1. INTRODUCTION
Periodontitis and osteoporosis are prevalent inflammation-associated skeletal disorders that pose significant public health challenges to our aging population. Periodontal diseases consist of a spectrum of disorders in the dento-gingival tissues with a predominant dysbiotic and inflammatory nature.1,2 Gingivitis, the most common form of periodontal disease is a reversible inflammatory response to the accumulation of bacterial plaque and is self-limiting upon removal of the plaque. On the other hand, periodontitis represents a chronic inflammation of the deeper periodontal tissue and alveolar bone, leading to progressive deepening of the gingival sulcus and further accumulation of plaque and calculus. In the advanced stage, periodontitis is associated with irreversible destruction of alveolar bone and loss of tooth support, causing tooth mobility and drifting. Current disease model suggests periodontitis results from multifactorial interactions between dysbiotic subgingival biofilm, host immune response and environmental/risk factors, such as genetic contribution, aging, nutritional deficiencies, hormonal balance, and tobacco use.3 According to the latest National Health and Nutrition Examination Survey ending in 2014, 42% of American adults 30 or older have periodontitis.4 The prevalence and susceptibility of periodontal diseases increase with age.3,4 While approximately 4.1% of US adults aged 30–44 have severe periodontitis, the prevalence rate doubles to 10.4% in the 45–65, and 9.05% in the 65 and older group, respectively.4
Osteoporosis is an age-related bone disease characterized by deterioration of bone density and architecture, along with increased fracture risk. Afflicting over 200 million worldwide,5 it is the most common metabolic bone disease, which places 1 in 3 women and 1 in 5 men over 50 at risk for fracture.6 Aging of the population likely accelerates the economic burden of the associated mortality and morbidity. In 2025, the cost of osteoporotic fractures is projected to rise over 50% from 17 billion dollars in 2005.7
Both periodontitis and osteoporosis are bone disorders closely associated with inflammation and aging. With multiple shared risk factors and links in pathogenic mechanisms, there has been consistent intrigue on whether a systemic skeletal disease like osteoporosis will amplify the alveolar bone loss in periodontitis. While the initial clinical findings up to the early 2000s were controversial at best, recent studies have provided more compelling evidence to suggest an association between these two disorders. In this review, we have evaluated the literature published in the past 25 years to provide an update on the association between osteoporosis and periodontitis, followed by a robust discussion of their mechanistic links, shared risk factors, and therapeutic implications.
2. CLINICAL CORRELATION BETWEEN PERIODONTITIS AND OSTEOPOROSIS
The link between periodontitis and osteoporosis was first drawn in 1960, where periodontitis was initially considered a local manifestation of systemic “pre-senile osteoporosis.”8 Although it was soon revealed that bacterial plaque is a primary etiological factor separating these two disorders, the intrigue has driven extensive studies and meta-analysis centered around two questions: (1) whether systemic weakening of bone mass in osteoporosis enhances the propensity for localized alveolar bone loss; and (2) whether osteoporosis exacerbates loss of attachment and other clinical manifestations of periodontitis. However, depending on the different clinical and radiological parameters used as determinants for periodontitis and osteoporosis, studies to date have yielded varied strength in the association between these two diseases. Before delving into the literature further, the evolving classification and diagnostic criteria for periodontitis and osteoporosis need to be clarified.
Over the past 4 decades, the classification of periodontal diseases has been through several major updates, given emerging evidence on pathogenic factors, host response, and association with systemic diseases.2,9,10 In the most recent 2017 world workshop, periodontitis has been reclassified into three main forms: necrotizing periodontitis, periodontitis as a manifestation of systemic disease, and periodontitis, which combines two previously separate “chronic” and “aggressive” categories.11 Several systemic and genetic disorders significantly compromise the intrinsic immune system, leading to early presentation of severe periodontitis. As a consequence of Down syndrome, patients experience altered T lymphocyte migration to the periodontium. The compromised immune response predisposes them to infections, and periodontal attachment loss begins as early as adolescence.12 Individuals with Papillon-Lefèvre syndrome may lose primary and permanent teeth at a young age because of defects in neutrophil function.13 These conditions should be grouped as “periodontitis as a manifestation of systemic disease,” and be classified based on the primary conditions.14 In contrast, several common systemic diseases, including osteoporosis, exert relatively moderate influence on the pathogenesis of periodontitis. While contributing to loss of periodontal apparatus, these systemic diseases do not lead to periodontal manifestations with unique onset or severity, thus should be considered as risk factors. Of note, the task force from the 2017 workshop concluded that osteoporosis is significantly associated with higher prevalence and severity of radiographic alveolar bone loss. However, there is no clear association with other clinical parameters of periodontitis.14
In general, the diagnosis and staging of periodontitis are based on a combination of examination of clinical attachment loss (CAL) and assessment of radiographic bone loss.10 Reduction of 1–2 mm CAL and <15% of root length in bone loss is characterized as mild periodontitis (Stage I), 3–4 mm CAL and 15%−30% alveolar bone loss as moderate periodontitis (Stage II), and CAL ≥ 5 mm with >30% bone loss as severe periodontitis (Stage III and IV).15 These measurements, when combined with parameters including probing depth (PD), bleeding on probing (BOP), tooth loss, pattern of bone loss, furcation involvement, and presence of systemic risk factors, further determine the progression rate and complexity for managing the periodontitis case.10,15
On the other hand, the gold standard for diagnosing osteoporosis is the evaluation of bone mineral density (BMD) of lumbar vertebrae and/or proximal femur using dual energy X-ray absorptiometry (DXA).16 Osteoporosis is defined by a BMD score (T score) of 2.5 standard deviations or more below the average BMD of a healthy young adult. While the diagnostic criteria for osteoporosis are well established, the assessment of fracture risk for patients with low BMD takes into consideration multiple risk factors. Other validated sites and techniques for BMD measurements such as quantitative computer tomography (QCT) may also be utilized to determine fracture risk. The 10-year probability of fracture, rather than BMD alone, is suggested to determine the threshold for intervention.16 However, in most clinical studies on the correlation with periodontitis, lumbar and/or femoral DXA was used as the indicator to represent systemic BMD associated with osteoporotic patients.
3. CORRELATION BETWEEN OSTEOPOROSIS AND ALVEOLAR BONE LOSS
As osteoporosis represents generalized thinning of trabecular and cortical bone, the long-standing hypothesis is that the alveolar bone surrounding the teeth in osteoporotic patients is more susceptible to periodontitis-related bone loss. In clinical studies examining the association between systemic and alveolar bone loss (ABL), all of the 10 studies published between 1996 and 2020 revealed an inverse correlation between systemic BMD and ABL17–26 (Table 1). All studies employed DXA of lumbar vertebrae and/or femoral neck as a determinant of osteoporosis. However, since DXA is limited to measuring alveolar BMD in edentulous subjects, a diverse range of alternative techniques were employed to assess the ABL, including linear assessment of alveolar crest height (ACH) or ABL using intraoral18,22,24,26,27 or panoramic radiographs,19,25 mandibular cortical width (MCW) using panoramic radiographs,17,19,20,25 and digital densitometry analysis of alveolar BMD.21,23,26 Since these parameters encompass both bone volume and density, as well as cortical and trabecular bone, the conclusion that ABL is inversely correlated with systemic BMD is particularly compelling.
TABLE 1.
Summary of literature on the correlation between alveolar bone loss and systemic BMD
| Study | Year | Sample size | Oral bone loss determinant | Osteoporosis determinant | Age (years) | Patient population | Correlation: ABL and systemic BMD |
|---|---|---|---|---|---|---|---|
| Okabe et al17 | 2008 | 659 | MCW (pano) | DXA heel | 80 | 262 men and 397 women | Yes |
| Brennan et al18 | 2008 | 1256 | ACH (intraoral) | DXA forearm, hip, spine | 66.6 ± 7.0 | Postmenopausal women | Yes (among women <70 years) |
| Ishii et al19 | 2007 | 54 | MCW, ABL (pano) | DXA femur | 56.8 ± 7.7 | Postmenopausal women | Yes (MCW); not strong (ABL) |
| Taguchi et al20 | 2007 | 450 | MCW (pano) | DXA spine | 57.2 ± 8.1 | Postmenopausal women | Yes |
| Takaishi et al21 | 2005 | 40 | alveolar BMD (intraoral) | DXA spine, ultrasound | 59.4 ± 5.6 | Postmenopausal women | Yes |
| Hilderbolt et al22 | 2002 | 49 | ACH (intraoral) | DXA spine, femur | 60 ± 5.5 | Postmenopausal women | Yes |
| Jonasson et al23 | 2001 | 80 | alveolar BMD (intraoral) | DXA forearm | 47 ± 27 | Mixed | Yes |
| Tezal et al24 | 2000 | 70 | ABL (intraoral) | DXA spine and femur | 62.1 ± 7.1 | Postmenopausal women | Yes |
| Taguchi et al25 | 1999 | 90 | MCW, ABL (pano) | QCT spine | 54.1 ± 7.4 | Mixed | Yes (MCW); no(ABL) |
| Payne et al26 | 1999 | 38 | ACH, alveolar BMD (intraoral) | DXA spine | 53.9 ± 0.4 | Postmenopausal women | Yes (ACH and alveolar BMD) |
The ratio of compact cortical bone and spongy trabecular bone varies significantly between maxillae and mandible. With approximately 10% cortical bone in the spine and maxillae, the mandible comprises up to 80% cortical bone and 20% trabecular bone.28 In the mandible, cortical bone is concentrated at the inferior cortex, and begins to decrease in width with time after the age of 50. Thus, the MCW is used as an index for cortical bone assessment using panoramic radiographs, where the inferior cortex is well captured. Studies showed that thinning MCW is significantly correlated with systemic BMD, and serves as a potential risk indicator for osteoporosis.17,29,30 However, the MCW is not associated with systemic fracture risk.17,30 In contrast to cortical bone, mandibular trabecular bone pattern is more closely related to fracture risk,31–33 and serves as a valuable predictor for osteoporosis in women.30,33,34 Of note, while mandibular trabecular pattern becomes more spaced and less connected with advanced age in most females, the trabecular pattern is preserved in most males.31,35 Given the challenge of directly measuring trabecular pattern, most studies resorted to assessment of the alveolar BMD through densitometry or measurement of alveolar crest height, where most periodontal bone loss was concentrated. Consistently, both ACH and alveolar BMD are correlated with systemic BMD. Particularly in the predominantly trabecular maxillary bone, alveolar BMD is also correlated to lumbar and hip BMD.36 Taken together, there is strong evidence supporting that osteoporosis is associated with higher susceptibility of alveolar bone loss in postmenopausal women.
As a caveat, only one study dating back to 1992 found no statistically significant correlation between alveolar bone height and systemic BMD in women between 46 and 55 years of age.37 It is possible that the results were confounded by the number of retained teeth in the subjects, as the study included a high percentage of edentulous subjects. And the relative young age of these perimenopausal women in the study could explain a lack of contribution from postmenopausal osteoporosis. While most of the studies were cross-sectional, a 5-year longitudinal study examining the effect of estrogen replacement therapy also demonstrated significant improvement in mandibular bone mass in association with BMD of the spine and wrist.38 As 70% of all fractures happen in osteoporotic females5 and with the traditional view that osteoporosis is predominantly associated with postmenopausal estrogen deficiency, it is not surprising that most studies focused on postmenopausal women. Despite limited findings supporting the trend,17 given the distinct gender-specific pattern of alveolar bone loss, further analysis is required to confirm if osteoporosis is associated with alveolar bone loss in men.
4. CORRELATION BETWEEN OSTEOPOROSIS AND PERIODONTAL ATTACHMENT LOSS
While radiographic assessment of alveolar bone loss is an important criterion, CAL reflects the lifetime experience of periodontitis and is a critical outcome measurement for diagnosing and staging of periodontitis.10 Of 23 studies published between 1995 and 2020, 17 revealed a significant correlation between CAL and osteoporosis18,24,25,39–60 (Table 2). These included 18 cross-sectional and 5 longitudinal studies, ranging from a sample size of 3054 to 2990.44 Most studies focused on postmenopausal women aged between 41 and 80 years old. Lumbar and/or femoral DXA was used to represent systemic BMD, but the clinical outcome measurements for CAL as determinants of periodontitis were more variable, especially considering the inclusion of other parameters, such as PD and BOP. The lack of a consistent CAL criteria could contribute to variability in the conclusions of these studies. However, the more recent studies since 2010 used CAL > 5 mm as a determinant for severe periodontitis, in accordance with the clinical classification criteria. In these 11 studies, all demonstrated positive association between CAL and systemic BMD, except for one study that utilized a much more stringent criteria of CAL > 7 mm.48 Consistently, two systematic reviews from 2010 and 2017 reached similar conclusions.61,62 Taking into consideration the bias and qualities of the evidence, Penoni et al suggested that 10 out of 11 studies with high quality evidence demonstrated positive correlation between CAL and systemic BMD.61 Particularly, compiling the data from all 11 studies, the low BMD group had 3.04% more sites with CAL > 4 mm and 5.07% sites with CAL > 6 mm, compared to normal BMD group. With 30% of sites as the threshold, this difference could create a significant clinical implication in a shift from localized to generalized periodontitis.4,61
TABLE 2.
Summary of literature on the correlation between periodontal attachment loss and systemic BMD
| Study | Year | Sample size | Periodontitis determinant | Osteoporosis determinant | Age (year) | Patient population | Correlation between CAL and BMD |
|---|---|---|---|---|---|---|---|
| Mashalkar et al39 | 2018 | 94 | CAL > 5 mm on 30% site | DXA spine | 45–60 | Postmenopausal women | Yes |
| Passos-Soares et al40 | 2017 | 492 | PD > 5 mm with CAL > 6 mm | None | 66.6 ± 7.4 | Postmenopausal women | Yes (osteoporosis treatment with CAL) |
| Penoni et al41 | 2016 | 134 | PD > 5 mm with CAL > 6 mm | DXA femur, spine | 69.8 ± 3.9 | Postmenopausal women | Yes |
| Juluri et al42 | 2015 | 100 | CAL, PD | DXA spine | 60.2 ± 2.1 | Postmenopausal women | Yes |
| Singh et al43 | 2014 | 78 | PD, CAL, tooth loss | DXA femur, spine | 46–54 | Postmenopausal women | Yes (CAL and PD), No (tooth loss) |
| Tak et al44 | 2014 | 2990 | CAL, tooth loss | DXA femur, spine | 64 ± 8 | Postmenopausal women | Yes (spine BMD only) |
| Gondim et al45 | 2013 | 148 | tooth loss, CAL | DXA femur, spine | 58.9 ± 4.3 | Postmenopausal women | Yes |
| Passos et al46 | 2013 | 521 | PD>5mm with CAL>6mm | DXA femur, spine | 60.6 ± 7.3 | Postmenopausal women | Yes |
| Iwasaki et al47 | 2013 | 397 | CAL, tooth loss, BOP | DXA femur, spine | 68.2 | Postmenopausal women | Yes |
| Marjanovic et al48 | 2013 | 380 | PD > 5.5 mm or CAL > 7 mm | DXA femur, spine | 58 ± 4.7 | Postmenopausal women | No |
| Moeintaghavi et al49 | 2013 | 60 | PD, CAL, tooth loss | DXA femur, spine | 50.8–56 | Postmenopausal women | No |
| Grocholewicz et al50 | 2012 | 37 | CAL, tooth loss | DXA femur, spine, and forearm | 59.4 ± 5.6 | Postmenopausal women | Yes |
| Al Habashneh et al51 | 2010 | 400 | PD > 5 mm with CAL > 6 mm | DXA femur, spine | 62.5 ± 6.4 | Postmenopausal women | Yes |
| Nicopoulou et al63 | 2009 | 665 | tooth loss | DXA spine, femur | 45–70 | Mixed | Yes |
| Brennan et al18 | 2007 | 1329 | CAL | DXA spine, femur, forearm | 66.6 ± 7.0 | Postmenopausal women | Yes (without subgingival calculus) |
| Gomes-Filho et al52 | 2007 | 139 | PD > 4 mm with CAL > 3 mm | DXA femur, spine | 58.8 ± 6.4 | Postmenopausal women | Yes |
| Taguchi et al53 | 2004 | 1298 | tooth loss | DXA femur, spine | 70.8 ± 9 | Mixed | Yes (femur BMD only) |
| Mohammad et al54 | 2003 | 30 | tooth loss; CAL | DXA calcenaous | 63.4 ± 8.6 | Postmenopausal women | Yes |
| Pilgram et al55 | 2002 | 135 | PD, CAL | DXA femur, spine | 41–70 | Postmenopausal women | No (weak) |
| Lundstrom et al56 | 2001 | 36 | BOP; PD; recession; | DXA femur | 70 | Mixed | No |
| Tezal et al24 | 2000 | 70 | CAL, PD, BOP | DXA femur, spine | 62.1 ± 7.1 | Postmenopausal women | No |
| Weyant et al57 | 1999 | 293 | CAL, PD, BOP | DXA femur and spine; | 75.5 ± 4.4 | Mixed | No |
| Taguchi et al25 | 1999 | 90 | tooth loss | CT spine | 54.1 ± 7.4 | 62 postmenopausal | Yes (posterior teeth only) |
| Mohammad et al58 | 1997 | 44 | tooth loss; CAL; PD | DXA spine | 65.2 ± 1.6 | Postmenopausal women | Yes (CAL) No (PD and tooth loss) |
| Hildebolt et al59 | 1997 | 135 | tooth loss; CAL; PD | DXA femur, spine | 59 ± 6.2 | Mixed | No |
| Mohammad et al60 | 1996 | 42 | CAL; recession | DXA spine | 68 ± 6.8 | Postmenopausal women | Yes (gingival recession) |
| Krall et al64 | 1996 | 189 | tooth loss | DXA femur, spine | 60 ± 6 | Postmenopausal women | Yes |
| May et al65 | 1995 | 874 | tooth loss | DXA femur, spine | 65–76 | Mixed | Yes in men, No in women |
Abbreviations: BOP, bleeding on probing; CAL, clinical attachment loss; DXA, dual energy X-ray absorptiometry; PD, probing depth.
Loss of clinical attachment in severe periodontitis culminates in the destruction of alveolar bone support and tooth loss. Of the 16 studies that evaluated correlation between tooth loss and systemic BMD, 11 showed a positive association,25,44,45,47,50,53,54,63–66 while 5 studies reported conflicting results.43,49,58,59,67 The lack of correlation could be due to a relatively small sample size and inclusion of confounding factors such as age in the earlier studies.43,49,58 When corrected for confounding factors including age and smoking, a large group multicenter study showed compelling association between tooth loss with systemic BMD.63 Of note, tooth loss is also a clinical endpoint associated with multiple factors beyond periodontitis. The contribution of osteoporosis may be of minor importance in comparison to other clinical and socioeconomic factors, such as access to dental care and oral hygiene habits.
Since the task force report from the 2017 world workshop, which perceived insufficient evidence to support a connection,14 there have been a remarkable number of studies and systematic reviews supporting the association between osteoporosis and CAL or other clinical parameters of periodontitis. Considering the numerous risk factors associated with both diseases, further confirmation of the association calls for correction for confounding factors in the data analysis and for well-controlled longitudinal studies in the future.
5. INFLAMMATION AND BONE HOMEOSTASIS
Although the pathogenesis and progression of periodontitis depend on the host interaction with the dysbiotic biofilm,68 the ensuing inflammation and its influence on bone homeostasis play critical roles in both osteoporosis and periodontitis, and could serve as the central mechanistic link between these disorders.
The healthy skeleton undergoes continuous remodeling process, which allows new bone to replace the old as part of physiological development and in response to factors, such as mechanical loading. Normal bone turnover relies on an orchestrated balance between bone resorption by osteoclasts and bone formation by osteoblasts, essential for the maintenance of a stable bone mass and mineral homeostasis.69
Inflammation is characterized by the activation of various immune cells in the innate and adaptive immunity, resulting in an elevation of immune cytokine production in the cellular environment. The cytokines activated during the course of inflammatory responses have profound effects on the differentiation and activity of osteoblasts and osteoclasts and, therefore, are considered to be mediators of inflammation-associated osteoporosis and periodontitis.
In periodontitis, persistent microbial biofilm on the tooth surface elicits the recruitment of polymorphonuclear neutrophils from the vasculature to the site of infection. As the first line of defense against the pathogens, neutrophil recruitment is influenced by a multitude of factors, including bacterial products, immune cytokines, chemokines, and lipid mediators.70,71 Detection of bacteria by toll-like receptors (TLRs) via binding of bacterial products to TLRs activates the innate immune system.72 Subsequent crosstalk between innate and adaptive host response stimulate lymphocyte activation and amplification of local inflammatory signaling cascade, including nuclear factor-kappa B (NF-κB), activator protein 1 (AP-1), and p38 pathways.70 The local alveolar bone loss appears to be at least partly mediated by bacteria-activated T lymphocytes stimulating receptor activator of nuclear factor kappa-B ligand (RANKL) signaling to promote osteoclastogenesis.73,74 In addition to osteoclasts, enhanced inflammation could also inhibit osteoblast lineage cells to further uncouple the balance in bone remodeling, culminating in a net bone loss.75
The uncoupling of bone remodeling also drives systemic inflammatory bone disorders, including osteoporosis. With enhanced cytokine secretions, inflammation, particularly activation of NF-κB signaling, promotes osteoclasts and simultaneously suppresses osteoblasts.76 The genetic or chemical inhibition of NF-κB signaling has been shown to attenuate bone loss in osteoporosis and arthritis.77 For instance, TNF blockers have been an effective approach to dramatically slow down the progression of local and systemic bone loss in rheumatoid arthritis.78,79 Growing evidence suggests that modulating the inflammatory responses have profound influence on the balance in bone remodeling and could represent an important therapeutic approach.
NF-κB signaling is a major signaling pathway activated during most inflammatory responses.80–82 NF-κB represents a family of five proteins (c-Rel, RelA/p65, RelB, NF-κB1/p50, and NF-κB2/p52), all of which play critical roles in osteoimmunology and aging.80 NF-κB proteins exist as homo- or heterodimers and form a complex with inhibitors of κB (IκBs) in the cytoplasm. Upon stimulation by various inflammatory cytokines, activation of IκB kinases (IKKs) phosphorylates, and degrade IκBs to release NF-κB, which translocate into the nucleus to activate transcription of various target genes. Genetic manipulation of various NF-κB pathway components in animal models have shown that NF-κB signaling has profound effects on bone formation and resorption. Double knockout mice of Nfkb1 and Nfkb2 did not form osteoclasts in vivo or in vitro.83 Conditional Ikkb knockout mice using a Mx1 promoter showed osteopetrotic phenotype and decreased osteoblast numbers.84 In the context of osteoporosis, mice overexpressing an Ikkϒ dominant-negative gene in mature osteoblasts showed enhanced osteoblast formation and suppressed osteoclast activation following ovariectomy (OVX), a mouse model of estrogen-deficiency induced osteoporosis.76 In the periodontal tissue, inhibition of NF-κB signaling in the osteoblast lineage cells directly impaired osteoclastic bone resorption and promoted bone formation,75 further affirming their regulatory roles in bone remodeling.
In addition to components of NF-κB signaling pathways, manipulation of proinflammatory cytokines, such as TNFα, interleukin-1 (IL-1), and IL-6, also profoundly impact systemic and periodontal bone loss. In humans, IL-1β level is elevated significantly in gingival crevicular fluid at sites of periodontal attachment loss.85 Chemical or genetic inhibition of IL-1 in mice resulted in significantly suppressed pathogenic bacterial load.86,87 Conversely, transgenic mice over-expressing IL-1α developed attachment loss and alveolar bone destruction paralleling periodontitis.88 Injection of recombinant human TNF-α ameliorated periodontal inflammation and bone loss in a rat ligature model.89 Interestingly, genetic ablation of TNF receptor p55 in mice increased bacterial load of A actinomycetemcomitans via reduced amount of neutrophilic antimicrobial myeloperoxidase.90 Despite the reduced host response to bacterial pathogens, the periodontal inflammation and alveolar bone loss were both reduced in the p55 knockout mice, suggesting that the critical role of cytokine amplification and NF-κB signaling, rather than pathogen load alone, in the progression of periodontitis. As TNF-α and IL-1 activate NF-κB signaling pathway, increased cytokine production effectively induces RANKL signaling, essential for osteoclastogenesis and subsequent periodontal bone resorption.91 As for osteoporosis, serum IL-1 level was higher in osteoporotic women for as long as 15 years after menopause, and the IL-1 level was inversely correlated to systemic BMD.92 Three recent large-cohort nested epidemiological studies confirmed this immunological link whereby a 1.5- to 3-fold increase in osteoporotic fracture risk was associated with higher level of inflammatory markers (TNF and IL-6 receptors).93 Mice lacking TNF-α are resistant to OVX-induced bone loss. In rodent OVX models, simultaneous administration of IL-1 and TNF-α was required to completely abrogate the osteoporotic bone loss in rats,94 implicating synergistic coordination between immune cytokines. These evidence from human and animal models suggest NF-κB–related cytokines play a central role in periodontitis and osteoporosis.
Apart from the inflammatory cytokines, the complement system represents a critical component of the innate immunity responsible for first-line host defense and further elicitation of adaptive immune response. This network of more than 50 proteins mediates response to microbial infections or tissue damage,95 thereby amplifying and modulating the host immune response via activation of NF-κB and AP-1 pathways to elevate the plasma levels of IL-6, TNF-α, and IL-1β.96 Three distinct pathways that activate the complement system (classical, lectin and alternative) converge at the C3 protein, a central component of the complement system.97 Multiple lines of evidence from clinical and animal studies have strongly supported the role of C3 and complement system in the progression of periodontitis. The amount of activated complement fragments in the gingival crevicular fluid (GCF) is elevated in periodontitis patients compared to healthy samples,98,99 and C3 activation decreases after periodontal therapy.100 C3-deficient mice showed significantly less bone loss and inflammation in three distinct models of periodontal bone loss induced by bacterial inoculation, ligature, and aging.101 Consistently, C3 inhibition in non-human primates also protects against the development of periodontitis, and the amount of NF-κB–related inflammatory cytokines dramatically decreased in the GCF of primates treated with C3 inhibitor.101 On the other hand, C3-deficient mice, compared to wild-type controls, show significantly lower trabecular loss and less cortical erosion after OVX, suggesting that C3 activation is required in osteoporotic bone loss as well.102 These studies collectively demonstrate that the complement system associated with NF-κB signaling activation play a key role in the regulation of systemic and periodontal bone loss.
Mechanistically, NF-κB signaling mediates the uncoupling of bone remodeling via simultaneous but opposite effects on osteoclasts and osteoblasts. Osteoclast differentiation and activation are regulated by both systemic hormones and cytokines secreted locally into the bone microenvironment. As the bone formation and resorption are coupled, osteoclasts can be regulated by osteoblasts, marrow stromal cells, osteocytes, as well as lymphocytes in the bone marrow. Two key transcription factors required for osteoclasts are c-Fos and nuclear factor of associated T-cells c1 (NFATc1). Mice lacking c-fos develop macrophages but cannot form osteoclasts.103 The activation of NFATc1 is essential for the transcription of various essential osteoclastic markers, including genes encoding the tartrate-resistant acid phosphatase (TRAP), matrix metalloproteinase-9 (MMP-9), and cathepsin K.104,105 The initial step of osteoclast differentiation from hematopoietic stem cells, a step toward monocytes and macrophages, requires macrophage colony-stimulating factor (M-CSF). Through the activation of extracellular signal-regulated kinases and anti-apoptotic serine/threonine kinase (AKT), M-CSF subsequently induces the proliferation and survival of osteoclast progenitor cells.106 The next step of osteoclast differentiation requires RANKL signaling. RANKL is a member of the TNFα superfamily and is primarily expressed by osteoblasts, marrow stromal cells, and lymphocytes. RANKL signaling can be modulated by systemic hormones, including parathyroid hormone (PTH), IL-11, prostaglandins, and 1,25-(OH)2D3.107,108 Interestingly, osteoclasts can also regulate their own formation via autocrine secretion of cytokines. For instance, IL-6 secreted by osteoclasts acts on the surrounding progenitors to promote osteoclast formation, independent of RANKL signaling.109
On the other hand, in inflammatory bone loss, such as rheumatoid arthritis and osteoporosis, the number and activity of osteoblasts are too low to compensate for the accelerated osteoclast action. Inflammatory responses could severely impair osteoblast differentiation via several pathways. For instance, TNFα inhibits RUNX2 expression, a master regulator for osteoblast differentiation110 and promotes RUNX2 degradation by upregulating its E3 ubiquitin ligases Smurf1/2.111 Conversely, inhibition of NF-κB pathway components such as IKKϒ led to increased AP-1 activation and enhanced osteoblastogenesis.76 Furthermore, as canonical WNT signaling promotes osteoblasts, NF-κB activation interferes with WNT signaling to exert inhibitory effects on osteoblasts. NF-κB-induced Smurf1/2 upregulation promotes β-catenin degradation in mesenchymal stem cells,112 while antagonists DKK1113 and sclerostin114 are induced by TNF to abrogate the osteoblastogenic effects of canonical WNT signaling.
Thus, chronic inflammation is a cardinal yet often neglected risk factor for both local and systemic bone loss.104,115 Systemic osteoporosis and increased fracture rates are found in correlation with various rheumatological diseases, including rheumatoid arthritis, systemic lupus erythematosus, psoriatic arthritis, as well as inflammatory bowel disease, celiac disease, cystic fibrosis, and periodontitis.115 In addition to observing the co-incidence of inflammatory conditions, there is a temporal link between inflammation and osteoporosis in aging, menopause, pregnancy, and steroid administration.116 Supporting evidence is also observed in animal models of arthritis and colitis.78,117 In summary, inflammation via the activation of NF-κB signaling uncouples the bone remodeling process. Closer examination of the shared risk factors further elucidates that systemic inflammation may also enhance immune response in the localized alveolar bone microenvironment, thereby serving as the mechanistic link underpinning the association between periodontitis and osteoporosis.
6. AGING IN OSTEOPOROSIS AND PERIODONTITIS
Advanced age is a well-established risk factor for osteoporosis.16 The classical estrogen-centric model of osteoporosis suggested a predominant role of estrogen deficiency, as postmenopausal women presented with high incidence of osteoporotic fractures and bone loss. However, in both males and females, trabecular bone loss occurred after reaching peak bone mass, even in the presence of sufficient sex steroids, thereby implicating intrinsic age-related mechanisms at play.69,118 Chronic inflammation is a hallmark of aging.119 With age, the bone marrow microenvironment becomes increasingly proinflammatory with accumulation of immune cytokines, such as IL-6 and IL-1.120 The increased production of immune cytokines and oxidative stress entail that bone metabolism is more susceptible to osteoimmunological influences. The inflammatory microenvironment may mediate the interactions between bone cells and immune cells, to directly influence bone metabolism and age-related bone loss. Studies have shown that while NF-κB signaling is activated in progeroid mice with accelerated aging,121 and suppression of NF-κB activation could attenuate physiological skeletal aging and development of symptoms associated with accelerated aging.121,122
While cross-sectional studies identified correlation between age and bone loss for up to 45 years of age, alveolar bone mass remained stable after 50 years of age.123,124 This suggests that increased prevalence and severity of periodontitis is not an obligatory consequence of aging. Rather, it is likely the result of an altered disease susceptibility and host response associated with the aging as a complicating factor. Recent evidence suggests that aging promotes pathogenic microbial colonization, while evoking a pro-inflammatory microenvironment to exacerbate periodontal inflammation and bone loss.125–127 Human studies revealed that older individuals not only had a more severe inflammatory response in an experimental gingivitis model, the gingival lesions from the older individuals contained greater composition of B-cells compared to polymorphonuclear neutrophils.128 In a non-human primate study, the levels of systemic inflammatory mediators were significantly elevated in older animals and that this was associated with gingival inflammation and periodontal tissue destruction.129 While the fundamental mechanisms remain unclear, emerging evidence suggests that accumulation of oxidative stress and cellular senescence are two shared, age-related mechanisms driving osteoporosis and exacerbation of periodontitis.
7. OXIDATIVE STRESS AND SENESCENCE AS SHARED MECHANISTIC LINKS
Oxidative stress in the skeletal tissue microenvironment is elevated during aging, as a result of excessive accumulation of intracellular reactive oxygen species (ROS), as well as a depletion of enzymes for antioxidant defense.130 ROS production takes place mostly in mitochondria-rich tissues during aerobic metabolism, fatty acid oxidation and in response to environmental stimuli, including immune cytokines.131 With age, mitochondrial dysfunction, DNA damage, and proinflammatory cytokines are associated with increased production of ROS. In concert, elevated oxidative stress, via NF-κB signaling, triggers enhanced osteoclastogenesis, increased osteoblast apoptosis, along with decreased osteoblastogenesis.
Mounting in vivo evidence suggests that age-induced oxidative stress may contribute to osteoporotic bone loss. In mouse skeleton, age-dependent decline of bone mass and strength is linked with increased ROS levels and decreased antioxidant glucothiane reductase activity in the bone marrow.132 Knockout of NADPH oxidase 4(Nox4), which is essential in ROS production, led to attenuation of osteoclastogenesis and OVX-induced bone loss, without significant impact on osteoblasts.133 Mice deficient in antioxidants such as glutathione exacerbated OVX-induced bone loss.134 Mice depleted with antioxidant foxhead box O (FoxO) family proteins showed rising oxidative stress that aggravated osteoclastogenesis and osteoblast apoptosis, contributing to an osteoporotic phenotype.135,136 In humans, elevated markers of oxidative stress were found to be associated with low BMD in postmenopausal women.137
During the initial periodontal response to bacterial pathogens, respiratory burst of the recruited polymorphonuclear neutrophils, the primary producers of ROS, contributes to elevated ROS level, which in turn is responsible for neutrophil priming for the microbial defense.138 However, persistent and excessive production of ROS leads to elevation of immune cytokines, triggering signaling cascades that culminates in the uncoupling of bone remodeling in periodontitis. Numerous case-control and longitudinal studies have established that the level of markers for oxidative stress or antioxidants are directly correlated to the presence, severity, or improvement of periodontitis, and have been extensively reviewed elsewhere.71,139,140 For instance, the levels of oxidant-induced DNA damage, as measured by the biomarker 8-hydroxy-2′-deoxyguanosine (8-OHdG), are higher in patients with chronic periodontitis than in healthy controls.141 Higher levels of total protein carbonyls, another indicator of ROS, were found in the patients with periodontal disease and correlated with increased loss of periodontal attachment.142 Thus, excess free radicals in conjunction with reduced host antioxidant defense play a central role in the pathogenesis and progression of periodontitis.
This imbalance is extended to diabetes mellitus, another established risk factor for periodontal bone loss. Conversely, periodontitis is ranked as the sixth most common end-stage complications for type 2 diabetes.143 Advanced glycation end (AGE) products, produced in general in conjunction with aging, are the most common product of chronic hyperglycemia. AGE products promote excessive ROS production and establish a proinflammatory state. In an environment characterized by the overproduction of free radicals, various molecules release enzymatic antioxidants in an attempt to prevent oxidative damage. Total antioxidant capacity was increased in both peripheral blood samples and gingival crevicular fluid samples from patients with type 2 diabetes and periodontitis.71 As such, the generation of oxidative stress may be an underlying systemic condition directly related to alveolar bone loss in periodontitis, in patients with type 2 diabetes.
Cellular senescence, the halting of proliferation for damaged and dysfunctional cells, is critical in the pathogenesis of age-related chronic diseases, including diabetes and osteoporosis.144–147 Mesenchymal stem cells (MSCs) possess self-renewal ability and multiple lineage potentials that contribute to osteoblasts and adipocytes in the adult bone marrow.148–150 Prolonged age-induced stressors, such as oxidative stress, drives chronic cellular senescence. The exhaustion of the MSC pool through senescence represents one of the hallmarks for skeletal aging.151–153 In the bone microenvironment, age-induced senescence was found in all stages of the osteoblast lineage in the vertebrae and long bones.154 Clearance of senescent osteocytes, but not osteoclast progenitors, significantly alleviated age-related bone loss, including osteoporosis.146,155 Therefore, targeting senescence in the mesenchymal lineage through senolytic agents is a promising strategy for treating osteoporosis.147,156
The negative impact of cellular senescence on tissue homeostasis is twofold: the loss of regenerative potential in progenitors and altered immunomodulation in the microenvironment. Senescent MSCs lose potential for proliferation, self-renewal, and osteogenic differentiation, contributing to the impaired bone mass and delayed repair in long-bone.122,152,153 Cellular senescence is also associated with inflammation and extracellular matrix remodeling through the secretion of proteins termed as senescence-associated secretory phenotype (SASP). The pro-inflammatory microenvironment would in turn increase the cellular stress to trigger senescence in the neighboring cells as a positive feedback loop. In the oral cavity, aging, hyperglycemia, and bacterial lipopolysaccharide (LPS) have been demonstrated to induce cellular senescence in various subpopulations of the periodontal tissues to exacerbate the adaptive immune response and periodontal inflammation.126,144,157
As a unitary theory, advanced age drives the establishment of proinflammatory tissue microenvironment through accumulation of oxidative stress and senescence. In the respective contexts of systemic and local bone microenvironments, the elevated inflammation disrupts the balance between osteoclasts and osteoblasts to cause uncoupling of bone remodeling (Figure 1).
FIGURE 1.
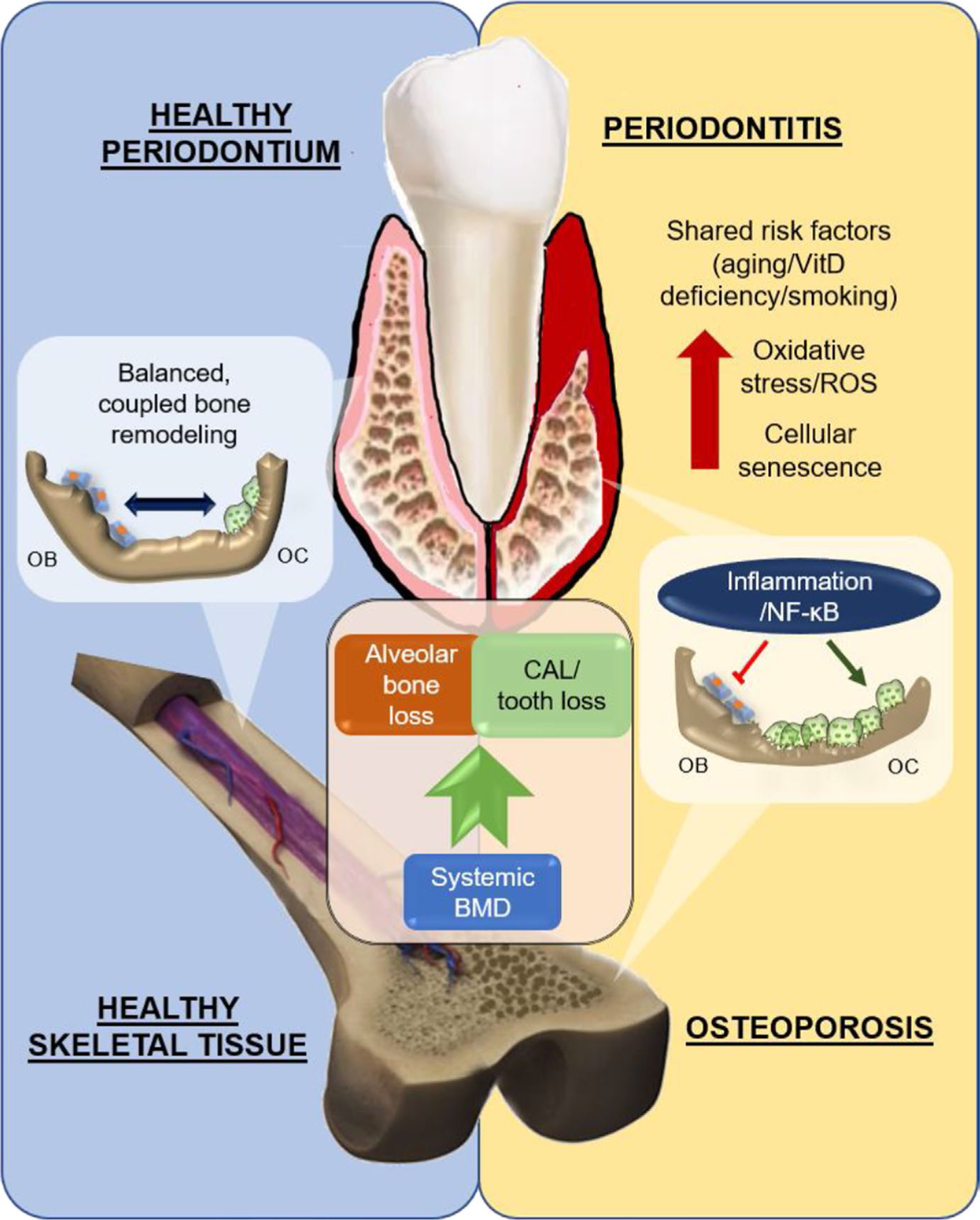
Schematic of the association between periodontitis and osteoporosis. Low systemic bone mineral density (BMD) is linked to alveolar bone loss and clinical attachment loss (CAL). In healthy periodontium and skeletal tissue, osteoblasts (OB) and osteoclasts (OC) function in an orchestrated balance to achieve bone homeostasis. In both periodontitis and osteoporosis, shared risk factors, such as aging, vitamin D deficiency, and smoking, escalate the oxidative stress and cellular senescence in the tissue microenvironment. Mechanistically, the exacerbation of inflammatory response, through activation of NF-κB signaling, inhibits osteoblasts while promoting osteoclasts, resulting in a net bone loss
8. NUTRITIONAL DEFICIENCY AND SMOKING AS SHARED RISK FACTORS
Calcium and vitamin D deficiency are major risk factors for osteoporosis158 and periodontitis.159,160 Inadequate vitamin D intake resulting in insufficiency (<25 nmol/L serum concentration) can lead to decreased calcium intestinal absorption and ultimately release of calcium from the skeleton in order to maintain calcium homeostasis.161 Serum level of 25-(OH)2D3 was reportedly associated with BMD.162 A total of 5 out of 9 interventional studies of vitamin D supplementation alone, and 16 of 22 studies of vitamin D combined with calcium supplementation, have reported positive effects on the systemic BMD and reduced fracture risks.163 On the other hand, large cohort cross-sectional studies revealed that women with a low intake of dietary calcium have more severe periodontal disease, and a more modest relationship is suggested for men.159
Vitamin D3 is a pleiotropic hormone, the main activities of which are the result of the interaction of its active metabolite (1,25-(OH)2D3, or calcitriol), in concert with PTH to modulate bone homeostasis and calcium/phosphate balance.164 Vitamin D stimulates intestinal absorption of calcium, regulates PTH release by the chief cells, and mediates PTH-induced bone reabsorption. Administration of vitamin D derivatives promoted osteoclastogenesis in vitro, but inhibited osteoclast fusion and function in vivo.161,165,166 Also, 1,25-(OH)2D3 is a potent inducer of RANKL secretion in immature osteoblasts and a suppressor of osteoprotegerin (OPG) synthesis.167 Through regulation of the RANKL/OPG ratio, vitamin D controls bone remodeling. On the other hand, PTH is well known to enhance differentiation of committed osteoblast precursors, prolonging osteoblast lifespan via inhibition of apoptosis.168 From an immunological perspective, 1,25-(OH)2D3 and intermittent administration of PTH downregulates cytokine production, including IL-6 and TNF-α, especially in postmenopausal women.169,170 Therefore, aside from their physiological roles, both vitamin D and PTH exert direct and indirect influences over the differentiation and function of bone cells to modulate bone remodeling.
Smoking is a dose-dependent risk factor for periodontal disease171 and has also been implicated in osteoporotic bone loss.172 The mechanisms through which cigarette smoking influences periodontal destruction are complex. Evidence is unclear on the effect of smoking on the gingival crevicular fluid cytokine profile, as related to periodontal inflammation.173,174 Nonetheless, a suppressive effect on OPG level in serum and gingival crevicular fluid was observed in smokers.175–178 The resultant imbalance in the RANKL/OPG ratio has been positively associated with increased osteoclastic resorption and bone loss in smoking-related periodontitis patients.
In addition to RANKL-induced destruction of tooth-supporting tissue, there is evidence of smoking-induced oxidative stress caused mainly by increased generation of ROS within gingival tissues.179 The results of screening tests for markers of DNA (8-OHdG) and protein (C-reactive protein) oxidation have been compared with the smoking status of periodontally diseased patients, as well as the formation of antioxidant compounds, such as superoxide dismutase, catalase, and glutathione peroxidase. Recent cohort studies showed that oxidative stress is higher in smokers with chronic periodontitis than in nonsmokers with chronic periodontitis.179,180 Conversely, when the effects of periodontal treatment on oxidative biomarkers were evaluated, a significant interaction between smoking status and salivary superoxide dismutase levels at baseline and after treatment was reported.181 Smokers had significantly lower reductions in superoxide dismutase levels after treatment in comparison with nonsmokers and former smokers. The authors implied that cigarette smoking influences redox homeostasis and alters antioxidant levels in favor of ROS.181 Consistently, superoxide dismutase levels were found to be significantly lower in smokers than in nonsmokers and, most interestingly, the antioxidant levels of heavy smokers differed from those of light smokers, implicating that tobacco consumption influences superoxide dismutase levels in a dose-dependent manner.182 In systemic bone health, smoking elicits similar effects on the RANKL/OPG ratio and, thus, disrupts the balance in bone remodeling.183 In summary, smoking accelerates bone turnover and resorption via increasing RANKL/OPG ratio in both systemic and alveolar bone; smoking is also associated with a build-up of oxidative stress from diminished antioxidant capacity, thereby rendering the periodontal tissue more susceptible to damage.
9. INTERDISCIPLINARY MANAGEMENT AND THERAPEUTIC IMPLICATIONS
While osteoporosis may be considered a risk modifying factor for periodontitis,184 there is insufficient evidence to suggest periodontitis influence systemic BMD. However, the correlation between alveolar bone loss/CAL and systemic BMD could serve as the basis for dentists to screen for potential fracture risk when treating patients with severe periodontitis. Osteoporosis is a “silent bone killer,” which is often undiagnosed until the first osteoporotic fracture. Early detection and diagnosis of osteoporosis will be instrumental for prevention of debilitating fractures. It should be advisable for dentists to identify patients with multiple shared risk factors, such as aging and smoking, and based on the periodontal status, recommend these patients to perform fracture risk assessment with their primary care physicians. Several groups have devised digital assessment tools to analyze intraoral and panoramic radiographs for alveolar bone loss, with the intent that routine dental radiographs could serve as a low-cost tool to screen and predict fracture risk.32,33 Conversely, it should also be recognized that patients with fracture risk beyond the intervention threshold are at greater risk for developing severe periodontitis and undergo tooth loss.185 Routine dental care should be recommended for patients who are under treatment for osteoporosis and possess shared risk factors for periodontitis.
Intriguingly, while osteoporosis and periodontitis are managed independently, treatment for osteoporosis has shown to improve alveolar bone loss and periodontal attachment loss, such as in a longitudinal study on the effect of hormone replacement therapy.186,187 Modifiable, shared risk factors, such as nutritional deficiency and smoking, could be proactively managed via supplementation of vitamin D and smoke cessation programs, respectively. While the optimal dosage is still unclear, the beneficial effect of oral supplementation of vitamin D and/or calcium on periodontal conditions is well supported.188–191 Smoke cessation has significant impact on both prevention of vertebral fracture172,192 and improvement of periodontal conditions.193
Other Food and Drug Administration (FDA) -approved treatment modalities for osteoporosis have, to various extent, shown positive impact on the treatment of periodontitis. Antiresorptive therapies such as oral bisphosphonates, as an adjuvant therapy to non-surgical management of periodontitis, showed promising efficacies.194,195 However, emerging evidence suggests significant risks for complications of medication-related osteonecrosis of the jaw (MRONJ) arising in patients using bisphosphonates or the RANKL antibody Denosumab who undergo invasive dental treatments or even occasionally non-surgical debridement.196,197 As such, the risks for these complications are deterrent for the therapeutic application of antiresorptives in periodontal treatment. Bone anabolic agents such as PTH (teriparatide) is approved for treatment of osteoporosis. Limited evidence on rodent models suggests that PTH administration may also attenuate periodontal inflammation and bone loss, suggesting a promising therapeutic option.198–200 Another bone anabolic agent Romosozumab, a SOST antibody was recently approved for osteoporosis treatment. In rodent models, Romosozumab stimulated periodontal bone regeneration after experimental periodontitis and promoted peri-implant osseointegration.201–204 While these anabolic agents could restore the inflammation-induced uncoupling of bone remodeling, further studies are required to assess their potentials in periodontal treatment as an adjunct therapy.
10. CONCLUSION
Osteoporosis and periodontitis are both inflammation-driven, age-related bone disorders. Increasing evidence strongly supports a correlation between systemic and alveolar bone loss, while moderately suggesting a correlation between systemic BMD and periodontal attachment loss. In both diseases, age-related oxidative stress and senescence are underlying mechanisms that drive a pro-inflammatory tissue microenvironment, thereby causing an uncoupling of bone remodeling process. These mechanistic links are at play in their shared risk factors including vitamin D deficiency and smoking. Understanding these factors and their interplay calls for well-controlled longitudinal studies to examine the interdisciplinary management and potential therapeutics to address both diseases.
REFERENCES
- 1.Demmer RT, Papapanou PN. Epidemiologic patterns of chronic and aggressive periodontitis. Periodontol 2000 2010;53:28–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Academy of Periodontology Task Force Report on the Update to the 1999 Classification of Periodontal Diseases and Conditions. J Periodontol 1999;2015(86):835–838. [DOI] [PubMed] [Google Scholar]
- 3.Genco RJ. Current view of risk factors for periodontal diseases. J Periodontol 1996;67:1041–1049. [DOI] [PubMed] [Google Scholar]
- 4.Eke PI, Thornton-Evans GO, Wei L, Borgnakke WS, Dye BA, Genco RJ. Periodontitis in US adults: national health and nutrition examination survey 2009–2014. J Am Dent Assoc 2018;149:576–588. e576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reginster JY, Burlet N. Osteoporosis: a still increasing prevalence. Bone 2006;38:S4–9. [DOI] [PubMed] [Google Scholar]
- 6.Kawai M, Modder UI, Khosla S, Rosen CJ. Emerging therapeutic opportunities for skeletal restoration. Nat Rev Drug Discov 2011;10:141–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Mineral Res 2007;22:465–475. [DOI] [PubMed] [Google Scholar]
- 8.Groen JJ, Duyvensz F, Halsted JA. Diffuse alveolar atrophy of the jaw (non-inflammatory form of paradental disease) and pre-senile osteoporosis. Gerontol Clin 1960;2:68–86. [DOI] [PubMed] [Google Scholar]
- 9.Armitage GC. Development of a classification system for periodontal diseases and conditions. Northwest Dent 2000;79:31–35. [PubMed] [Google Scholar]
- 10.Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J Periodontol 2018;89(Suppl 1):S159–s172. [DOI] [PubMed] [Google Scholar]
- 11.Caton JG, Armitage G, Berglundh T, et al. A new classification scheme for periodontal and peri-implant diseases and conditions - Introduction and key changes from the 1999 classification. J Periodontol 2018;89(Suppl 1):s1–s8. [DOI] [PubMed] [Google Scholar]
- 12.Ram G, Chinen J. Infections and immunodeficiency in Down syndrome. Clin Exp Immunol 2011;164:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vieira AR, Albandar JM. Role of genetic factors in the pathogenesis of aggressive periodontitis. Periodontol 2000 2014;65:92–106. [DOI] [PubMed] [Google Scholar]
- 14.Albandar JM, Susin C, Hughes FJ. Manifestations of systemic diseases and conditions that affect the periodontal attachment apparatus: Case definitions and diagnostic considerations. J Periodontol 2018;89(Suppl 1):s183–s203. [DOI] [PubMed] [Google Scholar]
- 15.Papapanou PN, Sanz M, Buduneli N, et al. Periodontitis: consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J Clin Periodontol 2018;45:S162–S170. [DOI] [PubMed] [Google Scholar]
- 16.Kanis JA. Diagnosis of osteoporosis and assessment of fracture risk. Lancet (London, England) 2002;359:1929–1936. [DOI] [PubMed] [Google Scholar]
- 17.Okabe S, Morimoto Y, Ansai T, et al. Assessment of the relationship between the mandibular cortex on panoramic radiographs and the risk of bone fracture and vascular disease in 80-year-olds. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008;106:433–442. [DOI] [PubMed] [Google Scholar]
- 18.Brennan RM, Genco RJ, Hovey KM, Trevisan M, Wactawski-Wende J. Clinical attachment loss, systemic bone density, and subgingival calculus in postmenopausal women. J Periodontol 2007;78:2104–2111. [DOI] [PubMed] [Google Scholar]
- 19.Ishii K, Taguchi A, Nakamoto T, et al. Diagnostic efficacy of alveolar bone loss of the mandible for identifying postmenopausal women with femoral osteoporosis. Dentomaxillofac Radiol 2007;36:28–33. [DOI] [PubMed] [Google Scholar]
- 20.Taguchi A, Ohtsuka M, Tsuda M, et al. Risk of vertebral osteoporosis in post-menopausal women with alterations of the mandible. Dentomaxillofac Radiol 2007;36:143–148. [DOI] [PubMed] [Google Scholar]
- 21.Takaishi Y, Okamoto Y, Ikeo T, et al. Correlations between periodontitis and loss of mandibular bone in relation to systemic bone changes in postmenopausal Japanese women. Osteoporos Int 2005;16:1875–1882. [DOI] [PubMed] [Google Scholar]
- 22.Hildebolt CF, Pilgram TK, Yokoyama-Crothers N, et al. The pattern of alveolar crest height change in healthy postmenopausal women after 3 years of hormone/estrogen replacement. Therapy 2002;73:1279–1284. [DOI] [PubMed] [Google Scholar]
- 23.Jonasson G, Bankvall G, Kiliaridis S. Estimation of skeletal bone mineral density by means of the trabecular pattern of the alveolar bone, its interdental thickness, and the bone mass of the mandible. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol 2001;92:346–352. [DOI] [PubMed] [Google Scholar]
- 24.Tezal M, Wactawski-Wende J, Grossi SG, Ho AW, Dunford R, Genco RJ. The relationship between bone mineral density and periodontitis in postmenopausal women. J Periodontol 2000;71:1492–1498. [DOI] [PubMed] [Google Scholar]
- 25.Taguchi A, Suei Y, Ohtsuka M, Otani K, Tanimoto K, Hollender LG. Relationship between bone mineral density and tooth loss in elderly Japanese women. Dentomaxillofac Radiol 1999;28:219–223. [DOI] [PubMed] [Google Scholar]
- 26.Payne JB, Reinhardt RA, Nummikoski PV, Patil KD. Longitudinal alveolar bone loss in postmenopausal osteoporotic/osteopenic women. Osteoporos Int 1999;10:34–40. [DOI] [PubMed] [Google Scholar]
- 27.Hausmann E, Allen K, Carpio L, Christersson LA, Clerehugh V. Computerized methodology for detection of alveolar crestal bone loss from serial intraoral radiographs. J Periodontol 1992;63:657–662. [DOI] [PubMed] [Google Scholar]
- 28.von Wowern N Bone mass of mandibles. In vitro and in vivo analyses. Dan Med Bull 1986;33:23–44. [PubMed] [Google Scholar]
- 29.Horner K, Devlin H, Harvey L. Detecting patients with low skeletal bone mass. J Dent 2002;30:171–175. [DOI] [PubMed] [Google Scholar]
- 30.Devlin H, Allen PD, Graham J, et al. Automated osteoporosis risk assessment by dentists: a new pathway to diagnosis. Bone 2007;40:835–842. [DOI] [PubMed] [Google Scholar]
- 31.Jonasson G, Sundh V, Hakeberg M, Hassani-Nejad A, Lissner L, Ahlqwist M. Mandibular bone changes in 24 years and skeletal fracture prediction. Clin Oral Invest 2013;17:565–572. [DOI] [PubMed] [Google Scholar]
- 32.Jonasson G, Sundh V, Ahlqwist M, Hakeberg M, Björkelund C, Lissner L. A prospective study of mandibular trabecular bone to predict fracture incidence in women: a low-cost screening tool in the dental clinic. Bone 2011;49:873–879. [DOI] [PubMed] [Google Scholar]
- 33.Hassani-Nejad A, Ahlqwist M, Hakeberg M, Jonasson G. Mandibular trabecular bone as fracture indicator in 80-year-old men and women. Eur J Oral Sci 2013;121:525–531. [DOI] [PubMed] [Google Scholar]
- 34.Lindh C, Horner K, Jonasson G, et al. The use of visual assessment of dental radiographs for identifying women at risk of having osteoporosis: the OSTEODENT project. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008;106:285–293. [DOI] [PubMed] [Google Scholar]
- 35.Jonasson G, Jonasson L, Kiliaridis S. Skeletal bone mineral density in relation to thickness, bone mass, and structure of the mandibular alveolar process in dentate men and women. Eur J Oral Sci 2007;115:117–123. [DOI] [PubMed] [Google Scholar]
- 36.Lindh C, Obrant K, Petersson A. Maxillary bone mineral density and its relationship to the bone mineral density of the lumbar spine and hip. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2004;98:102–109. [DOI] [PubMed] [Google Scholar]
- 37.Elders PJ, Habets LL, Netelenbos JC, van der Linden LW, van der Stelt PF. The relation between periodontitis and systemic bone mass in women between 46 and 55 years of age. J Clin Periodontol 1992;19:492–496. [DOI] [PubMed] [Google Scholar]
- 38.Jacobs R, Ghyselen J, Koninckx P, van Steenberghe D. Long-term bone mass evaluation of mandible and lumbar spine in a group of women receiving hormone replacement therapy. Eur J Oral Sci 1996;104:10–16. [DOI] [PubMed] [Google Scholar]
- 39.Mashalkar VN, Suragimath G, Zope SA, Varma SA. A cross-sectional study to assess and correlate osteoporosis and periodontitis among postmenopausal women: a dual energy X-ray absorptiometry study. J Mid-Life Health 2018;9:2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Passos-Soares JS, Vianna MIP, Gomes-Filho IS, et al. Association between osteoporosis treatment and severe periodontitis in post-menopausal women. Menopause (New York, NY) 2017;24:789–795. [DOI] [PubMed] [Google Scholar]
- 41.Penoni DC, Torres SR, Farias ML, Fernandes TM, Luiz RR, Leão AT. Association of osteoporosis and bone medication with the periodontal condition in elderly women. Osteoporos Int 2016;27:1887–1896. [DOI] [PubMed] [Google Scholar]
- 42.Juluri R, Prashanth E, Gopalakrishnan D, et al. Association of post-menopausal osteoporosis and periodontal disease: a double-blind case-control study. J Int Oral Health 2015;7:119–123. [PMC free article] [PubMed] [Google Scholar]
- 43.Singh A, Sharma RK, Siwach RC, Tewari S, Narula SC. Association of bone mineral density with periodontal status in postmeno-pausal women. J Invest Clin Dent 2014;5:275–282. [DOI] [PubMed] [Google Scholar]
- 44.Tak I-H, Shin M-H, Kweon S-S, et al. The association between periodontal disease, tooth loss and bone mineral density in a Korean population. J Clin Periodontol 2014;41:1139–1144. [DOI] [PubMed] [Google Scholar]
- 45.Gondim V, Aun J, Fukuda CT, et al. Severe loss of clinical attachment level: an independent association with low hip bone mineral density in postmenopausal females. J Periodontol 2013;84:352–359. [DOI] [PubMed] [Google Scholar]
- 46.Passos JS, Vianna MI, Gomes-Filho IS, et al. Osteoporosis/osteopenia as an independent factor associated with periodontitis in postmenopausal women: a case-control study. Osteoporos Int 2013;24:1275–1283. [DOI] [PubMed] [Google Scholar]
- 47.Iwasaki M, Taylor GW, Nakamura K, Yoshihara A, Miyazaki H. Association between low bone mineral density and clinical attachment loss in Japanese postmenopausal females. J Periodontol 2013;84:1708–1716. [DOI] [PubMed] [Google Scholar]
- 48.Marjanovic EJ, Southern HN, Coates P, et al. Do patients with osteoporosis have an increased prevalence of periodontal disease? A cross-sectional study. Osteoporos Int 2013;24:1973–1979. [DOI] [PubMed] [Google Scholar]
- 49.Moeintaghavi A, Pourjavad M, Dadgar S, Tabbakh NS. Evaluation of the association between periodontal parameters, osteoporosis and osteopenia in post menopausal women. J Dent (Tehran, Iran) 2013;10:443–448. [PMC free article] [PubMed] [Google Scholar]
- 50.Grocholewicz K, Bohatyrewicz A. Oral health and bone mineral density in postmenopausal women. Arch Oral Biol 2012;57:245–251. [DOI] [PubMed] [Google Scholar]
- 51.Al Habashneh R, Alchalabi H, Khader YS, Hazza’a AM, Odat Z, Johnson GK. Association between periodontal disease and osteoporosis in postmenopausal women in Jordan. J Periodontol 2010;81:1613–1621. [DOI] [PubMed] [Google Scholar]
- 52.Gomes-Filho IS, Passos JDS, Cruz SS, et al. The association between postmenopausal osteoporosis and periodontal disease. J Periodontol 2007;78:1731–1740. [DOI] [PubMed] [Google Scholar]
- 53.Taguchi A, Fujiwara S, Masunari N, Suzuki G. Self-reported number of remaining teeth is associated with bone mineral density of the femoral neck, but not of the spine, in Japanese men and women. Osteoporos Int 2004;15:842–846. [DOI] [PubMed] [Google Scholar]
- 54.Mohammad AR, Hooper DA, Vermilyea SG, Mariotti A, Preshaw PM. An investigation of the relationship between systemic bone density and clinical periodontal status in post-menopausal Asian-American women. Int Dent J 2003;53:121–125. [DOI] [PubMed] [Google Scholar]
- 55.Pilgram TK, Hildebolt CF, Dotson M, et al. Relationships between clinical attachment level and spine and hip bone mineral density: data from healthy postmenopausal women. J Periodontol 2002;73:298–301. [DOI] [PubMed] [Google Scholar]
- 56.Lundström A, Jendle J, Stenström B, Toss G, Ravald N. Periodontal conditions in 70-year-old women with osteoporosis. Swed Dent J 2001;25:89–96. [PubMed] [Google Scholar]
- 57.Weyant RJ, Pearlstein ME, Churak AP, Forrest K, Famili P, Cauley JA. The association between osteopenia and periodontal attachment loss in older women. J Periodontol 1999;70:982–991. [DOI] [PubMed] [Google Scholar]
- 58.Mohammad AR, Bauer RL, Yeh CK. Spinal bone density and tooth loss in a cohort of postmenopausal women. Int J Prosthodont 1997;10:381–385. [PubMed] [Google Scholar]
- 59.Hildebolt CF, Pilgram TK, Dotson M, et al. Attachment loss with postmenopausal age and smoking. J Periodont Res 1997;32:619–625. [DOI] [PubMed] [Google Scholar]
- 60.Mohammad AR, Brunsvold M, Bauer R. The strength of association between systemic postmenopausal osteoporosis and periodontal disease. Int J Prosthodont 1996;9:479–483. [PubMed] [Google Scholar]
- 61.Penoni DC, Fidalgo TK, Torres SR, et al. Bone density and clinical periodontal attachment in postmenopausal women: a systematic review and meta-analysis. J Dent Res 2017;96:261–269. [DOI] [PubMed] [Google Scholar]
- 62.Martínez-Maestre M, González-Cejudo C, Machuca G, Torrejón R, Castelo-Branco C. Periodontitis and osteoporosis: a systematic review. Climacteric 2010;13:523–529. [DOI] [PubMed] [Google Scholar]
- 63.Nicopoulou-Karayianni K, Tzoutzoukos P, Mitsea A, et al. Tooth loss and osteoporosis: the OSTEODENT study. J Clin Periodontol 2009;36:190–197. [DOI] [PubMed] [Google Scholar]
- 64.Krall EA, Garcia RI, Dawson-Hughes B. Increased risk of tooth loss is related to bone loss at the whole body, hip, and spine. Calcif Tissue Int 1996;59:433–437. [DOI] [PubMed] [Google Scholar]
- 65.Drozdzowska B, Pluskiewicz W, Michno M. Tooth count in elderly women in relation to their skeletal status. Maturitas 2006;55:126–131. [DOI] [PubMed] [Google Scholar]
- 66.Krall EA, Dawson-Hughes B, Papas A, Garcia RI. Tooth loss and skeletal bone density in healthy postmenopausal women. Osteoporos Int 1994;4:104–109. [DOI] [PubMed] [Google Scholar]
- 67.May H, Reader R, Murphy S, Khaw KT. Self-reported tooth loss and bone mineral density in older men and women. Age Ageing 1995;24:217–221. [DOI] [PubMed] [Google Scholar]
- 68.Hajishengallis G Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol 2014;35:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu B, Wang C-Y. Osteoporosis: the result of an ‘aged’ bone micro-environment. Trends Mol Med 2016;22:641–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Graves D Cytokines that promote periodontal tissue destruction. J Periodontol 2008;79:1585–1591. [DOI] [PubMed] [Google Scholar]
- 71.Sczepanik FSC, Grossi ML, Casati M, et al. Periodontitis is an inflammatory disease of oxidative stress: We should treat it that way. Periodontol 2000 2020;84:45–68. [DOI] [PubMed] [Google Scholar]
- 72.Mahanonda R, Pichyangkul S. Toll-like receptors and their role in periodontal health and disease. Periodontol 2000 2007;43(1):41–55. [DOI] [PubMed] [Google Scholar]
- 73.Kawai T, Matsuyama T, Hosokawa Y, et al. B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am J Pathol 2006;169:987–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Teng Y-TA, Nguyen H, Gao X, et al. Functional human T-cell immunity and osteoprotegerin ligand control alveolar bone destruction in periodontal infection. J Clin Investig 2000;106:R59–R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pacios S, Xiao W, Mattos M, et al. Osteoblast lineage cells play an essential role in periodontal bone loss through activation of nuclear factor-kappa B. Sci Rep 2015;5:16694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chang J, Wang Z, Tang E, et al. Inhibition of osteoblastic bone formation by nuclear factor-kappaB. Nat Med 2009;15:682–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jimi E, Aoki K, Saito H, et al. Selective inhibition of NF-[kappa] B blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nat Med 2004;10:617–624. [DOI] [PubMed] [Google Scholar]
- 78.Redlich K, Görtz B, Hayer S, et al. Repair of local bone erosions and reversal of systemic bone loss upon therapy with anti-tumor necrosis factor in combination with osteoprotegerin or parathy-roid hormone in tumor necrosis factor-mediated arthritis. Am J Pathol 2004;164:543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Haugeberg G, Conaghan PG, Quinn M, Emery P. Bone loss in patients with active early rheumatoid arthritis: infliximab and methotrexate compared with methotrexate treatment alone. Explorative analysis from a 12-month randomised, double-blind, placebo-controlled study. Ann Rheum Dis 2009;68:1898–1901. [DOI] [PubMed] [Google Scholar]
- 80.Ghosh S, Karin M. Missing Pieces in the NF-κB Puzzle. Cell 2002;109(2):S81–S96. [DOI] [PubMed] [Google Scholar]
- 81.Wang CY, Guttridge DC, Mayo MW, Baldwin AS Jr. NF-kappaB induces expression of the Bcl-2 homologue A1/Bfl-1 to prefer-entially suppress chemotherapy-induced apoptosis. Mol Cell Biol 1999;19:5923–5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang CY, Mayo MW, Baldwin AS Jr. TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science 1996;274:784–787. [DOI] [PubMed] [Google Scholar]
- 83.Franzoso G, Carlson L, Xing L, et al. Requirement for NF-κB in osteoclast and B-cell development. Genes & Development 1997;11(24):3482–3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ruocco MG, Maeda S, Park JM, et al. IκB kinase (IKK)β, but not IKKα, is a critical mediator of osteoclast survival and is required for inflammation-induced bone loss. J Exp Med 2005;201:1677–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee HJ, Kang IK, Chung CP, Choi SM. The subgingival microflora and gingival crevicular fluid cytokines in refractory periodontitis. J Clin Periodontol 1995;22:885–890. [DOI] [PubMed] [Google Scholar]
- 86.Delima AJ, Karatzas S, Amar S, Graves DT. Inflammation and tissue loss caused by periodontal pathogens is reduced by interleukin-1 antagonists. J Infect Dis 2002;186:511–516. [DOI] [PubMed] [Google Scholar]
- 87.Chiang CY, Kyritsis G, Graves DT, Amar S. Interleukin-1 and tumor necrosis factor activities partially account for calvarial bone resorption induced by local injection of lipopolysaccharide. Infect Immun 1999;67:4231–4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dayan S, Stashenko P, Niederman R, Kupper TS. Oral epithelial overexpression of IL-1alpha causes periodontal disease. J Dent Res 2004;83:786–790. [DOI] [PubMed] [Google Scholar]
- 89.Gaspersic R, Stiblar-Martincic D, Osredkar J, Skaleric U. Influence of subcutaneous administration of recombinant TNF-alpha on ligature-induced periodontitis in rats. J Periodontal Res 2003;38:198–203. [DOI] [PubMed] [Google Scholar]
- 90.Garlet GP, Cardoso CRB, Campanelli AP, et al. The dual role of p55 tumour necrosis factor-α receptor in Actinobacillus actinomycetemcomitans-induced experimental periodontitis: host protection and tissue destruction. Clin Exp Immuno 2006;147(1):128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. TNF-α induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest 2000;106:1481–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pacifici R, Rifas L, McCracken R, Avioli LV. The role of interleukin-1 in postmenopausal bone loss. Exp Gerontol 1990;25:309–316. [DOI] [PubMed] [Google Scholar]
- 93.Barbour KE, Lui LY, Ensrud KE, et al. Inflammatory markers and risk of hip fracture in older white women: the study of osteoporotic fractures. J Bone Mineral Res 2014;29:2057–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pacifici R Estrogen, cytokines, and pathogenesis of postmenopausal osteoporosis. J Bone Mineral Res 1996;11:1043–1051. [DOI] [PubMed] [Google Scholar]
- 95.Hajishengallis G, Reis ES, Mastellos DC, Ricklin D, Lambris JD. Novel mechanisms and functions of complement. Nat Immunol 2017;18:1288–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang X, Kimura Y, Fang C, et al. Regulation of Toll-like receptor–mediated inflammatory response by complement in vivo. Blood 2007;110:228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hajishengallis G, Kajikawa T, Hajishengallis E, et al. Complement-dependent mechanisms and interventions in periodontal disease. Front Immunol 2019;10. doi: 10.3389/fimmu.2019.00406 [DOI] [PMC free article] [PubMed]
- 98.Courts FJ, Boackle RJ, Fudenberg HH, Silverman M. Detection of functional complement components in gingival crevicular fluid from humans with periodontal disease. J Dent Res 1977;56:327–331. [DOI] [PubMed] [Google Scholar]
- 99.Patters M, Niekrash C, Lang NP. Assessment of complement cleavage in gingival fluid during experimental gingivitis in man. J Clin Periodontol 1989;16:33–37. [DOI] [PubMed] [Google Scholar]
- 100.Niekrash CE, Patters MR. Simultaneous assessment of complement components C3, C4, and B and their cleavage products in human gingival fluid: II. Longitudinal changes during periodontal therapy. J Periodontal Res 1985;20:268–275. [DOI] [PubMed] [Google Scholar]
- 101.Maekawa T, Abe T, Hajishengallis E, et al. Genetic and intervention studies implicating complement C3 as a major target for the treatment of periodontitis. J Immunol 2014;192:6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.MacKay DL, Kean TJ, Bernardi KG, et al. Reduced bone loss in a murine model of postmenopausal osteoporosis lacking complement component 3. J Orthopaedic Res 2018;3(36):118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Matsuo K, Owens JM, Tonko M, Elliott C, Chambers TJ, Wagner EF. Fosl1 is a transcriptional target of c-Fos during osteoclast differentiation. Nat Genet 2000;24:184–187. [DOI] [PubMed] [Google Scholar]
- 104.Redlich K, Smolen JS. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat Rev Drug Discov 2012;11:234–250. [DOI] [PubMed] [Google Scholar]
- 105.Takayanagi H, Kim S, Koga T, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell 2002;3:889–901. [DOI] [PubMed] [Google Scholar]
- 106.Teitelbaum SL. Osteoclasts: what do they do and how do they do it? Am J Pathol 2007;170:427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee SK, Lorenzo JA. Parathyroid hormone stimulates TRANCE and inhibits osteoprotegerin messenger ribonucleic acid expression in murine bone marrow cultures: correlation with osteoclast-like cell formation. Endocrinology 1999;140:3552–3561. [DOI] [PubMed] [Google Scholar]
- 108.Horwood NJ, Elliott J, Martin TJ, Gillespie MT. Osteotropic agents regulate the expression of osteoclast differentiation factor and osteoprotegerin in osteoblastic stromal cells. Endocrinology 1998;139:4743–4746. [DOI] [PubMed] [Google Scholar]
- 109.Han JH, Choi SJ, Kurihara N, Koide M, Oba Y, Roodman GD. Macrophage inflammatory protein-1alpha is an osteoclastogenic factor in myeloma that is independent of receptor activator of nuclear factor kappaB ligand. Blood 2001;97:3349–3353. [DOI] [PubMed] [Google Scholar]
- 110.Gilbert L, He X, Farmer P, et al. Expression of the osteoblast differentiation factor RUNX2 (Cbfa1/AML3/Pebp2αA) is inhibited by tumor necrosis factor-α. J Biol Chem 2002;277:2695–2701. [DOI] [PubMed] [Google Scholar]
- 111.Kaneki H, Guo R, Chen D, et al. Tumor necrosis factor promotes Runx2 degradation through up-regulation of Smurf1 and Smurf2 in osteoblasts. J Biol Chem 2006;281:4326–4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chang J, Liu F, Lee M, et al. NF-kappaB inhibits osteogenic differentiation of mesenchymal stem cells by promoting beta-catenin degradation. Proc Natl Acad Sci USA 2013;110:9469–9474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Diarra D, Stolina M, Polzer K, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Med 2007;13:156–163. [DOI] [PubMed] [Google Scholar]
- 114.Li X, Zhang Y, Kang H, et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem 2005;280:19883–19887. [DOI] [PubMed] [Google Scholar]
- 115.Mundy GR. Osteoporosis and inflammation. Nutr Rev 2007;65:S147–S151. [DOI] [PubMed] [Google Scholar]
- 116.Ginaldi L, Di Benedetto M, De Martinis M. Osteoporosis, inflammation and ageing. Immun Ageing 2005;2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lin CL, Moniz C, Chambers TJ, Chow JW. Colitis causes bone loss in rats through suppression of bone formation. Gastroenterology 1996;111:1263–1271. [DOI] [PubMed] [Google Scholar]
- 118.Khosla S, Melton LJ 3rd, Riggs BL. The unitary model for estrogen deficiency and the pathogenesis of osteoporosis: is a revision needed? J Bone Mineral Res 2011;26:441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell 2013;153:1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Manolagas SC, Parfitt AM. What old means to bone. Trends Endocrinol Metab 2010;21:369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tilstra JS, Robinson AR, Wang J, et al. NF-κB inhibition delays DNA damage–induced senescence and aging in mice. J Clin Investig 2012;122:2601–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yu B, Huo L, Liu Y, et al. PGC-1α controls skeletal stem cell fate and bone-fat balance in osteoporosis and skeletal aging by inducing TAZ. Cell Stem Cell 2018;23:193–209.e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Persson GR. Periodontal complications with age. Periodontol 2000 2018;78(1):185–194. [DOI] [PubMed] [Google Scholar]
- 124.Papapanou PN, Lindhe J. Preservation of probing attachment and alveolar bone levels in 2 random population samples. J Clin Periodontol 1992;19:583–588. [DOI] [PubMed] [Google Scholar]
- 125.Hajishengallis G Aging and its impact on innate immunity and inflammation: implications for periodontitis. J Oral Biosci 2014;56:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wu Y, Dong G, Xiao W, et al. Effect of aging on periodontal inflammation, microbial colonization, and disease susceptibility. J Dent Res 2016;95:460–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bodineau A, Folliguet M, Séguier S. Tissular senescence and modifications of oral ecosystem in the elderly: risk factors for mucosal pathologies. Curr Aging Sci 2009;2:109–120. [DOI] [PubMed] [Google Scholar]
- 128.Fransson C, Mooney J, Kinane DF, Berglundh T. Differences in the inflammatory response in young and old human subjects during the course of experimental gingivitis. J Clin Periodontol 1999;26:453–460. [DOI] [PubMed] [Google Scholar]
- 129.Ebersole JL, Steffen MJ, Gonzalez-Martinez J, Novak MJ. Effects of age and oral disease on systemic inflammatory and immune parameters in nonhuman primates. Clin Vaccine Immunol CVI 2008;15:1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Manolagas SC. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocrinol Rev 2010;31:266–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Newmeyer DD, Ferguson-Miller S. Mitochondria: releasing power for life and unleashing the machineries of death. Cell 2003;112:481–490. [DOI] [PubMed] [Google Scholar]
- 132.Almeida M, Han L, Martin-Millan M, et al. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem 2007;282:27285–27297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Goettsch C, Babelova A, Trummer O, et al. NADPH oxidase 4 limits bone mass by promoting osteoclastogenesis. J Clin Invest 2013;123:4731–4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lean JM, Davies JT, Fuller K, et al. A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J Clin Invest 2003;112:915–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bartell SM, Kim H-N, Ambrogini E, et al. FoxO proteins restrain osteoclastogenesis and bone resorption by attenuating H2O2 accumulation. Nat Commun 2014;5:3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ambrogini E, Almeida M, Martin-Millan M, et al. FoxO-mediated defense against oxidative stress in osteoblasts is indispensable for skeletal homeostasis in mice. Cell Metab 2010;11:136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cervellati C, Bonaccorsi G, Cremonini E, et al. Oxidative stress and bone resorption interplay as a possible trigger for postmenopausal osteoporosis. Biomed Res Int 2014;2014:569563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chapple IL, Matthews J. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol 2000 2007;43:160–232. [DOI] [PubMed] [Google Scholar]
- 139.Wang Y, Andrukhov O, Rausch-Fan X. Oxidative stress and antioxidant system in periodontitis. Front Physiol 2017;8:910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chapple IL, Matthews JB. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol 2000 2007;43(1):160–232. [DOI] [PubMed] [Google Scholar]
- 141.Konopka T, Król K, Kopeć W, Gerber H. Total antioxidant status and 8-hydroxy-2’-deoxyguanosine levels in gingival and peripheral blood of periodontitis patients. Arch Immunol Ther Exp 2007;55:417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Su H, Gornitsky M, Velly AM, Yu H, Benarroch M, Schipper HM. Salivary DNA, lipid, and protein oxidation in nonsmokers with periodontal disease. Free Radic Biol Med 2009;46:914–921. [DOI] [PubMed] [Google Scholar]
- 143.Löe H Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care 1993;16:329–334. [PubMed] [Google Scholar]
- 144.Aquino-Martinez R, Rowsey JL, Fraser DG, et al. LPS-induced premature osteocyte senescence: Implications in inflammatory alveolar bone loss and periodontal disease pathogenesis. Bone 2020;132:115220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Baker DJ, Wijshake T, Tchkonia T, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011;479:232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Farr JN, Xu M, Weivoda MM, et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat Med 2017;23:1072–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Palmer AK, Tchkonia T, LeBrasseur NK, Chini EN, Xu M, Kirkland JL. Cellular senescence in type 2 diabetes: a therapeutic opportunity. Diabetes 2015;64:2289–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhou Bo O, Yue R, Murphy Malea M, Peyer JG, Morrison SJ. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 2014;15:154–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bianco P, Cao X, Frenette PS, et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med 2013;19:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bianco P, Robey PG. Skeletal stem cells. Development 2015;142:1023–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Oh J, Lee YD, Wagers AJ. Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nat Med 2014;20:870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li H, Liu P, Xu S, et al. FOXP1 controls mesenchymal stem cell commitment and senescence during skeletal aging. J Clin Invest 2017;127:1241–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liu W, Zhang L, Xuan K, et al. Alpl prevents bone ageing sensitivity by specifically regulating senescence and differentiation in mesenchymal stem cells. Bone Res 2018;6:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Farr JN, Fraser DG, Wang H, et al. Identification of senescent cells in the bone microenvironment. J Bone Miner Res 2016;31:1920–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kim HN, Chang J, Iyer S, et al. Elimination of senescent osteoclast progenitors has no effect on the age-associated loss of bone mass in mice. Aging Cell 2019;18:e12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pignolo RJ, Samsonraj RM, Law SF, Wang H, Chandra A. Targeting cell senescence for the treatment of age-related bone loss. Curr Osteoporos Rep 2019;17:70–85. [DOI] [PubMed] [Google Scholar]
- 157.Zhang P, Wang Q, Nie L, et al. Hyperglycemia-induced inflammaging accelerates gingival senescence via NLRC4 phosphorylation. J Biol Chem 2019;294:18807–18819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supple-mentation: a meta-analysis of randomized controlled trials. J Am Med Assoc 2005;293:2257–2264. [DOI] [PubMed] [Google Scholar]
- 159.Shimazaki Y, Shirota T, Uchida K, et al. Intake of dairy products and periodontal disease: the Hisayama Study. J Periodontol 2008;79:131–137. [DOI] [PubMed] [Google Scholar]
- 160.Al-Zahrani MS. Increased intake of dairy products is related to lower periodontitis prevalence. J Periodontol 2006;77:289–294. [DOI] [PubMed] [Google Scholar]
- 161.Yoshida T, Stern PH. How vitamin D works on bone. Endocrinol Metab Clin North Am 2012;41:557–569. [DOI] [PubMed] [Google Scholar]
- 162.Bischoff-Ferrari HA, Dietrich T, Orav EJ, Dawson-Hughes B. Positive association between 25-hydroxy vitamin D levels and bone mineral density: a population-based study of younger and older adults. Am J Med 2004;116:634–639. [DOI] [PubMed] [Google Scholar]
- 163.Laird E, Ward M, McSorley E, Strain JJ, Wallace J. Vitamin D and bone health: potential mechanisms. Nutrients 2010;2:693–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kliewer SA, Umesono K, Mangelsdorf DJ, Evans RM. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature 1992;355:446–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Takahashi N, Udagawa N, Suda T. Vitamin D endocrine system and osteoclasts. Bonekey Rep 2014;3:495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zarei A, Morovat A, Javaid K, Brown CP. Vitamin D receptor expression in human bone tissue and dose-dependent activation in resorbing osteoclasts. Bone Res 2016;4:16030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Baldock PA, Thomas GP, Hodge JM, et al. Vitamin D action and regulation of bone remodeling: suppression of osteoclastogenesis by the mature osteoblast. J Bone Miner Res 2006;21:1618–1626. [DOI] [PubMed] [Google Scholar]
- 168.Jilka RL, Weinstein RS, Bellido T, Roberson P, Parfitt AM, Manolagas SC. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest 1999;104:439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Müller K, Haahr PM, Diamant M, Rieneck K, Kharazmi A, Bendtzen K. 1,25-Dihydroxyvitamin D3 inhibits cytokine production by human blood monocytes at the post-transcriptional level. Cytokine 1992;4:506–512. [DOI] [PubMed] [Google Scholar]
- 170.Inanir A, Ozoran K, Tutkak H, Mermerci B. The effects of calcitriol therapy on serum interleukin-1, interleukin-6 and tumour necrosis factor-alpha concentrations in post-menopausal patients with osteoporosis. J Int Med Res 2004;32:570–582. [DOI] [PubMed] [Google Scholar]
- 171.Grossi SG, Genco RJ, Machtei EE, et al. Assessment of risk for periodontal disease. II. Risk indicators for alveolar bone loss. J Periodontol 1995;66:23–29. [DOI] [PubMed] [Google Scholar]
- 172.Kanis JA, Johnell O, Oden A, et al. Smoking and fracture risk: a meta-analysis. Osteoporos Int 2005;16:155–162. [DOI] [PubMed] [Google Scholar]
- 173.Giannopoulou C, Kamma JJ, Mombelli A. Effect of inflammation, smoking and stress on gingival crevicular fluid cytokine level. J Clin Periodontol 2003;30:145–153. [DOI] [PubMed] [Google Scholar]
- 174.Tymkiw KD, Thunell DH, Johnson GK, et al. Influence of smoking on gingival crevicular fluid cytokines in severe chronic periodontitis. J Clin Periodontol 2011;38:219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Buduneli N, Buduneli E, Kütükçüler N. Interleukin-17, RANKL, and osteoprotegerin levels in gingival crevicular fluid from smoking and non-smoking patients with chronic periodontitis during initial periodontal treatment. J Periodontol 2009;80:1274–1280. [DOI] [PubMed] [Google Scholar]
- 176.César-Neto JB, Duarte PM, de Oliveira MC, Tambeli CH, Sallum EA, Nociti FH Jr. Smoking modulates interleukin-6:interleukin-10 and RANKL:osteoprotegerin ratios in the periodontal tissues. J Periodontal Res 2007;42:184–191. [DOI] [PubMed] [Google Scholar]
- 177.Lappin DF, Sherrabeh S, Jenkins WM, Macpherson LM. Effect of smoking on serum RANKL and OPG in sex, age and clinically matched supportive-therapy periodontitis patients. J Clin Periodontol 2007;34:271–277. [DOI] [PubMed] [Google Scholar]
- 178.Tang TH, Fitzsimmons TR, Bartold PM. Effect of smoking on concentrations of receptor activator of nuclear factor kappa B ligand and osteoprotegerin in human gingival crevicular fluid. J Clin Periodontol 2009;36:713–718. [DOI] [PubMed] [Google Scholar]
- 179.Aziz AS, Kalekar MG, Suryakar AN, et al. Assessment of some bio-chemical oxidative stress markers in male smokers with chronic periodontitis. Indian J Clin Biochem IJCB 2013;28:374–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fredriksson MI, Figueredo CM, Gustafsson A, Bergström KG, Asman BE. Effect of periodontitis and smoking on blood leukocytes and acute-phase proteins. J Periodontol 1999;70:1355–1360. [DOI] [PubMed] [Google Scholar]
- 181.Chang CH, Han ML, Teng NC, et al. Cigarette smoking aggravates the activity of periodontal disease by disrupting redox homeostasisan observational study. Sci Rep 2018;8:11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Agnihotri R, Pandurang P, Kamath SU, et al. Association of cigarette smoking with superoxide dismutase enzyme levels in subjects with chronic periodontitis. J Periodontol 2009;80:657–662. [DOI] [PubMed] [Google Scholar]
- 183.Al-Bashaireh AM, Haddad LG, Weaver M, Chengguo X, Kelly DL, Yoon S. The effect of tobacco smoking on bone mass: an overview of pathophysiologic mechanisms. J Osteoporos 2018;2018:1206235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Reddy MS, Morgan SL. Decreased bone mineral density and periodontal management. Periodontol 2000 2013;61:195–218. [DOI] [PubMed] [Google Scholar]
- 185.Penoni DC, Vettore MV, Torres SR, Farias MLF, Leão ATT. An investigation of the bidirectional link between osteoporosis and periodontitis. Arch Osteoporos 2019;14:94. [DOI] [PubMed] [Google Scholar]
- 186.Passos-Soares JDS, Vianna MIP, Gomes-Filho IS, et al. Association between osteoporosis treatment and severe periodontitis in post-menopausal women. Menopause 2017;24:789–795. [DOI] [PubMed] [Google Scholar]
- 187.Krall EA, Dawson-Hughes B, Hannan MT, Wilson PW, Kiel DP. Postmenopausal Estrogen Replacement and Tooth Retention. The Am J Med 1997;102(6):536–542. [DOI] [PubMed] [Google Scholar]
- 188.Garcia MN, Hildebolt CF, Miley DD, et al. One-year effects of vitamin D and calcium supplementation on chronic periodontitis. J Periodontol 2011;82:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Dixon D, Hildebolt CF, Miley DD, et al. Calcium and vitamin D use among adults in periodontal disease maintenance programmes. Br Dent J 2009;206:627–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Millen AE, Andrews CA, LaMonte MJ, et al. Vitamin D status and 5-year changes in periodontal disease measures among post-menopausal women: the Buffalo OsteoPerio Study. J Periodontol 2014;85:1321–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Pavlesen S, Mai X, Wactawski-Wende J, et al. Vitamin D status and tooth loss in postmenopausal females: the buffalo osteoporosis and periodontal disease (OsteoPerio) study. J Periodontol 2016;87:852–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Thorin MH, Wihlborg A, Åkesson K, Gerdhem P. Smoking, smoking cessation, and fracture risk in elderly women followed for 10 years. Osteoporos Int 2016;27:249–255. [DOI] [PubMed] [Google Scholar]
- 193.Tomar SL, Asma S. Smoking-attributable periodontitis in the United States: findings from NHANES III. National Health and Nutrition Examination Survey. J Periodontol 2000;71:743–751. [DOI] [PubMed] [Google Scholar]
- 194.Rocha M, Nava LE, Vázquez de la Torre C, Sánchez-Márin F, Garay-Sevilla ME, Malacara JM. Clinical and radiological improvement of periodontal disease in patients with type 2 diabetes mellitus treated with alendronate: a randomized, placebo-controlled trial. J Periodontol 2001;72:204–209. [DOI] [PubMed] [Google Scholar]
- 195.Akram Z, Abduljabbar T, Kellesarian SV, Abu Hassan MI, Javed F, Vohra F. Efficacy of bisphosphonate as an adjunct to nonsurgical periodontal therapy in the management of periodontal disease: a systematic review. Br J Clin Pharmacol 2017;83:444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Aghaloo TL, Kang B, Sung EC, et al. Periodontal disease and bisphosphonates induce osteonecrosis of the jaws in the rat. J Bone Miner Res 2011;26:1871–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Diniz-Freitas M, Fernández-Feijoo J, Diz Dios P, Pousa X, Limeres J. Denosumab-related osteonecrosis of the jaw following non-surgical periodontal therapy: A case report. J Clin Periodontol 2018;45:570–577. [DOI] [PubMed] [Google Scholar]
- 198.Barros SP, Silva MA, Somerman MJ, Nociti FH Jr. Parathyroid hormone protects against periodontitis-associated bone loss. J Dent Res 2003;82:791–795. [DOI] [PubMed] [Google Scholar]
- 199.Kim JH, Kim AR, Choi YH, et al. Intermittent PTH administration improves alveolar bone formation in type 1 diabetic rats with periodontitis. J Transl Med 2018;16:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yamashita J The therapeutic potential of parathyroid hormone in dental and oral medicine. Oral Sci Int 2020;17:3–14. [Google Scholar]
- 201.Taut AD, Jin Q, Chung J-H, et al. Sclerostin antibody stimulates bone regeneration after experimental periodontitis. J Bone Miner Res 2013;28:2347–2356. [DOI] [PubMed] [Google Scholar]
- 202.Yao Y, Kauffmann F, Maekawa S, et al. Sclerostin antibody stimulates periodontal regeneration in large alveolar bone defects. Sci Rep 2020;10:16217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Chen H, Xu X, Liu M, et al. Sclerostin antibody treatment causes greater alveolar crest height and bone mass in an ovariectomized rat model of localized periodontitis. Bone 2015;76:141–148. [DOI] [PubMed] [Google Scholar]
- 204.Yu SH, Hao J, Fretwurst T, et al. Sclerostin-Neutralizing Antibody Enhances Bone Regeneration Around Oral Implants. Tissue Eng Part A 2018;24:1672–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]


