Abstract
Aims
The incidence of respiratory failure and use of invasive or non-invasive mechanical ventilation (MV) in the cardiac intensive care units (CICUs) is increasing. While institutional MV volumes are associated with reduced mortality in medical and surgical ICUs, this volume–mortality relationship has not been characterized in the CICU.
Methods and results
National population-based data were used to identify patients admitted to CICUs (2005–2015) requiring MV in Canada. CICUs were categorized into low (≤100), intermediate (101–300), and high (>300) volume centres based on spline knots identified in the association between annual MV volume and mortality. Outcomes of interest included all-cause in-hospital mortality, the proportion of patients requiring prolonged MV (>96 h) and CICU length of stay (LOS). Among 47 173 CICU admissions requiring MV, 89.5% (42 200) required invasive MV. The median annual CICU MV volume was 43 (inter-hospital range 1–490). Compared to low-volume centres (35.9%), in-hospital mortality was lower in intermediate [29.2%, adjusted odds ratio (aOR) 0.84, 95% confidence interval (CI) 0.72–0.97, P = 0.019] and high-volume (18.2%; aOR 0.82, 95% CI 0.66–1.02, P = 0.076) centres. Prolonged MV was higher in low-volume (29.2%) compared to high-volume (14.8%, aOR 0.70, 95% CI 0.55–0.89, P = 0.003) and intermediate-volume (23.0%, aOR 0.85, 95% CI 0.68–1.06, P = 0.14] centres. Mortality and prolonged MV were lower in percutaneous coronary intervention (PCI)-capable and academic centres, but a shorter CICU LOS was observed only in subgroup of PCI-capable intermediate- and high-volume hospitals.
Conclusions
In a national dataset, we observed that higher CICU MV volumes were associated with lower incidence of in-hospital mortality, prolonged MV, and CICU LOS. Our data highlight the need for minimum MV volume benchmarks for CICUs caring for patients with respiratory failure.
Keywords: Coronary intensive care units, Mechanical ventilation
Graphical Abstract
Introduction
Since their inception in the 1960s, cardiac intensive care units (CICUs) have evolved to care for a growing proportion of patients with non-cardiovascular comorbidities and those requiring critical care therapies including mechanical ventilation (MV), vasoactive agents, and renal replacement therapy.1–6 Respiratory failure is increasingly common in patients presenting with primary cardiovascular conditions and it is the leading indication for CICU admission in over 25% of patients.7–9 Moreover, multiple studies have described temporal increases in invasive or non-invasive MV with utilization rates as high as 31% in tertiary CICUs and that MV is independently associated with in-hospital mortality.1,2,10
In medical and surgical intensive care unit (ICU) populations, respiratory failure requiring MV remains a risk factor for in-hospital mortality.11–13 Additionally, the annual institutional volume of mechanically ventilated patients admitted to ICUs has been inversely associated with in-hospital mortality. In one multicentre study, centres with over 400 patients receiving MV annually had a 37% reduction in ICU mortality of MV patients compared with centres with fewer than 150 patients, while other studies have reported higher institutional MV volumes were associated with worse outcomes.14–16 Although causal factors cannot be determined in these observational studies, the association may be attributable to greater provider-level experience and expertise, the implementation of guideline-directed ventilatory/weaning parameters and protocols, and the availability of multidisciplinary resources.5,14,17–19 Despite the growing provision of MV in CICUs, little is known about the relationship between annual institutional volume of MV on patient outcomes in this specialized setting. Accordingly, using a national population-based dataset, we sought to describe the association between annual institutional volumes of MV on in-hospital mortality and resource utilization in Canadian CICUs.
Methods
Data sources and study population
Study data were obtained from the Canadian Institute for Healthcare Information (CIHI), which is a national repository for healthcare data from nine Canadian provinces and three territories. The Discharge Abstract Data (DAD) describes the primary admitting diagnosis, up to 24 secondary diagnoses, patient demographics, dates of hospitalization, up to 20 diagnostic and therapeutic procedures, and the final disposition for all hospitalizations. Anonymous patient identifiers were used to track patients through multiple hospitalizations. The University of Alberta Research Ethics Board (Pro 00060335) approved the study.
The retrospective cohort of interest identified all patients ≥18 years of age admitted to Canadian CICUs who received MV between April 2005 and March 2016 (fiscal year 2005–2015) from a dataset of patients hospitalized with primary or secondary diagnoses of common cardiovascular conditions, cardiovascular risk factors, or cardiovascular procedures listed in the DAD [see Supplementary material online, Table S1 for list of International Classification of Diseases (ICD)-10 codes; see Supplementary material online, Figure S1 for flow diagram]. CICUs within this dataset were identified using hospital special care unit codes 40 (Cardiac Intensive Care Unit) and 45 (Coronary Intensive Care Unit) available within the CIHI dataset Patients admitted exclusively to general medical, surgical, or mixed ICUs were excluded. To avoid misclassification of cardiac surgical ICUs as CICUs, we used previously established methods to exclude all data from units with >15% of patients admitted with a primary diagnosis of coronary artery bypass grafting (CABG).1 Patients admitted to other critical care units during the same hospitalization were included. Canadian Classification of Health Interventions (CCI) codes available within the DAD were used to identify patients requiring invasive (1.GZ.31.CA) and non-invasive (1.GZ.31.CB) MV.
Primary discharge diagnoses were identified using ICD-10 codes. Medical comorbidities were identified using ICD-10 codes occurring in secondary diagnostic fields of the index CICU admission and all hospitalizations in the prior 1 year, while CCI codes were used to detect percutaneous coronary intervention (PCI), CABG, and critical care therapies performed during the hospitalization. The annual institutional volume of CICU admissions requiring MV was defined as the total number of CICU admissions requiring MV for each hospital/institute for each fiscal year. For the descriptive analysis, hospitals were categorized based on the annual institutional volume of CICU MV into three groups: low-volume (≤100), intermediate-volume (101–300), and high-volume (>300) centres based on qualitative analysis of inflexion points on the volume–mortality spline.
Outcomes
The primary outcome of interest was all-cause in-hospital mortality of patients who received MV. Secondary endpoints included prolonged MV (>96 h), and hospital and CICU length of stay (LOS).
Statistical analysis
Continuous variables were presented as mean and standard deviations or median and interquartile ranges and compared with analysis of variance or Kruskal–Wallis test across hospital groups, as appropriate. Categorical variables were presented as frequencies with percentages and compared across hospital groups with chi-square tests.
Multivariable mixed logistic regression models were used to assess the association of annual CICU volume on in-hospital mortality and MV duration >96 h including comorbidities and a random effect variable for hospital to account for the clustering. Hospital LOS and CICU LOS were analysed using generalized linear models with Poisson distribution and identity link. Annual institutional MV volume was used as a spline with two knots (100, 300) identified from the relationship between outcomes and annual MV volume. We used a stepwise variable selection approach with a prespecified P-value of 0.99 for variables to enter in the model and 0.20 to be included in the final model with MV volume categories and hospital characteristics being forced to stay in the model. Adjusted multivariable models included annual MV volume categories, hospital characteristics (PCI capability and academic vs. community status), admission category (emergency department vs. inter-hospital transfer), patient demographics/comorbidities (age, sex, diabetes, hypertension, heart failure, myocardial infarction, atrial fibrillation, cerebrovascular disease, peripheral vascular disease, renal disease, cancer, dementia), angiography/PCI requirements, and critical care therapies (MV modality, dialysis, resuscitation) interaction between MV volume categories and hospital characteristics. While assessing the outcome by PCI capability, hospital type (academic vs. community) was not included in the model and vice versa as these two variables were correlated. All statistical analyses were performed using SAS statistical software 9.4v.
Results
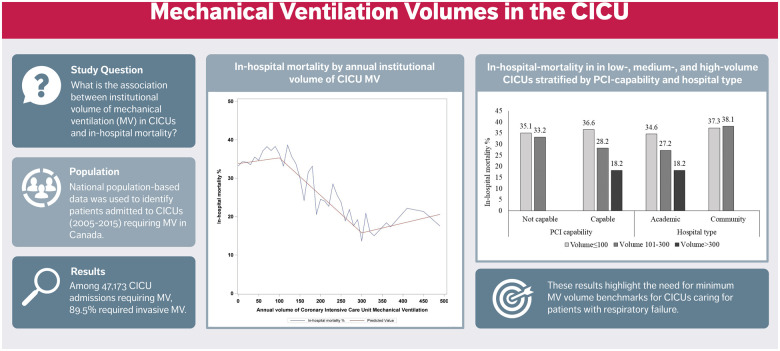
A total of 47 173 CICU admissions in 99 Canadian CICUs received MV between April 2005 and March 2016; among whom 42 200 (87.5%) patients exclusively received invasive MV and 4973 (10.5%) patients received non-invasive MV. A total of 1.9% received both invasive and non-invasive MV. Invasive MV was used in 88.4%, 89.3%, and 93.9% of cases in low-, intermediate-, and high-volume centres, respectively. The median annual number of patients receiving MV in CICUs was 43 (inter-hospital range 1–490). The non-linear relationship between annual CICU MV volume and in-hospital mortality is presented in Figure 1 with knots at 100 and 300 cases per year.
Figure 1.
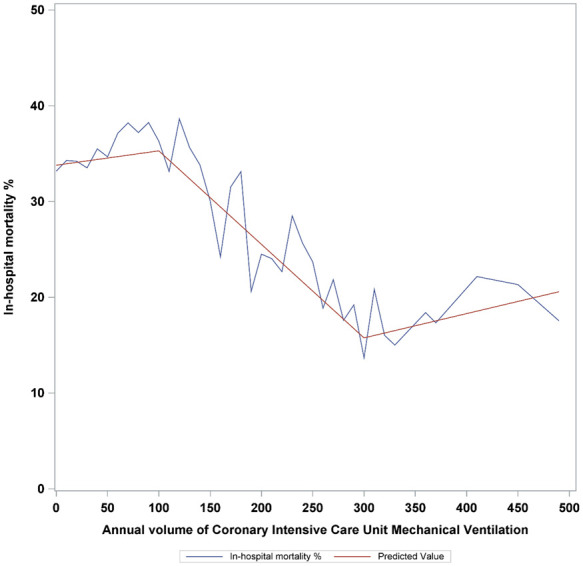
In-hospital mortality by annual institutional volume of cardiac intensive care unit mechanical ventilation.
Patient demographics and comorbidities stratified by CICU annual volume groups are summarized in Table 1. Larger MV volume centres were more frequently academic teaching centres with on-site PCI capabilities who admitted a higher proportion of inter-hospital transfers. Patients who received MV in higher volume CICUs were more frequently male, with a history of myocardial infarction but had a lower incidence of chronic kidney disease and chronic obstructive pulmonary disease. Higher volume CICUs more frequently had patients receiving MV with a primary discharge diagnosis of valvular heart disease or coronary artery disease (CAD), but fewer with acute coronary syndromes. Rates of invasive MV, cardiac catheterization, or PCI were higher in larger centres, while dialysis rates were lower.
Table 1.
Characteristics of Canadian cardiac intensive care unit patients requiring mechanical ventilation stratified by annual institutional mechanical ventilation volume
| CICU MV volume |
Total (n = 47 173) | P-value | |||
|---|---|---|---|---|---|
| ≤100 (n = 17 702) | 101–300 (n = 24 351) | >300 (5120) | |||
| Age at admission | |||||
| Median (IQR) | 70.0 (60.0–78.0) | 68.0 (59.0–77.0) | 68.0 (59.0–77.0) | 69.0 (59.0–78.0) | <0.0001 |
| Female sex (%) | 6884 (38.9) | 8431 (34.6) | 1605 (31.3) | 16 920 (35.9) | <0.0001 |
| MV type | |||||
| Invasive (%) | 15 405 (87.0) | 21 180 (87.0) | 4710 (92.0) | 41 295 (87.5) | <0.0001 |
| Non-invasive (%) | 2047 (11.6) | 2613 (10.7) | 313 (6.1) | 4973 (10.5) | |
| Both invasive and non-invasive (%) | 250 (1.4) | 558 (2.3) | 97 (1.9) | 905 (1.9) | |
| Admission categories | |||||
| Transferred (%) | 7555 (42.7) | 15 134 (62.1) | 4211 (82.2) | 26 900 (57.0) | <0.0001 |
| ED (%) | 10 147 (57.3) | 9217 (37.9) | 909 (17.8) | 20 273 (43.0) | |
| PCI capability | |||||
| Not capable (%) | 8471 (47.9) | 5274 (21.7) | 0 (0.0) | 13 745 (29.1) | <0.0001 |
| Capable (%) | 9231 (52.1) | 19 077 (78.3) | 5120 (100.0) | 33 428 (70.9) | |
| Hospital type | <0.001 | ||||
| Academic (%) | 8915 (50.4) | 19 820 (81.4) | 5120 (100.0) | 0 | |
| All community (%) | 8783 (49.6) | 4531 (18.6) | 0 | 0 | |
| Large community (%) | 7993 (91.0) | 4531 (100.0) | 0 | 0 | |
| Medium community (%) | 781 (8.9) | 0 | 0 | 0 | |
| Small community (%) | 9 (0.1) | 0 | 0 | 0 | |
| Comorbidities | |||||
| Diabetes (%) | 2981 (16.8) | 4021 (16.5) | 779 (15.2) | 7781 (16.5) | 0.0221 |
| Hypertension (%) | 2925 (16.5) | 4385 (18.0) | 867 (16.9) | 8177 (17.3) | 0.0003 |
| Dyslipidaemia (%) | 672 (3.8) | 1025 (4.2) | 216 (4.2) | 1913 (4.1) | 0.0867 |
| Myocardial infraction (%) | 1672 (9.4) | 2806 (11.5) | 760 (14.8) | 5238 (11.1) | <0.0001 |
| PCI (%) | 281 (1.6) | 342 (1.4) | 67 (1.3) | 690 (1.5) | 0.1895 |
| CABG (%) | 154 (0.9) | 147 (0.6) | 20 (0.4) | 321 (0.7) | 0.0001 |
| Heart failure (%) | 2336 (13.2) | 3081 (12.7) | 683 (13.3) | 6100 (12.9) | 0.17 |
| Atrial fibrillation (%) | 1321 (7.5) | 1772 (7.3) | 368 (7.2) | 3461 (7.3) | 0.7021 |
| Cerebrovascular disease (%) | 454 (2.6) | 530 (2.2) | 107 (2.1) | 1091 (2.3) | 0.0174 |
| Peripheral vascular disease (%) | 551 (3.1) | 667 (2.7) | 116 (2.3) | 1334 (2.8) | 0.0027 |
| Chronic obstructive pulmonary disease (%) | 1237 (7.0) | 1476 (6.1) | 210 (4.1) | 2923 (6.2) | <0.0001 |
| Renal disease (%) | 1033 (5.8) | 1381 (5.7) | 186 (3.6) | 2600 (5.5) | <0.0001 |
| Cancer (%) | 600 (3.4) | 636 (2.6) | 78 (1.5) | 1314 (2.8) | <0.0001 |
| Dementia (%) | 207 (1.2) | 182 (0.7) | 21 (0.4) | 410 (0.9) | <0.0001 |
| Charlson Comorbidity Index, mean (IQR) | 3 (1–4) | 2 (1–4) | 2 (1–4) | 3 (1–4) | <0.001 |
| Discharge diagnosis | |||||
| NSTEMI (%) | 1499 (8.5) | 1454 (6.0) | 203 (4.0) | 3156 (6.7) | <0.0001 |
| STEMI and complications (%) | 2780 (15.7) | 3849 (15.8) | 646 (12.6) | 7275 (15.4) | <0.0001 |
| Unstable angina (%) | 37 (0.2) | 78 (0.3) | 16 (0.3) | 131 (0.3) | 0.0891 |
| CAD and chest pain (%) | 680 (3.8) | 5009 (20.6) | 2012 (39.3) | 7701 (16.3) | <0.0001 |
| Valve disease (%) | 262 (1.5) | 1292 (5.3) | 717 (14.0) | 2271 (4.8) | <0.0001 |
| Shock (%) | 3898 (22.0) | 5606 (23.0) | 1056 (20.6) | 10 560 (22.4) | 0.0003 |
| HF and cardiomyopathies (%) | 2570 (14.5) | 2555 (10.5) | 377 (7.4) | 5502 (11.7) | <0.0001 |
| Cardiac arrest and ventricular arrhythmias (%) | 796 (4.5) | 961 (4.0) | 110 (2.2) | 1867 (4.0) | <0.001 |
| Supra ventricular arrhythmias (%) | 348 (2.0) | 305 (1.3) | 42 (0.8) | 695 (1.5) | <0.0001 |
| Syncope and conduction disease (%) | 390 (2.2) | 345 (1.4) | 49 (1.0) | 784 (1.7) | <0.001 |
| Aortic and arterial (%) | 301 (1.7) | 295 (1.2) | 95 (1.9) | 691 (1.5) | <0.0001 |
| Critical care therapies | |||||
| Resuscitation (%) | 2191 (12.4) | 2524 (10.4) | 343 (6.7) | 5058 (10.7) | <0.0001 |
| Dialysis including haemodialysis (%) | 2327 (13.1) | 3528 (14.5) | 435 (8.5) | 6290 (13.3) | <0.0001 |
| Catheterization (%) | 4991 (28.2) | 10 825 (44.5) | 2417 (47.2) | 18 233 (38.7) | <0.0001 |
| PCI (%) | 2737 (15.5) | 5116 (21.0) | 893 (17.4) | 8746 (18.5) | <0.001 |
| Catheterization or PCI (%) | 5223 (29.5) | 11 070 (45.5) | 2470 (48.2) | 18 763 (39.8) | <0.0001 |
| Intra-aortic balloon pump (%) | 1028 (5.8) | 2744 (11.3) | 521 (10.2) | 4293 (9.1) | <0.001 |
CABG, coronary artery bypass grafting; CAD, coronary artery disease; CICU, cardiac intensive care unit; ED, emergency department; ED, emergency department; HF, heart failure; IQR, interquartile range; MV, mechanical ventilation; NSTEMI, non-ST-elevation myocardial infarction; PCI, percutaneous coronary intervention; SD, standard deviation; STEMI, ST-elevation myocardial infarction.
In-hospital mortality
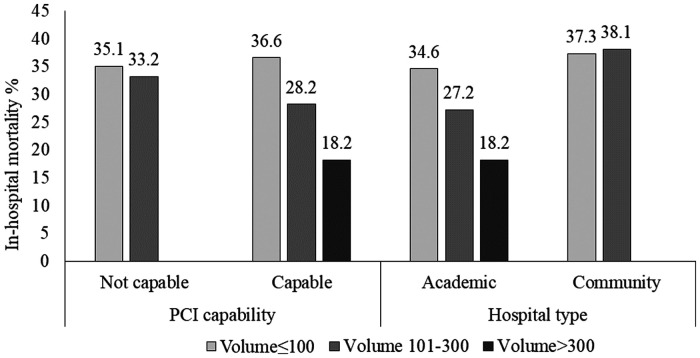
Unadjusted all-cause in-hospital mortality in low- (≤100 cases/year), intermediate- (101–300 cases/year), and high-volume centres (>300 cases/year) were 35.9%, 29.2%, and 18.2% (P < 0.0001; Table 2), respectively. In the overall cohort, after multivariable adjustment using the low-volume group as reference, the relationship was significant in the intermediate-volume centres [odds ratio (OR): 0.84, 95% confidence interval (CI) 0.72–0.97, P = 0.019; Table 3] and approached significance in the of high-volume centres (OR: 0.82, 95% CI 0.66–1.02, P = 0.076). In a subgroup analysis stratified by hospital type and on-site PCI capability, point estimates for in-hospital mortality were lower in intermediate- (OR: 0.81, 95% CI 0.67–0.97, P = 0.023) and high-volume (OR: 0.80, 95% CI 0.62–1.04, P = 0.10) academic centres, and in intermediate- (OR: 0.80, 95% CI 0.67–0.97, P = 0.019) and high-volume centres (OR: 0.79, 95% CI 0.62–1.01, P = 0.064) with on-site PCI (Figure 2). Sensitivity analysis of patients receiving exclusively non-invasive MV showed no significant volume mortality relationships in the overall cohort or in either PCI-capable or academic hospital subgroups.
Table 2.
Unadjusted outcomes by annual institutional volume of cardiac intensive care unit mechanical ventilation volumes
| CICU MV annual volume |
Total (n = 41 473) | P-value | |||
|---|---|---|---|---|---|
| ≤100 (n = 17 702) |
101–300 (n = 24 351) | >300 (n = 5120) | |||
| In-hospital mortality | 6357 (35.9) | 7122 (29.2) | 933 (18.2) | 14,412 (30.6) | <0.0001 |
| Hospital LOS (days) | |||||
| Mean (SD) | 21.4 (34.4) | 20.0 (29.9) | 16.7 (21.6) | 20.2 (31.0) | <0.0001 |
| Median (IQR) | 12.0 (6.0–24.0) | 12.0 (7.0–22.0) | 11.0 (7.0–18.0) | 12.0 (7.0–22.0) | <0.0001 |
| CICU LOS (h) | |||||
| Mean (SD) | 129.8 (171.4) | 121.6 (169.2) | 90.9 (100.2) | 121.3 (164.4) | <0.0001 |
| Median (IQR) | 85.0 (34.0–166.0) | 79.0 (33.0–151.0) | 66.0 (24.0–121.5) | 79.0 (32.0–153.0) | <0.0001 |
| MV duration (>96 h) (%) | 5161 (29.2) | 5608 (23.0) | 758 (14.8) | 11,527 (24.4) | <0.0001 |
CICU, cardiac intensive care unit; IQR, interquartile range; LOS, length-of-stay; MV, mechanical ventilation; SD, standard deviation.
Table 3.
Adjusted outcomes stratified by cardiac intensive care unit mechanical ventilation volumes in the overall cohort and hospital subtypes
| Outcomes | Group 1 | Group 2 | Group 3 |
|---|---|---|---|
| Annual volume ≤100 | Annual volume 101–300 | Annual volume >300 | |
| Mortality | Adjusted OR (95 % CI) | Adjusted OR (95% CI) | Adjusted OR (95% CI) |
| Overall cohort | Ref | 0.84 (0.72–0.97), P = 0.019 | 0.82 (0.66–1.02), P = 0.076 |
| Academic | Ref | 0.81 (0.67–0.97), P = 0.023 | 0.80 (0.62–1.04), P = 0.10 |
| Community | Ref | 0.99 (0.85–1.16), P = 0.93 | a |
| PCI-capable | Ref | 0.80 (0.67–0.97), P = 0.019 | 0.79 (0.62–1.01), P = 0.064 |
| Non PCI-capable | Ref | 0.92 (0.74–1.34), P = 0.42 | a |
| Prolonged MV (>96 h) | Adjusted OR (95% CI) | Adjusted OR (95% CI) | |
| Overall cohort | Ref | 0.85 (0.68–1.06), P = 0.14 | 0.70 (0.55–0.89), P = 0.003 |
| Academic | Ref | 0.80 (0.63–1.03), P = 0.084 | 0.66 (0.52–0.84), P = 0.001 |
| Community | Ref | 0.93 (0.64–1.34), P = 0.68 | a |
| PCI-capable | Ref | 0.69 (0.58–0.82), P < 0.001 | 0.58 (0.48–0.71), P < 0.001 |
| Non PCI-capable | Ref | 1.35 (0.90–2.04), P = 0.15 | a |
| CICU LOS, h | Adjusted risk difference (95% CI) | Adjusted risk difference (95% CI) | |
| Overall cohort | Ref | −9.69 (−28.27, 8.88), P = 0.31 | −10.42 (−30.54, 9.70), P = 0.31 |
| Academic | Ref | −18.37 (−39.23, 2.49), P = 0.084 | −18.73 (−41.50, 4.05), P = 0.11 |
| Community | Ref | 6.42 (−27.54, 40.37), P = 0.71 | a |
| PCI-capable | Ref | −25.44 (−38.21, −12.66), P < 0.001 | −25.18 (−42.84, −7.51), P = 0.005 |
| Non PCI-capable | Ref | 32.75 (−7.18, 72.67), P = 0.11 | a |
| Hospital LOS, h | Adjusted risk difference (95% CI) | Adjusted risk difference (95% CI) | |
| Overall cohort | Ref | −0.87 (−2.71, 0.97), P = 0.36 | −2.30 (−5.39, 0.78), P = 0.14 |
| Academic | Ref | −1.35 (−3.36, 0.66), P = 0.19 | −2.72 (−5.93, 0.48), P = 0.096 |
| Community | Ref | 0.04 (−3.26, 3.35), P = 0.98 | a |
| PCI-capable | Ref | −1.56 (−3.44, 0.32), P = 0.10 | −2.88 (−5.95, 0.19), P = 0.066 |
| Non PCI-capable | Ref | 1.20 (−1.97, 4.37), P = 0.46 | a |
Adjusted for age, sex, diabetes, hypertension, heart failure, myocardial infarction, atrial fibrillation, cerebrovascular disease, peripheral vascular disease, renal disease, cancer, dementia, admission category (emergency department vs. transferred), discharge diagnosis-valvular heart disease, heart failure and cardiomyopathies, arrhythmia, conduction disorder, pericardial disease, aortic and arterial disease, shock, and critical care therapy-resuscitation, dialysis, catheterization or PCI, and MV type (invasive and non-invasive).
CI, confidence interval; CICU, cardiac intensive care unit; LOS, length of stay; MV, mechanical ventilation; OR, odds ratio; PCI, percutaneous coronary intervention; ref, reference group.
There were no community or non-PCI capable hospitals in the high-volume group.
Figure 2.
In-hospital-mortality in in low-, medium-, and high-volume cardiac intensive care units stratified by percutaneous coronary intervention-capability and hospital type. PCI, percutaneous coronary intervention.
Secondary outcomes
The proportion of patients requiring prolonged (>96 h) MV in low-, intermediate-, and high-volume CICUs was 29.2%, 23.0%, and 14.8%, respectively. After multivariable adjustment, prolonged MV was lower in high-volume (OR: 0.70, 95% CI 0.55-0.89, P = 0.003) compared to low-volume centres. This relationship remained statistically significant in the academic high-volume, PCI-capable intermediate-volume and PCI-capable high-volume hospital subgroups.
CICU LOS was not significantly different in the overall cohort or in the subgroup of academic centres; however, after multivariable adjustment, CICU LOS in PCI-capable centres was significantly lower in intermediate- [relative difference (RD): −25.27 h, 95% CI −38.21 to −12.66 h, P < 0.001] and high-volume centres (RD: −25.18 h, 95% CI −42.84 to −7.51 h, P = 0.005). Unadjusted median hospital LOS in low-, intermediate-, and high-volume CICUs were 12, 12, and 11 days, respectively (P < 0.0001). In the overall cohort, as well as PCI-capable and academic subgroups, adjusted point estimates for LOS in higher-volume centres were not significantly different.
Discussion
In a national population-based analysis of Canadian CICU patients receiving MV, we observed associations between MV institutional volume and in-hospital mortality, CICU LOS, and duration of MV. Unadjusted in-hospital mortality was reduced at annual institutional volumes of >100, with no incremental improvement beyond 300 ventilated patients annually. In addition, the observed volume–mortality relationship was stronger in academic and PCI-capable CICUs.
Our finding that higher institutional MV volumes are associated with lower in-hospital mortality rates in the CICU is novel in this setting, yet consistent with findings published in the medical and surgical ICU literature. One large cohort study of 25 718 patients from 37 medical ICUs in the USA reported a significant volume-dependent mortality reduction; after multivariable adjustment for clinical risk factors, annual MV volumes of >276 patients per year were associated with lower mortality, compared to small-volume centres (<150 patients per year).14 Another study of 179 197 medical and surgical patients receiving MV in 294 French hospitals reported lower mortality in centres with the highest tertile of MV volumes (>282 patients/year) compared to the lowest tertile (<99 patients/year). Notably, not all studies have reported a significant volume–mortality relationship. A retrospective cohort study using administrative data from 126 hospitals in Ontario, Canada, found no significant volume-dependent change in the mortality of 13 846 medical and 6373 surgical ICU patients receiving MV.15 The difference may be due to the exclusion of patients receiving <48 h of MV which selects for patients at the extremes of illness severity, potentially attenuating the volume–mortality relationship.
In the present analysis, unadjusted in-hospital mortality rates declined between MV volumes of 100–300 patients per year. We hypothesize that this volume–mortality relationship is attributed to a combination of provider-level experience and expertise, better adherence of guideline-directed ventilatory/weaning parameters, or access to multidisciplinary resources including unit-based respiratory therapist or 24-h in-house staffing.1,14,17,19 PCI-capable CICUs demonstrated a pronounced volume–mortality relationship. Given that intermediate-volume CICUs have high proportions of patients admitted with shock (23%), CAD (20.6%), and ST-elevation myocardial infarction with complications (15.8%), the availability of on-site PCI may explain the amplification of the volume–mortality relationship within this subgroup. The attenuation of this signal in high-volume CICUs may be due to the smaller numbers of centres or the lack of adjustment for clinical risk factors (higher clinical severity and variable case mix) associated with high-volume CICUs.1,20 Future studies should be directed at confirming this association in an external dataset with more detailed haemodynamic and respiratory variables.
The association between MV volume and duration of MV has not been previously characterized in the CICU population. A retrospective cohort study of 25 718 patients from 37 medical ICUs in the USA by Kahn et al.14 reported unadjusted mean durations of MV of 3.1, 3.0, 3.6, and 4.5 days in centres with annual MV volumes of 87–150, 151–275, 276–400, and 401–617 patients, respectively. We observed a volume-dependent reduction in the proportion of prolonged MV in the overall cohort of high-volume centres and intermediate-volume centres in the PCI-capable subgroup. Given that this relationship is seen in high-volume academic and PCI-capable subgroups, we similarly hypothesize that this may reflect differences in experience, protocols and staffing, while on-site PCI may help facilitate timely revascularization and mitigate the additional time required for inter-hospital transfer. Though the CIHI dataset does not contain these variables, previously proposed Canadian CICU standard of care benchmarks for level 1 CICUs have targeted implementation of standardized multidisciplinary sedation, mobilization, and ventilatory weaning protocols that have reduced duration of MV, critical care LOS, and tracheostomy rates in the medical ICU population.21–25 Thus, lower rates of prolonged MV may be seen in larger volume centres may represent the clustering of resources designed to care for critically ill patients.21 These findings differ from those reported in medical and surgical ICU populations.14 This discrepancy between medical and surgical ICU literature and our findings in the CICU population may be explained by the differences in the indications for MV in the patient populations. One point-prevalence study of 412 medical and surgical ICUs found the leading indications for MV were acute respiratory failure caused by sepsis (16%), pneumonia (16%), post-operative respiratory failure (15%), chronic obstructive pulmonary disease (13%), acute respiratory distress syndrome (12%), and heart failure (12%).26 A prospective cohort study of 25 North American CICUs found that patients most likely to require invasive or non-invasive MV were those with admission diagnoses of cardiogenic shock (46%), acute heart failure (39%), valvular heart disease (27%), ventricular arrhythmias (35%), and general medical problems (42%). Differences in the volume-duration of MV relationship between ICU and CICU populations may in part be explained by the higher proportion of CICU patients requiring MV that can receive definitive therapy at high-volume centres, which are more likely to be PCI-capable.
Our population-based analysis of CICUs demonstrates lower in-hospital mortality and rates of prolonged MV at annual MV volumes >100 patients. Recognizing that in both cardiovascular and non-cardiovascular medical, surgical and emergency care studies, provider and hospital volumes have been consistency associated with improved patient outcomes , 27–31 these results suggest annual institutional volumes of >100 may be a minimum benchmark for MV in CICUs to promote quality care, supporting the developed of regional systems of cardiovascular critical care with a ‘spoke-and-hub’ model.21,32 Given the importance of properly understanding cardiopulmonary interactions in patients with cardiovascular disease undergoing MV, centralization of the care of high-risk patients with cardiac disease and respiratory failure into higher volumes CICUs with the staffing and multidisciplinary resources for comprehensive care may lead to improved patient outcomes, a reduction in complications associated with MV, and subsequent healthcare resource use; though we acknowledge the need for external validation of these findings.
Limitations
Limitations of this observational analysis include the lack of granular clinical information including haemodynamic, laboratory, MV parameter data, intensive care acuity scores, and the potential for residual confounding. In addition, practitioner differences including training, specialty, or individual MV case volumes were not captured in this population-based dataset. Finally, information on hospital staffing models and MV protocols were not available.
Conclusions
Our population-based analysis of Canadian CICUs demonstrates higher annual institutional MV volumes were significantly associated with lower in-hospital mortality and rates of prolonged MV. Moreover, point estimates were lower in the subgroup of PCI-capable and academic institutions. These data, pending future validation, suggest that annual institution MV volume of >100 MV per year may be a minimum quality benchmark for CICUs caring for patients with respiratory failure. Moreover, these data may help to inform the future design of regional system of cardiac critical care to ensure the care of patients requiring MV is centralized in high-volume centres.
Supplementary material
Supplementary material is available at European Heart Journal: Acute Cardiovascular Care online.
Supplementary Material
Acknowledgements
Parts of this material are based on data and information provided by the Canadian Institute for Health Information. However, the analyses, conclusions, opinions, and statements expressed herein are those of the authors and not those of the Canadian Institute for Health Information.
We would like to gratefully acknowledge Ms Lisa Soulard for copy-editing the manuscript.
Conflict of interest:
R.D.L. reports grants and personal fees from Bristol-Myers Squibb and Pfizer, personal fees from Boehringer Ingelheim and Bayer AG and grants from Amgen Inc, GlaxoSmithKline, Medtronic PLC, and Sanofi Aventis outside the submitted work. All other authors have declared no conflict of interest.
References
- 1.Woolridge S, Alemayehu W, Kaul P, Fordyce CB, Lawler PR, Lemay M, Jentzer JC, Goldfarb M, Wong GC, Armstrong PW, van Diepen S.. National trends in coronary intensive care unit admissions, resource utilization, and outcomes. Eur Heart J Acute Cardiovasc Care 2020;9:923–930. [DOI] [PubMed] [Google Scholar]
- 2.Sinha SS, Sjoding MW, Sukul D, Prescott HC, Iwashyna TJ, Gurm HS, Cooke CR, Nallamothu BK.. Changes in primary noncardiac diagnoses over time among elderly cardiac intensive care unit patients in the United States. Circ Cardiovasc Qual Outcomes 2017;10:e003616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holland EM, Moss TJ.. Acute noncardiovascular illness in the cardiac intensive care unit. J Am Coll Cardiol 2017;69:1999–2007. [DOI] [PubMed] [Google Scholar]
- 4.Katz JN, Turer AT, Becker RC.. Cardiology and the critical care crisis: a perspective. J Am Coll Cardiol 2007;49:1279–1282. [DOI] [PubMed] [Google Scholar]
- 5.Fordyce CB, Katz JN, Alviar CL, Arslanian-Engoren C, Bohula EA, Geller BJ, Hollenberg SM, Jentzer JC, Sims DB, Washam JB, van Diepen S; American Heart Association Council on Clinical Cardiology; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; and Stroke Council. Prevention of complications in the cardiac intensive care unit: a scientific statement from the American Heart Association. Circulation 2020;142:E379–E406. doi:10.1161/CIR.0000000000000909 [DOI] [PubMed] [Google Scholar]
- 6.Ferrer M, García-García C, El Ouaddi N, Rueda F, Serra J, Oliveras T, Labata C, Dégano IR, Montero S, De Diego O, Elosúa R, Lupón J, Bayes-Genis A; RUTI-ICCU Study. Transitioning from a coronary to a critical cardiovascular care unit: trends over the past three decades. Eur Heart J Acute Cardiovasc Care 2020;doi:10.1177/2048872620936038. [CVOCROSSCVO] [DOI] [PubMed] [Google Scholar]
- 7.Bohula EA, Katz JN, van Diepen S, Alviar CL, Baird-Zars VM, Park J-G, Barnett CF, Bhattal G, Barsness GW, Burke JA, Cremer PC, Cruz J, Daniels LB, DeFilippis A, Granger CB, Hollenberg S, Horowitz JM, Keller N, Kontos MC, Lawler PR, Menon V, Metkus TS, Ng J, Orgel R, Overgaard CB, Phreaner N, Roswell RO, Schulman SP, Snell RJ, Solomon MA, Ternus B, Tymchak W, Vikram F, Morrow DA; Critical Care Cardiology Trials Network. Demographics, care patterns, and outcomes of patients admitted to cardiac intensive care units: the Critical Care Cardiology Trials Network Prospective North American Multicenter Registry of Cardiac Critical Illness. JAMA Cardiol 2019;4:928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jentzer JC, van Diepen S, Murphree DH, Ismail AS, Keegan MT, Morrow DA, Barsness GW, Anavekar NS.. Admission diagnosis and mortality risk prediction in a contemporary cardiac intensive care unit population. Am Heart J 2020;224:57–64. [DOI] [PubMed] [Google Scholar]
- 9.Metkus TS, Miller PE, Alviar CL, Baird-Zars VM, Bohula EA, Cremer PC, Gerber DA, Jentzer JC, Keeley EC, Kontos MC, Menon V, Park J-G, Roswell RO, Schulman SP, Solomon MA, van Diepen S, Katz JN, Morrow DA.. Advanced respiratory support in the contemporary cardiac ICU. Crit Care Explor 2020;2:e0182.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jentzer JC, van Diepen S, Barsness GW, Katz JN, Wiley BM, Bennett CE, Mankad SV, Sinak LJ, Best PJ, Herrmann J, Jaffe AS, Murphy JG, Morrow DA, Wright RS, Bell MR, Anavekar NS.. Changes in comorbidities, diagnoses, therapies and outcomes in a contemporary cardiac intensive care unit population. Am Heart J 2019;215:12–19. [DOI] [PubMed] [Google Scholar]
- 11.Jones AE, Trzeciak S, Kline JA.. The Sequential Organ Failure Assessment score for predicting outcome in patients with severe sepsis and evidence of hypoperfusion at the time of emergency department presentation. Crit Care Med 2009;37:1649–1654. [CVOCROSSCVO] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Den Noortgate N, Vogelaers D, Afschrift M, Colardyn F.. Intensive care for very elderly patients: outcome and risk factors for in-hospital mortality. Age Ageing 1999;28:253–256. [DOI] [PubMed] [Google Scholar]
- 13.Ucgun I, Metintas M, Moral H, Alatas F, Yildirim H, Erginel S.. Predictors of hospital outcome and intubation in COPD patients admitted to the respiratory ICU for acute hypercapnic respiratory failure. Respir Med 2006;100:66–74. [DOI] [PubMed] [Google Scholar]
- 14.Kahn JM, Goss CH, Heagerty PJ, Kramer AA, O'Brien CR, Rubenfeld GD.. Hospital volume and the outcomes of mechanical ventilation. N Engl J Med 2006;355:41–50. [DOI] [PubMed] [Google Scholar]
- 15.Needham DM, Bronskill SE, Rothwell DM.. Hospital volume and mortality for mechanical ventilation of medical and surgical patients: a population-based analysis using administrative data. Crit Care Med 2006;34:2349–2354. [CVOCROSSCVO] [DOI] [PubMed] [Google Scholar]
- 16.Mehta AB, Walkey AJ, Curran-Everett D, Matlock D, Douglas IS.. Hospital mechanical ventilation volume and patient outcomes: too much of a good thing? Crit Care Med 2019;47:360–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen Y-L, Wallace DJ, Yordanov Y, Trinquart L, Blomkvist J, Angus DC, Kahn JM, Ravaud P, Guidet B.. The volume-outcome relationship in critical care: a systematic review and meta-analysis. Chest 2015;148:79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kahn JM, Ten Have TR, Iwashyna TJ.. The relationship between hospital volume and mortality in mechanical ventilation: an instrumental variable analysis. Health Serv Res 2009;44:862–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pronovost PJ, Angus DC, Dorman T, Robinson KA, Dremsizov TT, Young TL.. Physician staffing patterns and clinical outcomes in critically III patients: a systematic review. J Am Med Assoc 2002;288:2151–2162. [DOI] [PubMed] [Google Scholar]
- 20.Tsai AC, Votruba M, Bridges JFP, Cebul RD.. Overcoming bias in estimating the volume-outcome relationship. Health Serv Res 2006;41:252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le May M, van Diepen S, Liszkowski M, Schnell G, Tanguay J-F, Granger CB, Ainsworth C, Diodati JG, Fam N, Haichin R, Jassal D, Overgaard C, Tymchak W, Tyrrell B, Osborne C, Wong G.. From coronary care units to cardiac intensive care units: recommendations for organizational, staffing, and educational transformation. Can J Cardiol 2016;32:1204–1213. [DOI] [PubMed] [Google Scholar]
- 22.Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, Spears L, Miller M, Franczyk M, Deprizio D, Schmidt GA, Bowman A, Barr R, McCallister KE, Hall JB, Kress JP.. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009;373:1874–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kollef MH, Shapiro SD, Silver P.. A randomized, controlled trial of protocol-directed versus p… : critical care medicine. Crit Care Med 1997;25:567–574. [CVOCROSSCVO] [DOI] [PubMed] [Google Scholar]
- 24.Ely EW, Bennett PA, Bowton DL, Murphy SM, Florance AM, Haponik EF.. Large scale implementation of a respiratory therapist-driven protocol for ventilator weaning. Am J Respir Crit Care Med 1999;159:439–446. [DOI] [PubMed] [Google Scholar]
- 25.Brook AD, Ahrens TS, Schaiff R, et al. Effect of a nursing-implemented sedation protocol on the dur… : critical care medicine. Crit Care Med 1999;27:2609–2615. [CVOCROSSCVO] [DOI] [PubMed] [Google Scholar]
- 26.Esteban A, Anzueto A, Alía I, Gordo F, Apezteguía C, Pálizas F, Cide D, Goldwaser R, Soto L, Bugedo G, Rodrigo C, Pimentel J, Raimondi G, Tobin MJ.. How is mechanical ventilation employed in the intensive care unit? An international utilization review. Am J Respir Crit Care Med 2000;161:1450–1458. [DOI] [PubMed] [Google Scholar]
- 27.McGrath PD, Wennberg DE, Dickens JD Jr, Siewers AE, Lucas FL, Malenka DJ, Kellett MA Jr, Ryan TJ Jr. Relation between operator and hospital volume and outcomes following percutaneous coronary interventions in the era of the coronary stent. J Am Med Assoc 2000;284:3139–3144. [DOI] [PubMed] [Google Scholar]
- 28.Ross JS, Normand S-LT, Wang Y, Ko DT, Chen J, Drye EE, Keenan PS, Lichtman JH, Bueno H, Schreiner GC, Krumholz HM.. Hospital volume and 30-day mortality for three common medical conditions. N Engl J Med 2010;362:1110–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birkmeyer JD, Siewers AE, Finlayson EVA, Stukel TA, Lucas FL, Batista I, Welch HG, Wennberg DE.. Hospital volume and surgical mortality in the United States. N Engl J Med 2002;346:1128–1137. [DOI] [PubMed] [Google Scholar]
- 30.Finks JF, Osborne NH, Birkmeyer JD.. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med 2011;364:2128–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kocher KE, Haggins AN, Sabbatini AK, Sauser K, Sharp AL.. Emergency department hospitalization volume and mortality in the United States. Ann Emerg Med 2014;64:446–457.e6. [DOI] [PubMed] [Google Scholar]
- 32.van Diepen S, Fordyce CB, Wegermann ZK, Granger CB, Stebbins A, Morrow DA, Solomon MA, Soble J, Henry TD, Gilchrist IC, Katz JN, Cohen MG, Newby LK.. Organizational structure, staffing, resources, and educational initiatives in cardiac intensive care units in the United States: an American Heart Association Acute Cardiac Care Committee and American College of Cardiology Critical Care Cardiology Working. Circ Cardiovasc Qual Outcomes 2017;10:9–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.