Abstract
Post-traumatic stress disorder (PTSD) is a chronic and debilitating condition that is often refractory to standard frontline antidepressant therapy. A promising new approach to PTSD therapy is administration of a single sub-anesthetic dose of (R,S)-ketamine (Ket). The treatment produces rapid and significant therapeutic response, which lasts for only 4–7 days. In one of our studies, the mean duration of response was increased to 33 days when Ket administration was combined with a mindfulness-based cognitive therapy, Trauma Interventions using Mindfulness Based Extinction and Reconsolidation (TIMBER). We now report the results from a 20-patient study, which examined the duration of sustained response with combined TIMBER-Ket therapy, TIMBER-K arm, relative to the response observed in a placebo-controlled arm, TIMBER-P. A significant difference in the duration of response was observed between TIMBER-K and TIMBER-P arms: 34.44 ± 19.12 days and 16.50 ± 11.39 days, respectively (p = 0.022). Previous studies identified a negative correlation between antidepressant response to Ket and basal plasma concentrations of D-serine (DSR). In this study, the basal DSR levels positively correlated with the pre-treatment severity of PTSD symptoms (Pearson’s r = 0.42, p = 0.07) and patients with basal DSR level ≥3.5 μM displayed not only higher PTSD severity but also shorter duration of response. The data indicate that basal DSR levels may serve as a biomarker of the severity of PTSD symptoms and as a predictor of clinical response. This article is part of a Special Issue entitled: D-Amino acids: biology in the mirror, edited by Dr. Loredano Pollegioni, Dr. Jean-Pierre Mothet and Dr. Molla Gianluca.
Keywords: PTSD, Mindfulness, Ketamine, D-Serine
1. Introduction
Post-traumatic stress disorder (PTSD) is a debilitating, chronic and often difficult to treat condition that manifests as intrusive thoughts, flashbacks, avoidance, numbing and hyper-arousal [1,2]. PTSD is a worldwide problem that has reached a crisis level in the USA as 8% of the adult American population are at risk of suffering from PTSD during their life time, and currently is the fifth most prevalent mental disorder in the USA [3,4]. The personal and societal burden of PTSD is compounded by the lack of adequate pharmacotherapies and psychotherapies with over 42% of the subjects with PTSD receiving “minimally adequate care” [5,6].
The predominant frontline pharmacotherapy approach to PTSD is the administration of antidepressants such as the selective serotonin reuptake inhibitors (SSRI) sertraline and paroxetine [7], but < 60% of PTSD patients respond to these treatments and many have side effects [6–8]. This problem is being addressed in a variety of clinical trials utilizing a broad range of agents including cannabinoids, glucocorticoids, non-SRI antidepressants, opioids and riluzole [8]. One of the more promising agents is (R,S)-ketamine (Ket). A single intravenous administration of a low dose of Ket produces rapid (within a few hours) improvement in refractory PTSD symptoms [9] as well as in the symptoms of depression, suicidality and anxiety, which are major co-morbidities in PTSD [10,11]. However, while the therapeutic effects of Ket are rapid and positive, they are also short-lived, lasting, on average, from 4 to 7 days [9,11].
The poor therapeutic response in PTSD patients treated with pharmacotherapy indicates that a broader multi-modal clinical approach is necessary including combining them with psychotherapeutic interventions [12]. One potential psychotherapy approach is to specifically target the pathological trauma memories (TMs), which lay at the core of the etiopathogenesis of PTSD. In PTSD, TMs are ingrained into the brain through conditioned learning mechanisms mediated by the amygdala, hippocampus, pre-frontal cortex, and basal ganglia in conjunction with the brain stem and the hypothalamus-pituitary axis, and play a key role in the formation and maintenance of its major symptoms [1,2]. We have recently reported the development of a TMs-specific mindfulness based cognitive therapy, Trauma Interventions using Mindfulness Based Extinction and Reconsolidation (TIMBER), which attempts to close some of the existing treatment gaps in PTSD therapy [13]. In order to effectively target the TMs, TIMBER psychotherapy, in standardized ways, combines the Yoga and mindfulness based cognitive therapy (Y-MBCT) interventions with the mindfulness based graded exposure therapy (MB-GET, a type of cognitive behavioral therapy (CBT)), and allows the cognitive reprocessing and neutral/detached reappraisal of the TMs with the objective to change TMs and their expressions in clients’ daily life.
In our initial study in PTSD patients, we examined the combined effect of TIMBER psychotherapy and Ket pharmacotherapy in a 10-subject pilot study [14]. Ket was chosen as the pharmacotherapy arm of the study not only due to it efficacy in the treatment of depression and PTSD, but also for its ability to induce neurogenesis [15,16] and to effect synaptic plasticity by altering long-term potentiation (LTP) and long-term depression (LTD) [17]. The data demonstrated that 9 out of 10 patients with chronic and refractory PTSD experienced a robust positive clinical response, and that patients treated with TIMBER and Ket (TIMBER-K arm) had a more sustained response than the patients who were treated with TIMBER and placebo (TIMBER-P arm): 33 ± 22.98 days and 25 ± 16.8 days, respectively.
In the current study, the experimental cohorts were doubled and we now report the results from a 20-patient study, which examined the duration of sustained response in the TIMBER-K arm relative to the response observed in the TIMBER-P. The current study also explores the potential use of basal D-serine (DSR) plasma concentrations as a biomarker of clinical response. The analysis of DSR plasma concentrations was undertaken in this study based upon previous data from the use of Ket in the treatment of depression in which indicates an inverse relationship between endogenous DSR plasma concentrations and the antidepressant response to Ket, i.e. patients with lower basal DSR plasma concentrations exhibit a more robust therapeutic response [20]. In the treatment of PTSD, the determination of basal DSR plasma concentrations is reasonable as DSR is a key NMDA receptor co-agonist that plays a critical role in LTP and NMDA-induced neurotoxicity [18] and has been associated with acquisition and extinction of fear memory in animals and patients with PTSD [19]. Both of these processes are important in the TIMBER-K therapeutic approach. The results of this study indicate that basal DSR levels may serve as a predictor of the length of clinical response and lay the basis for the design of definitive clinical studies.
2. Materials and methods
2.1. Study design
This was a randomized, double-blind, placebo-controlled, parallel group study conducted in 20 subjects at the Department of Psychiatry and Department of Anesthesiology, Cooper University Hospital and Cooper Medical School of Rowan University, Camden, NJ, USA. The methodology of this study was guided by the methods employed in our initial study that examined the efficacy of the combined treatment protocol employing TIMBER psychotherapy in conjunction with Ket pharmacotherapy [14]. The study also determined the plasma concentrations of DSR before the initial dose (basal) of Ket/placebo and at the conclusion of the i.v. dosing (40 min).
The study was approved by the Cooper University Hospital Institutional Review Board, approval No. 13-078, and was performed in accordance with ethical guidelines laid out by the US National Institute of Health and the principles of the Declaration of Helsinki. Written informed consent was obtained from all patients.
2.2. Subjects
All 20 subjects were refractory for at least 6 months before their recruitment as defined by their lack of response to treatment as usual, i.e. trials of CBT and at least 2 antidepressants (SSRIs and SNRIs) for at least 6 months. A complete medical history and physical examination were performed at the initial assessment in the psychiatrist’s (BP) outpatient office in the Department of Psychiatry at Cooper University Hospital. After enrollment, the subjects were randomized by the statistician employing randomization with block technique using 1:1 ratio and assigned to one of the two arms of the study, TIMBER-K and TIMBER-P (see Section 2.3). Each arm contained 10 subjects. Except for the statistician and pharmacist, all study personnel were blinded to the composition of the two arms.
The subjects were assessed for the severity of PTSD symptoms using the PTSD Checklist (PCL) [21,22], Clinician Administered PTSD Scale for DSM-IV (CAPS, clinician rated) [23], Hamilton Depression Rating Scale (Ham-D, 17-item, clinician rated) [24], and Beck Anxiety Inventory (BAI, clinician rated) [25]. The effect of Ket on TMs and general memory was determined at baseline and 8 h after infusion using the Montreal Cognitive Assessment (MoCA, clinician rated) [26]. In this study, scores on CAPS and PCL scales were the primary outcome measure CAPS scale was administered at baseline, at 24 h and at relapse and the PCL scale was administered at baseline, at the end of the Ket infusion (40 min), at 24 h and during weekly follow up visits until relapse.
The mindfulness interventions in TIMBER were personalized based on subject’s scores on Assessment Scale for Mindfulness Interventions (ASMI: clinician rated 18-item scale, scores range 0–90: higher the scores, higher is the level of mindfulness) [13], which was administered at baseline, and after 5 sessions and 12 sessions (completion) of TIMBER. Also, a scripted narrative of the index trauma was prepared in a personalized way for each subject. To avoid re-traumatization in the subjects during the graded exposure to TMs while TIMBER therapy was being applied during the infusion, the arousal responses are kept brief and under control by using the STOPP module/mini-TIMBER therapy to deescalate and also by ongoing monitoring using a specific scale, the Arousal Response during Trauma Memory Reactivation (ART-MR: a clinician rated 11-item scale with scores ranging from 0 to 55; higher scores indicate higher level of arousal) [14]. Of note, the STOPP module of mindfulness is Pradhan’s behavioral adaptation of The Middle Way philosophy of mindfulness traditions, which he has successfully applied in clinical situations with age range of 6–80 years [14].
Recruitment was done over a 6-month period and the subjects were followed for 18 months after the initial infusion. During the first week after the initial infusion, 3 sessions of mini-TIMBER/STOPP module [14] was administered: first one during the infusion, second one at the 24 h visit and the third one at the 7th day visit. From the second day after the infusion until relapse, all subjects were assessed every week by face-to-face clinical evaluation using the appropriate rating scales. Once relapsed, they were assigned for 9 sessions of full-TIMBER therapy. Throughout the duration of the subjects’ participation in this study, the dosages of all medications were kept constant/unchanged.
2.3. Study interventions and outcome measures
In this study, there were two groups, each consisting of 10 subjects. The subjects in TIMBER-K arm of the study received (R,S)-ketamine (single infusion of 0.5 mg/kg dose, infused over 40-minutes) and TIMBER psychotherapy (total 12 sessions) whereas those in TIMBER-P arm received placebo (single infusion of normal saline same volume, infused over 40-minutes) and TIMBER psychotherapy (total 12 sessions). The total of 12 TIMBER sessions consisted of 3 mini-TIMBER sessions in first week (during infusion, on 2nd day and on 8th day) followed by 9 full-TIMBER sessions conducted on weekly basis, 45 min duration each.
The primary outcome measures were reduction in CAPS and PCL scores and response was defined as a 20-point decrease in the pre-treatment scores at 24 h post-infusion. To be a treatment responder, the reduction in PCL scores needed to be sustained for at least 7 days. Remission of PTSD was defined as no or minimal PTSD symptoms (total scores of ≤30 points in CAPS and ≤30 in PCL scales) and relapse was defined as total scores of > 50 points in the CAPS and ≥51 in PCL [21].
2.4. Sample size calculation
For this study, sample size calculation and power analysis was assessed using the mean PCL score of 72 (SD 5) and the difference of 20 points in the scores at 24-hour post-infusion revealed that for 80% power (2 tailed alpha = 0.05), the number of subjects needed per group is 10, so recruitment of 20 subjects was done who were assigned randomly into two groups.
2.5. DSR plasma concentrations
Plasma samples were collected prior to the initiation of the infusion (basal) and at the end of the infusion (40-min) and frozen at −80 °C until analysis. DSR plasma concentrations were determined using a previously reported validated assay employing liquid chromatography with mass spectrometric detection [20,27].
2.5.1. Instrumentation
The chromatographic experiments were carried out on a Shimadzu Prominence HPLC system (Shimadzu, Columbia, MD, USA). The samples were introduced to the analytical column using Shimadzu SIL-20A autosampler, maintained at 4 °C, and injections of 20 μl were made. The samples were run on a 5500 QTRAP triple quadruple mass spectrometer equipped with a Turbo V electrospray ionization source (AB Sciex, Concord, ON, Canada).
2.5.2. Plasma samples
Plasma samples (100 μl) were combined with 20 μl aliquot of the internal standard D-arginine (10 nmol/ml in acetone) and 400 μl acetone and then centrifuged at 13,000 ×g for 10 min at 4 °C. A 400 μl aliquot of the supernatant was subsequently derivatized with 300 μl (R)-1-Boc-2-piperidinecarbonyl chloride, the solution evaporated to dryness and the residue dissolved in 100 μl of methanol/water (10:90, v/v) and transferred to the autosampler for analysis.
2.5.3. Chromatographic conditions
Chromatographic separation was achieved on a Zorbax Eclipse XDB-C18 column (4.6 mm × 150 mm, 5 μm; Agilent Technologies, Santa Clara, CA, USA) protected with an Agilent C18 guard column at room temperature. The mobile phase consisted of water with 0.3% TFA (elute A) and methanol with 0.3% TFA (elute B). The gradient eluent at a flow rate of 0.4 ml/min was programmed as followed: 0–15 min, 5–9% B; 15–22 min, 15% B; 22–25 min, 5% B. The total run time was 25 min and the injection volume per sample was 20 μl.
2.5.4. Mass spectrometry conditions
MS/MS analysis was performed using a triple quadrupole mass spectrometer model API 5500Q system from Applied Biosystems/MDS Sciex equipped with Turbo V electrospray ionization source (TIS)® (Applied Biosystems, Foster City, CA, USA). The data was acquired and analyzed using Analyst version 1.5.1 (Applied Biosystems). Positive electrospray ionization data were acquired using multiple reaction monitoring (MRM). The TIS instrumental source settings for temperature, curtain gas, ion source gas 1 (nebulizer), ion source gas 2 (turbo ion spray), collision energy and ion spray voltage were 550 °C, 20 psi, 45 psi, 80 psi, 15 V and 4500 V, respectively. The TIS compound parameter settings for declustering potential, entrance potential, and collision cell exit potential were 80 V, 10 V, and 10 V, respectively. The standards were characterized using the following MRM ion transitions: DSR derivatization product (m/z 231.5 to 106.1) and D-Arg derivatization product (m/z 300.4 to 175.0).
2.6. Statistics
All study data were entered into an excel spreadsheet and were verified against the source documents by two members of the study team. For in-between group comparisons, quantitative test score data were analyzed using unpaired t-tests and within group comparisons were conducted using paired t-tests. Categorical data were analyzed using the Chi-square test. The responses in the TIMBER-K and TIMBER-P arms (in terms of % change in mean scores from baseline in various time frames) were compared using the Z-statistics as well. The Pearson correlation coefficient was used to evaluate the relationship between the PTSD scale (PCL) scores and duration of the sustained response. Data in the tables are presented as means ± SD and p < 0.05 was considered statistically significant. No changes to trial were made after the trial was commenced and intention-to-treat analysis was followed. Statistical analysis was performed using SPSS, Version 23 (IBM, Armonk, NY, USA).
3. Results
The socio-demographic and clinical data of the subjects are presented in Table 1. The severity of the PSTD symptoms, assessed using the PCL and CAPS total test scores at baseline for all 20 subjects in this study ranged from 60 to 82 (PCL) and 72 to 98 (CAPS), Table 1. The PCL scores were used as the primary measure to assess PTSD severity and response and the CAPS scores were used as confirmation of these assessments. In the PCL scale, the maximum score is 85 and represents the highest level of severity of PTSD, a score of < 30 (~35.29% of the maximum score) is considered as remission and scores above 51 (~60% of the maximum score) are considered as relapse [21]. Using this scale, we defined a score in between 51 and 60 as mild PTSD, a score in between 60 and 70 as moderate PTSD and a score in between 70 and 85 as severe PTSD. In this study, the basal PLC scores for 14 of the subjects were ≥70 and they were categorized as having severe PTSD, while the remaining 6 subjects were assessed to have moderate PTSD.
Table 1.
Socio-demographic and clinical data related to PTSD for the subjects enrolled in the study; where the PTSD Checklist (PCL) and Clinician Administered PTSD Scale for D-serine is a potential biomarker of clinical response to the treatment of post-traumatic stress disorder using (R,S)-ketamine infusion and TIMBER psychotherapy: A pilot study*.
| Subject | Age (years) | Gender | Years of education | Type of trauma | Duration of PTSD (years) | PCL | CAPS |
|---|---|---|---|---|---|---|---|
| 1 | 30 | F | 14 | Sexual abuse | 18 | 76 | 95 |
| 2 | 44 | F | 10 | Physical & emotional abuse | 6 | 77 | 86 |
| 3 | 57 | F | 9 | Sexual abuse | 34 | 80 | 84 |
| 4 | 38 | F | 12 | Physical & sexual abuse | 18 | 82 | 92 |
| 5 | 51 | M | 16 | Stabbing | 10 | 76 | 96 |
| 6 | 56 | F | 14 | Physical & sexual abuse | 29 | 77 | 81 |
| 7 | 44 | M | 18 | Sexual abuse | 28 | 60 | 72 |
| 8 | 37 | F | 11 | Physical & sexual abuse | 2 | 81 | 97 |
| 9 | 24 | F | 14 | Sexual abuse | 9 | 81 | 95 |
| 10 | 49 | M | 12 | Motor vehicle accident | 38 | 62 | 86 |
| 11 | 32 | M | 17 | Physical & sexual abuse | 5 | 60 | 74 |
| 12 | 40 | M | 11 | Physical & emotional abuse | 8 | 66 | 82 |
| 13 | 32 | M | 15 | Combat related injury | 5 | 75 | 96 |
| 14 | 28 | M | 10 | Physical & sexual abuse | 11 | 75 | 98 |
| 15 | 37 | F | 11 | Physical & sexual abuse | 12 | 75 | 93 |
| 16 | 32 | F | 15 | Physical & sexual abuse | 12 | 70 | 92 |
| 17 | 40 | M | 14 | Motor vehicle accident | 14 | 70 | 83 |
| 18 | 41 | F | 16 | Physical & sexual abuse | 20 | 67 | 89 |
| 19 | 42 | F | 13 | Physical & emotional abuse | 7 | 76 | 94 |
| 20 | 60 | F | 15 | Physical & sexual abuse | 18 | 67 | 92 |
The randomization of the subjects into the two arms of the study produced no significant differences in the baseline PCL scores, as the average ± SD values were 75.70 ± 5.81 (TIMBER-K) and 70.40 ± 7.74 (TIMBER-P), p = 0.0928, Tables 2A, 2B. In this study, all the patients experienced a definable and significant remission of symptoms at 24 h reflected by a > 60% reduction (p < 0.0001) in both the PCL and CAPS scores, Table 3. There were no significant differences in response between the two experimental groups indicating that the mindfulness protocol contributes to the clinical response. However, while there were no significant differences between the two arms of the study at 24 h, there was a wide difference between the number of days that the effect was sustained, Table 2A. A comparison of the duration of sustained response between the two treatment arms indicated that the effect was significantly greater in the TIMBER-K arm (34.44 ± 19.12 days) relative to the TIMBER-P arm (16.50 ± 11.39 days), p = 0.022, Table 2B. The data is consistent with the response observed in the previous pilot study [14].
Table 2A.
Pretreatment PCL scores (baseline) and plasma D-serine concentration (basal DSR) and the duration of response (see Materials and methods for definition of response). The plasma samples obtained from Subject 8 could not be analyzed.
| Treatment arm | Subject | PCL score Baseline | Basal DSR (μM) | Response (days) |
|---|---|---|---|---|
| TIMBER-K | 1 | 76 | 3.23 | 71 |
| 3 | 80 | 3.46 | 27 | |
| 4 | 82 | 5.01 | 21 | |
| 8 | 81 | ND | 11 | |
| 9 | 81 | 6.24 | 20 | |
| 13 | 75 | 2.61 | 25 | |
| 16 | 70 | 3.34 | 55 | |
| 17 | 67 | 2.99 | 14 | |
| 18 | 78 | 1.35 | 48 | |
| 20 | 67 | 1.59 | 29 | |
| TIMBER-P | 2 | 77 | 6.12 | 12 |
| 5 | 76 | 4.96 | 14 | |
| 6 | 77 | 5.36 | 4 | |
| 7 | 60 | 3.19 | 41 | |
| 10 | 62 | 2.25 | 28 | |
| 11 | 60 | 3.63 | 15 | |
| 12 | 66 | 1.64 | 4 | |
| 14 | 75 | 2.27 | 18 | |
| 15 | 75 | 4.46 | 8 | |
| 19 | 76 | 1.53 | 21 |
Table 2B.
Comparison of the combined data from TIMBER-K and TIMBER-P arms, presented as mean ± SD, p value, effect size (ES) and 95% confidence intervals (CI).
| TIMBER-K | TIMBER-P | p value | ES | CI | |
|---|---|---|---|---|---|
| PCL score | 75.70 ± 5.81 | 70.40 ± 7.44 | 0.0928 | 0.699 | −1.82 to 11.24 |
| Response (days) | 34.44 ± 19.12 | 16.50 ± 11.39 | 0.022 | 1.156 | 2.903 to 32.986 |
| DSR (basal) (μM) | 3.31 ± 1.54 | 3.54 ± 1.63 | 0.759 | −0.145 | −1.766 to 1.310 |
Table 3.
The individual and average PCL and CAPS scores determined before the initiation of treatment (pre-study) and at clinical relapse (relapse); where DR represents a patient who did not return for clinical assessment, # represents a significant difference between pre-study and 24 h scores with p < 0.0001 (paired t-test) and * represents a significant difference between pre-study and relapse scores with p < 0.0001 (paired t-test).
| Patient | PCL Pre-study | PCL 24 h | PCL Relapse | CAPS Pre-study | CAPS 24 h | CAPS Relapse |
|---|---|---|---|---|---|---|
| 1 | 76 | 25 | 67 | 95 | 12 | 62 |
| 2 | 77 | 36 | 65 | 86 | 38 | 68 |
| 3 | 80 | 32 | 64 | 84 | 19 | 66 |
| 4 | 82 | 25 | 52 | 92 | 16 | 58 |
| 5 | 76 | 28 | 55 | 96 | 26 | 72 |
| 6 | 77 | 17 | 71 | 81 | 16 | 52 |
| 7 | 60 | 31 | 57 | 72 | 18 | 54 |
| 8 | 81 | 22 | 68 | 97 | 26 | 72 |
| 9 | 81 | 24 | 69 | 95 | 16 | 68 |
| 10 | 62 | 21 | 57 | 86 | 19 | 54 |
| 11 | 60 | 22 | 66 | 74 | 10 | 64 |
| 12 | 66 | 36 | DR | 82 | 39 | DR |
| 13 | 75 | 18 | 52 | 96 | 16 | 52 |
| 14 | 75 | 26 | 56 | 98 | 28 | 56 |
| 15 | 75 | 28 | 56 | 93 | 27 | 56 |
| 16 | 70 | 21 | 55 | 92 | 18 | 55 |
| 17 | 67 | 29 | 52 | 83 | 26 | 52 |
| 18 | 78 | 17 | 54 | 89 | 18 | 54 |
| 19 | 76 | 18 | 64 | 94 | 16 | 64 |
| 20 | 67 | 45 | 58 | 92 | 48 | 58 |
| Average | 73.4 ± 7.0 | 26.1 ± 7.3# | 59.9 ± 6.4* | 89.2 ± 7.6 | 22.6 ± 9.8# | 63.1 ± 7.5* |
The PTSD Checklist (PCL) is a self-regulated scale, which was performed at home by the patient on a regular basis. A patient’s self-calculated score of above 50 indicates a relapse of PTSD symptoms and prompts a clinical visit and the administration of the Clinician Administered PTSD Scale for DSM-IV (CAPS) to confirm the relapse. In it of interest to note that in this study, the PCL and CAPS scores at relapse where significantly lower (p < 0.0001) than the corresponding scores assessed before treatment, Table 3. This may reflect a measurement taken at the initial stages of the PTSD relapse, which will increase during the course of the disease, or an effect produced by the patient’s daily practice of mindfulness meditation. These possibilities will be examined in a larger, extended clinical study.
Basal DSR plasma concentrations were determined in 19 of the 20 subjects, as there were insufficient volumes in the samples obtained for subject #8, Table 2A. The average basal DSR plasma concentration for all 19 subjects was 3.43 ± 1.55 μM, which was lower than, but not significantly different from, basal DSR plasma levels determined in 21 MDD patients, 4.05 ± 1.05 μM [20]. As shown in the Table 2B, there was no significant difference in the mean basal DSR plasma concentrations between the subjects enrolled in the TIMBER-K arm (3.31 ± 1.54 μM) and TIMBER-P arm (3.54 ± 1.63 μM) (p = 0.759). A comparison of the basal DSR plasma levels with the pre-treatment PTSD severity level (PCL scores) demonstrated a positive correlation between the two parameters (Pearson’s Correlation, r = 0.42, p = 0.07), although the relationship did not reach significance. In addition, the duration of treatment response was negatively correlated with basal DSR plasma concentration (Pearson’s Correlation, r = −0.02, p = 0.93) although the effect was not statistically significant.
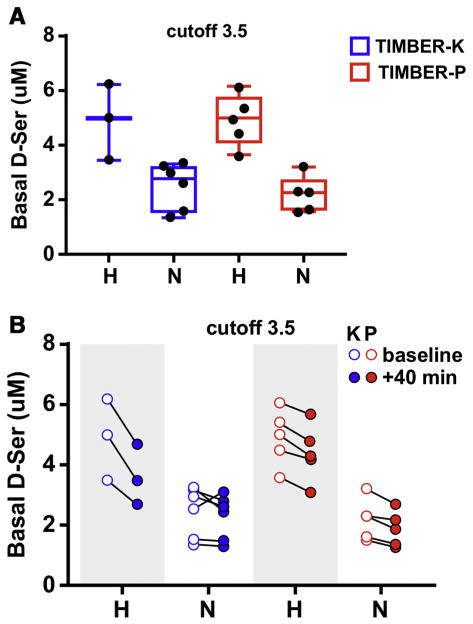
There was a wide distribution of basal DSR plasma concentrations in both arms of the study ranging from 6.24 μM to 1.35 μM, Table 2A. Previous studies in patients with treatment-resistant depression demonstrated that patients that responded to Ket antidepressant therapy had significantly (p < 0.001) lower basal DSR plasma concentrations than patients who did not respond to Ket, 3.0 ± 0.3 μM and 4.7 ± 0.8, respectively [20]. The data suggested that there was a bimodal distribution of basal DSR plasma concentrations in patients with depression [20]. An examination of the basal DSR plasma concentrations of the subjects enrolled in the current study suggested that a bimodal distribution of DSR plasma concentrations was also present in PTSD patients, which is represented in Fig. 1A. The basal DSR plasma concentration of 3.5 μM was used to divide the 19 patients into 2 groups, “high” DSR group (DSR(H), ≥3.5 μM) and “normal” DSR group (DSR (N), < 3.5 μM). There was a significant difference (p = 0.001) between the mean basal DSR plasma concentrations of the two groups, 4.91 ± 1.02 and 2.36 ± 0.75, respectively. The designation “normal” was used based upon previously reported DSR basal plasma concentrations in healthy controls and patients with MDD, which ranged from 2.06 ± 0.05 μM to 2.32 ± 0.92 μM [28–30]. The mean PCL score in the DSR(H) group (76.0 ± 6.9) was greater than the DSR(N) group (70.2 ± 6.2), but the difference did not reach significance (p = 0.0712), which is consistent with the trend observed when all 19 subjects were examined as a single group. There was an inverse relationship relative to the duration of the response as the DSR(N) group was in remission longer, 32.2 ± 19.7 days, than the DSR(H) group, 15.1 ± 7.4, p = 0.0333.
Fig. 1.
A. The distribution of patients according to basal D-serine plasma concentrations using 3.5 μM as the cutoff between “high” and “normal” concentrations where the subjects in the Timber-K arm of the are denoted in blue and the subjects in the TIMBER-P arm are denoted in red. B. The changes in the D-serine plasma concentrations at the conclusion of the 40-min intra venous infusion where the subjects receiving a 0.5 mg/kg dose of (R,S)-ketamine (K) are denoted in red and the subjects receiving normal saline (P) are denoted in blue and where basal plasma concentrations of D-serine are indicated by open circles (○) and the 40-min concentrations by filled circles (●).
The relationship between basal DSR plasma concentrations and response was further investigated by dividing each experimental arm of the study into DSR(H) and DSR(N) subgroups resulting in four subgroups, i.e. TIMBER-K(H), TIMBER-K(N), TIMBER-P(H) and TIMBER-P (N), Table 4A, Fig. 1A. There were significant differences (p = 0.001) between the basal DSR plasma concentrations in the “high” and “normal” subgroups in each arm but no differences between the TIMBER-K(H) and TIMBER-P(H) subgroups and the TIMBER-K(N) and TIMBER-P(N) subgroups, Tables 4A and 4B. The mean baseline PCL score in the TIMBER-K(H) group was significantly higher than the score for the TIMBER-K(N) group (p = 0.018) but no differences in baseline PCL scores were determined in any of the other subgroup comparisons. The mean duration of the response displayed the inverse effect as the TIMBER-K(N) subgroup was in remission, ~78% longer than the TIMBER-K(H), i.e. 40.3 days and 22.7 days, respectively, p = 0.017. There were no significant differences in the duration of response between the TIMBER-P(H) and TIMBER-P(N) subgroups or between the TIMBER-K(H), TIMBER-K(N) and TIMBER-P(N) subgroups. However, the length of response was significantly (p < 0.05) longer in the TIMBER-K(H) and TIMBER-K(N) subgroups relative to the TIMBER-P (H).
Table 4A.
The TIMBER-K and TIMBER-N arms of the study were divided into treatment subgroups using the basal DSR plasma concentration of 3.5 μM. The arms were divided into “high” DSR subgroups (≥3.5 μM), TIMBER-K(H) and TIMBER-P(H) and “normal” DSR subgroups (< 3.5 μM), TIMBER-K(N) and TIMBER-P(N). The effect of Ket on basal DSR plasma concentrations at the end of the Ket infusion (40 min DSR) was examined by the determination of the percent change (%) of DSR concentration in each subject.
| Groups | Subject | Basal DSR (μM) | PCL Baseline | Response (days) | 40 min DSR (μM) | Percent change (%) |
|---|---|---|---|---|---|---|
| TIMBER-K (H) | 3 | 3.5 | 80 | 27 | 2.7 | −21 |
| 4 | 5.0 | 82 | 21 | 3.5 | −30 | |
| 9 | 6.2 | 81 | 20 | 4.7 | −25 | |
| Mean ± SD | 4.9 ± 1.4 | 81.0 ± 1.0 | 22.7 ± 3.8 | 3.6 ± 1.0 | 25 ± 5 | |
| TIMBER-K (N) | 1 | 3.2 | 76 | 71 | 2.8 | −14 |
| 16 | 3.3 | 70 | 55 | 2.4 | −7 | |
| 18 | 1.4 | 78 | 48 | 1.3 | −3 | |
| 20 | 1.6 | 67 | 29 | 1.5 | −3 | |
| 13 | 2.6 | 75 | 25 | 3.1 | +8 | |
| 17 | 3.0 | 67 | 14 | 2.6 | −14 | |
| Mean ± SD | 2.5 ± 0.8 | 72.2 ± 4.8 | 40.3 ± 21.3 | 2.3 ± 0.7 | 8 ± 6 | |
| TIMBER-P (H) | 11 | 3.6 | 60 | 15 | 3.1 | −14 |
| 15 | 4.5 | 75 | 8 | 4.2 | −5 | |
| 5 | 5.0 | 76 | 14 | 4.3 | −14 | |
| 6 | 5.4 | 77 | 4 | 4.8 | −11 | |
| 2 | 6.1 | 77 | 12 | 5.7 | −7 | |
| Mean ± SD | 4.9 ± 0.9 | 73.0 ± 7.3 | 10.6 ± 4.6 | 4.4 ± 0.9 | 10 ± 4 | |
| TIMBER-P (N) | 19 | 1.5 | 76 | 21 | 1.3 | −15 |
| 12 | 1.6 | 66 | 4 | 1.4 | −12 | |
| 10 | 2.3 | 62 | 28 | 1.9 | −16 | |
| 14 | 2.3 | 75 | 18 | 2.2 | −3 | |
| 7 | 3.2 | 60 | 41 | 2.7 | −14 | |
| Mean ± SD | 2.2 ± 0.7 | 67.8 ± 7.4 | 22.4 ± 13.9 | 1.9 ± 0.6 | 12 ± 5 |
Table 4B.
Significant statistical differences between experimental subgroups before and after clinical treatment calculated using unpaired (UP) and paired (P) Student’s t-test. Only significant results, p < 0.05, are presented.
| Subgroups | Comparison | p value |
|---|---|---|
| TIMBER-K(H) v TIMBER-K(N) | Basal DSR plasma concentration | 0.001 (UP) |
| TIMBER-K(H) v TIMBER-K(N) | Baseline PCL | 0.018 (UP) |
| TIMBER-K(H) v TIMBER-K(N) | Length of response in days | 0.017 (UP) |
| TIMBER-K(H) + TIMBER-K(N) | Basal v 40-min DSR plasma concentration | 0.0313 (P) |
| TIMBER-K(H) | Basal v 40-min DSR plasma concentration | 0.0323 (P) |
| TIMBER-P(H) v TIMBER-P(N) | Basal DSR plasma concentration | 0.001 (UP) |
| TIMBER-P(H) + TIMBER-P(N) | Basal v 40-min DSR plasma concentration | 0.0001 (P) |
| TIMBER-P(H) | Basal v 40-min DSR plasma concentration | 0.00021 (P) |
| TIMBER-P(N) | Basal v 40-min DSR plasma concentration | 0.0289 (P) |
| TIMBER-K(H) v TIMBER-K(N) | Percent change in basal DSR plasma concentration in 40-min sample | 0.0015 (UP) |
| TIMBER-K(H) v TIMBER-P(H) | Percent change in basal DSR plasma concentration in 40-min sample | 0.0027 (UP) |
| TIMBER-K(H) v TIMBER-P(N) | Percent change in basal DSR plasma concentration in 40-min sample | 0.0108 (UP) |
Previous studies of Ket in depressed patients identified a biphasic effect on DSR plasma concentrations in both treatment non-responders (high basal DSR) and responders (low basal DSR) in which DSR concentrations were depressed relative to baseline at the end of the Ket infusion (40-min sample), followed by a recovery at 120 min, which was followed by a second decrease in the DSR plasma concentrations in the 4 h, 1-day and 7-day samples [20]. In the current study, we determined the change in plasma DSR concentrations at the end of the 40-min infusion and compared the data to the basal DSR plasma concentrations, Table 4A, Fig. 1B. The changes were probed within each subgroup of the study using a paired t-test and the results indicate that DSR plasma concentrations were significantly decreased in all of the groups, Table 4B, Fig. 1B. The difference in the effect between subgroups was probed by comparing the percent change in DSR plasma concentrations at 40 min relative to basal concentrations. The relative decrease in the DSR plasma concentrations in the TIMBER-K(H) subgroup was significantly greater than the effect in the TIMBER-K(N) subgroup, 25 ± 5% and 8 ± 6%, respectively, p = 0.0108. This difference is consistent with the previously observed effect in depressed patients in which there was a significant difference in the magnitude of the Ket effect between the non-responder and responders with 26% and 19% decreases, respectively, p < 0.001 [20]. The relative decrease in DSR plasma concentrations observed in the TIMBER-K(H) subgroup was also significantly greater than the effect in the TIMBER-P(H) and TIMBER-P(N) subgroups, Table 4B. No significant differences were observed between the TIMBER-K(N), TIMBER-P(H) and TIMBER-P(N) subgroups.
4. Discussion
In their recent review of the current state of PTSD therapy, Krystal and colleagues wrote that the “urgent need to find effective pharmacological treatments for PTSD should be considered a national mental health priority.” [8] One new and potentially effective treatment is the multimodal approach combining TIMBER mindfulness psychotherapy and Ket pharmacotherapy (TIMBER-K). The results of the current study reproduce the synergistic effects of the combined treatments observed in the pilot study [14]. In the current study, patients receiving TIMBER-K therapy experienced no or minimal PTSD symptoms for an average of 34 days, which is twice as long as the sustained remission achieved with mindfulness therapy alone and 5-fold longer than the reported response to Ket therapy alone [9,11].
The pharmacological basis for the synergistic effect of mindfulness therapy on the therapeutic effect of Ket is of interest. A key pharmacological effect of Ket is the non-competitive inhibition of the NMDA receptor and this property is associated with the efficacy of Ket in antidepressant therapy and, by extension, PTSD. However, recent studies have suggested that in mouse models of depression, the antidepressant effects observed after Ket administration may be due to the metabolic conversion of the drug to its (2S,6S)-hydroxynorketamine and (2R,6R)-hydroxynorketamine metabolites [31], which, respectively, have little or no NMDA receptor inhibitory effect [31,32]. An alternative explanation for the antidepressant effects of Ket is suggested by the Ket-associated reduction in the production and availability of the key NMDA receptor co-agonist DSR. Decreased DSR plasma concentrations are observed after Ket administration in depressed patients [20] and in the PTSD patients enrolled in this study. In addition, in vitro studies demonstrated that incubation with Ket and its major metabolites deceases intracellular and extracellular DSR concentrations in PC12, 1321N1 astrocytoma and cultured primary neuronal cells [33,34]. Thus, the Ket-associated reduction in DSR concentrations may lead to the indirect inhibition of NMDA receptor activity.
The potential association of DSR concentrations with clinical status and response is consistent with the role that DSR plays in long-term potentiation and NMDA-induced neurotoxicity [18,35,36]. Endogenous DSR levels have been correlated with a number of CNS diseases and pathological states, as increased levels of DSR are linked to amyotrophic lateral sclerosis (ALS) and Alzheimer’s disease while decreases in DSR concentrations have been associated with schizophrenia [35,37]. In schizophrenia, a single nucleotide polymorphism, SNP rs4523957, in the gene encoding the serine racemase enzyme is associated with the genetic risk for schizophrenia [19,37] and with reduced DSR production [19]. Recent data has determined that SNP rs4523957 is also associated with PTSD and identified a dual aspect of DSR, as it is a key component in fear learning and also facilitates the fear extinction [19].
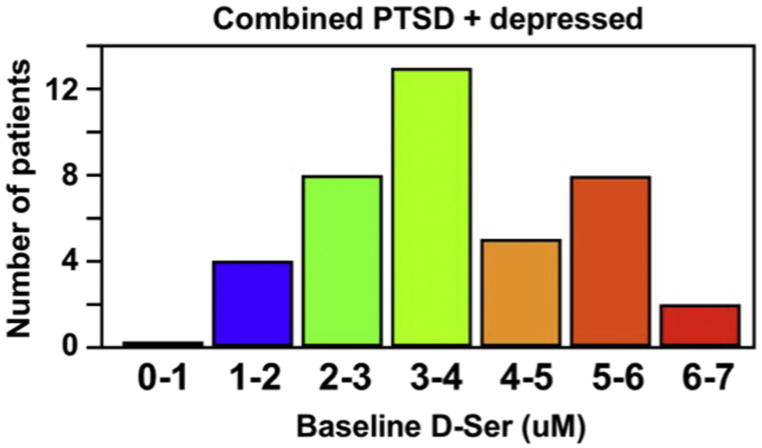
The observation that basal DSR plasma concentrations appear to be distributed into “high” and “normal” subgroups is consistent with the data from an earlier study in depressed patients [20]. The elevated severity of PTSD symptoms and shorter length of disease-free days observed in the TIMBER-K(H) subgroup relative to the TIMBER-K(N) subgroup is in accord with the hypothesis that elevated DSR plasma concentrations reflect increased NMDA receptor-based neurotoxicity although it is unknown whether this is a cause or a reflection of the condition. It is also unclear as to whether the bimodal distribution of basal DSR plasma concentrations in PTSD patients reflects genetic-based differences, such as differential expression of SNP4523957, or effects produced by the disease (PTSD, depression) itself, or both. When the measured basal DSR plasma concentrations of the PTSD subjects from this study and of the depressed patients in our earlier study were combined and displayed in histogram format, the result is consistent with a bimodal distribution within the two population, Fig. 2. In order to determine if the bimodal distribution of basal DSR plasma concentrations is an artifact or a reality, further studies with larger populations with PTSD and depressed patients are required as is the inclusion of healthy controls for comparison. These studies are currently in progress and the data will be reported elsewhere.
Fig. 2.
The distribution of basal D-serine plasma concentrations using the data from 21 patients with treatment resistant depression [20] and the 19 subjects in this study where the basal DSR plasma concentrations are grouped into 1 μM divisions (x axis) and the number of subjects within each division are presented (y axis).
The potential for the clinical manipulation of DSR plasma concentrations is evident from the effects of Ket on the plasma concentrations of patients receiving the drug for the treatment of depression. The clinical consequences are also evident as the Ket-associated decline in DSR plasma concentrations produces a mirror increase in dissociative effects as measured by the Clinician Administered Dissociative States Scale (CADSS) [20]. In addition, standard PTSD therapy involves the use of SSRIs of which fluoxetine and citalopram have been shown to decrease microglial release of DSR [38], and the antidepressant effects of the selective serotonin and noradrenaline reuptake inhibitor (SSNRI) duloxetine are reversed by administration of DSR [39]. The data suggest that the antidepressant effects are associated with an indirect attenuation of NMDA receptor activity. It is of interest to note that 13 of the 20 PTSD patients in the current study were receiving a SSRI during their treatment and no effects on clinical response or DSR basal plasma concentration were observed (data not shown). A SSRI-associated decrease in NMDA receptor activity and the dual role of DSR in fear learning and fear extinction may be associated with the observed partial response in PTSD patients produced by DSR administration [40]. Indeed, a similar effect may be responsible for the positive clinical response observed in PTSD patients treated with D-cycloserine combined with exposure psychotherapy [41]. D-Cycloserine is a partial NMDA receptor agonist and it is reasonable to expect that the administered drug would compete with endogenous DSR and lower overall NMDA receptor activity. This hypothesis has been previously proposed for the D-cycloserine-associated exacerbation of schizophrenic symptoms in patients treated with the drug [42].
In the current study, the relationship between the basal DSR plasma concentrations and the severity of the initial PTSD symptoms and the duration of treatment response is consistent with a pharmacologic mechanism in which decreased DSR concentrations result in an attenuation of NMDA receptor activity. A key observation in this study is that TIMBER psychotherapy affected DSR plasma concentrations. Mindfulness meditation and yoga have been previously shown to change brain chemistry, such as reducing concentrations of GABA, adrenocorticotropic hormone (ACTH) and pro-inflammatory cytokines, which has been related to the reduction in mental stress produced by the practices [43,44]. The data from this study indicate that TIMBER psychotherapy alone produced a significant (~10%) reduction in the 40-min DSR plasma concentrations relative to the basal concentrations, which is a further indication that mindfulness meditation produces changes in basic brain chemistry. This outcome was unexpected and the significance of this observation will be addressed in the full clinical study designed using the data from this pilot study. What is clear from the results of this study is that the TIMBER-associated decrease in plasma DSR concentrations is not sufficient to sustain the remission of PTSD symptoms. The data does show that TIMBER enhances the effect of Ket producing a profound and prolonged remission of PTSD symptoms.
The results of this study also suggest that there is a bimodal distribution of basal DSR plasma concentrations (Normal and High) and that PTSD patients within the High distribution tend to have more severe symptoms and lower duration of response in both arms of the study. It is not clear whether the elevated basal DSR plasma levels are a result of genetic polymorphisms or a PTSD-related cause or effect. These possibilities will be address in the larger clinical study and discussed elsewhere. Another source of the two populations of basal DSR plasma concentrations are the effect of co-administered medications. We conducted a thorough review of the concomitant medications used by the subjects in this study and there were no associations between basal DSR plasma concentrations and administered medications.
This study was designed to determine basal DSR plasma concentrations and the effect of the 40-min infusions on these concentrations in both the TIMBER-K and TIMBER-P arms of the study and to assess whether these parameters should be explored as potential response markers. Previous studies of the effect of Ket on DSR plasma concentration have shown that Ket produces a biphasic effect on DSR concentrations, which involves a rapid decrease (40 min) followed by a partial recovery (120 min) and then a slow decrease (7 days). The long range effects of Ket on DSR plasma concentration may be the source of the enhanced therapeutic response observed in the TIMBER-K arm relative to the TIMBER-P arm. In order to assess longitudinal effects of the TIMBER-K and TIMBER-P protocols on DSR plasma concentrations, serial plasma samples are being collected and the results will be reported elsewhere. The data also suggest that the synergism observed in the TIMBER-K arm of the study is in part due to TIMBER-enhanced attenuation of DSR plasma concentrations, which enhances Ket’s ability to induce neurogenesis and to effect synaptic plasticity by altering LTP and LTD. These are key aspects enabling the psychotherapeutic remodeling of TMs and their expression, which lay at the core of the psychopathology and dysfunctions in patients with PTSD. In addition, as stated above, this specific observation, i.e. the TIMBER-enhanced attenuation of DSR plasma concentrations, also suggests that changes in DSR plasma concentrations may reflect the effect of mindfulness meditation on stress-related symptoms and may represent a probe for physiological-psychological interactions in the PTSD diathesis. The applicability of this potential probe in healthy controls and in subjects with DSR-related conditions/anomalies is under investigation and the results will be reported elsewhere.
5. Conclusions
In conclusion, the data form this study support the earlier observation that the combination of Ket pharmacotherapy and TIMBER psychotherapy represents a valuable treatment option for refractory PTSD and a step forward in development of PTSD therapeutics. The results also indicate that DSR plasma concentrations may serve as a biomarker to predict response to TIMBER-K therapy and a means to individualize and optimize the clinical treatment of PTSD and depression. The objective of this pilot study was to set the experimental objectives for further studies. The results indicate that the investigation of relationship between basal DSR plasma levels and response in the TIMBER-K protocol should be included in larger inter-institutional studies. However, the initial results are promising and may represent a significant step towards fulfilling the “urgent need to find effective treatments for PTSD [8].”
Acknowledgments
The study was supported by funding from the Brain and Behavior Research Foundation (formerly NARSAD, Grant No. 24120) and by the Intramural Research Program of the NIA/NIH. We wish to thank Ms. Jessie Dotson, RN from the Department of Anesthesiology, Cooper University Hospital, Camden, New Jersey for her support in carrying out this research work and Nagendra S. Singh for the determination of DSR concentrations in the plasma samples.
Abbreviations
- PTSD
post-traumatic stress disorder
- Ket
(R,S)-ketamine
- TIMBER
Trauma Interventions using Mindfulness Based Extinction and Reconsolidation
- DSR
D-serine
- TM
trauma memory
- MB-GET
mindfulness based graded exposure therapy
- CBT
cognitive behavioral therapy
- LTP
long-term potentiation
- LTD
long-term depression
- PCL
PTSD Checklist
- CAPS
Clinician Administered PTSD Scale for DSM-IV
Footnotes
This article is part of a Special Issue entitled: D-Amino acids: biology in the mirror, edited by Dr. Loredano Pollegioni, Dr. Jean-Pierre Mothet and Dr. Molla Gianluca
Transparency document
The Transparency document associated with this article can be found, in the online version.
Conflicts of interest
The authors declare that there are no conflicts of interest.
References
- 1.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soeter M, Kindt M. Dissociating response systems: erasing fear from memory. Neurobiol Learn Mem. 2010;94:30–41. doi: 10.1016/j.nlm.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 4.Atwoli L, Stein DJ, Koenen KC, McLaughlin KA. Epidemiology of posttraumatic stress disorder: prevalence, correlates and consequences. Curr Opin Psychiatry. 2015;28:307–311. doi: 10.1097/YCO.0000000000000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoge CW, Grossman SH, Auchterlonie JL, Riviere LA, Milliken CS, Wilk JE. PTSD treatment for soldiers after combat deployment: low utilization of mental health care and reasons for dropout. Psychiatr Serv. 2014;65:997–1004. doi: 10.1176/appi.ps.201300307. [DOI] [PubMed] [Google Scholar]
- 6.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stein DJ, Ipser JC, Seedat S. Pharmacotherapy for post traumatic stress disorder (PTSD) Cochrane Database Syst Rev. 2006;1(1):CD002795. doi: 10.1002/14651858.CD002795.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krystal JH, Davis LL, Neylan TC, Raskind MA, Schnurr PP, Stein MB, Vessicchio J, Shiner B, Gleason TD, Huang GD. It is time to address the crisis in the pharmacotherapy of posttraumatic stress disorder: a consensus statement of the PTSD psychopharmacology working group. Biol Psychiatry. 2017;82(7):e51–e59. doi: 10.1016/j.biopsych.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Feder A, Parides MK, Murrough JW, Perez AM, Morgan J, Saxena S, Kirkwood K, aan het Rot M, Lapidus KAB, Wan L-B, Losifescu D, Charney DS. Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2014;71:681–688. doi: 10.1001/jamapsychiatry.2014.62. [DOI] [PubMed] [Google Scholar]
- 10.Aan het Rot M, Zarate CA, Jr, Charney DS, Mathew SJ. Ketamine for depression: where do we go from here? Biol Psychiatry. 2012;72:537–547. doi: 10.1016/j.biopsych.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, Iqbal S, Pillemer S, Foulkes A, Shah A, Charney DS, Mathew SJ. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170:1134–1142. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cukor J, Spitalnick J, Difede J, Rizzo A, Rothbaum BO. Emerging treatments for PTSD. Clin Psychol Rev. 2009;29:715–726. doi: 10.1016/j.cpr.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Pradhan BK. Yoga and Mindfulness Based Cognitive Therapy: A Clinical Guide. Springer International Publishing; Switzerland: 2015. [Google Scholar]
- 14.Pradhan BK, Wainer IW, Moaddel R, Torjman MC, Goldberg M, Sabia M, Parikh T, Pumariega AJ. Trauma Interventions using Mindfulness Based Extinction and Reconsolidation (TIMBER) psychotherapy prolong the therapeutic effects of single ketamine infusion on post-traumatic stress disorder and comorbid depression: a pilot randomized, placebo-controlled, cross-over clinical trial. Asia Pac J Clin Trials Nerv Syst Dis. 2017;2:80–90. [Google Scholar]
- 15.Dwyer JM, Duman RS. Activation of mammalian target of rapamycin and synaptogenesis: role in the actions of rapid-acting antidepressants. Biol Psychiatry. 2013;73:1189–1198. doi: 10.1016/j.biopsych.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paul RK, Singh NS, Khadeer M, Moaddel R, Sanghvi M, Green CE, O’Loughlin K, Torjman MC, Bernier M, Wainer IW. (R,S)-Ketamine metabolites (R,S)-norketamine and (2S,6S)-hydroxynorketamine increase the mammalian target of rapamycin (mTOR) function. Anesthesiology. 2014;121:149–159. doi: 10.1097/ALN.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Browne CA, Lucki I. Antidepressant effects of ketamine: mechanisms underlying fast-acting novel antidepressant. Front Pharmacol. 2013;4 doi: 10.3389/fphar.2013.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolosker H, Dumin E, Balan L, Foltyn V. D-Amino acids in the brain: D-serine in neurotransmission and neurodegeneration. FEBS J. 2008;275:3514–3526. doi: 10.1111/j.1742-4658.2008.06515.x. [DOI] [PubMed] [Google Scholar]
- 19.Balu DT, Presti KT, Huang CCY, Muszynski K, Radzishevesky I, Woloker H, Guffani G, Ressler J, Coyle JT. Serine racemase and D-serine in the amygdala are dynamically involved in fear learning. Biol Psychiatry. 2017. [DOI] [PMC free article] [PubMed]
- 20.Moaddel R, Luckenbaugh DA, Xie Y, Villaseñor A, Brutsche NE, Machado-Vieira R, Ramamoorthy A, Lorenzo MP, Garcia A, Bernier M, Torjman MC, Barbas C, Zarate CA, Jr, Wainer IW. D-Serine plasma concentration is a potential biomarker of (R,S)-ketamine antidepressant response in subjects with major depressive disorder. Psychopharmacology. 2014;232:399–409. doi: 10.1007/s00213-014-3669-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monson CM, Gradus JL, Young-Xu Y, Schnurr PP, Price JL, Schumm JA. Change in posttraumatic stress disorder symptoms: do clinicians and patients agree? Psychol Assess. 2008;20:131–138. doi: 10.1037/1040-3590.20.2.131. [DOI] [PubMed] [Google Scholar]
- 22.Weathers F, Litz B, Herman D, Huska J, Keane T. The PTSD Checklist-civilian Version (PCL-C) National Center for PTSD - Behavioral Sciences Division; Boston, MA: 1994. [Google Scholar]
- 23.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a clinician-administered PTSD scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 24.Beck AT, Steer RA. Beck Anxiety Inventory Manual. Harcourt Brace and Company; San Antonio, TX: 1993. [Google Scholar]
- 25.Bech P. The Bech, Hamilton, and Zung Scales for Mood Disorders: Screening and Listening: A Twenty Years Update With Reference to DSM-IV and ICD-10. Springer-Verlag; Berlin: 1995. [Google Scholar]
- 26.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 27.Xie Y, Alexander GM, Schwartzman RJ, Singh N, Torjman M, Goldberg M, Wainer IW, Moaddel R. Development and validation of a sensitive LC-MS/MS method for the determination of D-serine in human plasma. J Pharm Biomed Anal. 2014;89:1–5. doi: 10.1016/j.jpba.2013.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hashimoto K, Fukushima T, Shimizu E, Komatsu N, Watanabe H, Shinoda N, Nakazato M, Kumakiri C, Okada S, Hasegawa H, Imai K, Iyo M. Decreased serum levels of D-serine in patients with schizophrenia. Evidence in support of the N-methyl-D-aspartate receptor hypofunction hypothesis of schizophrenia. Arch Gen Psychiatry. 2003;60:572–576. doi: 10.1001/archpsyc.60.6.572. [DOI] [PubMed] [Google Scholar]
- 29.Mitani H, Shirayama Y, Yamada T, Maeda K, Ashby CR, Jr, Kawahara R. Correlation between plasma levels of glutamate, alanine and serine with severity of depression. Prog Neuro-Psychopharmacol Biol Psychiatry. 2006;30:1155–1158. doi: 10.1016/j.pnpbp.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 30.Alexander GM, Reichenberger E, Peterlin BL, Perreault MJ, Grothusen JR, Schwartzman R. Plasma amino acid changes in complex regional pain syndrome. Pain Res Treat. 2013;2013:742407. doi: 10.1155/2013/742407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ripke S, Neale BM, Corvin A, et al. Biological insights from 108 schizophrenia-associated genetic loci. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Nature. 2014;511(7510):421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moaddel R, Abdrakhmanova G, Kozak J, Jozwiak K, Toll L, Jimenez L, Rosenberg A, Tran T, Xiao Y, Zarate CA, Jr, Wainer IW. Sub-anesthetic concentrations of (R,S)-ketamine metabolites inhibit acetylcholine-evoked currents in α7 nicotinic acetylcholine receptors. Eur J Pharmacol. 2013;698:228–234. doi: 10.1016/j.ejphar.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh NS, Paul RK, Ramamoorthy A, Torjman MC, Moaddel R, Bernier M, Wainer IW. Nicotinic acetylcholine receptor antagonists alter the function and expression of serine racemase in PC-12 and 1321N1 cells. Cell Signal. 2013;25:2634–2645. doi: 10.1016/j.cellsig.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh NS, Rutkowska E, Plazinska A, Khadeer M, Moaddel R, Jozwiak K, Bernier M, Wainer IW. Ketamine metabolites enantioselectively decrease intracellular D-serine concentrations in PC-12 cells. PLoS One. 2016;11(4):e0149499. doi: 10.1371/journal.pone.0149499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jirásková-Vanícková J, Ettrich R, Vorlová B, Hoffman H, Lepšík M, Jansa P, Konvalinka J. Inhibition of human serine racemase, an emerging target for medicinal chemistry. Curr Drug Targets. 2011;12:1037–1055. doi: 10.2174/138945011795677755. [DOI] [PubMed] [Google Scholar]
- 36.Sethuraman R, Lee T, Tachibana S. D-Serine regulation: a possible therapeutic approach for central nervous diseases and chronic pain. Mini Rev Med Chem. 2009;9:813–819. doi: 10.2174/138955709788452630. [DOI] [PubMed] [Google Scholar]
- 37.Labrie V, Fukumura R, Rastogi A, Fick LJ, Wang W, Boutros PC, Kennedy JL, Semeralul MO, Lee FH, Baker GB, Belsham DD, Barger SW, Gondo Y, Wong AHC, Roder JC. Serine racemase is associated with schizophrenia susceptibility in humans and in a mouse model. Human Mol Genet. 2009;18:3227–3243. doi: 10.1093/hmg/ddp261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dhami KS, Churchward MA, Baker GB, Todd KG. Fluoxitine and citalopram decrease microglial release of glutamate and D-serine to promote cortical neuronal viability following ischemic insult. Mol Cell Neurosci. 2013;56:365–374. doi: 10.1016/j.mcn.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 39.Polezak E, Szewczyk Wlaz, Waiz A, Kasperek R, Wrobel A, Nowak G. A complex interaction between glycine/NMDA receptors and serotonergic/noradrenergic antidepressants in the forced swim test in mice. J Neural Transm. 2011;118:1535–1546. doi: 10.1007/s00702-011-0630-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heresco-Levy U, Vass A, Bloch B, Wolosker H, Dumin E, Balan L, Deutsch L, Kremer H. Pilot controlled trial of D-serine for the treatment of post-traumatic stress disorder. Int J Neuropsychopharmacol. 2009;12:1275–1282. doi: 10.1017/S1461145709000339. [DOI] [PubMed] [Google Scholar]
- 41.de Kleine RA, Rothbaum BO, van Minnen A. Pharmacological enhancement of exposure-based treatment in PTSD: a qualitative review. Eur J Psychotrauma. 2013;4:21626. doi: 10.3402/ejpt.v4i0.21626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Berckel BNM, Evenblj CN, van Loon BJAM, Maas MF, van der Geld MAM, Wynne HJ, van Ree JM, Kahn RS. D-Cycloserine increases positive symptoms in chronic schizophrenic patients when administered in addition to antripsychotics: a double-blind, parallel placebo-controlled study. Neuropsychopharmacology. 1999;21:203–210. doi: 10.1016/S0893-133X(99)00014-7. [DOI] [PubMed] [Google Scholar]
- 43.Streeter CC, Whitfield TH, Owen L, Rein T, Karri SK, Yakhkind A, Perlmutter R, Prescot A, Renshaw PF, Ciraulo DC, Jensen JE. Effects of yoga versus walking on mood, anxiety, and brain GABA levels: a randomized controlled MRS study. J Altern Complement Med. 2010;16:1145–1152. doi: 10.1089/acm.2010.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoge EA, Bui E, Palitz AP, Schwarz NR, Owens ME, Johnston JM, Pollack MH, Simone NM. The effect of mindfulness meditation training on biological acute stress responses in generalized anxiety disorder. Psychiatry Res. 2017. [DOI] [PMC free article] [PubMed]