ABSTRACT
Glioma-associated oncogene (Gli) antagonist-61 (GANT61) not only suppresses the malignant behavior of several cancers but also presents synergistic effects with other anticancer agents on suppressing the progression of cancers, while relevant information is rare in anaplastic thyroid carcinoma (ATC). This study aimed to explore the therapeutic effect of GANT61 in ATC and its molecular mechanism. ATC cells (8505C and CAL-62) were treated with GANT61, followed by detection of cell proliferation, apoptosis, invasion and epithelial-mesenchymal transition (EMT) markers. Subsequently, RNA sequencing was performed to explore the potential downstream pathway. Following that, rescue experiments were conducted by SC79 (AKT activator) or colivelin (STAT3 activator) monotreatment or combined with GANT61 in ATC cells. GANT61 reduced Gli1 expression, suppressed proliferation at several time settings, promoted apoptosis, inhibited invasion and increased E-cadherin while decreased Vimentin and Snail expressions (EMT markers) in ATC cells. The subsequent RNA sequence identified 85 upregulated differentially expressed genes (DEGs) and 71 downregulated DEGs in GANT61-treated ATC cells, which were mainly enriched in PI3K/AKT, JAK/STAT, Hedgehog and mTOR pathways. Next, the inactivation of AKT/mTOR and JAK/STAT3 pathways by GANT61 treatment was verified by western blot. The following rescue experiments showed that SC79 or colivelin treatment promoted the malignant behaviors of ATC cells. More importantly, SC79 or colivelin treatment compensated the effect of GANT61 treatment on cell proliferation at several time settings and apoptosis, invasion, and part of that on EMT in ATC cells. GANT61 suppresses cell survival, invasion and EMT through inactivating AKT/mTOR or JAK/STAT3 pathways in ATC.
KEYWORDS: GANT61, anaplastic thyroid carcinoma, cell function, AKT/mTOR, JAK/STAT3
Introduction
Anaplastic thyroid carcinoma (ATC) is one of the most fatal cancers with a high grade of malignancy.1 Unlike differentiated thyroid carcinoma, ATC develops fast and a huge part of patients with ATC are at end stage when diagnosed.2,3 Currently, several studies have illustrated that targeted drugs, such as dabrafenib and sorafenib, improve the prognosis of ATC patients.4,5 However, the overall survival of ATC patients remains unfavorable. A recently published study reveals that the 2-year overall survival rate of ATC patients ranges approximately from 18% to 42%.6,7 Therefore, searching for potential treatment options might be crucial to improve the management of ATC patients.
Glioma-associated oncogene (Gli) antagonist 61 (GANT61) has been reported to suppress the progression of several cancers.8–11 Mechanically, GANT61 binds to Gli1 and subsequently inhibits Gli1-mediated transcription to inactivate the downstream Hedgehog pathway, as well as multiple signaling pathways that crosstalk with the Hedgehog pathway, including mitogen-activated protein kinase (MAPK)/extracellular regulated protein kinases (ERK), phosphatidylinositol 3 kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR), signal transducer and activator of transcription 3 (STAT3), and Notch pathways.8 Previously, it has been proposed that GANT61 induces cell cycle arrest and inhibits the stemness of estrogen receptor-positive breast cancer cells.9 Meanwhile, it is also revealed that GANT61 promotes the radiosensitivity of both prostate cancer cells and xenografted prostate cancer mice.10 Besides, another interesting study discloses that GANT61 represses the growth of head and neck cancer cell lines through modulating glycogen synthesis kinase 3 β.11 However, the effect of GANT61 on ATC cell function and its molecular mechanisms remains largely unclear.
In the present study, ATC cells (8505C and CAL-62) were treated with GANT61, followed by the detection of cell proliferation, apoptosis and invasion. Subsequently, the molecular mechanisms of GANT61 on ATC cell function were explored by RNA sequence, western blot verification and rescue experiments.
Methods
Cell culture
Human ATC cells (8505C and CAL-62) were obtained from the National Collection of Authenticated Cell Cultures (Shanghai, China). 8505C cells were grown in Roswell Park Memorial Institute (RPMI) 1640 medium (Lonza, Belgium). CAL-62 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Lonza, Belgium). All culture media were supplemented with 10% fetal bovine serum (FBS) (Gibco, USA) and 1% penicillin/streptomycin (Sigma, USA). All cells were cultured at 37°C under 95% air and 5% CO2 conditions.
Cell treatments
To assess the effect of GANT61 (Merck, Germany), cells were cultured with 10 µM GANT6112,13 for 48 hours (h) and collected for RNA sequencing (RNA-seq). For the rescue experiments, cells were incubated with 10 µM SC7914,15 (Beyotime, China) or 0.5 μM colivelin16 (Tocris, UK). Reverse transcription-quantitative polymerase chain reaction (RT-qPCR), western blot, proliferation, apoptosis and invasion assays were performed after treatment.
RNA-seq and analysis
Cells were collected for RNA-seq after being cultured with GANT61 for 48 h. Total RNA was isolated using Trizol Reagent (Sangon, China). RNA-seq libraries were constructed using a TruSeq RNA library preparation kit (llumina, USA) per the manufacturers’ protocols. Paired-end RNA-seq was performed using the HiSeq 2000 platform (Illumina, USA) at Genergy Biotechnology (Shanghai, China). Quality control analysis was performed using FastQC (Version 0.11.5). RNA quantification, clustering analysis and differential expression analysis were performed using R-project (Version 3.6.3). Gene Ontology (GO) analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment were performed using the DAVID online tool (https://david.ncifcrf.gov/). In addition, the raw data of RNA-seq are uploaded to a public database: Bio-Med Big Data Center (https://www.biosino.org/node/) (affiliated by Chinese Academy of Sciences) with the project ID OEP003158.
RT-qPCR
At 48 h after being treated with GANT61, SC79 or colivelin, cells were collected for RNA isolation and RT-qPCR assay. Briefly, total RNA from cells was isolated using Trizol Reagent. The GeneAce Reverse Transcriptase Kit (Nippon, Japan) was used for the generation of cDNA. The TB Green™ Fast qPCR Mix (Takara, Japan) was adopted to perform qPCR. The 2−ΔΔCt method was used to calculate Gli1 mRNA expression. Primer sequences (5’->3’) were as follows: Gli1 forward, CCACCAAGCTAACCTCATGTC; Gli1 reverse, GCTTCTTGGTCAACTTGACTGC; GAPDH forward, GAGTCCACTGGCGTCTTCAC; and GAPDH reverse, ATCTTGAGGCTGTTGTCATACTTCT.
Western blot
Western blot analysis was performed after 48 h of treatment with GANT61, SC79 or colivelin. Briefly, cells were lysed using RIPA solution (Beyotime, China) supplemented with the protease inhibitor (Roche, Switzerland). After being quantified using a bicinchoninic acid (BCA) kit (Beyotime, China), 30 μg of total protein was separated using precast gel (Beyotime, China) and transferred to the nitrocellulose membrane (Bio-Rad, China). Then, the membranes were blocked with 5% fat-free dry milk (Beyotime, China) and incubated with primary antibodies. After being incubated with secondary antibodies, the bands were observed using an enhanced ECL chemiluminescence kit (Sangon, China). β-actin was used as the internal control. The primary antibodies were as follows: Gli1 (1:500, Abcam, UK), E-cadherin (1:1000, CST, USA), Vimentin (1:1000, CST, USA), Snail (1:1000, CST, USA), p-AKT (1:1000, Abcam, UK), AKT (1:1000, Abcam, UK), p-mTOR (1:3000, Abcam, UK), mTOR (1:2000, Abcam, UK), p-STAT3 (1:2000, Abcam, UK), STAT3 (1:1000, Abcam, UK), SOCS3 (1:1000, Abcam, UK), β-actin (1:5000, Abcam, UK), JAK2 (1:1000, Abcam, UK) and p-JAK2 (1:1000, Abcam, UK). The secondary antibody was Goat Anti-Rabbit IgG H&L (HRP) (1:10000, Abcam, UK). The grayscales of the blots were analyzed using ImageJ v1.5 (Version 1.8.0). Data were presented as mean standard deviation (SD).
Cell proliferation assay
Cell proliferation was assessed using Cell Counting Kit-8 (Dojindo, Japan). Briefly, cells were seeded in a 96-well plate (2 × 103 cells/well). At 0, 24, 48 and 72 h after treatment with GANT6A, SC79 or colivelin, 10 μl of reagent was added and incubated for 2 h. The optical density (OD) value was read using a microplate reader (Molecular Devices, USA).
Cell apoptosis assay
Cell apoptosis was assessed using a TUNEL apoptosis kit (Sangon, China) and an Annexin V-FITC Apoptosis Detection Kit (Beyotime, China). For TUNEL assay, cells were seeded and treated with GANT6A, SC79 or colivelin for 48 h in a 96-well plate. Cells were fixed with 4% paraformaldehyde (Sangon, China) and permeabilized with 0.1% Triton X-100 (Sangon, China). Then, cells were incubated with TUNEL working solution for 30 min. After DAPI (Beyotime, China) staining, images were acquired using a fluorescence microscope (Olympus, Japan). The apoptosis rate is calculated using the ratio of the number of TUNEL-positive cells to the number of DAPI-positive cells.17 For AV/PI assay, cells were treated with GANT61 for 48 h. After being harvested, the cells incubated with AV and PI for 20 min in the dark. Finally, the cells were detected using a FACSCalibur flow cytometer (BD, USA) and analyzed using Flowjo 7.6 (BD, USA).
Cell invasion assay
Cell invasion assay was performed using Matrigel-coated transwell insert (Corning, USA). Briefly, cells were harvested at 48 h after being treated with GANT6A, SC79 or colivelin and seeded into the upper chambers of transwell inserts with serum-free medium. Medium containing 10% FBS was added to the lower chamber. After being cultured for 24 h, the invasive cells were stained with crystal violet (Sangon, China) and counted to assess the invasion ability.
Statistical analysis
All experiments were triplicate. GraphPad Prism 7.0 software was adopted for data analysis. Comparison between two groups was assessed by the t test. Differences among groups were analyzed by One-way ANOVA followed by Tukey’s multiple comparisons test. P < .05 was considered statistically significant.
Results
GANT61 inhibited proliferation and invasion but promoted apoptosis in ATC
GANT61 treatment inhibited Gli1 expression at both mRNA and protein levels in 8505C and CAL-62 cells (Figure 1a–c) (all P < .01). Furthermore, it was observed that GANT61 treatment suppressed proliferation in 8505C and CAL-62 cells (all P < .05) (Figure 1d). In addition, GANT61 treatment enhanced apoptosis reflected by TUNEL assay (Figure 1e,f) and AV/PI assay (Supplementary Figure 1a–b) (all P < .05). Meanwhile, the transwell assay revealed that GANT61 repressed invasive ability in 8505C and CAL-62 cells (both P < .05) (Figure 2a–b). Besides, GANT61 also increased E-cadherin expression while decreased Vimentin and Snail expressions in 8505C and CAL-62 cells (all P < .05) (Figure 2c–d).
Figure 1.
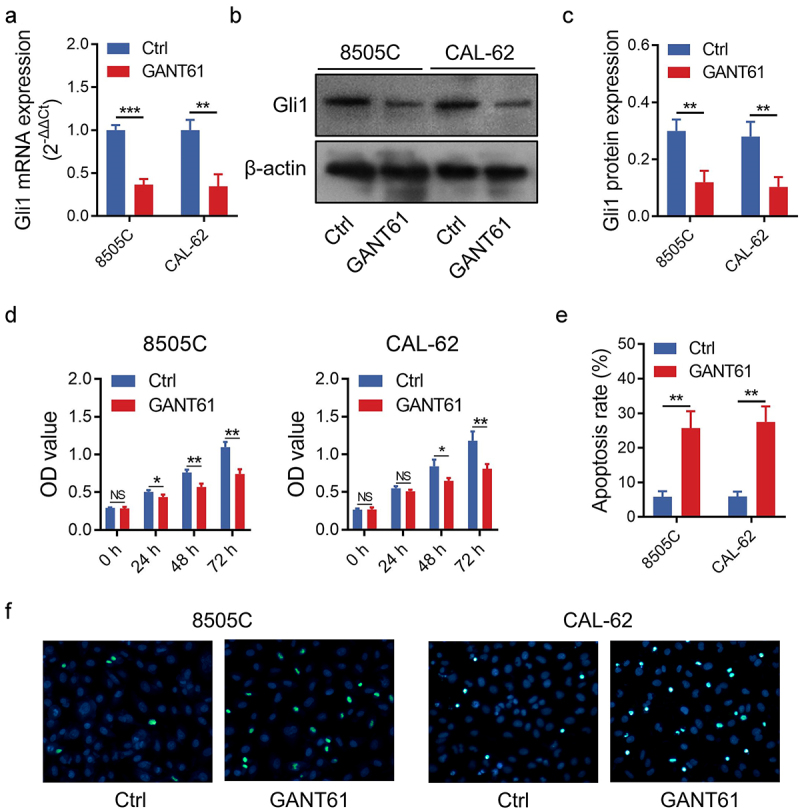
Effect of GANT61 on proliferation and apoptosis in ATC cells. Comparison of Gli1 mRNA expression between groups (a); Representative image of Gli1 protein expression detection by western blot (b); Comparison of Gli1 protein expression between groups (c); Comparison of cell proliferation (d) and apoptosis rate (e) between groups; Representative images of apoptosis detection by TUNEL assay (f). Gli1: glioma-associated oncogene; GANT61: glioma-associated oncogene antagonist 61; ATC: anaplastic thyroid carcinoma; Ctrl: control; TUNEL: Terminal deoxynucleotidyl transferase deoxyuridine 5’-triphosphate nick end labeling; h: hour; *: P < .05; **: P < .01; ***: P < .001; NS: not significant.
Figure 2.

Effect of GANT61 on invasion and EMT markers in ATC cells. Representative images of invasion detection by transwell assay (a); Comparison of invasive ability between groups (b); Representative images of EMT marker detection by western blot (c); Comparison of EMT marker levels between groups (d). GANT61: glioma-associated oncogene antagonist 61; Ctrl: control; ATC: anaplastic thyroid carcinoma; EMT: epithelial-mesenchymal transition; *: P < .05; **: P < .01.
GANT61 suppressed AKT/mTOR and Janus kinase/STAT3 (JAK/STAT3) pathways in ATC
To further explore the potential downstream pathway of GANT61, RNA sequence and bioinformatic analyses were conducted. The detailed principal component analysis, heatmap, volcano plot, GO enrichment and KEGG pathway enrichment in 8505C and CAL-62 cells are shown in Supplementary Figure 2a–e and listed in Supplementary Table 1, Supplementary Table 2, Supplementary Table 3 and Supplementary Table 4.
Moreover, a total of 85 upregulated differentially expressed genes (DEGs) and 71 downregulated DEGs were found in both GANT61-treated 8505C and CAL-62 cells (Figure 3a). The GO analysis revealed that these DEGs were mainly enriched in the biological processes of positive regulation of cell migration and epithelial-mesenchymal transition (EMT) (Figure 3b). Notably, the KEGG pathway enrichment disclosed that these DEGs were generally included in PI3K/AKT, JAK/STAT, Hedgehog and mTOR pathways (Figure 3c). The specific information is given in Supplementary Table 5 and Supplementary Table 6.
Figure 3.

RNA sequence and bioinformatic analysis. Venn diagram (a); GO analysis (b) and KEGG pathway enrichment (c). GO: Gene Ontology; KEGG: Kyoto Encyclopedia of Genes and Genomes.
Since GANT61 is a direct inhibitor of Gli1, a key component of the Hedgehog pathway, we did not further explore the effect of GANT61 on the Hedgehog pathway but focus on the AKT/mTOR and JAK/STAT3 pathways. Western blot assay confirmed that GANT61 treatment suppressed phosphorylation of AKT, mTOR, JAK2 and STAT3 in 8505C and CAL-62 cells, as well as SOCS3 expression in CAL-62 cells (all P < .05) (Figure 4a–b and Supplementary Figure 1c,d).
Figure 4.
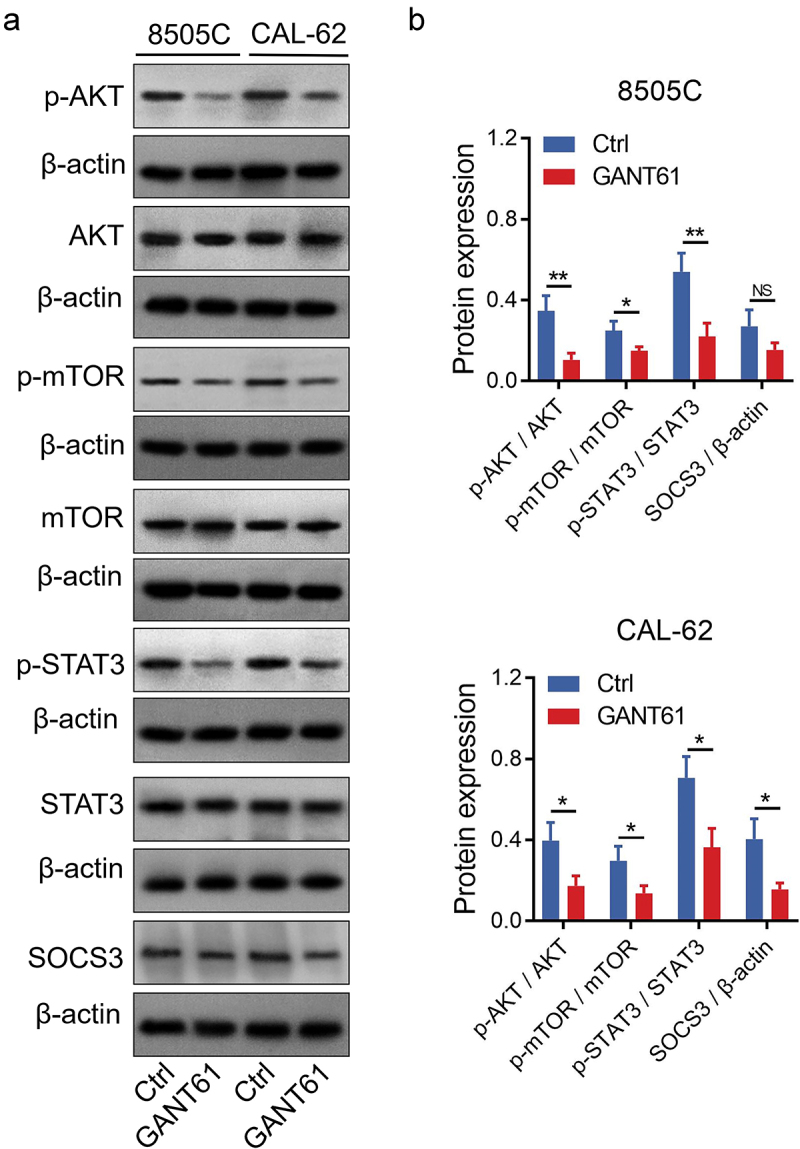
Verification of the downstream pathway by western blot. Representative images of AKT/mTOR and JAK/STAT3 pathways detection by western blot (a); Comparison of protein expressions between groups (b). AKT: protein kinase B; mTOR: mammalian target of rapamycin; JAK: Janus kinase; STAT3: signal transducer and activator of transcription 3; SOCS3: suppressor of cytokine signaling 3; Ctrl: control; GANT61: glioma-associated oncogene antagonist 61; *: P < .05; **: P < .01; NS: not significant.
Rescue experiments
In order to explore whether GANT61 regulated proliferation, migration and apoptosis in ATC cells through AKT/mTOR and JAK/STAT3 pathways, rescue experiments were conducted by treating 8505C and CAL-62 cells with SC79 (AKT activator) or colivelin (STAT3 activator) alone or in combination with GANT61. Data showed that SC79 or colivelin did not affect Gli1 expression (all P > .05) (Figure 5a–c); besides, the activation of AKT/mTOR and JAK/STAT3 pathways was confirmed by western blot (Figure 5d–e).
Figure 5.

Gli1 expression and AKT/mTOR and JAK/STAT3 pathways in rescue experiment. Comparison of Gli1 mRNA expression among groups in rescue experiment (a); Representative of Gli1 protein detection by western blot in rescue experiment (b); Comparison of Gli1 protein expression among groups in rescue experiment (c); Representative images of AKT/mTOR and JAK/STAT3 pathways detection by western blot in rescue experiment (d); Comparison of the expression of proteins involved in AKT/mTOR and JAK/STAT3 pathways among groups in rescue experiment (e). Ctrl: control; GANT61: glioma-associated oncogene antagonist 61; Gli1: glioma-associated oncogene; AKT: protein kinase B; mTOR: mammalian target of rapamycin; JAK: Janus kinase; STAT3: signal transducer and activator of transcription 3; SOCS3: suppressor of cytokine signaling 3; *: P < .05; **: P < .01; ***: P < .001; NS: not significant.
Moreover, SC79 or colivelin treatment promoted proliferation at several time settings, inhibited apoptosis, increased invasive ability and enhanced Vimentin and Snail levels in 8505C and CAL-62 cells; additionally, SC79 treatment also reduced the E-cadherin level in 8505C and CAL-62 cells (all P < .05) (Figure 6a–c, Figure 7a–d). More importantly, SC79 or colivelin treatment compensated the effect of GANT61 on proliferation at several time settings, apoptosis and invasive ability in 8505C and CAL-62 cells; SC79 or colivelin treatment also rescued the effect of GANT61 on E-cadherin, Vimentin and Snail in 8505C cells, as well as part of that in CAL-62 cells (Figure 6a–c, Figure 7a–d).
Figure 6.

Cell proliferation and apoptosis in rescue experiment. Comparison of cell proliferation (a) and apoptosis rate (b) among groups in rescue experiment; Representative images of apoptosis detection by TUNEL assay in rescue experiment (c). Ctrl: control; GANT61: glioma-associated oncogene antagonist 61; TUNEL: Terminal deoxynucleotidyl transferase deoxyuridine 5’-triphosphate nick end labeling; h: hour; *: P < .05; **: P < .01; NS: not significant.
Figure 7.

Cell invasion and EMT markers in rescue experiment. Representative images of invasion detection by transwell assay in rescue experiment (a); Comparison of invasive ability among groups in rescue experiment (b); Representative images of EMT marker detection by western blot in rescue experiment (c); Comparison of EMT marker levels among groups in rescue experiment (d). GANT61: glioma-associated oncogene antagonist 61; Ctrl: control; EMT: epithelial-mesenchymal transition; *: P < .05; **: P < .01; NS: not significant.
Discussion
Several previous studies have disclosed the effect of Gli1 on the progression of several cancers including ATC. For instance, Gli1 increases the stemness of colorectal adenocarcinoma through PI3K/AKT/nuclear factor-κB signaling.18 In addition, Gli1 contributes to the resistance of erlotinib in non-small-cell lung cancer.19 Apart from that, Gli1 promotes transforming growth factor-β-mediated EMT in hepatocellular carcinoma.20 Regarding ATC, it is revealed that Gli1 knockdown decreases stemness and radiosensitivity while increases Snail expression in ATC cell lines, but overexpression of Gli1 presents the reverse effects.21 Meanwhile, it is also indicated that silencing Gli1 suppresses tumor progression in cancer stem cells of ATC-bearing mice through modulating the expressions of B lymphoma Mo-MLV insertion region 1 homolog (BMI1) and SRY-Box Transcription Factor 2 (SOX2).22 Besides, another recently published study finds that silencing Gli1 induces autophagy by activating transforming growth factor-β-activated kinase 1 (TAK1) and its downstream protein c-Jun N-terminal kinase (JNK) and adenosine monophosphate-activated protein kinase (AMPK).12 According to these studies, Gli1 might be a potential target for inhibiting the progression of several cancers, including ATC.
Although the above-mentioned studies have shown the vital role of Gli1 in ATC progression, the effect and the molecular mechanisms of Gli1 inhibitor GANT61 on the cellular function of ATC are still largely unclear. The present study revealed that GANT61 treatment suppressed proliferation but promoted apoptosis in ATC cells. Furthermore, due to the fact that ATC is characterized by a high level of invasion and Gli1 modulates EMT (a critical pathway that regulates invasion in various cancers) in ATC,12,21,23 the regulation of GANT61 on invasion and EMT markers was also explored, which revealed that GANT61 repressed invasion and regulated EMT markers in ATC cells. These data highlighted the potential of GANT61 as a treatment option for ATC; however, further in vivo studies should be conducted to verify these findings. Previous studies also discover the effect of GANT61 on cell proliferation, invasion and EMT in other cancers, including breast cancer, gastric cancer and pancreatic cancer.24–26 Our findings were partly in line with these previous studies.
RNA sequence plus bioinformatic analysis is a powerful tool to understand the mechanisms of various biological processes, including carcinogenesis and the action of anticancer agents, through identifying transcriptome variations.27 For instance, an interesting previous study suggests that DEGs of ATC (versus normal thyroid samples) are enriched in cancer-related pathways, such as the PI3K/AKT pathway.28 However, the downstream signaling pathways of GANT61 revealed by RNA sequence and bioinformatic analysis in ATC have never been explored. In the present study, the upregulated and downregulated DEGs in GANT61-treated ATC cells were identified by RNA sequence and bioinformatic analysis; GO enrichment analysis revealed that these DEGs were enriched in regulation of cell migration and EMT, which conformed with the above-mentioned data; further KEGG enrichment analysis disclosed that these DEGs were enriched in PI3K/AKT, JAK/STAT, Hedgehog and mTOR signaling pathways. Besides, the PI3K/AKT/mTOR and JAK/STAT pathways contain a huge number of genes, while the Hedgehog pathway includes fewer genes; therefore, the RNA sequence showed that the DEGs were enriched in PI3K/AKT and JAK/STAT pathways with higher significance. Moreover, rescue experiments confirmed that GANT61 inhibited proliferation and invasion and modulated EMT markers through AKT/mTOR and JAK/STAT3 pathways in ATC cells. These findings potentially contributed to the acting mechanism of GANT61 on inhibiting ATC progression. Meanwhile, considering that the Hedgehog, AKT/mTOR and JAK/STAT pathways are involved in the carcinogenesis and progression of several cancers, GANT61 could serve as a potential treatment option for cancers, whereas this hypothesis needed further validation. In addition, SOCS3 is reported to take part in the feedback loop of the JAK/STAT3 pathway;29 therefore, it was observed that colivelin, activator of the JAK/STAT3 pathway, could modulate SOCS3 in the present study, which was partially in line with a previous study.16 Meanwhile, further studies should be conducted to verify the interaction among colivelin, JAK/STAT3 pathway and SOCS3
The crosstalk between the Hedgehog pathway and other signaling pathways in cancers, such as the mitogen-activated protein kinase (MAPK), AKT/mTOR and Wnt/β-catenin pathways, has been disclosed in previous studies. For example, Gli1 and S6K1 (downstream protein of mTOR) interact with each other, which further induces EMT in esophageal cancer cell lines;30,31 the activation of the Hedgehog pathway phosphorylates AKT in esophageal cells;32 the coinhibition of Hedgehog and AKT/mTOR pathways shows the synergistic effect on suppressing glioblastomas.33 Another important signaling pathway that correlates with the Hedgehog pathway is the Notch pathway. For instance, the Notch and Hedgehog pathways intercorrelate with each other, and both are critically involved in the self-renewal of breast cancer cells;34 the Notch pathway activates the STAT3 pathway and further promotes the Hedgehog pathway in neural stem cells.35 However, the crosstalk of the Hedgehog pathway with AKT/mTOR and JAK/STAT3 pathways is rarely reported in ATC. In the present study, it was observed that GANT61, an inhibitor of Gli1, exerted repression of proliferation and invasion through inactivating the AKT/mTOR and JAK/STAT3 pathways in ATC. These findings might contribute to the crosstalk of the Hedgehog pathway with AKT/mTOR and JAK/STAT3 pathways in ATC. However, since the main objective of the present study was to explore the effect and molecular mechanism of GANT61 on the progression of ATC, this potential crosstalk among Hedgehog, AKT/mTOR and JAK/STAT3 pathways should be further verified.
To be conclusive, GANT61 suppresses cell survival, inhibits invasion and represses EMT through inactivating AKT/mTOR and JAK/STAT3 pathways in ATC, suggesting the potential of GANT61 as a treatment option for ATC.
Supplementary Material
Funding Statement
The author(s) reported that there is no funding associated with the work featured in this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
References
- 1.Molinaro E, Romei C, Biagini A, Sabini E, Agate L, Mazzeo S, Materazzi G, Sellari-Franceschini S, Ribechini A, Torregrossa L, et al. 2017. Anaplastic thyroid carcinoma: from clinicopathology to genetics and advanced therapies. Nat Rev Endocrinol. 13(11):644–660. doi: 10.1038/nrendo.2017.76. [DOI] [PubMed] [Google Scholar]
- 2.Smallridge RC, Ain KB, Asa SL, Bible KC, Brierley JD, Burman KD, Kebebew E, Lee NY, Nikiforov YE, Rosenthal MS, et al. 2012. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 22(11):1104–1139. doi: 10.1089/thy.2012.0302. [DOI] [PubMed] [Google Scholar]
- 3.Rao SN, Zafereo M, Dadu R, Busaidy NL, Hess K, Cote GJ, Williams MD, William WN, Sandulache V, Gross N, et al. 2017. Patterns of Treatment Failure in Anaplastic Thyroid Carcinoma. Thyroid. 27(5):672–681. doi: 10.1089/thy.2016.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrari SM, Elia G, Ragusa F, Ruffilli I, La Motta C, Paparo SR, Patrizio A, Vita R, Benvenga S, Materazzi G, et al. 2020. Novel treatments for anaplastic thyroid carcinoma. Gland Surg. 9(Suppl 1):S28–S42. doi: 10.21037/gs.2019.10.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Subbiah V, Kreitman RJ, Wainberg ZA, Cho JY, Schellens JHM, Soria JC, Wen PY, Zielinski C, Cabanillas ME, Urbanowitz G, et al. 2018. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-Mutant anaplastic thyroid cancer. J Clin Oncol. 36(1):7–13. doi: 10.1200/JCO.2017.73.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin B, Ma H, Ma M, Zhang Z, Sun Z, Hsieh IY, Okenwa O, Guan H, Li J, Lv W.. The incidence and survival analysis for anaplastic thyroid cancer: a SEER database analysis. Am J Transl Res. 2019;11(9):5888–5896. [PMC free article] [PubMed] [Google Scholar]
- 7.Maniakas A, Dadu R, Busaidy NL, Wang JR, Ferrarotto R, Lu C, Williams MD, Gunn GB, Hofmann MC, Cote G, et al. 2020. Evaluation of overall survival in patients with anaplastic Thyroid Carcinoma, 2000-2019. JAMA Oncol. 6(9):1397–1404. doi: 10.1001/jamaoncol.2020.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doheny D, Manore SG, Wong GL, Lo HW.. 2020. Hedgehog Signaling and Truncated GLI1 in Cancer. Cells. 9(9):2114. doi: 10.3390/cells9092114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurebayashi J, Koike Y, Ohta Y, Saitoh W, Yamashita T, Kanomata N, Moriya T. 2017. Anti-cancer stem cell activity of a hedgehog inhibitor GANT61 in estrogen receptor-positive breast cancer cells. Cancer Sci. 108(5):918–930. doi: 10.1111/cas.13205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonnissen A, Isebaert S, McKee CM, Dok R, Haustermans K, Muschel RJ. 2016. The hedgehog inhibitor GANT61 sensitizes prostate cancer cells to ionizing radiation both in vitro and in vivo. Oncotarget. 7(51):84286–84298. doi: 10.18632/oncotarget.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zubcic V, Rincic N, Kurtovic M, Trnski D, Musani V, Ozretic P, Levanat S, Leovic D, Sabol M. 2020. GANT61 and lithium chloride inhibit the growth of head and neck cancer cell lines through the regulation of GLI3 processing by GSK3beta. Int J Mol Sci. 21(17):6410. doi: 10.3390/ijms21176410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li S, Wang J, Lu Y, Zhao Y, Prinz RA, Xu X. 2021. Inhibition of the sonic hedgehog pathway activates TGF-beta-activated kinase (TAK1) to induce autophagy and suppress apoptosis in thyroid tumor cells. Cell Death Dis. 12(5):459. doi: 10.1038/s41419-021-03744-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williamson AJ, Doscas ME, Ye J, Heiden KB, Xing M, Li Y, Prinz RA, Xu X. 2016. The sonic hedgehog signaling pathway stimulates anaplastic thyroid cancer cell motility and invasiveness by activating Akt and c-Met. Oncotarget. 7(9):10472–10485. doi: 10.18632/oncotarget.7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee NR, Kim DY, Jin H, Meng R, Chae OH, Kim SH, Park BH, Kim SM. Inactivation of the Akt/FOXM1 signaling pathway by panobinostat suppresses the proliferation and metastasis of gastric cancer cells. Int J Mol Sci. 2021;22(11). doi: 10.3390/ijms22115955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin Z, Cheng X, Feng H, Kuang J, Yang W, Peng C, Shen B, Qiu W. 2017. Apatinib inhibits angiogenesis via suppressing Akt/GSK3beta/ANG signaling pathway in anaplastic thyroid cancer. Cell Physiol Biochem. 44(4):1471–1484. doi: 10.1159/000485583. [DOI] [PubMed] [Google Scholar]
- 16.Li S, Xu Z, Guo J, Zheng J, Sun X, Yu J. 2020. Farnesoid X receptor activation induces antitumour activity in colorectal cancer by suppressing JAK2/STAT3 signalling via transactivation of SOCS3 gene. J Cell Mol Med. 24(24):14549–14560. doi: 10.1111/jcmm.16083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He J, Huang Z, He M, Liao J, Zhang Q, Wang S, Xie L, Ouyang L, Koeffler HP, Yin D, et al. 2020. Circular RNA MAPK4 (circ-MAPK4) inhibits cell apoptosis via MAPK signaling pathway by sponging miR-125a-3p in gliomas. Mol Cancer. 19(1):17. doi: 10.1186/s12943-019-1120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Z, Zhang C, Qi W, Cui Y, Xuan Y. 2018. GLI1 promotes cancer stemness through intracellular signaling pathway PI3K/Akt/NFkappaB in colorectal adenocarcinoma. Exp Cell Res. 373(1–2):145–154. doi: 10.1016/j.yexcr.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Dong Z, Wang Y, Ding V, Yan X, Lv Y, Zhong M, Zhu F, Zhao P, He C, Ding F, et al. 2020. GLI1 activation is a key mechanism of erlotinib resistance in human non-small cell lung cancer. Oncol Lett. 20(4):76. doi: 10.3892/ol.2020.11937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng X, Vittar NB, Gai X, Fernandez-Barrena MG, Moser CD, Hu C, Almada LL, McCleary-Wheeler AL, Elsawa SF, Vrabel AM, et al. 2012. The transcription factor GLI1 mediates TGFbeta1 driven EMT in hepatocellular carcinoma via a SNAI1-dependent mechanism. PLoS One. 7(11):e49581. doi: 10.1371/journal.pone.0049581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heiden KB, Williamson AJ, Doscas ME, Ye J, Wang Y, Liu D, Xing M, Prinz RA, Xu X. 2014. The sonic hedgehog signaling pathway maintains the cancer stem cell self-renewal of anaplastic thyroid cancer by inducing snail expression. J Clin Endocrinol Metab. 99(11):E2178–87. doi: 10.1210/jc.2014-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu Y, Zhu Y, Deng S, Chen Y, Li W, Sun J, Xu X. 2021. Targeting the sonic hedgehog pathway to suppress the expression of the cancer stem cell (CSC)-Related transcription factors and CSC-Driven thyroid tumor growth. Cancers (Basel). 13(3):418. doi: 10.3390/cancers13030418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alobuia W, Gillis A, Kebebew E. 2020. Contemporary management of anaplastic thyroid cancer. Curr Treat Options Oncol. 21(10):78. doi: 10.1007/s11864-020-00776-2. [DOI] [PubMed] [Google Scholar]
- 24.Riaz SK, Ke Y, Wang F, Kayani MA, Malik MFA. 2019. Influence of SHH/GLI1 axis on EMT mediated migration and invasion of breast cancer cells. Sci Rep. 9(1):6620. doi: 10.1038/s41598-019-43093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang M, Liu XC, Liu T, Li WJ, Xiang JG, Xiao D, Zhang YL, Zheng MH, Zhai C, Chen L, et al. 2018. GLI-1 facilitates the EMT induced by TGF-beta1 in gastric cancer. Eur Rev Med Pharmacol Sci. 22(20):6809–6815. doi: 10.26355/eurrev_201810_16148. [DOI] [PubMed] [Google Scholar]
- 26.Ghanbari A, Cheraghzadeh Z, Mahmoudi R, Zibara K, Hosseini E. 2019. GLI inhibitors overcome Erlotinib resistance in human pancreatic cancer cells by modulating E-cadherin. J Chemother. 31(3):141–149. doi: 10.1080/1120009X.2019.1584422. [DOI] [PubMed] [Google Scholar]
- 27.Hong M, Tao S, Zhang L, Diao LT, Huang X, Huang S, Xie SJ, Xiao ZD, Zhang H. 2020. RNA sequencing: new technologies and applications in cancer research. J Hematol Oncol. 13(1):166. doi: 10.1186/s13045-020-01005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu S, Liao Y, Chen L. 2018. Identification of key pathways and genes in anaplastic Thyroid Carcinoma via integrated bioinformatics analysis. Med Sci Monit. 24:6438–6448. doi: 10.12659/MSM.910088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poeggeler B, Schulz C, Pappolla MA, Bodo E, Tiede S, Lehnert H, Paus R. 2010. Leptin and the skin: a new frontier. Exp Dermatol. 19(1):12–18. doi: 10.1111/j.1600-0625.2009.00930.x. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Ding Q, Yen CJ, Xia W, Izzo JG, Lang JY, Li CW, Hsu JL, Miller SA, Wang X, et al. 2012. The crosstalk of mTOR/S6K1 and Hedgehog pathways. Cancer Cell. 21(3):374–387. doi: 10.1016/j.ccr.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pon YL, Zhou HY, Cheung AN, Ngan HY, Wong AS. 2008. p70 S6 kinase promotes epithelial to mesenchymal transition through snail induction in ovarian cancer cells. Cancer Res. 68(16):6524–6532. doi: 10.1158/0008-5472.CAN-07-6302. [DOI] [PubMed] [Google Scholar]
- 32.Wei L, Xu Z. 2011. Cross-signaling among phosphinositide-3 kinase, mitogen-activated protein kinase and sonic hedgehog pathways exists in esophageal cancer. Int J Cancer. 129(2):275–284. doi: 10.1002/ijc.25673. [DOI] [PubMed] [Google Scholar]
- 33.Filbin MG, Dabral SK, Pazyra-Murphy MF, Ramkissoon S, Kung AL, Pak E, Chung J, Theisen MA, Sun Y, Franchetti Y, et al. 2013. Coordinate activation of Shh and PI3K signaling in PTEN-deficient glioblastoma: new therapeutic opportunities. Nat Med. 19(11):1518–1523. doi: 10.1038/nm.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo S, Liu M, Gonzalez-Perez RR. 2011. Role of Notch and its oncogenic signaling crosstalk in breast cancer. Biochim Biophys Acta. 1815(2):197–213. doi: 10.1016/j.bbcan.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Androutsellis-Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, Rueger MA, Bae SK, Kittappa R, McKay RD. 2006. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 442(7104):823–826. doi: 10.1038/nature04940. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


