Abstract
Drug resistance has emerged as a major impediment in the treatment of leishmaniasis. Alkyl-lysophospholipids (ALP), originally developed as anticancer drugs, are considered to be the most promising antileishmanial agents. In order to anticipate probable clinical failure in the near future, we have investigated possible mechanisms of resistance to these drugs in Leishmania spp. The results presented here support the involvement of a member of the ATP-binding cassette (ABC) superfamily, the Leishmania P-glycoprotein-like transporter, in the resistance to ALP. (i) First, a multidrug resistance (MDR) Leishmania tropica line overexpressing a P-glycoprotein-like transporter displays significant cross-resistance to the ALP miltefosine and edelfosine, with resistant indices of 9.2- and 7.1-fold, respectively. (ii) Reduced expression of P-glycoprotein in the MDR line correlates with a significant decrease in ALP resistance. (iii) The ALP were able to modulate the P-glycoprotein-mediated resistance to daunomycin in the MDR line. (iv) We have found a new inhibitor of this transporter, the sesquiterpene C-3, that completely sensitizes MDR parasites to ALP. (v) Finally, the MDR line exhibits a lower accumulation than the wild-type line of bodipy-C5-PC, a fluorescent analogue of phosphatidylcholine that has a structure resembling that of edelfosine. Also, C-3 significantly increases the accumulation of the fluorescent analogue to levels similar to those of wild-type parasites. The involvement of the Leishmania P-glycoprotein-like transporter in resistance to drugs used in the treatment of leishmaniasis also supports the importance of developing new specific inhibitors of this ABC transporter.
Protozoan parasites are responsible for some of the most important and prevalent diseases of humans and domestic animals, threatening the lives of nearly one-quarter of the human population. World Health Organization statistics show that, with a 42-fold increase in the last 15 years, leishmaniasis has become the second worldwide cause of death among these diseases (20). In the absence of effective vaccines and vector control, chemotherapy still plays a critical role in the control of the infection. The recommended standard drugs for treatment are still the pentavalent antimonial drugs Pentostam and Glucantime, despite the requirement of long courses of parenteral administration (1) and increasing levels of resistance (13). Drug resistance has indeed emerged as a major problem in treating the disease. In fact, more than 50% of the cases of visceral leishmaniasis in India are resistant to Glucantime (44), due to the emergence of Leishmania donovani lines resistant to antimonials (27). Although alternative drugs or drug formulations have been proved to be effective (e.g., amphotericin B liposomes for visceral leishmaniasis and paramomycin ointment for cutaneous leishmaniasis), they present several drawbacks, such as their very high cost and their scant availability (1). On the other hand, alkyl-lysophospholipids (ALP) such as miltefosine and edelfosine, originally developed as anticancer drugs, have shown a significant antiproliferative activity against Leishmania spp., Trypanosoma cruzi, and Trypanosoma brucei parasites in vitro and in vivo in experimental models (7–9, 26, 40, 47). They only scarcely produce side effects at therapeutic doses, and no drug resistance has ever been described. Miltefosine is the first oral drug that has proved to be highly effective against visceral leishmaniasis in India, including antimony-resistant cases (23), and an antimony-resistant patient with human immunodeficiency virus-Leishmania coinfection (45). The leishmanicidal and trypanocidal activities of these compounds have been related to perturbation of the alkyl-lipid metabolism and the biosynthesis of alkyl-anchored glycolipids and glycoproteins (28), as well as damage to the flagellar membrane (39). Recently, it has been suggested that both miltefosine and edelfosine appear to induce a perturbation of ether-lipid (alkyl-phospholipids) remodeling through the inhibition of glycosomally located alkyl-specific acylcoenzyme A acyltransferase (29).
Resistance to ALP has already been observed in cancer cell lines induced in the laboratory (12, 15, 41, 51); besides, distinct cell types display different intrinsic sensitivities to them (43, 46, 50). Several mechanisms probably involved in such differences have been described: reduced drug uptake (46), faster drug metabolism (14), bcl-2 overexpression (15), and increased cholesterol content of plasma membranes (10), among others. It has recently been shown that P-glycoprotein (Pgp)-overexpressing cells transfected with the mdr1 gene are cross-resistant to the ALP ilmofosine (21). Pgp belongs to the ATP-binding cassette (ABC) superfamily of transporters (19). It is an ATP-dependent pump that exports a wide range of hydrophobic drugs from the cell, decreasing their intracellular concentration and preventing their cytotoxic activity, thus conferring a multidrug resistance (MDR) phenotype during the treatment of cancer. Pgp can be inhibited in vitro by compounds called reversal agents, that overcome the MDR phenotype. However, the role of Pgp in ilmofosine resistance could be indirect, being associated with Pgp-mediated alterations in membrane lipids (21). Besides, other Pgp-overexpressing MDR lines show a similar susceptibility to ALP as parental cells (16, 34, 35, 49).
An MDR phenotype due to Pgp-like transporters has also been characterized in Leishmania spp. (5, 6, 18), where the pump confers a cross-resistance to daunomycin, vinblastine, puromycin, and adriamycin. However, this entire spectrum of drugs are not used clinically as antileishmanial agents. Classical modulators of mammalian Pgp such as verapamil and cyclosporine poorly revert the MDR phenotype in Leishmania (5, 18, 32). Conversely, we have recently described that natural compounds such as sesquiterpenes and flavonoids, as well as hemisynthetic derivatives, constitute promising new classes of modulators due to their ability to increase drug accumulation and reverse the MDR phenotype in Leishmania parasites (31–33).
An understanding of the resistance mechanisms to ALP in Leishmania spp. can help us find strategies to avoid or overcome the problem before the widespread use of miltefosine for the treatment of leishmaniasis results in the appearance of clinical cases of resistance. In order to address this possibility, we have studied the ability of a Pgp-like transporter from Leishmania tropica to confer resistance to ALP miltefosine and edelfosine, as well as the ability of a new natural Pgp modulator to overcome this resistance phenotype.
MATERIALS AND METHODS
Chemical compounds.
Daunomycin was purchased from Pharmacia-Spain (Barcelona, Spain). Edelfosine (1-O-octadecyl-2-O-methyl-rac-glycero-3-phosphocholine, ET-18-OCH3, methyl-PAF) was obtained from Bachem AG (Bubendorf, Switzerland). Miltefosine (hexadecylphosphocholine) was obtained from Sigma Chemical (St. Louis, Mo.). Sesquiterpene C-3 (9α-benzoyloxy-8α,2- methylbutyroyloxy-1α,6β,15-triacetoxy-4β-hydroxydihydro-β-agarofuran) (see Fig. 3A) was isolated from Maytenus canariensis, as previously described (17). Bodipy-C5-PC (1-hexadecanoyl-2-[4,4-difluoro-5,7-dimethyl-4-bora-3α,4α-diaza-s-indacene-3-pentanoyl]-sn-glycero-3-phosphocholine) was obtained from Molecular Probes Europe BV (Leiden, the Netherlands).
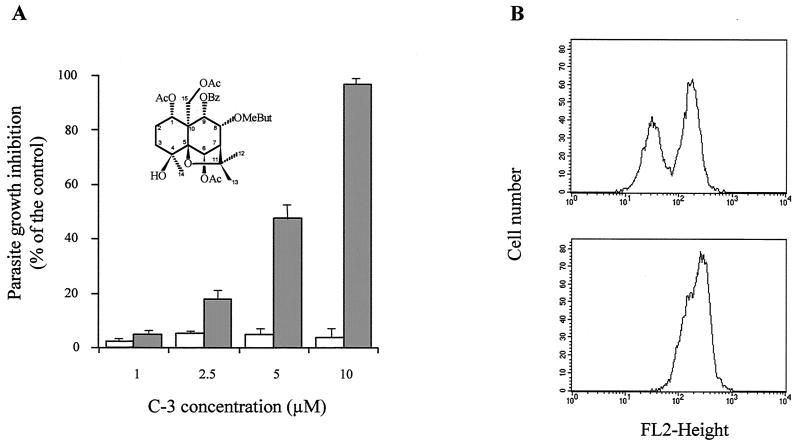
FIG. 3.
Reversal of the Pgp-mediated resistance to daunomycin in the MDR L. tropica line by the sesquiterpene C-3. (A) Sensitization of daunomycin resistance by the sesquiterpene C-3. Cell growth of either wild-type or resistant parasites was determined under conditions shown in Fig. 2. Wild-type parasites (open bars) were incubated in the presence of different concentrations of the sesquiterpene C-3 (chemical structure shown upper left) and used as control of modulator intrinsic cytotoxicity. Resistant parasites (solid bars) were incubated with the same concentrations of sesquiterpene in the presence of 150 μM daunomycin. Data are means with standard deviations of three independent experiments performed in duplicate. (B) Modulation of intracellular daunomycin accumulation by the sesquiterpene C-3. Fluorescence (FL2) histograms were obtained by flow cytometry, after a 1-h incubation of a mixture of wild-type and resistant parasites at 28°C with 8 μM daunomycin in the absence (top panel) or presence (bottom panel) of 50 μM sesquiterpene C-3. Experiments were repeated three times and gave essentially the same profiles as the typical experiment shown here.
Parasite culture and in vitro experiments.
Promastigote forms of a cloned L. tropica LRC strain (wild-type) were grown at 28°C in RPMI 1640 modified medium (Gibco) (22), supplemented with 20% heat-inactivated fetal bovine serum (FBS) (Gibco). A derivative line highly resistant to daunomycin at a 50% inhibitory concentration (IC50 [concentration of drug that decreases the rate of cell growth by 50%]) of 272 μM versus 2.6 μM in the wild-type line, was continuously maintained in the presence of 150 μM daunomycin, a concentration that does not produce any significant toxic effect. This resistant line was cloned from the MDR line DNM-R150 previously described (5) and presented an MDR phenotype similar to that described in tumor cells, with a profile of cross-resistance to several drugs due to an overexpressed Pgp-like transporter. A revertant line was obtained by maintenance of the resistant line in drug-free medium for 45 days, in order to decrease Pgp overexpression and daunomycin resistance to wild-type levels (IC50 9 μM), as previously described (5). The profile of cross-resistance of wild-type, resistant, and revertant parasites to ALP was ascertained as follows. Before each experiment, the MDR line was maintained in the absence of daunomycin during two passages (4 days). This treatment, which does not decrease Pgp overexpression as observed by Western blotting (data not shown), removes most of the intracellular daunomycin, as determined by flow cytometry analysis (not shown), in order to avoid any interference with other drugs. For each cell line, 4 × 106 parasites/ml were then incubated in 2 ml of fresh RPMI 1640 modified medium plus 20% FBS; afterwards, different concentrations of ALP were added to each tube in the presence or absence of 10 μM C-3. Three tubes lacking any drug, as controls for the ALP cytotoxicity, as well as three tubes only with the reversal agent, as a control of C-3 cytotoxicity during the sensitization to ALP, were maintained in parallel. After 72 h of incubation at 28°C, the numbers of cells per milliliter were determined on a Coulter counter model Z1. The initial cell density was then subtracted from the final cell density as described previously (18). The resulting difference was expressed as a percentage of growth in the control tubes, plotted as a function of ALP concentration, and the IC50s were graphically determined. Resistance indices were determined as the IC50 ratio among resistant and wild-type cells. The modulation of daunomycin resistance by reversal agents was monitored in a different way, as described previously (32). Briefly, the MDR parasites, adapted to normal growth in the presence of 150 μM daunomycin, were incubated at the cell density described above with this daunomycin concentration in fresh medium in the presence of different concentrations of the sesquiterpene C-3 or ALP. Three control tubes were incubated in parallel with the same daunomycin concentration, but lacking the reversal agent. After 72 h of incubation at 28°C, cell densities were determined as described above. The sensitization of the resistant parasites to daunomycin in the presence of the reversal agent was expressed as the percentage of growth inhibition with respect to the control cells, as described previously (31, 33). Control of the sesquiterpene toxicity in Leishmania was done in parallel tubes by incubating the wild-type parasite with the same concentration of reversal agent, as described previously (31–33).
Immunofluorescence studies.
Indirect immunofluorescence analysis was performed as previously described with some modifications (37). Parasite lines were washed three times with cold phosphate-buffered saline (PBS; 1.2 mM KH2PO4, 8.1 mM Na2HPO4, 130 mM NaCl, 2.6 mM KCl adjusted to pH 7.4) and resuspended at a concentration of 8 × 106 cells/ml. The parasite suspension was placed in a slide, air dried, and then fixed first in absolute ethanol for 5 min and later in acetone for 8 min at −20°C. Slides were incubated for 1 h at 37°C with the anti-Leishmania Pgp-like transporter serum (5) diluted 1:100 in PBS–0.1% bovine serum albumin (IFI buffer). Slides were then washed three times in IFI buffer prior to incubation with fluorescein-conjugated goat anti-rabbit immunoglobulin G (Sigma) diluted 1:200 in IFI buffer for 1 h at 37°C. Finally, slides were washed as described above and examined under a fluorescent microscope. Control experiments were performed by replacing the anti-Pgp serum with preimmune rabbit serum.
Accumulation of daunomycin and bodipy-C5-PC by flow cytometry.
The accumulation of daunomycin and bodipy-C5-PC in wild-type and resistant Leishmania lines was estimated by flow cytometry with a Becton Dickinson FacScan, as previously described with some modifications (5). In the case of daunomycin, a mixture of both parasite lines was incubated with 8 μM daunomycin for 1 h at 28°C in RPMI 1640 modified medium in the presence or in the absence of the sesquiterpene C-3. Cells were extensively washed, resuspended in cold PBS, and immediately analyzed. For bodipy-C5-PC, both lines were separately incubated with 3 μM bodipy-C5-PC for 1 h at 28°C in RPMI 1640 modified medium plus 20% FBS, with or without the reversal agent. Cells were first washed with the 20% FBS medium and then with PBS. In both cases, cells were gated on the basis of size and complexity to eliminate dead cells and debris from the analysis. Quantification of intracellular fluorescence was carried out by scanning the emission between 564 and 606 nm (FL-2) in the case of daunomycin, and between 515 and 545 nm (FL-1) for bodipy-C5-PC by using the Cell Quest Software application.
RESULTS
Resistance to ALP in an MDR L. tropica line overexpressing a Pgp-like transporter.
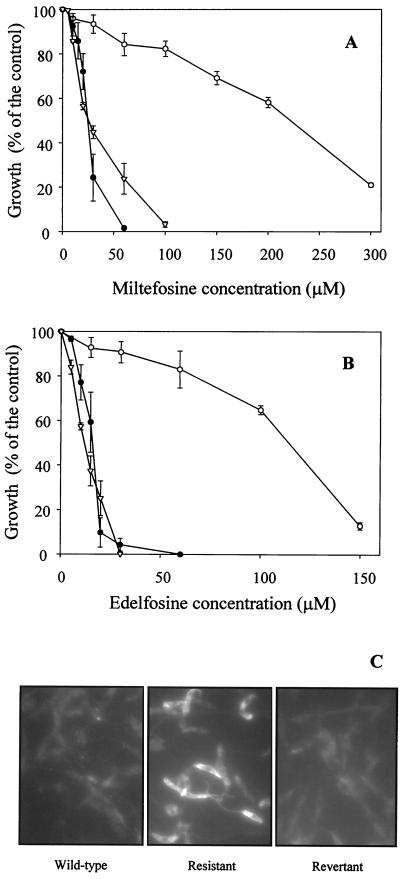
The cytotoxicities of the ALP miltefosine (Fig. 1A) and edelfosine (Fig. 1B) were assayed with an L. tropica line (wild type) and its MDR derivative line overexpressing a Pgp-like transporter. Edelfosine showed a slightly higher cytotoxic effect than miltefosine in the wild-type line, with IC50s of 16.2 and 24.7 μM, respectively (Fig. 1A and B). The MDR line was cross-resistant to these drugs, with IC50s of 227.3 μM for miltefosine (Fig. 1A) and 115.2 μM for edelfosine (Fig. 1B), exhibiting resistance indices of 9.2- and 7.1-fold, respectively. We also studied the correlation of Pgp expression with the resistance to ALP. For that purpose, the resistant line was incubated for 45 days in the absence of daunomycin to decrease Pgp-like transporter overexpression and daunomycin resistance to levels similar to those of wild-type parasites, as previously described (5) and demonstrated by indirect immunofluorescence (Fig. 1C) and Western blotting (not shown). The results showed that this revertant line displayed an ALP sensitivity similar to that found in the wild-type line (Fig. 1A and B).
FIG. 1.
Profile of cross-resistance of L. tropica lines to ALP. Cell growth of either wild-type (●), resistant (○), or revertant (▿) Leishmania lines was determined after incubation at 28°C for 72 h in the presence of different concentrations of the ALP miltefosine (A) and edelfosine (B) as described in Materials and Methods. The results are expressed as the percentage of growth observed in each case compared to that of the control cells (with no ALP). Data are the means of three independent experiments performed in duplicate, and standard deviations are represented by error bars. (C) Indirect immunofluorescence of wild-type (left), resistant (center), and revertant (right) Leishmania lines by using a polyclonal antibody directed to the Pgp-like transporter, as described in Materials and Methods.
Sensitization of the MDR Leishmania line to daunomycin by ALP.
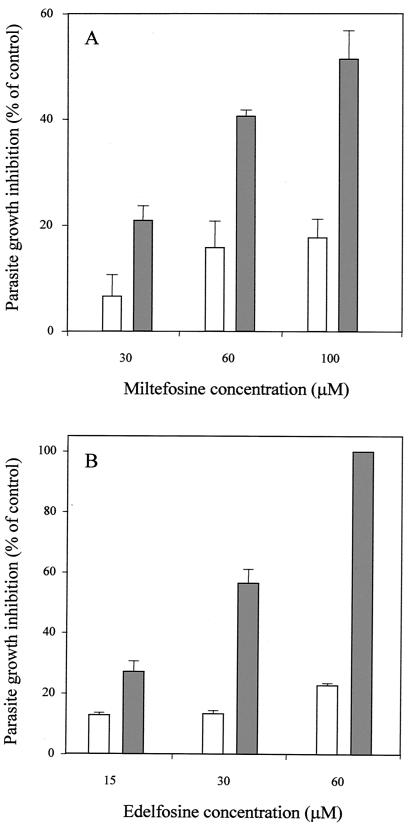
The MDR line, normally maintained in the presence of daunomycin (150 μM), shows a significant resistant index to daunomycin due to the activity of the Pgp-like transporter. To further establish the role of Pgp in the ALP resistance, we studied the ability of these ether-lipid analogs to sensitize the MDR parasites to daunomycin, as detailed in Materials and Methods. The results showed that both miltefosine (Fig. 2A) and, especially, edelfosine (Fig. 2B) were able to significantly revert the daunomycin resistance at 100 and 60 μM, respectively. Both ALP concentrations had limited toxic effects on the MDR line (Fig. 2A and B).
FIG. 2.
Sensitization by ALP in the daunomycin-resistant L. tropica line. Cell growth of MDR parasites was determined after 72 h of incubation at 28°C. Parasites were incubated with different concentrations of the ALP miltefosine (A) and edelfosine (B) in the presence (solid bars) or in the absence (open bars) of 150 μM daunomycin. The results are expressed as the percentage of growth inhibition observed in each case compared to that in the control cells (resistant parasites maintained in the presence of 150 μM daunomycin and with no ALP). Data are the means of three independent experiments performed in duplicate, and standard deviations are represented by error bars.
Reversion of Pgp-mediated ALP resistance by inhibition of the transporter.
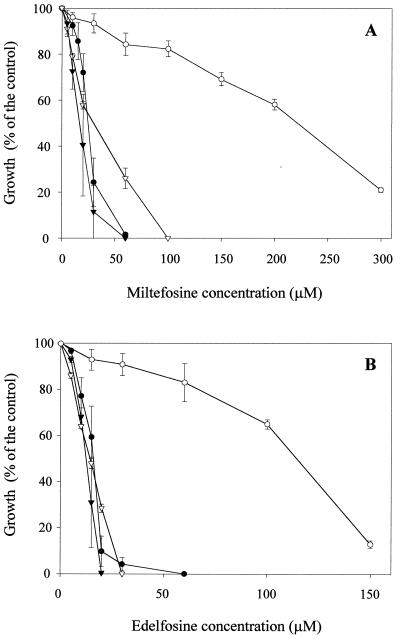
Contrary to conventional Pgp modulators, hemisynthetic flavonoids (32, 33) and natural sesquiterpenes (31) are able to efficiently overcome the daunomycin resistance phenotype in the MDR Leishmania line by increasing drug accumulation. If the Pgp-like transporter is responsible for ALP resistance, these modulators should be able to sensitize the MDR line to these antileishmanial drugs. Consequently, we studied the effect of a new natural sesquiterpene, called C-3 (Fig. 3A), that had previously shown a higher reversal effect of the MDR phenotype in mammalian cells overexpressing human Pgp than other sesquiterpenes previously analyzed (unpublished data). First, we studied the ability of C-3 to revert Pgp-mediated daunomycin resistance in the MDR Leishmania line. Figure 3A shows that C-3 efficiently overcame daunomycin resistance at low concentrations (10 μM), without any significant toxicity in the wild-type line. This reversal effect is due to an increase in daunomycin accumulation in the MDR line, as shown by flow cytometry assays (Fig. 3B). Thus, when a mixture of both lines was incubated for 1 h with 8 μM daunomycin, we observed the two expected peaks of fluorescence distribution (Fig. 3B, top panel) as a consequence of the lower drug accumulation in resistant (left peak) with respect to wild-type parasites (right peak). Treatment with 50 μM C-3 (Fig. 3B, bottom panel) resulted in a significant shift of the fluorescence peak corresponding to resistant parasites to the right, indicating an increase in daunomycin accumulation in these cells, and therefore, only one peak was observed in the mixed population. Afterwards, we studied the ability of this new Pgp inhibitor to overcome ALP resistance in the MDR line. Figure 4 shows that 10 μM C-3 almost completely sensitized the resistance of the Leishmania MDR line to miltefosine (Fig. 4A) and edelfosine (Fig. 4B), with no significant effect on wild-type parasites. This C-3 concentration did not produce any toxic effect on the resistant line in the absence of daunomycin (not shown). Treatment of MDR parasites with 10 μM C-3 over 72 h did not decrease the level of the Pgp-like transporter overexpression, as determined by Western blot analysis (data not shown).
FIG. 4.
Sensitization of the ALP resistance in the MDR L. tropica line by the sesquiterpene C-3. Cell growth of either wild-type (triangles) or resistant (circles) Leishmania lines was determined under the conditions described in the legend to Fig. 1, after incubation with different concentrations of the ALP miltefosine (A) and edelfosine (B) and in the presence (solid symbols) or absence (open symbols) of 10 μM sesquiterpene C-3. Control cells were incubated with the same C-3 concentration. Data are means of three independent experiments performed in duplicate, and standard deviations are represented by error bars.
Accumulation of bodipy-C5-PC in wild-type and MDR Leishmania lines.
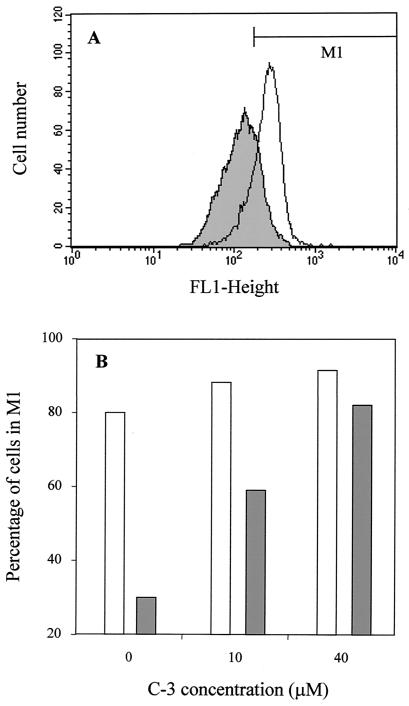
The accumulation of bodipy-C5-PC, a fluorescent analogue of edelfosine (46), was studied in both Leishmania cell lines (Fig. 5A). Flow cytometry analysis revealed that after 1 h of incubation with 3 μM bodipy-C5-PC, the accumulation of the fluorescent analogue was significantly lower in the MDR line than in the wild-type line. Interestingly, in the presence of 10 and especially 40 μM C-3, bodipy-C5-PC accumulation in the MDR line was significantly increased to the levels of wild-type parasites (Fig. 5B), suggesting that the differences found in both cell lines were due to the Pgp activity.
FIG. 5.
Cellular accumulation of bodipy-C5-PC in L. tropica lines. Fluorescence (FL1) intensity histograms (A) were obtained by flow cytometry after incubation of wild-type (open profile) and resistant (shaded profile) parasites with 3 μM bodipy-C5-PC for 1 h at 28°C, as described in Materials and Methods. The marker M1 was placed to include 80% of the wild-type cells. The percentages of wild-type (open bars) and resistant (solid bars) parasites in this region after incubation with different concentrations of the sesquiterpene C-3 are represented in panel B. A total of 10,000 cells were counted for each histogram. Experiments were repeated three times and gave essentially the same profiles as the experiment shown here.
DISCUSSION
Our main novel results concern the description in Leishmania of an in vitro case of resistance to ALP such as miltefosine and edelfosine, the most promising antileishmanial agents. We have also found a molecule probably involved in such resistance, which is the Pgp-like transporter, demonstrating the possibility of efficiently overcoming ALP resistance by using a new Pgp inhibitor.
In order to rationally design reversal agents that render the trypanosomatids sensitive to these new chemotherapeutic compounds, it is important to know the possible mechanisms involved in ALP resistance. We have found several indications that suggest the involvement of an ABC multidrug transporter in ALP resistance. (i) First of all, an MDR Leishmania line overexpressing a Pgp-like transporter displays a significant cross-resistance to ALP. (ii) Reduced Pgp expression in the resistant line maintained in the absence of the drug inducer of the MDR phenotype correlated with a significant decrease in ALP resistance. (iii) ALP were able to modulate the resistance to daunomycin produced by Pgp in the MDR line. (iv) We have observed that the sesquiterpene C-3, a new modulator of Pgp-mediated MDR phenotype described in this study, sensitizes MDR parasites to ALP. (v) Finally, the MDR line exhibits a lower accumulation of bodipy-C5-PC, a fluorescent analogue of phosphatidylcholine the structure of which resembles that of edelfosine (46) with respect to the wild-type line. As expected, if the Pgp-like transporter were involved in the resistance to ALP, the sesquiterpene C-3 produces a dose-dependent increase in bodipy-C5-PC accumulation in the resistant line to levels similar to those observed in the wild type. In agreement with these results, it is well established that mammalian Pgps and other ABC transporters are involved in phospholipid translocation, including that of phosphatidylcholine (2, 3, 38, 48). It is therefore tempting to suggest that the mechanism of ALP resistance by the Leishmania multidrug transporter could be related to the flippase mechanism of phosphatidylcholine transport by mammalian Pgps. Besides, as recently described (12), human Pgp is involved in the transport of the platelet-activating factor (PAF), an analogue of edelfosine (also called methyl-PAF) that could therefore be an endogenous substrate of the pump (12). In addition, human Pgp overexpression in different cell lines, including mdr1-transfected cells, induces a cross-resistance to ilmofosine (21), another ALP with a similar structure to edelfosine. However, contrary to our results, the resistance to ilmofosine in the cells described above cannot be reverted with Pgp inhibitors, nor can ilmofosine modulate their MDR phenotype. Besides, ilmofosine neither inhibits the Pgp labeling with azidopine nor affects its ATPase activity. The authors conclude that ilmofosine is not a Pgp substrate and suggest that the resistance could be mediated by modifications of the plasma membrane permeability induced by Pgp (21). Further cell lines selected for resistance to miltefosine overexpress the mdr1 gene, but this resistance could not be reverted with verapamil either (15). On the other hand, other cell lines with a MDR phenotype and overexpressing mammalian Pgps do not show a cross-resistance profile to ALP (16, 34, 35, 49). These contradictory results could be partly explained by the significant differences between human and Leishmania Pgp-like transporters, which share 37% identity at the amino acid level (5). In fact, classical modulators of MDR mammalian cells such as verapamil and cyclosporine do not efficiently revert the Pgp-like transporter-mediated Leishmania MDR phenotype (5, 18, 32). The possibility of overcoming ALP resistance by coadministration of modulators, such as the sesquiterpene C-3 described here, is of great significance for future clinical applications. Related sesquiterpenes are indeed known to reverse the Pgp-mediated MDR phenotype of Leishmania spp. (31) and, interestingly, also in mammalian cell lines (24, 25; unpublished results).
In spite of the evidence presented above, we cannot rule out some other possible mechanisms involved in the resistance to ALP in the MDR Leishmania line. In tumor cells, how ALP resistance could be determined by decreased uptake and accumulation (14, 41, 46, 51) or faster metabolism (14) of these drugs has been described, but little is known about what influences this different behavior. On the other hand, the results of Fleer and coworkers (14) have also shown that miltefosine-resistant cells could incorporate and tolerate a larger amount of the drug than the parental cells, indicating that mechanisms other than decreased drug accumulation are involved in this resistance. In addition, Pgp-like transporter overexpression could indirectly contribute to the ALP resistance, as suggested by Hoffman and coworkers (21), by inducing changes in the physical properties of the cell membrane. Indeed, mdr1 gene transfections are described to alter the fluidity of the membrane in mammalian cells (4), and this change has also been described as altering the ALP effects (42). On the other hand, the ability of ALP to overcome daunomycin resistance in the MDR Leishmania line could also be influenced by an ALP-mediated increase in membrane fluidity (11, 30), because membrane fluidization has been described to inhibit the mammalian Pgp ATPase activity (36). The finding of other specific genes involved in ALP resistance is of great significance, and we are therefore currently performing functional cloning studies with Leishmania spp. to this end.
We consider that the study of the molecular mechanisms involved in the resistance to ALP is of considerable interest for pharmaceutical and clinical purposes in the area of antiparasite chemotherapy. The increasingly widespread use of ALP in the treatment of visceral and cutaneous leishmaniasis could induce the appearance of resistance. Therefore, understanding how it arises could lead to strategies for new and more effective generation of antiprotozoal drugs. Finally, Leishmania multidrug transporters were thought to be involved in resistance to drugs not used to treat leishmaniasis; consequently, their clinical involvement had not been well established. However, their implication in ALP resistance together with the fact that many new potential leishmanicidal agents, such as azoles, are known substrates of ABC transporters, and thus could induce a drug resistance phenotype, strengthens the clinical relevance of this ABC transporter and supports the ever-increasing interest in the development of new specific inhibitors.
ACKNOWLEDGMENTS
This study was supported by Spanish grants CICYT-FEDER IFD97-0747-C04-01/03 (A.G. and S.C.) and PPQ2000-1655-C02-02 (F.G.). F.J.P.-V. was the recipient of a fellowship from the Ministerio de Educación y Cultura (Spain), and A.P.-T. was the recipient of a fellowship from the Agencia Española de Cooperación Internacional (Becas MUTIS).
We thank Pilar Navarro for help with parasite culture and Carmenza Spadafora for improving the English of the manuscript. We also acknowledge Pharmacia-Spain (Barcelona) for providing the daunomycin used in this study.
F.J.P.-V. and A.P.-T. contributed equally to this work.
REFERENCES
- 1.Berman J D. Human leishmaniasis: clinical, diagnostic, and chemotherapeutic developments in the last 10 years. Clin Infect Dis. 1997;24:684–703. doi: 10.1093/clind/24.4.684. [DOI] [PubMed] [Google Scholar]
- 2.Borst P, Zelcer N, van Helvoort A. ABC transporters in lipid transport. Biochim Biophys Acta. 2000;1486:128–144. doi: 10.1016/s1388-1981(00)00053-6. [DOI] [PubMed] [Google Scholar]
- 3.Bosch I, Dunussi-Joannopoulos K, Wu R L, Furlong S T, Croop J. Phosphatidylcholine and phosphatidylethanolamine behave as substrates of the human MDR1 P-glycoprotein. Biochemistry. 1997;36:5685–5694. doi: 10.1021/bi962728r. [DOI] [PubMed] [Google Scholar]
- 4.Callaghan R, van Gorkom L C, Epand R M. A comparison of membrane properties and composition between cell lines selected and transfected for multi-drug resistance. Br J Cancer. 1992;66:781–786. doi: 10.1038/bjc.1992.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiquero M J, Pérez-Victoria J M, O'Valle F, Gonzalez-Ros J M, del Moral R, Ferragut J A, Castanys S, Gamarro F. Altered drug membrane permeability in a multidrug-resistant Leishmania tropica line. Biochem Pharmacol. 1998;55:131–139. doi: 10.1016/s0006-2952(97)00385-7. [DOI] [PubMed] [Google Scholar]
- 6.Chow L M C, Wong A K C, Ullman B, Wirth D F. Cloning and functional analysis of an extrachromosomally amplified multidrug resistance-like gene in Leishmania enrietti. Mol Biochem Pharmacol. 1993;60:195–208. doi: 10.1016/0166-6851(93)90131-g. [DOI] [PubMed] [Google Scholar]
- 7.Croft S L, Neal R A, Pendergast W, Chan J H. The activity of alkyl phosphorylcholines and related derivatives against Leishmania donovani. Biochem Pharmacol. 1987;36:2633–2636. doi: 10.1016/0006-2952(87)90543-0. [DOI] [PubMed] [Google Scholar]
- 8.Croft S L, Neal R A, Thornton E A, Herrmann D B. Antileishmanial activity of the ether phospholipid ilmofosine. Trans R Soc Trop Med Hyg. 1993;87:217–219. doi: 10.1016/0035-9203(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 9.Croft S L, Snowdon D, Yardley V. The activities of four anticancer alkyllysophospholipids against Leishmania donovani, Trypanosoma cruzi and Trypanosoma brucei. J Antimicrob Chemother. 1996;38:1041–1047. doi: 10.1093/jac/38.6.1041. [DOI] [PubMed] [Google Scholar]
- 10.Diomede L, Colotta F, Piovani B, Re F, Modest E J, Salmona M. Induction of apoptosis in human leukemic cells by the ether lipid 1-octadecyl-2-methyl-rac-glycero-3-phosphocholine. A possible basis for its selective action. Int J Cancer. 1993;53:124–130. doi: 10.1002/ijc.2910530123. [DOI] [PubMed] [Google Scholar]
- 11.Diomede L, Bianchi R, Modest E J, Piovani B, Bubba F, Salmona M. Modulation of ATPase activity by cholesterol and synthetic ether lipids in leukemic cells. Biochem Pharmacol. 1992;43:803–807. doi: 10.1016/0006-2952(92)90246-f. [DOI] [PubMed] [Google Scholar]
- 12.Ernest S, Bello-Reuss E. Secretion of platelet-activating factor is mediated by MDR1 P-glycoprotein in cultured human mesangial cells. J Am Soc Nephrol. 1999;10:2306–2313. doi: 10.1681/ASN.V10112306. [DOI] [PubMed] [Google Scholar]
- 13.Faraut-Gambarelli F, Piarroux R, Deniau M, Giusiano B, Marty P, Michel G, Faugère B, Dumon H. In vitro and in vivo resistance of Leishmania infantum to meglumine antimoniate: a study of 37 strains collected from patients with visceral leishmaniasis. Antimicrob Agents Chemother. 1997;41:827–830. doi: 10.1128/aac.41.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleer E A, Berkovic D, Grunwald U, Hiddemann W. Induction of resistance to hexadecylphosphocholine in the highly sensitive human epidermoid tumour cell line KB. Eur J Cancer. 1996;32A:506–511. doi: 10.1016/0959-8049(95)00566-8. [DOI] [PubMed] [Google Scholar]
- 15.Fu D, Shi Z, Wang Y. Bcl-2 plays a key role instead of mdr1 in the resistance to hexadecylphosphocholine in human epidermoid tumor cell line KB. Cancer Lett. 1999;142:147–153. doi: 10.1016/s0304-3835(99)00146-9. [DOI] [PubMed] [Google Scholar]
- 16.Glasser L, Dalton W S, Fiederlein R L, Cook P, Powis G, Vogler W R. Response of human multiple myeloma-derived cell lines to alkyl-lysophospholipid. Exp Hematol. 1996;24:253–257. [PubMed] [Google Scholar]
- 17.González A G, Jiménez I A, Ravelo A G, Bazzocchi I L. β-Agarofuran sesquiterpenes from Maytenus canariensis. Phytochemistry. 1990;29:2577–2579. [Google Scholar]
- 18.Henderson D M, Sifri C D, Rodgers M, Wirth D F, Hendrickson N, Ullman B. Multidrug resistance in Leishmania donovani is conferred by amplification of a gene homologous to the mammalian mdr1 gene. Mol Cell Biol. 1992;12:2855–2865. doi: 10.1128/mcb.12.6.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins C F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 20.Hirst S I, Stapley L A. Parasitology: the dawn of a new millennium. Parasitol Today. 2000;16:1–3. doi: 10.1016/s0169-4758(99)01589-6. [DOI] [PubMed] [Google Scholar]
- 21.Hofmann J, Utz I, Spitaler M, Hofe S, Rybczynska M, Beck W T, Herrmann D B, Grunicke H. Resistance to the new anti-cancer phospholipid ilmofosine (BM 41 440) Br J Cancer. 1997;76:862–869. doi: 10.1038/bjc.1997.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson P R, Lawrie J M, Stiteler J M, Hawkins D W, Wohlhieter J A, Rowtin E D. Detection and characterization of Leishmania species and strains from mammals and vectors by hybridization and restriction endonuclease digestion of kinetoplast DNA. Vet Parasitol. 1986;20:195–215. doi: 10.1016/0304-4017(86)90100-7. [DOI] [PubMed] [Google Scholar]
- 23.Jha T K, Sundar S, Thakur C P, Bachmann P, Karbwang J, Fischer C, Voss A, Berman J. Miltefosine, an oral agent, for the treatment of Indian visceral leishmaniasis. N Engl J Med. 1999;341:1795–1800. doi: 10.1056/NEJM199912093412403. [DOI] [PubMed] [Google Scholar]
- 24.Kim S E, Kim Y H, Lee J J, Kim Y C. A new sesquiterpene ester from Celastrus orbiculatus reversing multidrug resistance in cancer cells. J Nat Prod. 1998;61:108–111. doi: 10.1021/np9702392. [DOI] [PubMed] [Google Scholar]
- 25.Kim S E, Kim H S, Hong Y S, Kim Y C, Lee J J. Sesquiterpene esters from Celastrus orbiculatus and their structure-activity relationship on the modulation of multidrug resistance. J Nat Prod. 1999;62:697–700. doi: 10.1021/np9804379. [DOI] [PubMed] [Google Scholar]
- 26.Le Fichoux Y, Rousseau D, Ferrua B, Ruette S, Lelièvre A, Grousson D, Kubar J. Short- and long-term efficacy of hexadecylphosphocholine against established Leishmania infantum infection in BALB/c mice. Antimicrob Agents Chemother. 1998;42:654–658. doi: 10.1128/aac.42.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lira R, Sundar S, Makharia A, Kenney R, Gam A, Saraiva E, Sacks D. Evidence that the high incidence of treatment failures in Indian kala-azar is due to the emergence of antimony-resistant strains of Leishmania donovani. J Infect Dis. 1999;180:564–567. doi: 10.1086/314896. [DOI] [PubMed] [Google Scholar]
- 28.Lux H, Hart D T, Parker P J, Klenner T. Ether lipid metabolism, GPI anchor biosynthesis, and signal transduction are putative targets for anti-leishmanial alkyl phospholipid analogues. Adv Exp Med Biol. 1996;416:201–211. doi: 10.1007/978-1-4899-0179-8_33. [DOI] [PubMed] [Google Scholar]
- 29.Lux H, Heise N, Klenner T, Hart D, Opperdoes F R. Ether-lipid (alkyl-phospholipid) metabolism and the mechanism of action of ether-lipid analogues in leishmania. Mol Biochem Parasitol. 2000;111:1–14. doi: 10.1016/s0166-6851(00)00278-4. [DOI] [PubMed] [Google Scholar]
- 30.Noseda A, Godwin P L, Modest E J. Effects of antineoplastic ether lipids on model and biological membranes. Biochim Biophys Acta. 1988;945:92–100. doi: 10.1016/0005-2736(88)90366-5. [DOI] [PubMed] [Google Scholar]
- 31.Pérez-Victoria J M, Tincusi B M, Jiménez I A, Bazzocchi I L, Gupta M P, Castanys S, Gamarro F, Ravelo A G. New natural sesquiterpenes as modulators of daunomycin resistance in a multidrug-resistant Leishmania tropica line. J Med Chem. 1999;42:4388–4393. doi: 10.1021/jm991066b. [DOI] [PubMed] [Google Scholar]
- 32.Pérez-Victoria J M, Chiquero M J, Conseil G, Dayan G, Di Pietro A, Barron D, Castanys S, Gamarro F. Correlation between the affinity of flavonoids binding to cytosolic site of Leishmania tropica multidrug transporter and their efficiency to revert parasite resistance to daunomycin. Biochemistry. 1999;38:1736–1743. doi: 10.1021/bi982455v. [DOI] [PubMed] [Google Scholar]
- 33.Pérez-Victoria J M, Pérez-Victoria F J, Conseil G, Maitrejean M, Comte G, Barron D, Di Pietro A, Castanys S, Gamarro F. High-affinity binding of silybin derivatives to the nucleotide-binding domain of a Leishmania tropica P-glycoprotein-like transporter and chemosensitization of a multidrug-resistant parasite to daunomycin. Antimicrob Agents Chemother. 2001;45:439–446. doi: 10.1128/AAC.45.2.439-446.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peters A C, Ahmad I, Janoff A S, Pushkareva M Y, Mayhew E. Growth inhibitory effects of liposome-associated 1-O-octadecyl-2-O-methyl-sn-glycero-3-phosphocholine. Lipids. 1997;32:1045–1054. doi: 10.1007/s11745-997-0135-8. [DOI] [PubMed] [Google Scholar]
- 35.Principe P, Faussat-Suberville A M, Coulomb H, Marie J P, Braquet P. Flow cytometric monitoring of anthracycline accumulation after anti-neoplastic ether phospholipid treatment. Anticancer Drugs. 1994;5:329–335. doi: 10.1097/00001813-199406000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Regev R, Assaraf Y G, Eytan G D. Membrane fluidization by ether, other anesthetics, and certain agents abolishes P-glycoprotein ATPase activity and modulates efflux from multidrug-resistant cells. Eur J Biochem. 1999;259:18–24. doi: 10.1046/j.1432-1327.1999.00037.x. [DOI] [PubMed] [Google Scholar]
- 37.Robello C, Dallagiovanna B, Engel J C, Castanys S, Gamarro F. A new member of YER057c family in Trypanosoma cruzi is adjacent to an ABC-transporter. Gene. 1998;220:1–12. doi: 10.1016/s0378-1119(98)00439-9. [DOI] [PubMed] [Google Scholar]
- 38.Ruetz S, Gros P. Phosphatidylcholine translocase: a physiological role for the mdr2 gene. Cell. 1994;77:1071–1081. doi: 10.1016/0092-8674(94)90446-4. [DOI] [PubMed] [Google Scholar]
- 39.Santa-Rita R M, Santos Barbosa H, Meirelles M N, de Castro S L. Effect of the alkyl-lysophospholipids on the proliferation and differentiation of Trypanosoma cruzi. Acta Trop. 2000;75:219–228. doi: 10.1016/s0001-706x(00)00052-8. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt-Ott R, Klenner T, Overath P, Aebischer T. Topical treatment with hexadecylphosphocholine (Miltex) efficiently reduces parasite burden in experimental cutaneous leishmaniasis. Trans R Soc Trop Med Hyg. 1999;93:85–90. doi: 10.1016/s0035-9203(99)90192-x. [DOI] [PubMed] [Google Scholar]
- 41.Small G W, Strum J C, Daniel L W. Characterization of an HL-60 cell variant resistant to the antineoplastic ether lipid 1-O-octadecyl-2-O-methyl-rac-glycero-3-phosphocholine. Lipids. 1997;32:715–723. doi: 10.1007/s11745-997-0091-3. [DOI] [PubMed] [Google Scholar]
- 42.Storme G A, Berdel W E, van Blitterswijk W J, Bruyneel E A, De Bruyne G K, Mareel M M. Antiinvasive effect of racemic 1-O-octadecyl-2-O-methylglycero-3-phosphocholine on MO4 mouse fibrosarcoma cells in vitro. Cancer Res. 1985;45:351–357. [PubMed] [Google Scholar]
- 43.Strassheim D, Shafer S H, Phelps S H, Williams C L. Small cell lung carcinoma exhibits greater phospholipase C-beta1 expression and edelfosine resistance compared with non-small cell lung carcinoma. Cancer Res. 2000;60:2730–2736. [PubMed] [Google Scholar]
- 44.Sundar S, Singh V P, Murray H W. Current epidemic of visceral leishmaniasis in India. Acta Parasitol Turcica. 1997;21:128. [Google Scholar]
- 45.Thakur C P, Sinha P K, Singh R K, Hassan S M, Narain S. Miltefosine in a case of visceral leishmaniasis with HIV co-infection and rising incidence of this disease in India. Trans R Soc Trop Med Hyg. 2000;94:696–697. doi: 10.1016/s0035-9203(00)90238-4. [DOI] [PubMed] [Google Scholar]
- 46.Tsutsumi T, Tokumura A, Kitazawa S. Undifferentiated HL-60 cells internalize an antitumor alkyl ether phospholipid more rapidly than resistant K562 cells. Biochim Biophys Acta. 1998;1390:73–84. doi: 10.1016/s0005-2760(97)00171-9. [DOI] [PubMed] [Google Scholar]
- 47.Urbina J A. Lipid biosynthesis pathways as chemotherapeutic targets in kinetoplastid parasites. Parasitology. 1997;114:S91–S99. [PubMed] [Google Scholar]
- 48.van Helvoort A, Smith A J, Sprong H, Fritzsche I, Schinkel A H, Borst P, van Meer G. MDR1 P-glycoprotein is a lipid translocase of broad specificity, while MDR3 P-glycoprotein specifically translocates phosphatidylcholine. Cell. 1996;87:507–517. doi: 10.1016/s0092-8674(00)81370-7. [DOI] [PubMed] [Google Scholar]
- 49.Verdonck L F, van Heugten H G. Ether lipids are effective cytotoxic drugs against multidrug-resistant acute leukemia cells and can act by the induction of apoptosis. Leuk Res. 1997;21:37–43. doi: 10.1016/s0145-2126(96)00047-1. [DOI] [PubMed] [Google Scholar]
- 50.Wagner B A, Buettner G R, Oberley L W, Burns C P. Sensitivity of K562 and HL-60 cells to edelfosine, an ether lipid drug, correlates with production of reactive oxygen species. Cancer Res. 1998;58:2809–2816. [PubMed] [Google Scholar]
- 51.Zoeller R A, Layne M D, Modest E J. Animal cell mutants unable to take up biologically active glycerophospholipids. J Lipid Res. 1995;36:1866–1875. [PubMed] [Google Scholar]