Abstract
An in vitro kinetic model was used to study the relation between pharmacokinetic and pharmacodynamic (PK-PD) parameters for antimicrobial effect, e.g., the time above MIC (T>MIC), maximum concentration in serum (Cmax), and area under the concentration-time curve (AUC). Streptococcus pyogenes and Escherichia coli were exposed to cefotaxime, and the activity of amoxicillin against four strains of Streptococcus pneumoniae with different susceptibilities to penicillin was studied. The drug elimination rate varied so that the T>MIC ranged from 20 to 100% during 24 h, while the AUC and/or the initial concentration (Cmax) were kept constant. For S. pyogenes and E. coli, the maximal antimicrobial effect (Emax) at 24 h occurred when the antimicrobial concentration exceeded the MIC for 50 and 80% of the strains tested, respectively. The penicillin-susceptible pneumococci (MIC, 0.03 mg/liter) and the penicillin-intermediate strain (MIC, 0.25 mg/liter) showed maximal killing by amoxicillin at a T>MIC of 50%. For a strain for which the MIC was 2 mg/liter, Cmax needed to be increased to achieve the Emax. Under the condition that Cmax was 10 times the MIC, Emax was obtained at a T>MIC of 60%, indicating that Cmax, in addition to T>MIC, may be an important parameter for antimicrobial effect on moderately penicillin-resistant pneumococci. For the strain for which the MIC was 4 mg/liter, the reduction of bacteria varied from −0.4 to −3.6 log10 CFU/ml at a T>MIC of 100%, despite an initial antimicrobial concentration of 10 times the MIC. Our studies have shown that the in vitro kinetic model is a useful complement to animal models for studying the PK-PD relationship for antimicrobial effect of antibiotics.
The optimal dosing regimen for antibiotics is still not fully understood. Obtaining clinical and microbiological efficacy is essential, but dosing regimens must also be tailored to minimize the risk for emergence of antibiotic-resistant strains. The prevailing method for determination of bacterial susceptibility, the MIC, is only a rough measure of antimicrobial activity. It gives no information about the time course or whether the bactericidal effect is dependent on drug concentration. Many additional factors need to be studied in order to increase knowledge about optimal dosing regimens, e.g., the relation between pharmacokinetic and pharmacodynamic parameters (PK-PD), interactions with the immune system of the host, and pharmacological determinants for selection of resistance at the site of infection and in the normal flora.
A number of reasons, e.g., economical and ethical, limit the possibilities for dose finding in clinical studies in which the PK-PD relationship can be defined for optimal efficacy. Another problem is that the pharmacokinetic parameters are interdependent; i.e., an increased dose leads to a higher maximum concentration (Cmax) and a larger area under the concentration-time curve (AUC), as a well as a longer time above the MIC (T>MIC) (7, 8, 13). Studies of animal and in vitro models give the possibility to minimize the interdependence between parameters. The aim of this study was to evaluate the usefulness of an in vitro kinetic model for defining the pharmacokinetic parameters that correlate to efficacy for β-lactam antibiotics. For this purpose, we studied the effect of cefotaxime against Streptococcus pyogenes and Escherichia coli. The AUC was kept constant, whereas the T>MIC and Cmax varied. To investigate if the parameters of efficacy were similar in strains with and without acquired resistance mechanisms, we studied the activity of amoxicillin against four strains of Streptococcus pneumoniae with different susceptibilities to penicillin.
MATERIALS AND METHODS
Bacteria and media.
The included strains were S. pyogenes M12 NCTC P1800; E. coli ATCC 25922; a penicillin-sensitive pneumococcus (PSP), S. pneumoniae ATCC 6306; two clinical isolates of penicillin-resistant pneumococcus (PRP), 508-1046 and 40932; and a penicillin-intermediate pneumococcus (PIP), 9506.07-126. Strain 508-1046 derives from the University Hospital, Uppsala, Sweden, and strain 40932 was obtained from the Centre Hospitalier Intercommunal, Créteil, France. The PIP 9506.07-126 was a clinical isolate from Reykjavik, Iceland. In addition to penicillin resistance, the PIP and the PRP 40932 were also resistant to trimethoprim-sulfamethoxazole. The PRP 508-1046 carried multidrug resistance to chloramphenicol, trimethoprim-sulfamethoxazole, and tetracycline but was sensitive to the macrolide-lincosamide-streptogramin B group. The gram-positive strains were cultured in Todd-Hewitt broth saturated with CO2. E. coli was cultured in Mueller-Hinton broth, supplemented with Ca2+ (50 mg/l) and Mg2+ (50 mg/l).
Antibiotics.
Cefotaxime (Claforan; Aventis) was dissolved in sterile distilled water to a concentration of 10 mg/ml. Amoxicillin trihydrate (Astra, Södertälje, Sweden) was dissolved in an equal volume of 0.1 M NaOH and phosphate-buffered saline, pH 7.2, to 10 mg/ml. The stock solutions were prepared before each experiment and diluted in broth to the desired concentration.
MIC determination.
MICs were determined by the macrodilution technique, at an inoculum of 1 × 105 to 2 × 105 CFU/ml, according to NCCLS standards (17). The MIC was defined as the lowest concentration inhibiting visible growth after 20 h. The MIC determinations were made in triplicate on separate occasions.
Determination of antibiotic concentrations.
The microbiological agar diffusion method was used with 1.5% nutrient agar (Difco Laboratories, Detroit Mich.). Plates were seeded with a standardized inoculum of Providencia rettgeri P66 (Swedish Institute for Infectious Disease Control, Solna, Sweden) for the determination of cefotaxime concentration (18) and a spore suspension of Bacillus stearothermophilus ATCC 3032 for the determination of amoxicillin concentration (5). Antibiotic standards and the samples were applied to agar wells. All assays were made in triplicate, and the plates were incubated overnight at 35 and 56°C, respectively. The limit of detection was 0.062 mg/liter for cefotaxime and 0.031 mg/liter for amoxicillin. The correlation coefficient for the standard curves was always >0.99.
The antibiotics were investigated for their stability under the experimental conditions. Dilutions of cefotaxime (10 mg/liter) and amoxicillin (1 mg/liter) in broth were incubated at 35°C for 24 h. Samples were collected after 0, 6, 20, and 24 h, and the concentrations were determined as described above. Degradation of cefotaxime was not taken into account (18% ± 6% at 24 h), whereas amoxicillin was degraded by 50% ± 1%. This implies a half-life (t1/2) of 24 h, giving a k of 0.0289 (see formula below). In the results with PSP, the T>MIC was recalculated to include the degradation in the graph. In the experiments with PIP and PRP strains, the degradation was included in the flow rate. The initial concentrations (C0) were determined in all experiments. Amoxicillin concentrations at 24 h were also determined to confirm the expected values.
In vitro kinetic model.
The in vitro kinetic model has previously been described (15). It consists of a spinner flask (110 ml) with an open bottom that was specially constructed to fit into a new holder that has an outlet connected to a pump (P-500; Pharmacia Biotech, Uppsala, Sweden). A filter membrane with a pore size of 0.45 μm, lying on a perforated metal support, was placed between the flask and the holder, impeding the elimination of bacteria. A magnetic stirrer ensured homogenous mixing and prevented membrane pore blockage. In one of the side arms, a silicone membrane enabled repeated sampling. A thin plastic tubing from a vessel containing fresh medium was connected to the other arm. The medium was drawn from the flask at a constant rate by the pump, while fresh sterile medium was sucked into the flask at the same rate by the negative pressure built up inside. The antibiotic was diluted according to first-order kinetics: C = C0 × e−kt, where C is the achieved concentration after a constant elimination rate (k) of the C0 during the course of time (t). The AUC at 24 h (AUC24) was calculated as follows: AUC = C0/k − C24/k, where C24 is the concentration after 24 h, depending upon the elimination constant k, determined from k = ln2/t1/2.
Experiments.
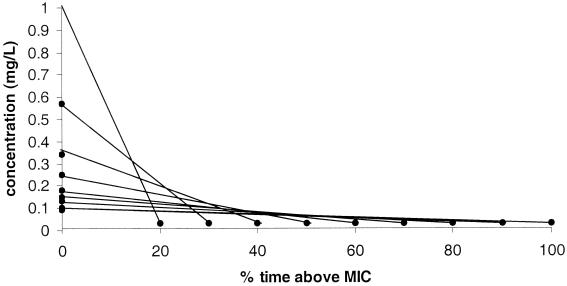
The intention during the experiments was to keep the AUC constant over 24 h, while the T>MIC and the Cmax varied (Fig. 1). Different T>MIC, ranging from 20 to 100% of 24 h, were investigated. The flask was prepared with appropriate broth and the desired initial antibiotic concentration and was installed in the thermostatic room (35°C). Bacteria from a 6- to 7-h broth culture were added, at an inoculum of 105 CFU/ml for S. pyogenes and S. pneumoniae and at 104 CFU/ml for E. coli. The flow rate of the pump was set to obtain the different t1/2s of the drugs (Table 1). C0, T>MIC, AUC, and the inoculum sizes for the different experiments are presented in Table 2.
FIG. 1.
Illustration of constant AUC with varied T>MIC and Cmax.
TABLE 1.
t1/2 and Cmax for the corresponding T>MIC
| T>MIC (%) |
S. pyogenes
|
E. coli
|
||
|---|---|---|---|---|
| t1/2 (h) | Cmax | t1/2 (h) | Cmax | |
| 20 | 0.95 | 32.5 | 1.03 | 26 |
| 30 | 1.72 | 19.1 | 1.90 | 13.8 |
| 40 | 2.80 | 11.2 | 3.02 | 9.0 |
| 50 | 4.04 | 8.3 | 4.65 | 6.0 |
| 60 | 5.75 | 5.8 | 6.74 | 4.4 |
| 70 | 7.42 | 5.1 | 9.88 | 3.2 |
| 80 | 9.36 | 4.8 | 12.6 | 2.9 |
| 90 | 13.05 | 3.2 | 15.1 | 2.7 |
| 100 | 14.55 | 3.1 | 18.2 | 2.5 |
TABLE 2.
Cmax, T>MIC, and AUC24h used in the experiments
| Strain | Antibiotic | MIC (mg/liter) | Cmaxa | T>MIC (%) | AUC24 (mg/liter · h) | Inoculumb (log CFU/ml) |
|---|---|---|---|---|---|---|
| S. pyogenes M12 | Cefotaxime | 0.031 | 32.5–3.1 | 20–100 | 1.4 | 5.8 ± 0.15 |
| E. coli ATCC 25922 | Cefotaxime | 0.125 | 26.0–2.4 | 20–100 | 4.8 | 4.6 ± 0.13 |
| 3.0 | 100 | 5.5 | 4.6 ± 0.13 | |||
| PSP ATCC 6303 | Amoxicillin | 0.031 | 14.4–2.5 | 28–57 | 1.1–0.9 | 5.3 ± 0.24 |
| PIP 9506.07-126 | Amoxicillin | 0.25 | 15.2–2 | 28–100 | 8.4–10 | 5.4 ± 0.26 |
| PRP 40932 | Amoxicillin | 2 | 3.3–3.0 | 90–100 | 88 | 5.5 ± 0.11 |
| 10 | 30–100 | 63–189 | 5.3 ± 0.14 | |||
| PRP 508–1046 | Amoxicillin | 4 | 12.0–2.4 | 30–80 | 141–111 | 5.2 ± 0.30 |
| 10 | 36–100 | 166–300 | 5.2 ± 0.30 | |||
| 20 | 100 | 600 | 5.2 ± 0.30 |
Initial concentration, expressed as number of times the MIC.
Mean ± standard deviation.
Samples for viable counts were withdrawn at 0, 3, 6, 12, and 24 h. Appropriate dilutions were plated (0.1 and 0.01 ml) on Columbia agar (Acumedia Manufacturers, Inc., Baltimore, Md.) with 5% horse blood and incubated overnight, and viable counts were determined. The sensitivity of the viable count was estimated at 50 CFU/ml.
S. pyogenes was exposed to a single dose of cefotaxime in a series of experiments. The T>MIC varied from 20 to 100%. The AUC was kept constant at 1.4 mg/liter · h. A similar series was performed with E. coli. The AUC was kept constant at 4.8 mg/liter · h, though it was increased to 5.5 mg/L · h for experiments with 100% T>MIC. The activity of amoxicillin was studied against four S. pneumoniae isolates with different susceptibilities to penicillin (Table 2). All experiments were performed in triplicate.
RESULTS
MICs.
The MICs are presented in Table 2.
Pharmacodynamics of cefotaxime.
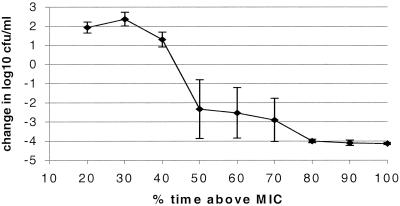
Figure 2 shows the killing of S. pyogenes as the difference between the initial inoculum and the number of bacteria at 24 h (change in log10 CFU per milliliter). There was a clear difference in killing when the T>MIC increased from 40 to 50%, although some regrowth occurred in single experiments at T>MIC of 50, 60, and 70%. The mean maximal antimicrobial effect (Emax) was achieved when the cefotaxime level exceeded the MIC for 50% of 24 h. Cmax continuously decreased at higher T>MIC (Fig. 1), indicating that Cmax was not the main parameter for efficacy.
FIG. 2.
S. pyogenes exposed to cefotaxime. Values are presented as the change in the number of CFU per milliliter at 24 h with a constant AUC of 1.4 mg/liter · h and different T>MIC. Error bars, standard deviation.
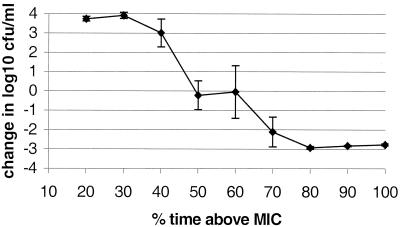
For E. coli exposed to cefotaxime, a T>MIC of 80% was needed to get a complete killing (Fig. 3). However, the lowest C0 (2.4 times the MIC) giving 100% T>MIC gave regrowth (data not shown). When the C0 and AUC were increased to three times the MIC and 5.5 mg/liter · h, respectively (Table 2), complete killing occurred.
FIG. 3.
E. coli exposed to cefotaxime. Values are presented as the change in the number of CFU per milliliter at 24 h with a constant AUC of 4.8 mg/liter · h ± standard deviation/error bars. At 100% T>MIC, Cmax was three times the MIC, giving an AUC of 5.5 mg/liter · h.
Pharmacodynamics of amoxicillin.
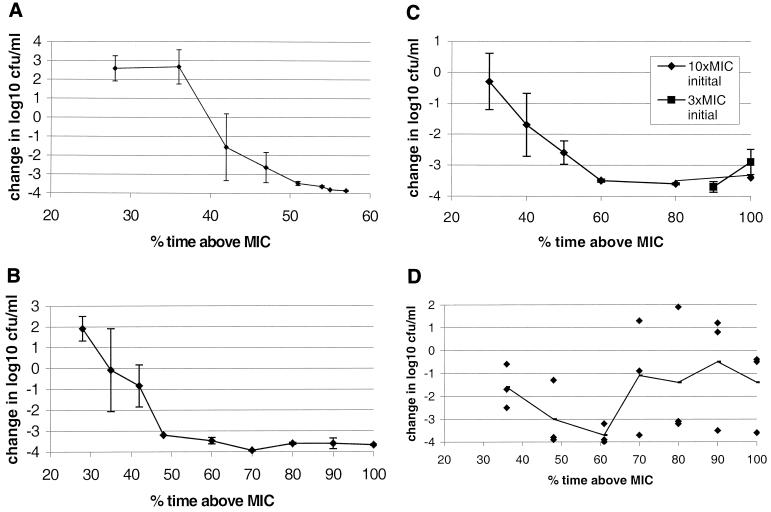
Emax was seen for the PSP and PIP strains with a T>MIC of approximately 50% (Fig. 4 A and B). When the PRP strain for which the MIC was 2 mg/liter was exposed to the same conditions as PSP and PIP, with a low initial dose for 100% T>MIC, regrowth occurred after 12 h in some experiments. The Cmax was therefore increased to a constant initial dose of 10 times the MIC. These conditions gave an Emax at 60% T>MIC (Fig. 4C).
FIG. 4.
The effect of amoxicillin at different T>MIC against S. pneumoniae. (A) PSP; (B) PIP; (C) PRP (MIC, 2 mg/liter), with an initial Cmax of 10 times the MIC and 3 times the MIC at 90 and 100%, respectively; (D) PRP (MIC, 4 mg/liter), with an initial Cmax of 10 times the MIC. Each dot represents an experiment, and the mean is shown as a line. The graphs illustrate change in number of bacterial CFU per milliliter at 24 h compared with the initial inoculum. Error bars, standard deviation.
The PRP for which the MIC was 4 mg/liter gave ambiguous results. In a series of experiments with the T>MIC ranging from 28 to 80% and the Cmax ranging from 2.4 to 12 times the MIC, the pattern was not clear. In all experiments an initial reduction of 2 to 3 log10 was noted during the first 6 h. Then, regrowth occurred irregularly, and the reduction of bacteria varied from 1 to 3.9 log10 at 100% T>MIC. The design of the experiments was therefore adjusted to see whether an increased Cmax would sustain the killing process. The initial concentration was set to 10 times the MIC, and the T>MIC was 36 to 100%. The results exhibited a similar pattern, shown in Fig. 4D. A predictable killing effect was not seen for this strain even with 100% T>MIC and an initial C0 of 20 times the MIC (not shown in Fig. 4). When bacteria, isolated at 24 h, were replicated onto plates containing amoxicillin at 4, 8, and 20 mg/liter there was no growth. Further studies on this strain showed an MIC/minimum bactericidal concentration ratio of 2, and lysis during 4 h did not fulfil the criteria for tolerance defined by Henriques Normark et al. (12).
DISCUSSION
The main objectives of this study were to evaluate the usefulness of an in vitro kinetic model in defining the PK-PD parameter for antimicrobial effect and to relate our results to those of other in vitro studies and animal models. Several animal models, in particular the thigh infection mice model, have been used for characterization of PK-PD relationships. Since the animals have a fixed elimination rate, different dosing regimens are used to achieve a variation in AUC and T>MIC. In the in vitro kinetic model, both the dose and the elimination rate can be varied, which makes it possible to minimize the interdependency of the different parameters for antimicrobial effect. Our results showed an Emax of 50% T>MIC of β-lactam antibiotics for S. pyogenes, PSP, and PIP. For E. coli a longer time of exposure, 80% T>MIC, was needed. Vogelman et al. showed, using the thigh infection mouse model, that the number of E. coli cells exposed to cefazolin was reduced when the concentration in serum exceeded the MIC for at least 60% of 24 h. To obtain Emax, 100% T>MIC was needed (21). A possible explanation of why E. coli requires a longer T>MIC for maximal killing is that β-lactam antibiotics give a pronounced effect at subinhibitory concentrations in the postantibiotic phase for gram-positive bacteria but not for gram-negative bacteria (6). However, the absolute figures on the T>MIC needed for maximal efficacy must be interpreted cautiously since the MIC may vary according to methodology, inoclum size, etc. For example, when tested with the E-test method (AB Biodisk, Solna, Sweden), the MIC for PRP strain 508-1046 was lower (MIC, 1.5 mg/liter) than that obtained by the macrodilution technique (4 mg/liter). An Emax achieved at 50% T>MIC with the MIC of 4 mg/liter would then correspond to 80% if a MIC of 1.5 were used instead.
In vivo studies of amoxicillin against pneumococci, in different animal models, have reported an Emax at 44 to 60% T>MIC for PSP and PIP (1, 2). Barry et al. demonstrated that amoxicillin was able to clear the infection of two resistant pneumococci (MICs 1 and 2 mg/liter) if the dose was increased (3). However, in a mouse pneumonia model, significant bactericidal effect was not achieved on PRP strains for which the MIC was ≥2 mg/liter, even with a dose/MIC ratio of 200 (2). The reason for these contradictory results is unclear but could be attributed to tolerance (2) or to the low growth rate of pneumococci in the pneumonia model (10). Experiments in vitro have also reported the need for high peak concentrations and repeated dosing for PRP (14, 19). Clinical studies of acute otitis media caused by pneumococci are difficult to evaluate and use for comparison, since self-limiting factors are involved (4). An extensive clinical study by Dagan et al. concluded that penicillin and amoxicillin-clavulanate susceptibilities of S. pneumoniae were not related to the bacteriological outcome in acute otitis media (9). In their study, the MIC of amoxicillin-clavulanate was >0.5 mg/liter for 24% of the strains, and among these only for a few strains was the MIC >2 mg/liter.
Penicillin resistance in S. pneumoniae is due to an altered configuration of the penicillin binding proteins in the bacterial cell wall that causes a lower affinity to the drug (11, 16, 20). Our results with the PRP for which the MIC was 2 mg/liter yielded the same Emax at 50 to 60% T>MIC under the condition that Cmax was increased to 10 times the MIC. The other PRP strain (MIC, 4 mg/liter) did not follow this pattern. The latter strain exhibited a killing of 2 to 3 log10 within the first 6 h, independent of Cmax ranging from 2 to 20 times the MIC. Regrowth occurred after 12 h in a majority of the experiments. Thus, an increased Cmax and larger AUC was not sufficient to achieve a predictable killing for this strain. Although the strain did not show tolerance, genetic alterations in the pathways that regulates autolysin activity or penicillin binding protein production may be reasons for this adaptation to the antibiotic environment. Additional highly resistant pneumococci strains need to be studied to seek an explanation for this phenomenon and determine its prevalence.
The in vitro kinetic model gave results comparable to those of different animal models for susceptible strains. It offers a cost-effective alternative as a screening model of the PK-PD parameters for the efficacies of new antibiotics. It has the advantages that the drug elimination rate and other pharmacokinetic parameters can be varied and also that bactericidal effect can be monitored continuously.
ACKNOWLEDGMENTS
We thank Marie Sandström at the Department of Pharmacy, Uppsala University, for her helpful assistance in the pharmacokinetics calculations and Birgitta Henriques-Normark at the Swedish Institute for Infection Disease Control, for valuable discussion.
REFERENCES
- 1.Andes D, Craig W A. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob Agents Chemother. 1998;42:2375–2379. doi: 10.1128/aac.42.9.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azoulay-Dupuis E, Moine P, Bedos J P, Rieux V, Vallee E. Amoxicillin dose-effect relationship with Streptococcus pneumoniae in a mouse pneumonia model and roles of in vitro penicillin susceptibilities, autolysis and tolerance properties of the strains. Antimicrob Agents Chemother. 1996;40:941–946. doi: 10.1128/aac.40.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barry B, Muffat-Joly M, Gehanno P, Pocidalo J-J. Effect of increased dosages of amoxicillin in treatment of experimental middle ear otitis due to penicillin-resistant Streptococcus pneumoniae. Antimicrob Agents Chemother. 1993;37:1599–1603. doi: 10.1128/aac.37.8.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barry B, Gehanno P, Blumen M, Boucot I. Clinical outcome of acute otitis media caused by pneumococci with decreased susceptibility to penicillin. Scand J Infect Dis. 1994;26:446–452. doi: 10.3109/00365549409008618. [DOI] [PubMed] [Google Scholar]
- 5.Cars O, Henning C, Holm S E. Penetration of ampicillin and dicloxacillin into tissue cage fluid in rabbits: relation to serum and tissue protein binding. Scand J Infect Dis. 1981;13:69–74. doi: 10.1080/00365548.1981.11690370. [DOI] [PubMed] [Google Scholar]
- 6.Cars O, Odenholt-Tornqvist I. The post-antibiotic sub-MIC effect in vitro and in vivo. Antimicrob Agents Chemother. 1993;31(Suppl. D):159–166. doi: 10.1093/jac/31.suppl_d.159. [DOI] [PubMed] [Google Scholar]
- 7.Cars O. Efficacy of beta-lactam antibiotics: integration of pharmacokinetics and pharmacodynamics. Diagn Microbiol Infec Dis. 1997;27:29–33. doi: 10.1016/s0732-8893(97)00020-5. [DOI] [PubMed] [Google Scholar]
- 8.Craig W A. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26:1–12. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 9.Dagan R, Johnson C E, McLinn S, Abughali N, Feris J, Leibovitz E, Burch D J, Jacobs M R. Bacteriologic and clinical efficacy of amoxicillin/clavulanate vs. azithromycin in acute otitis media. Pediatr Infect Dis J. 2000;19:95–104. doi: 10.1097/00006454-200002000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Erlendsdottir H, Dahl Knudsen J, Odenholt I, Cars O, Espersen F, Frimodt-Møller N, Fuursted K, Kristinsson K G, Gudmundsson S. Penicillin pharmacodynamics in four experimental pneumococcal infection models. Antimicrob Agents Chemother. 2001;45:1078–1085. doi: 10.1128/AAC.45.4.1078-1085.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hakenbeck R, Kaminski K, König A, Van der Linden M, Paik J, Reichmann P, Zähner D. Penicillin-binding proteins in β-lactam-resistant Streptococcus pneumoniae. Microb Drug Resist. 1999;5:91–99. doi: 10.1089/mdr.1999.5.91. [DOI] [PubMed] [Google Scholar]
- 12.Henriques Normark B, Novak R, Örtqvist A A, Källenius G, Tuomanen E, Normark S. Clinical isolates of Streptococcus pneumoniae exhibiting tolerance to vancomycin. Clin Infect Dis. 2001;32:552–558. doi: 10.1086/318697. [DOI] [PubMed] [Google Scholar]
- 13.Hyatt J M, McKinnon P S, Zimmer G S, Schentag J J. The importance of pharmacokinetic/pharmacodynamic surrogate makers to outcome. Focus on antibacterial agents. Clin Pharmacokinet. 1995;28:143–160. doi: 10.2165/00003088-199528020-00005. [DOI] [PubMed] [Google Scholar]
- 14.Lister P D, Pong A, Chartrand S A, Sanders C C. Rationale behind high-dose amoxicillin therapy for acute otitis media due to penicillin-nonsusceptible pneumococci: support from in vitro pharmcodynamic studies. Antimicrob Agents Chemother. 1997;41:1926–1932. doi: 10.1128/aac.41.9.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Löwdin E, Odenholt I, Bengtsson S, Cars O. Pharmacodynamic effect of sub-MICs of benzylpenicillin against Streptococcus pyogenes in a newly developed in vitro kinetic model. Antimicrob Agents Chemother. 1996;40:2478–2482. doi: 10.1128/aac.40.11.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moreillon P, Markiewicz Z, Nachman S, Tomasz A. Two bactericidal targets for penicillin in pneumococci: autolysis-dependent and autolysis-independent killing mechanisms. Antimicrob Agents Chemother. 1990;34:33–39. doi: 10.1128/aac.34.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Committee for Clinical Laboratory Standards. Methods for determining bactericidal activity of antimicrobial agents. Tentative guideline M26-T. Villnova, Pa: National Committee for Clinical Laboratory Standards; 1992. [Google Scholar]
- 18.Sjölin J, Eriksson N, Arneborn P, Cars O. Penetration of cefotaxime and desacetylcefotaxime into brain abcesses in humans. Antimicrob Agents Chemother. 1991;35:2606–2610. doi: 10.1128/aac.35.12.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thorburn C E, Knott S J, Edwards D I. In vitro activities of oral β-lactams at concentrations achieved in humans against penicillin-susceptible and -resistant pneumococci and potential to select resistance. Antimicrob Agents Chemother. 1998;42:1973–1979. doi: 10.1128/aac.42.8.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomasz A. Antibiotic resistance in Streptococcus pneumoniae. Clin Infect Dis. 1997;24(Suppl. 1):S85–S88. doi: 10.1093/clinids/24.supplement_1.s85. [DOI] [PubMed] [Google Scholar]
- 21.Vogelman B, Gudmundsson S, Leggett J, Turnidge J, Ebert S, Craig W A. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J Infect Dis. 1988;158:831–847. doi: 10.1093/infdis/158.4.831. [DOI] [PubMed] [Google Scholar]