ABSTRACT
The high metabolic activity and insufficient perfusion of tumors leads to the acidification of the tumor microenvironment (TME) that may inhibit the antitumor T cell activity. We found that pharmacological inhibition of the acid loader chloride/bicarbonate anion exchanger 2 (Ae2), with 4,4’-diisothiocyanatostilbene-2,2’-disulfonicacid (DIDS) enhancedCD4+ andCD8+ T cell function upon TCR activation in vitro, especially under low pH conditions. In vivo, DIDS administration delayed B16OVA tumor growth in immunocompetent mice as monotherapy or when combined with adoptive T cell transfer of OVA-specificT cells. Notably, genetic Ae2 silencing in OVA-specificT cells improvedCD4+/CD8+ T cell function in vitro as well as their antitumor activity in vivo. Similarly, genetic modification of OVA-specificT cells to overexpress Hvcn1, a selectiveH+ outward current mediator that prevents cell acidification, significantly improved T cell function in vitro, even at low pH conditions. The adoptive transfer of OVA-specificT cells overexpressing Hvcn1 exerted a better antitumor activity in B16OVA tumor-bearingmice. Hvcn1 overexpression also improved the antitumor activity of CAR T cells specific for Glypican 3 (GPC3) in mice bearing PM299L-GPC3tumors. Our results suggest that preventing intracellular acidification by regulating the expression of acidifier ion channels such as Ae2 or alkalinizer channels like Hvcn1 in tumor-specificlymphocytes enhances their antitumor response by making them more resistant to the acidic TME.
KEYWORDS: Lymphocytes, intracellular pH, tumor microenvironment, AE2, HVCN1, adoptive cell therapy
Graphical abstract

Introduction
Adoptive cell transfer (ACT) immunotherapy is one of the most promising advanced therapies for the treatment of cancer. Once adoptively transferred T cells reach the tumor site, the major challenge they face is overcoming the hostile TME and the multiple mechanisms the tumor can elicit to avoid immune-mediatedelimination. Tumors are characterized by having insufficient blood flow, which results in a hypoxic microenvironment and cancer cell metabolic shift from oxidative phosphorylation to glycolysis (Warburgeffect).1,2 The accumulation of glycolytic products such as lactate and protons in the TME causes the acidification of the cancer cell extracellularmilieu.3 Indeed, extracellular pH(pHe)in tumors can be as low as 5.6, although the most common values recorded are between 6.4 and 6.8 compared to healthy tissue, which maintains its pH around7–7.4.4 This acidicpHedirectly inhibitsCD8+ andCD4+ T cell function and T cell mediated antitumor immunity resulting in immuneescape.5,6 Previous studies showed that lowering the environmental pH to 6–6.5 induced a CD8+ T anergic state, which is characterized by impairment of cytotoxic ability, and reduced cytokine production and CD25 expression. This anergic state can be reversed by buffering pH to neutralvalues.7 ExposingCD8+ T cells to lactic acid caused a significant decrease in their proliferation, cytokine production, lytic activity, and tumorinfiltration.5 Furthermore, raising the TME pH by the administration of sodium bicarbonate (NaBi) was shown to enhance antitumor immune responses in mice andhumans.8,9 These data indicate that the negative effect the acidic TME has on infiltrating lymphocytes can be controlled and even reversed by basifying the TME.
Although T cells are highly sensitive to acidic pH, they have mechanisms to resist intracellular pH(pHi)acidification and achieve acid-basehomeostasis. To recover from intracellular acidification or alkalinization, cells are equipped with acid extruders and acid loaders to maintain thepHiwithin a narrow physiological range that is generally ~7.2. Notably, cancer cells exploit several proton pumps and transporters to neutralize metabolic acid loading and achieve higherpHivalues than normal cells, which promotes cell proliferation and evasion of apoptosis, therefore favoring tumorprogression.10,11 Accordingly, physiological pH sensors hold promise as targets in cancertherapeutics.12,13
They include the family of carbonic anhydrases(CAs),14 the vacuolar-typeH+-ATPaseprotonpump,15 anion exchangers such as AE1(SLC4A1)16 and AE2(SLC4A2),17Na+/HCO3− co-transporterssuch asSLC4A4,18 and theNa+/H+ exchanger 1 NHE1(SLC9A1).19 Among the SLC4 family of HCO3 – transporters, theNa+-independentCl–/HCO3 – anion exchanger 2 (AE2, SLC4A2) is considered a master acid loader in many celltypes.20,21 Under physiological conditions, AE2 favors the extrusion of intracellularHCO3− in exchange for extracellularCl−, resulting in an acid load. Previous studies showed that mice carrying a targeted deletion of Ae2(Ae2a,b–/ – mice) have lymphocytes with abnormalpHivalues, which eventually leads to an abnormal state of T cell activation andautoimmunity.22–24 We also found that AE2 inhibition with a synthetic peptide improved effector T cell functions in vitro.25 These data prompted us to investigate the role of the pH acidifier AE2 as a potential target for tumor immunotherapy. On the other hand, the voltage-gatedH+ channel (Hv1) encoded by the Hvcn1 gene is a highly selectiveH+ extruder that avoids cell acidification anddepolarization.26 Hv1 can restore cytoplasmic pH in seconds after heavy acid loads in different cell types including neurons, neutrophils, macrophages, and epithelial cells, amongothers.27–30 Moreover, in breast and colorectal cancers, Hv1 expression was correlated with worse prognosis, and the pharmacological inhibition of Hv1 caused the acidification of the tumor cellpHiand consequently decreased tumor proliferation andmigration.13,31 Hv1 was also shown to be necessary to regulate thepHiof Jurkatcells.12 In this scenario, we hypothesized that overexpression of Hv1 or AE2 silencing in T cells could facilitate the alkalinization of theirpHieven in the acidic TME and accordingly enhance the antitumor activity. These genetic modifications could be of interest in adoptive T cell therapies based on T cell receptor (TCR) transgenic T cells or in chimeric antigen receptor expressing T cells (CAR T cells).
Materials and methods
Cell lines, mice and reagents
B16OVA (ATCC, American Type Culture Collection), LLCOVA (a gift from Dr Daniel Ajona, CIMA, Universidad de Navarra), PM299L (kindly provided by Dr Lujambio, Mount Sinai, NY, USA) were cultured in RPMI medium (Gibco) supplemented with 10% FBS, antibiotics, 2 mM glutamine and 50 μM β-mercaptoethanol.Platinum Ecotropic cells (platE) (ATCC) were cultured in DMEM medium supplemented with 10% FBS and the selection antibiotics puromycin (100 μg/mL) and blasticidin (10 μg/mL).
The PM299L cell line was genetically modified to overexpress human glypican-3 (GPC3). PM299L cells were transduced with a pcDNA3.1 of hGPC3 using Lipofectamine 2000.GPC3+ cells were sorted via FACs using an anti-hGPC3-APCantibody (RD Systems) and maintained in culture with0.5 mg/mL of G418 antibiotic. These hGPC3-expressingPM299L tumor cells are referred as PM299L-GPC3.
Female C57B/l6 mice were purchased from Harlan Laboratories. CD45.1 (B6.SJL-PtprcaPep3b/BoyJ) (CD45.1), LSL-Cas9-GFP(B6;129-Gt(ROSA)26Sortm1(CAG-cas9*-EGFP)Fezh/J) and OT-1 (C57B/l6-Tg[Tcra/Tcrb]110Mjb/J) transgenic mice were purchased from Jackson Laboratories (Bar Harbor, ME). OT-1 mice were crossed with CD45.1 mice at our facility to obtain homozygous CD45.1 mice. OT-1× CD45.1 mice were crossed with LSLCas9GFP to obtain homozygous OT1/LSLCas9GFP mice. All mice were housed under specific pathogen-freeconditions, and the animal protocols were approved by the Ethics Committee of Animal Experimentation at the University of Navarra and The Local Authority for the use of laboratory animals (protocol R-131-16 GN).
DIDS (4,4ʹ-Diisothiocyanostilbene-2,2ʹ-disulfonicAcid) was obtained from SIGMA (309795) and diluted to50 mg/mL or 100 mM in 0.1 MKHCO3.The solution was aliquoted and stored at −20° degrees until experimental use.
Mouse and human T cell purification
MouseCD4+ andCD8+ T cells were obtained from the spleens and lymph nodes of C57Bl/6 mice. To obtain single-cellsuspensions of T cells, organs were first homogenized through a cell strainer in ACK lysis buffer and passed through a magnetic column labeled with negative selection microbeads (Miltenyi) following manufacturers’ instructions. CD8a+ and CD4 + T cell isolation kits (Miltenyi, Refs130–104-075 and130–104-454) were used to isolate mouse T cells. The same procedure was performed to purify OT-1CD8+ T cells from CD45.1 OT-1 mice. Purified lymphocytes were cultured in complete RPMI medium containing 10% FBS.
Human peripheral blood mononuclear cells (PBMCs) were isolated from healthy donor’s buffy coats by a centrifugation gradient in Ficoll-paque(GH Healthcare) and then,CD4+ andCD8+ T cells were purified using negative selection microbeads through a magnetic column. CD8a+ and CD4 + T cell isolation kits (Miltenyi130–096-495, and130–096-533) were used. Human T cells were cultured in Ex Vivo serum free medium (Gibco) containing 10% human serum AB (Sigma). The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Ethics Committee of the Universidad de Navarra (protocol R-131.16 (Ref 2019.162)).
Adjusting the pH of culture media
The pH of RPMI or Ex Vivo media was modified by adding different volumes of HEPES buffer (1 mM) pH 4.5 until the desired pH was reached. While adding HEPES pH 4.5, the pH of the complete media was measured using a pH-meterBasic20+ (CRISON). 6.5, 6.8, 7 and 7.4 pH media was prepared this way.
Retrovirus production and lymphocyte transduction
Ae2 silencing via CRISPR/Cas9: In order to silence the expression of Ae2, a retrovirus expression plasmid pRubiG containing CRE-Thy1.1 was modified to also contain an anti-murineAe2-specificsgRNA obtained using CHOPCHOP software (sgRNA forward: AGACTGGAGGAGCTGAAGACAG, sgRNA reverse: CTGTCTTCAGCTCCTCCAGTCT) or left unmodified as control to infect OT1/LSLCas9GFPCD8+ T cells. sgRNA efficiency at modifying Ae2 expression was measured by INDEL % using TIDE software. TheCD8+ T cells that were modified by the Ae2 sgRNA were sorted based onThy1.1.+ andGFP+ expression and are referred to from now on as Ae2 KO.
Hvcn1 overexpression: In order to overexpress Hvcn1, the mSCV-IRES-Hvcn1-Thy1.1 vector was created using Benchling and synthesized by GeneScript. The mSCV-IRES-Thy1.1 vector was used as a control to infect OT-1CD8+ T cells. Hvcn1-overexpressingor controlCD8+ T cells were sorted based on Thy1.1 expression.
CAR RV generation: We prepared a human GPC3 CAR containing the antihuman GPC3 ScFv (purified from GC33 hybridoma) and the murine 4–1BB-CD3ζendodomains linked through a F2A self-cleavingpeptide sequence to eGFP. This expression cassette was cloned in a pRubiG retroviral vector (produced by GeneScript) to produce GPC3 CAR T cells.CD8+ T cells were co-infectedwith pRubiG expressing GPC3 CAR and mSCV-IRES-Hvcn1-Thy1.1 retroviral vectors.Hvcn1+ GPC3 CAR T cells and GPC3 CAR T cells were purified by FACs sorting based on the expression ofThy1.1+GFP+ orGFP+ alone, respectively.
The PlatE cell line was used for retrovirus production. PlatE packaging cells were transfected with 5 μg of retroviral plasmids in combination with 2.5 μg pCL-Ecoplasmid DNA using Lipofectamine 2000 (ThermoFisher Scientific, MA, USA). Retroviral supernatants were collected at 48 and 72 h after transfection.CD8+ T cells were isolated from OT-1 or OT1/LSLCas9GFP spleens and purified by magnetic negative selection. Then,CD8+ T cells were activated with dynabeads CD3/CD28 at a 1:2 bead:T cell ratio for 24 h, at a 106 cells/mL density in 12-wellplates in RPMI complete media containing 100 IU/mL recombinant human interleukin-2 (rhIL-2). Twenty-four hourslater,CD8+ T cells were collected and resuspended in retroviral supernatants with 100 IU/mL rh IL-2 and 10 μg/mL protamine sulfate (Sigma) and spun at 2000xg at 32°C for 90 min in 12 well plates. Infection was repeated 1 d later using 72-h supernatant. After infection, lymphocytes were cultured in complete RPMI medium with IL-2until day 5 when they were used for functional in vitro or in vivo assays.
Tumor interstitial fluid (TIF) isolation and analysis
B16OVA tumors were harvested in cold saline and washed by placing them on a 10 μm cell strainer over a 50 mL conical tube and centrifuged at 50xg for 5 min. Then, tumors were punctured to break the tumor capsule and spun down at 400xg for 10 min. TIF was transferred to a 1.5 mL Eppendorf tube and spun down at6000 rpm for 10 min before analyzing the pH and lactate levels using a blood gas analyzer (RapidPoint 500, Siemens). Serum obtained from tumor bearing mice was used as control.
Functional activity of engineered lymphocytes
The functional activity of transduced (1) Ae2 KOCD8+ T cells and their corresponding pRubiG control (Ctrl) or (2) Hvcn1-overexpressingand Ctrl Thy1.1CD8+ T cells was measured by in vitro experiments. First, genetically modified OT-1CD8+ T cells were stimulated with SIINFEKL peptide at different concentrations from suboptimal (0.01 ng/ml) to optimal (1 ng/ml) concentrations in complete RPMI medium or RPMI medium at different pH (6.5, 6.8, 7, 7.4) for 48 h. The number of IFNγ or IL-2 producing cells was analyzed by ELISA. Briefly, culture supernatants were collected and diluted 1:10 in Assay Diluent (PBS + 10% FBS) and cultured in triplicate for 2 h in anti-IFNγor anti-IL-2 antibody coated plates. Then, the ELISAS were developed according to manufacturer’s instructions (BD Biosciences). T cells were cultured with SIINFEKL at different pH on anti-INFγcoated ELISPOT plates for 18 h before developing the plates according to the manufacturer’s instructions (BD Biosciences). The number of spot forming cells was automatically counted by an ELISPOT reader (CTL, Germany). Proliferation of genetically modified T cells was measured by 3H-thymidine incorporation (0.5 μCi per well) as previouslydescribed.32
Adoptive T cell transfer
C57Bl/6 mice (6 to 12 weeks of age) were injected with5 × 105 B16-OVAmelanoma cells or with1 × 106 LLCOVA lung cancer cells subcutaneously. Seven days later, mice were sublethally irradiated (total body irradiation) with 500 cGy and received5 × 106CD8+ T cells from OT1/LSLCas9GFP or OT-I mice retrovirally transduced to express pRubiG or AE2 sgRNA or Thy1.1 or Hvcn1-Thy1.1 respectively. ModifiedCD8+ T cells were purified by flow sorting ofGFP+Thy1.1+ cells before ACT experiments. The perpendicular diameters of the tumors were subsequently measured with a caliper. Mice were sacrificed when a tumor diameter reached a value greater than2 cm. Tumor volume was estimated using the formula1/2(LengthxWidth2). For characterization experiments, B16OVA tumor-bearingmice (expressing CD45.2 allele) were treated with genetically modifiedCD8+ T cells(5x106) from OT-IxCD45.1 mice and 7 d later, mice were sacrificed to analyze the presence of transferred cells into the tumors by flow cytometry. There was no exclusion of animals in the analyses.
For CAR T cell immunotherapy experiments, C57BL/6 mice were challenged with1 × 106 PM299L-GPC3cells subcutaneously in 100 μL matrigel (Merck), and 7 d later, when the tumor is5–7 mm in diameter, mice were sublethally irradiated (total body irradiation) with 500 cGy and received immunotherapy with5 × 106 Hvcn1-overexpressingCD8+ T cells, GPC3-CART cells, or Hvcn1-overexpressingGPC3 CAR T cells injected intravenously. Twenty-thousandIU/mouse of rhIL-2 were injected intraperitoneally once a day for 4 d after ACT. Tumor diameters were measured using calipers, and tumor volume was estimated using the formula1/2(LengthxWidth2).
Measurement of SIINFEKL-specificIFNγ producing T cells after ACT
To evaluate the behavior of the genetically modified T cells in vivo, tumor-bearingmice were sacrificed 7 d after T cell infusion, and IFNγ producing T cells were determined by ELISPOT (BD Biosciences) following the manufacturer´s instructions. Briefly, splenocytes(1x105/well) were stimulated with or without 1–10 μg/ml SIINFEKL peptide. After 1 d of culture, the number of spot-formingcells was enumerated with an automated ELISPOT reader (CTL, Aalen, Germany).
Flow cytometry
Excised tumors were digested with 400 U/mL collagenase D and 50 μg/mL DNase-(Roche) for 20 min at 37°C. After washing with PBS, red blood cells were lysed by ACK buffer (Sigma). Spleens were passed through a cell strainer in PBS. For functional analyses, cells were stimulated with the SIINFEKL peptide (1–10 μg/mL) in the presence of GolgiStop and GolgiPlug (BD Biosciences). After 5 h, cells were incubated with Zombie NIR Fixable viability dye (BioLegend). Subsequently, they were stained with fluorochrome-conjugatedmAbs against CD45.1-FITC (A20), CD45.2.-APC(104), CD8-PerCp-Cy5.5. (53–6.7), and CD4-BrilliantViolet 510 (RM4-5), CD25-PeCy7(3C7), CD62L-BrilliantViolet 421 (MEL-14), CD44-PerCp-Cy5.5. (IM7), F4/80- Brilliant Violet 421 (BM8), Ly6C-PerCp-Cy5.5. (HK1.4), CD11b-PeCy7(M1/70), CD11c-PE(N418) (BioLegend) and Ly6G-FITC(1A8) (BD Biosciences) in the presence of purified Fc block anti-CD16/32 mAb to prevent nonspecific binding. For intracellular and intranuclear staining, cells were fixed and permeabilized with the BD Fixation/Perm buffer (BD Biosciences) or the Foxp3 Transcription Factor Staining Buffer Kit (eBioSciences) following the manufacturers’ instructions and then stained with anti-IFNγ-PE(XMG1.2), anti-TNFα-PeCy7(16A8) (BioLegend), anti-GranzymeB-Brilliant Violet 421 (GB11) and anti-FoxP3-FITC(FJK16 F) (Invitrogen). Samples were acquired on a FACSCanto-IIcytometer (BD Biosciences). Data were analyzed using FlowJo software (TreeStar).
Hvcn1 overexpression on theCD8+ T cells’ membrane was detected via flow cytometry using an anti-Hvcn1polyclonal antibody (Invitrogen, PA5-77462) at a 1:25 dilution followed by a goat-anti-rabbit-APCsecondary antibody (Abcam, ab150083) at a 1:2000 dilution.
Intracellular pH measurement
The pHrodo Red AM fluorogenic probe (ThermoFisher) was used for pH measurement. First, human or mouse T cells were plated on 96 well plates at a 100,000 cell/well concentration and washed with HBSS (Gibco). Then, cells were loaded with pHrodo Red AM diluted in a PowerLoad concentrate according to manufacturer’s instructions and incubated at 37° for 30 min. Cells were washed with HBSS and then cultured in different pH media with indicated treatments for 6 h. pH measurements based on pHrodo Ex/Em were taken by flow cytometry in the PE-A channel in a Cytoflex Lx instrument (Beckman Coulter).
ThepHivalues were estimated from calibration curves plotted for each sample by using pHrodo Red AM-loadedT cells incubated in solutions containing 10 μM nigericin and valinomycin at different pH (4.5, 5.5, 6.5, and 7.5).
RNA isolation and quantitative real time polymerase time reaction(qRT-PCR)
Total RNA isolation from Ae2 KOCD8+ andCD4+ and their corresponding control T cells(1x106 cells) that had been activated with anti-CD3/CD28 Dynabeads for 0, 2, 6, or 24 h were carried out using the Maxwell RSC simplyRNA Tissue kit (Promega). After retrotranscription (Invitrogen), the expression of target genes was measured by a real-timePCR reaction using specific primers for Ae2 (Forward: GCTAAGATTTGGCCATGAGC, Reverse: CGGTGGTATTCAAAGTCTTCC), Ae1 (Forward: TTCAGAAACCACACTGGTGC, Reverse: TACAGTCCAGAAAGCTCTCC) and the iQ SYBR Green Supermix (Bio-Rad,Hercules, CA). GAPDH (Forward: TGCACCACCAACTGCTTA, Reverse: GGATGCAGGGATGATGTTC) was used to normalize gene expression. mRNA values were represented by the formula:2ΔCt, where ΔCt indicates the difference in the threshold cycle between GAPDH and target genes.
Statistical analysis
Data are represented as averages ± standard error of the mean (SEM). Student´s t tests were used for statistical comparisons between two groups of normally distributed variables, and one-wayor two-wayANOVA and subsequent Tukey’s post-hoctest were used for comparisons between more than two groups. For tumor growth, data were analyzed using nonlinear third-orderpolynomial (cubic) regression curves. Kaplan–Meier survival curves were evaluated for statistical significance with the Log-rankMantel–Cox test. For all tests, a p value <.05 was considered statistically significant. GraphPad PRISM software was used for statistical analysis.
Results
Pharmacological inhibition of anion exchangers enhances mouse and human T cell function
DIDS is a pharmacological inhibitor of anion exchangers(Ae)33 such as the chloride-bicarbonateexchanger Ae2, the most widely expressed member of the SLC4 family that contributes to the regulation of T cellpHiby extrudingHCO3− and loadingCl− into the cytoplasm in response to alkalinesignals.34 Previous studies showed that treatment of T lymphocytes with DIDS in vitro results in progressive intracellular alkalinization and increased TNFαrelease.35 Thus, we first wanted to evaluate by flow cytometry the alkalinizing effect of DIDS treatment on thepHiof T cells incubated in culture medium buffered at different pH (6.8, 7.0 and 7.4 pH units). To this end, murine C57BL/6CD8+ andCD4+ or OT-1CD8+ T cells were labeled with the pH-sensitivedye pHrodo Red-AMand stimulated with anti-CD3beads or SIINFEKL, respectively, for 6 h in the presence/absence of 50 μM DIDS and analyzed by flow cytometry to estimate thepHias described in the methods section (Supplementary Fig 1A, B, C). As expected, the results obtained from these experiments confirmed the DIDS-mediatedalkalinizing effect on thepHiof T cells. Similar results were obtained on humanCD4+ andCD8+ T cells stimulated with anti-CD3/CD28 beads (Supplementary Fig 1D, E). In all cases, with the exception ofCD4+ T cells cultured at high pH values (ph 7.4), DIDS treatment had a significant alkalinizing effect on thepHiof T cells.
To study the effect of DIDS on the functionality of T cells, mouse and humanCD4+ andCD8+ were activated for 48 h using anti-CD3/CD28 beads in the absence or presence of DIDS. DIDS treatment significantly enhanced IL-2 and IFNγ secretion as well as proliferation of mouse (Figure 1a,b) and human (Figure 1c,d)CD8+ andCD4+ T cells in a dose-dependentmanner.
Figure 1.
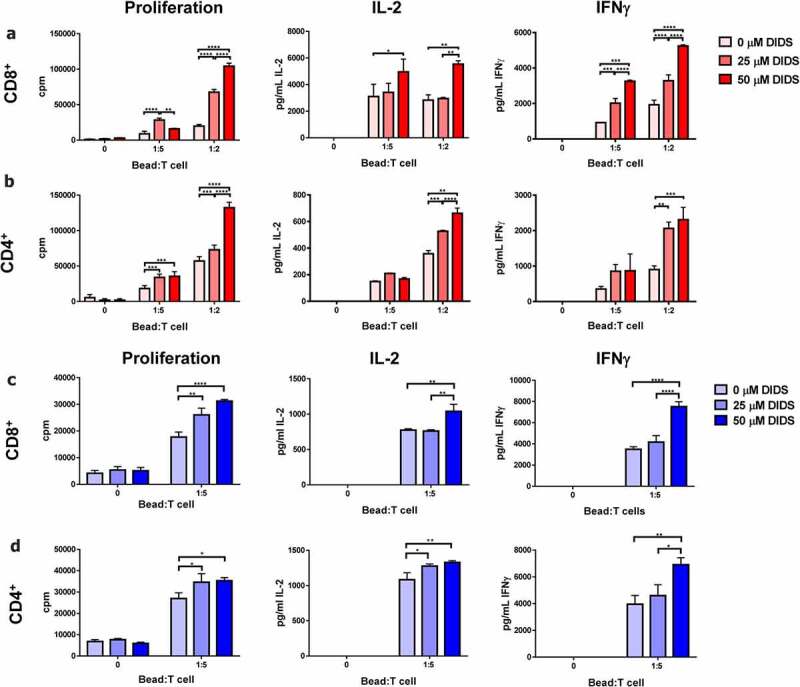
DIDS enhances T cell function. Murine and humanCD4+ andCD8+ T cells purified from splenocytes or healthy donor PBMCs respectively were activated using anti-CD3/CD28 dynabeads and treated with 0, 25, or 50 μM DIDs in unmodified RPMI (pH 7.4). After 48 h, their proliferation, IL-2 and IFNγ secretion were measured. DIDS treatment enhanced proliferation, IL-2 and IFNγ secretion of murine (a)CD4+ and (b)CD8+ T cells, and human (c)CD4+ and D)CD8+ T cells. n = 4. Data represent mean ± SEM. Analyzed using 2-way-ANOVAwith a post hoc Tukey test. **** = p < .0001, *** = p < .001, ** = p < .01, * = p < .05.
SinceCD8+ T cells critically rely on the cell acidifier Ae2 forpHihomeostasis after mitogenicstimulation,23 we decided to investigate if DIDS could improveCD8+ T cell function in physiological and acidic pH conditions. OT-1CD8+ T cells isolated from the spleen of OT-1 mice, and humanCD8+ T cells purified from the peripheral blood of healthy donors were activated with their cognate Ag SIINFEKL peptide or with anti-humanCD3/CD28 beads, respectively, at different pH (from 6.5 to 7.4 units) for 48 h in the presence of DIDS. It was observed that DIDS treatment was able to significantly increase IL-2 and IFNγ secretion of mouse OT-1CD8+ T cells (Figure 2a,b) and humanCD8+ T cells (Figure 2c, d) at all tested pH (6.5, 6.8, 7 and 7,4). Overall, these data indicate that pharmacological inhibition of Ae2 can enhance the function ofCD8+ T cells by regulating their physiologicalpHithrough alkalinization even under acidic pH conditions.
Figure 2.

DIDS enhancesCD8+ T cell function even at acidic pH. (a, b) Mouse OT-1CD8+ T cells and (c, d) humanCD8+ T cells were purified and activated with SIINFEKL cognate Ag or anti-CD3/CD28 dynabeads respectively at different pH (6.5, 6.8, 7, and 7.4) for 48 h. DIDS treatment enhanced the secretion of A, C) IL-2 and B, D) IFNγ ofCD8+ T cells even at acidic pH. n = 4. Data represent mean ± SEM. Analyzed using 2-way-ANOVAwith a post hoc Tukey test. **** = p < 0,0001, *** = p < .001, ** = p < .01, * = p < .05.
Pharmacological inhibition of anion exchangers enhances the endogenous T cell antitumor immune response
After finding that DIDS was able to enhance T cell function in vitro even at low pH, we hypothesized that DIDS treatment might also improve antitumor T cell immune responses by enhancing T cell function in the acidic TME. First, we measured the acidity of the B16OVA TME. Mice were challenged with B16OVA, and then the TIF was extracted, and the pH and lactate concentration were measured in comparison to serum of these mice. B16OVA TIF had a significantly more acidic pH and a higher lactate concentration than the serum of the animals (Figure 3a). In order to test if DIDS can enhance antitumor T cell immune responses in the acidic TME, we challenged mice with B16OVA melanoma cells and treated them daily with an intraperitoneal administration of 100 μM DIDS or PBS alone for 15 d (Figure 3b). We found that tumor growth was significantly delayed in DIDS-treatedmice compared to control mice (treated with PBS) (p < .0001) (Figure 3c,d). A similar trend of delayed tumor growth was observed in mice bearing Lewis lung carcinoma LLCOVA tumors after treatment with DIDS, although in this case statistically significance was not reached (p=.08) (Supplementary Fig 2). Interestingly, the DIDS-inducedtumor growth delay was not observed in immunedeficient mice (NGS) bearing B16OVA or LLCOVA tumors (Supplementary Fig 3), indicating that DIDS treatment specifically triggers an increase of the antitumor efficacy of the immune cell component.
Figure 3.

DIDS treatment enhances the endogenous T cell antitumor immune response. A) pH and lactate concentration in the tumor interstitial fluid (TIF) and serum of B16OVA tumor bearing mice. B) C57B/l6 mice were challenged with0.5 × 106 B16OVA melanoma cells subcutaneously and treated intraperitoneally with 100 μM DIDs or PBS for 15 d. C, D) Tumor growth was delayed in DIDs-treatedmice compared to mice that received PBS. E)At day 15, tumors from DIDs-treatedmice were significantly smaller than those that received PBS. Significantly more F, J)CD8+ and G, K)CD4+ T cells were found infiltrating the tumor and in the draining lymph nodes (DLN) of DIDs-treatedmice compared to control mice. A higher percentage of tumor infiltratingCD8+ T cells from DIDs treated mice expressed H) TNFα after being stimulated ex vivo with 10 μg/mL SIINFEKL for 5 h. I) Higher percentage ofCD4+ TILs expressed IFNγ in DIDs-treatedmice compared to control mice. L) Significantly more SIINFEKL Ag-specificT cells were found in the spleen of mice that received DIDs treatment compared to control mice. n = 6. Data analyzed with student t tests. **p < .01, *p < .05.
To characterize the immunomodulatory effects of DIDS treatment on immunocompetent mice bearing tumors, we studied the phenotype of tumor-infiltratinglymphocytes (TILs)at day 15 after DIDS treatment, when tumor weight was significantly lower in DIDS-treatedmice compared to control mice (Figure 3e). We found significantly moreCD8+ andCD4+ T cells infiltrating the tumor in DIDS-treatedmice compared to control mice (Figure 3f,g) (p < .05). Moreover,CD8+ TILs from DIDS-treatedmice secreted significantly more TNFα (Figure 3h), (p < .05), whereasCD4+ TILs from DIDS-treatedmice secreted significantly more IFNγ than those from control mice (Figure 3i) (p < .05). The number ofCD8+ andCD4+ T cells was also significantly higher in the tumor draining lymph nodes (DLN) of DIDS-treatedmice compared to control mice (Figure 3j,k) (p < .05). Similarly, significantly more IFNγ-producingAg-specificT cells were found in the spleen of DIDS-treatedmice compared to control mice when stimulated with SIINFEKL (Figure 3l), (p < .05). Overall, these data indicate that DIDS treatment enhances the T cell antitumor immune response.
To ensure that DIDS treatment was not directly acting on the tumor cells, we performed a proliferation assay in which different numbers of B16OVA and LLCOVA tumor cells were treated with 100 μM DIDS (Supplementary Fig 4a). Complementary, 10,000 tumor cells were treated with decreasing concentrations of DIDS (from 200 μM to 0.2 μM) (Supplementary Fig 4b). No significant differences of tumor cell proliferation were observed except for a slight decrease in proliferation when B16OVA were treated with 200 μM DIDS.
DIDS treatment enhances the T cell antitumor immune responses of adoptive cell therapy
Next, we wanted to assess if pharmacological inhibition of Aes using DIDS could enhance theCD8+ T cell antitumor immune response during ACT immunotherapy. Thus, mice were challenged with B16OVA melanoma cells and 7 d later, when tumors reached5 mm in diameter, they were given ACT immunotherapy with OT-1CD8+ T cells and subsequently treated intraperitoneally with 100 μM DIDS or PBS for 15 d daily (Figure 4a). DIDS treatment alone slightly delayed tumor growth as expected from previous experiments, but it did not significantly increase overall survival (Figure 4b–d). ACT immunotherapy with OT-1CD8+ T cells delayed tumor growth and increased overall survival, and more importantly, the efficacy of ACT immunotherapy was further enhanced when combined with DIDS treatment (Figure 4b–d). ACT immunotherapy combined with DIDS significantly increased overall survival compared to ACT immunotherapy alone (Figure 4d) (p < .05). Similar results were obtained when mice challenged with LLCOVA tumor cells were treated with ACT alone or a combination ACT immunotherapy and DIDS (Supplementary Fig 2) (p < .01).
Figure 4.

DIDS treatment enhances the T cell antitumor immune response during ACT immunotherapy. A) C57B/l6 mice were challenged with0.5 × 106 B16OVA melanoma cells and 7 d after mice were irradiated and received ACT of5 × 106 OT-1CD8+ T cells intravenously. Groups were treated with 100 μM DIDS or PBS alone intraperitoneally for 15 d after immunotherapy. B, C) DIDS monotherapy delayed tumor growth, and ACT immunotherapy combined with DIDS delayed tumor growth significantly more than immunotherapy alone as well as increased the D) overall survival. A significantly higher number of E) OT-1CD8+ T cells were found infiltrating the tumor of DIDS-treatedmice, these cells were significantly more effector memory (EffM) than those of mice that received ACT alone F). A significantly higher percentage of DIDS-treatedCD8+ OT-1 TILs showed expression of Granzyme B G), and IFNγ and TNFα simultaneously H) when stimulated with 10 μg/mL SIINFEKL ex vivo for 5 h compared to OT-1 T cells from untreated mice. n = 6. Data analyzed with student’s t-test. **p < .01, *p < .05.
The analysis of the tumor infiltratesat day 15 post-DIDStreatment revealed a significant increase in the absolute number of OT-1CD8+ T cells infiltrating the tumor in mice treated with DIDS and ACT combination therapy compared to mice that received ACT alone (Figure 4e). These tumor infiltrating OT-1CD8+ T cells were significantly more differentiated into effector memory (EffM) T cells(CD62L−CD44+) in mice treated with DIDS and ACT compared to mice treated with ACT alone, which had a higher percentage of naïve TILs(CD62L+CD44−) (Figure 4f). Equivalent percentages of central memory (CM) T cells(CD62L+CD44+) were observed in both groups (Figure 4f). Mice treated with the combination of DIDS and ACT had significantly higher percentages of TILs producing Granzyme B (Figure 4g), and IFNγ and TNFα simultaneously (Figure 4h) when activated ex vivo with SIINFEKL compared to TILs from mice that received ACT alone. Gating strategies for these experiments are represented in Supplementary Fig 5a, b. Overall, these data indicate that the pharmacological blockade of the Ae family ofCl−/HCO3− transporters using DIDS enhances the efficacy of ACT immunotherapy by increasing the functional capacities ofCD8+ T cells.
Additionally, we also analyzed the endogenous tumor infiltrate in these mice. The absolute number of endogenous(CD45.2+)CD8+ T cells infiltrating the tumor was significantly higher in mice treated with the combination of DIDS and ACT compared to the ones that received ACT alone (Supplementary Fig 6a). No significant differences were observed in the numbers of tumor infiltratingCD4+ T cells,Tregs(CD4+CD25+FoxP3+), DCs(CD11chigh), tumor associated macrophages(CD11c−CD11b+F480+), or monocytic myeloid-derivedsuppressor cells(CD11c−CD11b+Ly6c+Ly6g−) between groups of mice treated with OT-1 T cells alone or with OT-1 T cells + DIDS (Supplementary Fig 6b, c, D, E). Representative gating strategies for these data are presented in (Supplementary Fig 5c, d). These data indicate that DIDS treatment mainly affects theCD8+ TIL population, enhancing their numbers and antitumor function.
Genetic silencing of Ae2 inCD8+ T cells enhances T cell function in acidic pH and improves their antitumor efficacy in vivo
Pharmacological inhibition of Ae using DIDS-enhancedT cell function and antitumor immunity. While these results were interesting due toCD8+ T cells depending on Ae2 forCl−/HCO3− transport, DIDS is a pan inhibitor of the SLC4 family, and to our knowledge, there is no information on its potential use or toxicity in humans.
Therefore, we aimed to evaluate the impact of silencing Ae2 expression onCD8+ T cell function at different pH levels and antitumor immunity. We first studied the effect of Ae2 silencing on T cell function by using the CRISPR-Cas9technology. We engineered a pRubiG retroviral (RV) vector encoding chimeric single guide RNAs (sgRNA) containing a short sequence homologous to Ae2 exon 4, the recombinase CRE, and Thy1.1 as a reporter protein to evaluate transduction efficiency and to purify transduced cells. The pRubiG RV expressing only CRE and Thy1.1 was used as a control. These vectors were used to transduceCD8+ T cells from CRE-dependentCD45.1. OT1/LSLCas9GFP Cas9 knock-inmice. OT1/LSLCas9GFP mice are genetically modified to constitutively express the endonuclease Cas9 and GFP under the ubiquitous CAG promoter interrupted by a loxP-stop(33 polyA signal)-loxP(LSL) cassette to render Cas9 expression inducible byCRE.36 Using this system, successfully transducedCD8+ T cells were identified and sorted based on Thy1.1 and GFP expression. Three different sgRNAs were tested for their cleavage efficacy. The best sgRNA was #3 with an average 34.6% INDELs, so we used this sgRNA for the characterization experiments. First, we characterized the effect of Ae2 silencing on thepHiof T cells after TCR stimulation in culture medium buffered at different pH. We confirmed that, under all tested pH conditions, Ae2 silencing onCD8+ T cells resulted in a more alkalinepHiafter TCR stimulation (Supplementary Fig 7a) similar to the results obtained with its pharmacological inhibition. Notably, OT-1CD8+ T cells infected with Ae2 sgRNA#3 (AE2 KO) proliferated significantly more and produced significantly more IL-2 and IFNγ (Figure 5a–c) in response to SIINFEKL cognate Ag stimulation even at acidic pH 6.5 compared to control T cells. When Ae2 KOCD8+ T cells were co-culturedwith B16OVA, they were able to lyse tumor cells significantly more than control T cells (Figure 5d). Overall, these data indicate that genetic deletion of Ae2 improvesCD8+ T cell function at acidic pH levels and improves their cytotoxicity makingCD8+ T cells more resistant to acidic conditions.
Figure 5.

AE2 silencing enhancesCD8+ T cell function and antitumor immunity. AE2 KOCD8+ T cells A) proliferated, and secreted significantly more B) IL-2, and C) IFNγ than CtrlCD8+ T cells in response to SIINFEKL cognate Ag stimulation even at acidic pH 6.5. D) AE2 KOCD8+ T cells were able to lyse B16OVA cells significantly more than Ctrl OT-1CD8+ T cells at different effector:tumor ratios. E, F) AE2 KOCD8+ T cells delayed tumor growth significantly more than Ctrl T cells during ACT immunotherapy, and G) improved the overall survival of mice. H) Significantly more Ag-specificT cells were found on the spleen of mice that received AE2 KO T cells than Ctrl T cells. n = 4. Data represent mean ± SEM. Analyzed using 2-way-ANOVAwith a posthoc Tukey test. **** = p < .0001, *** = p < .001, ** = p < .01, * = p < .05.
To ensure that the deletion of Ae2 expression in T cells was not inducing a compensatory mechanism through other AE family members, we measured the mRNA activity of Ae1 in Ctrl and Ae2 KOCD8+ andCD4+ T cells activated overtime. Ae1 expression was not detected onCD8+ T cells as previously reported in Ae2 KOmice.23 InCD4+ T cells, the expression of Ae1 was increased at 2 and 6 h after stimulation, but there were no differences in its expression between Ctrl and Ae2 KO T cells (Supplementary Fig 8).
The effect of Ae2 genetic deletion was then tested in vivo during ACT immunotherapy using Ae2 KO or control OT-1CD8+ T cells against B16OVA. Mice bearing subcutaneous B16OVA tumors were treated with Ae2 KO OT-1CD8+ T cells or with unmodified control OT-1CD8+ T cells. Tumor growth was significantly delayed, and overall survival was improved in mice that received ACT of Ae2 KO OT-1CD8+ T cells compared to control OT-1CD8+ T cells (Figure 5e–g) (p < .0001 and p < .05, respectively). In a parallel experiment,on day 21 after tumor challenge and 14 d after ACT, mice were sacrificed to study the functionality of the transferredCD8+ cells. We found significantly more IFNγ-producingT cells in the spleens of mice that received Ae2 KOCD8+ T cells compared to control T cells in response to SIINFEKL (Figure 5h). Overall, these data indicate that silencing the expression of Ae2 inCD8+ T cells can enhance the efficacy of ACT immunotherapy.
Hvcn1 overexpression inCD8+ T cells enhances their function in acidic pH and improves the efficacy of T cell based ACT immunotherapy
An alternative way to silencing AE2 to avoid lymphocyte acidification could be the overexpression of hydrogen channels to maintain a basic intracellularpHieven in extracellular acid conditions. One of these basifier channels is Hvcn1. In order to overexpress Hvcn1 inCD8+ T cells, we modified the MSCV-IRES-Thy1.1-based RV vector (kindly provided by Dr A. Rao) to express murine Hvcn1 (MSCV-Hvcn1-IRES-Thy1.1). OT-1CD8+ T cells were retrovirally transduced and purified based on Thy1.1 expression. Then, Hvcn1 expression was measured via flow cytometry. It was observed that RV transduced T cells overexpressed Hvcn1 compared to control T cells that received the empty MSCV-IRES-Thy1.1 RV (Supplementary Fig. 9). To evaluate the functionality of the overexpressed Hvcn1 in the T cellpHi,CD8+ T cells transduced with Hvcn1 expressing RV or with the control were stimulated with anti-CD3beads in culture medium buffered at different pH. As occurred for Ae2 silencing, Hcvn1 overexpression onCD8+ T cells allowed a more alkalinepHiafter TCR stimulation (Supplementary Fig 7b). Importantly, Hvcn1 overexpressingCD8+ T cells proliferated more intensely (Figure 6a) and secreted significantly more IL-2 (Figure 6b) and IFNγ as measured by ELISA and ELISPOT (Figure 6c,d) than controlCD8+ T cells in response to the SIINFEKL peptide even at acidic pH 6.5. Indeed, the greatest differences were observed in the most unfavorable pH conditions tested for lymphocyte functionality (pH 6.5), where proliferation, IL-2 and IFN-γ production were significantly higher in the Hvcn1 OT-1CD8+ T cells compared to control T cells. When Hvcn1-overexpressingOT-1CD8+ T cells were co-culturedwith B16OVA, they were able to lyse tumor cells significantly more than control T cells (Figure 6e), indicating significantly higher cytotoxic potential. These data indicate that overexpressing Hvcn1 onCD8+ T cells enhances their function in acidic pH, putatively makingCD8+ T cells more resistant to intratumor acidic conditions.
Figure 6.

Hvcn1 overexpression enhancesCD8+ T cell function and antitumor immunity. Hvcn1 overexpressing OT-1CD8+ T cells A) proliferated, and secreted B) IL-2, and C, D) INFγ significantly more than CtrlCD8+ T cells in response to SIINFEKL cognate Ag even at acidic pH. E) Hvcn1-overexpressingOT-1CD8+ T cells were able to lyse B16OVA melanoma cells significantly better than CtrlCD8+ T cells at different effector:tumor (E:T) cell ratios. C57B/l6 mice were challenged with B16OVA and 7 d after, they were given ACT with5 × 106 Hvcn1-overexpressingor Ctrl OT-1CD8+ T cells intravenously. F, G) Tumor growth was significantly delayed by ACT immunotherapy with Hvcn1-overexpressingCD8+ T cells compared to CtrlCD8+ T cells. H) Overall survival was increased by ACT immunotherapy with Hvcn1-overexpressingOT-1CD8+ T cells compared to CtrlCD8+ T cells even though significance was not achieved. Significantly more Ag-specificT cells were found I) infiltrating the tumor and J) in the spleens of mice that received ACT with Hvcn1-overexpressingOT-1CD8+ compared to Ctrl T cells. n = 4. Data represent mean ± SEM. Analyzed using 2-way-ANOVAwith Tukey’s post hoc. **** = p < .0001, *** = p < .001, ** = p < .01, * = p < .05. In vivo data show two experiments combined, n = 16.
Next, we evaluated the effect of Hvcn1 overexpression onCD8+ T cells in vivo during ACT immunotherapy using Hvcn1-overexpressingor control OT-1CD8+ T cells in mice bearing B16OVA tumors. As was the case with Ae2 KOCD8+ OT-1 T cells, tumor growth was significantly delayed, and overall survival was slightly improved (two mice were cured, 12.5%) in mice treated with Hvcn1-CD8+OT-1 T cells, when compared to mice receiving control OT-1CD8+ T cells (Figure 6f-h). In addition, a significantly higher number of OT-1CD8+ T cells were found infiltrating the tumor of mice treated with Hvcn1-CD8+OT-1 T cells compared to control Ot-1CD8+ T cells (Figure 6i). We found significantly more IFNγ-producingT cells in the spleens of mice that received Hvcn1-overexpressingCD8+ T cells compared to control T cells in response to SIINFEKL (Figure 6j). Overall, these data indicate that increasing the expression of theH+ channel Hvcn1 onCD8+ T cells can enhance the efficacy of ACT immunotherapy and the antitumor function of transferred T cells.
Hvcn1 overexpression enhances the efficacy of CAR T cell immunotherapy
To study if Hvcn1 overexpression can also improve the efficacy of CAR T cell-basedtherapy, we combined these strategies by overexpressing Hvcn1 in CAR T cells against human GPC3. The antitumor efficacy of the GPC3 CAR T cells was tested in mice bearing PM299L-GPC3-tumors.Thus, mice were treated with5 × 106Hvcn1+ GPC3 CAR T cells or GPC3 CAR T cells purified by FACs sorting as described in methods.Hvcn1+ T cells were also used as control. GPC3 CAR T cells that overexpressed Hvcn1 delayed tumor growth significantly more efficiently than control GPC3 CAR T cells increasing overall survival (Figure 7a,b). These data indicate that overexpression of Hvcn1 can enhance CAR T cell immunotherapy.
Figure 7.

Hvcn1 overexpression improves the efficacy of CAR T cell immunotherapy. C57BL/6 mice were challenged subcutaneously with1 × 106 PM299L-GPC3embedded in matrigel and 7 d later they were irradiated and treated with5 × 106CD8+ T cells overexpressing Hvcn1, GPC3 CAR T cells or Hvcn1 overexpressing GPC3 CAR T cells. A) Tumor growth and B) survival were measured overtime. n = 6–8. **** = p < .0001.
Discussion
Tumor acidity is one of the key driving forces that renders the TME an immunosuppressive milieu for antitumor immune cells. The large amounts of protons and lactate produced by tumors as a consequence of anaerobic glycolysis lead to extracellular acidification (pH6.2–6.8) which has a suppressive effect on T cellfunction.5,6 Adoptive cell therapies based on ex vivo expanded tumor-infiltratinglymphocytes and genetically modified T cells to express tumor-specificchimeric receptors can be affected by this hostile microenvironment. As other groups have previouslydescribed,37,38 we found that a pH as low as 6.5 led to impaired proliferation and production of IFNγ and IL-2 in response to TCR stimulation in both murine and humanCD4+ andCD8+ T cells. These differences are even more pronounced when the strength of TCR stimulation is suboptimal (0.01–0.1 ng/ml of SIINFEKL peptide for murine OT-1CD8+ T cells, and an antiCD3/CD28 beads 1:10 ratio), suggesting that acidity might raise the activation threshold in T cells. The acidic pH-inducedimpairment of T cell function was significantly overcome by DIDS, a pharmacological inhibitor of theCl−/HCO3− exchangers (AEs). The alkalinizing effect of DIDS on T cellpHimight explain its capacity to improve T cell proliferation and function even at low pH conditions.
Despite the lack of data on the use of DIDS in vivo and regardless of the fact that its targets, the AEs, are widely expressed in many cell types, we assessed its potential immune-enhancingeffect in tumor-bearingmice. We previously confirmed that DIDS had no effect on the growth of tumor cell lines B16OVA or LLCOVA in vitro. However, administration of DIDS to tumor-bearingmice resulted in a significant delay in tumor growth. This in vivo effect was not observed when the tumor was implanted in NSG mice, indicating that the impact of DIDS on the immune system was relevant for the antitumor effect. DIDS treatment enhanced the endogenous T cell antitumor immune response, with a significant increase in the numbers of tumor-specificCD8+ andCD4+ T cells infiltrating the tumor and present in the tumor draining lymph nodes and the spleen. This beneficial effect of DIDS was also observed in combination with adoptive T cell transfer immunotherapy of antitumorCD8+ T cells. Mice treated with DIDS after ACT with OT-1CD8+ T cells showed a significant delay in tumor growth, and an increase in the overall survival of mice bearing B16OVA or LLCOVA tumors. This improvement in survival was concomitant to an increase in the number ofCD8+ T cells expressing granzyme B, IFNγ and TNFα suggesting that intratumoral pH acidification of transferred T cells impairs their antitumor activity. These results are in line with recent reports showing that neutralizing tumor acidity with bicarbonate monotherapy or in combination with other immunotherapies improved antitumor responses in murinemodels.8 There are other alternative pharmacologic approaches that could indirectly lead to the control of intracellular acidification. Potassium-sparingdiuretics (amiloride) for metabolic alkalosiscompensation,39 and the use of inhibitors for carbonic anhydraseIX40 or lactate dehydrogenase(LDH)41 are being considered to improve antitumor therapies. However, the efficacy of these agents to neutralize tumor pH or their effects on antitumor immunotherapy is not known. Other pathways associated with the control ofpHithat are also under consideration for intervention include the use of inhibitors of monocarboxylate transporters (MCT) (reviewedin42), the vacuolar-typeH+-ATPaseprotonpump,15 the sodium-hydrogenexchanger 1(NHE-1)43 orAe2.25 In preliminary experiments, we studiedCD8+ T cell function in the presence of MCT inhibitors (AR-C155858,α-cyano-4-hydroxycinnamic acid, AZD3965 and diclofenac sodium salt) that impair lactate transport. We could observe an improvement on T cell proliferation in the presence of diclofenac sodium salt (at 0.01 and 0.1 mM) after TCR stimulation, but not with the other inhibitors (not shown). A recent report showed that diclofenac lowers lactate secretion of tumor cells and improves anti-PD1-inducedT cell killing in vitro delaying tumor growth in vivo.44 However, other works have reported an inhibition of IFN-γ production and T cell proliferation in the presence of diclofenac during T cellactivation.45 It has been also reported that sodium lactate, can either enhance or impair T cell proliferation depending on thedose,46 suggesting a complex role for lactate during T cell activation. Further studies are needed to determine the role of MCT inhibitors in lactate transport across the membrane, thepHiand consequently on T cell function.
Moreover, the use of inhibitors for enzymes, ion channels and anion exchangers that are ubiquitously expressed in different cell types may pose toxicity problems that must be carefully considered. For these reasons, we planned to directly genetically modify lymphocytes to provide them with the tools to resist TME acidification and preserve their antitumor activity while minimizing the risk for treatment-relatedtoxicities.
Based on the beneficial effect of DIDS treatment in vivo, we focused on the Ae2 anion exchanger. Previous studies reported that Ae2-deficientmice exhibit marked splenomegaly, expansion of theCD8+ T cell compartment, and an increase in the production of cytokines, which favor the development ofautoimmunity.24 It was also reported that Ae2 deficiency favors the alkalinization ofCD8+ T cells after TCR stimulation enhancing cell proliferation and IL-2production.23 Although those mice were whole-bodyknockouts for Ae2, these data highlight the relevance of Ae2 forCD8+ T cells to maintainpHihomeostasis and modulate immune responses. These experiments prompted us to evaluate the effect of Ae2 silencing on the antitumor activity of Ag-specificCD8+ T cells. Using CRISPR/Cas9 technology we generated OT-1CD8+ T cells lacking Ae2. As observed in vitro with DIDS treatment, Ae2-deficientOT-1CD8+ T cells proliferated better than Ae2 proficient T cells and produced more IL-2 and IFNγ upon TCR stimulation with the SIINFEKL peptide. This improvement was observed at all pH levels tested, including the acidic pH 6.5 condition. When injected into tumor-bearingmice, Ae2-silencedOT-1CD8+ T cells delayed tumor growth more efficiently than Ae2-expressingT cells. When we isolated these cells from the spleen of tumor-bearingmice, we found higher numbers of SIINFEKL-specificT cells in mice treated with OT-1CD8+ T cells lacking Ae2. These results may indicate that Ae2 silencing makesCD8+ T cells more resistant to acidic conditions.
As an alternative to the silencing of the acidifier anion exchangers, we also tested the possibility of maintaining a more basicpHiby overexpressing proton extruder channels. Proton pump inhibitors (PPI) have been used to reduce proton extrusion by tumor cells and smooth the acidification of TME while inducing tumor cell apoptosis and inhibition of tumorgrowth.47–51 These studies elegantly described the effects of inhibiting acid extrusion in tumor cells but did not characterize the effects of PPIs on tumor infiltrating lymphocytes except formacrophages.50 These PPIs might involve the risk of inducing T cell apoptosis as was described for the inhibition of HVCN1 in Jurkat T cells.12 On the other hand, there are serious concerns about the increased cancer risk associated with the long-termuse of PPIs (reviewedin52). In our study, we considered a different alternative to protect the T lymphocytes from excessive intracellular acidification in the TME by exploiting the basifying effect that these proton pumps can exert. For this reason, we genetically modified T cells to overexpress Hvcn1. We found that overexpression of Hvcn1 in lymphocytes increases their proliferative and cytokine-producingcapacities even under acidic pH culture conditions. Notably, we observed that at pH as low as 6.5, where unmodified OT-1CD8+ T lymphocytes do not respond appropriately to antigenic stimulation with the SIINFEKL peptide, Hvcn1-overexpressingOT-1CD8+ T cells have a high functional activity, similar to that observed at physiological pH for unmodified T lymphocytes. These results suggest that Hvcn1 protects the cells from excessive acidification facilitating the TCR signaling that, as was previously described, is impaired by the acidic pH leading to an anergicstate.7 When mice bearing B16OVA tumors were treated with these Hcvn1-expressingCD8+ OT-1 T cells, we found a significant delay in tumor growth, although only 12.5% of mice were able to totally reject the tumor and survive. This is particularly relevant in a model in which adoptive T cell transfer experiments are not very efficient because OVA antigen expression is rapidly lost due to immunepressure.53,54 Interestingly, Hvcn1 overexpression improved the antitumor efficacy of GPC3 CAR-T cells in a murine model of hepatocellular carcinoma, suggesting that the control of excessive pHi acidification can also have a positive effect in CART cell-basedtherapies for solid tumors.
In conclusion, we have found that silencing of the acidifier Ae2 or the overexpression of the proton channel Hvcn1 may offer an advantage for lymphocytes thus improving their functionality in unfavorable acidic pH conditions present in the TME and might constitute an alternative for the improvement of cancer therapies based in the adoptive cell transfer of T lymphocytes.
Supplementary Material
Acknowledgments
We thank Elena Ciordia and Eneko Elizalde for excellent animal care, Uxua Mancheño for generating OT1/LSLCas9GFP mice and Dr Diego Alignani for his support in flow cytometry analyses. We also thank the Blood Bank of Navarra (Biobanco, IDISNA) for their collaboration.
Funding Statement
The work was supported by grants from Ministerio de Educación y Ciencia (SAF2016-78568-R to J.J.L. RTC-2017.6578-1 (Project AutoCAR), Ministerio de Ciencia e Innovación (PID2019-108989RB-I00, PLEC2021-008094 MCIN/AEI /10.13039/501100011033 (proyecto CARPanTu) and the European Union NextGenerationEU/PRTR), the European Union's Horizon 2020 research and innovation programme (Ner 945393, T2EVOLVE), Gobierno de Navarra (0011-1411-2019-000079 and 0011-1411-2019-000072 (Proyecto DESCARTHeS), Fundación Ramón Areces (to S.HS. and J.J.L) and Paula & Rodger Riney Foundation. TL is recipient of a Juan de la Cierva grant (IJCI-2017-34204).
Author contributions statement
Conceptualization, F.N, T.L. and J.J.L.; Methodology, F.N, N.C, C.M-O., T.L., M.G., P.S., D.LL., D.R., N.V., J.R, R-M. and J.J.L.; Investigation: F.N, N.C., T.L. and J.J.L; Writing – Original Draft, F.N. and J.J.L.; Writing –Review & Editing, all authors; Funding Acquisition, T.L., F.P, and J.J.L; Supervision, T.L. and J.J.L.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author [F.N and J.J.L.], upon reasonable request.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
References
- 1.Vaupel P, Hockel M.. Blood supply, oxygenation status and metabolic micromilieu of breast cancers: characterization and therapeuticrelevance. Int J Oncol. 2000;17(5):869–16. doi: 10.3892/ijo.17.5.869. [DOI] [PubMed] [Google Scholar]
- 2.Vaupel P. Physiological properties of malignanttumours. NMR Biomed. 1992;5(5):220–225. doi: 10.1002/nbm.1940050505. [DOI] [PubMed] [Google Scholar]
- 3.Gatenby RA, Gillies RJ. Why do cancers have high aerobicglycolysis? Nat Rev Cancer. 2004;4(11):891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 4.Griffiths JR. Are cancer cellsacidic? Br J Cancer. 1991;64(3):425–427. doi: 10.1038/bjc.1991.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, Gottfried E, Schwarz S, Rothe G, Hoves S, et al. Inhibitory effect of tumorcell-derived lactic acid on human Tcells. Blood. 2007;109(9):3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 6.Mendler AN, Hu B, Prinz PU, Kreutz M, Gottfried E, Noessner E. Tumor lactic acidosis suppresses CTL function by inhibition of p38 andJNK/c-Junactivation. Int J Cancer. 2012;131(3):633–640. doi: 10.1002/ijc.26410. [DOI] [PubMed] [Google Scholar]
- 7.Calcinotto A, Filipazzi P, Grioni M, Iero M, De Milito A, Ricupito A, Cova A, Canese R, Jachetti E, Rossetti M, et al. Modulation of microenvironment acidity reverses anergy in human and murinetumor-infiltrating Tlymphocytes. Cancer Res. 2012;72(11):2746–2756. doi: 10.1158/0008-5472.CAN-11-1272. [DOI] [PubMed] [Google Scholar]
- 8.Pilon-Thomas S, Kodumudi KN, El-Kenawi AE, Russell S, Weber AM, Luddy K, Damaghi M, Wojtkowiak JW, Mulé JJ, Ibrahim-Hashim A, et al. Neutralization of tumor acidity improves antitumor responses toimmunotherapy. Cancer Res. 2016;76(6):1381–1390. doi: 10.1158/0008-5472.CAN-15-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uhl FM, Chen S, O’Sullivan D, Edwards-Hicks J, Richter G, Haring E, Andrieux G, Halbach S, Apostolova P, Büscher J, et al. Metabolic reprogramming of donor T cells enhances graft-versus-leukGonzalezLeónemia effects in mice and humans. Sci Transl Med. 2020;12(567). doi: 10.1126/scitranslmed.abb8969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parks SK, Chiche J, Pouyssegur J. pH control mechanisms of tumor survival andgrowth. J Cell Physiol. 2011;226(2):299–308. doi: 10.1002/jcp.22400. [DOI] [PubMed] [Google Scholar]
- 11.Webb BA, Chimenti M, Jacobson MP, Barber DL. Dysregulated pH: a perfect storm for cancerprogression. Nat Rev Cancer. 2011;11(9):671–677. doi: 10.1038/nrc3110. [DOI] [PubMed] [Google Scholar]
- 12.Asuaje A, Smaldini P, Martin P, Enrique N, Orlowski A, Aiello EA, Docena G, Milesi V. The inhibition ofvoltage-gated H+ channel (HVCN1) induces acidification of leukemic Jurkat T cells promoting cell death byapoptosis. Pflugers Arch. 2017;469(2):251–261. doi: 10.1007/s00424-016-1928-0. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Wu X, Li Q, Zhang S, Li SJ. Humanvoltage-gated proton channel hv1: a new potential biomarker for diagnosis and prognosis of colorectalcancer. PLoS One. 2013;8(8):e70550. doi: 10.1371/journal.pone.0070550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mboge MY, Mahon BP, McKenna R, Frost S. Carbonic anhydrases: role in ph control andcancer. Metabolites. 2018;8(1):19. doi: 10.3390/metabo8010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Milito A, Canese R, Marino ML, Borghi M, Iero M, Villa A, Venturi G, Lozupone F, Iessi E, Logozzi M, et al. pH-dependentantitumor activity of proton pump inhibitors against human melanoma is mediated by inhibition of tumoracidity. Int J Cancer. 2010;127(1):207–219. doi: 10.1002/ijc.25009. [DOI] [PubMed] [Google Scholar]
- 16.Shiozaki A, Kudou M, Ichikawa D, Shimizu H, Arita T, Kosuga T, Konishi H, Komatsu S, Fujiwara H, Okamoto K, et al. Expression and role of anion exchanger 1 in esophageal squamous cellcarcinoma. Oncotarget. 2017;8(11):17921–17935. doi: 10.18632/oncotarget.14900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang LJ, Lu R, Song YN, Zhu J-Y, Xia W, Zhang M, Shao Z-Y, Huang Y, Zhou Y, Zhang H, et al. Knockdown of anion exchanger 2 suppressed the growth of ovarian cancer cells via mTOR/p70S6K1signaling. Sci Rep. 2017;7(1):6362. doi: 10.1038/s41598-017-06472-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parks SK, Pouyssegur J. TheNa(+)/HCO3(-)co-transporter SLC4A4 plays a role in growth and migration of colon and breast cancercells. J Cell Physiol. 2015;230(8):1954–1963. doi: 10.1002/jcp.24930. [DOI] [PubMed] [Google Scholar]
- 19.Amith SR, Fliegel L. Regulation of the Na+/H+ exchanger (NHE1) in breast cancermetastasis. Cancer Res. 2013;73(4):1259–1264. doi: 10.1158/0008-5472.CAN-12-4031. [DOI] [PubMed] [Google Scholar]
- 20.Alper SL. Molecular physiology of SLC4 anionexchangers. Exp Physiol. 2006;91(1):153–161. doi: 10.1113/expphysiol.2005.031765. [DOI] [PubMed] [Google Scholar]
- 21.Romero MF. Molecular pathophysiology of SLC4 bicarbonatetransporters. Curr Opin Nephrol Hypertens. 2005;14(5):495–501. doi: 10.1097/01.mnh.0000168333.01831.2c. [DOI] [PubMed] [Google Scholar]
- 22.Concepcion AR, Salas JT, Saez E, Sarvide S, Ferrer A, Portu A, Uriarte I, Hervás-Stubbs S, Oude Elferink RPJ, Prieto J, et al. CD8+ T cells undergo activation and programmeddeath-1 repression in the liver of agedAe2a,b-/- mice favoring autoimmunecholangitis. Oncotarget. 2015;6(30):28588–28606. doi: 10.18632/oncotarget.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Concepcion AR, Salas JT, Sarvide S, Sáez E, Ferrer A, López M, Portu A, Banales JM, Hervás-Stubbs S, Oude Elferink RPJ, et al. Anion exchanger 2 is critical for CD8 + T cells to maintain pH I homeostasis and modulate immune responses. Eur J Immunol. 2014;44(5):1341–1351. doi: 10.1002/eji.201344218. [DOI] [PubMed] [Google Scholar]
- 24.Salas JT, Banales JM, Sarvide S, Recalde S, Ferrer A, Uriarte I, Oude Elferink RPJ, Prieto J, Medina JF. Ae2a,b-deficient mice develop antimitochondrial antibodies and other features resembling primary biliarycirrhosis. Gastroenterology. 2008;134(5):1482–1493. doi: 10.1053/j.gastro.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 25.Celay J, Lozano T, Concepcion AR, Beltrán E, Rudilla F, García-Barchino MJ, Robles EF, Rabal O, de Miguel I, Panizo C, et al. Targeting the anion exchanger 2 with specific peptides as a new therapeutic approach in B lymphoidneoplasms. Haematologica. 2018;103(6):1065–1072. doi: 10.3324/haematol.2017.175687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Capasso M, DeCoursey TE, Dyer MJ. pH regulation and beyond: unanticipated functions for thevoltage-gated proton channel,HVCN1. Trends Cell Biol. 2011;21(1):20–28. doi: 10.1016/j.tcb.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seredenina T, Demaurex N, Krause KH. Voltage-gatedproton channels as novel drug targets: from NADPH oxidase regulation to spermbiology. Antioxid Redox Signal. 2015;23(5):490–513. doi: 10.1089/ars.2013.5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeCoursey TE, Cherny VV. Potential, pH, and arachidonate gate hydrogen ion currents in humanneutrophils. Biophys J. 1993;65(4):1590–1598. doi: 10.1016/S0006-3495(93)81198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapus A, Romanek R, Grinstein S. Arachidonic acid stimulates the plasma membrane H+ conductance ofmacrophages. J Biol Chem. 1994;269(7):4736–4745. doi: 10.1016/S0021-9258(17)37606-8. [DOI] [PubMed] [Google Scholar]
- 30.Murphy R, Cherny VV, Morgan D, DeCoursey TE. Voltage-gatedproton channels help regulate pHi in rat alveolarepithelium. Am J Physiol Lung Cell Mol Physiol. 2005;288(2):L398–408. doi: 10.1152/ajplung.00299.2004. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Li SJ, Wu X, Che Y, Li Q. Clinicopathological and biological significance of humanvoltage-gated proton channel Hv1 protein overexpression in breastcancer. J Biol Chem. 2012;287(17):13877–13888. doi: 10.1074/jbc.M112.345280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casares N, Rudilla F, Arribillaga L, Llopiz D, Riezu-Boj JI, Lozano T, López-Sagaseta J, Guembe L, Sarobe P, Prieto J, et al. A peptide inhibitor of FOXP3 impairs regulatory T cell activity and improves vaccine efficacy inmice. J Immunol. 2010;185(9):5150–5159. doi: 10.4049/jimmunol.1001114. [DOI] [PubMed] [Google Scholar]
- 33.Jessen F, Sjoholm C, Hoffmann EK. Identification of the anion exchange protein of Ehrlich cells: a kinetic analysis of the inhibitory effects of 4,4’-diisothiocyano-2,2’-stilbene-disulfonicacid (DIDS) and labeling of membrane proteins with 3H-DIDS.J Membr Biol. 1986;92(3):195–205. doi: 10.1007/BF01869388. [DOI] [PubMed] [Google Scholar]
- 34.Romero MF, Chen AP, Parker MD, Boron WF. The SLC4 family of bicarbonate (HCO₃⁻) transporters. Mol Aspects Med. 2013;34(2–3):159–182. doi: 10.1016/j.mam.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qian C, Diez J, Larrea E, Garciandia A, Arrazola A, Civeira MP, Medina JF, et al. The stilbene disulfonic acid DIDS stimulates the production ofTNF-alpha in humanlymphocytes. Biochem Biophys Res Commun. 1992;189(3):1268–1274. doi: 10.1016/0006-291x(92)90210-c. [DOI] [PubMed] [Google Scholar]
- 36.Platt RJ, Chen S, Zhou Y, Yim M, Swiech L, Kempton H, Dahlman J, Parnas O, Eisenhaure T, Jovanovic M, et al. CRISPR-Cas9knockin mice for genome editing and cancermodeling. Cell. 2014;159(2):440–455. doi: 10.1016/j.cell.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bosticardo M, Ariotti S, Losana G, Bernabei P, Forni G, Novelli F. Biased activation of human T lymphocytes due to low extracellular pH is antagonized by B7/CD28costimulation. Eur J Immunol. 2001;31(9):2829–2838. doi:. [DOI] [PubMed] [Google Scholar]
- 38.Carswell KS, Papoutsakis ET. Extracellular pH affects the proliferation of cultured human T cells and their expression of theinterleukin-2receptor. J Immunother. 2000;23(6):669–674. doi: 10.1097/00002371-200011000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Poon AC, Inkol JM, Luu AK, Mutsaers AJ. Effects of thepotassium-sparing diuretic amiloride on chemotherapy response in canine osteosarcomacells. J Vet Intern Med. 2019;33(2):800–811. doi: 10.1111/jvim.15382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Kuijk SJ, Gieling RG, Niemans R, Lieuwes NG, Biemans R, Telfer BA, Haenen GRMM, Yaromina A, Lambin P, Dubois LJ, et al. The sulfamate small molecule CAIX inhibitor S4 modulates doxorubicinefficacy. PLoS One. 2016;11(8):e0161040. doi: 10.1371/journal.pone.0161040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oshima N, Ishida R, Kishimoto S, Beebe K, Brender JR, Yamamoto K, Urban D, Rai G, Johnson MS, Benavides G, et al. Dynamic Imaging of LDH inhibition in tumors reveals rapid in vivo metabolic rewiring and vulnerability to combination therapy. Cell Rep. 2020;30(6):1798–1810 e4. doi: 10.1016/j.celrep.2020.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Payen VL, Mina E, Van Hee VF, Porporato PE, Sonveaux P. Monocarboxylate transporters incancer. Mol Metab. 2020;33:48–66. doi: 10.1016/j.molmet.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guan X, Hasan MN, Begum G, Kohanbash G, Carney KE, Pigott VM, Persson AI, Castro MG, Jia W, Sun D, et al. Blockade of Na/H exchanger stimulates glioma tumor immunogenicity and enhances combinatorial TMZ andanti-PD-1therapy. Cell Death Dis. 2018;9(10):1010. doi: 10.1038/s41419-018-1062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Renner K, Bruss C, Schnell A, Koehl G, Becker HM, Fante M, Menevse A-N, Kauer N, Blazquez R, Hacker L, et al. Restricting glycolysis preserves T cell effector functions and augments checkpointtherapy. Cell Rep. 2019;29(1):135–150 e9. doi: 10.1016/j.celrep.2019.08.068. [DOI] [PubMed] [Google Scholar]
- 45.Chirasani SR, Leukel Q P, Gottfried E, Hochrein J, Stadler K, Neumann B, Oefner PJ, Gronwald W, Bogdahn U, Hau P, et al. Diclofenac inhibits lactate formation and efficiently counteracts local immune suppression in a murine gliomamodel. Int J Cancer. 2013;132(4):843–853. doi: 10.1002/ijc.27712. [DOI] [PubMed] [Google Scholar]
- 46.Ostroukhova M, Goplen N, Karim MZ, Michalec L, Guo L, Liang, Alam R. The role oflow-level lactate production in airway inflammation inasthma. Am J Physiol Lung Cell Mol Physiol. 2012;302(3):L300–7. doi: 10.1152/ajplung.00221.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen M, Zou X, Luo H, Cao J, Zhang X, Zhang B, Liu W. Effects and mechanisms of proton pump inhibitors as a novel chemosensitizer on human gastric adenocarcinoma (SGC7901)cells. Cell Biol Int. 2009;33(9):1008–1019. doi: 10.1016/j.cellbi.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 48.Kim YJ, Lee JS, Hong KS, Chung JW, Kim JH, Hahm KB. Novel application of proton pump inhibitor for the prevention ofcolitis-induced colorectal carcinogenesis beyond acidsuppression. Cancer Prev Res (Phila). 2010;3(8):963–974. doi: 10.1158/1940-6207.CAPR-10-0033. [DOI] [PubMed] [Google Scholar]
- 49.Morimura T, Fujita K, Akita M, Nagashima M, Satomi A. The proton pump inhibitor inhibits cell growth and induces apoptosis in humanhepatoblastoma. Pediatr Surg Int. 2008;24(10):1087–1094. doi: 10.1007/s00383-008-2229-2. [DOI] [PubMed] [Google Scholar]
- 50.Vishvakarma NK, Singh SM. Immunopotentiating effect of proton pump inhibitor pantoprazole in alymphoma-bearing murine host: implication in antitumor activation oftumor-associatedmacrophages. Immunol Lett. 2010;134(1):83–92. doi: 10.1016/j.imlet.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 51.Yeo M, Kim DK, Kim YB, Oh TY, Lee JE, Cho SW, Kim HC, et al. Selective induction of apoptosis with proton pump inhibitor in gastric cancercells. Clin Cancer Res. 2004;10(24):8687–8696. doi: 10.1158/1078-0432.CCR-04-1065. [DOI] [PubMed] [Google Scholar]
- 52.Suissa S, Suissa A. Proton-pumpinhibitors and increased gastric cancer risk:time-relatedbiases. Gut. 2018;67(12):2228–2229. doi: 10.1136/gutjnl-2017-315729. [DOI] [PubMed] [Google Scholar]
- 53.Goldberger O, Volovitz I, Machlenkin A, Vadai E, Tzehoval E, Eisenbach L. Exuberated numbers oftumor-specific T cells result in tumorescape. Cancer Res. 2008;68(9):3450–3457. doi: 10.1158/0008-5472.CAN-07-5006. [DOI] [PubMed] [Google Scholar]
- 54.Lozano T, Chocarro S, Martin C, Lasarte-Cia A, Del Valle C, Gorraiz M, Sarrion P, et al. Genetic modification of CD8(+) T cells to express EGFR: potential application for adoptive T celltherapies. Front Immunol. 2019;10:2990. doi: 10.3389/fimmu.2019.02990. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author [F.N and J.J.L.], upon reasonable request.


