Abstract
Organoboron compounds are very important building blocks which can be applied in medicinal, biological and industrial fields. However, direct borylation in a metal free manner has been very rarely reported. Herein, we described the successful direct borylation of haloarenes under mild, operationally simple, catalyst-free conditions, promoted by irradiation with visible light. Mechanistic experiments and computational investigations indicate the formation of an excited donor–acceptor complex with a −3.12 V reduction potential, which is a highly active reductant and can facilitate single-electron-transfer (SET) with aryl halides to produce aryl radical intermediates. A two-step one-pot method was developed for site selective aromatic C–H bond borylation. The protocol's good functional group tolerance enables the functionalization of a variety of biologically relevant compounds, representing a new application of aryl radicals merged with photochemistry.
We reported a facile metal-free conversion of aryl halides to the corresponding boronic esters catalysed by an in situ formed donor–acceptor complex. A two-step one-pot method was also developed for site selective aromatic C–H bond borylation.
Introduction
Boronic esters are commonly encountered in pharmaceuticals, functional materials and agrochemicals.1 Aryl halides and related arenes are the most commonly employed precursors to produce them.2 Conventional routes for their synthesis include metalation (generally by Li or Mg reagents)3 followed by nucleophilic substitution and transition-metal catalysis,4 offering a broad substrate scope and good functional group compatibility if their disadvantages such as high cost, low reactivity and operational inconvenience could be ignored. Visible light catalysis5 has become a well-accepted and powerful method for the direct borylation of drug like molecules. Seminal studies by Larionov,6 Aggarwal,7 Wu,8 Schelter9 and others10 have established the viability and synthetic utility of this approach, circumventing the need for well recognized photocatalysts and metal additives. Studer11 developed radical borylation of aryl iodides with bis(catecholato)diboron (B2cat2) as the boron source under mild conditions. Recently, Jiao12 reported the borylation of aryl halides using 4-phenylpyridine as an efficient catalyst and sodium methanolate as a strong base with bis(pinacolato)diboron (B2pin2). Different from Jiao's work, Li,13 Larionov14 and Lin15 independently described simple and efficient methods to convert aryl triflates and aryl halides into aryl radicals under UV irradiation or reductive electrophotocatalysis. There is a constant quest for more efficient clean borylation strategies (e.g., metal-free visible light catalysis using an in situ formed photocatalyst) from simple and readily available starting materials.
In recent years, in situ formed donor–acceptor complexes have been successfully used in the single-electron-transfer (SET) process to produce radical species by hybridization and modulation of the relevant energy levels.16,17 As a result, visible light excitation can occur favoured by a red shift of the absorption band, although both donor and acceptor components are individually insensitive to visible light irradiation. This novel protocol can provide a new approach for aryl radical intermediates through C–X bond activation in a metal-free manner (Scheme 1).
Scheme 1. Synthetic approaches to aryl boronic esters.
In 2020, we reported a photoinduced dehalogenation of aryl halides.18 Spectroscopic, computational and chemical studies indicate that the formation of a twisted intramolecular charge-transfer (TICT) species enables the population of higher-energy doublet states. König19 developed a versatile photocatalytic strategy for the ipso-borylation of substituted arenes by using thiolate as a catalyst, forming a donor–acceptor complex between thiolate/B2pin2 and boryl-anion-activated substrates. Inspired by our previous work and related reports,20 we questioned whether borylation of aryl halides could be achieved with the photocatalytic intermediate formed in situ employing simple available substrates (e.g., N-containing heterocycles/organic base/boron source). Herein, we described the successful direct borylation of haloarenes under mild, operationally simple, catalyst-free conditions, promoted by irradiation with visible light.
Results and discussion
We made our initial discovery of the borylation reaction employing 4-bromoanisole as the model substrate (Table 1). First, under visible light photoirradiation, when the 2,2′-bipyridine (nitrogen containing heterocycle: NCH)/triethylamine (NEt3; final reductant)/B2pin2 (boron source) system was evaluated in acetonitrile, the desired product can be detected in 27% yield (entry 1). In order to generate the optimal ate-intermediate to facilitate the single-electron-transfer (SET) process, a series of NCHs were evaluated (entries 2–5). Only trace desired product was detected using the 2-phenyl pyridine/NEt3/B2pin2 system (entry 2) suggesting that the ate-intermediate formed with 2-phenyl pyridine couldn't deliver a single electron to the aromatic C–Br bond. To our delight, the yield was improved remarkably (49% yield) employing isoquinoline as the ate-intermediate precursor (entry 3). Neither acridine (entry 4) nor 4-trifluoromethyl pyridine (entry 5) could benefit the borylation reaction, indicating that steric resistance and electron-deficient factors of NCHs can decrease the reductive ability of the ate-intermediate. A brief investigation of the solvent effect demonstrates that DMSO (entry 6), DMF (entry 7) and DCE (entry 8) are not as efficient as acetonitrile. Gratifyingly, the desired product could be isolated with 81% yield via increasing the loading of B2pin2/NEt3 and prolonging the photoirradiation process (entry 9). Not surprisingly, control experiments without NCH (entry 10) and in the dark (entry 11) couldn't afford the desired product effectively.
Optimization of the reaction conditionsa.

| ||||||
|---|---|---|---|---|---|---|
| Entry | Solvent | B2pin2 (X eq.) | NEt3 (Y eq.) | NCH | Time (h) | Yield (%)b |
| 1 | AcN | X = 2 | Y = 3 | A | 12 | 27 |
| 2 | AcN | X = 2 | Y = 3 | B | 12 | Trace |
| 3 | AcN | X = 2 | Y = 3 | C | 12 | 49 |
| 4 | AcN | X = 2 | Y = 3 | D | 12 | 17 |
| 5 | AcN | X = 2 | Y = 3 | E | 12 | Trace |
| 6 | DMSO | X = 2 | Y = 3 | C | 12 | Trace |
| 7 | DMF | X = 2 | Y = 3 | C | 12 | NR |
| 8 | DCE | X = 2 | Y = 3 | C | 12 | 16 |
| 9 | AcN | X = 4 | Y = 5 | C | 36 | 87 (81) c |
| 10 | AcN | X = 4 | Y = 5 | — | 36 | 14 |
| 11 | AcN | X = 4 | Y = 5 | C | 36 | NRd |
Reactions were performed with 0.2 mmol 4-bromoanisole, 1.0 mmol NEt3, 20 mol% NCH, 0.5 ml solvent and suitable B2pin2 for the indicated period of time under 2 × 390 nm LED (40 W) irradiation.
NMR yields using pyrazine or hexamethyl disiloxane as the internal standard.
Isolated yields.
Without irradiation.
With the optimized reaction conditions in hand, we first examined the substrate scope of aryl bromides (Table 2). Various para-substituted aryl bromides, including electron donating group substituted (3a–3f), nonsubstituted (3g), unprotected –OH (3h)/–NH2 (3i), electron withdrawing group substituted (3j–3o) and even simple alkene moieties (3o), could be well tolerated in this system, delivering the corresponding arylboronates in good to excellent yields. However, bromo-styrene was not tolerated. A dual borylation product (3q) could be synthesized using dihaloarene or haloaryl boronic ester. Meta-(3r–3t) and ortho-(3u–3w) substituted arylboronates can both be obtained in good yields (e.g., 3w, 1-allyl-2-bromobenzene, 63% yield). Moreover, 2,5-(3x)/3,4-(3y–3ac)/3,5-disubstituted (3ad–3ae) aryl bromides produced borylation products in good yields (3aa and 3ac, 69% and 64%, respectively). Heteroaromatic bromides, such as benzothiophene (3ag) and indole (3ah) derivatives, can be employed to access the desired borylation products with good efficiencies other than brominated benzofuran (3af, acceptable 23% yield). This methodology also worked well with a series of diboron reagents to produce arylboronates 4a–6a with useful efficiencies (e.g., 4a, 69% yield). In contrast, B2(OH)4 was not a good borylation candidate with a poor solubility in acetonitrile. Notably, except for aryl halides, 3a could also be prepared through more challenging Ar–O (7a)/Ar–N (8a) bond cleavage under standard conditions. Surprisingly, reductive deoxygenation of fluorenone was accomplished with high efficiency (9a, 65%).
Substrate scope of aryl bromidesa.

|
Reactions were run on a 0.2 mmol scale; isolated yield.
Irradiation for 48 h.
Using 1-bromo-4-chlorobenzene as the starting material.
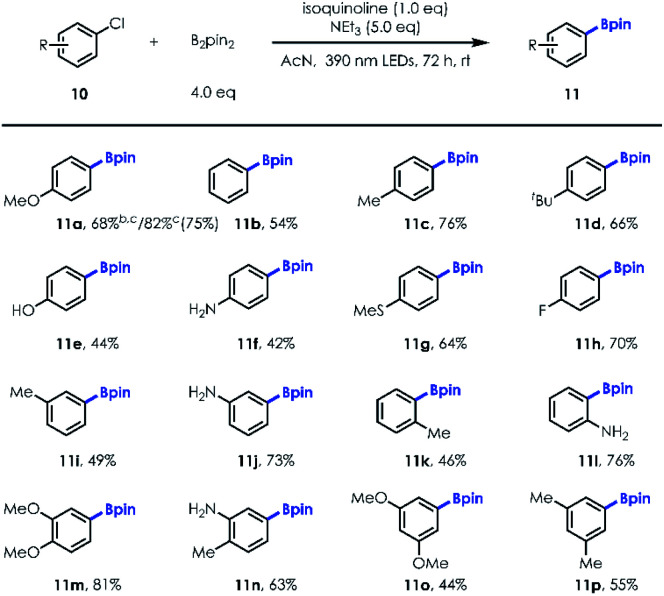
Next, we turned our attention to exploring the substrate scope of the borylation of unactivated aryl chlorides, since cleavage of the C–Cl bond in a metal-free manner remains quite challenging [e.g., for PhCl, Ered = −3.04 V vs. SCE].21 Our investigations revealed that both electron-rich and electron-deficient chlorobenzene derivatives could be employed to provide the corresponding arylboronates in good yields with a little more loading of NCH and longer irradiation time (Table 3; 68% NMR yield for 20 mol% NCH loading and 36 h irradiation vs. 82% NMR yield for 100 mol% NCH loading and 72 h irradiation). It's worth noting that the reaction was allowed for production of fluorinated boronic ester (11h). In addition, unprotected para, meta, and ortho –OH/–NH2 substituted aryl chlorides (10e–10f; 10j; 10l) were well tolerated, illustrating the immediate utility of this approach in preparing value-added boronic ester in a protecting group-free manner. Similar to aryl bromide substrates, more electron-rich and sterically hindered aryl chlorides (10m–10p) could also be transformed into desired products with good yields using this new protocol (Table 4).
Substrate scope of aryl chloridesa.
|
Reactions were run on a 0.2 mmol scale; isolated yield.
20 mol% NCH (isoquinoline) was used and irradiation for 36 h.
NMR yield.
Two step, one-pot method for Ar C–H bond borylationa.

|
Reactions were run on a 0.2 mmol scale; isolated yield.
Site selective aromatic C–H functionalization provides rapid access to useful structural and functional molecular complexity.22 Based on the optimized reaction conditions, we developed a two-step one-pot method for simple aromatic C–H borylation. In the first step, almost equivalent regioselective bromination is accomplished using N-bromosuccinimide (NBS) as the bromination reagent, controlled by the electrophilicity and steric hindrance effect of the aromatic ring.23 Subsequently, a series of aryl bromides without further purification were transformed into the corresponding boronic esters with high regioselectivities and efficiencies (e.g., 13a, 73% yield; rr up to 40 : 1). Aromatic silyl-ethers (13c–d), unprotected phenol (13e), aniline (13f) and polysubstituted benzenes (13g–h) were well tolerated, delivering site selective products with moderate to good yields (Table 5).
Late stage functionalization of complex moleculesa.

|
Reactions were run on a 0.2 mmol scale; isolated yield.
The broad functional group tolerance and two-step one-pot method for site selective aromatic C–H borylation encouraged us to test this mild strategy for late stage functionalization of complex molecules, such as natural products and active pharmaceutical ingredients (APIs). Boronates derived from methyl dehydroabietate (14), amino acid tyrosine (15), and a variety of APIs (16–18) containing polar groups, sterically hindered substituents and polycyclic frameworks were still readily produced under the optimized conditions, highlighting the utility of this methodology in the late-stage functionalization of biologically relevant compounds.
To obtain insights into the reaction mechanism, an N,N-diboronate complex24 (dimer A) was prepared through reductive coupling of isoquinoline and B2pin2 (Scheme 2a). Under photoirradiation, no reaction of 4-bromoanisole and dimer A was detected without addition of NEt3 (see the ESI†). In contrast, the potential excited donor–acceptor complex was able to slowly reduce 4-bromoanisole into anisole in the presence of NEt3; when additional B2pin2 (2.0 eq.) was applied as the trapping reagent without loading of NEt3, no dehalogenation product except boronic ester product was detected; furthermore, the reduction of 4-bromoanisole took place more efficiently in the presence of NEt3 and B2pin2, delivering the corresponding boronic ester as the major product (Scheme 2b). These observations indicated that the reduction ability of the coordination complex of acceptor dimer A and donor NEt3 has indeed been enhanced after photoexcitation. To obtain a clearer picture of the complex of acceptor dimer A and donor NEt3, density functional theory (DFT) calculations were carried out. First, the computational UV-Vis spectrum was employed to compare with the experimental UV-Vis spectrum (Scheme 2c). Without NEt3, there exist two smooth peaks in the computational UV-Vis spectrum of dimer A and the peak at 227 nm is higher than that at 301 nm, which is in agreement with the experiments (Scheme 2c(I) and d(III)). When one NEt3 molecule binds to dimer Ain silico, the curve between the two peaks becomes sharp, which is also consistent with the experimental UV-Vis spectrum (Scheme 2c(II) and d(IV)). However, when two NEt3 bind to dimer A, the computational UV-Vis spectrum reveals that the peak around 220 nm becomes lower than the peak around 300 nm, which is quite different from experiments (Scheme 2d(V)). Therefore, the computational results suggest that only one NEt3 binds to dimer A under the optimized reaction conditions. In addition, the binding energy between dimer A and one NEt3 is 11.6 kcal mol−1 by calculation. Considering that the ratio of dimer A (20 mol% isoquinoline → 10 mol% dimer A) to NEt3 (5.0 eq.) is 1 : 50 under the optimized reaction conditions, dimer A and complex B can equilibrate (Scheme 2d). After B is determined to be the complex combining acceptor dimer A and donor NEt3, time dependent TD-DFT calculations were conducted for the excited-state of B. The excited-state reduction potential of B* is −3.12 V, indicating that B* is a highly active reductant and can facilitate SET with aryl halides to produce aryl radical intermediates.
Scheme 2. Mechanistic and computational experiments: (a) preparation of dimer A; (b) mechanistic experiments using dimer A (0.15 mmol)/4-bromoanisole (0.2 mmol)/NEt3 (1.0 mmol) with or without B2pin2 under 2 × 390 nm LEDs (40 W) irradiation for 12 h; (c) experimental UV-Vis spectra (I: dimer A/II: dimer A + NEt3); (d) computational UV-Vis spectra (III: dimer A; IV: dimer A + NEt3; V: dimer A + 2NEt3); (e) binding between dimer A and one NEt3 and then photoexcitation of D–A complex B by 390 nm light.
With this mechanistic insight, a plausible mechanism for the conversion of aryl halides to their corresponding boronic esters is proposed in Scheme 3. Reductive coupling of isoquinoline and B2pin2 generates dimer A, which can coordinate with NEt3in situ to afford intermediate B. Donor–acceptor complex B is then excited by 390 nm light, populating a highly reducing excited state B*, which can undergo a single electron transfer process with an electronically matched aryl halide to generate a radical cation species C, aryl radical D, and regenerate isoquinoline/B2pin2. Radical cation C could be transformed into enamine E followed by hydrogen atom transfer and deprotonation processes. Finally, radical D is trapped by B2pin2 immediately, yielding the desired boronic ester.
Scheme 3. The plausible mechanism.
Conclusions
In summary, a facile transition-metal-free conversion of aryl halides to the corresponding boronic esters through a radical process has been developed. A series of aryl boronic esters were synthesized with good functional group tolerance in moderate to excellent yields under mild conditions. Mechanistic experiments and computational investigations indicate the formation of an excited donor–acceptor complex, serving as the super single electron reductant. Furthermore, the key role of the donor–acceptor complex formed in situ was confirmed by control experiments and DFT calculations. The protocol's functional group tolerance and two-step one-pot method for site selective aromatic C–H bond borylation enabled the functionalization of a variety of biologically relevant compounds, representing a new application of aryl radical merged with photochemistry.
Data availability
Data for all compounds in this manuscript are available in the ESI,† which includes experimental details, characterisation data, computational data and copies of 1H, 13C, 19F, 11B NMR spectra.
Author contributions
M.-H. L. performed the experiments with contributions from H.-S. B and Q.-N. L., S.-Q. L. and Y.-H. D. performed DFT calculations. T.-Y. S. and L.-F. W. supervised the project. All the authors analyzed the data, discussed results and contributed to the manuscript.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
We gratefully acknowledge the support of this research by the National Natural Science Foundation of China (No. 21933004), the Key-Area Research and Development Program of Guangdong Province (No. 2020B010188001), the Guangdong Basic and Applied Basic Research Foundation (No. 2022A1515011994), the Technology & Innovation Commission of Shenzhen Municipality (No. 75110-42100033), the Fundamental Research Funds for the Central Universities (No. 75110-30610023) and the Sun Yat-sen University Startup Fund (No. 75110-1841230 & 75110-18841290). The calculations were carried out at the SZBL supercomputing center.
Electronic supplementary information (ESI) available. CCDC 2144922. For ESI and crystallographic data in CIF or other electronic format see https://doi.org/10.1039/d2sc00552b
Notes and references
- (a) )Hall D. G., Boronic Acids: Preparation and Applications in Organic Synthesis Medicine and Materials, Wiley-VCH, Weinheim, Germany, 2nd edn, 2011 [Google Scholar]; (b) Cuenca A. B. Shishido R. Ito H. Fernández E. Chem. Soc. Rev. 2017;46:415–430. doi: 10.1039/C6CS00692B. [DOI] [PubMed] [Google Scholar]; (c) Tian Y.-M. Guo X.-N. Braunschweig H. Radius U. Marder T. B. Chem. Rev. 2021;121:3561–3597. doi: 10.1021/acs.chemrev.0c01236. [DOI] [PubMed] [Google Scholar]
- (a) Friese F. W. Studer A. Chem. Sci. 2019;10:8503–8518. doi: 10.1039/C9SC03765A. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Qiu Z.-H. Li C.-J. Chem. Rev. 2020;120:10454–10515. doi: 10.1021/acs.chemrev.0c00088. [DOI] [PubMed] [Google Scholar]; (c) Bose S. K. Mao L.-J. Kuehn L. Radius U. Nekvinda J. Santos W. L. Westcott S. A. Steel P. G. Marder T. B. Chem. Rev. 2021;121:13238–13341. doi: 10.1021/acs.chemrev.1c00255. [DOI] [PubMed] [Google Scholar]; (d) Larin E. M. Loup J. Polishchuk I. Ross R. J. Whyte A. Lautens M. Chem. Sci. 2020;11:5716–5723. doi: 10.1039/D0SC02421J. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Lim T. Ryoo J. Y. Han M. S. J. Org. Chem. 2020;85:10966–10972. doi: 10.1021/acs.joc.0c01065. [DOI] [PubMed] [Google Scholar]; (f) Gao G.-L. Yan J.-X. Yang K. Chen F.-E. Song Q.-L. Green Chem. 2017;19:3997–4001. doi: 10.1039/C7GC01161J. [DOI] [Google Scholar]; (g) Pu X.-H. Hu J.-F. Zhao Y. Shi Z.-Z. ACS Catal. 2016;6:6692–6698. doi: 10.1021/acscatal.6b01956. [DOI] [Google Scholar]; (h) Verma A. Tomar K. Bharadwaj P. K. Inorg. Chem. 2019;58:1003–1006. doi: 10.1021/acs.inorgchem.8b03015. [DOI] [PubMed] [Google Scholar]
- (a) Letsinger R. L. Skoog I. H. J. Org. Chem. 1953;18:895–897. doi: 10.1021/jo50013a019. [DOI] [Google Scholar]; (b) Légaré M. Rang M. Bélanger-Chabot G. Schweizer J. I. Krummenacher I. Bertermann R. Arrowsmith M. Holthausen M. C. Braunschweig H. Science. 2019;363:1329–1332. doi: 10.1126/science.aav9593. [DOI] [PubMed] [Google Scholar]; (c) Knöller J. A. Meng G.-Y. Wang X. Hall D. Pershin A. Beljonne D. Olivier Y. Laschat S. Zysman-Colman E. Wang S. Angew. Chem., Int. Ed. 2020;59:3156–3160. doi: 10.1002/anie.201912340. [DOI] [PubMed] [Google Scholar]; (d) Yang D.-T. Zheng J. Peng J.-B. Wang X. Wang S. J. Org. Chem. 2021;86:829–836. doi: 10.1021/acs.joc.0c02379. [DOI] [PubMed] [Google Scholar]
- For reviews, see: ; (a) Wang D. Astruc D. Chem. Soc. Rev. 2017;46:816–854. doi: 10.1039/C6CS00629A. [DOI] [PubMed] [Google Scholar]; (b) Song F. Gou T. Wang B.-Q. Shi Z.-J. Chem. Soc. Rev. 2018;47:7078–7115. doi: 10.1039/C8CS00253C. [DOI] [PubMed] [Google Scholar]; (c) Quan Y.-J. Xie Z. W. Chem. Soc. Rev. 2019;48:3660–3673. doi: 10.1039/C9CS00169G. [DOI] [PubMed] [Google Scholar]; (d) Huang H.-M. Bellotti P. Glorius F. Chem. Soc. Rev. 2020;49:6186–6197. doi: 10.1039/D0CS00262C. [DOI] [PubMed] [Google Scholar]; (e) Lu H. Yu T.-Y. Xu P.-F. Wei H. Chem. Rev. 2021;121:365–411. doi: 10.1021/acs.chemrev.0c00153. [DOI] [PubMed] [Google Scholar]; (f) Kim U. B. Jung D. J. Jeon H. J. Rathwell K. Lee S. Chem. Rev. 2020;120:13382–13433. doi: 10.1021/acs.chemrev.0c00245. [DOI] [PubMed] [Google Scholar]; (g) Trouvé J. Gramage-Doria R. Chem. Soc. Rev. 2021;50:3565–3584. doi: 10.1039/D0CS01339K. [DOI] [PubMed] [Google Scholar]; (h) Chan A. Y. Perry I. B. Bissonnette N. B. Buksh B. F. Edwards G. A. Frye L. I. Garry O. L. Lavagnino M. N. Li B.-X. Liang Y. Mao E. Millet A. Oakley J. V. Reed N. L. Sakai H. A. Seath C. P. MacMillan D. W. C. Chem. Rev. 2022;122:1485–1542. doi: 10.1021/acs.chemrev.1c00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For reviews, see: ; (a) Romero N. A. Nicewicz D. A. Chem. Rev. 2016;116:10075–10166. doi: 10.1021/acs.chemrev.6b00057. [DOI] [PubMed] [Google Scholar]; (b) Wang C.-S. Dixneuf P. H. Soulé J.-F. Chem. Rev. 2018;118:7532–7585. doi: 10.1021/acs.chemrev.8b00077. [DOI] [PubMed] [Google Scholar]; (c) Troian-Gautier L. Turlington M. D. Wehlin S. A. M. Maurer A. B. Brady M. D. Swords W. B. Meyer G. J. Chem. Rev. 2019;119:4628–4683. doi: 10.1021/acs.chemrev.8b00732. [DOI] [PubMed] [Google Scholar]; (d) Huang H.-M. Bellotti P. Glorius F. Chem. Soc. Rev. 2020;49:6186–6197. doi: 10.1039/D0CS00262C. [DOI] [PubMed] [Google Scholar]; (e) Yu X.-Y. Chen J.-R. Xiao W.-J. Chem. Rev. 2021;121:506–561. doi: 10.1021/acs.chemrev.0c00030. [DOI] [PubMed] [Google Scholar]; (f) Reed N. L. Yoon T. P. Chem. Soc. Rev. 2021;50:2954–2967. doi: 10.1039/D0CS00797H. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Bell J. D. Murphy J. A. Chem. Soc. Rev. 2021;50:9540–9685. doi: 10.1039/D1CS00311A. [DOI] [PubMed] [Google Scholar]; (h) Galliher M. S. Roldan B. J. Stephenson C. R. J. Chem. Soc. Rev. 2021;50:10044–10057. doi: 10.1039/D1CS00411E. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Lu F.-D. Chen J. Jiang X. Chen J.-R. Lu L.-Q. Xiao W.-J. Chem. Soc. Rev. 2021;50:12808–12827. doi: 10.1039/D1CS00210D. [DOI] [PubMed] [Google Scholar]; (j) Bryden M. A. Zysman-Colman E. Chem. Soc. Rev. 2021;50:7587–7680. doi: 10.1039/D1CS00198A. [DOI] [PubMed] [Google Scholar]; (k) Holmberg-Douglas N. Nicewicz D. A. Chem. Rev. 2022;122:1925–2016. doi: 10.1021/acs.chemrev.1c00311. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Pitre S. P. Overman L. E. Chem. Rev. 2022;122:1717–1751. doi: 10.1021/acs.chemrev.1c00247. [DOI] [PubMed] [Google Scholar]; (m) Kwon K. Simons R. T. Nandakumar M. Roizen J. L. Chem. Rev. 2022;122:2353–2428. doi: 10.1021/acs.chemrev.1c00444. [DOI] [PMC free article] [PubMed] [Google Scholar]; (n) Chang L. An Q. Duan L. Feng K. Zuo Z. Chem. Rev. 2022;122:2429–2486. doi: 10.1021/acs.chemrev.1c00256. [DOI] [PubMed] [Google Scholar]
- Jin S. Dang H. T. Haug G. C. He R. Nguyen V. D. Nguyen V. T. Arman H. D. Schanze K. S. Larionov O. V. J. Am. Chem. Soc. 2020;142:1603–1613. doi: 10.1021/jacs.9b12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble A. Mega R. S. Pflästerer D. Myers E. L. Aggarwal V. K. Angew. Chem., Int. Ed. 2018;57:2155–2159. doi: 10.1002/anie.201712186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J.-H. Cao J.-L. Wu X.-Y. Wang H. Yang X.-N. Tang X.-X. Toh R.-W. Zhou R. Yeow E. K. L. Wu J. J. Am. Chem. Soc. 2021;143:13266–13273. doi: 10.1021/jacs.1c05994. [DOI] [PubMed] [Google Scholar]
- Qiao Y.-S. Yang Q.-M. Schelter E. J. Angew. Chem., Int. Ed. 2018;57:10999–11003. doi: 10.1002/anie.201804022. [DOI] [PubMed] [Google Scholar]
- (a) Tian Y.-M. Guo X.-N. Krummenacher I. Wu Z. Nitsch J. Braunschweig H. Radius U. Marder T. B. J. Am. Chem. Soc. 2020;142:18231–18242. doi: 10.1021/jacs.0c08834. [DOI] [PubMed] [Google Scholar]; (b) Docherty J. H. Nicholson K. Dominey A. P. Thomas S. P. A. ACS Catal. 2020;10:4686–4691. doi: 10.1021/acscatal.0c00869. [DOI] [Google Scholar]; (c) Kuang Z.-J. Chen H.-H. Qiu J. Ou Z.-L. Lan Y. Song Q.-L. Chem. 2020;6:2347–2363. doi: 10.1016/j.chempr.2020.06.034. [DOI] [Google Scholar]; (d) Zhang G. Li M.-Y. Ye W.-B. He Z.-T. Feng C.-G. Lin G.-Q. Org. Lett. 2021;23:2948–2953. doi: 10.1021/acs.orglett.1c00617. [DOI] [PubMed] [Google Scholar]
- Cheng Y. Mück-Lichtenfeld C. Studer A. Angew. Chem., Int. Ed. 2018;57:16832–16836. doi: 10.1002/anie.201810782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Zhang L. Jiao L. J. Am. Chem. Soc. 2017;139:607–610. doi: 10.1021/jacs.6b11813. [DOI] [PubMed] [Google Scholar]; (b) Zhang L. Jiao L. J. Am. Chem. Soc. 2019;141:9124–9128. doi: 10.1021/jacs.9b00917. [DOI] [PubMed] [Google Scholar]; (c) Yang H. Zhang L. Zhou F.-Y. Jiao L. Chem. Sci. 2020;11:742–747. doi: 10.1039/C9SC05627K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Liu W.-B. Yang X.-B. Gao Y. Li C.-J. J. Am. Chem. Soc. 2017;139:8621–8627. doi: 10.1021/jacs.7b03538. [DOI] [PubMed] [Google Scholar]; (b) Liu W. Li J. Huang C.-Y. Li C.-J. Angew. Chem., Int. Ed. 2020;59:1786–1796. doi: 10.1002/anie.201909138. [DOI] [PubMed] [Google Scholar]
- (a) Mfuh A. M. Doyle J. D. Chhetri B. Arman H. D. Larionov O. V. J. Am. Chem. Soc. 2016;139:2985–2988. doi: 10.1021/jacs.6b01376. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Jin S.-F. Dang H. T. Haug G. C. He R. Nguyen V. D. Nguyen V. T. Arman H. D. Schanze K. S. Larionov O. V. J. Am. Chem. Soc. 2020;142:1603–1613. doi: 10.1021/jacs.9b12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. Kim H. Lambert T. H. Lin S. J. Am. Chem. Soc. 2020;142:2087–2092. doi: 10.1021/jacs.9b10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Takeuchi S. Nakagawa T. Yokoyama Y. Chem. Commun. 2020;56:6492–6494. doi: 10.1039/D0CC02411B. [DOI] [PubMed] [Google Scholar]; (b) Madhu M. Ramakrishnan R. Vijay V. Hariharan M. Chem. Rev. 2021;121:8234–8284. doi: 10.1021/acs.chemrev.1c00078. [DOI] [PubMed] [Google Scholar]; (c) Kohara K. Trowbridge A. Smith M. A. Gaunt M. J. J. Am. Chem. Soc. 2021;143:19268–19274. doi: 10.1021/jacs.1c09445. [DOI] [PubMed] [Google Scholar]
- (a) Wang Y. Zhu W. Du W. Liu X. Zhang X. Dong H. Hu W. Angew. Chem., Int. Ed. 2018;57:3963–3967. doi: 10.1002/anie.201712949. [DOI] [PubMed] [Google Scholar]; (b) Whittemore T. J. Millet A. Sayre H. J. Xue C. Dolinar B. S. White E. G. Dunbar K. R. Turro C. J. Am. Chem. Soc. 2018;140:5161–5170. doi: 10.1021/jacs.8b00599. [DOI] [PubMed] [Google Scholar]
- MacKenzie I. A. Wang L.-F. Onuska N. P. R. Williams O. F. Begam K. Moran A. M. Dunietz B. D. Nicewicz D. A. Nature. 2020;580:76–80. doi: 10.1038/s41586-020-2131-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. Wang H. König B. Chem. 2021;7:1653–1665. [Google Scholar]
- (a) Grommet A. B. Lee L. M. Klajn R. Acc. Chem. Res. 2020;53:2600–2610. doi: 10.1021/acs.accounts.0c00434. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Baroncini M. Silvi S. Credi A. Chem. Rev. 2020;120:200–268. doi: 10.1021/acs.chemrev.9b00291. [DOI] [PubMed] [Google Scholar]; (c) Witzel Si. Hashmi A. S. K. Xie J. Chem. Rev. 2021;121:8868–8925. doi: 10.1021/acs.chemrev.0c00841. [DOI] [PubMed] [Google Scholar]; (d) Guo W.-S. Wang Q. Zhu J.-P. Chem. Soc. Rev. 2021;50:7359–7377. doi: 10.1039/D0CS00774A. [DOI] [PubMed] [Google Scholar]; (e) Barham J. P. König B. Angew. Chem., Int. Ed. 2020;59:11732–11747. doi: 10.1002/anie.201913767. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Moreau L. M. Lapsheva E. Amaro-Estrada J. I. Gau M. R. Carroll P. J. Manor B. C. Qiao Y. Yang Q. Lukens W. W. Sokaras D. Schelter E. J. Maron L. Booth C. H. Chem. Sci. 2022;13:1759. doi: 10.1039/D1SC06623D. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Li H.-Y. Tang X.-X. Pang J.-H. Wu X.-Y. Yeow E. K. L. Wu J. Chiba S. J. Am. Chem. Soc. 2021;143:481–487. doi: 10.1021/jacs.0c11968. [DOI] [PubMed] [Google Scholar]; (h) Novaes L. F. T. Liu J.-J. Shen Y.-F. Lu L. X. Meinhardt J. M. Lin S. Chem. Soc. Rev. 2021;50:7941–8002. doi: 10.1039/D1CS00223F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pause L. Robert M. Savéant J.-M. J. Am. Chem. Soc. 1999;121:7158–7159. doi: 10.1021/ja991365q. [DOI] [Google Scholar]
- (a) Kelly C. B. Padilla-Salinas R. Chem. Sci. 2020;11:10047–10060. doi: 10.1039/D0SC03833D. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhang L. Ritter T. J. Am. Chem. Soc. 2022;144:2399–2414. doi: 10.1021/jacs.1c10783. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Berger F. Ritter T. Synlett. 2022;33:339–345. doi: 10.1055/s-0040-1706034. [DOI] [Google Scholar]; (d) Cannalire R. Pelliccia S. Sancineto L. Novellino E. Tron G. C. Giustiniano M. Chem. Soc. Rev. 2021;50:766–897. doi: 10.1039/D0CS00493F. [DOI] [PubMed] [Google Scholar]; (e) Prabagar B. Yang Y.-Q. Shi Z.-Z. Chem. Soc. Rev. 2021;50:11249–11269. doi: 10.1039/D0CS00334D. [DOI] [PubMed] [Google Scholar]
- (a) Xiong X. Tan F. Yeung Y.-Y. Org. Lett. 2017;19:4243–4246. doi: 10.1021/acs.orglett.7b01899. [DOI] [PubMed] [Google Scholar]; (b) Motati D. R. Uredi D. Watkins E. B. Chem. Sci. 2018;9:1782–1788. doi: 10.1039/C7SC04107A. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Tang R.-J. Milcent T. Crousse B. J. Org. Chem. 2018;83:930–938. doi: 10.1021/acs.joc.7b02920. [DOI] [PubMed] [Google Scholar]; (d) Wang W.-J. Li X.-Y. Yang X.-X. Ai L.-S. Gong Z.-W. Jiao N. Song S. Nat. Commun. 2021;12:3873–3882. doi: 10.1038/s41467-021-24174-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D.-P. Xu G.-Q. Zhou Q.-H. Chung L. W. Tang W.-J. J. Am. Chem. Soc. 2017;139:9767–9770. doi: 10.1021/jacs.7b04256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data for all compounds in this manuscript are available in the ESI,† which includes experimental details, characterisation data, computational data and copies of 1H, 13C, 19F, 11B NMR spectra.





