Abstract
Rheumatic heart disease (RHD) is a chronic valvular disease resulting after severe or repetitive episodes of acute rheumatic fever (ARF), an autoimmune response to group A Streptococcus infection. RHD has been almost eliminated with improved social and health infrastructure in affluent countries while it remains a neglected disease with major cause of morbidity and mortality in many low- and middle-income countries, and resource-limited regions of high-income countries. Despite our evolving understanding of the pathogenesis of RHD, there have not been any significant advances to prevent or halt progression of disease in recent history. Long-term penicillin-based treatment and surgery remain the backbone of a RHD control program in the absence of an effective vaccine. The advent of echocardiographic screening algorithms has improved the accuracy of diagnosing RHD and has shed light on the enormous burden of disease. Encouragingly, this has led to a rekindled commitment from researchers in the most affected countries to advocate and take bold actions to end this disease of social inequality.
Keywords: Rheumatic heart disease, Acute rheumatic fever
Introduction
Rheumatic heart disease (RHD) is an autoimmune phenomenon resulting in valvular damage secondary to a non-suppurative complication of Streptococcus pyogenes or group A streptococcal (GAS) infection. RHD, a disease of poverty, is one of the leading causes of morbidity and mortality from acquired cardiac diseases and disproportionately affects people in low- and middle-income countries (LMIC) and vulnerable populations in higher income countries (HIC) [1, 2, 3••].
RHD accounts for 1.4 million premature deaths annually [4, 5]. It has the highest cardiovascular disease-related loss of disability-adjusted-life-years (DALY) in children among 10–14 years worldwide [2, 6]. In a multicenter study of acute heart failure in sub-Saharan Africa, it was the second most common cause of hospitalization in patients with heart failure [7]. The paucity of high quality epidemiological studies prevents a precise assessment of the burden of the disease. Conservatively extrapolated data estimates 15.6 million people affected by RHD worldwide [8]. More recent data, based on echocardiographic screening, suggests that these figures may be 4- to 5-fold higher [9, 10].
There is a wide variation in the clinical manifestation of GAS infection. Pharyngitis (strep throat), mainly affecting children between the ages of 5 and 15 [1], is thought to be the most common clinical presentation linked to acute rheumatic fever (ARF). ARF is an autoimmune inflammatory process in which antibodies formed to target antigenic bacterial epitopes cross-react with proteins found in synovial, cardiac, and neuronal tissues. ARF can manifest with arthritis, carditis, erythema marginatum, and Sydenham chorea [11]. Most of ARF manifestations are self-limited; however, valvular disease resulting from repeated or severe episodes of rheumatic fever leads to irreversible valvular thickening and fibrosis. The long-term sequelae of valvular regurgitation and eventually valvular stenosis are the hallmark of RHD. Subsequently, RHD predisposes to high risk of morbidity and mortality from stroke, infective endocarditis, heart failure, and arrhythmias.
Epidemiology
The decline of ARF and RHD in HIC occurred prior to widespread use of penicillin for treatment of strep pharyngitis. Although the direct mechanisms remain poorly defined, better living conditions, nutrition, and access to care may have been fundamental to the significant disease reduction in industrialized countries [12]. The high incidence and prevalence of the disease in indigenous communities within affluent countries, such as Australia, New Zealand, Canada, and American Samoa, are examples of how individual or community socioeconomic status is a major determinant in the incidence of the disease [13, 14].
RHD is exceedingly rare in the USA (0.04 cases per 1000 children) [14]. In comparison, studies from LMIC have recorded prevalence of RHD ranging from 30.4 cases per 1000 children in Mozambique [15] to 51.0 per 1000 cases in India [16]. The worst affected regions are sub-Saharan Africa, indigenous populations in Australia and New Zealand, southcentral Asia, and the Pacific [17]. A recent meta-analysis of echocardiogram-based studies in RHD endemic countries estimated a combined prevalence of RHD of 12.9 per 1000 people [3••] and higher rates (21.1 per 1000 people) when clinically undetectable valvular disease visualized only on echocardiogram was incorporated into the definition of RHD. However, because rural areas are more likely to be severely affected by the disease than urban areas [9], reported estimates extrapolated mainly from studies of school children in urban communities are likely to be an underestimate. There is also great variation between and within countries limiting generalizability. The prevalence of RHD also increases with age [15, 18] and studies limited only to school children may merely reflect a small segment of a much more prevalent problem.
Pathophysiology
The current model of ARF pathogenesis is based on a complex interaction between rheumatogenic GAS strains, aberrant immune system, and host susceptibility.
ARF and Rheumatogenic Strains
Outbreaks of GAS pharyngitis have been associated with increased incidence of ARF, tying specific GAS strains (rheumatogenic strains) to the onset of the disease [1, 19, 20]. From a molecular basis, the surface M protein on GAS confers the bacteria its ability to attach to pharyngeal epithelial cells. Specific moieties of M protein in turn cause immune activation that target the host tissue. Molecular mimicry in which antibodies directed at bacterial antigens cross-react to similarly composed proteins in valvular tissue has been traditionally the accepted basis of valvular damage and RHD [21]. Antigenic α-helical moieties on the M protein are structurally homologous to cardiac helical proteins such as myosin, tropomyosin, actin, and laminin [22]. Recurrent GAS infection over time leads to reactivation of the primed immune system and a repetitive inflammatory process in which valvular damage ensues.
This widely accepted model of specific GAS rheumatogenic strains causing ARF is an active area of debate. Studies from ARF endemic areas in Australia revealed that typing of GAS did not match the classically recognized rheumatogenic strains of M serotypes, and group C and G Streptococcus were more common than group A Streptococcus [23, 24]. In addition, pharyngitis may be a rarer presentation of GAS infection with skin infection being the most common clinical manifestation [23].
ARF and the Immune System
There is an interval of approximately 2 to 5 weeks from the onset of GAS pharyngitis to the development of ARF. This latency is thought to be related to the period needed to mount immunity which correlates with the rise of antibodies to streptococcal enzymes such as antistreptolysin O (ASO) titers and anti-DNAse B. Eighty percent of patients with ARF have ASO titers elevated, and when additional GAS-specific antibodies, such as anti-DNAse B or anti-group A carbohydrate, are included, 95 % of ARF patients have elevated titers [22].
Current investigations suggest that antibodies produced as a result of GAS infection may not necessarily target the structural M protein but instead a group A carbohydrate, a component of GAS cell wall structure [25, 26]. Historically, levels of anti-group A carbohydrate antibodies have correlated with severity of valvular disease whereby surgical removal of affected valves results in decline of anti-group A carbohydrate antibodies [27]. Anti-group A carbohydrate antibodies also have high affinity for cardiac proteins such as myosin. However, valves are void of myosin, and myocardial damage, as detected by troponin elevation and myocardial dysfunction based on echocardiography finding, is absent during ARF [28]. Current studies hypothesize antibodies (which cross-react with myosin) bind to laminin and other basement membrane proteins found on endothelial surfaces of valves with similar helical structures to myosin [29]. The initial antibody mediated injury subsequently targets valvular endothelium leading to upregulation of in the inflammatory marker vascular cell adhesion protein 1 (VCAM-1) [30]. VCAM-1 causes the valvular endothelial surface to become porous, allowing T cells to infiltrate into the normally immune-privileged valvular tissue. These specific Tcells in turn recognize M protein and myosin as antigens and continue to proliferate leading to a cascade of inflammation and cytokine production causing valvular damage and scarring in RHD [31]. Although humoral response (mainly antibody mediated) is thought to be the inciting factor for endothelial dysfunction, newer studies point at specific CD4+ T cells [32] targeting connective tissue proteins [33]. These proteins, normally hidden from the immune system, become exposed following the endothelial damage that ensues from the initial inflammatory process. Despite decades of investigation, there is no unifying immunological pathway to explain the nuances of the disease.
ARF and Host Susceptibility
An important link between GAS infection and ARF is the regulating genetic factors determining the immune response to the infection. Only 3–6% [34, 35] of those who are infected with GAS develop ARF, implicating host susceptibility in the pathogenesis of ARF. This rate is also similar in communities where ARF is endemic, suggesting that genetic predisposition to ARF progression following a GAS infection is overall uniform in different populations [35].
Familial clustering and twin studies—showing increased concordance among monozygotic twins compared to dizygotic twins—support the role of genetic susceptibility in ARF [34, 36]. The major histocompatibility human leukocyte antigen (HLA) class II molecules, involved in antigen presentation to T cell receptors, have been associated with increased susceptibility to ARF [20]. Further studies are needed to identify the role of specific HLA haplotypes and their correlation to increased immunogenic predisposition to ARF and RHD in different ethnicities. On the whole, the evidence so far points to a complex interaction between humoral and cellular immune response triggered by GAS infection that is intricately connected to the host’s genetic makeup.
Clinical Manifestation of ARF
The peak incidence of ARF follows a similar pattern as GAS pharyngitis and occurs between the ages of 5–14 [13]. There is a female predominance which remains poorly understood. Increased exposure to GAS infection associated with child rearing and/or an underlying immunologic response could potentially be contributing factors [37].
The most common clinical features associated with ARF are arthritis, which occurs in 35–77 % of cases [13, 38], and carditis in 30–70 % [19, 39]. Other clinical manifestations with lower frequency include Syndeham chorea in 10–30 % (with female predominance) [13, 40], erythema nodosum in <6 %, and subcutaneous nodules in 0–10 % [41••]. All the above ARF manifestations compose the major Jones criteria, a guide established in 1944 [42] to aid in the diagnosis of ARF. In addition to these major criteria, other clinical manifestations such as fever, elevated inflammatory markers, and PR interval prolongation on electrocardiogram compose the minor criteria. The Jones criteria were most recently revised by the American Heart Association (AHA) in 2015 [41••]. The current AHA modified Jones criteria, in line with other international guidelines [43], recognize the variation in the clinical manifestation of the disease in endemic areas and takes into account newer studies that include Doppler echocardiography in the assessment of carditis in ARF [41••]. The updated guideline also differentiates ARF clinical manifestation between moderate/high risk population and low risk population. Furthermore, for all patients with evidence of preceding GAS infection, these criteria can be used to diagnose an initial or a recurrent episode of ARF (Table 1). Studies in endemic areas [44, 45] drew attention to the low sensitivity of Jones criteria in which under-diagnosis of ARF would have significant detrimental implications in RHD prevention. In contrast, the AHA guideline favors low sensitivity and high specificity in lower risk populations to minimize over-diagnosis of the disease.
Table 1.
Revised Jones criteria for diagnosis of ARF
| For all patients with evidence of preceding GAS infection | |
| 1. Diagnosis of initial ARF | |
| 2 Major manifestations or 1 major plus 2 minor manifestations | |
| 2. Diagnosis of recurrent ARF | |
| 2 Major or 1 major and 2 minor or 3 minor criteria | |
| Low risk population: ARF incidenceof ≤2 per 100,000 school-aged children or all-age RHD prevalence of ≤1 per 1000 population per year | |
| Major criteria | Minor criteria |
| □ Carditis clinical or subclinical (i.e., echocardiographic) □ Polyarthritis □ Chorea □ Erythema marginatum □ Subcutaneous nodules |
□ Polyarthralgia □ Fever >38.5 °C □ ESR ≥60 mm/h in the first hour and/or CRP ≥3.0 mg/dl □ Prolonged PR interval, after accounting for age variability (unless carditis is a major criterion) |
| Moderate/high risk population: | |
| Major criteria | Minor criteria |
| □ Carditis clinical or subclinical (i.e., echocardiographic) □ Polyarthritis or monoarthritis or polyarthralgia □ Chorea □ Erythema marginatum □ Subcutaneous nodules |
□ Monoarthralgia □ Fever >38 °C □ ESR ≥30 mm/h and or CRP ≥3.0 mg/dl □ Prolonged PR interval, after accounting for age variability (unless carditis is a major criterion) |
Adapted with permission from Gewitz et al. [41••]
Most ARF manifestations resolve without any further sequelae. Rheumatic valvular disease resulting from severe or repetitive episodes of ARF is the most significant complication. ARF tends to recur the most in the first 4 years after the initial attack [13]. Subsequently, approximately 60 % of ARF cases progress to RHD within 10 years with the incidence of RHD peaking between the age of 25 and 44 [13, 46, 47]. For the rest of this review, we will focus on the cardiac manifestations of ARF and RHD.
Cardiac Manifestation in ARF
In the acute phase of ARF, the inflammatory process typically involves the valves, pericardium, and the myocardium [1, 48]. Patients with acute rheumatic carditis can present with chest pain attributable to pericarditis, heart failure symptoms, and sinus tachycardia that tends to persist at night [38].
Valvulitis
The mitral valve is the most common site of rheumatic valvulitis (in >70–94 % of cases) [39, 49] with involvement of aortic valve in 20–25 % of those cases. Isolated aortic valvulitis is seen in less than 5–8 % of cases [50]. Inflammation of the valves and subsequent new mitral or aortic regurgitation murmur is central to the pathology of rheumatic carditis. In the absence of these signs, rheumatic carditis is unlikely [51]. It is exceedingly rare for rheumatic carditis to present as isolated right-sided valvular disease. Involvement of mainly mitral and aortic valves in rheumatic carditis is possibly related to the expression of specific endothelial surface proteins and the exposure to higher pressures than the right side [28].
In the acute setting, regurgitation is secondary to inflamed valves, mitral annular dilatation, and leaflet prolapse with or without chordal elongation [41••, 49]. Cellular infiltrate of the valves, edema, and neovascularization create a fibrinous vegetation on the valves [28]. As opposed to infectious vegetations, fibrinous vegetations do not embolize. Left untreated, mitral regurgitation leads to regurgitation-induced volume overload and left ventricular and atrial enlargement causing further dilation of the annulus, chordal tethering, and worsening mitral regurgitation; hence, the adage “mitral regurgitation begets mitral regurgitation” [52].
Traditionally, carditis was a clinical diagnosis based on pathological mitral regurgitation and/or aortic regurgitation on auscultation. An apical soft, blowing, pansystolic murmur is characteristic of mitral regurgitation and a basal early diastolic murmur for aortic regurgitation. With the advances in cardiac imaging and widespread use of echocardiography, we can now identify subclinical carditis—pathological valvular regurgitation on echocardiogram [41••, 44, 53] in the absence of clinical carditis. Echocardiography detects RHD up to ten times more frequently than clinical auscultation [15, 18, 54], and echocardiographic findings are now included as major Jones criteria [41••, 53]. The World Heart Federation (WHF) outlined the echocardiographic criteria that constitute RHD [41••, 55••] (Table 2).
Table 2.
WHF echocardiographic criteria for RHD in adults aged >20 years
| Definite RHD is met by any of the following criteria (A, B, C, or D) | |
|---|---|
|
| |
| A. Pathological MR and at least two morphological features of RHD of the MV | |
| B. MS mean gradient ≥4 mmHg | |
| C. Pathological AR and at least two morphological features of RHD of the AV, only in individuals aged <35 years | |
| D. Pathological AR and at least two morphological features of RHD of the MV | |
| Doppler findings | |
| Pathological mitral regurgitation (all 4 criteria must be met) | 1. Seen in at least 2 views 2. Jet length ≥2 cm in at least 1 view 3. Peak velocity ≥3 m/s 4. Pansystolic jet in at least 1 envelope |
| Pathological aortic regurgitation (all 4 criteria met) | 1.Seen in at least 2 views 2. Jet length ≥1 cm in at least 1 view 3. Peak velocity ≥3 m/s in early diastole 4. Pan-diastolic jet in at least one envelope |
| Morphological findings | |
| Mitral valve changes | 1. AMVL thickeninga 2. Chordal thickening 3. Restricted leaflet motion 4. Excessive leaflet tip motion during systole (only for individuals aged <35) |
| Aortic valve changes | 1. Irregular or focal leaflet thickening 2. Coaptation defect 3. Restricted leaflet motion 4. Leaflet prolapse |
Reprinted by permission from Macmillan Publishers Ltd, from Remenyi et al. [55••]
MR mitral regurgitation, MV mitral valve, MS mitral stenosis, AR aortic regurgitation, AV aortic valve
AMVL (anterior mitral valve leaflet) thickness is age-dependent: ≥4 mm for ages 21–40 years and ≥5 mm for individuals aged >40 years
Pericarditis
Pericarditis in ARF is unlikely to occur in the absence of valvular regurgitation [28] and does not cause constrictive physiology [56]. Pericardial effusion can be present—although rarely large enough to present with tamponade physiology—and should not be attributed to rheumatic carditis in the absence of valvulitis [1]. Clinically, friction rub and electrocardiogram changes consistent with pericarditis can be observed in the acute presentation. Pericarditis due to ARF resolves spontaneously without any sequelae.
Myocarditis
Myocarditis in the setting of ARF loosely describes “unexplained [congestive heart failure] or cardiomegaly” [1]. Myocardial necrosis is not a feature of ARF. Multiple studies have shown that inflammatory changes do not affect the myocytes per se but the perivascular and interstitial areas in the myocardium [25, 48]. Aschoff nodules—granulomatous bodies containing macrophages and T cells—although not a very sensitive finding, are pathognomonic for ARF carditis. These nodules tend to occur near the valves, below the endothelial layer or in the interstitium of the myocardium [57], and are thought to be associated with recurrent ARF. The lack of myocardial damage has been investigated in multiple studies. Troponin-I and creatinine kinase remain normal or minimally elevated [58] in rheumatic carditis and, even in the setting of overt symptoms of congestive heart failure (CHF), ventricular function is not affected [49].
Rheumatic Heart Disease
Following an episode of ARF, 60% of those affected progress to RHD [13]. Once the acute inflammatory course of ARF has subsided, patients should be evaluated for residual valvular damage. Pathological mitral regurgitation on auscultation and/or echocardiographic findings showing mitral regurgitation with thickened valve leaflets (dog leg or elbow deformity) are characteristic of RHD (Fig. 1 and Supplementary online Videos S1 and S2). Specific criteria (Table 2) have to be met to minimize the risk of over-diagnosis by excluding physiological regurgitant murmurs or non-specific valvular findings on echocardiogram [41••, 55••].
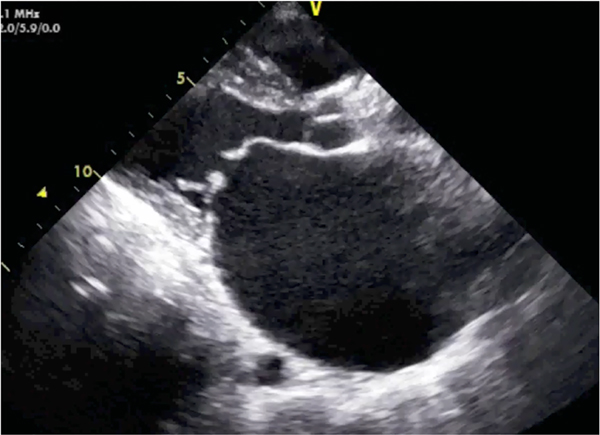
Fig. 1.
Rheumatic mitral valve disease on echocardiogram. Still image from an echocardiogram showing rheumatic mitral valve disease from the parasternal long-axis window in mid-diastole. Valve commissural thickening, dilated left atrium, and characteristic “hockey stick” deformity of the anterior mitral valve leaflet are shown
The rate of progression to stenotic valvular disease varies across geographical areas. In North America, there is a longer latency period of 10 to 40 years after initial manifestation of rheumatic carditis, whereas in developing countries patients often present with advanced disease in their late teens and early 20s [1, 59, 60]. The more rapid progression of the disease is presumably associated with recurrences of ARF, severity of carditis, inadequate prophylaxis with penicillin, and socioeconomic status [61, 62].
With echocardiogram-assisted diagnosis of RHD, the concept of latent RHD has been recognized—echocardiographic changes suggesting RHD in asymptomatic individuals without a known history of ARF. A subset of these individuals have subclinical RHD noted by echocardiographic valvular changes without clinically audible cardiac murmur. The natural progression of latent RHD is currently under investigation. A recent study [63] showed that half of patients with latent RHD reverse to normal in a 5-year follow-up with others either remaining stable or progressing. Valvular changes detected on echocardiogram may therefore be dynamic. More research is needed to determine factors associated with progression to definite RHD and the utility of interventions (such as BPG prophylaxis) in halting or reversing progression of disease.
Prevention and Treatment
In most developing countries that lack adequate healthcare infrastructure and preventative services, RHD patients present at an advanced stage with complications such as CHF, thromboembolic events, or infective endocarditis [62]. Even when RHD patients are suitable candidates for life-saving surgical valvular interventions, the services are often inaccessible. Shortage of skilled-personnel and access to those services are cost-prohibitive for the majority of patients affected by RHD. These patients are often identified in the prime wageearning and childbearing ages with attendant serious medical, social, and economical consequences [13, 17].
Prevention of ARF
RHD is a disease of social inequality. Efforts to eradicate poverty, limit overcrowding and implement environmental hygiene, and provide basic needs such as access to clean water, sanitation, and primary health care, would be the center of prevention. However, these interventions are notably transdisciplinary and complex. It should nevertheless raise the awareness of health sectors and disaster response groups to consider ARF, among other infectious diseases, as a potential outbreak threat, as seen in military and civilian communities in the USA in the 1980s and 1990s [11, 19, 64]. Outbreaks have also been seen in the setting of social unrest, urban slums, immigration, and overcrowding of refugee camps [65]. Public education centered at raising awareness of GAS pharyngitis and its many sequelae should be integrated in the preventative public health efforts in ARF/RHD endemic areas.
Primary Prevention
Primary prevention refers to treatment of GAS pharyngitis to prevent an initial episode of ARF. Diagnosis of GAS infection is based on rapid antigen detection or the gold standard of throat culture. Antibiotic therapy aims at eradicating GAS from the upper respiratory track. With its bactericidal activity, penicillin is the treatment of choice for GAS pharyngitis. A single dose of intramuscular injection of benzathine penicillin G (BPG) is effective in eradicating the infection. Alternatively, a 10-day course of oral penicillin can be used but patient compliance should be weighed. However, in countries that lack basic health infrastructure, implementation of sore-throat screening programs may not be a viable alternative. In addition, more than 60 % of ARF cases occur without a prior recollection of symptomatic GAS pharyngitis [11, 66] and may hinder a program focused on surveillance of disease for eradication of GAS pharyngitis. On the other hand, a cost-effective analyses using data from South Africa showed that treating all children presenting with pharyngitis with intramuscular penciling injections without culturing is the least expensive out of seven approaches considered, and that a strategy using a clinical decision rule (which considers the presence of cervical nodes, rhinitis, and rash without culturing) is the overall preferred strategy [67]. The prevalence of mainly skin infection, as opposed to upper respiratory GAS infection, in endemic regions also raises the question of the efficacy of the traditional primary prevention in many resource-poor settings. In a study in Australia, sore-throat screening and treatment programs have not been shown to be cost-effective [43] despite the demonstrated efficacy of penicillin to prevent ARF following GAS pharyngitis [68].
Vaccines
Vaccines for GAS would theoretically be the most cost-effective intervention in countries where ARF/RHD is endemic [66]. Interest and funding for vaccine development however dwindled with dramatic reduction of ARF and RHD in developed countries. There are currently no licensed vaccines. M-protein antigen-based polyvalent vaccines (mainly targeting serotypes in low-risk countries) and others containing conserved GAS antigens are currently being investigated [66, 69, 70•]. In addition to the dearth of funding, incomplete understanding of the pathogenesis of ARF and the lack of epidemiological data from the majority of ARF endemic countries hinders effective and significant advances in the development of a vaccine [37].
Secondary Prevention
Long-term management of rheumatic carditis should include secondary prophylaxis with penicillin to prevent recurrence of GAS infection and repetitive episodes of ARF—which increase the risk of progression to RHD [71]. As opposed to a one-time intramuscular dose of penicillin, secondary prophylaxis requires BPG every 3 to 4 weeks (Table 3). The duration of therapy is dependent on patient’s age, severity of valvular disease, and last recurrence of ARF [1, 72]. Adherence is problematic because of the burden imposed by the long-term need for prophylaxis in patients who often do not have easy access to health care facilities or ability to afford extended treatment. Nonetheless, secondary prophylaxis with BPG has been shown to be cost-effective and is central to preventing rheumatic fever recurrence and strep throat infections [1, 72, 73].
Table 3.
Antibiotic dose for secondary prophylaxis in RHD
| Antibiotic | Dose |
|---|---|
|
| |
| Benzathine penicillin G | 600,000 units intramuscular (≤27 kg) or 1,200,000 units intramuscular (>27 kg) every 3–4 weeks (every 3 weeks in endemic areas) |
| Penicillin V (phenoxymethyl penicillin) | 250 mg oral twice daily |
| Sulfadiazine | 500 mg oral daily (≤27 kg) |
| (For patients allergic to penicillin) | 1000 mg oral daily (>27 kg) |
| Macrolide | Variable dose |
| (For patients allergic to penicillin and sulfadiazine) | |
Although BPG prophylaxis is often the focus of secondary prevention, it heavily relies on RHD case detection. Active surveillance of RHD is fundamental in reaching individuals that would benefit most from BPG prophylaxis by preventing recurrence of GAS infection and potentially limiting progression to chronic RHD. The availability of handheld and portable echocardiography and the updated Jones criteria allow for active screening and identification of undiagnosed and subclinical carditis in ARF/RHD endemic areas. Most attention has been paid to school- or community-based RHD screening [74, 75••]. Handheld echocardiograms have been shown to be equally effective as standard echocardiograms in the diagnosis of definite RHD with comparable sensitivity and specificity [75••]. More recent studies focused on training non-expert healthcare workers (e.g., nurses, clinical officers) [76, 77] in using handheld echocardiograms for RHD detection show promise with this approach as a viable strategy in the implementation of a screening program in areas where there is a dearth of highly trained echocardiogram technicians or cardiologists.
Surgical Management of RHD
Surgical intervention is often needed in chronic RHD. The options for surgical management include mitral commissurotomy, valve repair, and valve replacement with either a mechanical or bioprosthetic valve. The 2014 AHA and American College of Cardiology (ACC) guidelines outline indications for surgical management in valvular disease including RHD-related valvular disease [78••]. Class 1 indications relevant to mitral and aortic RHD are shown in Table 4. Age, childbearing status, symptoms, ventricular function, and feasibility of repair versus valvular replacement also need to be considered in timing and type of surgical intervention. Resource-poor settings with inadequate access to anticoagulation services pose another consideration in choosing bioprosthetic or mechanical valves in patients with RHD who may need long-term anticoagulation.
Table 4.
Class 1 indications for surgical management of RHD-related valvular disease
| Lesion | Assessment of severity | Clinical status | Other considerations | Class 1 indication |
|---|---|---|---|---|
|
| ||||
| MS | Severe | Symptomatic | Favorable valve morphology | PMBC |
| MVA ≤1.5 cm2 | No left atrium clot | |||
| T½ ≥150 ms | No or mild MR | |||
| Very severe | NYHA Class III-IV symptoms | MVR | ||
| MVA ≤1 cm2 | Low surgical risk | |||
| T½ ≥220 ms | ||||
| MR | Severe | Symptomatic | LVEF >30 % | MVRa |
| Asymptomatic | LVEF 30–60 % or LVESD ≥40 mm |
|||
| AR | Severe | Symptomatic | AVR | |
| Asymptomatic | LVEF <50 % | |||
| Other cardiac surgery | ||||
MS mitral stenosis, MVA mitral valve area, T1/2 pressure half-time, PMBC percutaneous mitral balloon commissurotomy, NYHA New York Heart Association, MVR mitral valve surgery (replacement or repair), MR mitral regurgitation, LVEF left ventricular ejection fraction, LVESD left ventricular end-systolic dimension, AVR aortic valve replacement (valve repair may be appropriate in selected patients)
Mitral valve repair is preferred over mitral valve replacement (MVR) whenever possible
Mitral valve repair has better outcomes than mitral valve replacement in rheumatic mitral regurgitation [79, 80]. Repair is less invasive and avoids the need for long-term anticoagulation, but should be considered in light of the need for early reoperation (10 % need repair within 2 years) [72].
In the case of isolated symptomatic severe mitral stenosis, percutaneous balloon commissurotomy is the procedure of choice [78••, 81]. Younger patients with favorable valve morphology have better outcomes. Valve morphology can be assessed with a mitral valvuloplasty score [82] which considers leaflet mobility, valve thickening, calcification, and subvalvular thickening. However, patients in developing countries often present late with involvement of multiple valves precluding the option of these less invasive procedures. Patients with mitral stenosis are at risk of atrial fibrillation, increasing their risk for thromboembolic disease but also hemodynamic compromise due to abrupt loss of atrial contribution in ventricular filling. Therapy aimed at rate control and anticoagulation is key in mitral stenosis.
Although there have been advances in surgical therapy of valvular disease, it should be noted that the majority of patients living in these poor-resource settings will not be able to access these services. Surgical interventions in these communities are associated with high morbidity [4] and are not by any means a cost-effective solution to the great burden of RHD remaining in LMIC.
Eradication—the Final Frontier in RHD
While there is much work to be done at the bench and at the bedside for patients with and at risk for RHD, the twenty-first century issue with RHD relates to advocacy, awareness, and political will to finally eradicate this disease. The disproportionate burden of RHD in LMIC and impoverished regions of high-income countries is a social injustice. Advances in the social and ecological environments in many nations have virtually eradicated this disease from the practice of physicians in HIC. As the world focuses on sustainable development goals and achieving the World Heart Federation/World Health Organization “25 by 25” goals [83] of reducing premature deaths from cardiovascular diseases by 25 % by 2025, RHD must remain on the agenda.
The last decade has witnessed great attention to RHD in sub-Saharan Africa. There have been four All-Africa Workshops on Rheumatic Fever and RHD since 2005, producing compelling statements such as The Drakensburg Declaration [84] (2005), the Mosi-o-Tunya call to action [85] (2014) and, most recently, the Addis Abbaba communiqué [86] (2016). These workshops were convened with support, in part, by the South African government, the World Heart Federation, and the World Health Organization. Historically, recommended actions to eradicate RHD in Africa have been wide reaching encompassing vaccine development, ending poverty, educating teachers, and a number of other cross-cutting themes.
Although there has been progress on understanding the burden of RHD in at-risk regions of the world, much work remains in terms of establishing national prevention policies and programs. The historical challenges of translating principles and policy into practice notwithstanding, the Addis Ababa communiqué [86] outlines several specific bold recommendations to the African Union and member states. These include the following: (1) establishing prospective RHD registries at sentinel sites; (2) ensuring adequate supply of benzathine penicillin; (3) guaranteeing access to reproductive health services; (4) decentralizing of technical expertise; (5) establishing centers of excellence for cardiac surgery; (6) fostering multi-sectoral national RHD control programs; and (7) cultivating a communication framework and partnership within key stakeholders in the region. National action to combat RHD can be effective as demonstrated by concerted action in other nations [87].
Conclusion
RHD remains a major cause of morbidity and mortality mostly affecting LMIC. Despite advances in understanding the pathology of the disease, efforts at implementation of coordinated national programs to raise awareness, assess disease burden, and offer preventative services remain the major hindrance in eradication of the disease. Advocacy and action plans to address these issues are encouragingly being pursued by advocates from LMIC [86, 88, 89] and roadmaps with the ambitious goal of ending ARF and RHD are underway to being implemented in regions affected the most. For the twenty-first century, the remaining work related to RHD spans many disciplines; however, eradication of this disease remains the penultimate goal.
Supplementary Material
Acknowledgments
Bethel Woldu is supported by the National Institutes of Health (NIH) Research Training Grant R25 TW009337, funded by the Fogarty International Center, the NIH Office of the Director, the National Institute of Mental Health, and the National Heart, Lung, and Blood Institute.
Footnotes
Compliance with Ethical Standards
Conflict of Interest Bethel Woldu and Gerald S. Bloomfield declare that they have no conflict of interest.
Electronic supplementary material The online version of this article (doi:10.1007/s11886-016-0773-2) contains supplementary material, which is available to authorized users.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.WHO. Rheumatic fever and rheumatic heart disease. (2004). [cited 16 Feb 2016] Available from <http://www.who.int/cardiovascular_diseases/resources/en/cvd_trs923.pdf>. [Google Scholar]
- 2.GBD 2013 DALYs, HALE Collaborators, et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet. 2015;386:2145–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rothenbühler M et al. Active surveillance for rheumatic heart disease in endemic regions: a systematic review and meta-analysis of prevalence among children and adolescents. Lancet Glob Health. 2014;2:e717–26. •• A systematic review and meta-analysis examining prevalence of RHD in endemic areas and estimating the prevalence according to either clinical or echocardiogram based diagnosis.
- 4.Zühlke L et al. Characteristics, complications, and gaps in evidence-based interventions in rheumatic heart disease: the Global Rheumatic Heart Disease Registry (the REMEDY study). Eur Heart J. 2015;36:1115–22a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carapetis JR, Zühlke L, Taubert K, Narula J. Continued challenge of rheumatic heart disease: the gap of understanding or the gap of implementation? Glob Heart 2013;8:185–6. [DOI] [PubMed] [Google Scholar]
- 6.Lozano R et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damasceno A et al. The causes, treatment, and outcome of acute heart failure in 1006 Africans from 9 Countries. Arch Intern Med. 2012;172:1386–94. [DOI] [PubMed] [Google Scholar]
- 8.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5:685–94. [DOI] [PubMed] [Google Scholar]
- 9.Paar JA et al. Prevalence of rheumatic heart disease in children and young adults in Nicaragua. Am J Cardiol 2010;105:1809–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinberg J, Beaton A, Aliku T, Lwabi P, Sable C. Prevalence of rheumatic heart disease in African school-aged population: extrapolation from echocardiography screening using the 2012 World Heart Federation Guidelines. Int J Cardiol 2016;202:238–9. [DOI] [PubMed] [Google Scholar]
- 11.Wallace MR, Garst PD, Papadimos TJ, Oldfield EC. The return of acute rheumatic fever in young adults. JAMA. 1989;262:2557–61. [PubMed] [Google Scholar]
- 12.Gordis L The virtual disappearance of rheumatic fever in the United States: lessons in the rise and fall of disease. T. Duckett Jones memorial lecture. Circulation. 1985;72:1155–62. [DOI] [PubMed] [Google Scholar]
- 13.Lawrence JG, Carapetis JR, Griffiths K, Edwards K, Condon JR. Acute rheumatic fever and rheumatic heart disease: incidence and progression in the Northern Territory of Australia, 1997 to 2010. Circulation. 2013;128:492–501. [DOI] [PubMed] [Google Scholar]
- 14.Beaudoin A et al. Acute rheumatic fever and rheumatic heart disease among children—American Samoa, 2011–2012. MMWR Morb Mortal Wkly Rep 2015;64:555–8. [PMC free article] [PubMed] [Google Scholar]
- 15.Carapetis JR et al. Evaluation of a screening protocol using auscultation and portable echocardiography to detect asymptomatic rheumatic heart disease in Tongan schoolchildren. Nat Clin Pract Cardiovasc Med. 2008;5:411–7. [DOI] [PubMed] [Google Scholar]
- 16.Bhaya M, Panwar S, Beniwal R, Panwar RB. High prevalence of rheumatic heart disease detected by echocardiography in school children. Echocardiography. 2010;27:448–53. [DOI] [PubMed] [Google Scholar]
- 17.Jackson SJ, Steer AC, Campbell H. Systematic review: estimation of global burden of non-suppurative sequelae of upper respiratory tract infection: rheumatic fever and post-streptococcal glomerulonephritis. Trop Med Int Health. 2011;16:2–11. [DOI] [PubMed] [Google Scholar]
- 18.Marijon E et al. Prevalence of rheumatic heart disease detected by echocardiographic screening. N Engl J Med. 2007;357:470–6. [DOI] [PubMed] [Google Scholar]
- 19.Veasy LG, Tani LY, Hill HR. Persistence of acute rheumatic fever in the intermountain area of the United States. J Pediatr. 1994;124:9–16. [DOI] [PubMed] [Google Scholar]
- 20.Bryant PA, Robins-Browne R, Carapetis JR, Curtis N. Some of the people, some of the time: susceptibility to acute rheumatic fever. Circulation. 2009;119:742–53. [DOI] [PubMed] [Google Scholar]
- 21.Krisher K, Cunningham MW. Myosin: a link between streptococci and heart. Science. 1985;227:413–5. [DOI] [PubMed] [Google Scholar]
- 22.Cunningham MW. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev. 2000;13:470–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDonald M, Currie BJ, Carapetis JR. Acute rheumatic fever: a chink in the chain that links the heart to the throat? Lancet Infect Dis 2004;4:240–5. [DOI] [PubMed] [Google Scholar]
- 24.Bessen DE et al. Contrasting molecular epidemiology of group A streptococci causing tropical and nontropical infections of the skin and throat. J Infect Dis. 2000;182:1109–16. [DOI] [PubMed] [Google Scholar]
- 25.Tandon R et al. Revisiting the pathogenesis of rheumatic fever and carditis. Nat Rev Cardiol. 2013;10:171–7. [DOI] [PubMed] [Google Scholar]
- 26.Cunningham MW. Rheumatic fever, autoimmunity, and molecular mimicry: the streptococcal connection. Int Rev Immunol 2014;33: 314–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ayoub EM, Taranta A, Bartley TD. Effect of valvular surgery on antibody to the group A streptococcal carbohydrate. Circulation. 1974;50:144–50. [DOI] [PubMed] [Google Scholar]
- 28.Veasy LG, Tani LY. A new look at acute rheumatic mitral regurgitation. Cardiol Young 2005;15:568–77. [DOI] [PubMed] [Google Scholar]
- 29.Ellis NMJ, Li Y, Hildebrand W, Fischetti VA, Cunningham MW. T cell mimicry and epitope specificity of cross-reactive T cell clones from rheumatic heart disease. J Immunol. 2005;175:5448–56. [DOI] [PubMed] [Google Scholar]
- 30.Roberts S et al. Pathogenic mechanisms in rheumatic carditis: focus on valvular endothelium. J Infect Dis. 2001;183:507–11. [DOI] [PubMed] [Google Scholar]
- 31.Cunningham MW. Streptococcus and rheumatic fever. Curr Opin Rheumatol. 2012;24:408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guilherme L et al. Rheumatic fever: how S. pyogenes-primed peripheral T cells trigger heart valve lesions. Ann N Y Acad Sci. 2005;1051:132–40. [DOI] [PubMed] [Google Scholar]
- 33.Galvin JE, Hemric ME, Ward K, Cunningham MW. Cytotoxic mAb from rheumatic carditis recognizes heart valves and laminin. J Clin Invest. 2000;106:217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Engel ME, Stander R, Vogel J, Adeyemo AA, Mayosi BM. Genetic susceptibility to acute rheumatic fever: a systematic review and meta-analysis of twin studies. PLoS One. 2011;6:e25326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carapetis JR, Currie BJ, Mathews JD. Cumulative incidence of rheumatic fever in an endemic region: a guide to the susceptibility of the population? Epidemiol Infect. 2000;124:239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carapetis JR, McDonald M, Wilson NJ. Acute rheumatic fever. Lancet. 2005;366:155–68. [DOI] [PubMed] [Google Scholar]
- 37.Carapetis JR, Wolff DR, Currie BJ. Acute rheumatic fever and rheumatic heart disease in the top end of Australia’s Northern Territory. Med J Aust. 1996;164:146–9. [DOI] [PubMed] [Google Scholar]
- 38.Sanyal SK, Thapar MK, Ahmed SH, Hooja V, Tewari P. The initial attack of acute rheumatic fever during childhood in North India; a prospective study of the clinical profile. Circulation. 1974;49:7–12. [DOI] [PubMed] [Google Scholar]
- 39.Grassi A et al. Clinical characteristics and cardiac outcome of acute rheumatic fever in Italy in the last 15 years. Clin Exp Rheumatol. 2009;27:366–72. [PubMed] [Google Scholar]
- 40.Reeves BM, Kado J, Brook M. High prevalence of rheumatic heart disease in Fiji detected by echocardiography screening. J Paediatr Child Health. 2011;47:473–8. [DOI] [PubMed] [Google Scholar]
- 41. Gewitz MH. Revision of the Jones Criteria for the diagnosis of acute rheumatic fever in the era of Doppler echocardiography: a scientific statement from the American Heart Association. Circulation. 2015;131:1806–18. •• Discusses the newly revised Jones Criteria differentiating between low risk and high risk ARF population and the use of Doppler echocardiography in diagnosis of ARF carditis and RHD.
- 42.Jones TD. The diagnosis of rheumatic fever. JAMA. 1944;126: 481–4. [Google Scholar]
- 43.Carapetis JR, Brown A, Wilson NJ, Edwards KN. Rheumatic Fever Guidelines Writing Group. An Australian guideline for rheumatic fever and rheumatic heart disease: an abridged outline. Med J Aust. 2007;186:581–6. [DOI] [PubMed] [Google Scholar]
- 44.Cann MP, Sive AA, Norton RE, McBride WJH, Ketheesan N. Clinical presentation of rheumatic fever in an endemic area. Arch Dis Child. 2010;95:455–7. [DOI] [PubMed] [Google Scholar]
- 45.Carapetis JR, Currie BJ. Rheumatic fever in a high incidence population: the importance of monoarthritis and low grade fever. Arch Dis Child. 2001;85:223–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carapetis JR et al. Acute rheumatic fever and rheumatic heart disease. Nat Rev Dis Prim. 2016. doi: 10.1038/nrdp.2015.84.15084 EP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sliwa K et al. Contribution of the human immunodeficiency virus/acquired immunodeficiency syndrome epidemic to de novo presentations of heart disease in the Heart of Soweto Study cohort. Eur Heart J. 2012;33:866–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Narula J et al. Does endomyocardial biopsy aid in the diagnosis of active rheumatic carditis? Circulation. 1993;88:2198–205. [DOI] [PubMed] [Google Scholar]
- 49.Vasan RS et al. Echocardiographic evaluation of patients with acute rheumatic fever and rheumatic carditis. Circulation. 1996;94: 73–82. [DOI] [PubMed] [Google Scholar]
- 50.Kumar RK, Tandon R. Rheumatic fever & rheumatic heart disease: the last 50 years. Indian J Med Res. 2013;137:643–58. [PMC free article] [PubMed] [Google Scholar]
- 51.Mody GM & Mayosi BM in Rheumatology 1093–1102 (Elsevier, 2011). doi: 10.1016/B978-0-323-06551-1.00108-1. [DOI] [Google Scholar]
- 52.Perloff JK, Roberts WC. The mitral apparatus. Functional anatomy of mitral regurgitation. Circulation. 1972;46:227–39. [DOI] [PubMed] [Google Scholar]
- 53.Narula J, Chandrasekhar Y, Rahimtoola S. Diagnosis of active rheumatic carditis. The echoes of change. Circulation. 1999;100: 1576–81. [DOI] [PubMed] [Google Scholar]
- 54.Saxena A et al. Prevalence and outcome of subclinical rheumatic heart disease in India: the RHEUMATIC (Rheumatic Heart Echo Utilisation and Monitoring Actuarial Trends in Indian Children) study. Heart. 2011;97:2018–22. [DOI] [PubMed] [Google Scholar]
- 55. Remenyi B et al. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease—an evidence-based guideline. Nat Rev Cardiol. 2012;9:297–309. •• International guideline standardizing diagnosis of RHD by defining three different categories of RHD based on specific Doppler, two-dimensional and continuous-wave echocardiographic changes.
- 56.Przybojewski JZ. Rheumatic constrictive pericarditis. A case report and review of the literature. S Afr Med J. 1981;59:682–6. [PubMed] [Google Scholar]
- 57.Chopra P, Narula J, Kumar AS, Sachdeva S, Bhatia ML. Immunohistochemical characterisation of Aschoff nodules and endomyocardial inflammatory infiltrates in left atrial appendages from patients with chronic rheumatic heart disease. Int J Cardiol. 1988;20:99–105. [DOI] [PubMed] [Google Scholar]
- 58.Kamblock J et al. Does rheumatic myocarditis really exists? Systematic study with echocardiography and cardiac troponin I blood levels. Eur Heart J. 2003;24:855–62. [DOI] [PubMed] [Google Scholar]
- 59.Joswig BC, Glover MU, Handler JB, Warren SE, Vieweg WV. Contrasting progression of mitral stenosis in Malayans versus American-born Caucasians. Am Heart J. 1982;104:1400–3. [DOI] [PubMed] [Google Scholar]
- 60.Oli K, Asmera J. Rheumatic heart disease in Ethiopia: could it be more malignant? Ethiop Med J 2004;42:1–8. [PubMed] [Google Scholar]
- 61.Meira ZMA, Goulart EMA, Colosimo EA, Mota CCC. Long term follow up of rheumatic fever and predictors of severe rheumatic valvar disease in Brazilian children and adolescents. Heart. 2005;91:1019–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mirabel M et al. Newly diagnosed rheumatic heart disease among indigenous populations in the Pacific. Heart. 2015;101:1901–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zühlke L et al. The natural history of latent rheumatic heart disease in a 5 year follow-up study: a prospective observational study. BMC Cardiovasc Disord. 2016;16:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bisno AL. Group A streptococcal infections and acute rheumatic fever. N Engl J Med. 1991. [DOI] [PubMed] [Google Scholar]
- 65.Essop MR, Nkomo VT. Rheumatic and nonrheumatic valvular heart disease: epidemiology, management, and prevention in Africa. Circulation. 2005;112:3584–91. [DOI] [PubMed] [Google Scholar]
- 66.WHO. Status of vaccine research and development of vaccines for Streptococcus pyogenes prepared for WHO PD-VAC. 2014; 1–7. [Google Scholar]
- 67.Irlam J, Mayosi BM, Engel M, Gaziano TA. Primary prevention of acute rheumatic fever and rheumatic heart disease with penicillin in South African children with pharyngitis: a cost-effectiveness analysis. Circ Cardiovasc Qual Outcomes. 2013;6:343–51. [DOI] [PubMed] [Google Scholar]
- 68.Robertson KA, Volmink JA, Mayosi BM. Antibiotics for the primary prevention of acute rheumatic fever: a meta-analysis. BMC Cardiovasc Disord. 2005;5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dale JB et al. Group A streptococcal vaccines: paving a path for accelerated development. Vaccine. 2013;31 Suppl 2:B216–22. [DOI] [PubMed] [Google Scholar]
- 70. Steer AC et al. Status of research and development of vaccines for Streptococcus pyogenes. Vaccine. 2016. doi: 10.1016/j.vaccine.2016.03.073. • Provides an up-to-date review of vaccine development for GAS.
- 71.Tompkins DG, Boxerbaum B, Liebman J. Long-term prognosis of rheumatic fever patients receiving regular intramuscular benzathine penicillin. Circulation. 1972;45:543–51. [DOI] [PubMed] [Google Scholar]
- 72.Steer AC, Carapetis JR. Prevention and treatment of rheumatic heart disease in the developing world. Nat Rev Cardiol. 2009;6: 689–98. [DOI] [PubMed] [Google Scholar]
- 73.Manyemba J, Mayosi BM. Penicillin for secondary prevention of rheumatic fever. Cochrane Database Syst Rev 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lu JC et al. Simplified rheumatic heart disease screening criteria for handheld echocardiography. J Am Soc Echocardiogr. 2015;28: 463–9. [DOI] [PubMed] [Google Scholar]
- 75. Beaton A et al. The utility of handheld echocardiography for early rheumatic heart disease diagnosis: a field study. Eur Heart J Cardiovasc Imaging. 2015;16:475–82. •• A field study comparing handheld echocardiogram to standard echocardiogram for diagnosis of RHD. Handheld echocardiograms have good sensitivity and specificity especially in diagnosis of definite RHD.
- 76.Engelman D et al. Screening for rheumatic heart disease: quality and agreement of focused cardiac ultrasound by briefly trained health workers. BMC Cardiovasc Disord. 2016;16:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ploutz M et al. Handheld echocardiographic screening for rheumatic heart disease by non-experts. Heart. 2016;102:35–9. [DOI] [PubMed] [Google Scholar]
- 78. Nishimura RA et al. 2014 AHA/ACC Guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;129:2440–92. •• (Lippincott Williams & Wilkins, 2014). Latest guideline by AHA/ACC on surgical management of valvular heart disease and the evidence behind the guidelines.
- 79.Kim JB et al. Long-term outcomes after surgery for rheumatic mitral valve disease: valve repair versus mechanical valve replacement. Eur J Cardiothorac Surg. 2010;37:1039–46. [DOI] [PubMed] [Google Scholar]
- 80.Enriquez-Sarano M, Akins CW, Vahanian A. Mitral regurgitation. Lancet. 2009;373:1382–94. [DOI] [PubMed] [Google Scholar]
- 81.Yanagawa B, Butany J, Verma S. Update on rheumatic heart disease. Curr Opin Cardiol. 2016;31:162–8. [DOI] [PubMed] [Google Scholar]
- 82.Baumgartner H et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22:1–23. quiz 101–2. [DOI] [PubMed] [Google Scholar]
- 83.Remenyi B et al. Position statement of the World Heart Federation on the prevention and control of rheumatic heart disease. Nat Rev Cardiol. 2013;10:284–92. [DOI] [PubMed] [Google Scholar]
- 84.Mayosi B et al. The Drakensberg declaration on the control of rheumatic fever and rheumatic heart disease in Africa. S Afr Med J. 2006;96:246. [PubMed] [Google Scholar]
- 85.Mayosi BM, Gamra H, Dangou J-M, Kasonde J. Rheumatic heart disease in Africa: the Mosi-o-Tunya call to action. Lancet Glob Health. 2014;2:e438–9. [DOI] [PubMed] [Google Scholar]
- 86.Watkins D et al. Seven key actions to eradicate rheumatic heart disease in Africa: the Addis Ababa communiqué. Cardiovasc J Afr. 2016;27:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nordet P, Lopez R, Dueñas A, Sarmiento L. Prevention and control of rheumatic fever and rheumatic heart disease: the Cuban experience (1986–1996-2002). Cardiovasc J Afr 2008;19:135–40. [PMC free article] [PubMed] [Google Scholar]
- 88.Mayosi BM. The four pillars of rheumatic heart disease control. S Afr Med J. 2010. [DOI] [PubMed] [Google Scholar]
- 89.Beaton A, Sable C. Health policy: reducing rheumatic heart disease in Africa [mdash] time for action. Nat Rev Cardiol 2016;13:190–1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.