Abstract
The antibacterial activity of TAK-083 was tested against 54 clinical isolates of Helicobacter pylori and was compared with those of amoxicillin, clarithromycin, and metronidazole. The growth-inhibitory activity of TAK-083 was more potent than that of amoxicillin, clarithromycin, or metronidazole (the MICs at which 90% of the strains are inhibited were 0.031, 0.125, 64, and 8 μg/ml, respectively). The antibacterial activity of TAK-083 was highly selective against H. pylori; there was a >30-fold difference between the concentration of TAK-083 required to inhibit the growth of H. pylori and that required to inhibit the growth of common aerobic and anaerobic bacteria. Exposure of H. pylori strains to TAK-083 at the MIC or at a greater concentration resulted in an extensive loss of viability. When four H. pylori strains were successively subcultured in the medium containing subinhibitory concentrations of TAK-083, no significant change in the MICs of this compound was observed. TAK-083 strongly inhibited the formation of tryptophanyl-tRNA in H. pylori while exhibiting little effect on the same system in eukaryotes. TAK-083 was efficacious in the treatment of gastric infection caused by H. pylori in Mongolian gerbils. The results presented here indicate that TAK-083 is a promising candidate for the treatment of H. pylori infection.
Helicobacter pylori is the major causative agent of chronic active gastritis in humans, and infection with this organism is considered to be an important etiological factor in the pathogenesis of peptic ulcer disease and possibly of gastric cancer (22, 27, 30). As clinical evidence shows that eradication of H. pylori results in a significant reduction of ulcer relapse, cure of H. pylori infection has become an important goal in the healing and prevention of peptic ulcers (16). While in vitro experiments have shown that various antibacterial agents possess anti-H. pylori activity, it is clear that essentially none of these agents has demonstrated high enough bacterial eradication rates to be used routinely (1, 6, 7, 24, 26, 28).
Until recently, the most effective treatment regimens have been those using a combination of bismuth salts and a nitroimidazole together with amoxicillin or tetracycline. Although this therapy has achieved high eradication rates and suppression of ulcer recurrence, some drawbacks have been noted with this therapy; one is the emergence of metronidazole resistance, and another is low compliance related to the occurrence of side effects (9). More recently, proton pump inhibitor-based triple therapy has been introduced with improved efficacy (2, 17). However, the development of resistance in isolates to metronidazole and clarithromycin and the relatively high incidence of side effects make this regimen far from ideal.
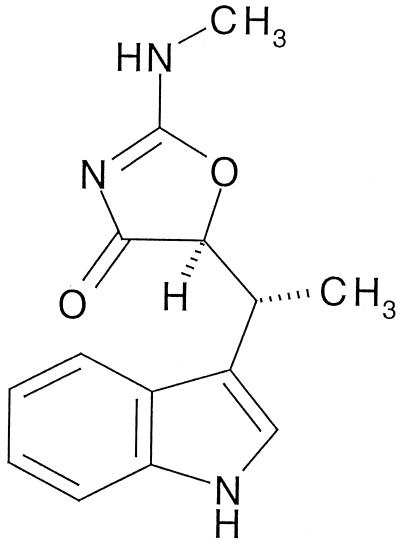
According to the present needs for simpler and more effective strategies for the treatment of H. pylori infection, antibiotics having the following characteristics have been sought among many secondary metabolites of microorganisms and synthetic compounds: first, highly selective antibacterial activity against H. pylori; second, stability in 0.1 N HCl. Finally, a promising substance was found in the fermentation broth of Streptomyces sp. strain HC-21, which was isolated from a soil sample from the city of Asahikawa, Hokkaido, Japan (13). An active substance designated TAK-083 was isolated and identified as indolmycin {(5S)-5-[(1R)-1-(indol-3-yl)ethyl]-2-methylamino-4-oxazolone} (Fig. 1) (18, 25). The mechanism of antibacterial activity of indolmycin lies in its ability to inhibit bacterial tryptophanyl-tRNA synthetase (26). Because of its insufficient activity against common pathogenic bacteria, the development of indolmycin for chemotherapeutic use was abandoned, but TAK-083 has a highly selective and potent anti-H. pylori activity and exhibits excellent efficacy in clearing H. pylori in experimentally infected animals. Therefore, TAK-083 is regarded as an attractive candidate for curing H. pylori infection.
FIG. 1.
Chemical structure of TAK-083.
MATERIALS AND METHODS
Bacterial strains.
Fifty-four clinical isolates of H. pylori and strain NCTC 11637 were used. The identification of clinical isolates was based on microaerophilic growth requirement, morphology, Gram stain and oxidase, catalase, and rapid urease reactions (23). The bacterial strain used for experimental infection was originally isolated from a human patient and was adapted to the gastric mucosa of Mongolian gerbils by four serial passages. Stock cultures were kept at −80°C in brucella broth (BBL, Becton Dickinson Microbiology Systems, Cockeysville, Md.) supplemented with 2.5% heat-inactivated fetal bovine serum (FBS) and 15% glycerol. H. pylori strains were grown in brucella broth supplemented with 2.5% FBS in GasPak jars (BBL) containing Campy Paks (BBL) with shaking at 37°C overnight. The laboratory standard strains of common aerobic and anaerobic bacteria were obtained from our culture collection.
Antibacterial agents.
TAK-083 and amoxicillin were prepared at the Pharmaceutical Research Division of Takeda Chemical Industries, Ltd., Osaka, Japan. The following antimicrobial agents were purchased commercially: clarithromycin (Taisho Pharmaceuticals, Ltd., Tokyo, Japan) and metronidazole (Sigma Chemical Co., St. Louis, Mo.).
Susceptibility testing.
The MICs for H. pylori were determined by an agar dilution method. Bacterial suspensions of approximately 106 CFU/ml, prepared with brucella broth supplemented with 2.5% FBS, were applied to the brucella agar (BBL) plates supplemented with 7% defibrinated horse blood containing twofold serial dilutions of test compounds using a multiple inoculator capable of delivering 5-μl samples. The plates were incubated at 37°C for 4 days in a microaerobic atmosphere consisting of 5% O2, 10% CO2, and 85% N2. MICs for common aerobic and anaerobic bacteria were also determined by the agar dilution method. Mueller-Hinton agar (BBL) alone or supplemented with 5% defibrinated horse blood was used for aerobic bacteria, and Gifu Anaerobic Medium (GAM) Agar Modified (Nissui Seiyaku Co., Ltd., Tokyo, Japan) was used for anaerobic bacteria. The inocula were prepared in Mueller-Hinton broth (BBL) for aerobic bacteria and in GAM broth (Nissui) for anaerobic bacteria from an overnight culture and adjusted to give approximately 106 CFU/ml. Plates were incubated at 37°C for 20 h in an aerobic atmosphere for aerobic bacteria, 24 h in an anaerobic glove box for anaerobic bacteria, and 4 days in a microaerobic atmosphere for Campylobacter jejuni. MICs were defined as the lowest concentrations of the compounds preventing visible bacterial growth. To examine the effects of pH variation on the susceptibility of H. pylori to antibacterial agents, brucella agar plates supplemented with 7% defibrinated horse blood were adjusted to a pH range of 4.5 to 7.5 by adding hydrochloric acid or sodium hydroxide.
Bactericidal activity.
Portions of brucella broth with 2.5% FBS (10 ml) containing TAK-083 at concentrations of 0.25, 1, 4, and 16 times the MIC were inoculated with bacteria from an overnight culture to yield an initial cell concentration of approximately 106 CFU/ml. The cultures were shaken at 37°C in a microaerobic atmosphere, and 100-μl portions were removed at various time points (0, 3, 6, and 24 h). Viable bacteria were counted after serial 10-fold dilutions were made in brucella broth with 2.5% FBS, and a 50-μl portion of each was inoculated in triplicate onto brucella agar supplemented with 7% defibrinated horse blood. Colonies were counted after 4 days of incubation in a microaerobic atmosphere.
Assay for development of resistance.
The standard strain and clinical isolates of H. pylori adjusted to a cell density of approximately 106 CFU/ml in brucella broth supplemented with 2.5% FBS were exposed to serial twofold dilutions of TAK-083 and metronidazole. Following incubation of the bacteria at 37°C for 24 h, the MIC, defined as the lowest concentration of the agent that allowed no visible growth, was recorded, and then the culture that attained a turbidity comparable to that of the untreated culture in the presence of the highest level of the test agent was further exposed to increasing concentrations of the test agent. These procedures were repeated for up to 10 cycles. The fluctuations of the MIC in the course of the repeated exposure of the bacteria to the test agent were determined.
Assay for the tryptophanyl-tRNA formation.
Aminoacylation of each tRNA (Escherichia coli and calf liver) was carried out in 100 μl of a reaction mixture containing 50 mM Tris-HCl (pH 8.0), 70 mM KCl, 10 mM Mg(OAc)2, 5 mM ATP, 1.25% glycerol, 1 μM l-[5-3H]tryptophan (specific activity, 1.07 TBq/mmol; Amersham Pharmacia Biotech), aminoacyl-tRNA synthetase (ARS), and serially diluted TAK-083. The following combinations of ARS with tRNA were examined: H. pylori ARS with E. coli tRNA (Roche Diagnostics), E. coli ARS (Sigma) with E. coli tRNA, and bovine ARS (Sigma) with calf liver tRNA (Roche Diagnostics). After incubation at 30°C for 10 min, the reaction was stopped by adding 10 μl of 0.5 M EDTA followed by a washing with ice-cold 5% trichloroacetic acid and distilled water. The precipitates collected on filtration plates (Millipore) were dried and counted in a liquid scintillation counter (Win Spectra α/β 1414 liquid scintillation counter; Wallac) by adding scintillator A (Wako Pure Chemical Ltd., Osaka, Japan). The sample was measured in triplicate. To compare the inhibitory activities of TAK-083, enzyme activities of three kinds of enzyme system were adjusted to the same level as 0.03 pmol of l-[5-3H]tryptophanyl-tRNA formed in 10 min.
H. pylori gastric infection of Mongolian gerbils.
Five-week-old male MON/Jms/Gbs Slc Mongolian gerbils (SLC Japan Inc., Shizuoka, Japan) which had been fasting for 20 h were inoculated intragastrically with a bacterial culture containing 107.11 CFU of H. pylori TN85GF4. TAK-083, suspended in 0.5% aqueous solution of methylcellulose, was given orally twice daily for 7 days starting at 13 days after infection. The doses were set based on the mean body weight determined on the day before administration and after the seventh administration. On the day after the final administration, the stomach of each infected Mongolian gerbil was excised and disrupted; a series of 10-fold dilutions of the stomach homogenate were each inoculated into modified Skirrow's medium (4) supplemented with activated charcoal and cultured under microaerobic conditions at 37°C for 4 days, after which the clearance effect was assessed on the basis of the presence or absence of bacterial growth. All animal experiments were conducted in accordance with the guidelines for the care and use of laboratory animals of the Pharmaceutical Research Division, Takeda Chemical Industries, Ltd., and approved by the ethical committee for the animal experiments of our division.
Statistics.
Differences in bacterial counts in the gastric wall between the control-treated and the TAK-083-treated groups were analyzed by Dunnett's ranked test. P values below 0.05 were considered statistically significant.
RESULTS
Antibacterial activity.
The ranges of the MICs for TAK-083, amoxicillin, clarithromycin, and metronidazole against 54 clinical isolates of H. pylori and the minimal concentrations required to inhibit 50% of isolates (MIC50) and 90% of isolates (MIC90) are shown in Table 1. TAK-083 inhibited the growths of all the tested strains at ≤0.031 μg/ml, and its activity was more than fourfold more potent than those of the currently available anti-H. pylori agents. No strain resistant against TAK-083 was noted among the clinical isolates of H. pylori. TAK-083 showed a moderate antibacterial activity against some gram-positive aerobes, C. jejuni, and Eubacterium alactolyticum (Table 2). The effects of medium pH on the antibacterial activities of TAK-083, amoxicillin, and clarithromycin against 29 clinical isolates of H. pylori were examined. As summarized in Table 3, the MICs of TAK-083 remained stable over the pH range of 4.5 to 7.5. The activity of clarithromycin, on the other hand, was considerably diminished under acidic environment, and that of amoxicillin was influenced slightly.
TABLE 1.
Antibacterial activity of TAK-083, amoxicillin, clarithromycin, and metronidazole against 54 clinical isolates of H. pylori
| Compound | MIC (μg/ml)
|
||
|---|---|---|---|
| Range | MIC50a | MIC90b | |
| TAK-083 | ≤0.008–0.031 | 0.016 | 0.031 |
| Amoxicillin | ≤0.008–0.5 | 0.031 | 0.125 |
| Clarithromycin | 0.016–128 | 0.063 | 64 |
| Metronidazole | 2–128 | 4 | 8 |
MIC required to inhibit 50% of strains.
MIC required to inhibit 90% of strains.
TABLE 2.
Antibacterial activity of TAK-083 against aerobic and anaerobic bacteria
| Organism | Strain | MIC (μg/ml) |
|---|---|---|
| Staphylococcus aureus | FDA 209P | 2 |
| Staphylococcus epidermidis | IFO 3762 | 4 |
| Streptococcus pneumoniae | Type I | >128 |
| Enterococcus faecalis | IFO 12580 | >128 |
| Moraxella catarrhalis | BN-2 | 4 |
| Escherichia coli | NIHJ JC-2 | 32 |
| Citrobacter freundii | IFO 12681 | >128 |
| Klebsiella pneumoniae | IFO 3321 | >128 |
| Enterobacter cloacae | CS4495 | >128 |
| Serratia marcescens | IFO 12648 | >128 |
| Proteus vulgaris | IFO 3988 | 64 |
| Morganella morganii | IFO 3168 | >128 |
| Pseudomonas aeruginosa | IFO 3445 | >128 |
| Acinetobacter calcoaceticus | IFO 13006 | >128 |
| Campylobacter jejuni | 455 | 0.5 |
| Peptostreptococcus anaerobius | B-30 | 32 |
| Clostridium perfringens | CW-2 | >128 |
| Lactobacillus acidophilus | IID-893 | >128 |
| Eubacterium aerofaciens | ATCC 25986 | 16 |
| Eubacterium alactolyticum | 1441 | 0.5 |
| Eubacterium limosum | ATCC 8486 | 4 |
| Bifidobacterium adolescentis | 15706 | 32 |
| Bifidobacterium bifidum | aE-319 | 64 |
| Bifidobacterium pseudolongum | Mo2-10 | >128 |
| Bacteroides fragilis | 2509 | >128 |
| Bacteroides vulgatus | W-6 | >128 |
| Fusobacterium varium | ATCC 8501 | 16 |
| Fusobacterium mortiferum | 15 | 16 |
| Fusobacterium glutinosum | Ju-21 | 16 |
TABLE 3.
Effects of medium pH on antibacterial activity of TAK-083, amoxicillin, and clarithromycin against 29 clinical isolates of H. pylori
| Compound pH | MIC (μg/ml)
|
||
|---|---|---|---|
| Range | MIC50a | MIC90b | |
| TAK-083 | |||
| 4.5 | ≤0.008–0.063 | 0.016 | 0.031 |
| 5.5 | ≤0.008–0.031 | 0.016 | 0.031 |
| 6.5 | ≤0.008–0.031 | 0.016 | 0.031 |
| 7.5 | ≤0.008–0.016 | 0.016 | 0.016 |
| Amoxicillin | |||
| 4.5 | 0.031–1 | 0.125 | 0.5 |
| 5.5 | 0.016–1 | 0.063 | 0.25 |
| 6.5 | ≤0.008–0.5 | 0.031 | 0.25 |
| 7.5 | ≤0.085–0.5 | 0.031 | 0.25 |
| Clarithromycin | |||
| 4.5 | 0.125–>128 | 1 | >128 |
| 5.5 | 0.125–>128 | 0.5 | 128 |
| 6.5 | 0.016–64 | 0.063 | 16 |
| 7.5 | ≤0.008–8 | 0.016 | 4 |
MIC required to inhibit 50% of strains.
MIC required to inhibit 90% of strains.
Bactericidal activity.
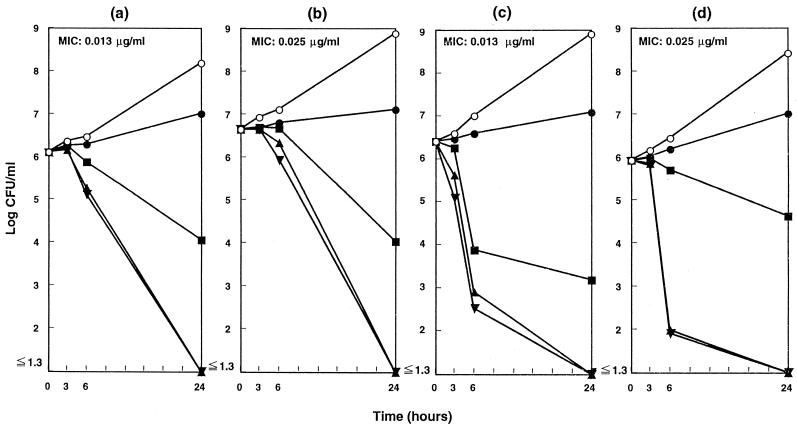
The killing kinetics of TAK-083 for H. pylori strains is summarized in Fig. 2. TAK-083 showed concentration-dependent bactericidal activity against all tested strains of H. pylori, and the number of viable organisms decreased progressively by exposure to it at the MIC or at greater concentrations.
FIG. 2.
Bactericidal activity of TAK-083 against H. pylori in a liquid medium. H. pylori NCTC 11637 (a), CPY433 (b), TN2 (c), and TN58 (d) were cultured microaerobically in brucella broth with 2.5% heat-inactivated FBS at 37°C with shaking and exposed to TAK-083 at concentrations of 0 (○), 0.25× MIC (●), 1× MIC (■), 4× MIC (▴), and 16× MIC (▾). After further incubation, the viability was determined at each time point.
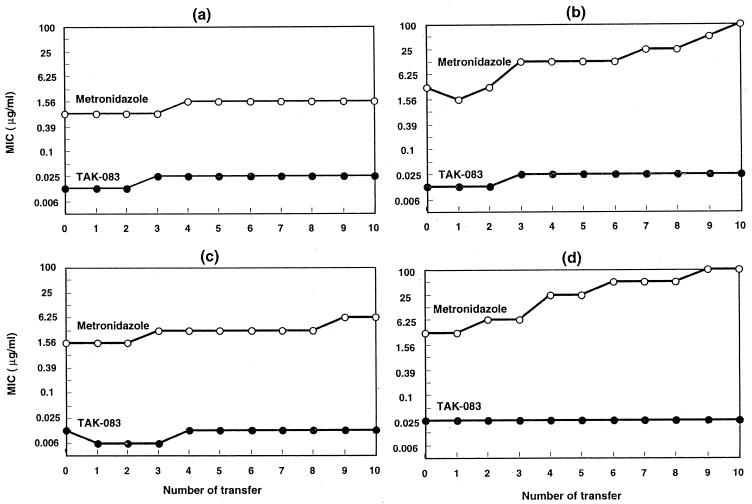
Effect on development of resistance.
There were no significant changes in the susceptibilities to TAK-083 of any of the tested H. pylori strains following repeated exposure to the drug. Metronidazole, on the other hand, induced a rapid emergence of resistance (Fig. 3).
FIG. 3.
Development of resistance to TAK-083 and metronidazole in H. pylori NCTC 11637 (a), CPY433 (b), TN2 (c), and TN58 (d).
Inhibitory effect against tryptophanyl-tRNA synthetase.
The inhibitory activity of TAK-083 against tryptophanyl-tRNA synthetase was comparatively examined by using three kinds of enzyme sources (H. pylori, E. coli, and bovine liver). TAK-083 selectively inhibited the formation of tryptophanyl-tRNA in prokaryotic systems such as H. pylori (with a 50% inhibitory concentration [IC50] of 12.2 nM) and E. coli (IC50, 9.25 nM), whereas the corresponding eukaryotic system (bovine liver) was hardly affected (IC50, 4.04 mM).
Therapeutic effect against experimental gastric infection by H. pylori in Mongolian gerbils.
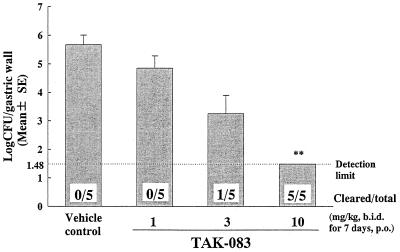
All of the vehicle-treated control Mongolian gerbils maintained gastric H. pylori at a level of approximately 105 CFU. TAK-083 was efficacious in reducing the numbers of infecting H. pylori in a dose-dependent fashion, and a complete clearance was obtained at a dose of 10 mg/kg of body weight (Fig. 4).
FIG. 4.
Therapeutic effects of TAK-083 against gastric infection caused by H. pylori TN85GF4 in MON/Jms/Gbs Slc Mongolian gerbils. The doses (in parentheses, dose ranges) (in milligrams per kilogram) are as follows: 1 (0.892 to 1.10), 3 (2.72 to 3.31), and 10 (8.57 to 11.1). ∗∗, P < 0.01 by Dunnett's test on ranked data; b.i.d., twice a day; p.o., orally.
DISCUSSION
Intensive research in the field of peptic ulcer disease led to the recognition that almost all duodenal ulcers and the majority of gastric ulcers are disorders induced by H. pylori infection (10). Moreover, persistent H. pylori infection has been shown to be closely related to relapse of peptic ulcers as well as ulcer complications (14, 16). Therefore, anti-H. pylori treatment seems to be a logical approach, promising rapid ulcer healing and a stable remission of ulcer disease. In contrast with conventional antibiotics, TAK-083 showed a potent anti-H. pylori activity with no noticeable effects on common aerobic and anaerobic bacteria. The selective anti-H. pylori activity of TAK-083 may be conferred by its preferential penetrability to the target site, as has been shown for hydrophobic compounds of small molecular size (3). Because antibiotics currently used in eradication therapy have broad antibacterial spectra and would therefore affect the natural gut flora, it is likely that their use would be accompanied by gastrointestinal side effects. As the antibacterial spectrum of TAK-083 is mainly restricted to H. pylori, its oral use would not cause the disturbance of normal gastrointestinal microflora.
There have been discrepancies between the in vitro antibacterial activities and the clinical efficacies of several antibacterial agents in the clearance of H. pylori from the gastric mucosa in the presence of peptic ulcer disease (20, 28). In spite of excellent anti-H. pylori activity in vitro, neither clarithromycin nor amoxicillin has been shown effective in clearing H. pylori in the Mongolian gerbil model (14, 21). One possible explanation for the discordance between in vitro activity and in vivo effectiveness of antibacterial agents is the diminished activity of these agents at a low pH (11). In the present study, the potent anti-H. pylori activity of TAK-083 was shown to remain stable under acidic conditions. Following oral administration of TAK-083 at 10 mg/kg, which enabled a complete clearance of gastric H. pylori in the Mongolian gerbils, the plasma and gastric mucosal levels of TAK-083 far surpassed the MIC (data not shown).
The increasing prevalence of H. pylori strains resistant to some of the most commonly used antibacterial agents is the major cause of failure to eradicate the infection (15). Some investigators have related that secondary resistance against clarithromycin and metronidazole develops rapidly and limits the usefulness of a number of potentially effective agents (7, 8, 26). We have found that all the tested clinical isolates were susceptible to TAK-083 and that repeated exposure of H. pylori strains to TAK-083 in vitro resulted in no selection of resistant mutants, unlike what is observed with metronidazole.
Eradication of H. pylori has been shown to be effective not only for preventing the recurrence of peptic ulcers but also for curing them (12, 29). H. pylori infection has been implicated in the pathogenesis of gastric cancer, and the interleukin-1 polymorphisms in the host have been shown to be associated with increased risk of gastric cancer (5). Therefore, application of H. pylori eradication therapy for high-risk individuals is rationalized to prevent subsequent development of gastric cancer. In the present study, administration of TAK-083 resulted in a complete clearance of H. pylori in experimentally infected Mongolian gerbils. In conclusion, TAK-083 is an attractive candidate for the cure of H. pylori infection, and further studies of its potential clinical use are warranted.
REFERENCES
- 1.Bayerdorfer E, Ottenjann R. The role of antibiotics in Campylobacter pylori associated peptic ulcer disease. Scand J Gastroenterol. 1988;23:93–100. [PubMed] [Google Scholar]
- 2.Bazzoli F, Zagari R M, Fossi S, Pozzato P, Alampi G, Simoni P, Sottili S, Ronda A, Ronda E. Short-term low dose triple therapy for the eradication of Helicobacter pylori. Eur J Gastroenterol. 1994;6:773–777. doi: 10.1046/j.1365-2036.1998.00330.x. [DOI] [PubMed] [Google Scholar]
- 3.Bina J E, Alm R A, Uria-Nickelsen M, Thomas S R, Trust T J, Hancock R E W. Helicobacter pylori uptake and efflux: basis for intrinsic susceptibility to antibiotics in vitro. Antimicrob Agents Chemother. 2000;44:248–254. doi: 10.1128/aac.44.2.248-254.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dent J C, McNulty C A M. Evaluation of a new selective medium for Campylobacter pylori. Eur J Clin Microbiol Infect Dis. 1988;7:555–558. doi: 10.1007/BF01962615. [DOI] [PubMed] [Google Scholar]
- 5.El-Omar E M, Carrington M, Chow W H, McColl K E L, Bream J H, Young H A, Herrera J, Lissowska J, Yuan C C, Rothman N, Lanyon G, Martin M, Faumeni J F, Jr, Rabkin C S. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 6.Glupczynski Y, Labbe M, Burette A, Delmee M, Avesani V, Bruck C. Treatment failure of ofloxacin in Campylobacter pylori infection. Lancet. 1987;i:1096. doi: 10.1016/s0140-6736(87)90527-7. [DOI] [PubMed] [Google Scholar]
- 7.Glupczynski Y, Burette A. Drug therapy for Helicobacter pylori infection: problems and pitfalls. Am J Gastroenterol. 1990;85:1545–1551. [PubMed] [Google Scholar]
- 8.Graham D Y, Borsch G M. The who's and when's of therapy for Helicobacter pylori. Am J Gastroenterol. 1990;85:1552–1555. [PubMed] [Google Scholar]
- 9.Graham D Y, Lew G M, Malaty H M, Evans D J, Klein P D, Alpert L C, Genta R M. Factors influencing the eradication of Helicobacter pylori with triple therapy. Gastroenterology. 1992;102:493–496. doi: 10.1016/0016-5085(92)90095-g. [DOI] [PubMed] [Google Scholar]
- 10.Graham D Y. Treatment of peptic ulcers caused by Helicobacter pylori. N Engl J Med. 1993;328:349–350. doi: 10.1056/NEJM199302043280512. [DOI] [PubMed] [Google Scholar]
- 11.Grayson M L, Eliopoulos G M, Ferraro M J, Moellering R C. Effect of varying pH on the susceptibility of Campylobacter pylori to antimicrobial agents. Eur J Clin Microbiol Infect Dis. 1989;8:888–889. doi: 10.1007/BF01963775. [DOI] [PubMed] [Google Scholar]
- 12.Hosking S W, Ling T K W, Chung S C S, Yung M Y, Cheng A F B. Duodenal ulcer healing by eradication of Helicobacter pylori without anti-acid treatment: randomized controlled trial. Lancet. 1994;343:508–510. doi: 10.1016/s0140-6736(94)91460-5. [DOI] [PubMed] [Google Scholar]
- 13.Kanamaru, T., M. Nakao, H. Tawada, and K. Kamiyama. December 1997. Oxazolone derivatives and their use. Patent, international publication no. WO97/49703.
- 14.Keto Y, Takahashi S, Okabe S. Healing of Helicobacter pylori-induced gastric ulcers in Mongolian gerbils: combined treatment with omeprazole and clarithromycin. Dig Dis Sci. 1999;44:257–265. doi: 10.1023/a:1026685929992. [DOI] [PubMed] [Google Scholar]
- 15.Labenz J, Gyenes E, Ruhl G H, Borsch G. Amoxicillin plus omeprazole versus triple therapy for eradication of Helicobacter pylori in duodenal ulcer disease: a prospective, randomized, controlled study. Gut. 1993;34:1167–1170. doi: 10.1136/gut.34.9.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Labenz J, Borsch G. Evidence of the essential role of Helicobacter pylori in gastric ulcer disease. Gut. 1994;35:19–22. doi: 10.1136/gut.35.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamouliatte H, Cayla R, Zerbib F, Talbi P, Mégraud F. Triple therapy using proton pump inhibitor-amoxicillin and clarithromycin for Helicobacter pylori eradication. Gut. 1995;37(Suppl. 1):A91. . (Abstract.) [Google Scholar]
- 18.Marsh W S, Garretson A L, Wesel E M. PA155A, B and X: antibiotics produced by a strain of Streptomyces albus. Antibiot Chemother. 1960;10:316–320. [PubMed] [Google Scholar]
- 19.Marshall B J, Goodwin C S, Warren J R, Murray R, Blincow C R. Prospective, double-blind trial of duodenal ulcer relapse after eradication of Campylobacter pylori. Lancet. 1988;ii:1437–1442. doi: 10.1016/s0140-6736(88)90929-4. [DOI] [PubMed] [Google Scholar]
- 20.McNulty C A M, Gearty J C, Crump B, Davis M, Donovan I A, Melikian V, Lister D M, Wise R. Campylobacter pyloridis and associated gastritis: investigator blind, placebo controlled trial of bismuth salicylate and erythromycin ethylsuccinate. Br Med J. 1986;293:645–649. doi: 10.1136/bmj.293.6548.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagahara N, Akiyama Y, Nakao M, Tada M, Kitano M, Ogawa Y. Mucoadhesive microspheres containing amoxicillin for clearance of Helicobacter pylori. Antimicrob Agents Chemother. 1998;42:2492–2494. doi: 10.1128/aac.42.10.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nomura A, Stemmermann G N, Chyou P H, Perez-Perez G I, Blaser M J. Helicobacter pylori infection and the risk for duodenal and gastric ulceration. Ann Intern Med. 1994;120:977–981. doi: 10.7326/0003-4819-120-12-199406150-00001. [DOI] [PubMed] [Google Scholar]
- 23.Penner J L. Campylobacter, Helicobacter and related spiral bacteria. In: Balows A, Hausler W J, Herrmann K L, Isenberg H D, Shadomy H J, editors. Manual of clinical microbiology. Washington, D.C.: American Society for Microbiology; 1991. pp. 402–409. [Google Scholar]
- 24.Peterson W L, Graham D Y, Marshall B, Blaser M J, Genta R M, Klein P D, Stratton C W, Drnec J, Prokocimer P, Siepman N. Clarithromycin as monotherapy for eradication of Helicobacter pylori: a randomized, double-blind trial. Am J Gastroenterol. 1993;88:1860–1864. [PubMed] [Google Scholar]
- 25.Ras K V. PA155A: new antibiotic. Antibiot Chemother. 1960;10:312–315. [PubMed] [Google Scholar]
- 26.Rautelin H, Seppala K, Renkonen O, Vainio U, Kosunen T U. Role of metronidazole resistance in therapy of Helicobacter pylori infection. Antimicrob Agents Chemother. 1992;36:163–166. doi: 10.1128/aac.36.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sipponen P, Hyvarinen H. Role of Helicobacter pylori in the pathogenesis of gastritis, peptic ulcer and gastric cancer. Scand J Gastroenterol. 1993;28(Suppl. 196):3–6. doi: 10.3109/00365529309098333. [DOI] [PubMed] [Google Scholar]
- 28.Stone J W, Wise R, Donovan I A, Gearty J. Failure of ciprofloxacin to eradicate Campylobacter pylori from the stomach. J Antimicrob Chemother. 1988;22:92–93. doi: 10.1093/jac/22.1.92. [DOI] [PubMed] [Google Scholar]
- 29.Sung J J Y, Chung S C S, Ling T K W, Yung M Y, Leung V K S, Ng E K W, Li M K K, Cheng A F B, Li A K C. Antibacterial treatment of gastric ulcers associated with Helicobacter pylori. N Engl J Med. 1995;332:139–142. doi: 10.1056/NEJM199501193320302. [DOI] [PubMed] [Google Scholar]
- 30.Webb P M, Forman D. Helicobacter pylori as a risk factor for cancer. Bailliere's Clin Gastroenterol. 1995;9:563–582. doi: 10.1016/0950-3528(95)90049-7. [DOI] [PubMed] [Google Scholar]
- 31.Werner R G, Thorpe L F, Reuter W, Nierhaus K H. Indolmycin inhibits prokaryotic tryptophanyl-tRNA ligase. Eur J Biochem. 1976;68:1–3. doi: 10.1111/j.1432-1033.1976.tb10758.x. [DOI] [PubMed] [Google Scholar]