Abstract
A continuous infusion of vancomycin (CIV) may provide an alternative mode of infusion in severe hospital-acquired methicillin-resistant staphylococcal (MRS) infections. A multicenter, prospective, randomized study was designed to compare CIV (targeted plateau drug serum concentrations of 20 to 25 mg/liter) and intermittent infusions of vancomycin (IIV; targeted trough drug serum concentrations of 10 to 15 mg/liter) in 119 critically ill patients with MRS infections (bacteremic infections, 35%; pneumonia, 45%). Microbiological and clinical outcomes, safety, pharmacokinetics, ease of treatment adjustment, and cost were compared. Microbiological and clinical outcomes and safety were similar. CIV patients reached the targeted concentrations faster (36 ± 31 versus 51 ± 39 h, P = 0.029) and fewer samples were required for treatment monitoring than with IIV patients (7.7 ± 2.2 versus 11.8 ± 3.9 per treatment, P < 0.0001). The variability between patients in both the area under the serum concentration-time curve (AUC24h) and the daily dose given over 10 days of treatment was lower with CIV than with IIV (variances, 14,621 versus 53,975 mg2/liter2/h2 [P = 0.026] and 414 versus 818 g2 [P = 0.057], respectively). The 10-day treatment cost per patient was $454 ± 137 in the IIV group and was 23% lower in the CIV group ($321 ± 81: P < 0.0001). In summary, for comparable efficacy and tolerance, CIV may be a cost-effective alternative to IIV.
In a recent report of antimicrobial use and bacterial resistance in U.S. hospitals, methicillin-resistant staphylococci (MRS) were found to represent 54% of nonduplicate clinical isolates of staphylococci in intensive care unit (ICU) patients and 51% of those in non-ICU inpatients (11). Intravenously administered vancomycin remains the drug of choice for treatment of MRS infections (8), and a conventional dosage for adults with normal renal function is either 500 mg every 6 h or 1,000 mg every 12 h adjusted on the basis of trough and peak vancomycin concentrations in serum. Dosage (9, 14) and treatment monitoring (23) have been recently re-evaluated for several reasons. First, vancomycin does not exhibit concentration-dependent killing and exceeding the MIC by more than four- to fivefold does not result in further activity (26). Second, the need for treatment monitoring has been debated because of the lack of definitive evidence linking concentrations to either outcome or toxicity (23). Finally, alternative dosages and monitoring strategies have been proposed to reduce the treatment cost and to improve the ease of treatment adjustment (15).
Continuous infusion of vancomycin (CIV) is an alternative mode of vancomycin administration which has been recently evaluated (14) and which may make treatment monitoring and adjustment easier and cheaper because vancomycin concentrations in serum are less variable (14). We designed a multicenter prospective randomized study to compare two parallel groups of ICU patients with severe MRS infections: one which received a conventional dose by intermittent infusion of vancomycin (IIV) and one which received CIV. The aim was to determine which of the two modalities is more efficient, safer, easier to adjust, and more cost-effective.
MATERIALS AND METHODS
In this multicenter prospective study, ICU patients with MRS infections were randomly assigned to the IIV or CIV group. Medico-surgical ICUs from 10 teaching or affiliated hospitals participated in the study, which complied with French legislation regarding human investigations and was approved by the ethics committee of the Henri Mondor hospital (Creteil, France). Written informed consent was obtained from either the patient or a close relative.
Patients.
Over a 36-month period, consecutive ICU patients were considered eligible if their physicians decided to give vancomycin for either a suspected or a well-established MRS infection acquired 72 h after admission. They were not eligible if they had received vancomycin 72 h before the current infection, if they had a β-lactam allergy, if they had been previously included in the same protocol, or if they were currently in another protocol. Eligible patients 18 years old or older having a serum creatinine concentration of less than 200 μM/liter (2.3 mg/dl), a neutrophil cell count above 1.103/mm3 (1.109/liter), no more than three organ failures according to the organ system failure score (16), and a direct microbiologic examination showing gram-positive cocci were selected and randomly assigned to receive either IIV or CIV. Selected and randomized patients remained in the study if microbiological confirmation of infection with methicillin-resistant Staphylococcus aureus or coagulase-negative staphylococcus (CNS) was made no later than 72 h after randomization.
Randomization.
Randomization was stratified by center using a random-number table and a block randomization method with a block size of 8. The infusion mode was contained in sealed opaque envelopes labeled consecutively with the randomization numbers.
Treatments.
All patients enrolled in the study received a purified commercial form of vancomycin hydrochloride (Vancocyn; Lilly, Saint-Cloud, France) which was diluted to a concentration of 0.25 mg/kg/ml in 5% dextrose in water and infused by an automatic volumetric device using 60-ml syringes.
Patients assigned to IIV received 15 mg/kg infused over 60 min every 12 h. Except for the first 15 mg/kg, which was adjusted according to the baseline serum creatinine concentration, the same initial dosage was given to everyone. The treatment was adjusted afterward, based on trough vancomycin concentrations in serum, by increasing or decreasing the daily dose by 500 mg. An increase in the number of infusions per day (maximum, three infusions per day) was allowed secondarily for more precise adjustment because the treatment objective was to rapidly attain a trough vancomycin concentration in serum of 10 to 15 mg/liter. This trough concentration is higher than the conventional 5 to 10 mg/liter (6) and was chosen because we expected to include patients with bacteremic MRS infections some of whom would have endocarditis, a situation in which therapeutic failures have been reported with trough values below 10 mg/liter (20). In addition, we also expected to include patients with pneumonia and to obtain therapeutic vancomycin concentrations in lung tissue (7) or in pulmonary lining fluid (17), where concentrations above 10 mg/liter may be required. Indeed, the time required to reach the target concentrations, one of the pharmacological study end points, is directly related to the values chosen for such concentrations; i.e., the time required to reach a trough concentration of 10 to 15 mg/liter will be longer than the time required to reach 5 to 10 mg/liter.
Patients assigned to the CIV group received vancomycin at 15 mg/kg infused over 60 min, followed by a continuous infusion of 30 mg/kg. Except for the first 15 mg/kg, which was adjusted according to the baseline serum creatinine concentration, the same initial dosage was given to everyone. The treatment was adjusted afterward, based on plateau vancomycin concentrations in serum, by either increasing or decreasing the speed of the volumetric device so that the daily dose was increased or decreased by 500 mg. The treatment was adjusted to obtain a plateau vancomycin concentration in serum of 20 to 25 mg/liter. This concentration was chosen by consensus by the investigators according to available data on the MIC of vancomycin against staphylococcus species (27), its protein binding (27), and its diffusion into tissues (21). It was also the target concentration previously proposed for deep infections (1, 25).
In the two treatment groups, for patients with serum creatinine concentrations of 100 to 200 μM/liter (1.1 to 2.3 mg/dl), the first 15-mg/kg infusion was adjusted according to the Moellering nomogram (21). Further adjustments were made afterward on the basis of trough and plateau concentrations.
Vancomycin concentrations in serum were measured by enzyme immunoassay (Emit Vancomycin assay on a Cobas Bio Centrifugal Analyzer). Trough (immediately before infusion) vancomycin concentrations for IIV and plateau (at 7 a.m.) vancomycin concentrations for CIV were measured daily until two consecutive concentrations in the targeted ranges were obtained. Thereafter, samples were taken only if a 10% change in body weight or in serum creatinine was noted. The routine practice in the participating centers was to measure trough and peak concentrations (postinfusion) together. However, no targeted peak concentration was required and it was clearly stated in the protocol that the objective was to reach the targeted trough concentration as soon as possible, whatever the peak values.
Treatment duration was 10 days or longer, depending on the site and severity of infection. Administration of nonglycopeptide antibiotics in combination with vancomycin was permitted, and those antibiotics administered for longer than 5 days were reported.
Microbiological analysis.
Antimicrobial susceptibilities of staphylococci isolated from infection sites were tested by a disk agar diffusion method recommended by the French Antibiogram Committee (24). In addition, for 40 randomly selected strains, the vancomycin MIC was measured on Mueller-Hinton agar. An inoculum of 103 to 104 CFU per spot was deposited with a Steer replicator. Plates were incubated for 18 h at 37°C in air prior to measurement.
Pharmacokinetic evaluation.
The time required to reach target concentrations was calculated as the time interval between the first administration and the first concentration in the target ranges. Because the time required to reach target concentrations is highly dependent on the values assigned to the target concentrations, we also calculated the time required to reach an equivalent range of concentrations with the two modalities. The area under the serum concentration-time curve (AUC24h) of vancomycin was calculated for 24-h intervals using the log-trapezoidal rule, assuming a monoexponential decrease in the drug concentration in serum when vancomycin was given intermittently and a constant drug concentration in serum for the 24 h when it was given continuously. For a given patient, over the treatment period, all of the 24-h interval values were averaged to give a the mean serum concentration-time integral value per treatment.
Definitions.
Vancomycin was prescribed for infections defined in accordance with the criteria of the Centers for Disease Control and Prevention (12), except for hospital-acquired pneumonia, for which we used the definition given by the 1992 International Conference Consensus, which requires a quantitative culture of protected specimens obtained from the lower respiratory tract (G. U. Meduri and W. G. Johanson, Jr., Editorial, Chest 102(5 Suppl. 1):551S–552S, 1992). Catheter-related infection was defined as a quantitative culture of the catheter tip yielding the same pathogen obtained from a peripheral-blood culture (two positive peripheral-blood cultures for CNS infections).
End points and analysis. (i) Efficacy.
Efficacy was assessed by the number of clinical failures at treatment end. Clinical failures were those patients who died from the infection or whose clinical, laboratory, and radiological statuses were unchanged or worsened by comparison with those at day 0. The number of clinical failures at treatment day 10, the number of patients who died while in intensive care, and the number of microbiological failures at treatment day 5 were also assessed. The latter was defined as the presence of Staphylococcus spp. in a day 5 specimen from the site of infection.
Clinical failure was first evaluated by local investigators, and since the treatment was not administered in a blinded fashion; a committee blinded to the infusion mode reviewed the charts from patients with clinical failure, as well as those of all of the study patients who died in the ICU. The committee members included the microbiologist (Y.P.), the statistician (A.R.), the principal investigator (M.W.), a coinvestigator not involved in patient care (F.D.), and at least two physicians not attending the patient under discussion. After reviewing clinical, laboratory, radiological, and pathological findings, the committee decided by consensus if death could reasonably be attributed to the staphylococcal infection. The committee met 10 times to review 53 charts.
(ii) Safety.
All side-effects attributed to vancomycin or which resulted in treatment discontinuation were reported on the case report form. Serum creatinine concentrations were measured, and creatinine clearance was calculated (4) on the day on which treatment was started, on day 10, and at the end of treatment. Nephrotoxicity was defined as a 50% increase in serum creatinine from the day treatment was started to the end of treatment. The concomitant use of nephrotoxic antibiotics (such as aminoglycosides) was also examined to analyze changes in serum creatinine concentrations. The number of patients in the study who required hemodialysis was also noted.
(iii) Pharmacokinetics, treatment adjustment, and monitoring.
The AUC24h values of the two treatment groups were compared. The ease of treatment adjustment and monitoring was examined by comparing the time required to reach targeted concentrations and the number of samples needed to adjust the treatment.
(iv) Cost.
The perspective for cost analysis was the hospital which, in France, receives a global allocation from health directories. It was considered that for comparable activity and allocation, a saving was achieved by reducing a treatment cost. The treatment cost was calculated for 10 days of treatment and defined as the cost of the amount of vancomycin infused (considering a price of $8.33/g in 1996) plus the cost of monitoring vancomycin concentrations in serum (considering the French Ministry of Health estimate of $25.20 per determination). To be able to compare our results with those of others, we also used cost variables published recently by Karam and coworkers (15). They estimated the vancomycin acquisition cost at $6.32 and the cost to measure vancomycin concentrations in serum at $5. Costs were not adjusted for inflation over the study period and were expressed in 1996 U.S. dollars. Charges for disposable materials, volumetric devices for infusion, and nurses' salaries were assumed to be comparable for the two modes of infusion.
Sample size and statistical analysis.
A preliminary controlled historical study (M. Wysocki, F. Thomas, M. A. Wolff, Y. Pean, Y. Ravaud, and B. Herman, Letter, J. Antimicrob. Chemother. 35:352–354, 1995) showing an 8% decrease in the mortality rate and a 23% decrease in the infection-related mortality rate with CIV indicated that detection of a 15% absolute difference in the number of treatment failures between the two groups with an alpha risk of 5% and a power of 90% required 320 patients. A priori, the study length was fixed at 36 months and a power analysis was designed to be used if the required number of patients was not obtained. Outcomes were evaluated in all included patients, and mortality was also evaluated in an intent-to-treat analysis. Quantitative results reported are the mean ± 1 standard deviation (SD), and qualitative results are expressed as percentages. A nonparametric Mann-Whitney U test was used to evaluate differences in quantitative values; Fisher's exact test was used to compare percentages. Variances were compared by using a variance analysis, and changes in the serum creatinine concentration with time were compared by using a Kruskal-Wallis test. A two-tailed P value of 0.05 was considered significant. Analyses were performed by using BMDP software (BMDP Statistical Software, Cork, Ireland).
RESULTS
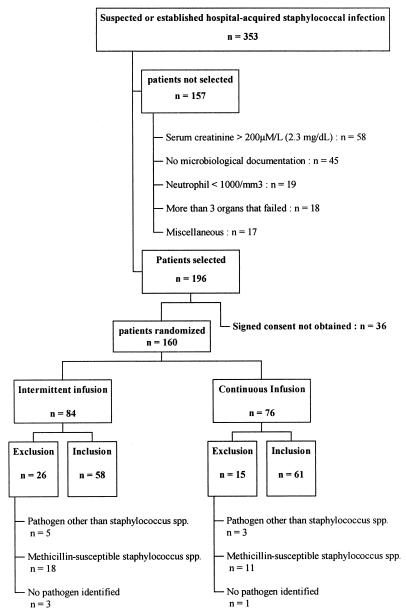
Over a 36-month period, 353 eligible patients had suspected or established hospital-acquired staphylococcal infections. One hundred fifty-seven were not selected, and 160 patients were randomized. Fourty-one patients (26 in the IIV group and 15 in the CIV group; P = 0.15) were later excluded because the pathogen was not a staphylococcus (n = 8), the staphylococcus was methicillin susceptible (n = 29), or the pathogen was not isolated (n = 4). Finally, 119 patients were included, 58 in the IIV group and 61 in the CIV group (Fig. 1).
FIG. 1.
Patient selection scheme.
Efficacy.
Demographic characteristics, severity of underlying disease, site of infection, and pathogens were similar in the two groups (Table 1). Treatment duration (14 ± 6 versus 13 ± 5 days) and concomitant antibiotics (fusidic acid, 22 versus 21%; aminoglycoside, 28 versus 26%) were also similar in the two treatment groups. The median follow-up period was 33 days for surviving patients of the IIV group and 39 days for those in the CIV group. Trough and plateau concentrations were, respectively, 15 ± 9 and 24 ± 8 mg/liter. Microbiological and clinical outcomes were not different in the two groups (Table 2). The study's power to detect a difference in clinical outcome between the two treatment groups was 23%. At treatment day 5, 35% of those in the IIV group and 28% of those in the CIV group were still bacteremic (no significant difference). Treatment failure was observed in 11 patients in the IIV group and in 13 in the CIV group (no significant difference). Severity of the underlying disease at admission, the number of organs failing, the number of patients with immunodrepression, and the day 10 serum creatinine concentrations (Table 1), trough concentrations, and AUC24h (Table 3) were higher for patients who failed in comparison with those of patients who succeed. A multivariate logistic regression found that the severity of the underlying disease at admission and the day 10 serum creatinine concentrations were independently associated with treatment failure.
TABLE 1.
Patient characteristics, infection sites, pathogens, and MICs of vancomycin between patients treated with IIV and those treated with CIVa and between patients treated successfully and those whose treatment failedb
| Parametera | IIV (n = 58) | CIV (n = 61) | Success (n = 95) | Failure (n = 24) |
|---|---|---|---|---|
| Mean age (yr) ± SD | 62 ± 16 | 64 ± 13 | 63 ± 15 | 64 ± 15 |
| No. (%) of males | 35 (60) | 42 (69) | 63 (66) | 14 (58) |
| Mean wt (kg) ± SD | 69 ± 17 | 73 ± 15 | 71 ± 17 | 68 ± 19 |
| Mean ht (cm) ± SD | 168 ± 8 | 168 ± 8 | 168 ± 8 | 169 ± 9 |
| Mean simplified acute physiologic score (19) ± SD | 13 ± 4 | 14 ± 5 | 13 ± 4 | 16 ± 4gh |
| No. (%) with more than one organ failure (16) | 17 (29) | 19 (31) | 24 (25) | 12 (50)g |
| Mean temp (°C) ± SD | 38.7 ± 0.9 | 38.7 ± 1.0 | 38.7 ± 0.9 | 38.5 ± 1.0 |
| Mean no. of leukocytes/mm3 ± SD | 16,736 ± 6,755 | 14,822 ± 6,311 | 15,667 ± 6,160 | 16,015 ± 8,110 |
| Mean plasma protein level (g/liter) ± SD | 56 ± 9 | 56 ± 10 | 57 ± 10 | 53 ± 12 |
| No. (%) mechanically ventilated | 44 (77) | 48 (79) | 68 (72) | 20 (83) |
| No. (%) with vasoactive supportc | 15 (26) | 17 (28) | 19 (20) | 6 (25) |
| No. (%) immunosuppressedd | 17 (29) | 18 (30) | 24 (25) | 11 (46)g |
| No. (%) with endocarditis | 1 (2) | 1 (2) | 2 (2) | 0 (0) |
| No. (%) with septicemia | 3 (5) | 7 (11) | 8 (8) | 2 (8) |
| No. (%) with catheter-related infections | 15 (26) | 12 (20) | 20 (21) | 7 (29) |
| No. (%) with pneumonia | 31 (53) | 26 (43) | 49 (52) | 8 (33) |
| No. (%) with mediastinitis | 2 (3) | 4 (6) | 4 (4) | 2 (8) |
| No. (%) with meningitis | 1 (2) | 0 (0) | 1 (1) | 0 (0) |
| No. (%) with miscellaneous infections | 5 (9) | 11 (18) | 11 (12) | 5 (21) |
| No. (%) with infections with bacteremia | 28 (48) | 25 (40) | 41 (43) | 12 (50) |
| No. (%) with Staphylococcus aureus infections | 46 (82) | 49 (80) | 74 (78) | 21 (87) |
| No. (%) with CNS infections | 12 (18) | 12 (20) | 12 (22) | 3 (13) |
| No. (%) with vancomycin MICf of: | ||||
| 0.5 mg/liter | 0 (0) | 3 (14) | 3 (10) | 0 (0) |
| 1 mg/liter | 5 (28) | 8 (36) | 9 (29) | 4 (44) |
| 2 mg/liter | 13 (72) | 11 (50) | 19 (61) | 5 (55) |
There were no significant differences between the two treatment groups.
Failures were those patients who died from the infection or whose clinical, laboratory, and radiological statuses were unchanged or worsened by comparison with day 0 of treatment.
Vasoactive support was defined as the need to infuse adrenaline, noradrenaline, or dopamine at >5 μg/kg/min.
Defined as corticosteroid at 1 mg/kg/day for over 3 months, AIDS or leukemia, or any immunosuppresive therapy other than corticosteroids.
See text for definitions of infection sites.
The MIC of vancomycin was measured in 40 randomly selected strains.
P < 0.05 between success and failure of treatment in univariate analysis.
P < 0.05 between success and failure of treatment in multivariate analysis.
TABLE 2.
Microbiologial and clinical outcomes, overall and infection-related mortality, risk factors for nephrotoxicity, changes over time in serum creatinine concentrations, and creatinine clearances between patients treated with IIV and those treated with CIVa
| Parameter | IIV (n = 58) | CIV (n = 61) |
|---|---|---|
| Efficacy | ||
| Day 5 microbiological evaluation | ||
| No. (%) with no pathogen | 30 (52) | 28 (46) |
| No. (%) with Staphylococcus sp. infections | 24 (41) | 24 (39) |
| No. (%) of infections with other species | 4 (7) | 9 (15) |
| No. (%) of treatment failure at: | ||
| Day 10 | 15 (26) | 13 (21) |
| End of treatment | 11 (19) | 13 (21) |
| No. (%) of infection-related deaths at: | ||
| Day 10 | 7 (12) | 5 (8) |
| End of treatment | 7 (12) | 6 (10) |
| Overall no. (%) of deaths at: | ||
| Day 10 | 7 (12) | 5 (8) |
| End of treatment | 7 (12) | 11 (18) |
| In intensive care | 19 (33) | 21 (37) |
| Nephrotoxicity | ||
| No. (%) with risk factors for nephrotoxicity | ||
| Diabete mellitus | 8 (13) | 7 (12) |
| Diuretics | 21 (34) | 18 (35) |
| Iodine | 6 (10) | 2 (3) |
| Aminoglycoside | 16 (28) | 6 (26) |
| Mean serum creatinine (μM/liter) ± SD at: | ||
| Baseline | 88 ± 35 | 98 ± 41 |
| Day 10 | 90 ± 54 | 107 ± 71 |
| End of treatment | 108 ± 61 | 120 ± 79 |
| Mean creatinine clearance (ml/min) ± SD at: | ||
| Baseline | 84 ± 42 | 77 ± 41 |
| Day 10 | 79 ± 48 | 73 ± 34 |
| End of treatment | 70 ± 49 | 68 ± 36 |
No statistically significant difference between and within the two treatment groups was found.
TABLE 3.
Mode of vancomycin infusion, associated antibiotics, and pharmacokinetics characteristics between treatment success and failure groups
| Parameter | Success (n = 95) | Failure (n = 24) |
|---|---|---|
| No. (%) who received: | ||
| IIV | 47 (49) | 11 (46) |
| CIV | 48 (51) | 13 (54) |
| No. (%) who also received: | ||
| No additional antibiotic | 20 (21) | 1 (4) |
| Fusidic acid | 23 (24) | 5 (21) |
| Aminoglycoside | 20 (21) | 3 (12) |
| Fosfomycin | 8 (8) | 3 (12) |
| Rifampin | 7 (7) | 2 (8) |
| Fluoroquinolone | 5 (5) | 4 (17) |
| Extended-spectrum cephalosporin | 4 (4) | 2 (8) |
| Other antibiotics | 8 (8) | 3 (12) |
| Mean serum creatinine (μM/liter) ± SD at: | ||
| Baseline | 91 ± 36 | 106 ± 46 |
| Day 10 | 91 ± 48 | 160 ± 122ab |
| Mean creatinine clearance (ml/min) ± SD at: | ||
| Baseline | 76 ± 39 | 61 ± 38 |
| Day 10 | 69 ± 39 | 36 ± 37a |
| Mean AUC24h (mg/liter/h) ± SD | 596 ± 159 | 685 ± 260a |
| Mean serum vancomycin concn (mg/liter) ± SD | ||
| Trough in IIV group | 14 ± 5 | 21 ± 9a |
| Plateau in CIV group | 23 ± 4 | 25 ± 5 |
| Mean time (h) needed to achieve target serum vancomycin concn ± SD | ||
| Trough or plateau of ≥5 mg/liter | 21 ± 24 | 22 ± 21 |
| Trough or plateau of ≥10 mg/liter | 34 ± 32 | 31 ± 29 |
P < 0.05 in univariate analysis.
P < 0.02 in multivariate analysis.
Safety.
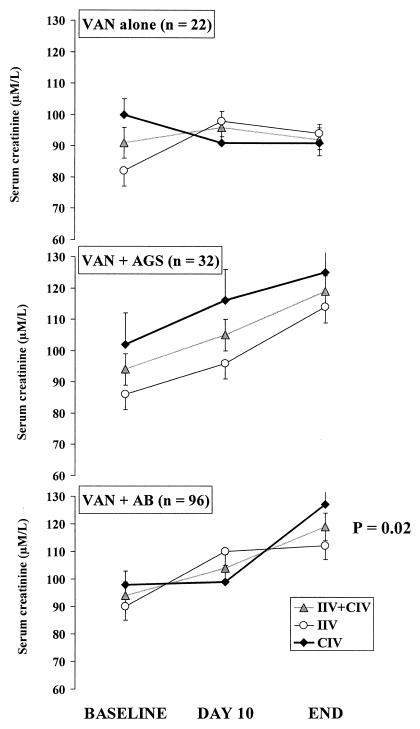
Serum creatinine concentrations and creatinine clearance increased nonsignificantly from the baseline to the end of treatment in the two treatment groups (Table 3). Nephrotoxicity occurred in 21 patients (20%) (11 in the IIV group and 10 in the CIV group [P = 0.64]). Vancomycin given with concomitant antibiotics was associated with a significant increased in the serum creatinine concentration. This was not the case when vancomycin was given alone (Fig. 2). Dialysis was required for three patients in the IIV group and for six in the CIV group (P = 0.31). A red-man syndrome was reported in one patient in the IIV group, and phlebitis and fever attributed to vancomycin were reported in two patients in the CIV group.
FIG. 2.
Changes in serum creatinine concentration during treatment with vancomycin (VAN) alone or with vancomycin given concomitantly with an aminoglycoside (AGS) or with any kind of antibiotic (AB). In the whole group (n = 96), vancomycin with any kind of antibiotic was associated with a significant (P = 0.02) increase in serum creatinine concentration but this was not the case when vancomycin was given alone. No interaction with treatment groups was found, suggesting that the infusion mode had no significant role in the observed effect. The total treatment duration was 14 ± 6 days in the IIV group and 13 ± 5 days in the CIV group.
Pharmacokinetics, treatment adjustment, and monitoring.
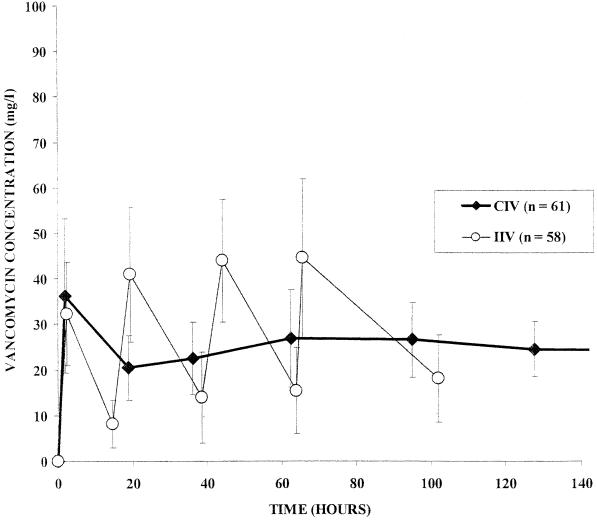
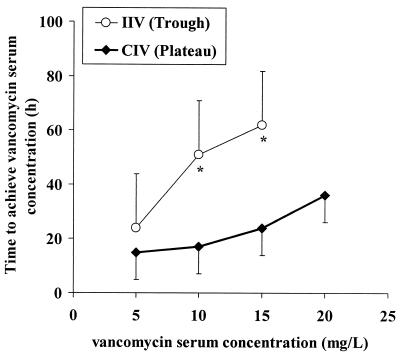
Changes in vancomycin concentrations in serum over time in the two treatment groups are shown in Fig. 3. The AUC24h values were comparable between the two groups, but variability between patients was lower in the CIV group than in the IIV group (variance, 14,621 versus 53,975 mg2/h2/liter2, respectively; P = 0.026). The daily doses of infused vancomycin were comparable between the two groups, but the variability between patients was lower in the CIV group than in the IIV group (variance, 414 versus 818 g2, respectively; P = 0.057). In the IIV group, targeted concentrations were reached within 51 ± 39 h. By contrast, it took 36 ± 31 h in the CIV group (P = 0.03). It required 51 ± 39 h to reach a concentration above 10 mg/liter in the IIV group and only 17 ± 14 h (P < 0.0001) in the CIV group (Fig. 4). Fewer samples per treatment were required to monitor vancomycin concentrations in serum for the CIV group (7.7 ± 2.2) than for the IIV group (11.8 ± 3.9; P < 0.0001). When peak determinations were not taken account, the number of samples collected was not significantly different between the two groups (6.1 ± 1.9 versus 6.7 ± 2.1).
FIG. 3.
Changes in serum vancomycin concentrations over time in the two treatment groups. The AUC24h was 577 ± 120 in the CIV group and 653 ± 232 mg/liter/h in the IIV group. The AUC24h and the daily dose given over 10 days of treatment have less variability between patients with CIV than with IIV (variance, 14,621 versus 53,975 mg2/liter2/h2 [P = 0.02] and 414 versus 818 g2 [P = 0.05], respectively).
FIG. 4.
Time required to reach target serum vancomycin concentrations (trough concentrations in the IIV group and plateau concentration in the CIV group). With IIV, the time required to reach concentrations above 10 or 15 mg/liter was significantly longer than with CIV (∗, P < 0.05).
Cost.
For 10 days of treatment, the drug cost per patient was $152 ± 76 in the IIV group and $127 ± 60 in the CIV group (P = 0.05). The cost of serum vancomycin determinations per patient was $301 ± 100 in the IIV group and $193 ± 56 in the CIV group (P < 0.0001). As a result, the 10-day treatment cost per patient ($454 ± 137 in the IIV group) was 23% lower in the CIV group ($321 ± 81; P < 0.0001). A similar result was obtained by using the cost variables reported by Karam and coworkers (15). These values gave a 10-day treatment cost per patient of $134 ± 46 with CIV by comparison with $175 ± 64 with IIV (P < 0.0002).
DISCUSSION
CIV was first used in postneurosurgical pediatric patients to attain bactericidal concentrations in infected cerebrospinal fluid (1) and subsequently a few historical controlled (M. Wysocki et al., letter, 1995) and uncontrolled studies have reported the use of CIV in various clinical situations (2, 3, 5, 25). This is the first prospective multicenter randomized study comparing the efficacy, safety, and costs of IIV and CIV in severe hospital-acquired MRS infections.
The present study found that in critically ill patients requiring vancomycin infusion for severe MRS infections, CIV to obtain plateau concentrations of 20 to 25 mg/liter and IIV to obtain trough concentrations of 10 to 15 mg/liter were comparable in clinical efficacy and safety. However, targeted concentrations were acquired faster with fewer samples, less variability in the daily infused dose, and reduced cost with CIV.
Efficacy.
Despite faster acquisition of target concentrations by CIV, we were unable to demonstrate a microbiological or clinical superiority of CIV. Indeed, modification of vancomycin delivery in severe infections may not be sufficient, by itself, to change the clinical outcome for such critically ill patients. In addition, despite a comparable randomization of patients to the two treatment groups, there was variability in the severity of infection (30% of patients were immunosuppressed) and variability in the site of infection (Table 1). Post-hoc analysis failed to find subgroups of patients in whom CIV might be beneficial, but the number of patients in each subgroup was relatively small. In addition, patients with S. aureus infections (80% of the population) and those with CNS infections were both included in the study and such heterogeneity might mask a beneficial effect of CIV in specific situations. Finally, policies intended to reduce MRS infections were successfully implemented during the study period and the number of patients included in the study was markedly lower than that required a priori.
Safety.
Vancomycin-related side effects were not frequently observed in the present study. In both treatment groups, there was a moderate increase in serum creatinine concentration (Table 2). In agreement with a previous report (22), we found that the increase in serum creatinine concentration was observed mainly in patients receiving concomitant aminoglycosides and more generally in those receiving any concomitant antibiotics (Fig. 2). Such patients may also have the most severe cases and may be those with uncontrolled infections. Interestingly, the multivariate analysis found that patients who failed treatment had a day 10 serum creatinine concentration which was higher than that of those who succeeded, suggesting that an increase in serum creatinine concentration during vancomycin treatment might be a marker of treatment failure rather than of vancomycin nephrotoxicity per se.
Pharmacokinetics.
In agreement with James and coworkers (14), we found that the AUC24h and the daily dose were comparable in the two treatment groups. In addition, we found that the target concentrations were reached faster with CIV (Fig. 4), and this may be clinically relevant in critically ill patients. Faster acquisition of targeted concentrations might be coupled to the ease of treatment adjustment with CIV, as suggested by the lower variability between patients in the AUC24h and in the daily dose that was seen with this infusion mode. Faster acquisition of targeted concentrations in the CIV group was observed mainly in patients with serum creatinine concentrations below 100 μM/liter (data not shown) and could not be explained by the higher baseline serum creatinine concentration in the CIV group (Table 2).
Monitoring.
Fewer samples per treatment were required to monitor vancomycin concentrations in serum in the CIV group, even in patients with a change in serum creatinine or in body weight of greater than 10% during the treatment period. However, the study was designed to assess the pharmacokinetics of the two modalities and the number of samples obtained in the present study is greater than that obtained with the usual standard of clinical care. For instance, in the recent report of Karam and coworkers (15), only two samples were obtained during 9 days of treatment and, in a population of cancer patients, Elting and coworkers (10) reported taking five samples over 8 days of treatment. In the present study, patients in the IIV group had 12 samples taken during 10 days of treatment. Since the algorithm for sampling of vancomycin concentrations in serum was comparable in the two treatment groups, the relative difference in the number of samples collected between the two groups (35% reduction in the CIV group) may be valid. In a recent study (15), a 45% reduction in the number of samples collected was obtained by shifting from a conventional pharmacokinetics-based to an alternative nomogram-based vancomycin regimen. It should be stressed that with IIV, the routine practice in the participating centers was to measure peak and trough concentrations together, and when only the numbers of trough and plateau determinations were compared (excluding peak determinations), the difference between the two treatment groups was no longer significant.
Treatment adjustment.
The ease with which the treatment could be adjusted was assessed by the variability between patients in the AUC24h and in the daily dose of vancomycin, both of which were lower (respectively, by 72 and 49%) in the CIV group than in the IIV group. In agreement with previous pharmacokinetic comparisons showing less variability in vancomycin concentrations in serum with CIV (14), the present results suggest that CIV may be a more efficient modality, given the highly variable pharmacokinetics of ICU patients. Within the treatment groups, these results were not dependent on whether the baseline serum creatinine was below or above 100 μM/liter (data not shown).
Cost.
Ten days of CIV was 23% less expensive than 10 days of IIV, both because less vancomycin was infused and because fewer samples were required to monitor the treatment. However, vancomycin not used is not equal to vancomycin saved and several aspects that could have an impact on the cost of vancomycin therapy (disposable materials, volumetric devices for infusion, and nurses' salaries) were not recorded in the present study. Despite this, our results are in agreement with previous publications suggesting that continuous infusion of β-lactam antibiotics may be cost saving (18) by reducing the amount of drug required (28). Additional savings could also come from the reduction in the number of samples required for serum drug level determination with CIV.
Target concentrations.
IIV was adjusted to maintain a trough concentration of 10 to 15 mg/liter but was, in fact, at the upper end of the targeted limit (15 ± 0.5 mg/liter). If the more conventional lower target concentrations of 5 to 10 mg/liter had been chosen, one could reasonably expect faster acquisition of the target concentration and less need for serum vancomycin determinations for treatment adjustment. In addition, less vancomycin might be required, hence reducing the cost of treatment. However, in endocarditis, therapeutic failures have been reported with minimal concentrations below 10 mg/liter (20) and concentrations above 10 mg/liter may be required to inhibit bacterial growth at the site of infection (7, 17). At the time the study was designed, all of the participating centers considered that targeted trough concentrations of 5 to 10 mg/liter were insufficient in critically ill patients with severe infections.
On the other hand, continuous infusion was adjusted to obtain a plateau of 20 to 25 mg/liter. As suggested by recent pharmacodynamic studies (9, 14), a lower plateau might be comparable in efficacy. This would further reduce the time needed to reach target concentrations, the number of determinations, and the cost of treatment with this modality. Considering the pharmacokinetic properties of vancomycin (21, 27) and the trend toward an increase in the concentrations deemed necessary to inhibit staphylococcus species, a target plateau of 20 to 25 mg/liter is probably a safe and reasonable goal. Finally, recent preliminary data (J. R. Aeschlimann, E. Hershberger, and M. J. Rybak, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-10, p. 3, 1998) suggested that CIV may be an alternative against staphylococcus species with reduced susceptibility to vancomycin.
In summary, in critically ill patients requiring vancomycin infusion for severe MRS infections, CIV to obtain plateau concentrations of 20 to 25 mg/liter and IIV to obtain trough concentrations of 10 to 15 mg/liter were comparable in clinical efficacy and safety. However, targeted concentrations were acquired faster with fewer samples, less variability in the daily infused dose, and reduced cost with CIV.
ACKNOWLEDGMENTS
This study was promoted and supported by the Association pour la Recherche Clinique et Infectieuse en Reanimation (ARCIR), a nonprofit organization sponsoring research on infectious disease in ICUs, and supported by a grant given by the Société de Réanimation de Langue Française.
We acknowledge the editorial assistance of Suzanne M. Kelly.
Appendix
The study group included Christine Cheval, Hôpital Saint-Joseph, Paris, France; Jean-Christophe Lucet, Jean-Pierre Bedos, and Michel Wolff, Hôpital Bichat-Claude Bernard, Paris, France; Marie-Jo Laisné and Claudette Briard, Hôpital Lariboisière, Paris, France; Thierry Lazard and Eric Maury, Hôpital Saint-Antoine, Paris, France; Christian Lamer, Jean-Luc Leguillou, Laurent Tric, and Michel A. Wolff, Institut Mutualiste Montsouris, Paris, France; Patrice Guérrini, Hôpital Henri Mondor, Creteil, France; and Claude Guerin, Centre Hospitalier Lyon-Sud, Pierre Benite, France.
REFERENCES
- 1.Barois A, Estournet B, Moranne J B, Piliot J, Chabenat C, Bataille J. Ventricular staphylococcal infections. Treatment with vancomycin by continuous venous infusion. Presse Med. 1986;15:1805–1808. [PubMed] [Google Scholar]
- 2.Borderon J C, Laugier J, Chamboux C, Saliba E, Mathieu A. Continuous infusion of vancomycin during the neonatal period. Pathol Biol (Paris) 1994;42:525–529. [PubMed] [Google Scholar]
- 3.Brinquin L, Rousseau J M, Boulesteix G, Diraison Y, Bonsignour J R. Continuous infusion of vancomycin in post-neurosurgical staphylococcal meningitis in adults. Presse Med. 1993;22:1815–1817. [PubMed] [Google Scholar]
- 4.Cockcroft D W, Gault M H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 5.Conil J M, Favarel H, Laguerre J, Brouchet A, Chabanon G, Cazal L, Bodnar M, Rouge D, Virenque C, Costagliola M. Continuous administration of vancomycin in patients with severe burns. Presse Med. 1994;23:1554–1558. [PubMed] [Google Scholar]
- 6.Cook F V, Farrar W E. Vancomycin revisited. Ann Intern Med. 1978;88:813–818. doi: 10.7326/0003-4819-88-6-813. [DOI] [PubMed] [Google Scholar]
- 7.Cruciani M, Gatti G, Lazzarini L, Furlan G, Broccali G, Malena M, Franchini C, Concia E. Penetration of vancomycin into human lung tissue. J Antimicrob Chemother. 1996;38:865–869. doi: 10.1093/jac/38.5.865. [DOI] [PubMed] [Google Scholar]
- 8.Duckworth G J. Diagnosis and management of methicillin resistant Staphylococcus aureus infection. BMJ. 1993;307:1049–1052. doi: 10.1136/bmj.307.6911.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duffull S B, Begg E J, Chambers S T, Barclay M L. Efficacies of different vancomycin dosing regimens against Staphylococcus aureus determined with a dynamic in vitro model. Antimicrob Agents Chemother. 1994;38:2480–2482. doi: 10.1128/aac.38.10.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elting L, Rubenstein E B, Kurtin D, Rolston K V, Fangtang J, Martin C G, Raad I I, Whimbey E E, Manzullo E, Bodey G P. Mississippi mud in the 1990s. Risk and outcomes of vancomycin-associated toxicity in general oncology practice. Cancer. 1998;83:2597–2607. doi: 10.1002/(sici)1097-0142(19981215)83:12<2597::aid-cncr27>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 11.Fridkin S K, Steward C D, Edwards J R, Pryor E R, McGowan J E, Jr, Archibald L K, Gaynes R P, Tenover F C. Surveillance of antimicrobial use and antimicrobial resistance in United States hospitals: project ICARE phase 2. Project Intensive Care Antimicrobial Resistance Epidemiology (ICARE) hospitals. Clin Infect Dis. 1999;29:245–252. doi: 10.1086/520193. [DOI] [PubMed] [Google Scholar]
- 12.Garner J S, Jarvis W R, Emori T G, Horan T C, Hughes J M. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 13.Houlihan H H, Mercier R C, Rybak M J. Pharmacodynamics of vancomycin alone and in combination with gentamicin at various dosing intervals against methicillin-resistant Staphylococcus aureus-infected fibrin-platelet clots in an in vitro infection model. Antimicrob Agents Chemother. 1997;41:2497–2501. doi: 10.1128/aac.41.11.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.James J K, Palmer S M, Levine D P, Rybak M J. Comparison of conventional dosing versus continuous-infusion vancomycin therapy for patients with suspected or documented gram-positive infections. Antimicrob Agents Chemother. 1996;40:696–700. doi: 10.1128/aac.40.3.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karam C M, McKinnon P S, Neuhauser M M, Rybak M J. Outcome assessment of minimizing vancomycin monitoring and dosing adjustments. Pharmacotherapy. 1999;19:257–266. doi: 10.1592/phco.19.4.257.30933. . (Erratum, 19:674, 1999.) [DOI] [PubMed] [Google Scholar]
- 16.Knauss W A, Draper E A, Wagner D P, Zimmerman J E. Prognosis in acute organ system failure. Ann Surg. 1985;202:685–692. doi: 10.1097/00000658-198512000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamer C, de Beco V, Soler P, Calvat S, Fagon J Y, Dombret M C, Farinotti R, Chastre J, Gilbert C. Analysis of vancomycin entry into pulmonary lining fluid by bronchoalveolar lavage in critically ill patients. Antimicrob Agents Chemother. 1993;37:281–286. doi: 10.1128/aac.37.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leder K, Turnidge J D, Korman T M, Grayson M L. The clinical efficacy of continuous-infusion flucloxacillin in serious staphylococcal sepsis. J Antimicrob Chemother. 1999;43:113–118. doi: 10.1093/jac/43.1.113. [DOI] [PubMed] [Google Scholar]
- 19.Le Gall J R, Loirat P, Alperovitch A, Glaser P, Granthil C, Mathieu D, Mercier P, Thomas R, Villers D. A simplified acute physiology score for ICU patients. Crit Care Med. 1984;12:975–977. doi: 10.1097/00003246-198411000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Levine D P, Fromm B S, Reddy B R. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med. 1991;115:674–680. doi: 10.7326/0003-4819-115-9-674. [DOI] [PubMed] [Google Scholar]
- 21.Moellering R C., Jr Pharmacokinetics of vancomycin. J Antimicrob Chemother. 1984;14:43–52. doi: 10.1093/jac/14.suppl_d.43. [DOI] [PubMed] [Google Scholar]
- 22.Rybak M J, Albrecht L M, Boike S C, Chandrasekar P H. Nephrotoxicity of vancomycin, alone and with an aminoglycoside. J Antimicrob Chemother. 1990;25:679–687. doi: 10.1093/jac/25.4.679. [DOI] [PubMed] [Google Scholar]
- 23.Saunders N J. Why monitor peak vancomycin concentrations? Lancet. 1994;344:1748–1750. doi: 10.1016/s0140-6736(94)92890-8. [DOI] [PubMed] [Google Scholar]
- 24.Soussy C J. Antibiogram committee of the French Society for Microbiology. 1996 statement. Pathol Biol (Paris) 1996;44:I–VIII. [Google Scholar]
- 25.Thomas F, Michel P, Ravaud Y. Traitement d'infections à staphylocoques chez l'adulte par perfusion veineuse continue de vancomycine en monothérapic. Sem Hôp Paris. 1988;64:215–218. [Google Scholar]
- 26.Watanabe T, Ohashi K, Matsui K, Kubota T. Comparative studies of the bactericidal, morphological and post-antibiotic effects of arbekacin and vancomycin against methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1997;39:471–476. doi: 10.1093/jac/39.4.471. [DOI] [PubMed] [Google Scholar]
- 27.Zeckel M L. A closer look at vancomycin, teicoplanin and antimicrobial resistance. J Chemother. 1997;9(5):311–335. doi: 10.1179/joc.1997.9.5.311. [DOI] [PubMed] [Google Scholar]
- 28.Zeisler J, McCarthy J, Richelieu W, Anderson J. Antibiotics by continuous infusion: saving money without sacrificing quality of care. Drug Benefit Trends. 1995;7(4):25–27. [Google Scholar]