Abstract
Rapidly progressive dementias (RPDs) are a group of heterogeneous disorders that include immune-mediated, infectious and metabolic encephalopathies, as well as prion diseases and atypically rapid presentations of more common neurodegenerative diseases. Some of these conditions are treatable, and some must be diagnosed promptly because of their potential infectivity. Prion disease is considered to be the prototypical RPD, but over the past two decades, epidemiological reports and the identification of various encephalitis-mediating antibodies have led to a growing recognition of other encephalopathies as potential causes of rapid cognitive decline. Knowledge of RPD aetiologies, syndromes and diagnostic work-up protocols will help clinicians to establish an early, accurate diagnosis, thereby reducing morbidity and mortality, especially in immune-mediated and other potentially reversible dementias. In this Review, we define the syndrome of RPD and shed light on its different aetiologies and on secondary factors that might contribute to rapid cognitive decline. We describe an extended diagnostic procedure in the context of important differential diagnoses, discuss the utility of biomarkers and summarize potential treatment options. In addition, we discuss treatment options such as high-dose steroid therapy in the context of therapy and diagnosis in clinically ambiguous cases.
Subject terms: Encephalopathy, Prion diseases, Alzheimer's disease
The term ‘rapidly progressive dementia’ (RPD) describes a cognitive disorder with fast progression, leading to dementia within a relatively short time. This Review discusses the wide range of RPD aetiologies, as well as the diagnostic approach and treatment options.
Key points
Definitions of rapidly progressive dementia (RPD) vary according to the aetiological background and relate to the speed of cognitive decline, time from first symptom to dementia syndrome and/or overall survival.
RPD can occur in rapidly progressive neurodegenerative diseases, such as prion diseases, or in primarily slowly progressive diseases as a consequence of intrinsic factors or concomitant pathologies.
Besides neurodegenerative diseases, inflammatory (immune-mediated and infectious), vascular, metabolic and neoplastic CNS diseases are important and frequent causes of RPD.
To identify treatable causes of RPD, the technical diagnostic work-up must include MRI and analyses of blood and cerebrospinal fluid, and further diagnostics might be indicated in unclear cases.
Therapeutic options for many non-neurodegenerative causes of RPD are already available; disease-modifying therapies for neurodegenerative RPDs are an important focus of current research and could become a treatment option in the near future.
Introduction
The term ‘rapidly progressive dementia’ (RPD) is commonly used to describe a cognitive disorder with fast progression leading to the clinical syndrome of dementia, as defined by the Diagnostic and Statistical Manual of Mental Disorders fourth edition1, within a relatively brief time period, which is commonly considered to be less than either 1 or 2 years2. This rather vague definition encompasses a large group of heterogeneous disorders, including immune-mediated, infectious and metabolic encephalopathies, as well as prion diseases and atypically rapid presentations of other neurodegenerative diseases. As RPD is one of the typical clinical characteristics of Creutzfeldt–Jakob disease (CJD) and has long been part of the diagnostic criteria for this condition3, prion diseases have been considered to be prototypical RPDs. However, the growing recognition of immune-mediated encephalitis4, rapidly progressive subtypes of classic dementias such as Alzheimer disease (AD)5 and various other mimics of prion diseases6,7 demands a thorough consideration of differential diagnoses, especially potentially reversible conditions8,9. Moreover, the potential infectivity of some diseases underlying RPD, such as HIV or prion diseases, must be considered as a matter of public health10.
In this Review, we discuss the definitions of RPD and shed light on its different aetiologies. We do not provide exhaustive lists of differential diagnoses because they can be readily found elsewhere2,9,11–16. Instead, we describe the most important entities, underlying pathophysiological mechanisms, disease categories and factors that might contribute to rapid cognitive decline in primarily slowly progressive neurodegenerative diseases. We also discuss the diagnostic procedure, the likelihood that certain diseases are related to the speed of disease progression, and the utility of biomarkers. Finally, we summarize current curative and palliative treatment options. Knowledge of the aetiologies, syndromes and complex diagnostic work-up of RPD will help clinicians to establish an early diagnosis and prevent morbidity and mortality.
Definition and prevalence of RPD
One of the earliest scientific articles to mention RPD, published in the 1950s, described this disorder in the context of demyelinating diseases17. In the intervening years, RPD has become increasingly recognized as a distinct clinical syndrome that occurs in atypical (non-AD) dementias11, human prion diseases and related disorders that are considered in the differential diagnosis of these conditions12,18. Although general definitions usually consider less than 1 or 2 years as the time span from the first disease-related symptom to development of the dementia syndrome2, some causes of RPD, such as encephalitis or metabolic encephalopathies, can lead to dementia within weeks. In addition, particular definitions of RPD in neurodegenerative diseases have been proposed, using either total disease duration19 or measures for speed of cognitive decline such as changes in Mini-Mental State Examination (MMSE) scores in rapidly progressive AD (rpAD)5,20. A summary of common definitions2,5,18–23 can be found in Table 1. Owing to these heterogeneous definitions, the overall incidence of RPD is difficult to determine or even to estimate, and data are scarce. According to a Brazilian study from a tertiary care centre, 3.7% of all patients referred to a neurological unit over 3 years were diagnosed with RPD24. In other single-centre studies, around one-quarter of hospitalized patients with dementia were classified as RPD (24% in Greece25 and 27% in India26).
Table 1.
Definitions of rapidly progressive dementia
| Study | Type of RPD | Definition of RPD | Additional diagnostic characteristics |
|---|---|---|---|
| Geschwind (2016)2 | General definition of RPD | Symptom onset to dementia: <1 or 2 years | NA |
| Degnan and Levy (2014)22 | General definition of RPD | Symptom onset to dementia: <6 months | NA |
| Josephs et al. (2009)19 | Rapidly progressive neurodegenerative dementia | Symptom onset to death: <4 years | Neuropathological diagnosis of neurodegenerative disease |
| Soto et al. (2008)5 | Rapidly progressive AD | Reduction of ≥3 points per 6 months in MMSE score | Clinical diagnosis of AD |
| Schmidt et al. (2011)20 | Rapidly progressive AD | Reduction of ≥6 points per year in MMSE score | Clinical diagnosis of AD |
| Gaig et al. (2011)21 | Rapidly progressive DLB | Symptom onset to death: ≤1.5 years | Neuropathological diagnosis of diffuse Lewy body disease |
| Garcia-Esparcia et al. (2017)23 | Rapidly progressive DLB | Symptom onset to death: ≤2 years | Neuropathological diagnosis of diffuse Lewy body disease |
| Zerr et al. (2009)18 | Possible sporadic CJD | Total duration <2 years | CJD typical clinical syndrome |
AD, Alzheimer disease; CJD, Creutzfeldt–Jakob disease; DLB, dementia with Lewy bodies; MMSE, Mini-Mental State Examination; NA, not applicable; RPD, rapidly progressive dementia.
In summary, quantification of the prevalence or incidence of RPD depends on its definition, the type of evaluating centre and probably also the demographic characteristics of the population. Analyses of the global disease burden have shown that 43.8 million people lived with dementia in 2016 (ref.27). Assuming that a substantial percentage of these patients presented with RPD, the syndrome has exceedingly high clinical relevance.
Aetiologies of RPD
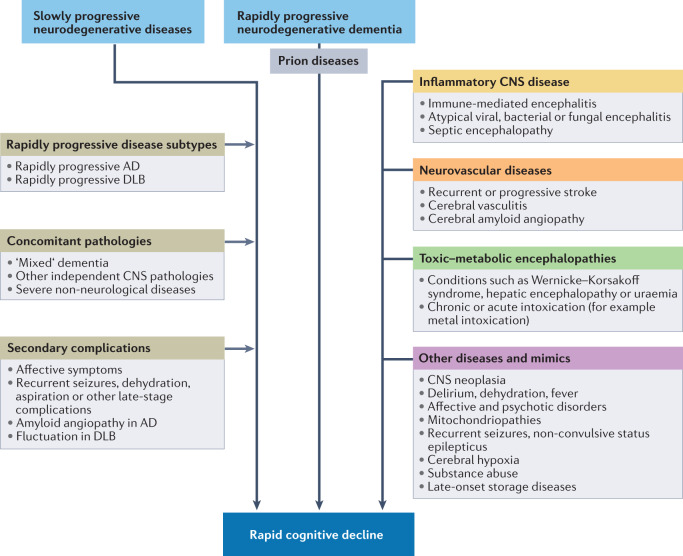
Rapid disease progression in dementia syndromes can be roughly categorized as primary or secondary. Primary rapid disease progression occurs in prion diseases, rapidly progressive types of other neurodegenerative dementias, encephalitis and other diseases that typically cause severe neuronal injury within a relatively brief time period. Secondary rapid disease progression can occur in primarily slowly progressive CNS diseases as a consequence of complications such as seizures or in the presence of concomitant CNS pathologies (for example, AD with cerebrovascular disease or Lewy body pathology). Furthermore, severe non-CNS pathologies might contribute to neuronal injury, to the overall clinical impression (for example, pain, dyspnoea or neoplasia) or to reduced survival times. The diseases and clinical factors that have been implicated in rapid cognitive decline are summarized in Fig. 1.
Fig. 1. Rapidly progressive dementia: disease entities and contributing factors.
The flow chart shows the diseases and other contributing clinical factors that have been implicated in rapid cognitive decline. AD, Alzheimer disease; DLB, dementia with Lewy bodies.
The distribution of different aetiologies of RPD has been evaluated in several studies from specialist6,28–32 and tertiary24–26,33–35 centres, and the observed frequencies are highly dependent on the study design (Table 2). According to data from CJD referral centres, prion diseases accounted for 53–76% of cases in autopsy series6,30–32, compared with 34% in a longitudinal multicentre study that used autopsy data or clinical diagnostic criteria including EEG, MRI and cerebrospinal fluid (CSF) analyses29. Among individuals without prion disease, AD was the most frequent differential diagnosis (16–51%), and potentially treatable conditions such as encephalitis (8–21%) or toxic–metabolic encephalopathies (1–10%) were less common6,28,30–32. By contrast, in a smaller, more recent study, inflammatory (immune-mediated and infectious) and cerebrovascular diseases were the most important differential diagnoses36 for CJD. Although these numbers might reflect the aetiologies of RPD to some degree, they must be interpreted cautiously. The studies included patients with suspected CJD and were not based on the presence of an RPD syndrome alone. In retrospective studies from tertiary centres, only 8% (ref.26) to 31% (ref.33) of referred RPD syndromes were caused by prion diseases. In these studies, inflammatory CNS diseases (35–66%)24,26,34 and neurodegenerative diseases (28–47%)25,33,35 were the most frequent differential diagnoses. These discrepancies might be attributable to differences in study design, type of referral centre or health-care system, or increased awareness of immune-mediated encephalitis as a potential cause of RPD over the years.
Table 2.
RPD aetiologies in routine clinical practice and specialist referral centres
| Study | Definition of RPD | Number of patients | Inflammatory (%) | Neurodegenerative (%) | Neurovascular (%) | Toxic–metabolic (%) | Other (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Infectious | Immune-mediated | CJD | AD | Other | ||||||
| Tertiary centre | ||||||||||
| Acosta et al. (2020)35 | First symptom to dementia: ≤2 years | 104 | 3 | 23 | 30 | 9 | 19 | 3 | 6 | 8 |
| Anuja et al. (2018)26 | First symptom to dementia: ≤1 year | 187 | 21 | 18 | 8 | 14 (AD or other) | 10 | 16 | 13 | |
| Neto et al. (2017)a (ref.24) | First symptom to MMSE score <20: ≤2 years | 61 | 20 | 46 | 12 | 8 (AD or other) | Counted as ‘other’ | Counted as ‘other’ | 15 | |
| Zhang et al. (2017)34 | First symptom to dementia: ≤2 years | 310 | 26 | 9 | 7 | 15 | 10 | Excluded | 10 | 23 |
| Sala et al. (2012)b (ref.33) | First symptom to dementia: ≤1 year | 49 | 2 | 4 | 31 | 14 | 23 | 8 | 8 | 12 |
| Papageorgiou et al. (2009)b (ref.25) | First symptom to dementia ≤1 year | 68 | 6 | 9 | 13 | 18 | 29 | 13 | Excluded | 12 |
| Outpatient memory clinic | ||||||||||
| Day et al. (2018)c (ref.28) | First symptom to dementia: ≤2 years | 67 | Counted as ‘other’ | Counted as ‘other’ | 6 | 66 | 27 | Counted as ‘other’ | Counted as ‘other’ | 5 |
| Single-centre CJD surveillance | ||||||||||
| Chitravas et al. (2011)6 | Initially suspected prion disease | 304 | 5 | 9 | NAd | 51 | 12 | 12 | 2 | 10 |
| Grau-Rivera et al. (2015)31 | Initially suspected prion disease | 52 | 8 | 13 | NAd | 29 | 23 | 13 | 6 | 8 |
| Maat et al. (2015)32 | Initially suspected prion disease | 181 | 4 | 12 | NAd | 34 | 17 | 11 | 1 | 21 |
| Peckeu et al. (2017)30 | Initially suspected prion disease | 483 | 8 (infectious or immune-mediated) | NAd | 36 | 12 | 9 | 10 | 25 | |
| Multicentre CJD surveillance | ||||||||||
| Stoeck et al. (2012)29 | Initially suspected prion disease | 7,115 | 11e (infectious or immune-mediated) | NAd | 16 | 24 | 11 | 7 | 31 | |
In tertiary centres and the outpatient clinic, diagnoses were based on clinical criteria24–26,28,31,33, whereas diagnosis in CJD surveillance centres was fully6,30–32 or partially29 based on neuropathology. AD, Alzheimer disease; CJD, Creutzfeldt–Jakob disease; MMSE, Mini-Mental State Examination; NA, not applicable; RPD, rapidly progressive dementia. aDelirium was an additional exclusion criterion. bAdditional exclusion criteria: acute cognitive disturbance associated with infections, metabolic disorder and intoxication. cAdditional inclusion criteria: increase of more than two Clinical Dementia Rating stages in ≤2 years. dOwing to selection bias, prion diseases were not considered for this table. eParaneoplastic disease and CNS neoplasia were not differentiated (both classed as ‘other’).
Despite the various caveats, these data highlight the importance of considering potentially reversible aetiologies and superimposed reversible conditions in the differential diagnosis of RPD. The clinical differentiation between truly progressive diseases, monophasic illnesses and recurring events that can lead to rapid cognitive decline can be challenging. Conditions that usually show immediate onset or stepwise progression might mimic other RPDs and are frequently considered in the differential diagnosis. An ongoing clinical review is required to assess the nature of disease progression. In the sections that follow, we describe the pathophysiology, epidemiology and clinical characteristics of specific conditions that frequently present with RPD in the clinic.
Prion diseases
Though rare in the general population, prion diseases are very important in the context of RPD. The incidence of these diseases is around two per million person-years and has increased over the past few decades, probably because of improved diagnostic techniques36,37. Sporadic CJD (sCJD) is the most common human prion disease and is generally regarded as a spontaneous neurodegenerative illness, arising from either spontaneous prion protein gene (PRNP) somatic mutation or stochastic prion protein structural change. sCJD is characterized clinically by RPD with ataxia, myoclonus or other neurological signs, and neuropathologically by the presence of aggregates of abnormal prion protein (PrPSc), spongiform change, neuronal loss and gliosis. Despite these common features, sCJD has long been recognized as encompassing a wide phenotypic spectrum with regard to age of onset, presenting features, rate of progression and emergence of other clinical manifestations.
The various clinicopathological phenotypes of sCJD have been linked at the molecular level with two distinct PrPSc protein glycotypes (type 1 and type 2) and the methionine (M)/valine (V) polymorphism at PRNP codon 129, resulting in six main disease subtypes38. The most common subtypes are MM1/MV1 and VV2, with MM1/MV1 — the ‘classic’ form of CJD — comprising about 65% of all cases. Typical symptoms and signs at onset of MM1/MV1 include rapid cognitive decline and cortical anopsia (the so-called Heidenhain variant), followed closely by ataxia, myoclonus or other involuntary movements38. The disease progresses very rapidly, leading to death within 4–5 months. The phenotype associated with the VV2 molecular subtype comprises about 15–20% of sCJD cases. This subtype is clinically characterized by early onset of cerebellar symptoms, followed by memory loss within 2–3 months. True cognitive decline (besides the initial memory impairment) and myoclonus are late features39. The MV2 subtype accounts for about 10% of cases. This subtype is characterized by a relatively prolonged disease duration and diverse symptomatology, especially at onset. Dementia is the first clinical sign in about 50% of patients40. However, the disease can also begin with ataxia or extrapyramidal signs and is challenging for the clinician because of the potential diagnostic overlap with neurodegenerative diseases, including various α-synucleinopathies. Rare subtypes of sCJD include the VV1 and MM2 subtypes, as well as variably protease-sensitive prionopathy (VPSPr). The diagnosis of these phenotypes is complicated not only by their low frequency but also by their relatively slow progression and long disease durations (often 2 years or more). The VV1 subtype, which occurs mostly — but not exclusively — in males at a relatively young age38,41, typically starts with psychiatric or cognitive abnormalities, followed by extrapyramidal signs and ataxia. In the MM2 thalamic subtype, insomnia, frequent arousals and enacted dreams can be reported at onset42, whereas the MM2 cortical subtype is characterized by progressive dementia and disturbances of higher cognitive function, a high frequency of aphasia and apraxia, and myoclonus in the later stages43.
The clinical diagnosis of a prion disease can be supported by CSF analysis, MRI and EEG. Although EEG might provide important information, classic periodic sharp and slow wave complexes are a late feature of prion disease. By contrast, MRI sequences such as fluid-attenuated inversion recovery (FLAIR) and diffusion-weighted imaging (DWI) can detect signal hyperintensities very early in the disease course44,45 and have become a valuable part of the commonly applied diagnostic criteria18,46. MRI might also help to define the clinicopathological disease subtype on the basis of specific patterns of alterations. A diagnosis of sCJD is further supported by the detection of biomarkers in blood or CSF47, including 14-3-3 protein and the phosphorylated tau (p-tau) to tau ratio as markers of neuronal damage. In the differential diagnosis of neurodegenerative disorders, elevated levels of 14-3-3 and/or tau support a diagnosis of sCJD with a sensitivity of 85–94% and a specificity of 80%47.
Another class of assay based on initial amplification of PrPSc protein shows considerable promise for specific and sensitive pre-mortem testing for CJD48. Real-time quaking-induced conversion (RT-QuIC) is an amplification system that mimics the conversion process from physiological PrP to PrPSc. This technique allows the amplification and detection of femtogram amounts of PrPSc from the CSF. Given its high sensitivity and specificity, it has recently become part of the clinical diagnostic criteria47 and could improve early diagnosis and surveillance of CJD36,49.
Some forms of human prion disease can present with different biomarker profiles and clinical characteristics. Inherited prion diseases such as genetic CJD, fatal familial insomnia (FFI) and Gerstmann–Sträussler–Scheinker syndrome should be considered, especially when the family has a history of such syndromes. Variant CJD, which was linked to bovine spongiform encephalopathy, has only affected about 232 patients since 1995 but is still a public health concern and should be considered in patients with inconclusive biomarker results or atypical clinical presentation10.
Neurodegenerative and vascular dementias
Rapidly progressive Alzheimer disease
Studies on prion disease diagnosis and RPD have revealed AD as a major differential diagnosis6,29,30. The heterogeneity of AD is increasingly recognized, and diagnostic standards have yet to be defined for atypical forms of AD characterized by rapid disease progression, a poor prognosis, a distinct clinical and neuropsychological syndrome and possibly a specific genetic background. Most such cases were discovered within the framework of clinical surveillance centres for prion diseases6,50,51. The patients exhibited a very rapid disease course with dementia and various early focal neurological signs and symptoms, and they died within 24 months of disease onset.
Although typical AD and rpAD seem to share core neuropathological features, individuals with rpAD show a higher prevalence of moderate to severe cerebral amyloid angiopathy52, which might contribute to additional neuronal injury and cognitive disturbance53. Clinically, patients with rapid disease progression present atypically28 and already have a high frequency of focal neurological signs in the early disease stages52,54. Neuropsychological assessment at baseline evaluation can aid the identification of patients with rapidly progressing disease55, with early executive and language impairment being highly predictive of rapid AD progression56. With respect to paraclinical parameters and CSF biomarkers, no differences in CSF tau, p-tau and amyloid-β (Aβ) levels, hippocampal volumes or amyloid deposition have been observed between patients with typical AD and those with rpAD, but a lower CSF p-tau to tau ratio and region-specific hypometabolism in the left angular and left temporal cortices on 18F-fluorodeoxyglucose (18F-FDG) PET might identify patients who are at risk57. The apolipoprotein E (APOE) genotype distribution has been controversially discussed in the literature20: most publications have reported no difference between typical AD and rpAD, but some reports have suggested that the APOE ε4 allele is less prevalent among patients with very fast progression54,58.
Various hypotheses have been put forward to explain disease heterogeneity in AD. From a biological perspective, disease progression has been linked to distinct conformers of Aβ59. Nuclear magnetic resonance data indicate that Aβ40 fibrils from individuals with rpAD exhibit significantly greater structural variability than those from individuals with typical AD60. In addition, specific expression patterns of isoforms such as Aβ11–42 and differences in Aβ aggregate seeding characteristics have been reported61, and proteomic differences in amyloid plaques have been identified62. In particular, rpAD plaques showed significantly higher levels of neuronal proteins and lower levels of astrocytic proteins than those from individuals with typical AD. Plaques from patients with rpAD have a particular abundance of synaptic proteins, especially those involved in synaptic vesicle release. Other cofactors, such as prion protein oligomers, have also been found to be potentially associated with rpAD63. With regard to AD-related tau pathology, the colocalization of SFPQ protein with tau oligomers in the brain of individuals with rpAD suggests a possible role for SFPQ in the oligomerization and subsequent misfolding of tau protein64. In addition, an increased proportion of the four-repeat (4R) tau isoform was recently found to be associated with rapid cognitive decline in AD65.
Rapidly progressive types and subtypes of other neurodegenerative dementias
Cases of RPD have also been described among individuals with α-synucleinopathies, in particular, dementia with Lewy bodies (DLB)21. The mean disease duration of DLB is more than 8 years, but advanced age at onset, Lewy body disease of the diffuse type and alterations in markers of the innate immune system23,66 were found to be associated with substantially shorter survival times. However, no distinct neuropathological subtype of rapidly progressive DLB has been identified to date67. Other factors that might give a clinical impression of RPD or lead to a shortened survival time include the characteristic symptom fluctuations, recurrent falls and frequent occurrence of delirium in DLB.
With regard to tauopathies and the frontotemporal lobar degeneration (FTLD) spectrum, a rapid disease course with relatively short survival has been reported in progressive supranuclear palsy (mean 2.9 years)68 and FTLD with motor neuron disease (mean 2.3 years)69. In the latter condition, bulbar involvement or respiratory insufficiency, as well as specific types of mutation, such as C9orf72 expansion70, might be associated with accelerated disease progression.
Rapid progression in cerebrovascular disease and vascular dementia
Cerebrovascular disease is associated with various dementia phenotypes, including post-stroke dementia, multiple cortical infarct dementia and subcortical ischaemic dementia71. Cognitive impairment directly following a stroke might manifest as RPD, but in the absence of additional vascular events, the disease course is usually not progressive. Many studies have reported vascular events and chronic vascular dementia as relevant causes of RPD25,26 or differential diagnoses of CJD6,29,36, and recurring cerebrovascular events can cause further rapid cognitive decline. Even after a single stroke, secondary complications such as seizures might contribute to further cognitive deterioration.
In patients who present with RPD, unusual mechanisms of cerebrovascular disease and dysfunction, other than age-related and risk factor-related arteriosclerosis, must be considered. These mechanisms include cerebral amyloid angiopathy with or without AD-related Aβ pathology72, general presence of microbleeds73, vessel malformations and posterior reversible encephalopathy syndrome74. Primary angiitis of the CNS (PACNS), also known as primary CNS vasculitis, should always be considered in patients with RPD. Cognitive decline is observed in about 50% of patients with PACNS75, and the outcome is usually favourable if appropriate treatment is administered76. Besides PACNS, CNS vasculitis can also occur secondarily to various infectious (for example, varicella zoster virus (VZV) or HIV), other immune-mediated conditions (for example, giant-cell arteritis, Behçet disease or lupus erythematosus) and neoplastic diseases77. Vascular inflammation with related Aβ deposition is also relevant in this context: cognitive symptoms are even more common in Aβ-related angiitis than in PACNS78.
Mixed pathologies
The co-occurrence of two or more CNS pathologies is not a rare phenomenon, especially in older populations79. A study that evaluated neuropathological results from a brain bank series found that 73% of individuals with RPD had concomitant pathologies, most of which involved AD-related pathology, Lewy bodies or cerebrovascular disease31. However, although recent diagnostic criteria for AD and vascular dementia take concomitant pathologies into account71,80, the nature of their relationship is still a matter of controversy, and potential dependencies, synergies and effects on disease progression are still poorly understood. Moreover, the phenotype of slowly progressive dementias such as AD might be modified by synergistic effects involving concomitant α-synuclein, TAR DNA-binding protein or, in rare cases, even PrPSc-related pathology. Such superimposition might be difficult to detect in a clinical context because specific in vivo biomarkers for many of these proteinopathies are not available.
Inflammatory CNS diseases
Infectious encephalitis
Dementia-causing bacterial, viral, fungal and protozoan infections of the brain are a well-known phenomenon. The spectrum of the most important pathogens has changed over time and differs between geographical regions and populations with distinct socioeconomic characteristics.
Although neurosyphilis (late-stage Treponema pallidum infection) has become rare since the introduction of contact tracing and penicillin, the global prevalence of syphilis has been on the rise again since the turn of the millennium, and might be overlooked and undertreated81,82. Untreated syphilis can lead to various neurological symptoms at the meningovascular syphilis stage, and in the later stage of parenchymatous manifestation (‘general paresis’ or ‘dementia paralytica’), it typically causes dementia and other neuropsychiatric symptoms83.
Subacute sclerosing panencephalitis is an important cause of RPD in some countries with low current or past rates of vaccination for the measles virus26. In European countries and North America, herpes simplex virus (HSV) and VZV infections are the most prevalent causes of encephalitis84,85. Owing to their typically acute onset with seizures and altered conscious state, these infections are not usually considered in the differential diagnosis of RPD, but are reported to be potential mimics24,26 and are important to exclude in patients with severe RPD with an accelerated disease course. A meta-analysis found that neurocognitive disorder has a global prevalence of 42.6% among HIV-infected adults with and without antiretroviral therapy86. Although RPD seems to be a rare clinical finding among these patients, we recommend that an HIV test should be part of the basic diagnostic work-up for dementia. However, physicians should also pay attention to less frequent CNS infections with good treatment options (for example, Whipple disease87), regionally highly frequent CNS infections (for example, Japanese encephalitis in Asia88) and emerging pathogens (for example, Borna virus89). Progressive multifocal leukoencephalopathy is a demyelinating disease that is associated with reactivation of the JC virus (not to be confused with CJD or other occasionally used abbreviations for CJD such as JCD or JD) and typically affects patients with immunodeficiency. This condition can cause various progressive neurological symptoms, affecting cognition or behaviour in 36–54% of patients90.
The COVID-19 pandemic has brought new challenges, not only regarding internal medicine and public health but also for the differential diagnosis of RPD. It seems probable that SARS-CoV-2 infection can cause encephalopathy with cognitive disturbance, either directly or delayed through immune-mediated processes. The frequency of these conditions and potential disease mechanisms, as well as the pandemic’s effects on primary care and surveillance of dementia syndromes, are still under investigation91–98 (Box 1).
Box 1 Rapidly progressive dementia during the COVID-19 pandemic.
The COVID-19 pandemic, which began in early 2020, has affected almost all aspects of health care, the economy, politics and culture in a global context. The pandemic poses several challenges to rapidly progressive dementia (RPD) diagnosis, treatment and surveillance.
The SARS-CoV-2 virus itself has been linked to various neurological symptoms and pathologies that might cause or mimic RPD, such as delirium, toxic–metabolic encephalopathies, post-infectious and para-infectious encephalitis, cerebral haemorrhage or thrombosis and encephalomyelitis91–93. Considering the extremely high incidence of the infection, associated encephalopathies should be considered in cases where the cause of RPD is unclear. Moreover, an accelerating or even causal effect of SARS-CoV-2 infection or its complications on neurodegenerative disorders is a current topic of discussion94–96, and these potential long-term effects of the virus merit further investigation.
Additional effects of the pandemic include worsening of pre-existing cognitive deficits and other neuropsychiatric symptoms as the result of lockdown measures, as well as the increased risk of severe COVID-19 among patients with dementia97. Health-care systems, including surveillance systems for prion diseases, might also be affected by lockdown measures or regional peaks of COVID-19 incidence. To overcome these difficulties, innovative concepts such as telehealth98 need to be developed and established.
Immune-mediated encephalitis
Immune-mediated encephalitis accounts for a substantial proportion of encephalitis cases (21% in a 2010 study from England84) and is among the most important differential diagnoses for RPD24,26. This heterogeneous group of conditions includes antibody-mediated paraneoplastic and non-paraneoplastic encephalitis and CNS manifestations of systemic autoimmune disorders.
The ‘classic’ paraneoplastic encephalopathies are caused by antibodies that target intracellular onconeuronal antigens such as Hu, Yo and Ri. These encephalopathies are predominantly associated with small cell lung cancer and gynaecological neoplasia but occasionally occur with other tumour entities. The most common syndrome caused by paraneoplastic encephalopathy is cerebellar degeneration, but other syndromes such as panencephalitis or stiff-person syndrome have also been observed4.
Immune-mediated encephalitides associated with antibodies against neuronal cell-surface proteins, ion channels or receptors, such as the NMDA receptor (NMDAR) or leucine-rich glioma inactivated protein 1 (LGI1)4,99, are related to neoplasia to varying degrees (frequently in the case of anti-NMDAR antibodies but rarely in the case of anti-LGI1 antibodies). Structures of the limbic system are often affected, and the characteristic neuropsychiatric syndrome includes prominent memory deficits4,100. These syndromes typically show acute or subacute onset and early occurrence of altered consciousness and seizures. Some specific types have been reported to be important mimics of CJD (for example, anti-LGI1 encephalitis)101 or other rapidly progressive neurodegenerative diseases (for example, anti-IGLON5 encephalitis). Anti-IGLON encephalitis might even mimic neurodegenerative disease at the neuropathological level by inducing deposition of hyperphosphorylated tau protein in the brain102. In addition, distinct syndromes with anterograde or global amnesia have been reported to be manifestations of anti-adenylate kinase 5 (AK5)103 and anti-AMPA receptor (AMPAR)104 encephalitis, respectively.
The diagnosis of immune-mediated encephalitis can be challenging. A study from Spain reported a lack of CSF pleocytosis and signs of inflammation on MRI in 23% of older patients with limbic encephalitis105, and another study showed that in 7% of patients with limbic encephalitis, no associated antibody could be identified106. Neoplasia99,107 and viral encephalitides (especially herpes encephalitis)108 are the most important diseases associated with antibody-mediated encephalitis, and accurate and early diagnosis is crucial because the outcomes are generally favourable with appropriate treatment109. An important example of immune-mediated encephalitis with good treatment options is steroid-responsive encephalopathy associated with autoimmune thyroiditis (SREAT). Unfortunately, related thyroid antibodies are not disease-specific and have a high prevalence in the general population. Therefore, clinicians must be extremely cautious in considering this diagnosis, especially in patients without specific signs of CNS inflammation110.
Other causes of RPD
Metabolic and toxic encephalopathies
Metabolic and toxic encephalopathies have been found to be frequent causes (6–16%) of RPD in several studies26,35. Alcohol abuse and related dementia is probably the most important of these encephalopathies. Alcohol-related dementia is common, accounting for up to 10% of cases early-onset dementia111. Alcoholism is often associated with malnutrition and organ injuries, potentially leading to Wernicke encephalopathy, other vitamin deficiency-related encephalopathies or hepatic encephalopathy. In addition, alcohol abuse is often related to the occurrence of seizures and is a major precipitant of status epilepticus112.
Other potential metabolic causes of RPD include — but are not restricted to — recurrent hypoglycaemia, severe hypothyroidism, hyponatraemia and osmotic extrapontine myelinolyses, highlighting the importance of including basic metabolic markers in the initial diagnostic process. Depending on the patient’s history and clinical impression, acute or chronic intoxications with drugs, metals or other toxic agents should also be considered.
CNS neoplasia
Besides paraneoplastic immune-mediated encephalopathies, primary CNS neoplasias, such as glioma and meningioma, and intracranial manifestations of peripheral neoplastic diseases, such as cerebral metastases and neoplastic meningitis, are also potential causes of RPD. These neoplasias can affect cortical and subcortical structures through space occupation, infiltration and destruction of brain tissue, secondary oedema or disturbance of CSF circulation resulting in intracranial hypertension. Among the different tumour entities that have been linked to RPD, primary CNS lymphoma and the increasingly recognized intravascular lymphoma113 are of major importance. The incidence of primary CNS lymphoma is increasing, and these tumours have profound effects on cognitive functions and are challenging to diagnose114. However, treatment options that prolong survival and might improve cognitive performance are becoming available115.
Mitochondriopathies and other hereditary diseases
Mitochondriopathies and storage diseases usually show a chronic or stepwise disease course and are not commonly considered as RPDs. However, these conditions can show accelerated progression under the influence of secondary complications or concomitant pathologies. In addition, the onset of mitochondriopathies might mimic RPDs such as CJD, and several reports from prion disease centres mention these diseases among the differential diagnoses6,24,34.
The typical onset of mitochondriopathies such as mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes (MELAS) is before the age of 20 years, and only 10–24% of patients initially present with cognitive impairment116. Nonetheless, late-onset manifestations are possible and must be considered in patients with RPD with additional syndromes matching the MELAS criteria. Similarly, various types of lysosomal, glycogen and other storage diseases and leukodystrophies can show late-life onset and can be accelerated by other systemic diseases. In particular, disorders of metal metabolism such as Wilson disease and neurodegeneration with brain iron accumulation are known to cause neuropsychiatric symptoms, although they predominantly present as movement disorders.
Mimics of RPD
Several CNS disorders and secondary conditions can present with or mimic RPD. Delirium, in which cognitive impairment is a clinical key feature117, and non-convulsive status epilepticus are important differential diagnoses. These potentially reversible conditions might be superimposed upon, aggravate or even mimic an RPD syndrome, and should be considered during both initial and ongoing clinical investigations. The concept of ‘pseudodementia’, a syndrome in which cognitive impairment manifests in people with severe affective disorders, was introduced in the 1980s118 and has been a matter of controversy ever since. However, a multinational European study found that 4% of patients with RPD and initially suspected CJD were finally diagnosed with a psychiatric disease29.
Similar to some of the other mentioned disease groups, demyelinating inflammatory disorders do not characteristically cause an RPD syndrome. However, the term RPD was first used in this context17, and multiple sclerosis and acute disseminated encephalomyelitis have been recognized as rare differential diagnoses of RPD30,34.
The diagnostic process in RPD
Owing to the wide spectrum of differential diagnoses and manifold underlying disease mechanisms, the diagnostic work-up for RPDs is complex and a linear decision tree is unlikely to be applicable to various potential clinical scenarios. In this section, we describe the different diagnostic steps, tools, procedures and implications. A summary based on the published literature and the clinical experience of the authors in provided in Box 2. This framework is intended as an initial screening tool but it is not exhaustive, and critical re-evaluation by the clinician will be required in patients with negative test results.
Box 2 Differential diagnosis of rapidly progressive dementia.
Step 1: Patient history and clinical examination
Patient history:
Age at onset
Speed of cognitive decline
Medical history
Type of cognitive deficit
Other symptoms
Physical examination:
State of consciousness
Focal neurological signs
Other physical symptoms
Identify or rule out acute conditions such as delirium, intoxication or stroke
Search for characteristics of specific rapidly progressive dementias to determine the sequence of further investigations
Step 2: Standard technical procedures
Blood tests:
Standard markers of inflammation, organ function and metabolism, including electrolytes (especially sodium), kidney and liver markers, thyroid hormones and B vitamins
Test for HIV, syphilis or other specific pathogens or antibodies, depending on the clinical presentation
Imaging:
Search for inflammation, vascular pathology, tumours, atrophy, restricted diffusion and metal deposition
CT (brain): exclude acute intracranial pressure, if necessary
CT (thorax and abdomen): search for tumours
MRI: T2-weighted fluid-attenuated inversion recovery, diffusion-weighted imaging plus apparent diffusion coefficient, susceptibility-weighted or T2*-weighted imaging and T1-weighted imaging with gadolinium administration
Cerebrospinal fluid:
Basic analyses to identify inflammatory processes
Specific autoantibody or other pathogen tests
Protein biomarkers for Alzheimer disease (tau, phosphorylated tau and amyloid-β42) and Creutzfeldt–Jakob disease (14-3-3 protein and real-time quaking-induced conversion (RT-QuIC))
Cytopathology
EEG:
Abnormal patterns
Non-convulsive status epilepticus
Step 3: Advanced diagnostics
Biomaterials:
Skin biopsy, genetic or enzyme diagnostics to identify rare storage and other hereditary diseases
Imaging:
18F-Fluorodeoxyglucose PET
Amyloid and tau PET
Whole-body PET–CT to detect non-CNS neoplasia
Anti-inflammatory therapy:
Immunoglobulin or high-dose steroids in patients with suspected but unproven encephalitis (caveat: CNS tumours might also respond to steroids)
Last resort:
Brain or leptomeningeal biopsy
Clinical presentation
The first steps in the diagnostic process for RPD, as with any other diseases, are to evaluate the clinical presentation, the demographic background and the medical history. In patients with cognitive impairment, history of onset and speed of decline might set the course for further investigations. The patient history is best assessed in the presence of the next of kin or caregivers, and should include the history of any neuropsychiatric diseases or substance abuse, as well as the occupational background to identify potential exposures to toxins. An initial assessment of the disease onset and prediagnostic course might provide clues to the nature of symptom evolution (rapid progression versus stable condition), but the patient should continue to be monitored throughout the diagnostic process.
Differentiation of RPD from delirium117 is one of the most important considerations in the initial diagnostic phase. Immediate or acute onset of severe cognitive deficits within minutes to hours points towards acute events such as stroke, seizures or high intracranial pressure. However, some inflammatory CNS diseases and metabolic encephalopathies can also develop within a very short time period. By contrast, individuals with prion diseases usually present with dementia weeks to months after onset of the first symptoms, and other RPDs can present even later. Figure 2 displays the different aetiological categories of RPD and their association with the speed of symptom development. The age at onset is another important factor for the initial clinical evaluation. For prion diseases, the peak of onset is between 60 and 69 years of age, and onset before the sixth decade of life is rare (5% of cases)119. Many other neurodegenerative disorders that underlie RPD are typical diseases of the older population, but in younger patients with RPD, the diagnostic spectrum is very broad120. In reports from tertiary centres, the mean age of individuals with RPD in tertiary centres ranged from 48 to 63 years, and patients with encephalitis in particular tended to be at the younger end of the age spectrum24,26,34,35.
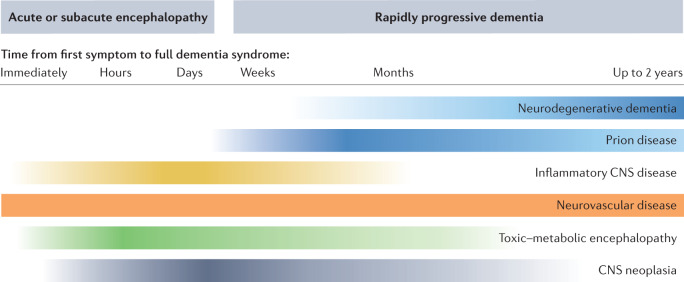
Fig. 2. Probable rapidly progressive dementia aetiologies in relation to time from onset to dementia.
The chart reflects the likelihood of particular disease categories in light of the rapidity of cognitive decline. The time of symptom progression (indicated above disease categories) ranges from immediate onset of relevant cognitive dysfunction (left) to 2 years from onset of first symptoms to presence of dementia (right). Higher colour intensity indicates a typical time frame for each disease category. Patients with Creutzfeldt–Jakob disease usually develop a full dementia syndrome within a time span of a few weeks or a few months after onset. In other rapidly progressive neurodegenerative dementias, this process usually takes from several months up to 2 years. Encephalitides and metabolic encephalopathies are likely to show immediate or subacute onset with very rapid disease progression, whereas vascular encephalopathies are extremely heterogeneous and can show immediate onset of dementia (after stroke, for example), rapid disease progression (vasculitis), or moderate or stepwise progression (classic vascular dementia). This chart is intended to provide a rough overview and exceptions can occur. It is based largely on the literature reviewed in this article, as well as on the authors’ personal experience from their clinical work in a dementia referral centre and in prion disease surveillance.
Neurological and psychiatric assessments are other important steps in the differential diagnosis of RPD. The type of cognitive impairment or the state of consciousness, as well as the presence of focal neurological signs, might help differentiate conditions such as delirium, seizures and stroke. Although individuals with rpAD might present with focal neurological signs in the earlier stages of the disease54, the presence of these signs generally points towards non-AD dementia. Some signs may even be considered to be characteristic of certain diseases, such as stimulus-sensitive myoclonus in CJD, asterixis (flapping tremor) in hepatic encephalopathy, faciobrachial dystonic seizures in anti-LGI1 limbic encephalitis121 or aphasia in herpes encephalitis. Importantly, however, even these characteristics do not exclude other causes of RPD.
Blood-based biomarkers
Routine blood tests conducted at the first admission of a patient with RPD can provide valuable information and identify potentially reversible conditions. These conditions include systemic inflammation, as detected through tests such as leukocyte counts and C-reactive protein levels, and various metabolic disorders. Besides disturbance of electrolytes, markers of liver, kidney and thyroid function are of special interest. Further information can be gained by testing for specific infectious diseases such as HIV and syphilis and searching for systemic autoantibodies and vitamin deficiencies (for example vitamin B1, vitamin B12 and folic acid). Depending on the clinical presentation, patient history and other diagnostic results, additional tests such as specific hormone levels, drug screens or coeruloplasmin and copper blood levels might be indicated. In the presence of a positive or ambiguous family history, or a clinical syndrome that indicates a hereditary disease, various genetic analyses might be warranted as part of the diagnostic process. Blood-based biomarkers for neurodegenerative diseases and CJD are currently undergoing validation and are expected to be available in the next few years122.
CT and MRI
CT has relevance in the emergency setting to exclude intracerebral bleeding or acute hydrocephalus, but in most patients with RPD, differential diagnosis requires cranial MRI. The MRI protocol should include T1-weighted images with gadolinium injection to identify tumorous and inflammatory lesions with blood–brain barrier leakage. Atrophy patterns can also provide information on potential neurodegenerative diseases. T2 and FLAIR images might reveal gliosis resulting from chronic vascular or inflammatory encephalopathy, or oedema with numerous causes. DWI lesions with corresponding hypointensities on apparent diffusion coefficient maps can identify restricted diffusion in acute ischaemia and CJD. Susceptibility or T2*-weighted images can show chronic microbleeds and metal deposits. In addition, magnetic resonance angiography is useful to identify vessel occlusion and also to visualize stenosis in PACNS123,124.
MRI is of particular importance for the diagnosis of CJD. Although no standard protocols currently exist, evidence indicates that DWI is more sensitive than FLAIR for the early detection of signal abnormalities in CJD, especially in the cerebral cortex45,46. However, the evaluation of MRI scans in CJD and its differential diagnoses is not trivial and the typical lesions are occasionally overlooked123. Involvement of subcortical structures (other than the basal ganglia), oedema and T1 abnormalities or absence of the typical radiological features of CJD might point to ischaemia, inflammation or metabolic disorders125,126. The MRI lesions in encephalitis have a wide spectrum, with signal abnormalities including an increased FLAIR signal and/or gadolinium enhancement, both typically involving the temporal lobe in viral encephalitides and structures of the limbic system in immune-mediated encephalitis. Numerous other lesion patterns can be present, depending on the type of encephalitis. MRI abnormalities are reported to be completely absent in the majority (70% or more) of patients with anti-NMDAR109 or anti-DPPX limbic encephalitis4, whereas individuals with other types of encephalitis are likely to show increased signal in the medial temporal lobes (anti-LGI1, anti-CASPR2, anti-AMPAR and anti-GABAB receptor), basal ganglia (anti-dopamine receptor 2) or multiple cortical and subcortical regions (anti-GABAA receptor)4.
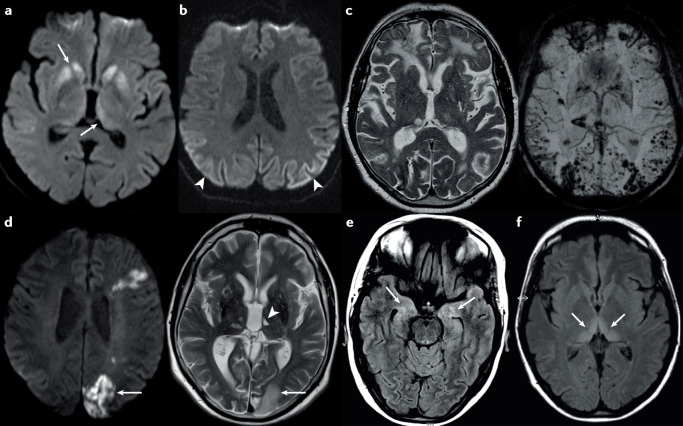
Figure 3 provides examples of MRI results in the most important diagnostic groups of RPD: prion diseases (Fig. 3a and b), AD (with cerebral amyloid angiopathy; Fig. 3c), cerebrovascular disease (PACNS; Fig. 3d), encephalitis (anti-NMDAR; Fig. 3e) and metabolic encephalopathy (Wernicke encephalopathy127; Fig. 3f).
Fig. 3. MRI findings in rapidly progressive dementia.
a | Diffusion-weighted imaging (DWI) in a 62-year-old woman with the MV2 subtype of sporadic Creutzfeldt–Jakob disease (sCJD), showing hyperintensities of the basal ganglia including the thalamus (arrows). The patient had a history of progressive movement disorder and rapidly progressive cognitive decline that had started about 6 months before the scan. b | DWI in a 54-year-old man with the MM2 sCJD subtype, showing hyperintensities in several posterior cortical regions (arrowheads). At the time of the scan, the patient displayed a moderate dementia syndrome and aphasia without other neurological signs. The symptoms had started about 8 months previously. c | MRI scans in a 67-year-old man with Alzheimer disease and severe cerebral amyloid angiopathy. The T2-weighted image (left) shows several cortical and subcortical post-haemorrhagic lesions, as well as bitemporal atrophy. The susceptibility-weighted image (right) reveals multiple residual signs of microbleeds and macrobleeds. Before the scan, the patient had exhibited mild cognitive impairment for an unknown period. The symptoms progressed to a severe dementia syndrome within 1 year of the scan. d | MRI scans in a 53-year-old man with primary angiitis of the CNS. The DWI (left) shows cortical and subcortical hyperintensities in regions with acute and subacute ischaemia (arrow). The T2-weighted image (right) shows cortical and subcortical ischaemic lesions and lacunes of differing ages. The arrow indicates the same region of subacute ischaemia as indicated in the left image, and the arrowhead indicates an older lacunar ischaemic lesion in the left thalamus. At the time of the scan, the patient showed a mild to moderate dementia syndrome, gait disturbance and dysarthria. The symptoms had started about 1 year previously and worsened continuously with stepwise accelerations. e | MRI scan in a 31-year-old woman with anti-NMDA receptor encephalitis. The arrows indicate diffuse fluid-attenuated inversion recovery (FLAIR) signal hyperintensities of the hippocampal regions in both hemispheres. At the time of the scan, the patient was experiencing recurrent seizures, which had been preceded by a 2-week period of episodic memory impairment and psychotic symptoms. f | MRI scan in a 39-year-old woman with Wernicke encephalopathy. The arrows indicate FLAIR signal hyperintensities of the medial thalamus and pulvinar nucleus in both hemispheres, which are similar to the ‘hockey stick’ sign in variant CJD. At the time of the scan, the patient was experiencing memory impairment, hallucinations, eye movement disturbance and flaccid tetraparesis. These symptoms had started 3 weeks previously127. Part f adapted with permission from ref.127, Cambridge University Press.
Cerebrospinal fluid analysis
Lumbar puncture and CSF analyses are key tools in identifying inflammatory CNS diseases and are essential for diagnostic evaluation of RPDs. In initial or emergency evaluation, elevated white blood cell (WBC) counts can rapidly identify encephalitis and meningitis. It is important to recognize that despite the transmissibility of prion diseases between individuals and across species, the CSF lacks a typical inflammatory response in people with these conditions. Therefore, routine analyses of CSF samples from patients with CJD is usually unrevealing, although a slight non-specific increase in total protein is observed in about one-third of patients, and oligoclonal bands can occasionally be found128,129. Thus, any specific sign of inflammation on CSF analysis probably points towards a non-neurodegenerative cause of RPD.
An elevated WBC count is usually a clear sign of encephalitis, although some neoplastic CNS diseases such as lymphoma or neoplastic meningitis can also cause mild to moderate WBC elevation130, necessitating further cytopathological investigation. A normal WBC count does not exclude the presence of tumour cells in the CSF, and cytopathology should be considered in all patients with unclear RPD. A so-called reactive WBC count elevation (six or more leukocytes per microlitre) resulting from the lumbar puncture itself has been reported but is thought to be rare131. Similarly, elevation of WBC count is uncommon after status epilepticus without inflammatory aetiology132 and rarely occurs within the first 24 h after onset133. Conversely, around 20% of patients with immune-mediated encephalitides can lack inflammatory signs in the CSF134. We recommend performing a basic search for infectious agents (for example, HSV and VZV) and a panel test for the most important autoantibodies4 in all patients with evidence of inflammation on CSF analysis. As individuals with atypical and immune-mediated encephalitides, such as anti-LGI1 encephalitis, might lack inflammatory signs in the CSF, such tests may also be reasonably performed on the basis of clinical presentation or imaging results. In unclear cases, an advanced search for atypical agents (for example, Tropheryma whipplei), systemic autoimmune disease and neoplasia is indicated.
With respect to neurodegenerative dementia, highly sensitive and specific protein biomarker tests have been established for the early diagnosis of AD (p-tau, Aβ42 and the Aβ42 to Aβ40 ratio)135 and CJD (RT-QuIC)47. CJD is also associated with extreme elevation of neuronal injury markers such as CSF total tau protein and neurofilament light chain47. However, markers of neuronal injury and neurodegeneration are not disease-specific and can be altered in various RPD aetiologies136. The possibility that very high levels of neurofilament light chain or tau can predict rapid progression in common neurodegenerative diseases is currently under investigation.
EEG
EEG can detect abnormal brain function, latent epileptic activity and seizures and should be part of the standard diagnostic work-up for RPD, at least to exclude non-convulsive status epilepticus, which might mimic RPD. Historically, EEG has been included in the diagnostic criteria for sCJD, but the typical pattern, periodic sharp and slow wave complexes, is not easy to distinguish from active seizures137 and usually occurs at a late disease stage. EEG abnormalities of any nature have a high sensitivity for immune-mediated diseases138, but again they are not specific for these conditions.
Other imaging techniques
Cerebral 18F-FDG PET can reveal glucose hypometabolism in specific brain regions and might be indicated in some suspected differential diagnoses for RPD; for example, to detect thalamic hypometabolism in FFI or sporadic fatal insomnia139, or to characterize tumour entities140,141. In the case of neurodegenerative diseases, 18F-FDG PET can detect tissue hypometabolism as a stage of neurodegeneration that precedes MRI changes such as atrophy. Molecular tracers that bind to cerebral Aβ (amyloid-PET) and tau (tau-PET) deposits can identify AD-related pathology at an early stage135. In addition, bilateral mesial temporal signal abnormalities on 18F-FDG PET have been reported to be a sign of immune-mediated encephalitis142, but no specific patterns or tracers have been identified for this disease type.
An important step in the differential diagnosis of RPD is the search for neoplastic diseases, either cerebral or peripheral, as potential source of paraneoplastic syndromes. Thus, we recommend that thoracic and abdominal CT is performed in unclear cases. Whole-body PET to search for neoplasia might also be considered.
Brain biopsy
Though rare, complications after brain biopsy may be serious143, and the potential presence of prion disease requires specific hygiene considerations because surgical instruments are a potential source of transmission144. Therefore, brain biopsy or even leptomeningeal biopsy should be considered as a last resort for the differential diagnosis of RPD. However, specific diseases such as CNS neoplasia or vasculitis might still require histopathological or tissue biochemical data to determine or optimize treatment procedures.
Therapeutic options
The therapeutic options for patients with RPD are as diverse as the underlying aetiologies. For neurodegenerative diseases such as AD, established medications include cholinesterase inhibitors and memantine145. The recent FDA approval of aducanumab might constitute a breakthrough for the treatment of AD but remains controversial146. Data on the efficacy of these drugs in rapidly progressive neurodegenerative dementias are not yet available. No causal therapies are available for prion diseases, although immunotherapies147 or protein expression-modifying therapies148 could become an option in the near future. For the treatment of infectious encephalitides, various antimicrobiotic and antiviral drugs, depending on the identified infectious agent, are well established and effective. The treatment options for immune-mediated encephalitis include steroids, immunoglobulins or plasmapheresis as first-line or bridging therapeutics, and other immunotherapeutics such as rituximab as maintenance therapy149. In paraneoplastic syndromes, identification and treatment of the underlying neoplasia is crucial. In the context of suspected encephalitis without evidence of specific antibodies and with or without signs of inflammation in the CSF, high-dose steroid therapy might not only show beneficial effects but also offer potential insights for the diagnosis, and we suggest that this option should be considered in all patients with unclear RPD. However, the symptoms of CNS tumours may also partially respond to steroids. In particular, in the case of a suspected lymphoma, steroid therapy might lead to clinical improvement but could also impair the diagnostic value of a subsequent biopsy. Moreover, short-term application of high-dose steroids is not sufficient to achieve a sustained clinical benefit in most types of immune-mediated encephalitis.
RPDs caused by inflammatory, metabolic or neoplastic diseases can be reversible with appropriate treatment, and many aggravating factors that contribute to cognitive deficits, such as seizures or affective symptoms, might also respond to therapeutic intervention. Nonetheless, the clinical management might reach a point where a palliative therapeutic regimen has to be considered. In patients with an irreversible condition, the treatment of secondary complications and application of life-extending procedures such as percutaneous endoscopic gastrostomy tubes determine the survival time. These measures must be balanced with regard to prognosis, the patient’s wishes and legal considerations.
Conclusions
In recent years, we have seen tremendous advances in knowledge of the pathogenesis of various forms of dementias. The focus on rapidly progressive forms of dementia has allowed potentially treatable pathologies to be identified. Early diagnosis is essential for the efficacy of potential therapies, because the chances of treatment success are better the earlier the diagnosis is made. The continuous improvement of imaging techniques, the development of biomarkers — including blood-based biomarkers — for the diagnosis of brain pathologies and the recognition of the expanding spectrum of immune-mediated disorders, together with refinement of diagnostic options, provide valuable tools to enable clinicians to achieve an early differential diagnosis. Using this knowledge, we need to study RPDs systematically to build a basis for rational diagnostic work-up and treatment decisions.
Acknowledgements
The work of I.Z. is supported by grants from the Robert Koch Institute through funds of the German Federal Ministry of Health (grant no. 1369-341). The authors wish to thank all employees at the German National Reference Center for TSE for performing the Creutzfeldt–Jakob disease surveillance and counselling physicians all over Germany with regard to the differential diagnosis of prion diseases and other rapidly progressive dementias.
Author contributions
The authors contributed equally to all aspects of the article.
Peer review
Peer review information
Nature Reviews Neurology thanks the anonymous reviewers for their contribution to the peer review of this work.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 4th edn (American Psychiatric Association, 1994).
- 2.Geschwind MD. Rapidly progressive dementia. Continuum. 2016;22:510–537. doi: 10.1212/CON.0000000000000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masters CL, et al. Creutzfeldt-Jakob disease: patterns of worldwide occurrence and the significance of familial and sporadic clustering. Ann. Neurol. 1979;5:177–188. doi: 10.1002/ana.410050212. [DOI] [PubMed] [Google Scholar]
- 4.Dalmau J, Graus F. Antibody-mediated encephalitis. N. Engl. J. Med. 2018;378:840–851. doi: 10.1056/NEJMra1708712. [DOI] [PubMed] [Google Scholar]
- 5.Soto ME, et al. Rapid cognitive decline in Alzheimer’s disease. Consensus paper. J. Nutr. Health Aging. 2008;12:703–713. doi: 10.1007/BF03028618. [DOI] [PubMed] [Google Scholar]
- 6.Chitravas N, et al. Treatable neurological disorders misdiagnosed as Creutzfeldt-Jakob disease. Ann. Neurol. 2011;70:437–444. doi: 10.1002/ana.22454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mead S, Rudge P. CJD mimics and chameleons. Pract. Neurol. 2017;17:113–121. doi: 10.1136/practneurol-2016-001571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geschwind MD, Murray K. Differential diagnosis with other rapid progressive dementias in human prion diseases. Handb. Clin. Neurol. 2018;153:371–397. doi: 10.1016/B978-0-444-63945-5.00020-9. [DOI] [PubMed] [Google Scholar]
- 9.Zerr I, Hermann P. Diagnostic challenges in rapidly progressive dementia. Expert. Rev. Neurother. 2018;18:761–772. doi: 10.1080/14737175.2018.1519397. [DOI] [PubMed] [Google Scholar]
- 10.Watson N, et al. The importance of ongoing international surveillance for Creutzfeldt–Jakob disease. Nat. Rev. Neurol. 2021;17:362–379. doi: 10.1038/s41582-021-00488-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woodruff BK. Evaluation of rapidly progressive dementia. Semin. Neurol. 2007;27:363–375. doi: 10.1055/s-2007-985337. [DOI] [PubMed] [Google Scholar]
- 12.Geschwind MD, Haman A, Miller BL. Rapidly progressive dementia. Neurol. Clin. 2007;25:783–807. doi: 10.1016/j.ncl.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geschwind MD, Shu H, Haman A, Sejvar JJ, Miller BL. Rapidly progressive dementia. Ann. Neurol. 2008;64:97–108. doi: 10.1002/ana.21430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Appleby BS, Lyketsos CG. Rapidly progressive dementias and the treatment of human prion diseases. Expert. Opin. Pharmacother. 2011;12:1–12. doi: 10.1517/14656566.2010.514903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenbloom MH, Atri A. The evaluation of rapidly progressive dementia. Neurologist. 2011;17:67–74. doi: 10.1097/NRL.0b013e31820ba5e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahajan S, Appleby BS. Comprehensive and methodical: diagnostic and management approaches to rapidly progressive dementia. Curr. Treat. Options Neurol. 2017;19:40. doi: 10.1007/s11940-017-0474-1. [DOI] [PubMed] [Google Scholar]
- 17.Bergin JD. Rapidly progressing dementia in disseminated sclerosis. J. Neurol. Neurosurg. Psychiatry. 1957;20:285–292. doi: 10.1136/jnnp.20.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zerr I, et al. Updated clinical diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Brain. 2009;132:2659–2668. doi: 10.1093/brain/awp191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Josephs KA, et al. Rapidly progressive neurodegenerative dementias. Arch. Neurol. 2009;66:201–207. doi: 10.1001/archneurol.2008.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt C, et al. Rapidly progressive Alzheimer disease. Arch. Neurol. 2011;68:1124–1130. doi: 10.1001/archneurol.2011.189. [DOI] [PubMed] [Google Scholar]
- 21.Gaig C, et al. Rapidly progressive diffuse Lewy body disease. Mov. Disord. 2011;26:1316–1323. doi: 10.1002/mds.23506. [DOI] [PubMed] [Google Scholar]
- 22.Degnan AJ, Levy LM. Neuroimaging of rapidly progressive dementias, part 1: neurodegenerative etiologies. Am. J. Neuroradiol. 2014;35:418–423. doi: 10.3174/ajnr.A3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Esparcia P, et al. Dementia with Lewy bodies: molecular pathology in the frontal cortex in typical and rapidly progressive forms. Front. Neurol. 2017;8:89. doi: 10.3389/fneur.2017.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neto AS, et al. Rapidly progressive dementia: prevalence and causes in a neurologic unit of a tertiary hospital in Brazil. Alzheimer Dis. Assoc. Disord. 2017;31:239–243. doi: 10.1097/WAD.0000000000000170. [DOI] [PubMed] [Google Scholar]
- 25.Papageorgiou SG, et al. Rapidly progressive dementia: causes found in a Greek tertiary referral center in Athens. Alzheimer Dis. Assoc. Disord. 2009;23:337–346. doi: 10.1097/WAD.0b013e31819e099b. [DOI] [PubMed] [Google Scholar]
- 26.Anuja P, et al. Rapidly progressive dementia: an eight year (2008–2016) retrospective study. PLoS ONE. 2018;13:e0189832. doi: 10.1371/journal.pone.0189832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:459–480. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Day GS, Musiek ES, Morris JC. Rapidly progressive dementia in the outpatient clinic: more than prions. Alzheimer Dis. Assoc. Disord. 2018;32:291–297. doi: 10.1097/WAD.0000000000000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stoeck K, et al. Cerebrospinal fluid biomarker supported diagnosis of Creutzfeldt-Jakob disease and rapid dementias: a longitudinal multicentre study over 10 years. Brain. 2012;135:3051–3061. doi: 10.1093/brain/aws238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peckeu L, et al. Accuracy of diagnosis criteria in patients with suspected diagnosis of sporadic Creutzfeldt-Jakob disease and detection of 14-3-3 protein, France, 1992 to 2009. Eur. Surveill. 2017;22:16-00715. doi: 10.2807/1560-7917.ES.2017.22.41.16-00715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grau-Rivera O, et al. Clinicopathological correlations and concomitant pathologies in rapidly progressive dementia: a brain bank series. Neurodegener. Dis. 2015;15:350–360. doi: 10.1159/000439251. [DOI] [PubMed] [Google Scholar]
- 32.Maat P, et al. Pathologically confirmed autoimmune encephalitis in suspected Creutzfeldt-Jakob disease. Neurol. Neuroimmunol. Neuroinflamm. 2015;2:e178. doi: 10.1212/NXI.0000000000000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sala I, et al. Rapidly progressive dementia: experience in a tertiary care medical center. Alzheimer Dis. Assoc. Disord. 2012;26:267–271. doi: 10.1097/WAD.0b013e3182368ed4. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Gao T, Tao QQ. Spectrum of noncerebrovascular rapidly progressive cognitive deterioration: a 2-year retrospective study. Clin. Interv. Aging. 2017;12:1655–1659. doi: 10.2147/CIA.S144821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Acosta JN, et al. Diagnosis of rapidly progressive dementia in a referral center in Argentina. Alzheimer Dis. Assoc. Disord. 2020;34:54–58. doi: 10.1097/WAD.0000000000000337. [DOI] [PubMed] [Google Scholar]
- 36.Hermann P, et al. Validation and utilization of amended diagnostic criteria in Creutzfeldt-Jakob disease surveillance. Neurology. 2018;91:e331–e338. doi: 10.1212/WNL.0000000000005860. [DOI] [PubMed] [Google Scholar]
- 37.Uttley L, Carroll C, Wong R, Hilton DA, Stevenson M. Creutzfeldt-Jakob disease: a systematic review of global incidence, prevalence, infectivity, and incubation. Lancet Infect. Dis. 2020;20:e2–e10. doi: 10.1016/S1473-3099(19)30615-2. [DOI] [PubMed] [Google Scholar]
- 38.Parchi P, et al. Classification of sporadic Creutzfeldt-Jakob disease based on molecular and phenotypic analysis of 300 subjects. Ann. Neurol. 1999;46:224–233. doi: 10.1002/1531-8249(199908)46:2<224::AID-ANA12>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 39.Baiardi S, et al. Towards an early clinical diagnosis of sporadic CJD VV2 (ataxic type) J. Neurol. Neurosurg. Psychiatry. 2017;88:764–772. doi: 10.1136/jnnp-2017-315942. [DOI] [PubMed] [Google Scholar]
- 40.Krasnianski A, et al. Clinical findings and diagnostic tests in the MV2 subtype of sporadic CJD. Brain. 2006;129:2288–2296. doi: 10.1093/brain/awl123. [DOI] [PubMed] [Google Scholar]
- 41.Meissner B, et al. Sporadic Creutzfeldt-Jakob disease: clinical and diagnostic characteristics of the rare VV1 type. Neurology. 2005;65:1544–1550. doi: 10.1212/01.wnl.0000184674.32924.c9. [DOI] [PubMed] [Google Scholar]
- 42.Puoti G, et al. Sporadic human prion diseases: molecular insights and diagnosis. Lancet Neurol. 2012;11:618–628. doi: 10.1016/S1474-4422(12)70063-7. [DOI] [PubMed] [Google Scholar]
- 43.Krasnianski A, et al. Clinical features and diagnosis of the MM2 cortical subtype of sporadic Creutzfeldt-Jakob disease. Arch. Neurol. 2006;63:876–880. doi: 10.1001/archneur.63.6.876. [DOI] [PubMed] [Google Scholar]
- 44.Alvarez FJ, Bisbe J, Bisbe V, Dávalos A. Magnetic resonance imaging findings in pre-clinical Creutzfeldt-Jakob disease. Int. J. Neurosci. 2005;115:1219–1225. doi: 10.1080/00207450590914491. [DOI] [PubMed] [Google Scholar]
- 45.Satoh K, et al. Early detection of sporadic CJD by diffusion-weighted MRI before the onset of symptoms. J. Neurol. Neurosurg. Psychiatry. 2011;82:942–943. doi: 10.1136/jnnp.2008.155242. [DOI] [PubMed] [Google Scholar]
- 46.Vitali P, et al. Diffusion-weighted MRI hyperintensity patterns differentiate CJD from other rapid dementias. Neurology. 2011;76:1711–1719. doi: 10.1212/WNL.0b013e31821a4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hermann P, et al. Biomarkers and diagnostic guidelines for sporadic Creutzfeldt-Jakob disease. Lancet Neurol. 2021;20:235–246. doi: 10.1016/S1474-4422(20)30477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McGuire LI, et al. Real time quaking-induced conversion analysis of cerebrospinal fluid in sporadic Creutzfeldt-Jakob disease. Ann. Neurol. 2012;72:278–285. doi: 10.1002/ana.23589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rhoads D, et al. Diagnosis of prion diseases by RT-QuIC results in improved surveillance. Neurology. 2020;95:e1017–e1026. doi: 10.1212/WNL.0000000000010086. [DOI] [PubMed] [Google Scholar]
- 50.Heinemann U, et al. Creutzfeldt-Jakob disease in Germany: a prospective 12-year surveillance. Brain. 2007;130:1350–1359. doi: 10.1093/brain/awm063. [DOI] [PubMed] [Google Scholar]
- 51.Gelpi E, et al. Creutzfeldt-Jakob disease in Austria: an autopsy-controlled study. Neuroepidemiology. 2008;30:215–221. doi: 10.1159/000126915. [DOI] [PubMed] [Google Scholar]
- 52.Abu-Rumeileh S, Capellari S, Parchi P. Rapidly progressive Alzheimer’s disease: contributions to clinical-pathological definition and diagnosis. J. Alzheimers Dis. 2018;63:887–897. doi: 10.3233/JAD-171181. [DOI] [PubMed] [Google Scholar]
- 53.Hecht M, Krämer LM, von Arnim CAF, Otto M, Tal DR. Capillary cerebral amyloid angiopathy in Alzheimer’s disease: association with allocortical/hippocampal microinfarcts and cognitive decline. Acta Neuropathol. 2018;135:681–694. doi: 10.1007/s00401-018-1834-y. [DOI] [PubMed] [Google Scholar]
- 54.Schmidt C, et al. Clinical features of rapidly progressive Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2010;29:371–378. doi: 10.1159/000278692. [DOI] [PubMed] [Google Scholar]
- 55.Seidl JN, Massman PJ. Rapidly versus slowly progressing patients with Alzheimer’s disease: differences in baseline cognition. Am. J. Alzheimers Dis. Other Dement. 2016;31:318–325. doi: 10.1177/1533317515617720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tosto G, et al. Neuropsychological predictors of rapidly progressive Alzheimer’s disease. Acta Neurol. Scand. 2015;132:417–422. doi: 10.1111/ane.12415. [DOI] [PubMed] [Google Scholar]
- 57.Ba M, et al. The prevalence and biomarkers’ characteristic of rapidly progressive Alzheimer’s disease from the Alzheimer’s Disease Neuroimaging Initiative database. Alzheimers Dement. 2017;3:107–113. doi: 10.1016/j.trci.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cohen ML, et al. Rapidly progressive Alzheimer’s disease features distinct structures of amyloid-β. Brain. 2015;138:1009–1022. doi: 10.1093/brain/awv006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu H, et al. Distinct conformers of amyloid beta accumulate in the neocortex of patients with rapidly progressive Alzheimer’s disease. J. Biol. Chem. 2021;29:101267. doi: 10.1016/j.jbc.2021.101267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qiang W, Yau WM, Lu JX, Collinge J, Tycko R. Structural variation in amyloid-β fibrils from Alzheimer’s disease clinical subtypes. Nature. 2017;541:217–221. doi: 10.1038/nature20814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Noor A, et al. Molecular profiles of amyloid-β proteoforms in typical and rapidly progressive Alzheimer’s disease. Mol. Neurobiol. 2022;59:17–34. doi: 10.1007/s12035-021-02566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Drummond E, et al. Proteomic differences in amyloid plaques in rapidly progressive and sporadic Alzheimer’s disease. Acta Neuropathol. 2017;133:933–954. doi: 10.1007/s00401-017-1691-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shafiq M, et al. Prion protein oligomers cause neuronal cytoskeletal damage in rapidly progressive Alzheimer’s disease. Mol. Neurodegener. 2021;6:11. doi: 10.1186/s13024-021-00422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Younas N, et al. SFPQ and Tau: critical factors contributing to rapid progression of Alzheimer’s disease. Acta Neuropathol. 2020;140:317–339. doi: 10.1007/s00401-020-02178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim C, et al. Distinct populations of highly potent TAU seed conformers in rapidly progressing Alzheimer’s disease. Sci. Transl. Med. 2022;14:eabg0253. doi: 10.1126/scitranslmed.abg0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Graff-Radford J, et al. Duration and pathologic correlates of Lewy body disease. JAMA Neurol. 2017;74:310–315. doi: 10.1001/jamaneurol.2016.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Geut H, et al. Neuropathological and genetic characteristics of a post-mortem series of cases with dementia with Lewy bodies clinically suspected of Creutzfeldt-Jakob’s disease. Parkinsonism Relat. Disord. 2019;63:162–168. doi: 10.1016/j.parkreldis.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 68.Coyle-Gilchrist IT, et al. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology. 2016;86:1736–1743. doi: 10.1212/WNL.0000000000002638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Josephs KA, et al. Survival in two variants of tau-negative frontotemporal lobar degeneration: FTLD-U vs FTLD-MND. Neurology. 2005;65:645–647. doi: 10.1212/01.wnl.0000173178.67986.7f. [DOI] [PubMed] [Google Scholar]
- 70.Moore KM, et al. Age at symptom onset and death and disease duration in genetic frontotemporal dementia: an international retrospective cohort study. Lancet Neurol. 2020;19:145–156. doi: 10.1016/S1474-4422(19)30394-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Skrobot OA, et al. Progress toward standardized diagnosis of vascular cognitive impairment: guidelines from the Vascular Impairment of Cognition Classification Consensus Study. Alzheimers Dement. 2018;14:280–292. doi: 10.1016/j.jalz.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 72.Gatti L, et al. Understanding the pathophysiology of cerebral amyloid angiopathy. Int. J. Mol. Sci. 2020;21:3435. doi: 10.3390/ijms21103435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chung CP, et al. Cerebral microbleed burdens in specific brain regions are associated with disease severity of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. J. Am. Heart Assoc. 2020;9:e016233. doi: 10.1161/JAHA.120.016233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gao B, Lyu C, Lerner A, McKinney AM. Controversy of posterior reversible encephalopathy syndrome: what have we learnt in the last 20 years? J. Neurol. Neurosurg. Psychiatry. 2018;89:14–20. doi: 10.1136/jnnp-2017-316225. [DOI] [PubMed] [Google Scholar]
- 75.Salvarani C, Brown RD, Jr, Hunder GG. Adult primary central nervous system vasculitis. Lancet. 2012;9843:767–777. doi: 10.1016/S0140-6736(12)60069-5. [DOI] [PubMed] [Google Scholar]
- 76.Schuster S, et al. Relapse rates and long-term outcome in primary angiitis of the central nervous system. J. Neurol. 2019;266:1481–1489. doi: 10.1007/s00415-019-09285-1. [DOI] [PubMed] [Google Scholar]
- 77.John S, Hajj-Ali RA. CNS vasculitis. Semin. Neurol. 2014;34:405–412. doi: 10.1055/s-0034-1390389. [DOI] [PubMed] [Google Scholar]
- 78.Salvarani C, et al. Aβ-related angiitis: comparison with CAA without inflammation and primary CNS vasculitis. Neurology. 2013;81:1596–1603. doi: 10.1212/WNL.0b013e3182a9f545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 80.Dubois B, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- 81.Ramachandran PS, et al. Neurosyphilis: still prevalent and overlooked in an at risk population. PLoS ONE. 2020;15:e0238617. doi: 10.1371/journal.pone.0238617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tang W, et al. Late neurosyphilis and tertiary syphilis in Guangdong Province, China: results from a cross-sectional study. Sci. Rep. 2017;7:45339. doi: 10.1038/srep45339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N. Engl. J. Med. 2020;382:845–854. doi: 10.1056/NEJMra1901593. [DOI] [PubMed] [Google Scholar]
- 84.Granerod J, et al. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect. Dis. 2010;10:835–844. doi: 10.1016/S1473-3099(10)70222-X. [DOI] [PubMed] [Google Scholar]
- 85.Boucher A, et al. Epidemiology of infectious encephalitis causes in 2016. Med. Mal. Infect. 2017;47:221–235. doi: 10.1016/j.medmal.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 86.Wang Y, et al. Global prevalence and burden of HIV-associated neurocognitive disorder: a meta-analysis. Neurology. 2020;95:e2610–e2621. doi: 10.1212/WNL.0000000000010752. [DOI] [PubMed] [Google Scholar]
- 87.Compain C, et al. Central nervous system involvement in Whipple disease: clinical study of 18 patients and long-term follow-up. Medicine. 2013;92:324–330. doi: 10.1097/MD.0000000000000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Turtle L, Solomon T. Japanese encephalitis–the prospects for new treatments. Nat. Rev. Neurol. 2018;14:298–313. doi: 10.1038/nrneurol.2018.30. [DOI] [PubMed] [Google Scholar]
- 89.Niller HH, et al. Zoonotic spillover infections with Borna disease virus 1 leading to fatal human encephalitis, 1999-2019: an epidemiological investigation. Lancet Infect. Dis. 2020;20:467–477. doi: 10.1016/S1473-3099(19)30546-8. [DOI] [PubMed] [Google Scholar]
- 90.Berger JR, et al. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology. 2013;80:1430–1438. doi: 10.1212/WNL.0b013e31828c2fa1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Helms J, et al. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Paterson RW, et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143:3104–3120. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Frontera JA, et al. A prospective study of neurologic disorders in hospitalized patients with COVID-19 in New York City. Neurology. 2021;96:e575–e586. doi: 10.1212/WNL.0000000000010979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Young MJ, O’Hare M, Matiello M, Schmahmann JD. Creutzfeldt-Jakob disease in a man with COVID-19: SARS-CoV-2-accelerated neurodegeneration? Brain Behav. Immun. 2020;89:601–603. doi: 10.1016/j.bbi.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baker HA, Safavynia SA, Evered LA. The ‘third wave’: impending cognitive and functional decline in COVID-19 survivors. Br. J. Anaesth. 2021;126:44–47. doi: 10.1016/j.bja.2020.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arnold C. Could COVID delirium bring on dementia? Nature. 2020;588:22–24. doi: 10.1038/d41586-020-03360-8. [DOI] [PubMed] [Google Scholar]
- 97.Numbers K, Brodaty H. The effects of the COVID-19 pandemic on people with dementia. Nat. Rev. Neurol. 2021;17:69–70. doi: 10.1038/s41582-020-00450-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Watson N, et al. Application of telehealth for comprehensive Creutzfeldt-Jakob disease surveillance in the United Kingdom. J. Neurol. Sci. 2021;420:117221. doi: 10.1016/j.jns.2020.117221. [DOI] [PubMed] [Google Scholar]
- 99.Lancaster E, Dalmau J. Neuronal autoantigens–pathogenesis, associated disorders and antibody testing. Nat. Rev. Neurol. 2012;8:380–390. doi: 10.1038/nrneurol.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hansen N, et al. Autoantibody-associated psychiatric symptoms and syndromes in adults: a narrative review and proposed diagnostic approach. Brain Behav. Immun. Health. 2020;9:100154. doi: 10.1016/j.bbih.2020.100154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Geschwind MD, et al. Voltage-gated potassium channel autoimmunity mimicking Creutzfeldt-Jakob disease. Arch. Neurol. 2008;65:1341–1346. doi: 10.1001/archneur.65.10.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sabater L, et al. A novel non-rapid-eye movement and rapid-eye-movement parasomnia with sleep breathing disorder associated with antibodies to IgLON5: a case series, characterisation of the antigen, and post-mortem study. Lancet Neurol. 2014;13:575–586. doi: 10.1016/S1474-4422(14)70051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Do LD, et al. Characteristics in limbic encephalitis with anti-adenylate kinase 5 autoantibodies. Neurology. 2017;88:514–524. doi: 10.1212/WNL.0000000000003586. [DOI] [PubMed] [Google Scholar]
- 104.Ricken G, et al. Autoimmune global amnesia as manifestation of AMPAR encephalitis and neuropathologic findings. Neurol. Neuroimmunol. Neuroinflamm. 2021;8:e1019. doi: 10.1212/NXI.0000000000001019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Escudero D, et al. Antibody-associated CNS syndromes without signs of inflammation in the elderly. Neurology. 2017;89:1471–1475. doi: 10.1212/WNL.0000000000004541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Graus F, et al. Syndrome and outcome of antibody-negative limbic encephalitis. Eur. J. Neurol. 2018;25:1011–1016. doi: 10.1111/ene.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gozzard P, et al. Paraneoplastic neurologic disorders in small cell lung carcinoma: a prospective study. Neurology. 2015;85:235–239. doi: 10.1212/WNL.0000000000001721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Armangue T, et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol. 2018;17:760–772. doi: 10.1016/S1474-4422(18)30244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Titulaer MJ, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12:157–165. doi: 10.1016/S1474-4422(12)70310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Valencia-Sanchez C, et al. Brain dysfunction and thyroid antibodies: autoimmune diagnosis and misdiagnosis. Brain Commun. 2021;3:fcaa233. doi: 10.1093/braincomms/fcaa233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Picard C, Pasquier F, Martinaud O, Hannequin D, Godefroy O. Early onset dementia: characteristics in a large cohort from academic memory clinics. Alzheimer Dis. Assoc. Disord. 2011;25:203–205. doi: 10.1097/WAD.0b013e3182056be7. [DOI] [PubMed] [Google Scholar]
- 112.Hillbom M, Pieninkeroinen I, Leone M. Seizures in alcohol-dependent patients: epidemiology, pathophysiology and management. CNS Drugs. 2003;17:1013–1030. doi: 10.2165/00023210-200317140-00002. [DOI] [PubMed] [Google Scholar]
- 113.Panda AK, Malik S. CNS intravascular lymphoma: an underappreciated cause of rapidly progressive dementia. BMJ Case Rep. 2014;2014:bcr2014203772. doi: 10.1136/bcr-2014-203772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Van der Meulen M, et al. Cognitive functioning and health-related quality of life in patients with newly diagnosed primary CNS lymphoma: a systematic review. Lancet Oncol. 2018;19:e407–e418. doi: 10.1016/S1470-2045(18)30356-5. [DOI] [PubMed] [Google Scholar]
- 115.Van der Meulen M, et al. Neurocognitive functioning and radiologic changes in primary CNS lymphoma patients: results from the HOVON 105/ALLG NHL 24 randomized controlled trial. Neuro Oncol. 2021;23:1315–1326. doi: 10.1093/neuonc/noab021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.El-Hattab AW, Adesina AM, Jones J, Scaglia F. MELAS syndrome: clinical manifestations, pathogenesis, and treatment options. Mol. Genet. Metab. 2015;116:4–12. doi: 10.1016/j.ymgme.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 117.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 5th edn (American Psychiatric Association, 2013).
- 118.McAllister TW, Price TR. Severe depressive pseudodementia with and without dementia. Am. J. Psychiatry. 1982;139:626–629. doi: 10.1176/ajp.139.5.626. [DOI] [PubMed] [Google Scholar]
- 119.Collins SJ, et al. Determinants of diagnostic investigation sensitivities across the clinical spectrum of sporadic Creutzfeldt-Jakob disease. Brain. 2006;129:2278–2287. doi: 10.1093/brain/awl159. [DOI] [PubMed] [Google Scholar]
- 120.Shrestha R, Wuerz T, Appleby BS. Rapidly progressive young-onset dementias: neuropsychiatric aspects. Psychiatr. Clin. North. Am. 2015;38:221–232. doi: 10.1016/j.psc.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 121.Irani SR, et al. Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann. Neurol. 2011;69:892–900. doi: 10.1002/ana.22307. [DOI] [PubMed] [Google Scholar]
- 122.Zetterberg H, Bendlin BB. Biomarkers for Alzheimer’s disease–preparing for a new era of disease-modifying therapies. Mol. Psychiatry. 2021;26:296–308. doi: 10.1038/s41380-020-0721-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Carswell C, et al. MRI findings are often missed in the diagnosis of Creutzfeldt-Jakob disease. BMC Neurol. 2012;12:153. doi: 10.1186/1471-2377-12-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Boulouis G, et al. Primary angiitis of the central nervous system: magnetic resonance imaging spectrum of parenchymal, meningeal, and vascular lesions at baseline. Stroke. 2017;48:1248–1255. doi: 10.1161/STROKEAHA.116.016194. [DOI] [PubMed] [Google Scholar]
- 125.Fragoso DC, et al. Imaging of Creutzfeldt-Jakob disease: imaging patterns and their differential diagnosis. Radiographics. 2017;37:234–257. doi: 10.1148/rg.2017160075. [DOI] [PubMed] [Google Scholar]
- 126.Rudge P, Hyare H, Green A, Collinge J, Mead S. Imaging and CSF analyses effectively distinguish CJD from its mimics. J. Neurol. Neurosurg. Psychiatry. 2018;89:461–466. doi: 10.1136/jnnp-2017-316853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schmidt C, Plickert S, Summers D, Zerr I. Pulvinar sign in Wernicke’s encephalopathy. CNS Spectr. 2010;15:215–218. doi: 10.1017/S1092852900000043. [DOI] [PubMed] [Google Scholar]
- 128.Janssen JC, Godbolt AK, Ioannidis P, Thompson EJ, Rossor MN. The prevalence of oligoclonal bands in the CSF of patients with primary neurodegenerative dementia. J. Neurol. 2004;251:184–188. doi: 10.1007/s00415-004-0296-4. [DOI] [PubMed] [Google Scholar]
- 129.Jacobi C, et al. Immunoglobulins and virus-specific antibodies in patients with Creutzfeldt-Jakob disease. Acta Neurol. Scand. 2005;111:185–190. doi: 10.1111/j.1600-0404.2005.00330.x. [DOI] [PubMed] [Google Scholar]
- 130.Djukic M, et al. Cerebrospinal fluid abnormalities in meningeosis neoplastica: a retrospective 12-year analysis. Fluids Barriers CNS. 2017;14:7. doi: 10.1186/s12987-017-0057-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Neumann B, et al. Reactive increase of cerebrospinal fluid white blood cell count after lumbar puncture: fact or fiction? J. Neurol. Sci. 2020;414:116876. doi: 10.1016/j.jns.2020.116876. [DOI] [PubMed] [Google Scholar]
- 132.Malter MP, Choi S, Fink GR. Cerebrospinal fluid findings in non-infectious status epilepticus. Epilepsy Res. 2018;140:61–65. doi: 10.1016/j.eplepsyres.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 133.Bajaj S, Griesdale D, Agha-Khani Y, Moien-Afshari F. Cerebrospinal fluid pleocytosis not attributable to status epilepticus in first 24 h. Can. J. Neurol. Sci. 2022;49:210–217. doi: 10.1017/cjn.2021.83. [DOI] [PubMed] [Google Scholar]
- 134.Dalmau J, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jack CR, Jr, et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lehmann S, et al. Diagnosis associated with Tau higher than 1200 pg/mL: insights from the clinical and laboratory practice. Clin. Chem. Acta. 2019;495:451–456. doi: 10.1016/j.cca.2019.04.081. [DOI] [PubMed] [Google Scholar]
- 137.Marquetand J, et al. Periodic EEG patterns in sporadic Creutzfeld-Jakob-disease can be benzodiazepine-responsive and be difficult to distinguish from non-convulsive status epilepticus. Seizure. 2017;53:47–50. doi: 10.1016/j.seizure.2017.10.023. [DOI] [PubMed] [Google Scholar]
- 138.Sonderen AV, et al. Predictive value of electroencephalography in anti-NMDA receptor encephalitis. J. Neurol. Neurosurg. Psychiatry. 2018;89:1101–1106. doi: 10.1136/jnnp-2018-318376. [DOI] [PubMed] [Google Scholar]
- 139.Cortelli P, et al. Pre-symptomatic diagnosis in fatal familial insomnia: serial neurophysiological and 18FDG-PET studies. Brain. 2006;29:668–675. doi: 10.1093/brain/awl003. [DOI] [PubMed] [Google Scholar]
- 140.Albano D, Bosio G, Bertoli M, Giubbini R, Bertagna F. 18F-FDG PET/CT in primary brain lymphoma. J. Neurooncol. 2018;136:577–583. doi: 10.1007/s11060-017-2686-3. [DOI] [PubMed] [Google Scholar]
- 141.Katsanos AH, et al. Performance of 18F-FDG, 11C-methionine, and 18F-FET PET for glioma grading: a meta-analysis. Clin. Nucl. Med. 2019;44:864–869. doi: 10.1097/RLU.0000000000002654. [DOI] [PubMed] [Google Scholar]
- 142.Graus F, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15:391–404. doi: 10.1016/S1474-4422(15)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Riche M, et al. Complications after frame-based stereotactic brain biopsy: a systematic review. Neurosurg. Rev. 2021;44:301–307. doi: 10.1007/s10143-019-01234-w. [DOI] [PubMed] [Google Scholar]
- 144.Brown P, et al. Iatrogenic Creutzfeldt-Jakob disease, final assessment. Emerg. Infect. Dis. 2012;18:901–907. doi: 10.3201/eid1806.120116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.National Institute for Health and Care Excellence. Donepezil, galantamine, rivastigmine and memantine for the treatment of Alzheimer’s disease. Technology appraisal guidance [TA217] (NICE, 2018).
- 146.Liu KY, Howard R. Can we learn lessons from the FDA’s approval of aducanumab? Nat. Rev. Neurol. 2021;17:715–722. doi: 10.1038/s41582-021-00557-x. [DOI] [PubMed] [Google Scholar]
- 147.Roettger Y, et al. Immunotherapy in prion disease. Nat. Rev. Neurol. 2013;9:98–105. doi: 10.1038/nrneurol.2012.258. [DOI] [PubMed] [Google Scholar]
- 148.Raymond GJ, et al. Antisense oligonucleotides extend survival of prion-infected mice. JCI Insight. 2019;5:e131175. doi: 10.1172/jci.insight.131175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Abboud H, et al. Autoimmune encephalitis: proposed recommendations for symptomatic and long-term management. J. Neurol. Neurosurg. Psychiatry. 2021;92:897–907. doi: 10.1136/jnnp-2020-325302. [DOI] [PMC free article] [PubMed] [Google Scholar]