Abstract
Mutations in GBA1, the gene encoding the lysosomal hydrolase glucocerebrosidase (GCase), are a risk factor for parkinsonism. Pursuing the potential mechanisms underlying this risk in aging neurons, we propose a new network uniting three major lysosomal proteins: (i) cathepsin D (CTSD), which plays a major role in α-synuclein (SNCA) degradation and prosaposin (PSAP) cleavage; (ii) PSAP, essential for GCase activation and progranulin (PGRN) transport; and (iii) PGRN, impacting lysosomal biogenesis, PSAP trafficking, and CTSD maturation. We hypothesize that alterations to this network and associated receptors modify lysosomal function and subsequently impact both SNCA degradation and GCase activity. By exploring the interactions between this protein trio and each of their respective transporters and receptors, we may identify secondary risk factors that provide insight into the relationship between these lysosomal proteins, GCase, and SNCA, and reveal novel therapeutic targets.
Seeking the Missing Links in the Association between GBA1 Mutations and the Synucleinopathies
More than a decade of research exploring the association between mutations in GBA1, the gene encoding the lysosomal hydrolase glucocerebrosidase (GCase) (see Glossary), and the development of synucleinopathies like Parkinson disease (PD) and dementia with Lewy bodies (DLB), has expanded our understanding of genetic risks and disruptions of lysosomal function in neurodegenerative disorders [1–4]. There is increasing evidence that mutations in different lysosomal genes are implicated in the etiology of PD [5,6]. Biallelic mutations in GBA1 cause the Mendelian lysosomal storage disorder Gaucher disease (GD), characterized by moderate to severe loss of GCase activity. As a result, the metabolism and degradation of glucosylceramide (GluCer) and other glycosphingolipids are significantly impaired, causing substrate accumulation in the lysosomes of macrophages and other cells. Furthermore, multiple lines of evidence have demonstrated that both heterozygous and homozygous mutations in GBA1 are an important risk factor for PD and DLB, although the penetrance is relatively low [1,2,7]. Nonetheless, depending on patient’s ethnicity, between 2 and 25% of patients with PD or DLB carry a mutation in GBA1, with an overall odds ratio of over 5. Thus, insights related to this rare disease have broad implications, potentially yielding new therapeutic targets for synucleinopathies.
In contrast to monogenic GD, DLB and PD are complex disorders, where factors including genetic predisposition, environment, aging, and epigenetics contribute to disease development. Of the many genes now known to contribute to these synucleinopathies, mutations in GBA1 confer the highest risk. However, as more is learned about this association, it is evident that other genes, pathways, and nongenetic factors must impact lysosomal function as it relates to α-synuclein (SNCA) degradation.
SNCA is a small and abundant protein present in neuronal cells. The aggregation and accumulation of oligomeric and fibrillar SNCA species is the pathological hallmark observed in brains of patients with PD and DLB, present in structures called Lewy bodies or Lewy neurites [8]. Two types of proteolytic pathways are involved in SNCA degradation: the cytosolic ubiquitin/proteasome pathway and, more importantly, the autophagic/lysosomal pathways, including macroautophagy and chaperone-mediated autophagy [9,10]. Here, we suggest the link between the synucleinopathies and GBA1 mutations to be a direct result of impaired lysosomal function. The machinery involved in lysosomal recycling and degradation of macromolecules depends on several functional pathways and regulatory systems, including endocytic membrane trafficking, autophagic pathways, and lysosomal biogenesis [11,12]. Dysfunction of any of the lysosomal proteins or pathways involved in these processes can disrupt lysosomal homeostasis, leading to the accumulation of toxic metabolites and subsequent cell death.
Based on the literature and some experimental evidence, we hypothesize that three multifunctional lysosomal proteins, procathepsin D (Pro-CTSD), prosaposin (PSAP), and progranulin (PGRN), together create a unique intralysosomal network, which we refer to as the Pro-CTSD, PSAP, and PGRN network (PPPN). Studying the role and function of this network can provide new insights into secondary risk factors contributing to the association between GBA1 mutations and parkinsonism. We maintain that this network is not only involved in the degradation of SNCA, but also modulates GCase activity. This potentially explains the findings of altered GCase activity among patients with PD without GBA1 mutations [13,14]. Furthermore, exploring the role of the PPPN can have vast clinical significance by uncovering new drug targets (see Clinician’s Corner). To better understand how this network functions, it is necessary to explore the connections and interfunctional relationships among these three proteins, whose interactions in the lysosome are reminiscent of the three intertwined witches in Shakespeare’s Macbeth.
Clinician’s Corner.
The identification of genes associated with Parkinson disease (PD) has illuminated new pathways involved in the disease pathogenesis that may yield targeted treatments [38]. Therapeutic strategies aimed at increasing levels of glucocerebrosidase (GCase) are already under development or in clinical trials [80,81].
While mutations in GBA1, the gene mutated in Gaucher disease (GD), are an important risk factor for the development of parkinsonism, the vast majority of patients with GD and heterozygous mutation carriers do not develop PD, implicating the contribution of additional pathways or proteins in this association. Genomic evaluations of cohorts of patients with PD with and without GBA1 mutations have already identified the lysosomal protein cathepsin B as a potential risk allele for parkinsonism [6].
Elucidating the role of GCase in PD has directed attention to the role of the acidic organelle, the lysosome, in the pathogenesis of different synucleinopathies, including PD and dementia with Lewy bodies.
The PPPN is a network comprised of three multifunctional proteins, the protease procathepsin D, the preactivator prosaposin, and the lysosomal biogenesis protein progranulin (PGRN). All three are active in the acidic lysosomal environment and influence the activity of GCase, an essential lysosomal enzyme, as well as the degradation of α-synuclein (SNCA), a protein implicated in the different synucleinopathies. Each member of the PPPN requires the other members for homeostasis. This interdependency introduces potential feedback mechanisms to protect and support cells under stress. Since interactions between the PPPN proteins directly impact GCase activity and SNCA degradation, they may provide new targets for therapeutic development for PD.
Several new therapeutic strategies to enhance brain PGRN levels or secretion are already under investigation for FTLD. These include histone deacetylase inhibitors (ClinicalTrials.gov Identifier NCT02249160) and the calcium channel blocker nimodipine (ClinicalTrials.gov Identifier NCT01835665). Other strategies under consideration are those blocking the sortilin–PGRN axis, inhibitors of lysosomal proteases, autophagy activators, and gene therapy [82,83].
The PPPN
Research exploring the role of each of these three PPPN proteins individually reveals their interconnectedness influencing trafficking, maturation, and activation of the precursor proteins at the acidic pH of the lysosome. Under normal circumstances, these interactions impact both GCase activity and SNCA degradation [15]. However, a deficiency in any of the three proteins can be deleterious, resulting in changes in both enzyme activation and protein degradation.
‘Double, Double, Toil and Trouble’: The Interdependency of PGRN and PSAP
PGRN is a conserved secreted glycoprotein found in most mammalian tissues. While this protein has multiple functions, its role in the lysosome remains unclear [16,17] (Box 1). PGRN contains seven and a half copies of 12 nonidentical cysteine granulin peptides connected by short linkers. The extracellular form of PGRN is highly stable in cortical neurons, protecting them from toxic and oxidative stress [18]. The trafficking of PGRN from the extracellular space to lysosomes is mediated by the cell surface sortilin1 receptor encoded by SORT1 [19]. It is proposed that in the lysosome, PGRN is cleaved by cathepsin L, generating seven active 10-kDa granulin peptides. The function of these cleaved granulin peptides is still undefined, yet it is speculated that they may modulate cathepsin activity or interact with other proteins in the lysosome [20].
Box 1. Structure and Biological Activity of Progranulin (PGRN).
PGRN
GRN, located on 17q21.31, consists of 593 amino acid residuals that contain a signal sequence and seven and a half granulin repeats of a 60-amino acid domain that includes 12 cysteine residues in each repeat, separated by a linker sequence [71]. It is an evolutionarily conserved secreted glycoprotein, initially identified as a growth factor protein [72]. Mature PGRN, a protein of 63.5 kDa, is a neurotrophic factor required to maintain lysosomal function in neurons [18]. Secreted PGRN can be cleaved in the cytosol or transported to the lysosome via the sortilin receptor and cleaved by cathepsin-L into seven active 8–10-kDa granulin peptides [20]. Heterozygous loss-of-function mutations in GRN cause frontotemporal lobar degeneration (FTLD), the second most common form of early-onset dementia. Patients with homozygous or compound heterozygous GRN mutations exhibit the lysosomal storage disorder neuronal ceroid lipofuscinosis (NCL), a severe neurodegenerative disease with lysosomal lipofuscin accumulation [73]. Brain samples and cells from patients with FTLD and NCL as well as GRN knockout cell lines demonstrate that GRN mutations are associated with lysosomal dysfunction, serving as a common mechanism in both diseases [73,74]. The lysosomal cleavage of PGRN into the seven stable granulins suggests that each granulin may have a functional role in lysosomal biogenesis.
PSAP is a glycosylated protein with dual function (Box 2). The 65-kDa form is the precursor for saposin peptides A–D and is shuttled from the endoplasmic reticulum (ER), entering into the lysosome via the mannose-6-phosphate receptor (M6PR). A second 70-kDa form binds to G protein-coupled receptors 37 (GPR37) and GPR37L and serves as a neuroprotective protein that is secreted extracellularly [21–24]. When neuronal cells are stressed as a result of lysosomal dysfunction, aging, oxidative stress, and/or inflammation [25], an extralysosomal interaction between PGRN and PSAP is activated, facilitating increased delivery of each protein to the lysosome [16,26,27]. This interaction occurs both extracellularly and in the cytoplasm. PSAP binds to PGRN at a linker region between saposins B and C, aiding the transport of both proteins into the lysosome via the endosomal system. The interaction between the proteins allows them to use both the M6PR and the sortilin receptor to cross the lysosomal membrane [16,26,28] (Figure 1A). Together, PSAP and PGRN arrive at the acidic environment of the lysosome as inactive proteins. Once in the endolysosomal system, PGRN facilitates the activation of the third PPPN protein, pro-CTSD [29], generating cathepsin D (CTSD). CTSD, in turn, facilitates the hydrolysis and conversion of PSAP into a functional activator protein [30].
Box 2. Structure and Biological Activity of Cathepsin D and Prosaposin Cathepsin D (CTSD).
CTSD (EC 3.4.23.5), encoded by CTSD on 11p15.5, is synthesized and translocated into the rough ER as an inactive pre-proenzyme (52 kDa) [31]. It is then glycosylated, phosphorylated, and subsequently converted to a 48-kDa proenzyme [32]. Pro-CTSD is sorted to the endosome/lysosome by the mannose-6-phosphate receptor (M6PR). Inside the lysosome, the 44-amino acid N terminal propeptide signal chain is cleaved and the 34-kDa mature active CTSD functions to degrade denatured or mutated proteins, facilitating their further cleavage by other lysosomal endopeptidases and exopeptidases [33,75]. CTSD is widely expressed in the cortex, hippocampus, striatum, and in dopaminergic neurons in the substantia nigra. Mutations and polymorphisms in CTSD affect its transport, maturation, and enzymatic activity [35]. Severe mutations in CTSD result in different forms of neuronal ceroid lipofuscinoses. CTSD is the principal endopeptidase responsible for SNCA degradation [34] and is considered neuroprotective against SNCA aggregation and toxicity [76].
Prosaposin (PSAP, Sulfated Glycoprotein-1, Sphingolipid Activator Protein-1)
PSAP, located on 10q22.1, encodes the 524-amino acid prosaposin precursor protein. Functionally, the protein exists in two forms, a 65-kDa lysosomal form and a 70-kDa extracellular form with neuroprotective and glioprotective effects [21,24]. PSAP is transported from the ER to the lysosome via the M6PR and is cleaved by CTSD into saposins A–D, four small glycoproteins of about 8–11 kDa each [30]. Inside the lysosome, saposin C does not directly bind lipid, but rather initiates the activation of the enzyme GCase, assisting in glucosylceramide degradation. The phenotype of saposin C deficiency in humans resembles GD type 3 or presents as rare cases of GD type 1 with normal GCase activity [77–79].
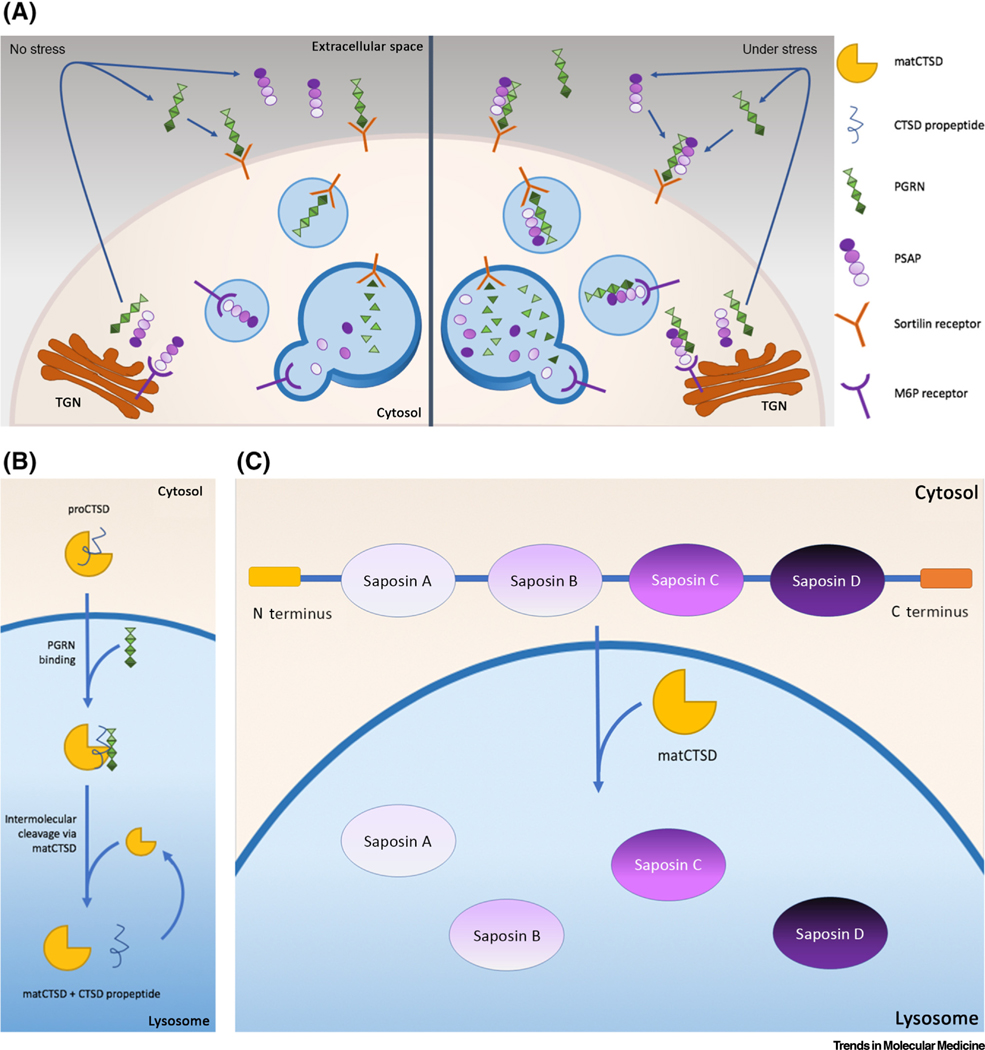
Figure 1. Pro-CTSD, PSAP, and PGRN Network (PPPN) Proteins Interact with and Help Activate Each Other within the Lysosome.
(A) Interaction between Prosaposin (PSAP) and Progranulin (PGRN) amplifies their response. Left: in the nonstressed state PSAP is transported to the endosome/lysosome via mannose-6-phosphate receptor (M6PR) and secreted PGRN is transported bound to the sortilin1 receptor. Once in the lysosome, cathepsin D (CTSD) cleaves PSAP, creating four different activators for enzyme hydrolysis, and PGRN is cleaved into seven active granulins. Right: under stress conditions, more lysosomal activity is required. Both PSAP, for activation of several enzymes, and granulins, for lysosomal biogenesis, are mobilized. They interact in order to use each other’s respective receptors. This enables both proteins to be transported to the lysosome via both M6PR and sortilin receptors, amplifying their endocytosis and lysosomal levels. The feedback mechanism remains unknown. (B) PGRN activates pro-CTSD at the acidic lysosomal pH. In the lysosome, PGRN binds to the N terminal signal chain of pro-CTSD, initiating its proteolytic cleavage in order to generate mature and active CTSD. Mature CTSD (matCTSD) accelerates the proteolytic cleavage to enhance production of activated CTSD [29]. (C) Processing of PSAP to generate mature saposins in the lysosome. PSAP, a 524-amino acid polypeptide, contains four conserved saposin domains (A–D) with six cysteines and one or two glycosylation sites, connected by linker sequences. PSAP is transported to the lysosome via M6PR and under stress conditions by both M6PR and sortilin1. PSAP is then cleaved by mature CTSD at the linker sites to produce the four saposins, which function as activators of sphingolipid hydrolases. Abbreviation: TGN, trans-Golgi network.
‘When Shall We Three Meet Again?’ The Trio Rendezvous in the Lysosome: Activation of Pro-CTSD, PSAP, and PGRN
CTSD, a soluble lysosomal aspartic endopeptidase, is synthesized and translocated into the rough ER as pre-pro-CTSD [31] (Box 2). Inactive pre-proenzyme is sorted to the endosome by M6PR molecules and is subsequently converted into the 48-kDa pro-CTSD [32,33] (Figure 1B). Once pro-CTSD is delivered to the endolysosome, PGRN becomes directly involved in its maturation [29]. PGRN binds to the active site of pro-CTSD and facilitates the cleavage of the signal chain, resulting in its conversion to mature CTSD [29]. CTSD then functions as one of the key endopeptidases responsible for protein cleavage and clearance, including the degradation of overexpressed wild type and/or mutated SNCA in neurons [34,35].
In the lysosome, active mature CTSD is also essential for the hydrolysis and activation of PSAP. CTSD cleaves PSAP into four lysosomal activator proteins known as saposins A–D [30] (Figure 1C). Each saposin then activates hydrolases specific for the cleavage of different sphingolipids. Saposin C is the activator of GCase and catalyzes the cleavage of GluCer and glucosylsphingosine. It also mediates the contact and binding of active GCase with its substrate [36]. This dual action of saposin C identifies three potential steps that can result in GCase dysfunction when GBA1 is mutated; first, the binding of saposin C with subsequent activation of GCase may not occur, secondly, the binding may not be sufficiently stable for activation, and/or lastly, saposin C may fail to act as a chaperone to facilitate the binding of GCase to its substrate. Thus, the interaction with saposin C is vital for normal GCase activity.
The PPPN Is a Potential Source of Secondary Risk Factors for GBA1-Associated PD
Despite significant research, many, if not most of the genetic factors contributing to PD remain unknown. Moreover, the few genes known to confer an increased PD risk identified through genome-wide association studies (GWAS), whole exome sequencing, whole genome sequencing, SNP chips, and neuro-chips, including GBA1, clearly require other genetic, epigenetic, and nongenetic factors working in concert with the primary gene to result in the eventual development of PD [1,37–42]. These secondary genetic risk factors may or may not be directly related to the primary ‘PD genes’. The secondary genes, variants, or epigenetic changes may impact protein expression, synthesis, degradation, and/or other pathways related to known risk genes. In the case of GBA1, there are several approaches to identify the additional determinants impacting penetrance, including: (i) genomic, whole transcriptome, and epigenetic analyses, which require a reasonably large sample size [6]; and (ii) evaluation of genes in lysosomal pathways and networks impacting GCase activity and SNCA degradation through cellular and molecular analyses [43,44]. PPPN-related proteins have been identified by both approaches. Multiple genetic studies have implicated genes in lysosomal pathways involving the PPPN [5,45,46]. Furthermore, cellular and molecular studies of the PPPN trio have demonstrated a direct effect of each PPPN protein on SNCA aggregation and degradation, as well as on GCase activity [47,48]. Interestingly, not only the PPPN genes but also their respective receptors have been shown to play a role in neurodegenerative diseases. Variants in and around SCARB2, the gene encoding the GCase receptor LIMP-2, have been identified in several PD GWAS studies [49–52] and studies of the M6PR in neurons demonstrate that levels of this protein influence the overexpression of SNCA [53].
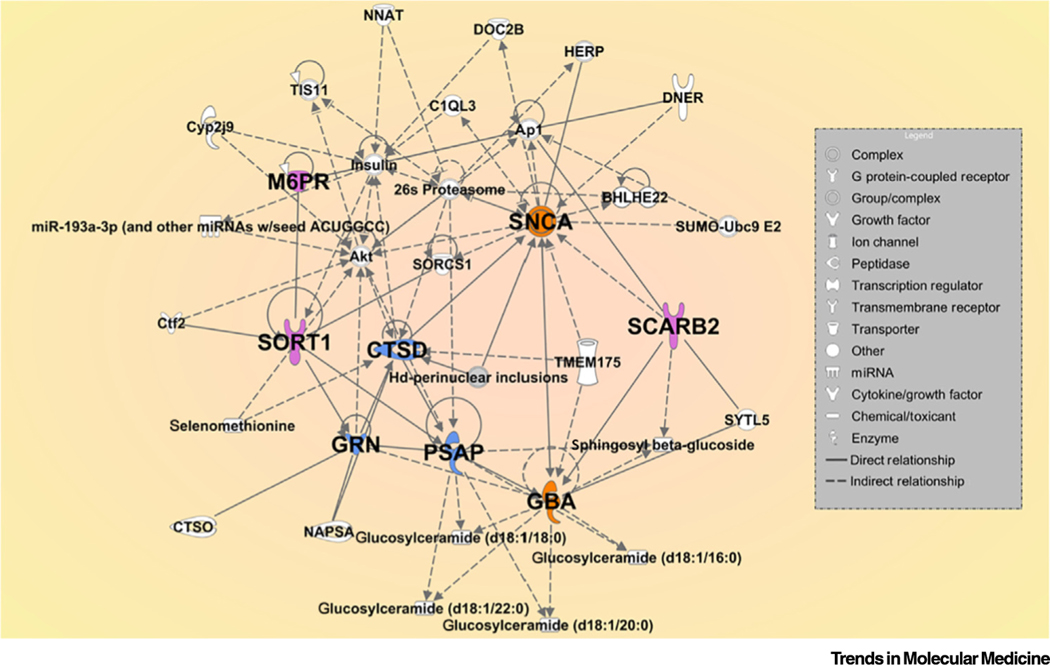
Gene–gene analyses of large PD GWAS studies have not been performed yet. Thus, to better visualize the relationship between variants identified from GWAS and other large datasets, specifically between the PPPN, SNCA, GBA1, and their related receptors, we applied Ingenuity Pathway Analysis (IPA), which integrates genomics, metabolomics, proteomics, and RNA-seq data. Through this analysis (Figure 2), we were able to show direct or indirect interactions among the proposed genes and identified several intermediate genes, including some known PD-associated genes.
Figure 2. Ingenuity Pathway Gene Network Analysis of the Procathepsin D-Prosaposin-Progranulin Network (PPPN) and Related Genes.
This analysis covers the PPPN genes (blue), SNCA and GBA1 (orange), and related receptors (lavender). The intermediate genes are illustrated in white. The direct (unbroken arrows) and indirect (dashes) relationships between the genes are shown. The results show an effect of GBA1 and PSAP on glucosylceramides with different length carbon chains. Some miRNA that may directly affect the network are also shown. The different symbols shown in the legend indicate the function of many of the genes or proteins. This pathway analysis illustrates not only the effect of PPPN proteins on each other, but also their direct influence on SNCA degradation and GCase activity.
Each Member of the PPPN Is Essential for GCase and SNCA Homeostasis
All three PPPN proteins are active at the acidic pH of the lysosome, where they exert their influence on both GCase activity and SNCA degradation. Moreover, since the M6PR and sortilin receptors mediate the vesicular transport of the three proteins from the cell membrane and trans-Golgi network (TGN) to endosomes, alterations to these receptors may also impact GCase and SNCA homeostasis (Figure 3).
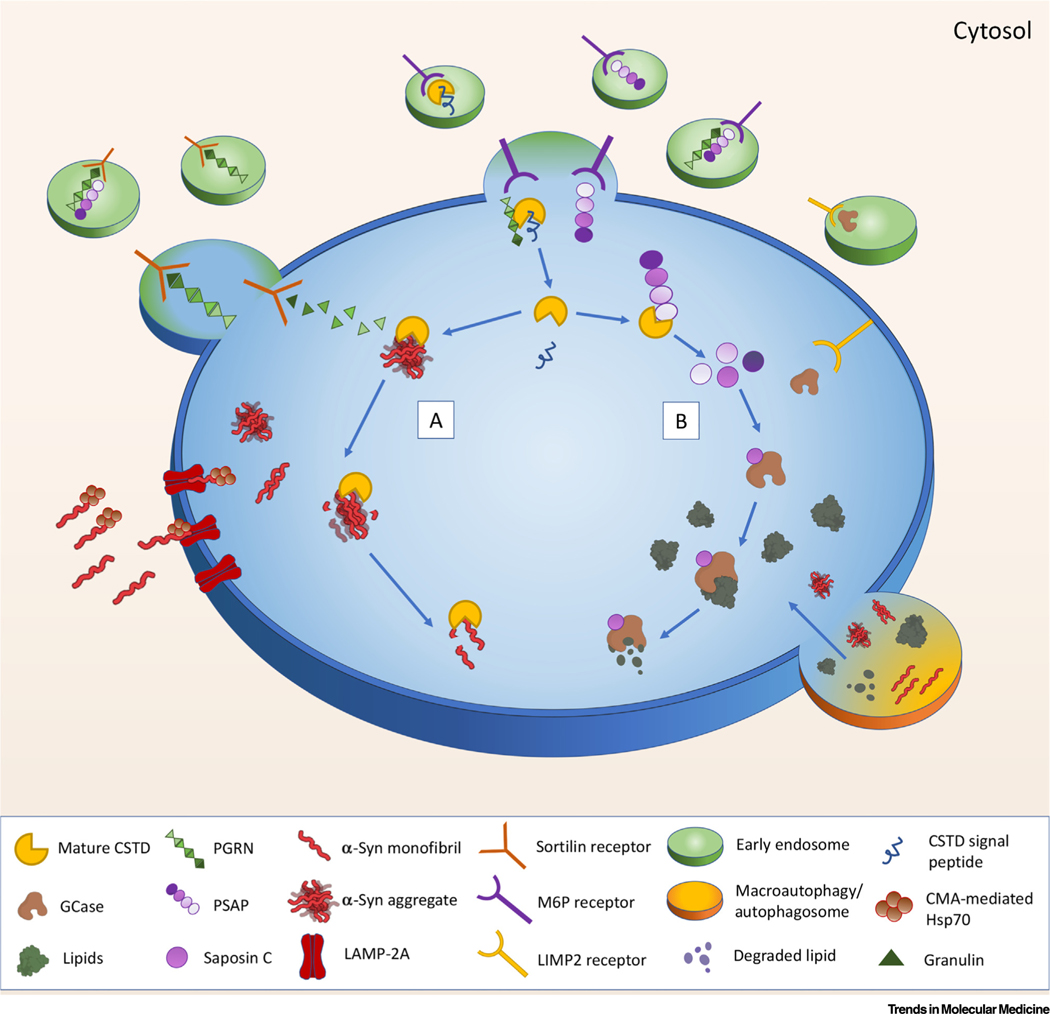
Figure 3. How the Procathepsin D (CTSD)-Prosaposin (PSAP)-Progranulin (PGRN) Network (PPPN) Can Impact α-Synuclein (SNCA) Degradation and Glucocerebrosidase (GCase) Activity.
For a Figure360 author presentation of Figure 3, see the figure legend at https://doi.org/10.1016/j.molmed.2020.07.004. Proposed model of the interactions of the PPPN within lysosomes of stressed (or aging) cells. With aging, GCase activity is reduced, more SNCA is expressed and/or aggregated, and CTSD levels are diminished. Under these conditions, the potential PGRN/PSAP feedback system is activated, enabling more PGRN and PSAP to enter the lysosome using both mannose-6-phosphate receptor (M6PR) and sortilin1. PGRN interacts with and cleaves proCSTD, initiating CSTD maturation. Meanwhile, monomeric and aggregated SNCA are transported to the lysosome via the LAMP-2A receptor and/or chaperone-mediated autophagy (CMA-Hsp70; lower left) for degradation (lower right). The specific lysosomal targeting protein for GCase, LIMP-2, may also play a role in this network (upper right). In the lysosome, mature CTSD serves to: (A) break down SNCA aggregates and degrade SNCA monomers, and (B) cleave PSAP to generate the four saposin activators. Saposin C activates GCase, enabling the hydrolysis of the lipids glucosylceramide and glucosylsphingosine.
The Impact of CTSD Deficiency on GCase and SCNA
Given the role of CTSD in PSAP processing and SNCA degradation, increasing CTSD expression and activity may enhance GCase activity, while concomitantly augmenting SNCA clearance. However, CTSD activity must be tightly regulated, as it could prove harmful if uncontrolled. CTSD can be modulated by cellular regulation of its expression, post-translational modifications, zymogen activation, pH modification, endogenous and exogenous inhibitors, or by a combination of each [54]. When CTSD is scarce, saposin C is deficient, and GCase activity is impaired, enhanced SNCA aggregation would be expected, contributing to the observed inverse relationship between the two proteins. Furthermore, it has been proposed that active GCase is necessary to enable CTSD to degrade and remove neuronal SNCA and that GBA1 mutations, in part, increase monomeric SNCA by negatively impacting CTSD activity [55].
The Impact of PGRN Deficiency on GCase and SNCA
While the complete function of PGRN is still not fully understood, it contributes to the pathogenesis of several neurodegenerative disorders [56,57] (Box 1). One function among its many roles is the cleavage of the pro-CTSD signal chain. Thus, by altering an upstream event involved in CTSD maturation, PGRN deficiency can result in outcomes similar to those described with CTSD deficiency. There is also evidence that PGRN may bind directly to GCase while the enzyme is being transported to the lysosome via its transporter LIMP-2. The binding of GCase to LIMP-2 is necessary to deliver the enzyme to the lysosome [58]. Based on new evidence that PGRN directly binds to GCase as it enters the lysosome, PGRN may be necessary for the disassociation of GCase and LIMP-2 once in the lysosome. This is supported by evidence demonstrating that in the absence of PGRN, GCase accumulates in the cytoplasm [59,60]. In addition, based on studies demonstrating decreased GCase activity in PGRN-deficient mice [61], it appears that PGRN deficiency also leads to reduced GCase activity [56,62]. In PGRN mutant cortical neurons derived from patients with frontotemporal lobar degeneration (FTLD), reduced GCase activity and increased insoluble SNCA levels were observed relative to isogenic controls [57]. Additionally, studies performed in brain samples from patients with FTLD demonstrated that the reduction in protein levels and enzymatic activity was unique to GCase and not seen with other lysosomal enzymes [62]. Conversely, in GD, PGRN levels tend to be lower than normal [63]. Furthermore, under stress conditions, the codependence of PGRN and PSAP during their entry into the lysosome would suggest that PGRN deficiency could generate a cascade of impairment that would negatively impact the levels of lysosomal PSAP, the production of saposin C, and ultimately the activity of GCase.
The Impact of PSAP Deficiency on GCase and SNCA
PSAP deficiency or mutations in the saposin C region of PSAP result in deficient levels of saposin C. As saposin C is a crucial activator of GCase, this decrease would, in turn, result in reduced GCase activity and function [64]. Thus, saposin C deficiency also results in the toxic accumulation of GluCer and glucosylsphingosine in the endolysosomal compartment [65]. In addition, as PSAP, under stress conditions, facilitates the transport of PGRN into the lysosome via M6PR (Figure 1A), PSAP deficiency would result in diminished lysosomal PGRN [26]. This would create additional dysfunction as the limited lysosomal PGRN would be unable to adequately convert pro-CTSD into mature CTSD (Figure 1B). The unique codependence between PGRN and PSAP suggests that in times of stress, as with PGRN deficiency, reduced PSAP may also result in decreased CTSD activity, further impairing the processing of PSAP by CTSD hydrolysis. This chain of PPPN inactivation ultimately impacts GCase function and SNCA homeostasis.
‘Fair Is Foul and Foul Is Fair’: Dual Relationships between the PPPN
Potential Competition
With the intricate relationships among the members of this protein trio, it is also possible that competition for protein activation could occur. For example, both PSAP and SNCA might compete for CTSD activity (Figure 3A,B). When the amount of active CTSD is inadequate, GCase activity would be compromised due to saposin C deficiency, while SNCA degradation could also be impaired. If the amount of active CTSD is limited, its preferential role in GCase activation or SNCA degradation would reduce its secondary function. In patients with PD without GBA1 mutations, decreased GCase activity could be a result of inadequate saposin C production due to the preferential action of CTSD on SNCA degradation [13,14].
The Role of Aging
Because the risk of PD increases with age, molecular and genetic changes associated with aging would be expected to contribute to altered activity of any of these three proteins. As we age, lysosomal function is compromised and this may change the intricate homeostasis between lysosomal proteins. Reduced lysosomal protein levels and activity, as well as the accumulation of toxic proteins, have been observed in age-dependent neurodegenerative diseases [18,66]. GCase activity and protein levels are also shown to naturally decrease with age [67], highlighting the possibility that other age-related factors or pathways affecting the three PPPN proteins, their interactions, and/or their receptors may also be involved.
Concluding Remarks
We have shown how the three proteins comprising the PPPN, PSAP, PGRN, and CTSD, play an essential role in the maturation and activation of several proteolytic enzymes and activators, which function to regulate lysosomal homeostasis in neurons. Ultimately, this network acts to facilitate the degradation and recycling of intracellular materials, including proteins, lipids, polysaccharides, DNA, and RNA. The impact of aging, genetics, epigenetics, and environmental factors on the multiple functions of the lysosome is still not fully understood. This is a field requiring further attention, as outlined later (see Outstanding Questions). Oxidative stress, mitochondrial dysfunction, inflammation, and other cellular disruptions caused by genetics, epigenetics, and environmental factors specifically influence neuronal lysosomal function [68]. We propose that among the different lysosomal-autophagy pathways activated during cellular stress, the feedback activity of the PPPN specifically helps neuronal survival in the following ways:
Outstanding Questions.
What are the best in vitro and in vivo models to study the PPPN inside the lysosome? Is there a model system where each PPPN protein can be knocked-down individually to assess its impact on GCase and SNCA levels? What cell type should be studied?
How does one best evaluate possible feedback occurring within this network?
Is PGRN the key protein in this network? Does a specific granulin contribute more to this network than others?
What other factors and proteins might be involved in or compete with proteins of the PPPN?
How does aging impact each protein in the PPPN and how might it influence lysosomal GCase activity and SNCA degradation in dopaminergic neurons?
Will therapies enhancing the levels of different PPPN proteins prevent the accumulation of toxic SNCA aggregates and increase GCase activity? Which protein would be the best to target?
Ordinarily, PSAP and PGRN are transported separately from the TGN and/or cell membrane to the lysosome via the M6PR and SORT1, respectively. However, during neuronal cell stress, their transport into the lysosome is augmented. Under our proposed PPPN, such stress leads to the cytosolic and/or extracellular binding between PSAP and PGRN through a feedback mechanism. This interaction, which takes place between granulin D and E in PGRN and the linker between saposins B and C on PSAP, allows both proteins to use each other’s receptors to enter the lysosome, quickly mobilizing the two PPPN proteins [26] (Figure 1A). As a result, the levels of critical lysosomal activators (saposins A–D) are enhanced and the increased PGRN present can serve as a mediator in lysosomal biogenesis and CTSD activation.
PGRN plays an active role in regulating CTSD activity in neurons. The direct interaction between PGRN and CTSD, which appears to occur at granulin E, facilitates proper functioning of CTSD, as well as neuronal cell homeostasis by degrading aggregated proteins in the lysosome [26,69].
Under conditions with PGRN insufficiency, the maturity and activity of the other PPPN proteins, as well as GCase, are significantly reduced [59,61,62,70].
The complex interactions between the PPPN proteins both at rest and under stress impact, mediate, and activate several lysosomal proteins and pathways. These proteins and their lysosomal transporters play a major role in maintaining GCase activity and in degrading toxic compounds such as SNCA. This intricate relationship between the three proteins indicates that they have a more nuanced role in lysosomal stability than previously appreciated, serving as secondary risk factors underlying the enigmatic relationship between GBA1 mutations and parkinsonism. Elucidating the contributions of the different PPPN proteins to the pathogenesis of PD may be clinically relevant, leading to novel therapeutic targets.
Supplementary Material
Highlights.
Mutations in GBA1, the gene encoding glucocerebrosidase (GCase), confer an increased risk of parkinsonism, directing attention to lysosomal pathways and proteins in Parkinson pathogenesis.
Other lysosomal proteins functioning at the acidic lysosomal pH influence GCase function and/or α-synuclein clearance.
Three multifunctional proteins (the PPPN): the protease procathepsin D, the preactivator prosaposin, and the lysosomal biogenesis protein progranulin, along with their respective transporters, are all interconnected. Their interdependency creates potential feedback mechanisms to protect and support cells under stress.
The interactions between the PPPN proteins directly impact GCase activity and α-synuclein degradation and lead to the identification of new risk factors and therapeutic targets for Parkinson disease.
Acknowledgments
This work was supported by the intramural research programs of the National Human Genome Research Institute and the National Institutes of Health. The authors thank Mr Eric J. Garcia for the IPA analysis, and Ms Julia Fekecs and Mr Darryl Leja for their assistence with the Figure360.
Glossary
- Dementia with Lewy bodies (DLB)
neurodegenerative disorder with progressive cognitive decline, ‘fluctuations’ in alertness and attention, visual hallucinations, and parkinsonian motor symptoms. While variants in three genes APOE, SNCA, and GBA1 are associated with an increased risk of DLB, in most cases, the cause is unknown
- G protein-coupled receptor 37 (GPR37)
receptor exclusively expressed in the nervous system that binds to and activates orphan neuropeptides, including neuroprotective and glioprotective factors like prosaposin
- Gaucher disease (GD)
the most common lysosomal storage disease due to mutations in GBA1, resulting in the deficiency of glucocerebrosidase and the accumulation of the glycolipids, glucosylceramide and glucosylsphingosine. GD has wide phenotypic heterogeneity. Patients have lipid-laden macrophages; rare forms also have neurological involvement. Patients with GD and heterozygous GBA1 mutations have an increased risk of PD and DLB
- Glucocerebrosidase (GCase)
the lysosomal enzyme deficient in patients with GD that catalyzes the cleavage of glucocerebroside to ceramide and glucose
- Glycosphingolipids and glucosylceramide (or glucocerebroside: GluCer)
the four types of glycosphingolipids include the cerebrosides, sulfatides, globosides, and gangliosides. Cerebrosides have a single sugar group linked to ceramide. Glucosylceramide is an intermediate in the synthesis or degradation of more complex glycosphingolipids and accumulates in patients with GD
- Ingenuity Pathway Analysis (IPA)
IPA is a web-based software application that analyzes data from gene expression studies, SNP microarrays, metabolomics, proteomics, miRNA, and RNA-seq data, building interactive models
- LIMP-2
lysosomal integral membrane protein 2 is a multifunctional protein and is expressed in different tissues, mainly in the membrane of lysosomes. This protein transports GCase to the lysosome independent of M6PR. The encoded gene is SCARB2
- Mannose-6-phosphate receptor (M6PR)
transporter of proteins between the trans-Golgi network (TGN) and endosome. Several lysosomal acid hydrolases are delivered to endosomes by M6PR. M6PR is a 46-kDa phosphorylated protein, encoded by M6PR gene
- Parkinson disease (PD)
a common neurodegenerative disorder affecting predominately dopamine-producing neurons in the substantia nigra. The defining symptoms are bradykinesia (slowed movements) and at least one of the following: rest tremor, rigidity, and postural instability. The histological hallmark is the presence of inclusions called Lewy bodies (LBs)
- Sortilin1 receptor
sortilins, receptors sharing a 700-amino acid extracellular domain, regulate intracellular transport by shunting proteins through secretory or endocytic pathways. The sortilin receptor traffics different proteins to the cell surface or subcellular compartments such as lysosomes and endosomes
- Synucleinopathies
a group of neurodegenerative disorders characterized by the intracytoplasmic accumulation of α-synuclein primarily in neurons and glia. They include Parkinson disease, dementia with Lewy bodies, the Lewy body variant of Alzheimer disease, multiple system atrophy, and neurodegeneration with brain iron accumulation
- α-Synuclein (SNCA)
a small, 140-amino acid, highly soluble cytosolic protein, encoded by the SNCA gene that aggregates and accumulates in Lewy bodies and neurites in PD and other synucleinopathies. SNCA, a presynaptic protein, is involved in synaptic vesicle mobility and recycling and the storage and compartmentalization of neurotransmitters. Mutations in and duplication or triplication of SNCA are associated with PD
References
- 1.Sidransky E. et al. (2009) Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N. Engl. J. Med 361, 1651–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nalls MA et al. (2013) A multicenter study of glucocerebrosidase mutations in dementia with Lewy bodies. JAMA Neurol. 70, 727–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mazzulli JR et al. (2011) Gaucher disease glucocerebrosidase and alpha-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell 146, 37–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazzulli JR et al. (2016) Alpha-synuclein-induced lysosomal dysfunction occurs through disruptions in protein trafficking in human midbrain synucleinopathy models. Proc. Natl. Acad. Sci. U. S. A 113, 1931–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robak LA et al. (2017) Excessive burden of lysosomal storage disorder gene variants in Parkinson’s disease. Brain 140, 3191–3203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blauwendraat C. et al. (2020) Genetic modifiers of risk and age at onset in GBA associated Parkinson’s disease and Lewy body dementia. Brain 143, 234–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruskey JA et al. (2019) Increased yield of full GBA sequencing in Ashkenazi Jews with Parkinson’s disease. Eur. J. Med. Genet 62, 65–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spillantini MG et al. (1998) Alpha-synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc. Natl. Acad. Sci. U. S. A 95, 6469–6473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gan-Or Z. et al. (2015) Genetic perspective on the role of the autophagy-lysosome pathway in Parkinson disease. Autophagy 11, 1443–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yasuda T. et al. (2013) Alpha-synuclein and neuronal cell death. Mol. Neurobiol 47, 466–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein AD and Mazzulli JR (2018) Is Parkinson’s disease a lysosomal disorder? Brain 141, 2255–2262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffmann AC et al. (2019) Extracellular aggregated alpha synuclein primarily triggers lysosomal dysfunction in neural cells prevented by trehalose. Sci. Rep 9, 544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gegg ME et al. (2012) Glucocerebrosidase deficiency in substantia nigra of parkinson disease brains. Ann. Neurol 72, 455–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy KE et al. (2014) Reduced glucocerebrosidase is associated with increased alpha-synuclein in sporadic Parkinson’s disease. Brain 137, 834–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou X. et al. (2017) Regulation of cathepsin D activity by the FTLD protein progranulin. Acta Neuropathol. 134, 151–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou X. et al. (2015) Prosaposin facilitates sortilin-independent lysosomal trafficking of progranulin. J. Cell Biol 210, 991–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paushter DH et al. (2018) The lysosomal function of progranulin, a guardian against neurodegeneration. Acta Neuropathol. 136, 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu J. et al. (2011) Extracellular progranulin protects cortical neurons from toxic insults by activating survival signaling. Neurobiol. Aging 32, 2326 [Google Scholar]
- 19.Hu F. et al. (2010) Sortilin-mediated endocytosis determines levels of the frontotemporal dementia protein, progranulin. Neuron 68, 654–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou X. et al. (2017) Lysosomal processing of progranulin. Mol. Neurodegener 12, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer RC et al. (2014) The protective role of prosaposin and its receptors in the nervous system. Brain Res. 1585, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Islam F. et al. (2020) Age- and sex-associated changes in prosaposin and its receptors in the lacrimal glands of rats. Histol. Histopathol 35, 69–81 [DOI] [PubMed] [Google Scholar]
- 23.Carvelli L. et al. (2017) Targeting exogenous beta-defensin to the endolysosomal compartment via a vehicle guided system. Histol. Histopathol 32, 1017–1027 [DOI] [PubMed] [Google Scholar]
- 24.Lundius EG et al. (2014)GPR37 protein trafficking to the plasma membrane regulated by prosaposin and GM1 gangliosides promotes cell viability. J. Biol. Chem 289, 4660–4673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castelli V. et al. (2019) Neuronal cells rearrangement during aging and neurodegenerative disease: metabolism, oxidative stress and organelles dynamic. Front. Mol. Neurosci 12, 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou X. et al. (2017) The interaction between progranulin and prosaposin is mediated by granulins and the linker region between saposin B and C. J. Neurochem 143, 236–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeng J. et al. (2009) The inactivation of the sortilin gene leads to a partial disruption of prosaposin trafficking to the lysosomes. Exp. Cell Res 315, 3112–3124 [DOI] [PubMed] [Google Scholar]
- 28.Van Damme P. (2017) Another piece in the progranulin puzzle: special binding between progranulin and prosaposin creates additional lysosomal access: an editorial comment for ‘The interaction between progranulin and prosaposin is mediated by granulins and the linker region between saposin B and C’ on page 236. J. Neurochem 143, 154–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Butler VJ et al. (2019) Progranulin stimulates the in vitro maturation of pro-cathepsin D at acidic pH. J. Mol. Biol 431, 1038–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hiraiwa M. et al. (1997) Lysosomal proteolysis of prosaposin, the precursor of saposins (sphingolipid activator proteins): its mechanism and inhibition by ganglioside. Arch. Biochem. Biophys 341, 17–24 [DOI] [PubMed] [Google Scholar]
- 31.Erickson AH and Blobel G. (1983) Carboxyl-terminal proteolytic processing during biosynthesis of the lysosomal enzymes beta-glucuronidase and cathepsin D. Biochemistry 22, 5201–5205 [DOI] [PubMed] [Google Scholar]
- 32.Conner GE (1992) The role of the cathepsin D propeptide in sorting to the lysosome. J. Biol. Chem 267, 21738–21745 [Google Scholar]
- 33.Benes P. et al. (2008) Cathepsin D – many functions of one aspartic protease. Crit. Rev. Oncol. Hematol 68, 12–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sevlever D. et al. (2008) Cathepsin D is the main lysosomal enzyme involved in the degradation of alpha-synuclein and generation of its carboxy-terminally truncated species. Biochemistry 47, 9678–9687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vidoni C. et al. (2016) The role of cathepsin D in the pathogenesis of human neurodegenerative disorders. Med. Res. Rev 36, 845–870 [DOI] [PubMed] [Google Scholar]
- 36.Berent SL and Radin NS (1981) Mechanism of activation of glucocerebrosidase by co-beta-glucosidase (glucosidase activator protein). Biochim. Biophys. Acta 664, 572–582 [DOI] [PubMed] [Google Scholar]
- 37.Nalls MA et al. (2014) Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat. Genet 46, 989–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang D. et al. (2017) A meta-analysis of genome-wide association studies identifies 17 new Parkinson’s disease risk loci. Nat. Genet 49, 1511–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blauwendraat C. et al. (2017) NeuroChip, an updated version of the NeuroX genotyping platform to rapidly screen for variants associated with neurological diseases. Neurobiol. Aging 57, 247 [Google Scholar]
- 40.Jansen IE et al. (2017) Discovery and functional prioritization of Parkinson’s disease candidate genes from large-scale whole exome sequencing. Genome Biol. 18, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Billingsley KJ et al. (2018) Genetic risk factors in Parkinson’s disease. Cell Tissue Res. 373, 9–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blauwendraat C. et al. (2019) Parkinson’s disease age at onset genome-wide association study: defining heritability, genetic loci, and α-synuclein mechanisms. Mov. Disord 34, 866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gan-Or Z. et al. (2018) GBA-associated Parkinson’s disease and other synucleinopathies. Curr. Neurol. Neurosci. Rep 18, 44. [DOI] [PubMed] [Google Scholar]
- 44.Senkevich K. and Gan-Or Z. (2020) Autophagy lysosomal pathway dysfunction in Parkinson’s disease; evidence from human genetics. Parkinsonism Relat. Disord 73, 60–71 [DOI] [PubMed] [Google Scholar]
- 45.Oji Y. et al. (2020) Variants in saposin D domain of prosaposin gene linked to Parkinson’s disease. Brain 143, 1190–1205 [DOI] [PubMed] [Google Scholar]
- 46.Ibanez L. et al. (2018) Pleiotropic effects of variants in dementia genes in Parkinson disease. Front. Neurosci 12, 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bae EJ et al. (2015) Haploinsufficiency of cathepsin D leads to lysosomal dysfunction and promotes cell-to-cell transmission of α-synuclein aggregates. Cell Death Dis. 6, e1901 [Google Scholar]
- 48.Moors TE et al. (2019) Characterization of brain lysosomal activities in GBA-related and sporadic Parkinson’s disease and dementia with Lewy bodies. Mol. Neurobiol 56, 1344–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Do CB et al. (2011) Web-based genome-wide association study identifies two novel loci and a substantial genetic component for Parkinson’s disease. PLoS Genet. 7, e1002141 [Google Scholar]
- 50.Michelakakis H. et al. (2012) Evidence of an association between the scavenger receptor class B member 2 gene and Parkinson’s disease. Mov. Disord 27, 400–405 [DOI] [PubMed] [Google Scholar]
- 51.Maniwang E. et al. (2013) Is Parkinson disease associated with lysosomal integral membrane protein type-2?: challenges in interpreting association data. Mol. Genet. Metab 108, 269–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alcalay RN et al. (2016) SCARB2 variants and glucocerebrosidase activity in Parkinson’s disease. NPJ Parkinsons Dis. 2, 16004 [Google Scholar]
- 53.Matrone C. et al. (2016) Mannose 6-phosphate receptor is reduced in α-synuclein overexpressing models of Parkinsons disease. PLoS One 11, e0160501 [Google Scholar]
- 54.Lopez-Otin C. and Bond JS (2008) Proteases: multifunctional enzymes in life and disease. J. Biol. Chem 283, 30433–30437 [Google Scholar]
- 55.Yang SY et al. (2020) Glucocerebrosidase activity, cathepsin D and monomeric alpha-synuclein interactions in a stem cell derived neuronal model of a PD associated GBA1 mutation. Neurobiol. Dis 134, 104620 [Google Scholar]
- 56.Almeida S. et al. (2011) Progranulin, a glycoprotein deficient in frontotemporal dementia, is a novel substrate of several protein disulfide isomerase family proteins. PLoS One 6, e26454 [Google Scholar]
- 57.Valdez C. et al. (2017) Progranulin-mediated deficiency of cathepsin D results in FTD and NCL-like phenotypes in neurons derived from FTD patients. Hum. Mol. Genet 26, 4861–4872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reczek D. et al. (2007) LIMP-2 is a receptor for lysosomal mannose6-phosphate-independent targeting of beta-glucocerebrosidase. Cell 131, 770–783 [DOI] [PubMed] [Google Scholar]
- 59.Jian J. et al. (2016) Progranulin recruits HSP70 to beta-glucocerebrosidase and is therapeutic against Gaucher disease. EBioMedicine 13, 212–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jian J. et al. (2016) Association between progranulin and Gaucher disease. EBioMedicine 11, 127–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou X. et al. (2019) Progranulin deficiency leads to reduced glucocerebrosidase activity. PLoS One 14, e0212382 [Google Scholar]
- 62.Arrant AE et al. (2019) Impaired beta-glucocerebrosidase activity and processing in frontotemporal dementia due to progranulin mutations. Acta Neuropathol. Commun 7, 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Afinogenova Y. et al. (2019) Aberrant progranulin, YKL-40, cathepsin D and cathepsin S in Gaucher disease. Mol. Genet. Metab 128, 62–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lieberman RL (2011) A guided tour of the structural biology of Gaucher disease: acid-beta-glucosidase and saposin C. Enzyme Res. 2011, 973231 [Google Scholar]
- 65.Tatti M. et al. (2015) BCM-95 and (2-hydroxypropyl)-beta-cyclodextrin reverse autophagy dysfunction and deplete stored lipids in Sap C-deficient fibroblasts. Hum. Mol. Genet 24, 4198–4211 [DOI] [PubMed] [Google Scholar]
- 66.Pang SY et al. (2019) The interplay of aging, genetics and environmental factors in the pathogenesis of Parkinson’s disease. Transl. Neurodegener 8, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hallett PJ et al. (2018) Glycosphingolipid levels and glucocerebrosidase activity are altered in normal aging of the mouse brain. Neurobiol. Aging 67, 189–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lawrence RE and Zoncu R. (2019) The lysosome as a cellular centre for signalling, metabolism and quality control. Nat. Cell Biol 21, 133–142 [DOI] [PubMed] [Google Scholar]
- 69.Beel S. et al. (2017) Progranulin functions as a cathepsin D chaperone to stimulate axonal outgrowth in vivo. Hum. Mol. Genet 26, 2850–2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Valdez C. et al. (2019) Progranulin mutations result in impaired processing of prosaposin and reduced glucocerebrosidase activity. Hum. Mol. Genet 29, 716–726 [Google Scholar]
- 71.Cenik B. et al. (2012) Progranulin: a proteolytically processed protein at the crossroads of inflammation and neurodegeneration. J. Biol. Chem 287, 32298–32306 [Google Scholar]
- 72.Tolkatchev D. et al. (2008) Structure dissection of human progranulin identifies well-folded granulin/epithelin modules with unique functional activities. Protein Sci. 17, 711–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou X. et al. (2017) Impaired prosaposin lysosomal trafficking in frontotemporal lobar degeneration due to progranulin mutations. Nat. Commun 8, 15277 [Google Scholar]
- 74.Holler CJ et al. (2017) Intracellular proteolysis of progranulin generates stable, lysosomal granulins that are haploinsufficient in patients with frontotemporal dementia caused by GRN mutations. eNeuro 4, ENEURO.0100–17.2017 [Google Scholar]
- 75.Minarowska A. et al. (2008) Human cathepsin D. Folia Histochem. Cytobiol 46, 23–38 [DOI] [PubMed] [Google Scholar]
- 76.Qiao L. et al. (2008) Lysosomal enzyme cathepsin D protects against alpha-synuclein aggregation and toxicity. Mol. Brain 1, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rafi MA et al. (1993) Mutational analysis in a patient with a variant form of Gaucher disease caused by SAP-2 deficiency. Somat. Cell Mol. Genet 19, 1–7 [DOI] [PubMed] [Google Scholar]
- 78.Pampols T. et al. (1999) Neuronopathic juvenile glucosylceramidosis due to sap-C deficiency: clinical course, neuropathology and brain lipid composition in this Gaucher disease variant. Acta Neuropathol. 97, 91–97 [DOI] [PubMed] [Google Scholar]
- 79.Tylki-Szymanska A. et al. (2011) Gaucher disease due to saposin C deficiency, previously described as non-neuronopathic form–no positive effects after 2-years of miglustat therapy. Mol. Genet. Metab 104, 627–630 [DOI] [PubMed] [Google Scholar]
- 80.Chen Y. et al. (2020) Glucocerebrosidase as a therapeutic target for Parkinson’s disease. Expert Opin. Ther. Targets 24, 287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sidransky E. et al. (2020) Substrate reduction therapy for GBA1-associated Parkinsonism: are we betting on the wrong mouse? Mov. Disord 35, 228–230 [DOI] [PubMed] [Google Scholar]
- 82.Chitramuthu BP et al. (2017) Progranulin: a new avenue towards the understanding and treatment of neurodegenerative disease. Brain 140, 3081–3104 [DOI] [PubMed] [Google Scholar]
- 83.Cui Y. et al. (2019) Progranulin: a conductor of receptors orchestra, a chaperone of lysosomal enzymes and a therapeutic target for multiple diseases. Cytokine Growth Factor Rev. 45, 53–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.