ABSTRACT
Trillions of bacteria reside within our gastrointestinal tract, ideally forming a mutually beneficial relationship between us. However, persistent changes in diet and lifestyle in the western diet and lifestyle contribute to a damaging of the gut microbiota-host symbiosis leading to diseases such as obesity and irritable bowel syndrome. Many symptoms and comorbidities associated with these diseases stem from dysfunctional signaling in peripheral neurons. Our peripheral nervous system (PNS) is comprised of a variety of sensory, autonomic, and enteric neurons which coordinate key homeostatic functions such as gastrointestinal motility, digestion, immunity, feeding behavior, glucose and lipid homeostasis, and more. The composition and signaling of bacteria in our gut dramatically influences how our peripheral neurons regulate these functions, and we are just beginning to uncover the molecular mechanisms mediating this communication. In this review, we cover the general anatomy and function of the PNS, and then we discuss how the molecules secreted or stimulated by gut microbes signal through the PNS to alter host development and physiology. Finally, we discuss how leveraging the power of our gut microbes on peripheral nervous system signaling may offer effective therapies to counteract the rise in chronic diseases crippling the western world.
KEYWORDS: Gut microbiota/ microbiota metabolites/PNS/neuronal sensing/obesity
1. Introduction
The gut microbiota consists of a dynamic community of trillions of bacteria, archaea, virus, and fungi, which is primarily established at birth from interaction with the mother’s microbiota. Environmental factors such as geographical location, diet, antibiotic exposure, and infection continue to gradually shape microbiota composition during first few years of life.1,2 The bacterial community that ultimately colonizes the gut should ideally be evolved to symbiotically function with the host, aiding in digestion and proper immune response. However, dramatic changes in diet and lifestyle within the last century have contributed to the explosion of non-communicable diseases such as obesity, diabetes, nonalcoholic fatty liver disease, and irritable bowel syndrome (IBS), and a disconnection between the gut microbiota and the host physiology likely contributes.3,4 With increased processing of foods and usage of antibiotics, preservatives, and other additives, our diet is apparently no longer suited for our gut bacteria, and over time the western diet may actively disrupt the balance and diversity of microbes within our gut5 (Figure 1). Additionally, insults such as infection disrupt the gut microbiome composition predisposing individuals to gastrointestinal (GI) issues.6
Figure 1.
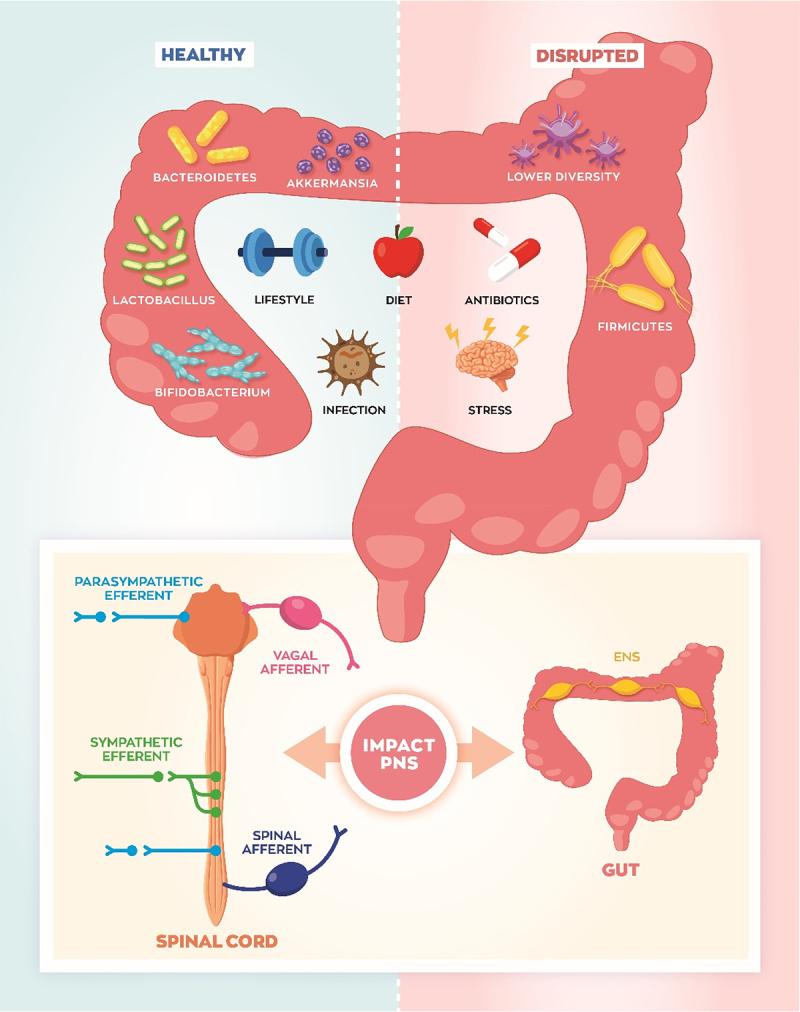
The gut microbiome composition is established at birth and is continually shaped by environmental factors such as lifestyle, diet, antibiotic use, infection, and stress. Generally, healthy individuals have a highly diverse microbiome, enriched in Bacteroidetes, Lactobacillus, Bifidobacterium, and Akkermansia. In chronic disease, microbiome diversity is often reduced and Firmicutes are expanded. The composition of gut microbes drastically impacts the peripheral nervous system (PNS) development and function. Vagal and spinal afferent (sensory) neurons relay microbial signals to the brain, and autonomic output is carried by sympathetic and parasympathetic efferent neurons. Enteric neurons form their own network in the gut, and they are ideally positioned to detect gut microbe signaling and reflexively alter gastrointestinal functions. The afferent, efferent, and enteric nervous systems are interconnected to respond to gut microbe signaling and cooperatively control a variety of homeostatic functions such as digestion, immunity, and visceral perception.
Dr. George Fox and Dr. Carl Woese were among the first to leverage the 16S ribosomal RNA gene as a unique marker to sequence the microbiome composition.7 Subsequent advances in genomic sequencing technology sparked an explosion of studies proposing associations between various bacteria and host processes or diseases. In the past two decades thousands of studies have aimed to identify microbial signatures, genes, or key species which underly the increased prevalence of various diseases, or to identify potential bacteria with therapeutic potential. Elegant studies performed by Jeff Gordon’s research team identified associations between the Firmicutes and Bacteroidetes phyla and adiposity, postulating the Firmicutes/Bacteroidetes ratio as a putative signature of the obese microbiome8–10 (Figure 1). They showed that transplanting bacteria from genetically obese mice (ob/ob) caused wild-type littermates to gain more fat mass, as compared to mice transplanted with a lean wild-type microbiota.8 Accompanying studies by Gordon’s group found that the microbiota of obese mice and overweight humans is comprised of higher percentage of Firmicutes.10 Lean individuals displayed higher abundance of Bacteroidetes, and as people lost weight via different diet regiments the population of Bacteroidetes expanded.9 However, associations between the Firmicutes/Bacteroidetes ratio and body weight have not been consistently supported.11 Prevotella species ferment fiber and often correlate with healthy metabolic outcomes and reduced inflammation,12–14 however there are conflicting findings on whether or not Prevotella are expanded in IBS patients.15,16 Reduced bacterial diversity, richness, and stability are often reported in obesity and IBS,10,17 but overall, identifying reliable microbial disease signatures has been a major challenge.11 Nonetheless, therapeutic candidates such as Akkermansia,18–20 Lactobacillus,21,22 Bifidobacterium,23–25 and Dysosmobacter welbionis26 have emerged as promising targets for obesity or IBS.
Mechanistically there are three general ways in which the gut microbiota and host can communicate to regulate metabolism and digestion: immunological, hormonal, and neuronal.26–29 Interestingly, there is a high degree of intercommunication between these modalities within the gut, and the peripheral nervous system (PNS) participates in the immunological and hormonal responses to gut bacterial biochemical processes. As shown in Figures 1 and 2, gut microbe signals can be “sensed” via vagal30,31 and spinal neurons,32 integrated in the brainstem and hypothalamus, and this ultimately influences the efferent signals to peripheral organs. Increasing efforts have been placed on understanding the molecular interactions between the gut microbiota and host PNS to identify causes of diseases, such as obesity and IBS, and to find novel therapeutic targets. Several recent studies manipulating the gut microbiota composition illustrate the importance of the interaction between gut microbes and the PNS in regulating host physiology. Antibiotics treated mice exhibit reduced innervation and disrupted excitability of enteric neurons, which contributes to slowed intestinal transit time and reduced motility.33–36 Maternal gut microbiota dramatically influences the development and maturation of the PNS, influencing host physiology.33–38 Furthermore, different components of the peripheral nervous system express nuclear and G-protein coupled receptors (GPCRs) which allow these neurons to “sense” gut microbe signaling.
Figure 2.
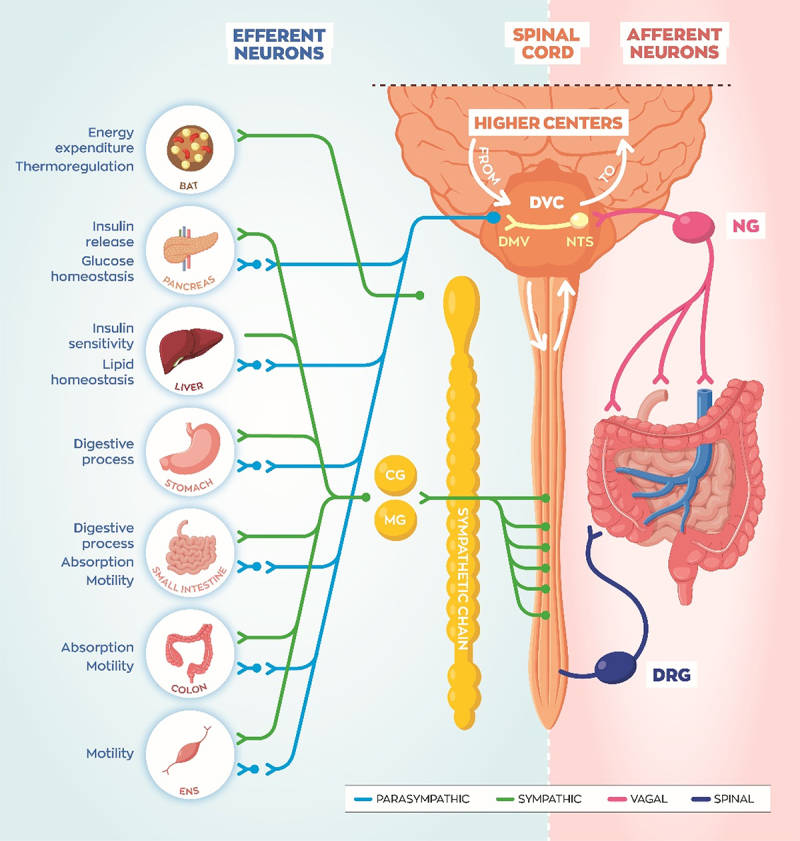
Spinal and vagal sensory neurons innervate the gastrointestinal (GI) tract and portal vein probing activities of the gut microbiota. Vagal sensory neurons with cell bodies in the nodose ganglia (NG) project to the nucleus tractus solitarius (NTS). The NTS and dorsal motor vagus (DMV), as well as the area postrema, comprise the dorsal vagal complex (DVC) in the hindbrain. Spinal sensory neurons with cell bodies in the dorsal root ganglia (DRG) project into the spinal cord to relay visceral signals to the brain. The vagal efferent system is comprised of long preganglionic neurons projecting out from the DMV connecting with short postganglionic neurons which then reach target organs. Short sympathetic efferent neurons leave the spinal cord and connect with postganglionic neurons in the sympathetic chain or discrete peripheral ganglia such as the celiac ganglia (CG) and mesenteric ganglia (MG). Sympathetic projections to brown adipose expends energy to produce heat (thermogenesis) during cold exposure or after a meal. Sympathetic innervation of pancreas and liver mobilizes glucose for energy, while GI innervation halts digestion, during a “fight or flight” state of arousal. Parasympathetic efferent projections generally oppose these actions to return the body back to baseline, during an internal state of “rest and digest”.
In this review we will focus on describing how the PNS serves as a relay between the gut microbiota and the host to regulate digestion, gastrointestinal function, and energy balance. We will begin by detailing the general function and anatomy of the peripheral nervous system. We will then highlight the key microbial signaling molecules that signal through the PNS to regulate physiology. Finally, we will discuss promising therapeutic targets for various chronic diseases and comorbidities. Furthering our mechanistic understanding of these interactions could provide insights into disease pathology and identify new treatment strategies.
2. Function and anatomy of the peripheral and enteric nervous systems
Neuronal transmission allows for nearly instantaneous processing of sensory input or generation of motor output. This rapid signaling of peripheral neurons in the gut is critical for homeostatic mechanisms such as GI motility, secretion, and even immune response modulation.39 The peripheral nervous system (PNS) consists of vagal and spinal sensory (afferent) neurons, autonomic motor (efferent) neurons, and enteric neurons (Figure 2). Afferent neurons send information from the periphery to the brain or spinal cord, while efferent neurons project out from the central nervous system (CNS) to peripheral organs. Classifying by anatomical distribution, the twelve cranial nerves project from the brain/brainstem and spinal nerves from the spinal cord. The autonomic system is divided into sympathetic, parasympathetic, and enteric nervous systems (ENS).
2.1. Afferent neurons
As illustrated in Fig.1 and 2, vagal and spinal afferent neurons innervate the digestive tract, monitoring mechanical, chemical, thermal, and nociceptive signals related to the diet and microbiota.40–45 It is important to note that some enteric neurons are also characterized as afferent and they are labeled as “intrinsic”, while spinal and vagal neurons which originate outside of the gut are “extrinsic”. Vagal afferent neurons transmit signals up from the viscera, their cell bodies are located in the nodose ganglia (NG), and they synapse into the solitary nucleus (NTS) in the brainstem (Figure 2). The NTS integrates vagal afferent signals and relays the information up to higher brain regions such as the hypothalamus, or reflexes back down to the dorsal motor nuclei of the brainstem where vagal efferent neurons project out to effector organs.46 Spinal neurons, with cell bodies in the dorsal root ganglia (DRG), project into the dorsal horn of the spinal cord. These signals are relayed up to the brain and integrated, or they induce reflex activation of motor neurons which may bypass the brain. The spinal nerves can be subdivided into 5 divisions: cervical, thoracic, lumbar, sacral, and coccygeal, based on their projections into and out of the vertebrae.
2.2. Efferent neurons and target tissues
The afferent system provides critical information to the CNS, which integrate the visceral information and produces an effect on peripheral organs via autonomic efferent fibers. Although oversimplified, the sympathetic and parasympathetic efferent neurons are generically segregated based on functions aiding in stress responses (fight or flight) or returning to baseline (rest and digest), respectively. As illustrated Figure 2, sympathetic preganglionic neurons are short and release acetylcholine onto postganglionic neurons triggering the release of mainly norepinephrine onto peripheral organs. Conversely, parasympathetic preganglionic neurons send long projections out to postganglionic neurons which are often located within the target tissue. Both pre- and postganglionic parasympathetic neurons release acetylcholine. Autonomic neurotransmitter release onto peripheral tissues is crucial for regulating key metabolic functions, which are often disrupted in chronic disease.
Sympathetic drive to brown adipose tissue (BAT) burns calories, dissipating heat, in a process called thermogenesis. Parasympathetic and sympathetic innervation of the pancreas controls glucose homeostasis through insulin release.47 Liver innervation regulates de novo production of glucose (gluconeogenesis), glycogen storage or breakdown, as well as lipid homeostasis.48,49 Sympathetic modulation of the ENS generally dampens motility, and sympathetic vasoconstrictor neurons can directly halt blood flow to the GI.39,50 Parasympathetic neurons stimulate pancreatic and gall bladder digestive secretions, relax sphincters, and accelerate motility.39
The coordinated regulation of these tissues carried out by autonomic nerves is crucial for proper delivery of nutrients into the circulation during “fighting or flight”, or to reabsorb and store nutrients during “resting or digesting.” Muller et al. beautifully demonstrated how vagal afferent neurons respond to alterations in the gut microbiota and modulate the sympathetic outflow through celiac (CG) and superior mesenteric ganglia (SMG) to control GI motility.30
2.3. Enteric neurons
The enteric nervous system is comprised of sensory, motor, and interneurons organized into networks or plexuses located within the gut, which are capable of operating independently of the CNS. The submucosal plexus lies between the mucosa and circular muscle, and it regulates secretion and blood flow.39 Enteric neurons between the circular and longitudinal muscle make up the myenteric plexus (Auerbach plexus), which controls gut motility by action on smooth muscle. Enteric sensory neurons known as IPANs (intrinsic primary afferent neurons) detect various chemicals or distension caused by a food bolus, and then coordinate the electrical activity of submucosal and myenteric neurons. Finally, interneurons link the activity of ascending and descending motor networks to allow the “little brain” of the gut to function autonomously (Figure 3).39,51 The enteric nervous system is also supported by local glial cells, which also respond to changes in gut microbiota signaling,52 but we will focus on enteric neurons in this review.
Figure 3.
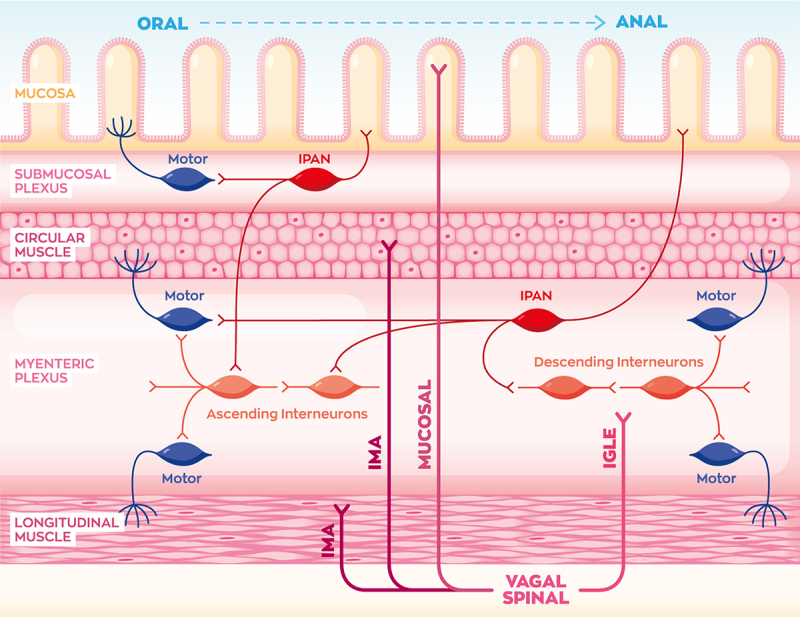
Vagal and spinal afferents are categorized based on their projections within the walls of the gastrointestinal (GI) tract. Intramuscular arrays (IMAs) terminate in the circular and longitudinal muscle, intraganglionic laminar ending (IGLEs) contact the myenteric plexus, and mucosal afferent neurons reach into the mucosa. In the enteric nervous system, intrinsic primary afferent neurons (IPANs) coordinate GI contraction and motility by sensing mechanical distension of the lumen and stimulating motor and interneurons. Motor neurons in the submucosal plexus mainly control blood flow and absorption, while myenteric interneurons and motor neurons control circular and longitudinal muscle contraction to propel food through the gut lumen. Although not shown in this image, parasympathetic and sympathetic efferent neurons also contact enteric neurons to modulate GI function.
As mentioned, the enteric nervous system can work autonomously to digest a meal, but the function of these neurons is modulated by autonomic nerves, depending on internal state.51 The parasympathetic nervous system communicates with the enteric neurons to increase motility, secretions, and blood flow, thus aiding in “rest and digestion”. Conversely, sympathetic neurons oppose these actions to halt digestion during a “fight or flight” state of arousal.
2.4. Morphology of extrinsic gut innervation
As illustrated Figure 3, vagal and spinal afferent neurons can be categorized by the morphology of innervation within the layers of the gastrointestinal tract. They are classified as intraganglionic laminar endings (IGLEs), intramuscular arrays (IMAs), or mucosal innervating neurons. IGLEs project into the myenteric plexus functioning as mechanoreceptors, and IMAs innervate the circular and longitudinal muscle layers. Mucosal primary afferents project all the way into the lamina propria and intestinal villi, likely functioning as nutrient-sensing chemoreceptors.53 Recent work has identified mucosal afferents forming synapses with enteroendocrine cells,54,55 but most are thought to terminate as free nerve endings inside the villi.40,56 Enteroendocrine cells synapsing onto vagal afferent neurons are known as “neuropod” cells, which likely regulate feeding behavior.55 Future studies assessing how these “neuropod” cells sense microbial signals would be of high interest.
The different morphologies of vagal afferents appear to express distinct GPCR or neuropeptide markers. For instance, most IGLEs in the stomach express GLP-1 receptor (GLP-1 R), while small intestine IGLEs express oxytocin receptor (OxtR). Mucosal afferent neurons generally are identified by GPR65 expression,53 stomach mucosal neurons express calcitonin-gene regulated peptide (CGRP), and small intestine mucosal afferent neurons express VIP. Interestingly, IGLEs appear to control food intake,57,58 while mucosal afferents regulate glucose production in the liver.58 “Neuropod” cells are glutamatergic and express Peptide YY (PYY) and Cholecystokinin (CCK), so the vagal afferents synapsing with “neuropod” cells likely express the receptors for these neurotransmitters and hormones. More and more studies are beginning to elucidate the molecular mechanisms of how these different populations of neurons are relaying microbial signals to regulate host physiology,59 which we will now discuss.
3. Molecular mechanisms mediating gut microbiota-PNS communication
As previously mentioned, the signaling between the gut microbiota and peripheral neurons occurs through three interacting pathways. Specialized endothelial cells in the lining of the gut are called enteroendocrine cells (EECs) and they represent a major part of the hormonal pathway. EECs respond to chemical byproducts and signaling molecules released by the gut bacteria, and in turn they release neuropeptides capable of activating vagal sensory neurons or circulating to directly target effector tissues (Figure 4). The receptors for EEC hormones are expressed in vagal sensory neurons innervating the gut, and various physiological processes are controlled via this pathway such as gastric emptying, gut motility, insulin release, satiety, and hunger.31,51,57,58,60,61 We will breakdown different molecules secreted, modified, or stimulated by gut microbes and discuss the mechanisms by which they interact with peripheral neurons. We will also briefly discuss the reverse interaction where peripheral neurons release neurotransmitters and neuropeptides to modulate microbial activity directly or via immune activation.
Figure 4.
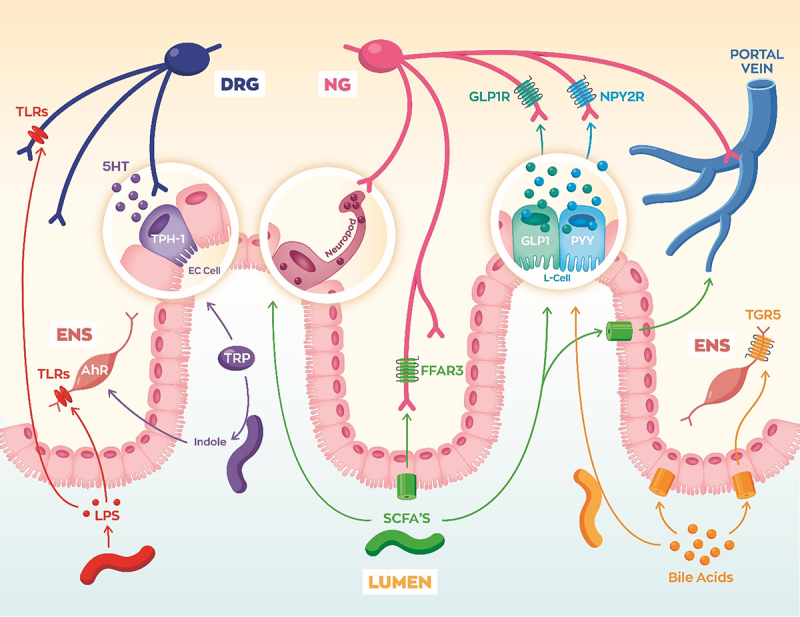
Gut microbes signal to vagal, spinal, and enteric neurons via a variety of mechanisms. Lipopolysaccharide (LPS) from gram-negative bacteria can activate neuronal toll-like receptors (TLRs). Bacteria convert tryptophan (Trp) into indole metabolites which can alter gene programming of enteric neurons via aryl hydrocarbon receptor (Ahr) signaling. Trp can also be converted into serotonin (5-HT), which is release by enterochromaffin cells (EC). Bacterial fermentation of fiber produces short-chain fatty acids (SCFAs) which can bind free fatty acid receptor 3 (FFAR3). SCFAs can also trigger L-cells to release neuropeptides glucagon-like peptide 1 (GLP-1) and peptide YY (PYY). Gut microbe production of secondary bile acids binds Takeda G-protein receptor 5 (TGR5) on L-cells to trigger GLP-1 and PYY release. Secondary bile acids can also signal to TGR5 on enteric neurons to regulate motility.
3.1. Lipopolysaccharide (LPS) and Lipoteichoic Acid (LTA)
Lipopolysaccharide and lipoteichoic acid are surface proteins found on gram-negative and gram-positive bacteria, respectively. LPS and LTA are considered pathogen-associated molecular patterns (PAMPs) because they can trigger innate immune responses through toll-like receptors (TLRs). Circulating LPS levels are altered based on diet,62 with increased levels in high fat diet (HFD) rodent models,63,64 and in people with a subset of IBS (diarrhea-predominant).65 LPS levels also correlate with weight gain,62 and chronic LPS injections can induce adipose macrophage infiltration66 and weight gain.63
Along with immune cells, enteric, spinal, and vagal neurons express toll-like receptors (TLRs). Disrupted TLR signaling in peripheral neurons may contribute to deficits in enteric nervous development, gut motility, immunity, and visceral perception (Figure 4). LPS binds to toll-like receptor 4 (TLR4) and LTA binds TLR2. TLR2 expressed in spinal sensory neurons and activation can alter the reflex release of neuropeptides capable of increasing cytokines.67 TLR4 is expressed in vagal neurons which play a key role in the inflammatory reflex where the brain senses potential proinflammatory signals and regulates the immune responses.67,68 This is accomplished by vagal efferent release of acetylcholine onto immune cells in target tissues which can dampen the local immune response. Additionally, vagal TLR4 signaling mediates LPS-induced release of the neuropeptide calcitonin gene-regulated peptide (CGRP).69 CGRP is a vasodilatory neuropeptide released by spinal and vagal afferent neurons, as well as enteric neurons, which contributes to inflammation resolution and pain.70
Enteric neurons express both TLR2 and TLR4. Surprisingly, TLR2 agonists have been shown to promote neurogenesis and restore myenteric neurons in the colon of GF mice and in mice after antibiotic-induced depletion of microbiota.36 TLR4 signaling also promotes enteric neuron survival and regulates proper gastrointestinal motility.71 While TLR signaling appears to be crucial for proper GI development and function, unresolved TLR signaling likely contributes to pain from infection or dysbiosis.72 Future studies likely need to segregate developmental vs. acute signaling roles for TLRs. Furthermore, many studies do not distinguish direct effects of TLR signaling in neurons from immune cell TLR signaling, which indirectly activates peripheral neurons.
3.2. Tryptophan metabolites
Tryptophan (Trp) is an essential amino acid implicated in various diseases such as IBS, metabolic disease, and potentially neurological disease as well.73–75 Trp is metabolized into three major pathways: serotonin (5-HT), kynurenine, and indole. Humans and rodents express enzymes for the conversion of tryptophan into serotonin and kynurenine metabolites, but these pathways are highly influenced by gut microbes, as reviewed extensively by Agus et al.75 Kynurenic acid activates GPR35,76 which is highly expressed in vagal sensory neurons,77 although the function of vagal GPR35 has yet to be directly demonstrated. Trp conversion into indole metabolites mostly requires bacterial enzymes. Indoles can freely diffuse through enterocytes and bind the aryl hydrocarbon receptor (Ahr), which is a nuclear receptor controlling gene expression. Immune Ahr signaling regulates intestinal inflammation, however different studies contradict in whether Ahr is pro- or anti-inflammatory.78
Focusing on PNS Ahr signaling, Obata and colleagues beautifully demonstrated that gene expression of enteric neurons is regulated by local gut bacteria, and the transcriptional programming varies along the intestine due to the bacteria colonized in that region.79 Germ-free (GF) and mice with deletion of enteric neuron Ahr show similarly reduced intestinal transit time compared to specific-pathogen-free (SPF) and Ahr expressing controls. Viral vector expression of Ahr was able to partially rescue disrupted intestinal transit in antibiotic treated mice. Interestingly, the authors suspected that depletion of microbiota-induced serotonin32 likely explained the limited rescue of intestinal transit after viral overexpression of Ahr.
3.3. Serotonin (5-HT)
Gut bacteria regulate the production and release of serotonin (5-HT) and 5-HT signaling is disrupted in GF mice,80,81 obesity models,82 as well as IBS patients.83 Enterochromaffin cells (EC) are the most common enteroendocrine cell in the gut, distributed throughout the entire GI tract, and they produce approximately 90% of the 5-HT in our body. While the gut microbiota does not produce 5-HT directly, SCFAs and secondary bile acids from the gut microbiota can stimulate 5-HT production and release.80 Proper 5-HT production and signaling is crucial for enteric neuron development, maturation, and adult function.33,84 De Vadder et al. found that colonizing GF mice with conventional gut bacteria restored serotonin levels and induced the proliferation of enteric neurons. They demonstrated that the 5-HT4 receptor was key to enteric neuron survival by showing that a specific agonist for this receptor was able to restore ENS innervation and intestinal transit time.33 5-HTR3 is expressed in intrinsic primary afferent neurons (IPANs), which are crucial for converting chemical and mechanical stimuli from the gut lumen to the submucosal and myenteric plexus for proper GI motility, secretion, and blood flow.85 Serotonin can also bind receptors expressed in vagal neurons to slow gastric emptying and delay motility.84 Enterochromaffin cells also form synaptic connections directly with spinal neurons, similar to “neuropod” cells. This connection may serve as a mechanism to fine tune motility, intestinal inflammation, and possibly visceral pain.54
3.4. Gamma-aminobutyric acid (GABA)
Several Lactobacillus and Bifidobacterium species are capable of producing the inhibitory neurotransmitter gamma-aminobutyric acid (GABA).86 They do so by converting glutamate into GABA via the enzyme glutamate decarboxylase (GAD), which functions to reduce the local acidity.87 While GAD-expressing bacteria apparently benefit from the lower pH, the host has clearly evolved to respond to the GABA by-product via a variety of mechanisms. GABAB receptors are GPCRs expressed in enteric, spinal, and vagal afferents,88,89 and activation of this receptor is generally inhibitory.90 For instance, one group found that a GABA-producing probiotic reduced spinal neuron excitability in a rat model of visceral hypersensitivity. Gavage of a Bifidobacterium species lacking GABA-producing enzymes failed to reduce neuronal excitability in their model.24 Activation of GABAB receptors on vagal neurons reduces the sensitivity to mechanical stimuli such as stretch or brushing.89,91 Additionally, vagal GABAB signaling controls gastric emptying, motility, and digestive secretions making it an interesting therapeutic target for functional GI disorders.90 Fecal microbiota transplantation (FMT) from lean donors dramatically increased circulating GABA levels in individuals with metabolic syndrome, which may have contributed to improved insulin sensitivity.92
3.5. Bile acids
Primary bile acids, cholic acid (CA) and chenodeoxycholic acid (CDCA), are produced by the liver and secreted into the small intestine to emulsify dietary fat and cholesterol, which is critical for digestion and nutrient absorption. Bacteria residing in the gut can modify bile acids into lithocholic acid (LCA) and deoxycholic acid (DCA) which are known as secondary bile acids. While these bile acids play an essential role in dietary lipid absorption, and they also function as signaling molecules for farnesoid X receptor (FXR) and Takeda G-protein receptor 5 (TGR5). TGR5 is a GPCR expressed in spinal neurons,93 enteric neurons,94 and vagal neurons,95 and is primarily activated by LCA.
In vagal afferent neurons, TGR5 is co-expressed with cholecystokinin (CCK) receptor+ neurons which are known to regulate feeding behavior. One study in rats demonstrated that bile acids reduce food intake, dependent upon TGR5 expression in vagal neurons. They also showed that TGR5 and CCK synergistically activate nodose neurons to reduce food intake.95 TGR5 activation induces calcium (Ca2+) responses in L-cells and stimulates their release of GLP-1,96 which regulates glucose homeostasis and feeding behavior via vagal and/or hormonal routes.97 TGR5 is also expressed in the hypothalamus, and bile acids can circulate directly to the brain and prevent diet-induced obesity.98 LCA and DCA also activate TGR5 in enteric neurons and enterochromaffin cells (EC) in the colon to control motility.99,100
3.6. Short chain fatty acids (SCFAs)
SCFAs are monocarboxylic acids (acetate, propionate, butyrate, and valerate) produced by fermentation of fiber by various genera of bacteria including Lactobacillus, Bifidobacterium, Prevotella, and Bacteroides.101 Interestingly, these bacterial metabolites exert pleiotropic effects on the host via multiple signaling mechanisms and are an intriguing signal between the gut and the brain.102–104 Focusing on their signaling roles in peripheral neurons, SCFA-binding G-protein coupled receptors are expressed in various peripheral ganglia. For instance, free fatty acid receptor 3 (FFAR3) is expressed in several sympathetic ganglia, enteric neurons, vagal ganglia, celiac/superior mesenteric, and dorsal root ganglia.77,105–107 Distinct roles have been suggested for FFAR3 in spinal, autonomic, and enteric neurons in regulating intestinal gluconeogenesis,32,104 heart rate,106 energy expenditure,108 and food intake.59 FFAR3 signaling also influences fetal nervous system development. The gut microbiota is established after birth, however production of SCFA from the pregnant mother can signal to the developing fetus via FFAR3 and promote sympathetic neuron innervation and neurite outgrowth.38 Ultimately, lack of FFAR3 signaling in utero predisposed the mice to HFD-induced obesity and metabolic syndrome via reduced energy expenditure.38 Using a novel FFAR3 flox mouse model, we found that vagal FFAR3 signaling regulates short-term feeding behavior in adults.59 More studies utilizing cell-type specific and inducible knockout models are necessary to clearly elucidate adult vs. developmental signaling roles for peripheral neurons in sensing fiber fermentation by the gut microbiota.
SCFAs also bind Olfactory receptor 78 (Olfr78) expressed in a subset of vagal sensory neurons,57 however, supraphysiological levels are required for activation. Physiologically, vagal Olfr78 likely is predominantly activated by lactate, which regulates respiration via innervation of the heart and lungs.109 FFAR2 and hydroxycarboxylic acid receptor 2 (HCAR2) also bind SCFAs, but to our knowledge, no studies have clearly defined neuronal populations expressing these receptors.
3.7. Cocaine-amphetamine regulated transcript (CART)
Cocaine-amphetamine regulated transcript (CART) is a neuropeptide expressed in vagal and enteric neurons, as well as in the CNS. Vagal neuron release and expression of CART is modulated based on feeding status via leptin and CCK.110,111 CART+ enteric neurons are modulated by the microbiota and regulate glucose homeostasis via direct communication with the pancreas and liver.112 Muller et al. also identified other ENS transcripts dysregulated in GF mice, including somatostatin (SST) and agouti-related peptide (Agrp),112 which warrants further investigation.
3.8. Substance P and calcitonin gene-regulated peptide (CGRP)
Excitation of spinal and vagal afferent neurons can induce the release of neuropeptides such as Substance P and CGRP, which control blood flow and immune cell function,113 while possibly possessing antimicrobial properties.114 Both peptides are also implicated in visceral and peripheral pain.115 Increased levels of Substance P have been seen in the colon after antibiotic treatment21 and in colitis models.116 As previously mentioned, vagal neurons release CGRP in response to TLR4 activation,69 but more work is needed to determine the physiological consequences of this signaling.
4. Gut microbiota to PNS communication as a therapeutic target
Numerous lines of evidence point to various members of the gut microbiota as contributors in chronic diseases, and microbiota-targeted therapies may provide benefits. Sensory and autonomic nerves innervating the GI tract are sensitive to infection, inflammation and local metabolites which can lead to changes in energy and glucose homeostasis, motility deficits and hypersensitivity commonly seen in IBS, functional dyspepsia, diabetes, or obesity.117,118 Microbiota-targeted treatments for these ailments come in the form of prebiotics, probiotics, postbiotics, fecal microbiota transplantations (FMT), and antibiotics (Figure 5). Prebiotics consist of nutrients directly targeted to feed or promote certain bacterial growth. For instance, dietary fibers like inulin are fermented by our gut bacteria and can provide therapeutic benefits to people who are overweight,119 diabetic,120 or suffering from IBS.121 Probiotics and postbiotics are viable or dead cultures of bacteria, respectively. Combinations of pre- and probiotics are referred to as synbiotics. FMT is a less specific approach where healthy donor bacteria are transferred to a diseased recipient in the hopes of restoring the perturbed microbiota back to healthy form. Different studies and treatment strategies utilize various combinations of pre-, pro-, and/or or postbiotics with antibiotics and/or FMT.
Figure 5.
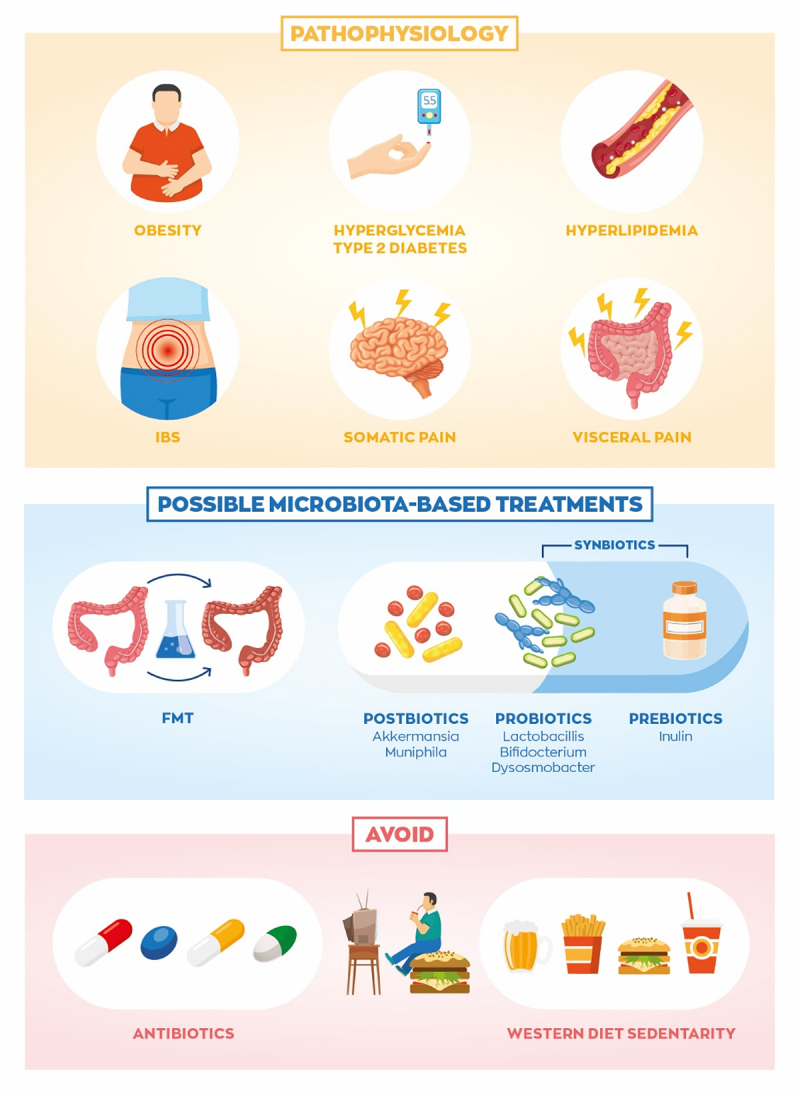
Disrupted gut microbe to peripheral nervous system signaling can lead to obesity, irritable bowel syndrome, and associated comorbidities. Microbiota-targeted therapies such as fecal microbiome transplantation (FMT), postbiotics, probiotics, prebiotics, and combinations may help improve obesity- and IBS-associated complications. Additionally, avoiding antibiotics and western diet may prevent progression of these diseases.
4.1. Obesity and accompanying comorbidities
Obesity is defined by a body mass index (BMI) of 30 kg/m2 or higher, and is associated with increased risk of several comorbidities, particularly heart disease and diabetes. Although studies have been inconsistent in identifying a clear microbiome signature associated with obesity pathogenesis, many recent studies have found effective microbiota-targeted therapies, and we are beginning to uncover some of the signaling mechanisms. For instance, probiotic supplementation of the commensal Dysosmobacter welbionis induced weight loss in mice, likely via activation brown adipose thermogenesis via unknown mechanisms25. The authors speculated that production of the SCFA butyrate was the driver of increased energy expenditure. SCFAs target autonomic circuits regulating glucose homeostasis, motility, feeding behavior, and energy expenditure, which offers promise for anti-obesity therapies. Li et al. demonstrated the thermogenic capability of butyrate to restore energy expenditure after antibiotics depletion.122 SCFAs directly activate FFAR3 in sympathetic neuron to increase energy expenditure106 and vagal sensory neuron to reduce food intake,31,59 thus bidirectionally improving energy balance. Therefore, therapies increasing gut microbe SCFA production could help obese individuals lose weight.123 In addition to SCFAs directly activating autonomic neurons, they can also regulate the release of EEC hormones such as GLP-1124 and GIP.125 These hormones induce potent effects on the host to decrease food intake126 and stimulate insulin release,127 resulting in a huge effort to produce GLP1 and GIP mimetics for obesity and diabetes.97,128 Endogenous release of these peptides could also be therapeutically targeted via modulation of the gut microbiota. Conversely, other studies show that SCFAs increase dietary energy harvest and weight gain, leading to disrupted glucose homeostasis.129,130 Finding the right dose and method of delivery may be necessary to establish SCFAs as a safe and effective dietary supplement.
One major complication of obesity is dysfunctional glucose homeostasis and increased comorbidity of type II diabetes. FMT, probiotic supplements, and dietary regiments have all been shown to improve host glucose homeostasis.92,131,132 A series of brilliant studies from Dr. Cani’s lab demonstrated Akkermansia municiphila to be beneficial for alleviating obesity-associated glucose and lipid disruptions in rodents20 and humans.18 The same group later discovered that a membrane protein that activating TLR2 was responsible for the metabolic improvements, and viable bacteria was not needed.19 Thus, Akkermansia muciniphila is a promising postbiotic supplement to improve metabolic disturbances in obesity. Further work examining the signaling between Akkermansia and TLR2 in peripheral neurons may provide interesting insights into future therapies for pain and motility issues, as well.
Pain also presents as a common feature in obesity and type II diabetes,133 which primarily manifests as somatic pain in the periphery, rather than in the viscera. In a case report, a diabetic woman received FMT from a healthy donor which improved glucose levels and improved indices of pain.134 FMT from lean mice to obese mice also shows efficacy in improving peripheral hypersensitivity caused by a western diet, independent of glucose lowering or weight loss.135 The downfall of FMT-based therapies is the lack of insight provided into how the disease symptoms are alleviated, or which bacteria are providing benefits. More work is needed to elucidate the molecular mechanisms linking obesity-associated pain and the microbiota. While we are still discovering the precise signaling processes involved, microbiota-targeted therapies show great promise for obese individuals by improving glucose intolerance, insulin sensitivity, satiety, appetite, and weight management.
4.2. Irritable bowel syndrome
Irritable bowel syndrome (IBS) is currently the most common functional GI disorder affecting between four and ten percent of the population worldwide, based on conservative estimates.136 Rates of IBS are potentially even higher in the western world, and a “western diet” high in fat and sugar may increase IBS risk137 (Figure 5). The underlying causes of IBS remain a mystery, however symptoms often develop after an acute bout of bacterial gastroenteritis. Later, hallmark features develop, including chronic low-grade inflammation, gastrointestinal motility issues and visceral pain.136 Thus, the interaction between peripheral neurons and the gut microbiota is highly implicated and serves as a promising therapeutic target.11,138,139 IBS can be divided into subtypes depending on the symptoms including bloating, diarrhea or constipation, cramping, and sometimes mood disruptions.6 Again, many of these symptoms correlate with disrupted PNS signaling, whether it be aberrant afferent signaling contributing to intestinal discomfort,41,139 disrupted enteric neuron excitability,140 or dysregulated autonomic signaling to the gut.26,141,142
Interestingly, FMT improved patient outcomes in several IBS trials, while actually worsening symptoms in other studies.143 Given the heterogeneity of symptoms across different types of IBS, a one-size fits all therapy is not likely realistic. Finding ways to individualize therapies for each patient’s unique symptoms and microbiota may be necessary to provide effective treatment.143 As opposed to obesity-associated peripheral pain, IBS often presents with intestinal pain. In animal models, antibiotic treatment causes visceral hypersensitivity,21,144 which can be prevented by probiotic supplementation of Lactobacillus.21,22 Several Lactobacillus and Bifidobacterium23,25 probiotics have been developed and show promise for the treatment of visceral pain in humans, as well. While the mechanisms have not been clearly demonstrated, Lactobacillus and Bifidobacterium produce GABA and SCFAs,24,86,88,102,135 and these molecules appear to exert inhibitory effects on enteric and spinal neurons,24,86,88 which may underly the alleviation of painful symptoms. Some studies show the prebiotic inulin improving constipation in people with IBS,145 however inulin can also cause gas. Combining another prebiotic, psyllium, counteracted this side effect of inulin.146 Numerous lines of evidence also point to disrupted tryptophan metabolism in IBS.75,80 IBS patients also exhibit psychiatric comorbidities, such as heightened stress response mediated by the hypothalamic-pituitary-adrenal (HPA) axis.26,142 There is a vicious cycle of bi-directional dysfunction of the gut brain axis where disruptions in microbiome signaling cause aberrant sensory signals, but perturbations in autonomic outflow can exacerbate intestinal inflammation and dysbiosis. Breaking this cycle through personalized treatments targeting gut microbe to peripheral neuron interactions may provide relief to the many people suffering from IBS.
5. Obstacles, conclusions and future directions
There is still large gap in the knowledge of molecular mechanisms driving the interaction between the gut microbiota and host physiology. The shortfall of microbiome sequencing approaches is that there likely exist a high level of redundancy in the genes and metabolic pathways across different genus of bacteria. Conversely, different species within the same genus may express distinct genes that affect the microbiota landscape or directly signal to the host in a unique way. Furthermore, it is difficult to identify a clear microbial signature driving disease or physiological process when the microbiome differs in individuals across different regions. The functional output of an individual’s microbiota may provide more physiological information than purely microbiome composition. Thus, future studies utilizing proteomic approaches to determine alterations in metabolites or peptides produced by the gut microbiota may prove very insightful.
Although many promising microbiota therapies have been demonstrated in rodent studies, translating to human application has been more difficult for several reasons. Studies tend to use only male mice, yet sex is a key genetic variable in metabolism and GI function,147–149 and IBS prevalence may be higher in females.136 Additionally, laboratory rodents live in excessively sterile environments that prevent the animals from proper immune150,151 and nervous system development,152 rendering them less relevant as model organisms. Paradoxically, dirtier laboratory environments may yield results that offer more translational value to humans.151 Another obstacle preventing us from understanding PNS/microbiota signaling has been identifying the specific cell populations involved. The gut is comprised of complex interactions of neurons, glia, immune, and other cells making it difficult determine the culprit of disease pathogenesis. Continuing with cell-type specific studies in relevant disease models will help us develop more effective treatments.
Despite these obstacles, it is becoming increasingly clear that interaction between gut microbiota and our nervous system is critical for our health and wellness. The microbes in our gut produce a variety of molecules capable of signaling to our peripheral neurons. From the very beginning of life in the womb, the mother’s gut microbes are already regulating fetal nervous system development. Disruptions in gut microbiome composition after infection, antibiotic use, dietary changes, and other environmental influences contribute to dysregulated host functions mediated by peripheral neurons. Continuing our understanding of the molecular mechanisms by which the host senses and responds to the gut microbiota signaling will guide improved development of therapies to combat the epidemics of obesity and gastrointestinal disease.
Funding Statement
This work was supported by the National institute of diabetes and digestive and kidney diseases [DK126441]; National institute of diabetes and digestive and kidney diseases [DK117404].
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Derrien M, Alvarez A-S, De Vos WM.. The Gut Microbiota in the First Decade of Life. Trends in Microbiology. 2019;27(12):997–20. doi: 10.1016/j.tim.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Dominguez-Bello MG, Blaser MJ, Ley RE, Knight R. Development of the Human Gastrointestinal Microbiota and Insights From High-Throughput Sequencing. Gastroenterology. 2011;140(6):1713–1719. doi: 10.1053/j.gastro.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lynch SV, Pedersen O, Phimister EG. The Human Intestinal Microbiome in Health and Disease. New England Journal of Medicine. 2016;375(24):2369–2379. doi: 10.1056/nejmra1600266. [DOI] [PubMed] [Google Scholar]
- 4.Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, Costea PI, Godneva A, Kalka IN, Bar N, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555:210–215. doi: 10.1038/nature25973. [DOI] [PubMed] [Google Scholar]
- 5.Derrien M, Veiga P. Rethinking Diet to Aid Human-Microbe Symbiosis. Trends Microbiol. 2017;25(2):100–112. doi: 10.1016/j.tim.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Enck P, Aziz Q, Barbara G, Farmer AD, Fukudo S, Mayer EA, Niesler B, Quigley EMM, Rajilić-Stojanović M, Schemann M, et al. Irritable bowel syndrome. Nature Reviews Disease Primers. 2016;2:16014. doi: 10.1038/nrdp.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woese CR, Fox GE. Phylogenetic structure of the prokaryotic domain: the primary kingdoms. Proc Natl Acad Sci U S A. 1977;74(11):5088–5090. doi: 10.1073/pnas.74.11.5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 9.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 10.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simrén M, Barbara G, Flint HJ, Spiegel BMR, Spiller RC, Vanner S, Verdu EF, Whorwell PJ, Zoetendal EG. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62(1):159–176. doi: 10.1136/gutjnl-2012-302167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diener C, Qin S, Zhou Y, Patwardhan S, Tang L, Lovejoy JC, Magis AT, Price ND, Hood L, Gibbons SM, et al. Baseline Gut Metagenomic Functional Gene Signature Associated with Variable Weight Loss Responses following a Healthy Lifestyle Intervention in Humans. mSystems. 2021;6(5):e0096421. doi: 10.1128/mSystems.00964-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kovatcheva-Datchary P, Nilsson A, Akrami R, Lee YS, De Vadder F, Arora T, Hallen A, Martens E, Björck I, Bäckhed F. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab. 2015;22(6):971–982. doi: 10.1016/j.cmet.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Van Den Munckhof ICL, Kurilshikov A, ter Horst R, Riksen NP, Joosten LAB, Zhernakova A, Fu J, Keating ST, Netea MG, de Graaf J, et al. Role of gut microbiota in chronic low-grade inflammation as potential driver for atherosclerotic cardiovascular disease: a systematic review of human studies. Obesity Reviews. 2018;19(12):1719–1734. doi: 10.1111/obr.12750. [DOI] [PubMed] [Google Scholar]
- 15.Tap J, Derrien M, Törnblom H, Brazeilles R, Cools-Portier S, Doré J, Störsrud S, Le Nevé B, Öhman L, Simrén M, et al. Identification of an Intestinal Microbiota Signature Associated With Severity of Irritable Bowel Syndrome. Gastroenterology. 2017;152(1):111–123.e118. doi: 10.1053/j.gastro.2016.09.049. [DOI] [PubMed] [Google Scholar]
- 16.Su T, Liu R, Lee A, Long Y, Du L, Lai S, Chen X, Wang L, Si J, Owyang C, et al. Altered Intestinal Microbiota with Increased Abundance of Prevotella Is Associated with High Risk of Diarrhea-Predominant Irritable Bowel Syndrome. Gastroenterol Res Pract. 2018;2018:6961783. doi: 10.1155/2018/6961783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim MH, Yun KE, Kim J, Park E, Chang Y, Ryu S, Kim HL, Kim HN. Gut microbiota and metabolic health among overweight and obese individuals. Sci Rep. 2020;10(1):19417. doi: 10.1038/s41598-020-76474-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, Falony G, Raes J, Maiter D, Delzenne NM, et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nature Medicine. 2019;25(7):1096–1103. doi: 10.1038/s41591-019-0495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, Chilloux J, Ottman N, Duparc T, Lichtenstein L, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nature Medicine. 2017;23(1):107–113. doi: 10.1038/nm.4236. [DOI] [PubMed] [Google Scholar]
- 20.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proceedings of the National Academy of Sciences. 2013;110(22):9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verdú EF, Bercik P, Verma-Gandhu M, Huang XX, Blennerhassett P, Jackson W, Mao Y, Wang L, Rochat F, Collins SM. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut. 2006;55(2):182–190. doi: 10.1136/gut.2005.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rousseaux C, Thuru X, Gelot A, Barnich N, Neut C, Dubuquoy L, Dubuquoy C, Merour E, Geboes K, Chamaillard M, et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med. 2007;13(1):35–37. doi: 10.1038/nm1521. [DOI] [PubMed] [Google Scholar]
- 23.Guglielmetti S, Mora D, Gschwender M, Popp K. Randomised clinical trial: bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life–a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33(10):1123–1132. doi: 10.1111/j.1365-2036.2011.04633.x. [DOI] [PubMed] [Google Scholar]
- 24.Pokusaeva K, Johnson C, Luk B, Uribe G, Fu Y, Oezguen N, Masunami RK, Lugo M, Major A, Mori-Akiyama Y, et al. GABA-producing Bifidobacterium dentium modulates visceral sensitivity in the intestine. Neurogastroenterol Motil. 2017;29(1). doi: 10.1111/nmo.12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whorwell PJ. Dysosmobacter welbionis is a newly isolated human commensal bacterium preventing diet-induced obesity and metabolic disorders in mice. Gut. 2021;71(3):534–543. doi: 10.1136/gutjnl-2020-323778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Palma G, Collins SM, Bercik P. The microbiota-gut-brain axis in functional gastrointestinal disorders. Gut Microbes. 2014;5(3):419–429. doi: 10.4161/gmic.29417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raskov H, Burcharth J, Pommergaard H-C, Rosenberg J. Irritable bowel syndrome, the microbiota and the gut-brain axis. Gut Microbes. 2016;7(5):365–383. doi: 10.1080/19490976.2016.1218585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torres-Fuentes C, Schellekens H, Dinan TG, Cryan JF. The microbiota-gut-brain axis in obesity. Lancet Gastroenterol Hepatol. 2017;2(10):747–756. doi: 10.1016/S2468-1253(17)30147-4. [DOI] [PubMed] [Google Scholar]
- 29.Fülling C, Dinan TG, Cryan JF. Gut Microbe to Brain Signaling: what Happens in Vagus …. Neuron. 2019;101(6):998–1002. doi: 10.1016/j.neuron.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Muller PA, Schneeberger M, Matheis F, Wang P, Kerner Z, Ilanges A, Pellegrino K, Del Mármol J, Castro TBR, Furuichi M, et al. Microbiota modulate sympathetic neurons via a gut–brain circuit. Nature. 2020;583(7816):441–446. doi: 10.1038/s41586-020-2474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Z, Yi C-X, Katiraei S, Kooijman S, Zhou E, Chung CK, Gao Y, van den Heuvel JK, Meijer OC, Berbée JFP, et al. Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut. 2018;67(7):1269–1279. doi: 10.1136/gutjnl-2017-314050. [DOI] [PubMed] [Google Scholar]
- 32.De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Bäckhed F, Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156(1–2):84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 33.De Vadder F, Grasset E, Mannerås Holm L, Karsenty G, Macpherson AJ, Olofsson LE, Bäckhed F. Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc Natl Acad Sci U S A. 2018;115(25):6458–6463. doi: 10.1073/pnas.1720017115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collins J, Borojevic R, Verdu EF, Huizinga JD, Ratcliffe EM. Intestinal microbiota influence the early postnatal development of the enteric nervous system. Neurogastroenterol Motil. 2014;26(1):98–107. doi: 10.1111/nmo.12236. [DOI] [PubMed] [Google Scholar]
- 35.McVey Neufeld KA, Mao YK, Bienenstock J, Foster JA, Kunze WA. The microbiome is essential for normal gut intrinsic primary afferent neuron excitability in the mouse. Neurogastroenterol Motil. 2013;25(2):183–e188. doi: 10.1111/nmo.12049. [DOI] [PubMed] [Google Scholar]
- 36.Yarandi SS, Kulkarni S, Saha M, Sylvia KE, Sears CL, Pasricha PJ. Intestinal Bacteria Maintain Adult Enteric Nervous System and Nitrergic Neurons via Toll-like Receptor 2-induced Neurogenesis in Mice. Gastroenterology. 2020;159(1):200–213.e208. doi: 10.1053/j.gastro.2020.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vuong HE, Pronovost GN, Williams DW, Coley EJL, Siegler EL, Qiu A, Kazantsev M, Wilson CJ, Rendon T, Hsiao EY, et al. The maternal microbiome modulates fetal neurodevelopment in mice. Nature. 2020;586(7828):281–286. doi: 10.1038/s41586-020-2745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kimura I, Miyamoto J, Ohue-Kitano R, Watanabe K, Yamada T, Onuki M, Aoki R, Isobe Y, Kashihara D, Inoue D, et al. Maternal gut microbiota in pregnancy influences offspring metabolic phenotype in mice. Science. 2020;367(6481). doi: 10.1126/science.aaw8429 [DOI] [PubMed] [Google Scholar]
- 39.Furness JB, Callaghan BP, Rivera LR, Cho HJ. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol. 2014;817:39–71. doi: 10.1007/978-1-4939-0897-4_3. [DOI] [PubMed] [Google Scholar]
- 40.Spencer NJ, Zagorodnyuk V, Brookes SJ, Hibberd T. Spinal afferent nerve endings in visceral organs: recent advances. Am J Physiol Gastrointest Liver Physiol. 2016;311(6):G1056–G1063. doi: 10.1152/ajpgi.00319.2016. [DOI] [PubMed] [Google Scholar]
- 41.Smith-Edwards KM, Najjar SA, Edwards BS, Howard MJ, Albers KM, Davis BM. Extrinsic Primary Afferent Neurons Link Visceral Pain to Colon Motility Through a Spinal Reflex in Mice. Gastroenterology. 2019;157(2):522–536.e522. doi: 10.1053/j.gastro.2019.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berthoud HR. The vagus nerve, food intake and obesity. Regul Pept. 2008;149(1–3):15–25. doi: 10.1016/j.regpep.2007.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berthoud HR. Vagal and hormonal gut-brain communication: from satiation to satisfaction. Neurogastroenterol Motil. 2008;20(Suppl 1):64–72. doi: 10.1111/j.1365-2982.2008.01104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85(1–3):1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- 45.Bonaz B, Bazin T, Pellissier S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front Neurosci. 2018;12:49. doi: 10.3389/fnins.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berthoud H-R, Albaugh VL, Neuhuber WL. Gut-brain communication and obesity: understanding functions of the vagus nerve. Journal of Clinical Investigation. 2021;131(10). doi: 10.1172/jci143770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwartz MW, Seeley RJ, Tschöp MH, Woods SC, Morton GJ, Myers MG, D’Alessio D. Cooperation between brain and islet in glucose homeostasis and diabetes. Nature. 2013;503(7474):59–66. doi: 10.1038/nature12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pocai A, Obici S, Schwartz GJ, Rossetti L. A brain-liver circuit regulates glucose homeostasis. Cell Metab. 2005;1(1):53–61. doi: 10.1016/j.cmet.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Yi CX, la Fleur SE, Fliers E, Kalsbeek A. The role of the autonomic nervous liver innervation in the control of energy metabolism. Biochim Biophys Acta. 2010;1802(4):416–431. doi: 10.1016/j.bbadis.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 50.Browning KN, Travagli RA. Central Nervous System Control of Gastrointestinal Motility and Secretion and Modulation of Gastrointestinal Functions. Comprehensive Physiology. 2014:1339–1368. doi: 10.1002/cphy.c130055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Furness JB. Integrated Neural and Endocrine Control of Gastrointestinal Function. Adv Exp Med Biol. 2016;891:159–173. doi: 10.1007/978-3-319-27592-5_16. [DOI] [PubMed] [Google Scholar]
- 52.Vicentini FA, Keenan CM, Wallace LE, Woods C, Cavin J-B, Flockton AR, Macklin WB, Belkind-Gerson J, Hirota SA, Sharkey KA, et al. Intestinal microbiota shapes gut physiology and regulates enteric neurons and glia. Microbiome. 2021;9(1). doi: 10.1186/s40168-021-01165-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams EK, Chang R, Strochlic D, Umans B, Lowell B, Liberles S. Sensory Neurons that Detect Stretch and Nutrients in the Digestive System. Cell. 2016;166(1):209–221. doi: 10.1016/j.cell.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bellono NW, Bayrer JR, Leitch DB, Castro J, Zhang C, O’Donnell TA, Brierley SM, Ingraham HA, Julius D. Enterochromaffin Cells Are Gut Chemosensors that Couple to Sensory Neural Pathways. Cell. 2017;170(1):185–198.e116. doi: 10.1016/j.cell.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaelberer MM, Buchanan KL, Klein ME, Barth BB, Montoya MM, Shen X, Bohórquez DV. A gut-brain neural circuit for nutrient sensory transduction. Science. 2018;361(6408). doi: 10.1126/science.aat5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang YB, de Lartigue G, Page AJ. Dissecting the Role of Subtypes of Gastrointestinal Vagal Afferents. Front Physiol. 2020;11:643. doi: 10.3389/fphys.2020.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bai L, Mesgarzadeh S, Ramesh KS, Huey EL, Liu Y, Gray LA, Aitken TJ, Chen Y, Beutler LR, Ahn JS, et al. Genetic Identification of Vagal Sensory Neurons That Control Feeding. Cell. 2019;179(5):1129–1143.e1123. doi: 10.1016/j.cell.2019.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Borgmann D, Ciglieri E, Biglari N, Brandt C, Cremer AL, Backes H, Tittgemeyer M, Wunderlich FT, Brüning JC, Fenselau H, et al. Gut-brain communication by distinct sensory neurons differently controls feeding and glucose metabolism. Cell Metab. 2021;33(7):1466–1482.e7. doi: 10.1016/j.cmet.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cook TM, Gavini CK, Jesse J, Aubert G, Gornick E, Bonomo R, Gautron L, Layden BT, Mansuy-Aubert V. Vagal neuron expression of the microbiota-derived metabolite receptor, free fatty acid receptor (FFAR3), is necessary for normal feeding behavior. Molecular Metabolism. 2021;54:101350. doi: 10.1016/j.molmet.2021.101350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen J, Cheng M, Wang L, Zhang L, Xu D, Cao P, Wang F, Herzog H, Song S, Zhan C, et al. A Vagal-NTS Neural Pathway that Stimulates Feeding. Curr Biol. 2020;30(20):3986–3998.e3985. doi: 10.1016/j.cub.2020.07.084. [DOI] [PubMed] [Google Scholar]
- 61.Varin EM, Mulvihill EE, Baggio LL, Koehler JA, Cao X, Seeley RJ, Drucker DJ. Distinct Neural Sites of GLP-1R Expression Mediate Physiological versus Pharmacological Control of Incretin Action. Cell Rep. 2019;27(11):3371–3384.e3373. doi: 10.1016/j.celrep.2019.05.055. [DOI] [PubMed] [Google Scholar]
- 62.Troseid M, Nestvold TK, Rudi K, Thoresen H, Nielsen EW, Lappegård KT. Plasma Lipopolysaccharide Is Closely Associated With Glycemic Control and Abdominal Obesity: evidence from bariatric surgery. Diabetes Care. 2013;36(11):3627–3632. doi: 10.2337/dc13-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes. 2007;56(7):1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 64.Kim K-A, Gu W, Lee I-A, Joh E-H, Kim D-H, Chamaillard M. High Fat Diet-Induced Gut Microbiota Exacerbates Inflammation and Obesity in Mice via the TLR4 Signaling Pathway. PLoS ONE. 2012;7(10):e47713. doi: 10.1371/journal.pone.0047713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dlugosz A, Nowak P, D’Amato M, Mohammadian Kermani G, Nyström J, Abdurahman S, Lindberg G. Increased serum levels of lipopolysaccharide and antiflagellin antibodies in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterology & Motility. 2015;27(12):1747–1754. doi: 10.1111/nmo.12670. [DOI] [PubMed] [Google Scholar]
- 66.Caesar R, Reigstad CS, Bäckhed HK, Reinhardt C, Ketonen M, Östergren Lundén G, Cani PD, Bäckhed F. Gut-derived lipopolysaccharide augments adipose macrophage accumulation but is not essential for impaired glucose or insulin tolerance in mice. Gut. 2012;61(12):1701–1707. doi: 10.1136/gutjnl-2011-301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tracey KJ. The inflammatory reflex. Nature. 2002;420(6917):853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 68.Pavlov VA, Tracey KJ. The vagus nerve and the inflammatory reflex–linking immunity and metabolism. Nat Rev Endocrinol. 2012;8(12):743–754. doi: 10.1038/nrendo.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jia L, Lee S, Tierney JA, Elmquist JK, Burton MD, Gautron L. TLR4 Signaling Selectively and Directly Promotes CGRP Release from Vagal Afferents in the Mouse. eneuro. 2021;8(1):ENEURO.0254–20.2020. doi: 10.1523/eneuro.0254-20.2020. ENEURO.0254-0220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pinho-Ribeiro FA, Baddal B, Haarsma R, O’Seaghdha M, Yang NJ, Blake KJ, Portley M, Verri WA, Dale JB, Wessels MR, et al. Blocking Neuronal Signaling to Immune Cells Treats Streptococcal Invasive Infection. Cell. 2018;173(5):1083–1097.e1022. doi: 10.1016/j.cell.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anitha M, Vijay–Kumar M, Sitaraman SV, Gewirtz AT, Srinivasan S. Gut Microbial Products Regulate Murine Gastrointestinal Motility via Toll-Like Receptor 4 Signaling. Gastroenterology. 2012;143(4):1006–1016.e1004. doi: 10.1053/j.gastro.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lacagnina MJ, Watkins LR, Grace PM. Toll-like receptors and their role in persistent pain. Pharmacology & Therapeutics. 2018;184:145–158. doi: 10.1016/j.pharmthera.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dong F, Perdew GH. The aryl hydrocarbon receptor as a mediator of host-microbiota interplay. Gut Microbes. 2020;12(1):1859812. doi: 10.1080/19490976.2020.1859812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ma N, He T, Johnston LJ, Ma X. Host–microbiome interactions: the aryl hydrocarbon receptor as a critical node in tryptophan metabolites to brain signaling. Gut Microbes. 2020;11(5):1203–1219. doi: 10.1080/19490976.2020.1758008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Agus A, Planchais J, Sokol H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe. 2018;23(6):716–724. doi: 10.1016/j.chom.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 76.Wang J, Simonavicius N, Wu X, Swaminath G, Reagan J, Tian H, Ling L. Kynurenic Acid as a Ligand for Orphan G Protein-coupled Receptor GPR35. Journal of Biological Chemistry. 2006;281(31):22021–22028. doi: 10.1074/jbc.m603503200. [DOI] [PubMed] [Google Scholar]
- 77.Egerod KL, Petersen N, Timshel PN, Rekling JC, Wang Y, Liu Q, Schwartz TW, Gautron L. Profiling of G protein-coupled receptors in vagal afferents reveals novel gut-to-brain sensing mechanisms. Mol Metab. 2018;12:62–75. doi: 10.1016/j.molmet.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barroso A, Mahler JV, Fonseca-Castro PH, Quintana FJ. The aryl hydrocarbon receptor and the gut–brain axis. Cellular & Molecular Immunology. 2021;18(2):259–268. doi: 10.1038/s41423-020-00585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Obata Y, Castaño Á, Boeing S, Bon-Frauches AC, Fung C, Fallesen T, de Agüero MG, Yilmaz B, Lopes R, Huseynova A, et al. Neuronal programming by microbiota regulates intestinal physiology. Nature. 2020;578(7794):284–289. doi: 10.1038/s41586-020-1975-8. [DOI] [PubMed] [Google Scholar]
- 80.Yano M,J, Yu K, Donaldson G, Shastri G, Ann P, Ma L, Nagler C, Ismagilov R, Mazmanian S, Hsiao E, et al. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell. 2015;161(2):264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proceedings of the National Academy of Sciences. 2009;106(10):3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Martin AM, Jones LA, Jessup CF, Sun EW, Keating DJ. Diet differentially regulates enterochromaffin cell serotonin content, density and nutrient sensitivity in the mouse small and large intestine. Neurogastroenterology & Motility. 2020;32(8). doi: 10.1111/nmo.13869. [DOI] [PubMed] [Google Scholar]
- 83.Dunlop SP, Coleman NS, Blackshaw E, Perkins AC, Singh G, Marsden CA, Spiller RC. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2005;3(4):349–357. doi: 10.1016/s1542-3565(04)00726-8. [DOI] [PubMed] [Google Scholar]
- 84.Fukumoto S, Tatewaki M, Yamada T, Fujimiya M, Mantyh C, Voss M, Eubanks S, Harris M, Pappas TN, Takahashi T. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284(5):R1269–1276. doi: 10.1152/ajpregu.00442.2002. [DOI] [PubMed] [Google Scholar]
- 85.McLean PG, Borman RA, Lee K. 5-HT in the enteric nervous system: gut function and neuropharmacology. Trends Neurosci. 2007;30(1):9–13. doi: 10.1016/j.tins.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 86.Strandwitz P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018;1693:128–133. doi: 10.1016/j.brainres.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Feehily C, Karatzas KA. Role of glutamate metabolism in bacterial responses towards acid and other stresses. J Appl Microbiol. 2013;114(1):11–24. doi: 10.1111/j.1365-2672.2012.05434.x. [DOI] [PubMed] [Google Scholar]
- 88.Page AJ, O’Donnell TA, Blackshaw LA. Inhibition of mechanosensitivity in visceral primary afferents by GABAB receptors involves calcium and potassium channels. Neuroscience. 2006;137(2):627–636. doi: 10.1016/j.neuroscience.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 89.Page AJ, Blackshaw LA. GABA B Receptors Inhibit Mechanosensitivity of Primary Afferent Endings. The Journal of Neuroscience. 1999;19(19):8597–8602. doi: 10.1523/jneurosci.19-19-08597.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hyland NP, Cryan JF. A Gut Feeling about GABA: focus on GABA(B) Receptors. Front Pharmacol. 2010;1:124. doi: 10.3389/fphar.2010.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Andrews PL, Bingham S, Wood KL. Modulation of the vagal drive to the intramural cholinergic and non-cholinergic neurones in the ferret stomach by baclofen. The Journal of Physiology. 1987;388(1):25–39. doi: 10.1113/jphysiol.1987.sp016599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kootte RS, Levin E, Salojärvi J, Smits LP, Hartstra AV, Udayappan SD, Hermes G, Bouter KE, Koopen AM, Holst JJ, et al. Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metabolism. 2017;26(4):611–619.e616. doi: 10.1016/j.cmet.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 93.Alemi F, Kwon E, Poole DP, Lieu T, Lyo V, Cattaruzza F, Cevikbas F, Steinhoff M, Nassini R, Materazzi S, et al. The TGR5 receptor mediates bile acid-induced itch and analgesia. J Clin Invest. 2013;123(4):1513–1530. doi: 10.1172/JCI64551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Poole DP, Godfrey C, Cattaruzza F, Cottrell GS, Kirkland JG, Pelayo JC, Bunnett NW, Corvera CU. Expression and function of the bile acid receptor GpBAR1 (TGR5) in the murine enteric nervous system. Neurogastroenterol Motil. 2010;22(7):814–825, e227–818. doi: 10.1111/j.1365-2982.2010.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu X, Li J-Y, Lee A, Lu Y-X, Zhou S-Y, Owyang C. Satiety induced by bile acids is mediated via vagal afferent pathways. JCI Insight. 2020;5(14). doi: 10.1172/jci.insight.132400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brighton CA, Rievaj J, Kuhre RE, Glass LL, Schoonjans K, Holst JJ, Gribble FM, Reimann F. Bile Acids Trigger GLP-1 Release Predominantly by Accessing Basolaterally Located G Protein–Coupled Bile Acid Receptors. Endocrinology. 2015;156(11):3961–3970. doi: 10.1210/en.2015-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Drucker DJ. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018;27(4):740–756. doi: 10.1016/j.cmet.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 98.Castellanos-Jankiewicz A, Guzmán-Quevedo O, Fénelon VS, Zizzari P, Quarta C, Bellocchio L, Tailleux A, Charton J, Fernandois D, Henricsson M, et al. Hypothalamic bile acid-TGR5 signaling protects from obesity. Cell Metab. 2021;33(7):1483–1492.e1410. doi: 10.1016/j.cmet.2021.04.009. [DOI] [PubMed] [Google Scholar]
- 99.Alemi F, Poole DP, Chiu J, Schoonjans K, Cattaruzza F, Grider JR, Bunnett NW, Corvera CU. The Receptor TGR5 Mediates the Prokinetic Actions of Intestinal Bile Acids and Is Required for Normal Defecation in Mice. Gastroenterology. 2013;144(1):145–154. doi: 10.1053/j.gastro.2012.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lund ML, Egerod KL, Engelstoft MS, Dmytriyeva O, Theodorsson E, Patel BA, Schwartz TW. Enterochromaffin 5-HT cells - A major target for GLP-1 and gut microbial metabolites. Mol Metab. 2018;11:70–83. doi: 10.1016/j.molmet.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Markowiak-Kopeć P, Śliżewska K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients. 2020;12(4):1107. doi: 10.3390/nu12041107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 2019;16(8):461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- 103.Jameson KG, Olson CA, Kazmi SA, Hsiao EY. Toward Understanding Microbiome-Neuronal Signaling. Mol Cell. 2020;78(4):577–583. doi: 10.1016/j.molcel.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 104.Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From Dietary Fiber to Host Physiology: short-Chain Fatty Acids as Key Bacterial Metabolites. Cell. 2016;165(6):1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 105.Nøhr MK, Egerod KL, Christiansen SH, Gille A, Offermanns S, Schwartz TW, Møller M. Expression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory ganglia. Neuroscience. 2015;290:126–137. doi: 10.1016/j.neuroscience.2015.01.040. [DOI] [PubMed] [Google Scholar]
- 106.Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S, Kobayashi M, Hirasawa A, Tsujimoto G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci U S A. 2011;108(19):8030–8035. doi: 10.1073/pnas.1016088108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nøhr MK, Pedersen MH, Gille A, Egerod KL, Engelstoft MS, Husted AS, Sichlau RM, Grunddal KV, Seier Poulsen S, Han S, et al. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology. 2013;154(10):3552–3564. doi: 10.1210/en.2013-1142. [DOI] [PubMed] [Google Scholar]
- 108.Bellahcene M, O’Dowd JF, Wargent ET, Zaibi MS, Hislop DC, Ngala RA, Smith DM, Cawthorne MA, Stocker CJ, Arch JRS, et al. Male mice that lack the G-protein-coupled receptor GPR41 have low energy expenditure and increased body fat content. Br J Nutr. 2013;109(10):1755–1764. doi: 10.1017/S0007114512003923. [DOI] [PubMed] [Google Scholar]
- 109.Chang AJ, Ortega FE, Riegler J, Madison DV, Krasnow MA. Oxygen regulation of breathing through an olfactory receptor activated by lactate. Nature. 2015;527(7577):240–244. doi: 10.1038/nature15721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.de Lartigue G, Lur G, Dimaline R, Varro A, Raybould H, Dockray GJ. EGR1 Is a target for cooperative interactions between cholecystokinin and leptin, and inhibition by ghrelin, in vagal afferent neurons. Endocrinology. 2010;151(8):3589–3599. doi: 10.1210/en.2010-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.de Lartigue G, Ronveaux CC, Raybould HE. Deletion of leptin signaling in vagal afferent neurons results in hyperphagia and obesity. Mol Metab. 2014;3(6):595–607. doi: 10.1016/j.molmet.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Muller PA, Matheis F, Schneeberger M, Kerner Z, Jové V, Mucida D. Microbiota-modulated CART + enteric neurons autonomously regulate blood glucose. Science. 2020;370(6514):314–321. doi: 10.1126/science.abd6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jacobson A, Yang D, Vella M, Chiu IM. The intestinal neuro-immune axis: crosstalk between neurons, immune cells, and microbes. Mucosal Immunology. 2021;14(3):555–565. doi: 10.1038/s41385-020-00368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Aresti Sanz J, El Aidy S. Microbiota and gut neuropeptides: a dual action of antimicrobial activity and neuroimmune response. Psychopharmacology. 2019;236(5):1597–1609. doi: 10.1007/s00213-019-05224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Iyengar S, Ossipov MH, Johnson KW. The role of calcitonin gene–related peptide in peripheral and central pain mechanisms including migraine. Pain. 2017;158(4):543–559. doi: 10.1097/j.pain.0000000000000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Miampamba M, Sharkey KA. Distribution of calcitonin gene-related peptide, somatostatin, substance P and vasoactive intestinal polypeptide in experimental colitis in rats. Neurogastroenterol Motil. 1998;10(4):315–329. doi: 10.1046/j.1365-2982.1998.00111.x. [DOI] [PubMed] [Google Scholar]
- 117.Bercík P, De Giorgio R, Blennerhassett P, Verdú EF, Barbara G, Collins SM. Immune-mediated neural dysfunction in a murine model of chronic Helicobacter pylori infection. Gastroenterology. 2002;123(4):1205–1215. doi: 10.1053/gast.2002.36024. [DOI] [PubMed] [Google Scholar]
- 118.Cervi AL, Lukewich MK, Lomax AE. Neural regulation of gastrointestinal inflammation: role of the sympathetic nervous system. Auton Neurosci. 2014;182:83–88. doi: 10.1016/j.autneu.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 119.Martinez TM, Meyer RK, Duca FA. Therapeutic Potential of Various Plant-Based Fibers to Improve Energy Homeostasis via the Gut Microbiota. Nutrients. 2021;13(10):3470. doi: 10.3390/nu13103470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chambers ES, Byrne CS, Morrison DJ, Murphy KG, Preston T, Tedford C, Garcia-Perez I, Fountana S, Serrano-Contreras JI, Holmes E, et al. Dietary supplementation with inulin-propionate ester or inulin improves insulin sensitivity in adults with overweight and obesity with distinct effects on the gut microbiota, plasma metabolome and systemic inflammatory responses: a randomised cross-over t. Gut. 2019;68(8):1430–1438. doi: 10.1136/gutjnl-2019-318424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gruenwald J, Busch R, Bentley C. Clinical and Experimental Gastroenterology. Efficacy and tolerability of Laxatan Granulat in patients with chronic constipation. 2009;2:95–100. doi: 10.2147/ceg.s6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li B, Li L, Li M, Lam SM, Wang G, Wu Y, Zhang H, Niu C, Zhang X, Liu X, et al. Microbiota Depletion Impairs Thermogenesis of Brown Adipose Tissue and Browning of White Adipose Tissue. Cell Reports. 2019;26(10):2720–2737.e2725. doi: 10.1016/j.celrep.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 123.Byrne CS, Chambers ES, Morrison DJ, Frost G. The role of short chain fatty acids in appetite regulation and energy homeostasis. International Journal of Obesity. 2015;39(9):1331–1338. doi: 10.1038/ijo.2015.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM, et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61(2):364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee EY, Zhang X, Miyamoto J, Kimura I, Taknaka T, Furusawa K, Jomori T, Fujimoto K, Uematsu S, Miki T. Gut carbohydrate inhibits GIP secretion via a microbiota/SCFA/FFAR3 pathway. J Endocrinol. 2018;239(3):267–276. doi: 10.1530/JOE-18-0241. [DOI] [PubMed] [Google Scholar]
- 126.Zhang Q, Delessa CT, Augustin R, Bakhti M, Colldén G, Drucker DJ, Feuchtinger A, Caceres CG, Grandl G, Harger A, et al. The glucose-dependent insulinotropic polypeptide (GIP) regulates body weight and food intake via CNS-GIPR signaling. Cell Metab. 2021;33(4):833–844.e835. doi: 10.1016/j.cmet.2021.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hutch CR, Roelofs K, Haller A, Sorrell J, Leix K, D’Alessio DD, Augustin R, Seeley RJ, Klein T, Sandoval DA, et al. The role of GIP and pancreatic GLP-1 in the glucoregulatory effect of DPP-4 inhibition in mice. Diabetologia. 2019;62(10):1928–1937. doi: 10.1007/s00125-019-4963-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Campbell E,J, Drucker J,D. Pharmacology, Physiology, and Mechanisms of Incretin Hormone Action. Cell Metabolism. 2013;17(6):819–837. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 129.Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U S A. 2008;105(43):16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tirosh A, Calay ES, Tuncman G, Claiborn KC, Inouye KE, Eguchi K, Alcala M, Rathaus M, Hollander KS, Ron I, et al. The short-chain fatty acid propionate increases glucagon and FABP4 production, impairing insulin action in mice and humans. Sci Transl Med. 2019;11(489). doi: 10.1126/scitranslmed.aav0120 [DOI] [PubMed] [Google Scholar]
- 131.Ruan Y, Sun J, He J, Chen F, Chen R, Chen H. Effect of Probiotics on Glycemic Control: a Systematic Review and Meta-Analysis of Randomized, Controlled Trials. PLOS ONE. 2015;10(7):e0132121. doi: 10.1371/journal.pone.0132121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.De Groot P, Nikolic T, Pellegrini S, Sordi V, Imangaliyev S, Rampanelli E, Hanssen N, Attaye I, Bakker G, Duinkerken G, et al. Faecal microbiota transplantation halts progression of human new-onset type 1 diabetes in a randomised controlled trial. Gut. 2021;70(1):92–105. doi: 10.1136/gutjnl-2020-322630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Callaghan BC, Xia R, Reynolds E, Banerjee M, Rothberg AE, Burant CF, Villegas-Umana E, Pop-Busui R, Feldman EL. Association Between Metabolic Syndrome Components and Polyneuropathy in an Obese Population. JAMA Neurology. 2016;73(12):1468. doi: 10.1001/jamaneurol.2016.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cai T-T, Ye X-L, Yong H-J, Song B, Zheng X-L, Cui B-T, Zhang F-M, Lu Y-B, Miao H, Ding D-F, et al. Fecal microbiota transplantation relieve painful diabetic neuropathy. Medicine. 2018;97(50):e13543. doi: 10.1097/MD.0000000000013543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bonomo RR, Cook TM, Gavini CK, White CR, Jones JR, Bovo E, Zima AV, Brown IA, Dugas LR, Zakharian E, et al. Fecal transplantation and butyrate improve neuropathic pain, modify immune cell profile, and gene expression in the PNS of obese mice. Proc Natl Acad Sci U S A. 2020;117(42):26482–26493. doi: 10.1073/pnas.2006065117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Oka P, Parr H, Barberio B, Black CJ, Savarino EV, Ford AC. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: a systematic review and meta-analysis. The Lancet Gastroenterology & Hepatology. 2020;5(10):908–917. doi: 10.1016/s2468-1253(20)30217-x. [DOI] [PubMed] [Google Scholar]
- 137.Buscail C, Sabate J-M, Bouchoucha M, Kesse-Guyot E, Hercberg S, Benamouzig R, Julia C. Western Dietary Pattern Is Associated with Irritable Bowel Syndrome in the French NutriNet Cohort. Nutrients. 2017;9(9):986. doi: 10.3390/nu9090986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Saulnier DM, Ringel Y, Heyman MB, Foster JA, Bercik P, Shulman RJ, Versalovic J, Verdu EF, Dinan TG, Hecht G, et al. The intestinal microbiome, probiotics and prebiotics in neurogastroenterology. Gut Microbes. 2013;4(1):17–27. doi: 10.4161/gmic.22973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Coss-Adame E, Rao SSC. Brain and Gut Interactions in Irritable Bowel Syndrome: new Paradigms and New Understandings. Current Gastroenterology Reports. 2014;16(4). doi: 10.1007/s11894-014-0379-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li S, Fei G, Fang X, Yang X, Sun X, Qian J, Wood JD, Ke M. Changes in Enteric Neurons of Small Intestine in a Rat Model of Irritable Bowel Syndrome with Diarrhea. J Neurogastroenterol Motil. 2016;22(2):310–320. doi: 10.5056/jnm15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cheng P, Shih W, Alberto M, Presson AP, Licudine A, Mayer EA, Naliboff BD, Chang L. Autonomic response to a visceral stressor is dysregulated in irritable bowel syndrome and correlates with duration of disease. Neurogastroenterology & Motility. 2013. n/a-n/a 10.1111/nmo.12177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chang L. The Role of Stress on Physiologic Responses and Clinical Symptoms in Irritable Bowel Syndrome. Gastroenterology. 2011;140(3):761–765.e765. doi: 10.1053/j.gastro.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Benech N, Sokol H. Fecal microbiota transplantation in gastrointestinal disorders: time for precision medicine. Genome Medicine. 2020;12(1). doi: 10.1186/s13073-020-00757-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Aguilera M, Vergara P, Martínez V. Stress and antibiotics alter luminal and wall-adhered microbiota and enhance the local expression of visceral sensory-related systems in mice. Neurogastroenterol Motil. 2013;25(8):e515–529. doi: 10.1111/nmo.12154. [DOI] [PubMed] [Google Scholar]
- 145.Gruenwald J, Busch R, Bentley C. Efficacy and tolerability of Laxatan® Granulat in patients with chronic constipation. Clinical and Experimental Gastroenterology. 2009:95. doi: 10.2147/ceg.s6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gunn D, Abbas Z, Harris HC, Major G, Hoad C, Gowland P, Marciani L, Gill SK, Warren FJ, Rossi M, et al. Psyllium reduces inulin-induced colonic gas production in IBS: MRI and in vitro fermentation studies. Gut. 2021;71(5):919–927. gutjnl-2021-2324. doi: 10.1136/gutjnl-2021-324784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mauvais-Jarvis F, Arnold AP, Reue K. A Guide for the Design of Pre-clinical Studies on Sex Differences in Metabolism. Cell Metabolism. 2017;25(6):1216–1230. doi: 10.1016/j.cmet.2017.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rastelli D, Robinson A, Lagomarsino VN, Matthews LT, Hassan R, Perez K, Dan W, Yim PD, Mixer M, Prochera A, et al. Diminished androgen levels are linked to irritable bowel syndrome and cause bowel dysfunction in mice. Journal of Clinical Investigation. 2022;132(2). doi: 10.1172/jci150789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Meleine M. Gender-related differences in irritable bowel syndrome: potential mechanisms of sex hormones. World Journal of Gastroenterology. 2014;20(22):6725. doi: 10.3748/wjg.v20.i22.6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Abolins S, King EC, Lazarou L, Weldon L, Hughes L, Drescher P, Raynes JG, Hafalla JCR, Viney ME, Riley EM, et al. The comparative immunology of wild and laboratory mice, Mus musculus domesticus. Nature Communications. 2017;8(1):14811. doi: 10.1038/ncomms14811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Beura LK, Hamilton SE, Bi K, Schenkel JM, Odumade OA, Casey KA, Thompson EA, Fraser KA, Rosato PC, Filali-Mouhim A, et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature. 2016;532(7600):512–516. doi: 10.1038/nature17655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Koizumi R, Kiyokawa Y, Mikami K, Ishii A, Tanaka KD, Tanikawa T, Takeuchi Y. Structural differences in the brain between wild and laboratory rats (Rattus norvegicus): potential contribution to wariness. Journal of Veterinary Medical Science. 2018;80(7):1054–1060. doi: 10.1292/jvms.18-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]


