmics package in R software, shows separation between groups in both 2-dimensional space (shown) and 3-dimensional space (not shown). (C) LEfSe enrichment plots display significantly enriched (P < .05; LDA > 2) functional KEGG genes within the groups. The logs of LDA scores are displayed on the x-axis and quantify the strength of enrichment within each respective categorical group. LEfSe assesses significance using Kruskal-Wallis tests.
LEfSe comparisons of KEGG (Kyoto Encyclopedia of Genes and Genomes) Orthologs revealed 31 genes were enriched after exercise training, including genes that may be involved in nonalcoholic fatty liver disease and NASH: K01633 (folB) and K19265 (gpr) oxidoreductase genes; K01966 (pccB), an amino acid degradation gene; K03269 (lpxH), a lipopolysaccharide biosynthesis gene; K04068 (nrdG), an organic free radical activating gene; and K04028 (eutN), an ethanolamine utilization gene (Figure 1C).
Post Hoc Power Calculation
Assuming the abundance of each bacterial taxon is normally distributed and independent, a post hoc power calculation revealed that a per-group sample size of 6 resulted in 80% power to detect 2-fold differences in bacterial taxa abundance (α = 0.01).
Discussion
This is the first study to investigate the effects of exercise training on the gut microbiome in patients with NASH. Our findings advance our understanding of how exercise training impacts NASH in 3 ways. First, exercise training reverses gut dysbiosis in NASH because both 16S rRNA and metatranscriptomics data demonstrated greater microbial and functional diversity. Second, exercise training enriches certain bacteria, including P distasonis, for which low abundance leads to NASH development.6,7 P distasonis enrichment created multiple positive co-occurring relationships with commensal gut organisms, including Lachnospiraceae and Ruminococcaceae, each of which may lessen “leaky gut” and promote gastrointestinal health. Third, this is the first study to apply metatranscriptomics to evaluate dysbiosis in patients with NASH. This is important because metatranscriptomics allowed for more both a more sensitive quantification of functional α-diversity and also identification of multiple genes involved in NASH pathogenesis that were enriched, offering novel explanations for how exercise training improves NASH through changes in the microbiome.
In conclusion, our findings demonstrate proof of concept that exercise training reverses gut dysbiosis. These findings require validation in a randomized controlled trial. If validated, new treatments beyond exercise that target microbiome manipulation may be discovered for NASH patients.
Acknowledgments
The authors thank the following individuals for their contributions: Chris Sicca and Jeff Vesek at the Penn State Center for NMR Research; Megan Beyer, Tiffany Myers, and Heather Tressler in the Penn State Division of Gastroenterology and Hepatology; David Gater and Kristin Slavoski in the Penn State Physical Medicine and Rehabilitation Research Laboratory; and Deborah Tregea in the Penn State University Fitness Center.
Funding
This grant was funded in part by National Institutes of Health grant L30 DK118601. This project is also funded, in part, under a grant with the Pennsylvania Department of Health using Tobacco CURE Funds. The department specifically disclaims responsibility for any analyses, interpretations or conclusion. The study was supported by National Institutes of Health/National Center for Advancing Translational Sciences Grants UL1TR000127 and UL1TR002014.
Footnotes
Conflict of Interest
The authors disclose no conflicts.
References
- 1.Diehl AM, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med 2017; 377:2063–2072. [DOI] [PubMed] [Google Scholar]
- 2.Carbajo-Pescador S, Porras D, García-Mediavilla MV, et al. Beneficial effects of exercise on gut microbiota functionality and barrier integrity, and gut-liver crosstalk in an in vivo model of early obesity and non-alcoholic fatty liver disease. Dis Model Mech 2019;12:dmm039206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan X, Saarinen A, Mikkola TM, et al. Effects of exercise and diet interventions on obesity-related sleep disorders in men: study protocol for a randomized controlled trial. Trials 2013;14:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stine JG, Schreibman I, Navabi S, et al. Nonalcoholic steatohepatitis Fitness Intervention in Thrombosis (NASHFit): study protocol for a randomized controlled trial of a supervised aerobic exercise program to reduce elevated clotting risk in patients with NASH. Contemp Clin Trials Commun 2020;18:100560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stine JG, Xu D, Schmitz K, et al. Exercise attenuates ribosomal protein six phosphorylation in fatty liver disease. Dig Dis Sci 2020;65:3238–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Xu J, Wang X, et al. Changes of intestinal bacterial microbiota in coronary heart disease complicated with nonalcoholic fatty liver disease. BMC Genomics 2019;20:862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bashiardes S, Shapiro H, Rozin S, et al. Non-alcoholic fatty liver and the gut microbiota. Mol Metab 2016;5:782–794. [DOI] [PMC free article] [PubMed] [Google Scholar]