Abstract
Impulsivity and sensation seeking are stimulus-oriented traits. Because they differ in degree of intention and planning, they may have distinct neurophysiological mechanisms. Impulsivity is prominent in bipolar disorder, and may be related to pre-attentional information filtering and stimulus-orientation. We investigated specificity of relationships between impulsivity and sensitivity to stimulus intensity in bipolar disorder and controls, using intensity-sensitivity of auditory evoked potentials. Seventy-six subjects (37 healthy controls, 39 with bipolar disorder) were administered an intensity-sensitivity paradigm. Additional measures included Barratt Impulsiveness Scale (BIS-11) and Eysenck Impulsivity and Venturesomeness scores. State-dependent rapid-response impulsivity was measured using the Immediate Memory Task. Intensity-sensitivities of the auditory evoked P1N1, N1P2, P1, N1, and P2 potentials were assessed as the slope of amplitude relative to loudness. Analyses used general linear models (GLM) with impulsivity-related measures as dependent variables and age, gender, education, and diagnosis as dependent variables. BIS-11 total, motor, and attentional impulsivity scores correlated positively with pre-attentional N1 and P1N1 intensity-sensitivity slopes in bipolar disorder, but not in controls. BIS-11 nonplanning and Eysenck Venturesomeness scores did not correlate with intensity-sensitivity. Intensity-sensitivity slopes did not correlate with rapid-response impulsivity. Correlations between N1 or P1N1 slopes and BIS-11 scores in bipolar disorder were not affected by age, education, WAIS, treatment, symptoms, or gender. Trait impulsivity in bipolar disorder may be related to poorly modulated stimulus-driven late pre-attentional responses to stimuli, potentially resulting in exaggerated responses to intense stimuli even before conscious awareness. Components of trait impulsivity are physiologically heterogeneous relative to intensity-sensitivity.
Keywords: Impulsive behavior, Sensation seeking, Personality, Bipolar disorder, Evoked potentials, loudness perception
Introduction
The initiation of action is an important aspect of normal and abnormal brain function. Impulsivity and sensation-seeking, related but potentially independent traits linking stimulus and action (Barratt et al., 2004;Patton et al., 1995), cross psychiatric diagnoses (Zuckerman & Kuhlman, 2000;Moeller et al., 2001;Fossati et al., 2007;Barratt et al., 2004). Impulsivity is increased in bipolar disorder, regardless of clinical state and pharmacological treatment, and is related to severe course of illness, including recurrent course, substance-use or personality disorder (Swann et al., 2004;Swann et al., 2009b;Swann et al., 2010), crime (Swann et al., 2011), or suicidal behavior (Swann et al., 2005). Neurophysiological bases of this increased impulsivity are not well understood. An important characteristic of bipolar disorder is that it is generally recurrent and can be progressive, and it is important to distinguish characteristics underlying or predisposing to the illness from those resulting from progression of the illness or state-dependent changes.
Impulsivity can be measured as a trait-like predisposition to impulsive behavior, or as behavioral responses sensitive to physiological state, environmental conditions, or pharmacological manipulations (Swann et al., 2002). The latter includes rapid-response impulsivity, representing inability to withhold a response to a stimulus that has not been fully appraised and measurable by suitably designed continuous performance or stop-signal tasks (Swann et al., 2002;Dougherty et al., 2003). Trait and state aspects of impulsivity may differ physiologically and have different roles in bipolar disorder.
In bipolar disorder, trait-like impulsivity is increased even in euthymic subjects, is not affected significantly by most treatments (Swann et al., 2009a), and is increased in unaffected first-degree relatives (Sanches et al., 2014). Therefore, increased trait-like impulsivity may represent a marker of susceptibility, or an endophenotype, for bipolar disorder. Neurophysiological correlates of trait-like impulsivity in bipolar disorder have not been identified.
On the other hand, rapid-response impulsivity is normal in euthymic patients with uncomplicated bipolar disorder but is increased with a recurrent course of illness (Swann et al., 2009b) or during mania (Swann et al., 2003;Strakowski et al., 2010), and is associated with complications of bipolar disorder including addiction (Swann et al., 2004), crime (Swann et al., 2011), and severe suicidal behavior (Swann et al., 2005), suggesting a relationship with illness progression.
Trait impulsivity may reflect poorly modulated stimulus-orienting (Zuckerman, 1979;Zuckerman, 1991;Barratt, 1985;Dickman, 2000). Stimulus-orienting can be studied as change in amplitude of auditory evoked potentials relative to change in loudness of the stimulus (intensity-sensitivity). N1 and P2 event-related potentials form a complex that peaks between 60 and 250 ms after an auditory stimulus. Both increase in amplitude with increased loudness, expressed as intensity-amplitude slopes reflecting loudness-dependent changes in N1 and P2 amplitude. The combined N1P2 is widely used to measure intensity-sensitivity.
Intensity-sensitivity of N1P2 may have trait-like characteristics (Norra et al., 2008), and underlying mechanisms are under debate. N1P2 slope has been shown not to be influenced by acute pharmacological manipulation of serotonergic function (Norra et al., 2008;O’neill et al., 2008). Genetic studies have suggested relationships to serotonergic 5HT-1B receptors (Juckel et al., 2008a), catechol O-methyltransferase (COMT) (Juckel et al., 2008b), Brain-derived Neurotrophic Factor (BDNF) (Park et al., 2013), and nitric oxide synthase (Kawohl et al., 2008), but not serotonin transporter (Juckel et al., 2007) regulation. These findings suggest involvement of integrated systems across neurotransmitters, and appear to be related to a chronic predisposition rather than being state-dependent (Norra et al., 2008).If that is the case, trait-like impulsivity in bipolar disorder may correlate with intensity-sensitivity.
Studies on relationships between impulsivity and intensity-sensitivity have focused mostly on the N1P2. Results have been inconsistent, with positive correlations (Barratt et al., 1987;Carrillo-de-la-Pena MT & Barratt, 1993;Brocke et al., 1999;Juckel et al., 1995;Herrmann et al., 2002;Norra et al., 2003) and insignificant or negative correlations (Wang et al., 1999a;Brocke et al., 1999;Tuchtenhagen et al., 2000;Carrillo-de-la-Pena MT & Barratt, 1993;Schwerdtfeger & Baltissen, 2002;Wang et al., 1999b). The amplitude-intensity slope of N1P2 may be lower in bipolar disorder than in controls (Park et al., 2010), whereas in healthy controls N1P2 slope correlated with hypomanic or hyperthymic traits (Hensch et al., 2007). These inconsistencies might be related to the fact that N1 and P2, which combine to form the N1P2 complex, have different functional significances.
The N1, generated in primary auditory cortex and anterior cingulate cortex (Enge et al., 2011) reflects involuntary, stimulus-driven triggering of attention (Naatanen & Picton, 1987;Rinne et al., 2006);(Naatanen, 1992), pp 113-135); P2 reflects early allocation of attention with initial conscious awareness of the stimulus (Naatanen, 1992), p 222). This may be relevant to potential relationships between the N1 or P2 and trait impulsivity or associated characteristics.
Impulsivity and sensation-seeking are related but potentially independent traits linking stimulus and action (Barratt et al., 2004;Patton et al., 1995) across psychiatric diagnoses (Zuckerman & Kuhlman, 2000;Moeller et al., 2001;Fossati et al., 2007;Barratt et al., 2004). Trait impulsivity and sensation seeking may relate differently to neurophysiological correlates of stimulus-intensity assessed by N1 and P2. We know of only three reports investigating relationships between impulsivity or sensation-seeking and intensity-sensitivity of N1 and P2 independently. In a study combining controls and prisoners, trait impulsivity correlated positively with the visual N1 slope (Carrillo-de-la-Pena MT & Barratt, 1993). In studies of healthy controls that did not measure impulsivity, sensation-seeking correlated more strongly with the slope for P2 than for N1 (Brocke et al., 2000;Brocke et al., 1999).
Therefore, trait impulsivity may reflect increased late pre-attentional orientation to intense stimuli, with increased N1 amplitude-intensity slopes with higher trait impulsivity, suggesting augmenting of the N1 in people with high trait impulsivity compared to those with lower impulsivity. Sensation-seeking may reflect early attention allocation to and initial conscious awareness of intense stimuli, with increased amplitude-sensitivity of P2 with higher venturesomeness, suggesting augmenting of the P2 in people with high sensation-seeking or venturesomeness. To address the specificity of relationships between impulsivity and pre-attentive stimulus orientation in bipolar disorder, this study focuses on N1 and P2 rather than the N1P2 complex. In addition to comparing intensity-amplitude slopes in bipolar disorder to healthy controls, we addressed the relationship between those slopes and impulsivity.
We used the Barratt Impulsiveness Scale (BIS-11) (Patton et al., 1995) and I7 Impulsiveness scale (Eysenck et al., 1985) to assess trait impulsivity. Both measures are designed to address impulsivity independent from sensation seeking, assessed with the I7 Venturesomeness scale (Eysenck et al., 1985;Miller et al., 2004;Luengo et al., 1991;Eysenck, 1993).
We hypothesized that (1) impulsivity is more strongly related to the N1 than to the P2 slope, whereas venturesomeness is more strongly related to the P2 slope, (2) these characteristics are stronger in bipolar disorder than in controls, reflecting the relative importance of impulsivity in the pathology of bipolar disorder, (3) relationships between intensity-amplitude slopes and impulsivity involve trait-like impulsivity rather than state-dependent impulsivity as measured by performance tests.
Methods
Participants
The study complied with the Declaration of Helsinki and was approved by the Committee for the Protection of Human Subjects, IRB for the University of Texas Health Science Center at Houston (protocol HSC-MS-05-0036). Subjects, recruited through print advertisements, received thorough descriptions of the study, with full opportunity for questions, and signed informed consent before research-related procedures. Inclusion criteria included: age 18-55, good hearing by self-report, (corrected-to-) normal vision, and no history of traumatic brain injury or epilepsy. Structured Clinical Interviews for DSM-IV axis-I and axis-II disorders (SCID-I and SCID-II) (First et al., 1996;First et al., 1997) were administered by trained staff to establish diagnosis, confirmed by consensus of co-investigators (ACS, JLS, FGM).
Subjects were 37 healthy controls without history or known family history of Axis I or Axis II psychiatric disorder, and 39 with bipolar disorder. Among those with bipolar disorder, 36 had bipolar I disorder, 3 had bipolar II disorder, and none had bipolar disorder NOS (not otherwise specified). Subjects with bipolar II disorder resembled bipolar I disorder (within 1 standard deviation of the mean) in age, education, age of illness onset, current symptom scores, BIS-11 scores, and intensity-amplitude slopes. Large international or prospective studies support the idea that bipolar I and II disorders are subtypes of the same illness rather than distinct conditions (Merikangas & Lamers, 2012;Angst et al., 2013). Table 1 summarizes demographics, WAIS scores, years of education, and scores for impulsivity and venturesomeness. Two subjects with bipolar disorder were prescribed lithium, eleven an anticonvulsant, 14 an antipsychotic, six an antidepressant, and seven another drug type (usually benzodiazepine). Four were receiving no psychotropic medicines (all were required to have active contact with a clinician); four, one medicine type; five, two types; five, three types; and four subjects, four types or more. No treatments or combinations significantly affected intensity-sensitivity slopes (F(1,34)<1.2, p>0.17), and there were no relationships between slopes and number of psychotropic medicine types prescribed (F(4,28)<1.9, p>0.15), confirming the trait-like nature of intensity-sensitivity slopes.
Table 1. Subject characteristics.
| Group | Gender | N | Age | Educ. | WAIS | BIS-11 | IVE Imp. | IVE Vent. |
|---|---|---|---|---|---|---|---|---|
| Controls | Men | 18 | 31.5 ± 7.2 |
13.9 ± 2.5 |
106.1 ± 11.5 |
55.9 ± 8.8 |
4.54 ± 3.99 |
7.83 ± 3.89 |
| Women | 19 | 31.5 ± 9.5 |
13.7 ± 1.8 |
108.3 ± 10.0 |
54.7 ± 9.2 |
4.12 ± 3.47 |
5.31 ± 2.78 |
|
| Bipolar disorder |
Men | 22 | 39.9 ± 9.7 |
12.2 ± 2.7 |
101.8 ± 15.5 |
75.4 ± 10.7 |
12.26 ± 3.57 |
8.68 ± 3.85 |
| Women | 17 | 35.2 ± 10.9 |
12.4 ± 2.5 |
102.9 ± 12.2 |
80.1 ± 15.3 |
11.86 ± 3.85 |
6.52 ± 4.21 |
|
| F, df= 1,72 | Group |
7.74
(0.007) |
7.39
(0.008) |
2.66 (0.1) |
90.7
(10−6) |
95.7
(10−6) |
1.7 | |
| Gender | 1.18 | 0.01 | 0.30 | 0.6 | 0.3 |
8.9
(0.004) |
||
| Group x gender interaction |
1.21 | 0.06 | 0.03 | 1.6 | 0 | 0.1 | ||
Significant effects are in bold face with p-values in parentheses. BIS-11 nonplanning, motor, and attentional subscales resembled total score in relationships to diagnosis and gender.
Procedure
On test days, subjects were required to have negative screens for drugs (RediCup®, Redwood biotech, Santa Rosa) and alcohol (Alco-Sensor III, Intoximeters Inc., Saint Louis), and to refrain from consuming caffeine for eight hours, and from smoking for one hour, before testing. Symptoms of bipolar disorder were rated on the day of testing using the Change version of the Schedule for Affective Disorders and Schizophrenia (SADS-C), designed to measure depressive, manic, anxiety, and psychotic symptoms concomitantly (Endicott & Spitzer, 1978). We used the adapted version of the SADS-C (Bowden et al., 1994), including all ten mania rating scale items from the full SADS (Spitzer & Endicott, 1978a), rather than only the five items in the conventional SADS-C (Spitzer & Endicott, 1978b).
Subjects were administered one block of the intensity-sensitivity paradigm and three blocks of a paired-click paradigm (results reported elsewhere). Subjects completed personality questionnaires on the day they signed informed consent.
Instruments
Barratt Impulsiveness Scale (BIS-11). The BIS-11 has three oblique factors (Patton et al., 1995): non-planning (lack of future sense, 11 items), motor (acting on the spur of the moment, 11 items), attentional (distractibility, lack of sustained attention, 8 items), scored on a four-point Likert scale. Reliability of the BIS-11 total score was good (α > 0.79) across different populations (Stanford et al., 2009). The factor structure of the BIS-10 is not compatible with the BIS-11, though the total BIS-10 score is still a valid measure of overall trait impulsivity (Patton et al., 1995;Stanford et al., 2009). This should be kept in mind regarding results published before development of the BIS-11 (Patton et al., 1995) or more recent studies using the BIS-10 (Norra et al., 2003). Impulsiveness-Venturesomeness-Empathy (IVE). The I7 (Eysenck & Eysenck, 1985;Eysenck, 1993) measures two orthogonal action-oriented traits: Impulsiveness (19 items), and Venturesomeness (16 items). Reliability for Impulsiveness and Venturesomeness are good (α > 0.83).
Rapid-response impulsivity: Immediate Memory Task (IMT) is a Continuous Performance Test (Dougherty et al., 2000). Subjects are shown 5-digit numbers, for 0.5 s, 0.5 s apart, and instructed to respond as quickly as possible when a number matches the previous one. Responses (each 1/3 of trials) are correct detections (matching number); commission errors (4 of 5 digits match); and random errors (no digits match; less than 2% for all subjects and not reported here). Commission errors are considered impulsive responses (Dougherty et al., 2003;Swann et al., 2002). Other measurements were reaction times and signal detection parameters including signal/noise discriminability (A’), and response bias (β) (Donaldson, 1992;Green & Swets, 1966).
Event-Related Potentials: Intensity-sensitivity Paradigm
There were 500 trials of 50, 60, 70, 80, and 90 dB tones (1000 Hz, 40 ms, 10 ms rise/fall), each presented 100 times to optimize reliability at lower intensities (Hensch et al., 2008). Intensities were randomly presented. Interstimulus interval was 1.6 or 2.1s. Subjects were instructed to sit quietly with gaze fixed on a fixation-cross attached to the wall, to keep their eyes open, and to listen passively to the tones.
Data Collection and Analysis
Data collection, analysis, and reporting are consistent with guidelines proposed by the Society for Psychophysiological Research (Pivik et al., 1993). EEG was recorded with the Acquire module of SCAN 4.3 (Compumedics NeuroScan) from electrodes Fz,3,4, FCz,3,4, Cz,3,4, CPz,3,4, and Pz,3,4 attached in a Quik-cap (Compumedics NeuroScan) and arranged according to the international 10-20 system (Jasper, 1958). AFz served as ground electrode. Signals were referenced to linked mastoids, sampled at 1000 Hz, filtered between 0.1-100 Hz, and amplified with Synamps amplifiers. Electrooculograms were assessed with electrodes above and below the right eye, and both outer canthi. Impedances were kept below 5 kΩ.
Raw signals were analyzed off-line using the Edit module of SCAN 4.3 and EEGLAB (Delorme & Makeig, 2004). Blinks were detected and corrected semi-automatically with a regression algorithm (Semlitsch et al., 1986) in Edit. Data were then imported in EEGLAB, epoched between −100 ms and 400 ms relative to stimulus onset, and baseline-corrected. Trials with non-repetitive artifacts were rejected. The remaining signal underwent Independent Component Analysis (ICA), calculating components reflecting different, stationary, underlying sources contributing linearly to the measured evoked potential signal (Jung et al., 2000;Makeig et al., 1997;Onton et al., 2006). ICA allows separation of signal and artifact, including eye movements and muscle activity, which were removed from the signal. Next, the artifact-free signal was averaged per intensity, filtered with a 30 Hz low-pass filter (24 dB/oct roll-off, zero-phase shift) and baseline-corrected.
Detection of P1, N1, and P2 peaks and latencies was confined to Cz, consistent with other studies on intensity-sensitivity (Beauducel et al., 2000) (Table 1). Cz had the most pronounced auditory evoked potentials (Carrillo-de-la-Pena MT, 1999;Hensch et al., 2008) and intensity-sensitivity function (Hensch et al., 2008), and the highest reliability for N1, P2, and N1P2 amplitudes and slopes (Beauducel et al., 2000;Hensch et al., 2008).
Some subjects had one or two intensity-averages with an evoked potential signal slightly differing from those of the remaining intensity averages and the grand average for that subject. These differences appeared random. To optimize consistency in peak detection across intensities, P1, N1, and P2 peaks were determined first for the evoked potential averaged per subject across the 5 intensities (grand average). For grand average peak detection per subject, N1 was identified as the most pronounced negativity between 60 and 150 ms, P2 as the most pronounced positivity between 150 and 250 ms, and P1 as the most pronounced positivity between 20 and 85 ms. If more than one peak was detected in a window, components were selected with clear frontocentral distribution based on the topography of the peak. Next, peaks were detected per intensity using grand-average peaks as templates: peaks were selected that were temporally closest to equivalent grand average peaks. Amplitudes were expressed as peak-to-peak between P1 and N1 (P1N1 complex) or N1 and P2 (N1P2 complex), and as baseline-to-peak for P1, N1, and P2. Latencies to P1, N1, and P2 were also assessed.
Intensity-sensitivity slopes were calculated as: (1) linear slope by linear regression of amplitude (dependent variable) against stimulus intensity (independent variable) (amplitude-stimulus function, or ASF slope), and (2) median slope of all possible slopes that connected the amplitudes between each available pair of intensities. Although the ASF slope is potentially more reliable than the median slope (Hensch et al., 2008), this may be limited to true linear amplitude-intensity relationships. ASF and median slopes were essentially identical for all components for our study, so we report outcomes of and analyses with ASF slopes only.
Statistical Analysis
Group differences in demographics and personality were examined with analysis of variance (ANOVA). Bipolar disorder and controls were compared using general linear models (GLM) analysis, with diagnosis and gender as categorical independent variables; age, years of education, BIS-11 and venturesomeness scores as independent continuous variables; and intensity-sensitivity slopes for P1N1, N1P2, P1, N1, and P2 as dependent variables. Distributions were tested for normality with significance set at p = 0.01 suitable for small sample sizes (Tabachnick & Fidell, 1989) (pp 72-73). Distributions deviating from normality were normalized by logarithmic transformation. Effects were considered significant if p < 0.05 (two-tailed). Testing was performed with Statistica, v.7 (Statsoft, Tulsa, OK).
Results
Demographics and Personality Measures
Table 1 shows that subjects with bipolar disorder were older than controls, had fewer years of education, and similar WAIS scores. BIS-11 and IVE impulsivity scores were substantially elevated (Standardized Effect Size about 2) in bipolar disorder without gender effect. IVE Venturesomeness was higher in men than in women but was not significantly elevated in bipolar disorder.
There were pronounced intercorrelations among impulsivity measures (r > 0.675, p < .001), but no significant correlations between Venturesomeness and impulsivity scales (r < 0.17). Age correlated negatively with Venturesomeness (r = −0.3, p < .05), and Venturesomeness correlated positively with education (r=0.283) and WAIS (r=0.326). BIS-11 total and subscale scores correlated negatively with education (r≤−0.272) but not with WAIS. Thus, in this sample, IVE Venturesomeness and impulsivity measures assessed independent constructs.
Intensity-sensitivity slopes, trait impulsivity, and diagnostic group
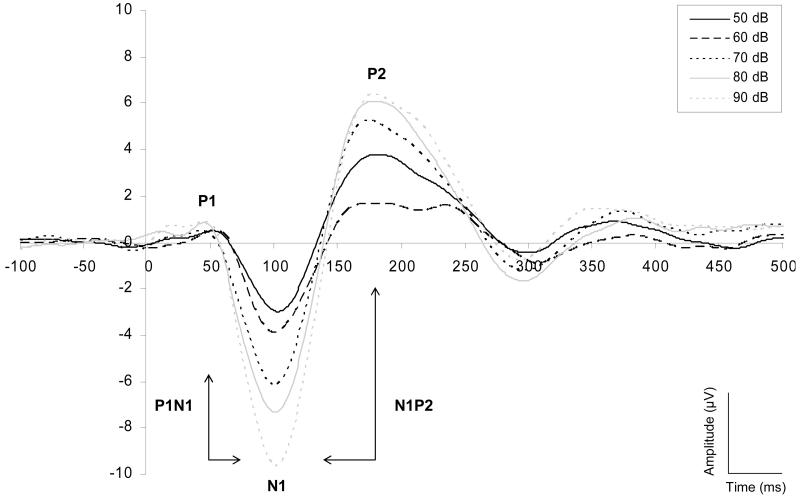
Figure 1 shows auditory evoked potentials at a range of intensities, averaged across subject groups. Slopes were calculated from amplitude-intensity relationships as described in Methods. P1 and N1 slopes did not correlate significantly (r=0.022 in controls and 0.186 in bipolar disorder). N1 and P2 slopes were significantly correlated (r=0.49, p=0.002 in controls and 0.33, p=0.04 in bipolar disorder). P1N1 and N1P2 slopes were strongly correlated with their components (r>0.8), but are included, as they are widely reported.
Figure 1.
Grand average across total group of the auditory evoked potentials for 50, 60, 70, 80, and 90 dB tones at Cz. Marked are the P1, N1, and P2 baseline-to-peak components, and the P1N1 and N1P2 peak-to-peak complexes.
P1 slope correlated positively with SADS-C factor scores for Depression (r=0.515, p<0.001) and Anxiety (r=0.383, p<0.05); P1N1 slope correlated with Depression score (r=0.329, P<0.05). Affective symptoms (SADS-C factors) did not correlate with N1 or P2 slopes (r<0.21, p>0.23), and had no effect on correlations between BIS-11 scores and intensity-amplitude slopes.
Table 2 compares intensity-sensitivity slopes in controls and bipolar disorder. GLM analyses were conducted with intensity-sensitivity slopes as dependent variables, age, BIS-11 total and IVE venturesomeness scores, and education (since it differed between bipolar disorder and controls) as continuous independent variables, and gender and diagnosis (control or bipolar disorder) as dichotomous independent variables. Mean slopes did not differ between the bipolar and control groups.
Table 2. Effects of diagnostic group, impulsivity, and subject characteristics on intensity-amplitude slopes.
| P1 | N1 | P2 | P1N1 | N1P2 | ||
|---|---|---|---|---|---|---|
| Control men (18) | 0.00337± 0.026 |
-0.125± 0.069 |
0.108± 0.075 |
-0.128± 0.082 |
0.233± 0.119 |
|
| Control women (19) | 0.0081 ± 0.0275 |
-0.155 ± 0.119 |
0.118 ± 0.087 |
-0.163 ± 0.115 |
0.273 ± 0.182 |
|
| Bipolar men (21) | 0.00152 ± 0.0369 |
-0.106 ± 0.073 |
0.074 ± 0.075 |
-0.107 ± 0.079 |
0.180 ± 0.114 |
|
| Bipolar women (17) | 0.0145 ± 0.0491 |
-0.186 ± 0.099 |
0.134 ± 0.098 |
-0.200 ± 0.091 |
0.320 ± 0.156 |
|
| F, df = 1, 68 |
Age | 0.05 | 3.73 (0.06) | 1.62 | 3.71 (0.06) | 4.17 (0.044) |
| BIS-11 | 0.71 | 4.17 (0.044) | 0.49 | 5.25 (0.026) | 0.75 | |
| Vent. | 1.89 | 0.11 | 0.45 | 0.07 | 0.41 | |
| Educ. | 0.06 | 0.43 | 0.35 | 0.52 | 0.63 | |
| Group | 0.04 | 0.86 | 0.60 | 0.94 | 0.01 | |
| Gender | 0.48 | 4.04 (0.049) | 3.28 (0.076) | 4.83 (0.033) | 5.92 (0.02) | |
| Group X Gender |
0.59 | 0.77 | 0.24 | 0.26 | 0.76 | |
| Multiple R2 | 0.099 | 0.267 (0.02) | 0.153 | 0.290 (0.01) | .270 (0.02) | |
Significant F ratios or values for R2 are shown in bold; p-values less than 0.1 are in parentheses. Vent. = IVE Venturesomeness score; Educ. = years of education.
BIS-11 score was significantly related to N1 and P1N1 slopes; venturesomeness was not. Women had higher N1 and N1P2 slopes than men, as previously reported (Bruneau et al., 1986); there were no interactions between gender and diagnosis.
Intensity sensitivity-trait impulsivity relationships in controls and bipolar disorder
To examine influence of stimulus orientation on trait impulsivity, we conducted GLM analyses with BIS-11 score as dependent variable and N1 slope, age, education, and gender as independent variables. Table 3 shows slopes for regressions between BIS-11 total, nonplanning, motor, and attentional scores (dependent variables) and N1 intensity-sensitivity slopes (independent variable) for controls and subjects with bipolar disorder. After accounting for age, gender, and education, BIS-11 total, motor, and attentional scores were significantly related to N1 intensity-sensitivity slopes in bipolar disorder, but not in controls. BIS-11 nonplanning score did not correlate significantly with intensity-amplitude slopes. The pattern with P1N1 slopes was similar, without significant relationships to P1, P2, or N1P2 slopes.
Table 3. Relationships between BIS-11 Scores and N1 Intensity-amplitude Slopes: in Controls and Bipolar Disorder.
| BIS-11 Score (p) | Bipolar Disorder (n=39) | Controls (n=37) |
|---|---|---|
| Total | 56.4 ± 22.4 (0.016) | 4.58 ± 15.06 |
| Nonplanning | 6.32 ± 9.36 | 1.53 ± 7.47 |
| Motor | 27.4 ± 9.5 (0.006) | 2.29 ± 6.06 |
| Attentional | 22.7 ± 7.6 (0.005) | 1.52 ± 5.05 |
The Table shows regression slope ± standard error of the mean for the relationship between N1 intensity-amplitude slope and BIS-11 scores for controls and for subjects with bipolar disorder, based on GLM with BIS-11 score as dependent variable and N1 slope, age, and education as independent variables. Significant relationships (p < 0.05) are shown in bold face with p in parentheses.
Intensity-sensitivity slopes and state-related impulsivity
A GLM analysis similar to that for trait impulsivity was conducted with amplitude-intensity slopes as dependent variables, gender and diagnosis as categorical variables, and impulsive IMT errors (commission errors), age, and education as continuous independent variables. There were no significant relationships between intensity-sensitivity slopes and commission errors (F(1,68)<2, p>0.15).
Discussion
We studied relationships between stimulus-orientation and impulsivity or sensation seeking in controls and subjects with bipolar disorder. Relative to our main hypotheses, results were:
Trait impulsivity correlated with the N1 but not with the P2 slope, as hypothesized. However, venturesomeness did not correlate with the P2 or N1 slope. These outcomes suggest that impulsivity and venturesomeness can be distinguished neurophysiologically (Table 2).
As hypothesized, the N1-impulsivity relationship was stronger in bipolar disorder than in controls (Table 3), consistent with the importance of impulsivity in the pathology of bipolar disorder.
Intensity-sensitivity slopes were related to trait impulsivity but not to a performance measure of impulsivity, consistent with the idea that intensity-sensitivity slopes reflect trait-like characteristics (Norra et al., 2008;Carrillo-de-la-Pena MT, 2001).
Intensity-sensitivity, Impulsivity, and Sensation Seeking
Impulsivity is multifactorial (Patton et al., 1995) (Gorlyn et al., 2005;Swann et al., 2002) (Evenden, 1999;Pattij & Vanderschuren, 2008). Its correlation with N1 slope implies a relationship to mechanisms triggering the initial attentional response to an auditory stimulus (Naatanen & Picton, 1987;Naatanen, 1992). Table 3 shows that this relationship is strongest for aspects of trait impulsivity that are directly related to responses to stimuli (attentional and motor) rather than more habitual patterns of thought (nonplanning). This corroborates the idea that trait impulsivity as represented by BIS-11 total score is physiologically heterogeneous, consistent with a recent two-factor formulation of the BIS-11 (Reise et al., 2013).
The BIS-11 measures trait-related impulsivity as a pattern of attitudes or recalled behaviors (Stanford et al., 2009). Performance measures, including modified Continuous Performance Tests (the IMT), are more state-dependent (Dougherty et al., 2003;Swann et al., 2002). We showed that impulsive IMT commission errors were increased by noradrenergic stimulation (Swann et al., 2013a) and correlated with poor P50 sensory gating (Swann et al., 2013b), suggesting that poor pre-attentional suppression of irrelevant information (P50 gating) is related to a tendency to respond to irrelevant stimuli that resemble relevant information (commission errors).
In this paper we showed that there were no significant relationships between IMT commission errors and intensity-amplitude slopes. On the other hand, intensity-amplitude slope of N1 was related to trait impulsivity, suggesting that specific aspects of impulsivity in patients with bipolar disorder differ in time course of stimulus processing, with failure of inhibition related to events at about 50 msec and a trait-like pattern of impulsivity associated with exaggerated responses to intense stimuli at 100 msec.
Impulsivity has been formulated in terms of attention (Dickman, 1993;Dickman, 2000), where, rather than being related to arousal (Barratt, 1985), impulsivity could be related to the intensity of stimulus fixation: subjects with high self-reported impulsivity would ‘readily shift attention’ between stimuli, whereas subjects scoring low could remain fixed on a stimulus for a longer time (Dickman, 1993) (p. 176). Our experiment required no active attention, suggesting that the relationship between impulsivity and stimulus-orientation is at least partly pre-attentive, involuntary (stimulus-driven), and automatic, involving the trigger for attention rather than allocated attention itself. Thus, in bipolar disorder, high trait-impulsivity may reflect rapid and involuntary shift of attention to intense stimuli due to a strong or disinhibited stimulus-driven, pre-attentive, involuntary trigger signal.
Impulsivity, intensity-sensitivity, and bipolar disorder
Slopes correlated positively with impulsivity in bipolar disorder, but not in controls (Table 3), suggesting that, in controls, there may be a protective mechanism against impulsive behavior following intense stimuli, perhaps reducing the stimulus-driven call for attention to intense stimuli reflected by N1. Impulsivity in bipolar disorder may be associated with failure of this mechanism (Swann et al., 2009b), resulting in a stronger relationship between impulsivity and N1 slope. As increased trait impulsivity was also related to more severe course-of-illness in bipolar disorder (Swann et al., 2009a), our results suggest that high pre-attentional sensitivity to intense stimuli enhances the risk of responding to them with unwanted, or unconsidered, overt actions.
Intensity-sensitivity, Personality, and Functional Significance of N1 and P2
Zuckerman suggested that increased intensity-sensitivity could underlie more pronounced ‘impulsive unsocialized sensation seeking’ (Zuckerman, 1991) (p. 407; reviews in (Zuckerman, 1979;Carrillo-de-la-Pena MT, 1992), a combination of impulsivity and sensation-seeking. These traits contrast in their relationship to reflection and intentional action. Impulsivity reflects behavior without opportunity for reflection or regard for consequences (Moeller et al., 2001). This implies a relationship between impulsivity and the automatic, pre-conscious trigger for attention (N1) (Naatanen & Picton, 1987;Rinne et al., 2006;Naatanen, 1992). Sensation-seeking reflects planned, intentional behavior, with potential consequences integrated into the decision to act (Eysenck & Eysenck, 1985). We found no relationship between sensation-seeking and P2, suggesting that this relationship might express later processing of information.
Conclusions
BIS-11-measured impulsivity correlated significantly with intensity-sensitivity of N1 and P1N1 complexes in bipolar disorder but not in controls, while I7 venturesomeness did not correlate with intensity-sensitivity slopes. These data suggest that trait impulsivity in bipolar disorder is related to exaggerated pre-attentional responses to intense stimuli. Sensation-seeking was not related to this early phase of information processing.
Highlights.
Amplitude-intensity slopes are a physiological measure of responses to stimuli
Slope of the pre-attentional N1 potential correlated with impulsivity in bipolar disorder
This relationship appeared independent of clinical state or treatment
Impulsivity was not related to later attentional responses
Impulsivity may be related to exaggerated pre-attentional responses to stimuli
Acknowledgements
This study was supported in part by the Pat R Rutherford, Jr Chair in Psychiatry (ACS) and by NIH grants RO1-MH 69944 (ACS), KO2-DA00403 (FGM), and UL1-RR024148 (CTSA) (General Clinical Research Center UT Houston). This work was also supported with resources and use of facilities at the Michael E. DeBakey VA Medical Center, Houston, Texas. We thank Lorina Maili, MS, Blake Cox, BA, Leslie M. Paith, BA, Irshad N. Prasla, BSc, and Antony G. Zamudio, RN, BC, MSN, CRM, APRN, APMH-NP, for their help with recruitment and study of subjects and the General Clinical Research Center for providing research facilities and excellent nursing support.
Role of funding source:
The work in this paper was funded by NIH grants and an endowed chair. The funding source was not involved in the choice of topics, study design, data analysis or interpretation, or preparation/submission of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
Within the past 12 months, Dr. Swann has acted as a consultant (DSMB) for Teva Pharmaceuticals, has received grant support from Elan pharmaceuticals, and has acted as a consultant and organized/participated in symposia sponsored by Otsuka and Lundbeck. None of these activities was related in any way to the current publication.
The other authors report no potential conflicts of interest.
Contributors
Marijn Lijffijt PhD: Formulating research question, designing and conducting neurophysiological studies, data analysis, writing the manuscript
Scott Lane PhD: Formulating research question, design and interpretation of behavioral studies, consulting on data analysis, assisting with manuscript
Joel Steinberg MD: Formulating research question, consulting on data analysis, assisting with manuscript
F. Gerard Moeller MD: Formulating research question, consulting on data analysis, assisting with manuscript
Alan Swann MD: Formulating research question, overall design, interpretation, data analysis, writing the manuscript, submitting the manuscript
All authors are familiar with the results and approve the final version of the manuscript. Drs. Lijffijt and Swann wrote the first draft of the paper.
References
- Angst J, Gamma A, Bowden CL, Azorin JM, Perugi G, Vieta E, Young AH. Evidence-based definitions of bipolar-I and bipolar-II disorders among 5,635 patients with major depressive episodes in the Bridge Study: validity and comorbidity. European Archives of Psychiatry and Clinical Neuroscience. 2013;263:663–673. doi: 10.1007/s00406-013-0393-4. [DOI] [PubMed] [Google Scholar]
- Barratt ES. Impulsiveness subtraits: arousal and information processing. In: Spence JT, Izard CE, editors. Motivation, emotion, and personality. Elsevier Science publishers; Amsterdam: 1985. pp. 137–146. [Google Scholar]
- Barratt ES, Orozco-Cabal LF, Moeller FG. Impulsivity and sensation-seeking: A historical perspective in current challenges. In: Telmack RM, editor. On the psychobiology of personality. Elsevier Scientific Publishers; Amsterdam: 2004. pp. 3–15. [Google Scholar]
- Barratt ES, Pritchard WS, Faulk DM, Brandt ME. The relationship between impulsiveness subtraits, trait anxiety, and visual N100 augmenting/reducing: A topographic analysis. Personality and Individual Differences. 1987;8:43–51. [Google Scholar]
- Beauducel A, Debener S, Brocke B, Kayer J. On the reliability of augmenting/reducing. Journal of Psychophysiology. 2000;14:226–240. [Google Scholar]
- Bowden CL, Brugger AM, Swann AC, Calabrese JR, Janicak PG, Petty F, Dilsaver SC, Davis JM, Rush AJ, Small JG, Garza Trevino ES, Risch SC, Goodnick PJ, Morris DD. Efficacy of divalproex vs lithium and placebo in the treatment of mania. JAMA. 1994;271:918–924. [PubMed] [Google Scholar]
- Brocke B, Beauducel A, John R, Debener S, Heilemann H. Sensation seeking and affective disorders: characteristics in the intensity dependence of acoustic evoked potentials. Neuropsychobiology. 2000;41:24–30. doi: 10.1159/000026629. [DOI] [PubMed] [Google Scholar]
- Brocke B, Beauducel A, Tasche KG. Biopsychological bases and behavioral correlates of sensation seeking: contributions to a multilevel validation. Personality and Individual Differences. 1999;26:1103–1123. [Google Scholar]
- Bruneau N, Barthelemy C, Jouve J, Lelord G. Frontal auditory-evoked potential augmenting-reducing and urinary homovanillic acid. Neuropsychobiology. 1986;16:78–84. doi: 10.1159/000118302. [DOI] [PubMed] [Google Scholar]
- Carrillo-de-la-Pena MT. ERP augmenting/reducing and sensation seeking: a critical review. Int.J Psychophysiol. 1992;12:211–220. doi: 10.1016/0167-8760(92)90059-k. [DOI] [PubMed] [Google Scholar]
- Carrillo-de-la-Pena MT. Effects of intensity and order of stimuli presentation on AEPs: an analysis of the consistency of EP augmenting/reducing in the auditory modality. Clin.Neurophysiol. 1999;110:924–932. doi: 10.1016/s1388-2457(99)00041-3. [DOI] [PubMed] [Google Scholar]
- Carrillo-de-la-Pena MT. One-year test-retest reliability of auditory evoked potentials (AEPs) to tones of increasing intensity. Psychophysiology. 2001;38:417–424. [PubMed] [Google Scholar]
- Carrillo-de-la-Pena MT, Barratt ES. Impulsivity and ERP augmenting/reducing. Personality and Individual Differences. 1993;15:25–32. [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J.Neurosci.Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dickman SJ. Impulsivity and information processing. In: McCown WG, Johnson JL, Shure MB, editors. The impulsive client: Theory, research, and treatment. American psychological Association; Washington,D.C.: 1993. pp. 151–184. [Google Scholar]
- Dickman SJ. Impulsivity, arousal, and attention. Personality and Individual Differences. 2000;28:563–581. [Google Scholar]
- Donaldson W. Measuring recognition memory. J Exp.Psychol.Gen. 1992;121:275–277. doi: 10.1037//0096-3445.121.3.275. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Bjork JM, Harper RA, Marsh DM, Moeller FG, Mathias CW, Swann AC. Behavioral impulsivity paradigms: A comparison in hospitalized adolescents with disruptive behavior disorders. J Child Psychol.Psychiatry. 2003;44:1145–1157. doi: 10.1111/1469-7610.00197. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Bjork JM, Marsh DM, Moeller FG. A comparison between adults with conduct disorder and normal controls on a Continuous Performance Test: Differences in impulsive response characteristics. Psychological Record. 2000;50:203–219. [Google Scholar]
- Endicott J, Spitzer RL. A diagnostic interview: the schedule for affective disorders and schizophrenia. Archives of General Psychiatry. 1978;35:837–844. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- Enge S, Fleischhauer M, Lesch KP, Strobel A. On the role of serotonin and effort in voluntary attention: evidence of genetic variation in N1 modulation. Behav.Brain Res. 2011;216:122–128. doi: 10.1016/j.bbr.2010.07.021. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ, Eysenck SBG. Personality and individual differences. Plenum Press; New York: 1985. [Google Scholar]
- Eysenck SBG. The development of a measure of impulsivity and its relationship to the superfactors of personality. In: McCown WG, Johnson JL, Shure MB, editors. The impulsive client: theory, research, and treatment. Elsevier Scientific Publishers; Amsterdam: 1993. pp. 141–149. [Google Scholar]
- Eysenck SBG, Pearson PR, Easting G, Allsopp JF. Age norms for impulsiveness, venturesomeness, and empathy in adults. Psychological Reports. 1985;43:1247–1255. [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JB, Benjamin L. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) Biometrics Research Institute, New York State Psychiatric Institute; New York: 1997. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders Patient Edition. Biometrics Research Institute, New York State Psychiatric Institute; New York: 1996. [Google Scholar]
- Fossati A, Barratt ES, Borroni S, Villa D, Grazioli F, Maffei C. Impulsivity, aggressiveness, and DSM-IV personality disorders. Psychiatry Res. 2007;149:157–167. doi: 10.1016/j.psychres.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Gorlyn M, Keilp JG, Tryon WW, Mann JJ. Performance test correlates of component factors of impulsiveness. Personality and Individual Differences. 2005;38:1549–1559. [Google Scholar]
- Green DM, Swets JA. Signal Detection Theory and Psychophysics. Wiley; New York: 1966. [Google Scholar]
- Hensch T, Herold U, Brocke B. An electrophysiological endophenotype of hypomanic and hyperthymic personality. J.Affect.Disord. 2007;101:13–26. doi: 10.1016/j.jad.2006.11.018. [DOI] [PubMed] [Google Scholar]
- Hensch T, Herold U, Diers K, Armbruster D, Brocke B. Reliability of intensity dependence of auditory-evoked potentials. Clin.Neurophysiol. 2008;119:224–236. doi: 10.1016/j.clinph.2007.09.127. [DOI] [PubMed] [Google Scholar]
- Herrmann MJ, Sonnek G, Weijers HG, Wiesbeck GA, Boning J, Fallgatter AJ. Electrophysiological indication for a link between serotonergic neurotransmission and personality in alcoholism. Progress in Neuropsychopharmacology and Biological Psychiatry. 2002;26:157–161. doi: 10.1016/s0278-5846(01)00241-x. [DOI] [PubMed] [Google Scholar]
- Jasper HH. Report of the committee on methods of clinical examination in electroencephalography. Electroencephalography & Clinical Neurophysiology. 1958;10:370–371. [Google Scholar]
- Juckel G, Hegerl U, Giegling I, Mavrogiorgou P, Gallinat J, Augustin H, Mulert C, Pogarell O, Rujescu D. Loudness dependence of auditory evoked potentials is not associated with polymorphisms or haplotypes in the serotonin transporter gene in a community-based sample of German healthy volunteers. Psychiatry Res. 2007;153:183–187. doi: 10.1016/j.psychres.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Juckel G, Hegerl U, Giegling I, Mavrogiorgou P, Wutzler A, Schuhmacher C, Uhl I, Brune M, Mulert C, Pogarell O, Rujescu D. Association of 5-HT1B receptor polymorphisms with the loudness dependence of auditory evoked potentials in a community-based sample of healthy volunteers. Am J Med.Genet.B Neuropsychiatr.Genet. 2008a;147B:454–458. doi: 10.1002/ajmg.b.30628. [DOI] [PubMed] [Google Scholar]
- Juckel G, Kawohl W, Giegling I, Mavrogiorgou P, Winter C, Pogarell O, Mulert C, Hegerl U, Rujescu D. Association of catechol-O-methyltransferase variants with loudness dependence of auditory evoked potentials. Hum Psychopharmacol. 2008b;23:115–120. doi: 10.1002/hup.906. [DOI] [PubMed] [Google Scholar]
- Juckel G, Schmidt LG, Rommelspacher H, Hegerl U. The Tridimensional Personality Questionnaire and the intensity dependence of auditory evoked dipole source activity. Biological Psychiatry. 1995;37:311–317. doi: 10.1016/0006-3223(94)00118-M. [DOI] [PubMed] [Google Scholar]
- Jung TP, Makeig S, Westerfield M, Townsend J, Courchesne E, Sejnowski TJ. Removal of eye activity artifacts from visual event-related potentials in normal and clinical subjects. Clin.Neurophysiol. 2000;111:1745–1758. doi: 10.1016/s1388-2457(00)00386-2. [DOI] [PubMed] [Google Scholar]
- Kawohl W, Giegling I, Mavrogiorgou P, Pogarell O, Mulert C, Moller HJ, Hegerl U, Rujescu D, Juckel G. Association of functional polymorphisms in NOS1 and NOS3 with loudness dependence of auditory evoked potentials. Int.J.Neuropsychopharmacol. 2008;11:477–483. doi: 10.1017/S1461145708008420. [DOI] [PubMed] [Google Scholar]
- Luengo MA, Carrillo-de-la-Pena MT, Otero JM. The components of impulsiveness: A comparison of the I7 impulsiveness questionnaire and the Barratt Impulsiveness Scale. Personality and Individual Differences. 1991;12:657–667. [Google Scholar]
- Makeig S, Jung TP, Bell AJ, Ghahremani D, Sejnowski TJ. Blind separation of auditory event-related brain responses into independent components. Proc.Natl.Acad.Sci.U.S.A. 1997;94:10979–10984. doi: 10.1073/pnas.94.20.10979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, Lamers F. The ‘true’ prevalence of bipolar II disorder. Curr.Opin.Psychiatry. 2012;25:19–23. doi: 10.1097/YCO.0b013e32834de3de. [DOI] [PubMed] [Google Scholar]
- Miller E, Joseph S, Tudway J. Assessing the component structure of four self-report measures of impulsivity. Personality and Individual Differences. 2004;37:349–358. [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. American Journal of Psychiatry. 2001;158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Naatanen R. Attention and brain function. Lawrence Erlbaum Associates; Hillsdale: 1992. [Google Scholar]
- Naatanen R, Picton T. The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiology. 1987;24:375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Norra C, Becker S, Brocheler A, Kawohl W, Kunert HJ, Buchner H. Loudness dependence of evoked dipole source activity during acute serotonin challenge in females. Hum Psychopharmacol. 2008;23:31–42. doi: 10.1002/hup.880. [DOI] [PubMed] [Google Scholar]
- Norra C, Mrazek M, Tuchtenhagen F, Gobbele R, Buchner H, Sass H, Herpertz SC. Enhanced intensity dependence as a marker of low serotonergic neurotransmission in borderline personality disorder. J.Psychiatr.Res. 2003;37:23–33. doi: 10.1016/s0022-3956(02)00064-x. [DOI] [PubMed] [Google Scholar]
- O’Neill BV, Guille V, Croft RJ, Leung S, Scholes KE, Phan KL, Nathan PJ. Effects of selective and combined serotonin and dopamine depletion on the loudness dependence of the auditory evoked potential (LDAEP) in humans. Human Psychopharmacology. 2008;23:301–312. doi: 10.1002/hup.926. [DOI] [PubMed] [Google Scholar]
- Onton J, Westerfield M, Townsend J, Makeig S. Imaging human EEG dynamics using independent component analysis. Neuroscience and Biobehavioral Reviews. 2006;30:808–822. doi: 10.1016/j.neubiorev.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Park YM, Lee SH, Kim S, Bae SM. The loudness dependence of the auditory evoked potential (LDAEP) in schizophrenia, bipolar disorder, major depressive disorder, anxiety disorder, and healthy controls. Progress in Neuropsychopharmacology and Biological Psychiatry. 2010;34:313–316. doi: 10.1016/j.pnpbp.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Park YM, Lee SH, Lee HJ, Kang SG, Min JA, Chae JH. Association between BDNF gene polymorphisms and serotonergic activity using loudness dependence of auditory evoked potentials in healthy subjects. PLoS.ONE. 2013;8:e60340. doi: 10.1371/journal.pone.0060340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattij T, Vanderschuren LJ. The neuropharmacology of impulsive behaviour. Trends Pharmacol.Sci. 2008;29:192–199. doi: 10.1016/j.tips.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin.Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Pivik RT, Broughton RJ, Coppola R, Davidson RJ, Fox N. Nuwer.M.R. Guidelines for the recording and quantitative analysis of electroencephalographic activity in research contexts. Psychophysiology. 1993;30:547–558. doi: 10.1111/j.1469-8986.1993.tb02081.x. [DOI] [PubMed] [Google Scholar]
- Reise SP, Moore TM, Sabb FW, Brown AK, London ED. The Barratt Impulsiveness Scale-11: reassessment of its structure in a community sample. Psychol.Assess. 2013;25:631–642. doi: 10.1037/a0032161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne T, Sarkka A, Degerman A, Schroger E, Alho K. Two separate mechanisms underlie auditory change detection and involuntary control of attention. Brain Research. 2006;1077:135–143. doi: 10.1016/j.brainres.2006.01.043. [DOI] [PubMed] [Google Scholar]
- Sanches M, Scott-Gurnell K, Patel A, Caetano SC, Zunta-Soares GB, Hatch JP, Olvera R, Swann AC, Soares JC. Impulsivity in children and adolescents with mood disorders and unaffected offspring of bipolar parents. Comprehensive Psychiatry. 2014;55:1337–1341. doi: 10.1016/j.comppsych.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwerdtfeger A, Baltissen R. Augmenting-reducing paradox lost? A test of Davis et al.’s (1983) hypothesis. Personality and Individual Differences. 2002;32:257–271. [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Endicott J. Schedule for Affective Disorders and Schizophrenia. New York State Psychiatric Institute Biometrics Inst.; New York: 1978a. [Google Scholar]
- Spitzer RL, Endicott J. Schedule for Affective Disorders and Schizophrenia: Change Version. Biometrics Research, New York State Psychiatric Institute; New York: 1978b. [Google Scholar]
- Stanford MS, Mathias CW, Dougherty DM, Lake SL, Anderson NE, Patton JH. Fifty years of the Barratt Impulsiveness Scale: An update and review. Personality and Individual Differences. 2009;47:385–395. [Google Scholar]
- Strakowski SM, Fleck DE, DelBello MP, Adler CM, Shear PK, Kotwal R, Arndt S. Impulsivity across the course of bipolar disorder. Bipolar.Disord. 2010;12:285–297. doi: 10.1111/j.1399-5618.2010.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann AC, Bjork JM, Moeller FG, Dougherty DM. Two models of impulsivity: Relationship to personality traits and psychopathology. Biological Psychiatry. 2002;51:988–994. doi: 10.1016/s0006-3223(01)01357-9. [DOI] [PubMed] [Google Scholar]
- Swann AC, Dougherty DM, Pazzaglia PJ, Pham M, Moeller FG. Impulsivity: A link between bipolar disorder and substance abuse. Bipolar.Disord. 2004;6:204–212. doi: 10.1111/j.1399-5618.2004.00110.x. [DOI] [PubMed] [Google Scholar]
- Swann AC, Dougherty DM, Pazzaglia PJ, Pham M, Steinberg JL, Moeller FG. Increased impulsivity associated with severity of suicide attempt history in patients with bipolar disorder. American Journal of Psychiatry. 2005;162:1680–1687. doi: 10.1176/appi.ajp.162.9.1680. [DOI] [PubMed] [Google Scholar]
- Swann AC, Lijffijt M, Lane SD, Cox B, Steinberg JL, Moeller FG. Norepinephrine and impulsivity: effects of acute yohimbine. Psychopharmacology (Berl) 2013a;229:83–94. doi: 10.1007/s00213-013-3088-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann AC, Lijffijt M, Lane SD, Kjome KL, Steinberg JL, Moeller FG. Criminal conviction, impulsivity, and course of illness in bipolar disorder. Bipolar Disord. 2011;13:173–181. doi: 10.1111/j.1399-5618.2011.00900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann AC, Lijffijt M, Lane SD, Steinberg JL, Acas MD, Cox B, Moeller FG. Pre-attentive information processing and impulsivity in bipolar disorder. J.Psychiatr.Res. 2013b;47:1917–1924. doi: 10.1016/j.jpsychires.2013.08.018. [DOI] [PubMed] [Google Scholar]
- Swann AC, Lijffijt M, Lane SD, Steinberg JL, Moeller FG. Increased trait-like impulsivity and course of illness in bipolar disorder. Bipolar.Disord. 2009a;11:280–288. doi: 10.1111/j.1399-5618.2009.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann AC, Lijffijt M, Lane SD, Steinberg JL, Moeller FG. Severity of bipolar disorder is associated with impairment of response inhibition. J Affect.Disord. 2009b;116:30–36. doi: 10.1016/j.jad.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann AC, Lijffijt M, Lane SD, Steinberg JL, Moeller FG. Interactions between bipolar disorder and antisocial personality disorder in trait impulsivity and severity of illness. Acta Psychiatrica Scandinavica. 2010;121:453–461. doi: 10.1111/j.1600-0447.2009.01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann AC, Pazzaglia P, Nicholls A, Dougherty DM, Moeller FG. Impulsivity and phase of illness in bipolar disorder. J.Affect.Disord. 2003;73:105–111. doi: 10.1016/s0165-0327(02)00328-2. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using Multivariate Statistics. Harper Collins Publishers; New York: 1989. [Google Scholar]
- Tuchtenhagen F, Daumann J, Norra C, Gobbele R, Becker S, Pelz S, Sass H, Buchner H, Gouzoulis-Mayfrank E. High intensity dependence of auditory evoked dipole source activity indicates decreased serotonergic activity in abstinent ecstasy (MDMA) users. Neuropsychopharmacology. 2000;22:608–617. doi: 10.1016/S0893-133X(99)00140-2. [DOI] [PubMed] [Google Scholar]
- Wang W, Mei XF, Du L, Lu SW, Fu XM, Wang YH. Personality correlates of auditory augmenting response to clicks repeated around 2Hz. J Neural Transm. 1999a;106:559–568. doi: 10.1007/s007020050179. [DOI] [PubMed] [Google Scholar]
- Wang W, Wang Y, Fu X, Sung Z, Schoenen J. Auditory evoked potentials and multiple personality measures in migraine and post-traumatic headaches. Pain. 1999b;79:235–242. doi: 10.1016/s0304-3959(98)00168-7. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. Sensation seeking: Beyond the optimal level of arousal. Erlbaum; Hillsdale, N.J.: 1979. [Google Scholar]
- Zuckerman M. Psychobiology of personality. Cambridge University Press; Cambridge England: 1991. [Google Scholar]
- Zuckerman M, Kuhlman DM. Personality and risk-taking: common biosocial factors. J.Pers. 2000;68:999–1029. doi: 10.1111/1467-6494.00124. [DOI] [PubMed] [Google Scholar]