Abstract
In order to improve the leishmanicidal activity of the synthetic cecropin A-melittin hybrid peptide CA(1-7)M(2-9) (KWKLFKKIGAVLKVL-NH2), a systematic study of its acylation with saturated linear fatty acids was carried out. Acylation of the Nɛ-7 lysine residue led to a drastic decrease in leishmanicidal activity, whereas acylation at lysine 1, in either the α or the ɛ NH2 group, increased up to 3 times the activity of the peptide against promastigotes and increased up to 15 times the activity of the peptide against amastigotes. Leishmanicidal activity increased with the length of the fatty acid chain, reaching a maximum for the lauroyl analogue (12 carbons). According to the fast kinetics, dissipation of membrane potential, and parasite membrane permeability to the nucleic acid binding probe SYTOX green, the lethal mechanism was directly related to plasma membrane permeabilization.
The protozoal mammalian parasite Leishmania is the causative agent of the set of clinical manifestations known as leishmaniasis, which afflicts 12 million to 14 million people worldwide (24). To date, the only treatments available rely on chemotherapy, and this is threatened by the growing incidence of strains resistant to first- and second-line drugs and by the often severe secondary effects caused by them (7).
A large number of eukaryotic peptides and small proteins with antimicrobial activity have been discovered over the last two decades (see references 3, 21, 34, and 39 and references cited therein). Therapy based on the rational use of such peptides appears to be a feasible alternative, and successful preliminary results have been reported for natural peptides such as dermaseptins (19, 23), SPYY (47), cecropin A (1), and gomesin (42), all of which have been shown to be active against different forms of the parasite. In our own work, we have expanded the repertoire of peptides with activity against Leishmania with cecropin-melittin hybrids such as CA(1-8)M(1-18) and CA(1-7)M(2-9), both of which are active in the micromolar range (15).
Fatty acid acylation is a common posttranslational modification for a wide variety of viral, bacterial, and eukaryotic proteins and peptides involved in either functional roles (for reviews, see references 17 and 38) or structural roles (26). The acylation process is enzymatic, with a variable degree of specificity for both the fatty acid and the primary structure of the protein (17). A major role for protein acylation is to increase membrane association, although other effects such as improved proteolytic stability (6) and sorting into specific subcellular localizations have also been described (30, 38).
In contrast to frequent C-terminal amidation and to other less common posttranslational modifications such as glycosylation or incorporation of d-amino acids or halogenated amino acids (for a review, see reference 3), fatty acid acylation of antimicrobial peptides is quite rare and is mostly confined to non-gene-encoded structures of fungal or bacterial origin such as the polymyxins (33) or peptides from different pathovars of Pseudomonas syringae, such as syringomycin, syringotoxin, and syringopeptin (8), or echinocandin (13). Only a very few examples of acylation of synthetic versions of antimicrobial peptides have been reported, such as lactoferricin B (48) and a heptapeptide from human cathepsin G (40), but no general conclusions have been derived from those studies.
In the study described in this paper we have studied in considerable detail the effect of fatty acid acylation on peptide antimicrobial activity, using CA(1-7)M(2-9), a synthetic cecropin A-melittin hybrid (9), as the template. This peptide exhibits substantial antibacterial (4, 9, 32), antiparasitic (4, 9, 15), and antifungal (10) activities only slightly inferior to those of the larger hybrid, CA(1-8)M(1-18), also referred to as CEME (36), one of the most extensively studied antimicrobial peptides. Our study, which has focused mostly on Leishmania but which has also been extended to a few representative bacterial targets, explores structural parameters such as the length and the position of the acylating chain and provides some insights into the mechanism of antimicrobial action of these fatty acid-modified peptides.
MATERIALS AND METHODS
Reagents.
The reagents used in the present study were of the highest purity available and were obtained from Sigma-Aldrich (St. Louis, Mo.) or Merck (Darmstadt, Germany). Fetal calf serum was obtained from Gibco-BRL (Paisley, United Kingdom). Fluorescent probes and caged luciferin were purchased from Molecular Probes (Leiden, The Netherlands). Propylene plasticware (Corning, Acton, Mass.) was used for peptide assays, as recommended elsewhere (http://www.cmdr.ubc.ca/bobh/MIC.htm).
tert-Butyloxycarbonyl (Boc) amino acids and resins for peptide synthesis were from Neosystems (Strasbourg, France). Solvents for synthesis and high-pressure liquid chromatography (HPLC) were from Scharlau (Barcelona, Spain).
Organisms.
Leishmania donovani strain MHOM/SD/00/1S-2D was kindly provided by S. Turco (School of Medicine, University of Kentucky, Lexington). Parasites were grown at 25°C in RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum (HIFCS), 2 mM l-glutamine, and penicillin-streptomycin (RPMI-HIFCS). The 3-luc strain was derived from the strain described above by transfection with the pX63Neo expression vector, which contains a C-terminal truncated Photinus pyralis luciferase gene that retains the enzyme at the cytoplasm and allows semiquantitative monitoring of changes in ATP levels (28). This strain was maintained under the same conditions as the parent strain, but under antibiotic pressure (50 μg of G418 per ml; Gibco-BRL). Control experiments to rule out a contribution of adsorbed cationic antibiotics from the growth medium (gentamicin and/or G418) on the permeability effects caused by the peptides were carried out with parasites grown in the absence of these antibiotics 48 h prior to the assay, and growth medium devoid of antibiotics was used for proliferation experiments.
Leishmania pifanoi axenic amastigotes were a kind gift from A. A. Pan (35). Amastigotes were maintained in medium 199 (Gibco-BRL) supplemented with 20% HIFCS and 50 μg of hemin per ml at 32°C (35). Amastigotes from L. donovani were obtained from the infected J774.1 murine macrophage line as described elsewhere (11), except that growth was at 35°C.
An Acinetobacter baumannii reference strain (strain ATCC 19606) and another clinical isolate (isolate Ac157) resistant to amikacin, cefotaxime, doxycycline, imipenem, ofloxacin, and ticarcillin were kindly provided by M. López-Brea (Microbiology Unit, Hospital de la Princesa, Madrid, Spain). An Escherichia coli strain (strain ML-35) that constitutively produces β-galactosidase was kindly donated by R. Gennaro (University of Trieste, Trieste, Italy). A Micrococcus luteus reference strain (strain ATCC 15307) was kindly provided by R. López, (Centro de Investigaciones Biológicas, Madrid, Spain). All strains except M. luteus were grown in Mueller-Hinton broth at 37°C. M. luteus was grown at 30°C. For E. coli ML-35, 50 μg of ampicillin per ml was added to Luria-Bertani medium for maintenance of the plasmid; antibiotic was omitted for experiments dealing with the bactericidal effect.
Peptides.
Solid-phase synthesis of CA(1-7)M(2-8) and acylated analogues was done manually on p-methylbenzhydrylamine resin (0.45 mmol/g) at a 0.1-mmol scale. In situ neutralization protocols (2) and dicyclohexylcarbodiimide-mediated coupling of Boc amino acids (0.4 mmol each in CH2Cl2, 50 min) were used throughout the synthesis, with formyl and 2-chlorobenzyloxycarbonyl used as protecting groups for Trp and Lys, respectively. In the acylated sequences, the Lys residue to be used for fatty acid coupling was protected with the 9-fluorenylmethyloxycarbonyl (Fmoc) group. After assembly of the complete peptide sequence, the Fmoc-protected Lys residue was selectively deprotected with piperidine-dimethyl formamide (DMF) (1:4 [vol/vol], 20 min) and the fatty acid was coupled by means of (benzotriazole-1-yl-oxy)tris(dimethylamino) phosphonium hexafluorophosphate in the presence of ethyldiisopropylamine ( 0.4, 0.4, and 0.8 mmol, respectively, in DMF; 90 min). The lipopeptide resins were first treated with piperidine-DMF (1:1, 30 min) to remove the formyl protection and then with HF-anisole (9:1 [vol/vol], 0°C, 1 h) for full deprotection and cleavage of the peptide from the resin. The resulting products showed purities in the 80 to 90% range by analytical HPLC and were further purified (>95% purity by HPLC) by preparative reverse-phase chromatography on a Vydac C18 silica column (4.6 by 250 mm; particle size, 5 μm) by using linear acetonitrile gradients in the 30 to 60% range in water (both eluents contained 0.05% trifluoroacetic acid) over 30 min at 1 ml/min. Peptides were detected at 220 nm. Combined synthesis and purification yields were in the 18 to 35% range, with lower yields consistently observed for peptides bearing larger fatty acid moieties. All peptides were further characterized by amino acid analysis and matrix-assisted laser desorption-ionization time-of-flight mass spectrometry (MALDI-TOF-MS) and had the expected compositions and molecular weights.
For circular dichroism measurements, peptides were dissolved to a concentration of 50 μM in 5 mM phosphate (pH 7) buffer containing different amounts of hexafluoroisopropanol. Spectra were acquired at 5°C in a Jasco 720 spectropolarimeter with quartz cylindrical cells with path lengths of 1 mm. For each solvent concentration, three acquisitions were performed in the 260- to 190-nm interval by using a 4-s time constant, a 10-nm/min scan speed, and a bandwidth of 0.2 nm. The α-helical content was calculated from the molar ellipticity at 222 nm by the method of Yang et al. (51).
Leishmanicidal activity.
Parasites were harvested at the late exponential phase, washed twice in Hanks medium supplemented with 20 mM d-glucose (Hanks-Glc), and resuspended to a final concentration of 2 × 107 parasites/ml. Aliquots of this suspension (120 μl) were incubated with the peptide for 4 h at 25°C and were then divided into two further aliquots (100 and 10 μl), which were used in the two assays described below.
(i) Assay 1.
Inhibition of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction to insoluble formazan by mitochondrial dehydrogenases was used as a parasite viability parameter (15). The 100-μl aliquot was added to 1.3 ml of Hanks-Glc at 4°C to slow the peptide action. The parasites were then collected by centrifugation, resuspended in 100 μl of a solution with 0.5 mg of MTT per ml in Hanks-Glc, and transferred to a 96-microwell plate; the plate was then incubated for 2 h at 25°C. The reduced formazan was solubilized with sodium dodecyl sulfate (final concentration, 5%), and the absorbance was measured in a Bio-Rad 450 microplate reader with a 595-nm filter.
(ii) Assay 2.
For inhibition of parasite proliferation, the 10-μl aliquot was added to 200 μl of RPMI-HIFCS devoid of phenol red. The surviving parasites were allowed to proliferate for 72 h, and then 100 μl of 0.5 mg of MTT per ml in Hanks-Glc was added and its reduction was measured as described above. Minor variations of this standard protocol are indicated in Fig. 3 and Table 5. All assays were performed in triplicate, and the experiments were repeated twice. The results were normalized to those for the corresponding control in the absence of the peptide. Axenic L. pifanoi amastigotes were assayed as described above, but all the procedures were performed at 32°C, the growth medium was that of Pan (35), and proliferation was allowed for 1 week instead of the 72 h allowed for the promastigotes. Amastigotes were washed with Hanks-Glc to remove hemin from the medium before the MTT assay. For L. donovani amastigotes, MTT reduction was done at 35°C. Amastigote proliferation was evaluated by allowing the amastigotes to convert into promastigotes by culturing them in RPMI-HIFCS at 25°C and further proliferation of this form as described above, except that the incubation time was extended to 7 days by culturing the parasites at 25°C in RPMI-HIFCS devoid of phenol red (27).
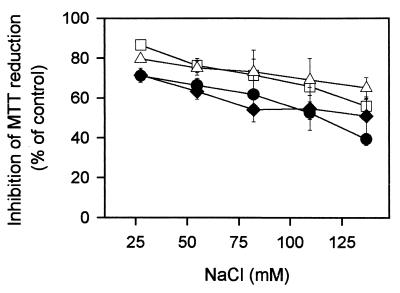
FIG. 3.
Variation with ionic strength of MTT reduction inhibition by α1-acylated CA(1-7)M(2-9) analogues on L. donovani promastigotes. Symbols: ●, 1.0 μM CA(1-7)M(2-9); □; 1.0 μM Acα1-CA(1-7)M(2-9); ♦, 0.7 μM Octα1-CA(1-7)M(2-9); ▵, 0.7 μM Lauα1-CA(1-8)M(2-9). Isosmolarity was maintained with d-sorbitol. Data are expressed as means ± standard deviations for a single experiment, conducted in triplicate, representative of three independent experiments.
TABLE 5.
Effectsa of calcium, heparin, and BSA on the inhibition of MTT reduction by 1Nα-acylated analogues of CA(1-7)M(2-9) on L. donovani promastigotes
| Assayb | % Inhibition
|
||
|---|---|---|---|
| CA(1-7)M(2-9) (1 μM) | Octα1-CA(1-7)M(2-9) (0.7 μM) | Lauα1-CA(1-7)M(2-9) (0.7 μM) | |
| Standard conditions | 82.1 ± 1.5 | 70.1 ± 7.5 | 78.9 ± 3.7 |
| With Ca2+ at: | |||
| 0.5 mM | 60.6 ± 6.7 | 59.1 ± 3.5 | 58.2 ± 9.0 |
| 1.0 mM | 41.2 ± 8.8 | 57.8 ± 9.3 | 60.4 ± 9.4 |
| 5.0 mM | 17.2 ± 3.4 | 43.5 ± 9.8 | 52.4 ± 6.2 |
| With heparin at: | |||
| 0.01 mg/ml | 5.4 ± 3.6 | 25.5 ± 6.9 | 53.5 ± 2.1 |
| 0.1 mg/ml | 6.4 ± 5.3 | 26.5 ± 6.1 | 42.5 ± 3.7 |
| 1.0 mg/ml | 3.3 ± 4.9 | 6.1 ± 1.3 | 31.4 ± 5.3 |
| With BSA at: | |||
| 0.1 mg/ml | 78.5 ± 5.0 | 56.3 ± 4.3 | 46.5 ± 2.5 |
| 1.0 mg/ml | 52.4 ± 1.9 | 32.7 ± 7.4 | 15.4 ± 3.9 |
| 10 mg/ml | 17.9 ± 2.6 | 10.5 ± 1.7 | 4.1 ± 2.1 |
Expressed as percent inhibition of MTT reduction compared to the respective control in the absence of peptides. Values are means ± standard deviations.
The peptide and the corresponding agent were preincubated together for 15 min before being added to the parasite suspension.
Promastigote membrane permeabilization.
The procedure described by Thevissen et al. (45) was adapted to assess the permeability of Leishmania promastigote membranes. Briefly, after peptide incubation, the parasites were washed with Hanks-Glc and incubated in 50 μl of 2 μM SYTOX green in Hanks-Glc for 30 min in the dark. The increase in fluorescence due to binding of the dye to intracellular DNA was measured in a Fluorostat Galaxy microplate reader (BMG Labotechnologies, Offenburg, Germany) with 485- and 520-nm filters as excitation and emission wavelengths, respectively, and a 10-nm slit, and the readings were normalized by subtracting parasite scattering and the basal fluorescence of the dye. Maximal membrane permeabilization was defined as that caused by 5 μM CA(1-8)M(1-18) (15) or 0.05% Triton X-100.
Modification of bioenergetic parameters.
Three assays were performed. (i) In vivo monitoring of changes in intracellular ATP levels is described in detail elsewhere (28). Briefly, promastigotes transfected with the 3-luc pX63Neo expression vector containing the gene for a cytoplasmic P. pyralys luciferase were incubated at 25°C with the membrane-permeant caged luciferase substrate d- luciferin–[1-(4,5-dimethoxy-2-nitrophenyl)ethyl ester] (DMNEP-luciferin) at 25 μM. Luminescence was measured in a BioOrbit 1250 LKB luminometer, with readings averaged every 10 s. When the luminescence reached a plateau, peptide was added and the luminescence decrease was monitored continuously for 30 min.
(ii) Collapse of membrane potential was estimated with the potential-sensitive dye bis-(1,3-diethylthiobarbituric) trimethine oxonol (bisoxonol), whose fluorescence increased after it was inserted into the membrane once the cell was depolarized. Assays were performed under standard conditions but in the presence of 0.2 μM bisoxonol. Peptide was added, and the changes in fluorescence were monitored for 15 min after the addition of peptide to the Fluorostat Galaxy microplate reader. Emission and excitation wavelengths were 544 and 584 nm, respectively. Maximal depolarization was considered to be that obtained with 5 μM CA(1-8)M(1-18) (15) or 0.5 μM valinomycin.
(iii) Mitochondrial membrane potential (ΔΨm) in L. donovani promastigotes was estimated by rhodamine 123 accumulation, determined by cytofluorometry as described previously (15). Parasites were loaded with the probe at 0.3 μg/ml for 5 min at 32°C prior to the standard assay. Dye incorporation was measured in a Coulter XL EPICS cytofluorometer (excitation and emission wavelengths, 488 and 525 nm, respectively). As negative controls, parasites were depolarized by treatment with 7.5 μM carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP).
Bactericidal activity.
Freshly grown bacteria were diluted in Hanks-Glc at a density of 107 cells/ml and incubated with the peptide for 1 h at 37°C for E. coli and both A. baumannii strains, whereas for M. luteus incubation was at 30°C for 2 h. Afterward, bacterial suspensions were diluted to 106 cell/ml in Mueller-Hinton broth (Oxoid, Basingstoke, United Kingdom). Bacterial growth was checked by measurement of an increase in culture turbidimetry at 600 nm at different times in a Bio-Rad 410 microplate reader by previously described procedures (44). Only readings within the linear range of the absorbance with respect to CFU were considered for calculation.
Statistical methods.
Data represent the means ± standard deviations for triplicate samples. The 50% lethal doses (LD50s) were calculated by the Lichfield and Wilcoxon procedure, and the 95% confidence intervals were calculated. Significance was assessed by the paired t test.
RESULTS
Synthetic peptides.
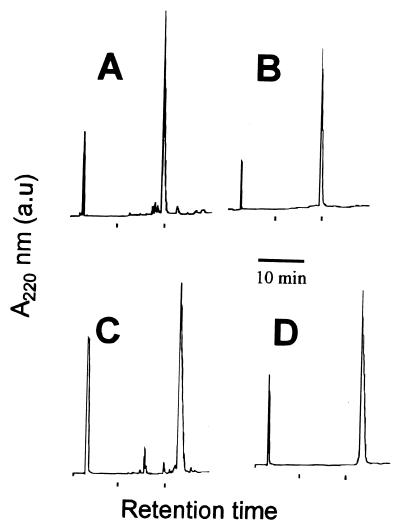
The parent CA(1-7)M(2-9) peptide, its Nα- and Nɛ-acetyl (Ac) derivatives, and a set of analogues (Table 1) substituted with C8 (octanoyl [Oct]), C12 (lauryl [Lau]), and C14 (myristoyl [Myr]) fatty acid residues at positions 1Nα, 1Nɛ, and 7Nɛ were prepared by solid-phase methods by using Boc-based synthetic chemistry with selective Fmoc protection of the amino group to be acylated. This ensured the unequivocal positioning of the acyl chain at a fixed position rather than a random postsynthetic acylation. After purification of all the peptides used in the assay by HPLC and further analysis by MALDI-TOF-MS, purities were found to be higher than 97% (Fig. 1 and Table 2). Derivatives acylated with palmitic acid (palmitoyl [Pam] group; C16) were also prepared but were difficult to handle due to their tendency to aggregate under diverse experimental conditions. They were tested for their antimicrobial activities (Table 3), with anomalous results. In any event, since their activities were lower than those of shorter, easier to handle analogues, they were not further tested. Circular dichroism analysis of the myristoylated analogues (Table 4) showed that they had a clear tendency to adopt the α-helical conformation even in aqueous buffer, where the parent molecule is unstructured. Upon the addition of a structure-inducing solvent such as hexafluoroisopropanol (HFIP), the helical content increased to almost 100% for all peptides except the 7Nɛ derivative. Acylation with Oct and Lau fatty acids produced similar behaviors (data not shown).
TABLE 1.
General structures of peptides used in this studya
| Name | Primary structure |
|---|---|
| CA(1-7)M(2-9) | KWKLFKKIGAVLKVL-NH2 |
| Acα1-CA(1-7)M(2-9) | Ac-KWKLFKKIGAVLKVL-NH2 |
| Acɛ1-CA(1-7)M(2-9) | KWKLFKKIGAVLKVL-NH2 |
| | | |
| Ac | |
| FAα1-CA(1-7)M(2-9) | FA-KWKLFKKIGAVLKVL-NH2 |
| FAɛ1-CA(1-7)M(2-9) | KWKLFKKIGAVLKVL-NH2 |
| | | |
| FA | |
| FAɛ7-CA(1-7)M(2-9) | KWKLFKKIGAVLKVL-NH2 |
| | | |
| FA |
The fatty acid (FA) residues used were Oct (C8), Lau (C12) Myr (C14), and Pam (C16). Ac, acetyl.
FIG. 1.
Representative examples of synthetic fatty acid-acylated derivatives of the peptide CA(1-7)M(2-9) before and after HPLC purification. The results of HPLC analysis of Lauα1-CA(1-7)M(2-9) (A and B) and Myrɛ7-CA(1-7)M(2-9) (C and D) and the chromatographic patterns of the synthetic crude material (A and C) and of the material after preparative reverse-phase purification (B and D) are shown. Detection was at 220 nm.
TABLE 2.
MALDI-TOF mass spectra of CA(1-7)M(2-9) and acylated derivatives
| Peptidea | Mass spectrum or spectra (m/z)
|
|
|---|---|---|
| Theoreticalb | Found | |
| CA(1-7)M(2-9) | 1,769.181 | 1,770.21 (MH+), 1,792.14 (MNa+) |
| Acα1-CA(1-7)M(2-9) | 1,811.191 | 1,812.44 (MH+) |
| Acε1-CA(1-7)M(2-9) | 1,811.191 | 1,812.32 (MH+) |
| Octα1-CA(1-7)M(2-9) | 1,895.286 | 1,896.73 (MH+), 1,918.46 (MNa+) |
| Octɛ1-CA(1-7)M(2-9) | 1,895.286 | 1,918.53 (MNa+) (no MH+ observed) |
| Octɛ7-CA(1-7)M(2-9) | 1,895.286 | 1,918.63 (MH+) |
| Lauα1-CA(1-7)M(2-9) | 1,951.348 | 1,952.13 (MH+) |
| Lauɛ1-CA(1-7)M(2-9) | 1,951.348 | 1,952.29 (MH+), 1,974.46 (MNa+) |
| Lauɛ7-CA(1-7)M(2-9) | 1,951.348 | 1,952.65 (MNa+), 1,991.06 (MK+) (no MH+ observed) |
| Myrα1-CA(1-7)M(2-9) | 1,979.379 | 2,002.54 (MNa+) (no MH+ observed) |
| Myrɛ1-CA(1-7)M(2-9) | 1,979.379 | 2,001.98 (MNa+) (no MH+ observed) |
| Myrɛ7-CA(1-7)M(2-9) | 1,979.379 | 2,002.33 (MNa+) (no MH+ observed) |
| Pamα1-CA(1-7)M(2-9) | 2,007.410 | 2,008.01 (MH+), 2,030.42 (MNa+) |
| Pamɛ1-CA(1-7)M(2-9) | 2,007.410 | 2,030.45 (MNa+), 2,046.88 (MK+) (no MH+ observed) |
| Pamɛ7-CA(1-7)M(2-9) | 2,007.410 | 2,030.27 (MNa+) (no MH+ observed) |
Ac, acetyl; Oct, C8; Lau, C12, Myr, C14; Pam, C16.
Calculated for monoisotopic composition.
TABLE 3.
LD50s of the acylated CA(1-8)M(2-9) for different microorganismsa
| Peptideb | LD50 (μM)
|
|||||
|---|---|---|---|---|---|---|
|
Leishmania spp.
|
M. luteus ATCC 15307 | E. coli ML-35 |
A. baumannii
|
|||
| Promastigotes | Amastigotes | ATCC 19606 | Ac157 | |||
| CA(1-7)M(2-9) | 4.4 (3.0-6.3)c | 14.4 (6.2-34.8) | 0.8 (0.4-1.6) | 1.4 (0.6-3.4) | 0.7 (0.4-1.3) | 1.9 (1.2-3.0) |
| Lauɛ7-CA(1-7)M(2-9) | 20.4 (11.5-40.3) | 32.5 (25.9-40.8) | >50 | >50 | ND | ND |
| Myrɛ7-CA(1-7)M(2-9) | 32.1 (27.3-37.8) | NDd | >50 | >50 | ND | ND |
| Pamɛ7-CA(1-7)M(2-9) | 35.2 (24.2-51.6) | ND | >50 | >50 | ND | ND |
| Acα1-CA(1-7 )M(2-9) | 2.5 (1.5-4.2) | 10.4 (2.7-39.3) | 0.9 (0.3-3.5) | 0.9 (0.5-1.7) | 0.9 (0.6-1.2) | 1.8 (1.4-2.4) |
| Octα1-CA(1-7)M(2-9) | 1.1 (0.6-2.1) | 2.7 (0.4-20.3) | 1.2 (0.6-2.2) | 1.0 (0.5-2.3) | 0.6 (0.4-0.9) | 1.3 (0.9-2.1) |
| Lauα1-CA(1-7)M(2-9) | 0.9 (0.6-1.4) | 1.3 (0.6-2.5) | 1.0 (0.5-2.0) | 0.7 (0.4-0.8) | 0.6 (0.5-0.9) | 0.9 (0.8-1.0) |
| Myrα1-CA(1-7)M(2-9) | 0.8 (0.4-1.5) | 0.9 (0.3-2.9) | 1.9 (0.8-4.5) | 1.3 (0.3-5.6) | ND | ND |
| Pamα1-CA(1-7)M(2-9) | 1.2 (0.5-2.9) | 3.9 (2.7-5.63) | 2.9 (2.5-3.1) | 1.6 (1.3-2.0) | ND | ND |
| Acɛ1-CA(1-7)M(2-9) | 3.2 (1.8-5.7) | 12.1 (9.6-15.3) | 1.2 (0.7-2.4) | 0.7 (0.6-0.8) | ND | ND |
| Lauɛ1-CA(1-7)M(2-9) | 1.1 (0.7-1.7) | 1.2 (0.7-2.05) | 0.9 (0.7-1.2) | 0.8 (0.5-1.3) | ND | ND |
| Myrɛ1-CA(1-7)M(2-9) | 0.7 (0.6-0.8) | 2.1 (1.0-4.2) | ND | ND | ND | ND |
| Pamɛ1-CA(1-7)M(2-9) | 1.4 (0.5-3.9) | 3.4 (2.1-5.5) | ND | ND | ND | ND |
Calculated from inhibition of proliferation data by the algorithm of Litchfield and Wilcoxon.
The nomenclature follows that described in footnote a of Table 1. Ac, acetyl.
Values in parentheses are 95% confidence intervals.
ND, not determined.
TABLE 4.
Helical contents of CA(1-7)M(2-9) and its myristoylated derivatives in aqueous and HFIP medium
| Name | % α-helixa
|
|
|---|---|---|
| 5 mM phosphate | 20% HFIP | |
| CA(1-7)M(2-9) | 0.0 | 92.2 |
| Myrα1-CA(1-7)M(2-9) | 42.2 | 99.8 |
| Myrɛ1-CA(1-7)M(2-9) | 27.3 | 100.0 |
| Myrɛ7-CA(1-7)M(2-9) | 25.6 | 60.6 |
Calculated according to molar ellipticity at 222 nm (32).
Modification of leishmanicidal activity of CA(1-7)M(2-9) by fatty acid acylation.
The two assays used to assess the effect of acylation on leishmanicidal activity, inhibition of proliferation and MTT reduction, evaluate the long-term and the medium- to short-term effects of the peptide on antiparasitic activity, respectively. Both assays consistently had comparable results for all tested organisms and peptides (Fig. 2), with only slight quantitative differences between them. The results of experiments carried out in parallel with parasites grown in the absence of antibiotics 48 h prior to the assay and tested in the absence or the presence of antibiotics at the same concentration as that in the growth medium did not show significant variations (P > 0.5). Thus, contributions by the antibiotics on their own or by synergy with the peptides were ruled out.
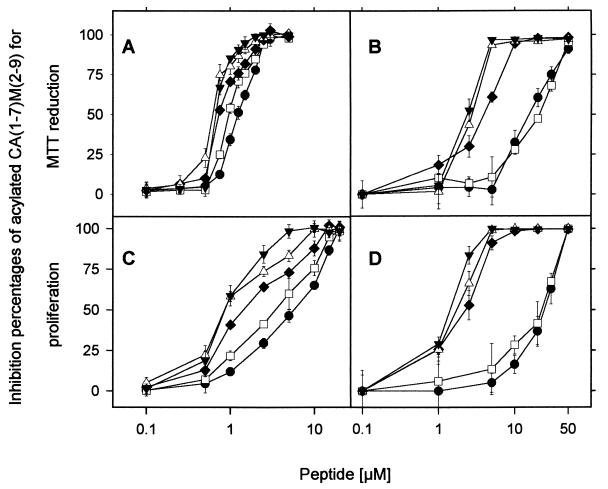
FIG. 2.
Inhibition of MTT reduction (A and B) and Leishmania parasite proliferation (C and D) by α1-acylated CA(1-7)M(2-9) analogues. (A and C) L. donovani promastigotes; (B and D) L. pifanoi amastigotes. Symbols: ●, CA(1-7)M(2-9); □, Acα1-CA(1-7)M(2-9); ♦, Octα1-CA(1-7)M(2-9); ▵; Lauα1-CA(1-7)M(2-9); ▾, Myrα1-CA(1-7)M(2-9). Data are expressed as means ± standard deviations. Data are from a single experiment, representative of three separate experiments.
The effect of the position of the acyl chain was first investigated. Acylation of the ɛ-amino group of Lys7 with any of the three long fatty acid residues (Lau, Myr, or Pam fatty acids) led to substantial losses of both leishmanicidal and bactericidal activities (Table 3). In contrast, acylation at the Nα position of Lys1 with the Oct, Lau, Myr, or Pam fatty acid gave up to fivefold increases in leishmanicidal activities against promastigotes (Fig. 2 and Table 3). Acylation of the 1Nɛ group of Lys1 with the same fatty acid residues did not produce significant differences in activities relative to the activities of the 1Nα derivatives (Table 3). The potential impact of the loss of a positive charge caused by the acylation process was evaluated with the corresponding acetylated analogues; no appreciable change (P > 0.1) in activity relative to that of the parent CA(1-7)M(2-9) peptide was observed.
The effect of the length of the fatty acid was also investigated, and a clear correlation of leishmanicidal activity with chain length was found. At 1 μM, analogues with longer fatty acid chains caused fivefold inhibition of promastigote proliferation relative to the level of inhibition caused by the parent peptide (Fig. 2C). These differences were more substantial against L. pifanoi amastigotes, the pathogenic form of the parasite. Lau and Myr analogues were especially active against this form of the parasite (Fig. 2B and D and Table 3), and the differences between Oct and larger acyl groups also increased. When assayed against L. donovani amastigotes obtained from infected J774 macrophages, the results differed for less than 5% of the corresponding values obtained for L. pifanoi; thus, the leishmanicidal activities were similar against these two species. Assays with L. pifanoi were carried out routinely, as their reproducibilities were always higher that those for the other amastigotes.
Inhibition of leishmanicidal activity.
For a better understanding of the modifications introduced by acylation, we next explored the effects on peptide activity of different parameters involved in the peptide-membrane interaction for promastigotes. On the basis of the results obtained in the experiments described in the previous section, assays were restricted only to inhibition of MTT reduction by the 1Nα analogues.
Figure 3 illustrates the inhibitory effect of ionic strength (NaCl) on MTT reduction. By normalizing peptide concentrations so that antibiotic activities were closely equivalent under standard conditions (140 mM NaCl), an inverse relationship between activity and ionic strength was found for all peptides assayed. Calcium and polyanions were even better inhibitors of antibiotic activity (Table 5), with the effect being most marked for the parent peptide and less for the 1Nα-Lau derivative (the 1Nα-Myr analogue gave spurious results due to aggregation under the assay conditions used). Levels of inhibition of MTT reduction for heparin were consistently higher than those for Ca2+, as previously found for a larger analogue, CA(1-8)M(1-18) (11). As expected from the increase in the overall hydrophobicity of the peptide, addition of bovine serum albumin (BSA) to the incubation medium had an inhibitory effect, with an inverse relationship between activity and chain length being found (Table 5).
Biological effects of acylated CA(1-7)M(2-9) analogues on Leishmania promastigotes.
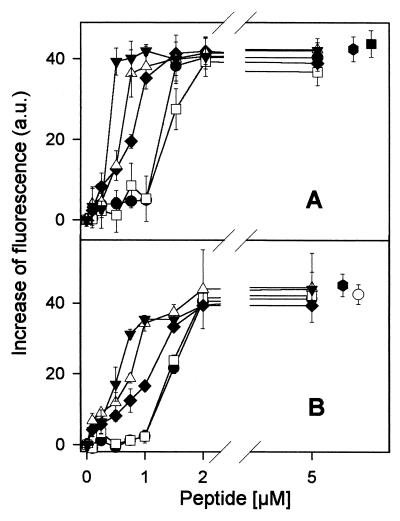
The membrane distortion caused by CA(1-7)M(2-9) and its fatty acid analogues on the promastigote can be monitored by measurement of the increase in fluorescence of the SYTOX green dye, which results from the interaction with intracellular nucleic acids. Figure 4A illustrates the dependence of fluorescence with peptide concentration, which follows a trend similar to that for the inhibition of MTT reduction and which hints toward a mechanism of simple membrane permeabilization by the peptides. The increase in bisoxonol fluorescence (Fig. 4B) reflected insertion of dye into the hydrophobic core of the lipid bilayer following depolarization and supported the previous conclusion. CA(1-8)M(1-18), another cecropin A-melittin hybrid peptide, was used as a control for both experiments; the values obtained for this peptide were similar to those obtained for other controls such as 0.05% Triton X-100 (for SYTOX green assay) or 0.5 μM valinomycin (for depolarization).
FIG. 4.
Membrane permeabilization of L. donovani promastigotes by α1-acylated CA(1-7)M(2-9) analogues. Parasites were incubated with peptides by the standard assay in the presence of the corresponding fluorescent probe. (A) Increase in 2 μM SYTOX green fluorescence by interaction with internal nucleic acids after membrane permeabilization by the peptides. Excitation and emission wavelengths were 485 and 520 nm, respectively. (B) Collapse of plasma membrane potential by acylated peptides as measured by increase in 0.2 μM bisoxonol fluorescence. Excitation and emission wavelengths were 544 and 584 nm, respectively. Symbols: ●, CA(1-7)M(2-9); □, Acα1-CA(1-7)M(2-9); ♦, Octα1-CA(1-7)M(2-9); ▵, Lauα1-CA(1-7)M(2-9); ▾, Myrα1-CA(1-7)M(2-9). Values obtained with 5 μM CA(1-8)M(1-18) (●), 0.05% Triton X-100 (▪), and 0.5 μM valinomycin (○) are shown for comparison. Data are expressed as means ± standard deviations.
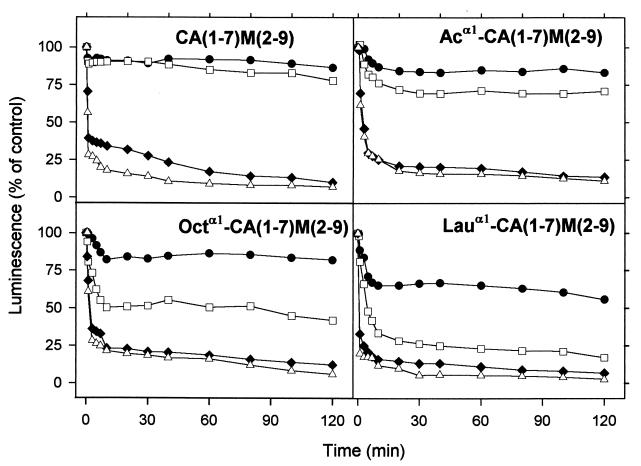
L. donovani promastigotes transfected with a mutated version of the luciferase gene (from the American firefly, P. pyralis) that causes the enzyme to be retained in the cytoplasm allow easy monitoring of relative changes in ATP levels in the presence of the membrane-permeant DMNPE-luciferin analogue (28). The loss of luminescence induced by the acylated CA(1-7)M(2-9) analogues is shown in Fig. 5A to D. Again, a pattern similar to those found for SYTOX green permeation or leishmanicidal activity is observed; i.e., luminescence inhibition at a given concentration increases with the length of the acyl chain.
FIG. 5.
In vivo monitoring of ATP levels in L. donovani promastigotes after addition of α1-acylated CA(1-7)M(2-9) analogues. Promastigotes were transfected with firefly luciferase mutated at its last three amino acids, and variations in ATP levels were monitored by using 25 μM DMNPE-luciferin as the luciferase substrate. Peptide concentrations were as follows: 0.5 μM (●), 1.0 μM (□), 2.0 μM (♦), and 2.5 μM (▵). Data are representative of four independent experiments and are normalized for the luminescence decay of parasites in the absence of peptide. Ac, acetylated.
Finally, to test whether the loss of ATP could be due to a direct effect of the peptides on mitochondria, either by permeabilization of the inner membrane or by alteration of the respiratory chain, and since both parameters are directly related to a loss of the mitochondrial membrane potential, variations of this potential were monitored by accumulation of rhodamine 123, a cationic dye whose incorporation is mainly dependent on such potential. Control parasites have fluorescence values of 87.2 ± 2.9 arbitrary units (a.u.), whereas for the 1Nα acetyl, Oct, Lau, and Myr analogues, the values were 90.7 ± 3.8 (P = 0.2), 84.0 ± 2.6 (P = 0.7), 87.8 ± 2.6 (P = 0.7), and 81.1 ± 5.0 (P = 0.05), respectively, when 3 μM peptide was tested; these values indicate that parasite viability is fully compromised. These values are in sharp contrast to the 8.8 ± 2.0 (P < 0.01) a.u. obtained when parasites were treated with 7.5 μM FCCP, one of the strongest mitochondrial uncouplers, thus ruling out a major role for mitochondria in the lethal hit of the active analogues. This agreed with the fluorescence patterns for parasites observed by confocal microscopy (data not shown).
Bactericidal activities.
In order to extend the differences in leishmanicidal activities observed for the different analogues, their bactericidal activities were tested against some representative gram-positive and -negative bacteria (Table 3). All analogues were active at the low micromolar range, but some interesting differences were observed. Thus, while all peptides behaved quite similarly against the reference strain of Acinetobacter, the LD50 of the 1Nα-Lau derivative for the multidrug-resistant strain was lower than those of analogues with shorter fatty acid chains and half of that of the parent peptide. The same trend was observed against E. coli. Increases in the LD50s of the Pam group-substituted peptides were observed, which was against the general trend. Aggregation effects (see the “Synthetic peptides” section above) might explain this anomaly. M. luteus was more resistant than E. coli and A. baumannii to all peptides except the 1Nα acetyl derivative.
DISCUSSION
Cecropin-melittin hybrids designed to combine in a single, smaller molecule the structural elements related to antibiotic activity in each respective parent sequence have become useful templates to test different strategies for improving the potency or expanding the spectrum of antimicrobial peptides. For active analogues with a polypeptide chain of 15 residues or longer, the mechanism of action appears to be clearly related to a process of permeabilization of the plasma membrane of the pathogen, leading to bioenergetic collapse and subsequent death, similar to that described for the longer analogue CA(1-8)M(1-18) (15). A number of these analogues have been successfully tested by us and other groups as antitumoral, bactericidal, and antifungal agents in both animals and plants (4, 9, 20, 25, 32, 41).
A number of strategies have been devised to improve the antibiotic activities of peptide lead compounds, including modulation and/or reduction of the size of the original sequence, total or partial replacement by d-amino acids, use of retro- and retroenantiomeric versions, cyclization, and linearization. Remarkably, acylation with fatty acid residues has only rarely been explored in this respect, a situation somehow surprising considering the relative simplicity of the synthesis procedure and its well-known possibilities in other areas of pharmacological and biochemical interest (see reference 18 and references cited therein).
In the present work we have used the cecropin A-melittin hybrid CA(1-7)M(2-9) as a template to evaluate the effect of acylation of the hybrid with fatty acid residues on activity against Leishmania and a few representative bacteria. The presence of the Lys-7 residue in the mostly hydrophilic C-terminal moiety of CA(1-7)M(2-9) allowed assessment of the effect of the position on activity. The decreased activity observed for all analogues acylated at this position (Table 3) might be due to the adoption of an unfavorable conformation or to the loss of the positive charge upon acylation. The first possibility would seem to be supported by circular dichroism data (Table 4), which show that 7Nɛ-acylated CA(1-7)M(2-9) does not achieve substantial α-helix levels even in the presence of high concentrations of structure-promoting solvent, in contrast to the results obtained for either the parent nonlipidated peptide or the 1Nα- or 1Nɛ-acylated analogues (see below). Since the membrane activities of linear cationic peptides is often associated with an amphipathic helical conformation (12, 46), this inability of 7Nɛ-acylated CA(1-7)M(2-9) to achieve a full helix might be detrimental to its activity.
In contrast to position 7, the leishmanicidal activities of CA(1-7)M(2-9) peptides acylated at either the α- or the ɛ-amino groups of Lys-1 are significantly enhanced. A direct relationship is found between antimicrobial activity and the length of the acyl chain up to 12 to 14 carbons; for longer chains, the effect of chain length on activity is less clear, possibly due to aggregation effects. Similar behavior has recently been reported for a lactoferricin B-derived nonapeptide acylated at the N-terminal position, with the highest activity found for an 11-carbon fatty acid chain (48). Also, Piazza et al. (36) have studied fatty acid length and the position of acylation on the lipopeptaibol trikoningin B and found that effective membrane permeabilization is related more to the length of the alkyl chain than to its location at either end of the sequence. Acylation is also required for polymyxin, another non-gene-encoded antibiotic peptide, with the deacylated nonapeptide being less active than the natural acylated molecule (33).
Although several killing mechanisms other than membrane permeabilization are being discovered for eukaryotic antibiotic peptides (50), for CA(1-7)M(2-9) and its acylated analogues membrane permeabilization is an essential step, if not the only one, in their lethal action. Thus, totally comparable responses have been obtained when parameters such as SYTOX green permeation, plasma membrane depolarization, intracellular ATP levels, MTT reduction, or parasite proliferation have been monitored. The last parameter required peptide concentrations slightly higher than those required for the other parameters to achieve comparable results, which was also previously reported for CA(1-8)M(1-18) (15) and cecropin A (43), and may reflect either some membrane repair activity or some mechanism partially counteracting the loss of internal homeostasis by the organism. In any event, membrane permeabilization is an essential step in the killing process, in agreement with the fast kinetics (>90% completion 5 min after peptide addition) observed for all active analogues in this work (data not shown), and the rapid drop in intracellular ATP levels, likely due to homeostatic damage in the parasite cell with release of internal metabolites along with the increased levels of wasting of ATP by ion pumps attempting to restore gradients. Alternatively, it could result from a direct effect of the peptide on mitochondria, as described previously for the effects of these peptides on isolated rat liver mitochondria (16) or for the effects of histatins in vivo (22). However, this option is not supported by measurements of mitochondrial membrane potential by rhodamine 123 incorporation; therefore, mitochondria are not targets of the peptides.
The perturbation of the lipid bilayer by specific peptide sequences is governed by a subtle equilibrium of electrostatic and hydrophobic interactions (for reviews, see references 12 and 46). Peptide acylation shifts this equilibrium toward a higher hydrophobic contribution. This is reflected by the decreased level of membrane activity in the presence of either heparin or Ca2+. In the first case, the anionic polysaccharide competes electrostatically with the membrane head groups for the polycationic peptide; Ca2+, on the other hand, competitively binds to these head groups with the peptide. This effect appears only at high charge densities, as it is not reflected in the variation in ionic strength. As expected, inhibition of peptide activity by BSA, a typical quencher of hydrophobic compounds, is higher for analogues with a longer hydrophobic tail. Interestingly, analogues acetylated at Lys-1 were as active as the parent peptide, despite the loss of one positive charge (5).
Amastigotes, the pathological form of Leishmania for vertebrates, are highly resistant to linear antibiotic peptides, including CA(1-8)M(1-18) (14). Remarkably, they become rather susceptible upon acylation with Oct, Lau, or Myr chains at the 1Nα position (Fig. 2). The reported differences between amastigotes and promastigotes in terms of both their lipooligosaccharide compositions and their plasma membrane proteins could account for this improved behavior; unfortunately, the composition of the amastigote lipids is not yet known in detail, and thus, no definite explanation can be given at this time.
In contrast to the effects against Leishmania, the effect of peptide acylation on antibacterial activity is less pronounced. Thus, some improvement in activity against E. coli is found for all analogues acylated at position 1, as well as for the Myr analogue against a multidrug-resistant strain of Acinetobacter, while the effects of acylated and nonacylated CA(1-7)M(2-9) against the gram-positive organism M. luteus were much alike. Further study is required to provide a reasonable explanation for this behavior.
While the present results, as well as results from another group presented previously (48), demonstrate the usefulness of acylation in improving the activity of synthetic antimicrobial peptides, acylation is not without difficulties of its own. For instance, low yields are usually found during the purification of hydrophobic peptides due to aggregation or unspecific absorption to chromatographic supports. In addition, lipophilicity may enhance peptide binding to neutral phospholipids, thus narrowing differences in activity between pathogen and mammalian cells, as reported for magainin analogues with a high percentage of hydrophobic residues (49); or it may result in increased toxicity, as was found for polymyxin B relative to the toxicity of its deacylated analogue (33). On the prospective side, a number of possibilities for acylation remain to be fully explored, such as the use of multiple acylation sites on a single peptide chain or use of unsaturated (e.g., farnesylated) residues or even cholesterol (29). Further potential benefits might include minimal peptide leakage upon liposome incorporation, thus reducing systemic toxicity, a desirable feature for peptide antibiotics targeting intraphagocytic pathogens such as Leishmania, Histoplasma, Legionella, or Mycobacterium.
ACKNOWLEDGMENTS
This work was supported by grants from the Fondo de Investigaciones Sanitarias, (grant 99/0025-02), Comunidad Autónoma de Madrid (Programa General de Grupos Estratégicos and grant 08-2/0029.2), and EU (grants IC 18-CT97-0213 and QLRT-2000-01404) (to L.R.) and from the Generalitat de Catalunya (CERBA) (to D.A.).
REFERENCES
- 1.Akuffo H, Hultmark D, Engstöm Å, Frohlich D, Kimbrell D. Drosophila antibacterial protein, cecropin A, differentially affects nonbacterial organisms such as Leishmania in a manner different from other amphipathic peptides. Int J Mol Med. 1998;1:77–82. doi: 10.3892/ijmm.1.1.77. [DOI] [PubMed] [Google Scholar]
- 2.Alewood P, Alewood D, Miranda L, Love S, Meutermans W, Wilson D. Rapid in situ neutralization protocols for Boc and Fmoc solid-phase chemistries. Methods Enzymol. 1997;289:14–29. doi: 10.1016/s0076-6879(97)89041-6. [DOI] [PubMed] [Google Scholar]
- 3.Andreu D, Rivas L. Animal antimicrobial peptides: an overview. Biopolymers. 1998;47:415–433. doi: 10.1002/(SICI)1097-0282(1998)47:6<415::AID-BIP2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 4.Andreu D, Ubach J, Boman A, Wåhlin B, Wade D, Merrifield R B, Boman H G. Shortened cecropin A-melittin hybrids. Significant size reduction retains potent antibiotic activity. FEBS Lett. 1992;296:190–194. doi: 10.1016/0014-5793(92)80377-s. [DOI] [PubMed] [Google Scholar]
- 5.Andreu D, Merrifield R B, Steiner H, Boman H G. Solid-phase synthesis of cecropin A and related peptides. Proc Natl Acad Sci USA. 1983;80:6475–6479. doi: 10.1073/pnas.80.21.6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnould S, Takahashi M, Camadro J M. Acylation stabilizes a protease-resistant conformation of protoporphyrinogen oxidase, the molecular target of diphenyl ether-type herbicides. Proc Natl Acad Sci USA. 1999;96:14825–14830. doi: 10.1073/pnas.96.26.14825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balaña-Fouce R, Reguera R M, Cubría J C, Ordoñez D. The pharmacology of leishmaniasis. Gen Pharmacol. 1998;30:435–443. doi: 10.1016/s0306-3623(97)00268-1. [DOI] [PubMed] [Google Scholar]
- 8.Bender C L, Alarcón-Chaidez F, Gross D C. Pseudomonas syringae phytotoxins: mode of action, regulation, and biosynthesis by peptide and polyketide synthetases. Microbiol Mol Biol Rev. 1999;63:266–292. doi: 10.1128/mmbr.63.2.266-292.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boman H G, Wade D, Boman I A, Wåhlin B, Merrifield R B. Antibacterial and antimalarial properties of peptides that are cecropin-melittin hybrids. FEBS Lett. 1989;259:103–106. doi: 10.1016/0014-5793(89)81505-4. [DOI] [PubMed] [Google Scholar]
- 10.Cavallarin L, Andreu D, San Segundo B. Cecropin A-derived peptides are potent inhibitors of fungal plant pathogens. Mol Plant Microbe Interact. 1998;11:218–227. doi: 10.1094/MPMI.1998.11.3.218. [DOI] [PubMed] [Google Scholar]
- 11.Chang K P. Human cutaneous leishmania in a mouse macrophage line: propagation and isolation of intracellular parasites. Science. 1980;209:1240–1242. doi: 10.1126/science.7403880. [DOI] [PubMed] [Google Scholar]
- 12.Dathe M, Wieprecht T. Structural features of helical antimicrobial peptides: their potential to modulate activity on model membranes and biological cells. Biochim Biophys Acta. 1999;1462:71–87. doi: 10.1016/s0005-2736(99)00201-1. [DOI] [PubMed] [Google Scholar]
- 13.Denning D W. Echinocandins and pneumocandins—a new antifungal class with a novel mode of action. J Antimicrob Chemother. 1997;40:611–614. doi: 10.1093/jac/40.5.611. [DOI] [PubMed] [Google Scholar]
- 14.Díaz-Achirica P, Guinea A, Ubach J, Andreu D, Rivas L. Mechanism of action of cecropin A-melittin hybrid peptides on Leishmania parasites. In: Kaumaya P, Hodges R S, editors. Peptides 95. Kingswinford, United Kingdom: Mayflowers Scientific Ltd.; 1996. pp. 183–185. [Google Scholar]
- 15.Díaz-Achirica P, Ubach J, Guinea A, Andreu D, Rivas L. The plasma membrane of Leishmania donovani promastigotes is the main target for CA(1-8)M(1-18), a synthetic cecropin A-melittin hybrid peptide. Biochem J. 1998;330:453–460. doi: 10.1042/bj3300453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Díaz-Achirica P, Prieto S, Ubach J, Andreu D, Rial E, Rivas L. Permeabilization of the mitochondrial inner membrane by short cecropin-A-melittin hybrid peptides. Eur J Biochem. 1994;224:257–263. doi: 10.1111/j.1432-1033.1994.tb20019.x. [DOI] [PubMed] [Google Scholar]
- 17.Dunphy J T, Linder M E. Signaling functions of protein palmitoylation. Biochim Biophys Acta. 1998;1436:245–261. doi: 10.1016/s0005-2760(98)00130-1. [DOI] [PubMed] [Google Scholar]
- 18.Epand R M. Biophysical studies of lipopeptide-membrane interactions. Biopolymers. 1997;43:15–24. doi: 10.1002/(SICI)1097-0282(1997)43:1<15::AID-BIP3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Feder R, Dagan A, Mor A. Structure-activity relationship study of antimicrobial dermaseptin S4 showing the consequences of peptide oligomerization on selective cytotoxicity. J Biol Chem. 2000;275:4230–4238. doi: 10.1074/jbc.275.6.4230. [DOI] [PubMed] [Google Scholar]
- 20.Friedrich C, Scott M G, Karunaratne N, Yan H, Hancock R E. Salt-resistant α-helical cationic antimicrobial peptides. Antimicrob Agents Chemother. 1999;43:1542–1548. doi: 10.1128/aac.43.7.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ganz T, Lehrer R I. Antibiotic peptides from higher eukaryotes: biology and applications. Mol Med Today. 1999;5:292–297. doi: 10.1016/s1357-4310(99)01490-2. [DOI] [PubMed] [Google Scholar]
- 22.Helmerhorst E J, Breeuwer P, van't Hof W, Walgreen-Weterings E, Oomen L C, Veerman E C, Amerongen A V, Abee T. The cellular target of histatin 5 on Candida albicans is the energized mitochondrion. J Biol Chem. 1999;274:7286–7291. doi: 10.1074/jbc.274.11.7286. [DOI] [PubMed] [Google Scholar]
- 23.Hernández C, Mor A, Dagger F, Nicolas P, Hernández A, Benedetti E L, Dunia I. Functional and structural damage in Leishmania mexicana exposed to the cationic peptide dermaseptin. Eur J Cell Biol. 1992;59:414–424. [PubMed] [Google Scholar]
- 24.Herwaldt B L. Leishmaniasis. Lancet. 1999;354:1191–1199. doi: 10.1016/S0140-6736(98)10178-2. [DOI] [PubMed] [Google Scholar]
- 25.Houston M E, Jr, Kondejewski L H, Karunaratne D N, Gough M, Fidai S, Hodges R S, Hancock R E. Influence of preformed α-helix and α-helix induction on the activity of cationic antimicrobial peptides. J Pept Res. 1998;52:81–88. doi: 10.1111/j.1399-3011.1998.tb01361.x. [DOI] [PubMed] [Google Scholar]
- 26.Johansson J. Structure and properties of surfactant protein C. Biochim Biophys Acta. 1998;1408:161–172. doi: 10.1016/s0925-4439(98)00065-9. [DOI] [PubMed] [Google Scholar]
- 27.Kiderlen A F, Kaye P M. A modified colorimetric assay of macrophage activation for intracellular cytotoxicity against Leishmania parasites. J Immunol Methods. 1990;127:11–18. doi: 10.1016/0022-1759(90)90334-r. [DOI] [PubMed] [Google Scholar]
- 28.Luque-Ortega J R, Rivero-Lezcano O M, Croft S L, Rivas L. In vivo monitoring of intracellular ATP levels in Leishmania donovani promastigotes as a rapid method to screen drugs targeting bioenergetic metabolism. Antimicrob Agents Chemother. 2001;45:1121–1125. doi: 10.1128/AAC.45.4.1121-1125.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mann R K, Beachy P A. Cholesterol modification of proteins. Biochim Biophys Acta. 2000;1529:188–202. doi: 10.1016/s1388-1981(00)00148-7. [DOI] [PubMed] [Google Scholar]
- 30.McCabe J B, Berthiaume L G. Functional roles for fatty acylated amino-terminal domains in subcellular localization. Mol Biol Cell. 1999;10:3771–3786. doi: 10.1091/mbc.10.11.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Committee for Clinical Laboratory Standards. Approved standard ML-A5. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically. Wayne, Pa: National Committee for Clinical Laboratory Standards; 2000. [Google Scholar]
- 32.Nos-Barberá S, Portolés M, Morilla A, Ubach J, Andreu D, Paterson C A. Effect of hybrid peptides of cecropin A and melittin in an experimental model of bacterial keratitis. Cornea. 1997;16:101–106. [PubMed] [Google Scholar]
- 33.Ofek I, Cohen S, Rahmani R, Kabha K, Tamarkin D, Herzig Y, Rubinstein E. Antibacterial synergism of polymyxin B nonapeptide and hydrophobic antibiotics in experimental gram-negative infections in mice. Antimicrob Agents Chemother. 1994;38:374–377. doi: 10.1128/aac.38.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Otvos L., Jr Antibacterial peptides isolated from insects. J Pept Sci. 2000;6:497–511. doi: 10.1002/1099-1387(200010)6:10<497::AID-PSC277>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 35.Pan A A. Leishmania mexicana: serial cultivation of intracellular stages in a cell-free medium. Exp Parasitol. 1984;58:72–80. doi: 10.1016/0014-4894(84)90022-5. [DOI] [PubMed] [Google Scholar]
- 36.Piazza C, Formaggio F, Crisma M, Toniolo C, Kamphuis J, Kaptein B, Broxterman Q B. Total synthesis and membrane modifying properties of the lipopeptaibol trikoningin KB II and its analogues with acyl chains of different length at the N- and C-termini. J Pept Sci. 1999;5:96–102. doi: 10.1002/(SICI)1099-1387(199902)5:2<96::AID-PSC185>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 37.Piers K L, Hancock R E. The interaction of a recombinant cecropin/melittin hybrid peptide with the outer membrane of Pseudomonas aeruginosa. Mol Microbiol. 1994;12:951–958. doi: 10.1111/j.1365-2958.1994.tb01083.x. [DOI] [PubMed] [Google Scholar]
- 38.Resh M D. Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim Biophys Acta. 1999;1451:1–16. doi: 10.1016/s0167-4889(99)00075-0. [DOI] [PubMed] [Google Scholar]
- 39.Scott M G, Hancock R E. Cationic antimicrobial peptides and their multifunctional role in the immune system. Crit Rev Immunol. 2000;20:407–431. [PubMed] [Google Scholar]
- 40.Shafer W M, Pohl J, Onunka V C, Bangalore N, Travis J. Human lysosomal cathepsin G and granzyme B share a functionally conserved broad spectrum antibacterial peptide. J Biol Chem. 1991;266:112–116. [PubMed] [Google Scholar]
- 41.Shin S Y, Lee M K, Kim K L, Hahm K S. Structure-antitumor and hemolytic activity relationships of synthetic peptides derived from cecropin A-magainin 2 and cecropin A-melittin hybrid peptides. J Pept Res. 1997;50:279–285. doi: 10.1111/j.1399-3011.1997.tb01469.x. [DOI] [PubMed] [Google Scholar]
- 42.Silva P I, Jr, Daffre S, Bulet P. Isolation and characterization of gomesin, an 18-residue cysteine-rich defense peptide from the spider Acanthoscurria gomesiana hemocytes with sequence similarities to horseshoe crab antimicrobial peptides of the tachyplesin family. J Biol Chem. 2000;275:33464–33470. doi: 10.1074/jbc.M001491200. [DOI] [PubMed] [Google Scholar]
- 43.Silvestro L, Gupta K, Weiser J N, Axelsen P H. The concentration-dependent membrane activity of cecropin A. Biochemistry. 1997;36:11452–11460. doi: 10.1021/bi9630826. [DOI] [PubMed] [Google Scholar]
- 44.Skerlavaj B, Romeo D, Gennaro R. Rapid membrane permeabilization and inhibition of vital functions of gram-negative bacteria by bactenecins. Infect Immun. 1990;58:3724–3730. doi: 10.1128/iai.58.11.3724-3730.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thevissen K, Terras F R, Broekaert W F. Permeabilization of fungal membranes by plant defensins inhibits fungal growth. Appl Environ Microbiol. 1999;65:5451–5458. doi: 10.1128/aem.65.12.5451-5458.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tossi A, Sandri L, Giangaspero A. Amphipathic, α-helical antimicrobial peptides. Biopolymers. 2000;55:4–30. doi: 10.1002/1097-0282(2000)55:1<4::AID-BIP30>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 47.Vouldoukis I, Shai Y, Nicolas P, Mor A. Broad spectrum antibiotic activity of the skin-PYY. FEBS Lett. 1996;380:237–240. doi: 10.1016/0014-5793(96)00050-6. [DOI] [PubMed] [Google Scholar]
- 48.Wakabayashi H, Matsumoto H, Hashimoto K, Teraguchi S, Takase M, Hayasawa H. N-Acylated and d-enantiomer derivatives of a nonamer core peptide of lactoferricin B showing improved antimicrobial activity. Antimicrob Agents Chemother. 1999;43:1267–1269. doi: 10.1128/aac.43.5.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wieprecht T, Dathe M, Beyermann M, Krause E, Maloy W L, MacDonald D L, Bienert M. Peptide hydrophobicity controls the activity and selectivity of magainin 2 amide in interaction with membranes. Biochemistry. 1997;36:6124–6132. doi: 10.1021/bi9619987. [DOI] [PubMed] [Google Scholar]
- 50.Wu M, Maier E, Benz R, Hancock R E. Mechanism of interaction of different classes of cationic antimicrobial peptides with planar bilayers and with the cytoplasmic membrane of Escherichia coli. Biochemistry. 1999;38:7235–7242. doi: 10.1021/bi9826299. [DOI] [PubMed] [Google Scholar]
- 51.Yang J T, Chuen-Shang C W, Martínez H M. Calculation of protein conformation from circular dichroism. Methods Enzymol. 1986;130:208–269. doi: 10.1016/0076-6879(86)30013-2. [DOI] [PubMed] [Google Scholar]