Abstract
Methamphetamine (meth) dependence is often characterized by persistent and chronic relapse (i.e., return to drug use). Previous work suggests females may be at greater risk to relapse. In this study, we extended this limited evidence and identified sex-dependent neural substrates related to meth-triggered reinstatement. Male and female Sprague-Dawley rats were implanted with indwelling jugular catheters. Half of the rats were then trained to self-administer meth (0.05 mg/kg/inf); the other half self-administered saline during 21 daily sessions (2 h). Rats were then given 12 extinction sessions. Twenty-four hours after the last extinction session, rats received reinstatement testing. Half of the rats received a meth-prime (0.3 mg/kg, IP) injection and the remaining rats received a saline injection. This design resulted in 4 separate groups for each sex, allowing for careful investigation of brain regions related to meth-triggered reinstatement. Brains were harvested following the reinstatement session and c-Fos immunoreactivity was measured in multiple brain regions. Meth triggered reinstatement in both sexes and this effect was more robust in females compared to males. Significant sex differences were detected. Females showed greater c-Fos immunoreactivity in the cingulate cortex area 1, lateral orbitofrontal cortex, prelimbic cortex, caudate-putamen, nucleus accumbens core and shell, and central nucleus of the amygdala following meth-primed reinstatement.
Keywords: Amphetamine, c-Fos, Drug self-administration, Immunohistochemistry, Relapse
1. Introduction
Methamphetamine (meth) use and dependence is a serious public health concern (National Institute on Drug Abuse, 2013). A major impediment to meth dependence treatment is relapse, or a return to drug use following a prolonged period of drug abstinence. Relapse can occur months and even years after drug cessation (Baicy and London, 2007; Bamford et al., 2008; Grant et al., 2012). Relapse is often triggered by drug craving, an intense urge or desire to use a drug, induced by drug-associated cues, by stress, or by the drug itself (Blum et al., 2009; Carter and Tiffany, 1999; Chornock et al., 1992; Kaplan et al., 1985; Katz and Higgins, 2003; Paulus and Stewart, 2020; Preston et al., 1992; Self and Nestler, 1998; Stockwell et al., 1982; Walsh et al., 2000).
Drug-associated cues, stress, and drug-primes can also precipitate drug-seeking behavior in animal models (termed reinstatement). Researchers have utilized these pre-clinical reinstatement models to elucidate brain areas associated with drug relapse. Previous studies have implicated the prefrontal cortex (Ciccocioppo et al., 2001; Coordie and McFadden, 2019; Kufahl et al., 2009; Neisewander et al., 2000; Recinto et al., 2012; Thomas and Everitt, 2001; Wexler et al., 2001; Zhou et al., 2014), dorsal and ventral striatum (Di Ciano and Everitt, 2001; Kufahl et al., 2009; Neisewander et al., 2000; Rocha and Kalivas, 2010; Rubio et al., 2015; Zhou et al., 2014; Quinn et al., 2018), hippocampus (Kufahl et al., 2009; Sun and Rebec, 2003; Taepavarapruk and Phillips, 2003; Takashima et al., 2018; Vorel et al., 2001; Zhou et al., 2014), amygdala (Di Ciano and Everitt, 2004; Fuchs et al., 2005; Kufahl et al., 2009; McLaughlin and See, 2003; Rich et al., 2019; See et al., 2001; Whitelaw et al., 1996), hypothalamus (Zhou et al., 2014; Blacktop and Sorg, 2018), ventral tegmental area (VTA: Mahler et al., 2019; McFarland and Kalivas, 2001; Neisewander et al., 2000; Zhou et al., 2014), and substantia nigra (SNR: Bortz and Grace, 2018; Ilango et al., 2014; Kufahl et al., 2009; Neisewander et al., 2000; Rossi et al., 2013) in drug-seeking behavior during reinstatement.
A number of differences between males and females have been documented, including differences in the patterns of meth use and relapse in both clinical and pre-clinical studies. In humans, for example, differences between males and females arise as early as the reported motivation for the initiation of meth use (Simpson et al., 2016). In one study, more women reported using meth for weight control and to increase energy, whereas more men reported being motivated by the desire to work more hours (Brecht et al., 2004). Brecht et al. (2004), as well as several other studies (Dluzen and Liu, 2008; Hser et al., 2005; Lin et al., 2004; Westermeyer and Boedicker, 2000; Wu et al., 2007), found that women initiate use at a younger age than men. Once meth use is initiated, women also tend to transition to regular use more quickly than men (1.6 years for females vs 2.56 years for males; Brecht et al., 2004; Rawson et al., 2005).
Despite evidence that women may be more susceptible to meth dependence, until recently, animal models rarely use female subjects. This leaves a critical need for empirical research on meth-taking/seeking in preclinical models employing females. Some of the limited animal research that has investigated such sex differences in meth effects is consistent with work in humans. In self-administration studies, a greater percentage of female than male rats self-administered a low dose of meth during 6 h sessions, acquired meth self-administration quicker than their male counterparts, and responded more on progressive ratio (PR) schedules (Roth and Carroll, 2004; Reichel et al., 2012). Additionally, females show greater reinstatement induced by a meth-priming injection compared to males (Cox et al., 2013; Holtz et al., 2012; Reichel et al., 2012), suggesting that under certain conditions females may be more vulnerable to meth relapse (see Discussion). This literature, along with our preliminary research, led us to examine meth-primed reinstatement in the present study.
In the present report, we are interested in the increased vulnerability to reinstatement found in females and potential neural factors that contribute to this sex difference. To this end, we used the immediate early gene c-Fos as a measure of neural activation (Curran and Morgan, 1985a, 1985b; Kovacs, 1998; Greenberg and Ziff, 1984). This approach will provide critical foundational knowledge on brain regions that may be associated with the behavioral sex differences found during reinstatement. The brain areas examined in this study were the cingulate cortex area 1 and 2 (Cg1; Cg2), prelimbic cortex (PrL), infralimbic cortex (IL), lateral orbital cortex (LO), dorsal medial caudate-putamen (dmCPu), dorsal lateral caudate-putamen (dlCPu), ventral medial caudate-putamen (vmCPu), ventral lateral caudate-putamen, (vlCPu), nucleus accumbens core (NAcC), nucleus accumbens shell (NAcSh), hippocampus proper (CA1; CA2; and CA3) and ventral subiculum (VS), amygdala [central (CEA); basolateral (BLA)], lateral hypothalamus (LH), ventral tegmental area (VTA), and substantia nigra (SNR).
2. Materials and methods
2.1. Subjects
Sprague-Dawley rats were purchased from Harlan Laboratories (Indianapolis, IN, USA) at approximately 9 weeks of age (n = 140). Rats were housed individually in clear polycarbonate cages (35.5 × 32 × 18 cm; length × width × depth) with TEK-Fresh® cellulose bedding. The colony room was temperature- and humidity-controlled and maintained on a 6:00 AM light/6:00 PM dark cycle. Rats were allowed to acclimate to the colony room for 3 days. At that time, 90% free-feeding weights were calculated and maintained for the duration of the experiment. Rats received ad libitum access to water in the home cages. All experimental procedures were conducted during light phase of the cycle. Protocols were approved by the University of Nebraska-Lincoln Institutional Animal Care and Use Committee.
2.2. Apparatus
Behavioral testing was conducted in chambers purchased from Med Associates (ENV-008CT; Georgia, VT, USA). Each chamber measured 30.5 × 24.1 × 21 cm and was enclosed in a sound-attenuating cubicle. A variable-speed syringe pump (PMH-100VS; Med-Associates) was located outside each cubicle. Tygon® tubing was threaded from the pump syringe, through a leash, into the chamber to be attached to the catheter port that exited below the scapula of the rat. A recessed receptacle (5.2 × 5.2 × 3.8 cm) was centered on one sidewall of each chamber. A dipper arm, when raised, provided access to 0.1 ml of 26% (w/v) sucrose in this recessed receptacle. A retractable lever was located on each side of the receptacle. A white cue-light (2.54 cm diameter; 28 V, 100-mA) was mounted 7 cm above each lever. A house-light (two white 28 V, 100-mA lamps) was located in the cubicle, 10 cm above the Perspex chamber ceiling.
2.3. Drugs
(+)-Methamphetamine hydrochloride (Sigma-Aldrich, St. Louis, MO) was dissolved in sterile saline. Meth was infused intravenous (IV) at a volume of 35.74 μl over 1 s at 0.05 mg/kg/infusion. For reinstatement, 0.3 mg/kg meth was injected intraperitoneal IP at 1 ml/kg. Meth doses are reported in salt form. These meth doses were based on published (Duryee et al., 2009; Reichel et al., 2009) and unpublished research from our lab indicating robust maintenance of self-administration and potential sex differences in reinstatement.
2.4. Preliminary lever training
Following acclimation to the colony room and food restriction to maintain 90% of free-feeding weight, rats were trained to lever press on both levers following previously reported procedures (Charntikov et al., 2015; Charntikov et al., 2013; Pittenger et al., 2016). The start of each session was signaled by illumination of the house light and insertion of a randomly selected lever (right or left). A lever press or a lapse of 15 s resulted in 4-s access to sucrose, retraction of the lever, and commencement of a timeout (average = 60 s; range = 30 to 89 s). Following the timeout, a randomly selected lever was again inserted with the condition that the same lever could not be presented more than twice in a row. This protocol was repeated for 60 sucrose deliveries. Daily sessions range from 65 to 80 min depending on individual performance. Training continued until a lever press was made on at least 80% of the lever insertions for two consecutive days. All rats met criterion between sessions 3 to 5. This autoshaping protocol ensured rats were pressing at robust levels but neither lever was differentially associated with sucrose.
2.5. Catheter surgery and recovery
Indwelling jugular catheters were implanted using previously reported protocols (Charntikov et al., 2015; Charntikov et al., 2013; Pittenger et al., 2016). Rats were anesthetized with a 2:1 ketamine HCl (100 mg/kg; MWI, Boise, ID) plus xylazine HCl (20 mg/kg; MWI, Boise, ID) cocktail (intramuscular; IM). Following surgery, rats were administered buprenorphine (0.1 mg/kg, subcutaneous: SC; MWI, Boise, ID) for pain management and atipamezole (0.5 mg/kg, IM; MWI, Boise, ID) to terminate anesthesia. Buprenorphine was again administered 24-h post-surgery. Rats were allowed to recover for 7 days. During recovery, they remained in their home cages and catheters were flushed daily with a cocktail of 0.2-ml baytril (5.0 mg/ml; MWI, Boise, ID) to prevent infections and heparin (30 Units/ml; MWI, Boise, ID) to minimize blood clotting blocking fluid flow through the catheter. Catheter patency was checked on the last day of recovery by IV infusion of 0.05 ml xylazine (20 mg/ml). Rats that displayed motor ataxia within 20 s were considered patent (cf. Charntikov et al., 2015; Charntikov et al., 2013; Reichel et al., 2008; Pittenger et al., 2016). Patency was again checked upon the completion of the self-administration phase. Twenty eight rats were excluded from the study for failing the patency test.
2.6. Post-surgery lever training
Following recovery, rats were placed on a variable ratio 3 (VR3) schedule of sucrose reinforcement. Under the VR3 schedule, on average every 3rd lever press (range 1 to 5) was followed by 4-s access to sucrose. Levers were again inserted individually with the condition that the same lever was not inserted >2 times in a row. These procedures ensured robust responding with both levers having a similar reinforcement history (Charntikov et al., 2015; Charntikov et al., 2013; Pittenger et al., 2016). This training lasted for 3 daily 1-h sessions conducted on consecutive days.
2.7. Self-administration
Following post-surgery lever press training, male and female rats were separated into two self-administration conditions: MethSA or SalineSA. Rats in the MethSA conditions began self-administration of meth during daily 2-h sessions. Rats in the SalineSA condition began daily 2-h sessions identical to those received by the MethSA condition except saline was available in lieu of meth. Drug (meth or saline) was available on a VR3 schedule of reinforcement. During training, two levers were present, active and inactive. Rats were randomly assigned which of the two levers served as the active (meth or saline infusion) vs inactive lever. Before a rat was attached to the leash/tubing at the start of each session, the catheter was flushed with 0.2-ml heparin (30 Units/ml) in sterile saline. The session commenced with insertion of both levers and priming of the catheter with meth or saline [ca. 31 μl (90% of internal catheter volume)]. Requisite VR3 responding on the active lever initiated an infusion of meth or saline, retraction of both levers, and illumination of the house light for a 20-s timeout. Following the timeout, both levers were extended and the house light was terminated. Responding on the inactive lever was recorded but had no programed outcome. After each session, the catheter was flushed with a cocktail of 0.2-ml baytril (5 mg/ml) and heparin (30 Units/ml) in sterile saline. Sessions were conducted 7 days per week for 21 days.
2.8. Extinction
Extinction sessions commenced 24 h after the last self-administration session. Extinction sessions were identical to self-administration sessions except drug was no longer infused. Requisite VR3 responding on the active lever still produced the same cues and the timeout. Responding on the inactive lever was recorded but held no programed consequence. Sessions were 2 h and conducted daily for 12 days.
2.9. Reinstatement
At the end of extinction, the MethSA and SalineSA conditions were split further into 2 different reinstatement-trigger groups: SalineT or MethT. Rats were pseudo-randomly assigned with the caveat that the groups did not differ statistically in responding at the end of extinction. This created 4 groups of males and 4 groups of females: SalineSA/SalineT, SalineSA/MethT, MethSA/SalineT, MethSA/MethT. The first part of each name indicates the drug each group self-administered (Saline or Meth) and the second part of each name indicates the drug that “triggered” reinstatement (Saline or Meth; n = 14 per group). Each group provided vital information regarding the activation of brain regions associated with reinstatement. The SalineSA/SalineT group provided baseline c-Fos activation for both sexes in the absence of meth self-administration or an acute meth injection. The MethSA/SalineT groups allowed for examination of c-Fos activation that is related to an extinction (i.e., drug abstinence) period following self-administration of meth. The SalineSA/MethT groups were used to detect neural substrates associated with acute meth injection while controlling for exposure to chambers, handling, etc. Finally, the MethSA/MethT groups allowed us to identify neural substrates related with meth-triggered reinstatement following meth self-administration. Rats in the MethT groups were administered a 0.3 mg/kg injection of meth (IP) 15 min before a 70-min reinstatement session. The SalineT groups received a saline injection (IP) 15 min before their 70-min reinstatement session. The reinstatement session was identical to extinction sessions (i.e., no available infusions) except for the truncated time; decreased from 2 h to 70 min. The session length was shortened given preliminary data showing that lever pressing peaked during the first 10 min of reinstatement sessions. c-Fos is primarily expressed approximately 60–90 min after neuronal activation (Kovacs, 1998). Limiting the reinstatement session to 70 min allowed for sufficient time to gather the crucial behavioral data, as well as identify brain regions associated with the meth trigger and the reinstatement behavior.
2.10. Perfusion and brain extraction
Immediately following the reinstatement session, rats were deeply anesthetized by injection with Fatal-Plus® (25 mg/kg; MWI, Boise, ID). Rats were then transcardially perfused with 200 ml of ice cold 0.9% saline followed by 200 ml of ice cold 4% paraformaldehyde. Brains were rapidly removed and post-fixed in 4% paraformaldehyde for 24 h at 4 °C. Brains were then cryoprotected in 30% sucrose for 72 h at 4 °C. Following cryoprotection, brains were frozen on dry ice and stored at −80 °C until sectioning.
2.11. Histology
Brains were sectioned at 40 μm on a freezing microtome. Brain regions were identified according to the atlas of Paxinos and Watson (2007). Coronal sections were collected at 3.24 mm bregma to assess the Cg1, PrL, IL, and LO (see Fig. 1A). Coronal sections at 1.80 mm bregma were used to examine the Cg2, dmCPu, dlCPu, vmCPu, vlCPu, NAcC, and NAcSh (see Fig. 1B). Regions of the hippocampus (CA1; CA2; and CA3), amygdala (CEA; BLA) and LH were identified on sections collected at −2.64 mm bregma (see Fig. 1C). Finally, sections at −5.88 mm bregma contained the VTA, SNR, and VS (see Fig. 1D). Each area of interest was examined in a single hemisphere from 3 separate tissue sections per rat (cf. Zhao and Li, 2010).
Fig. 1.
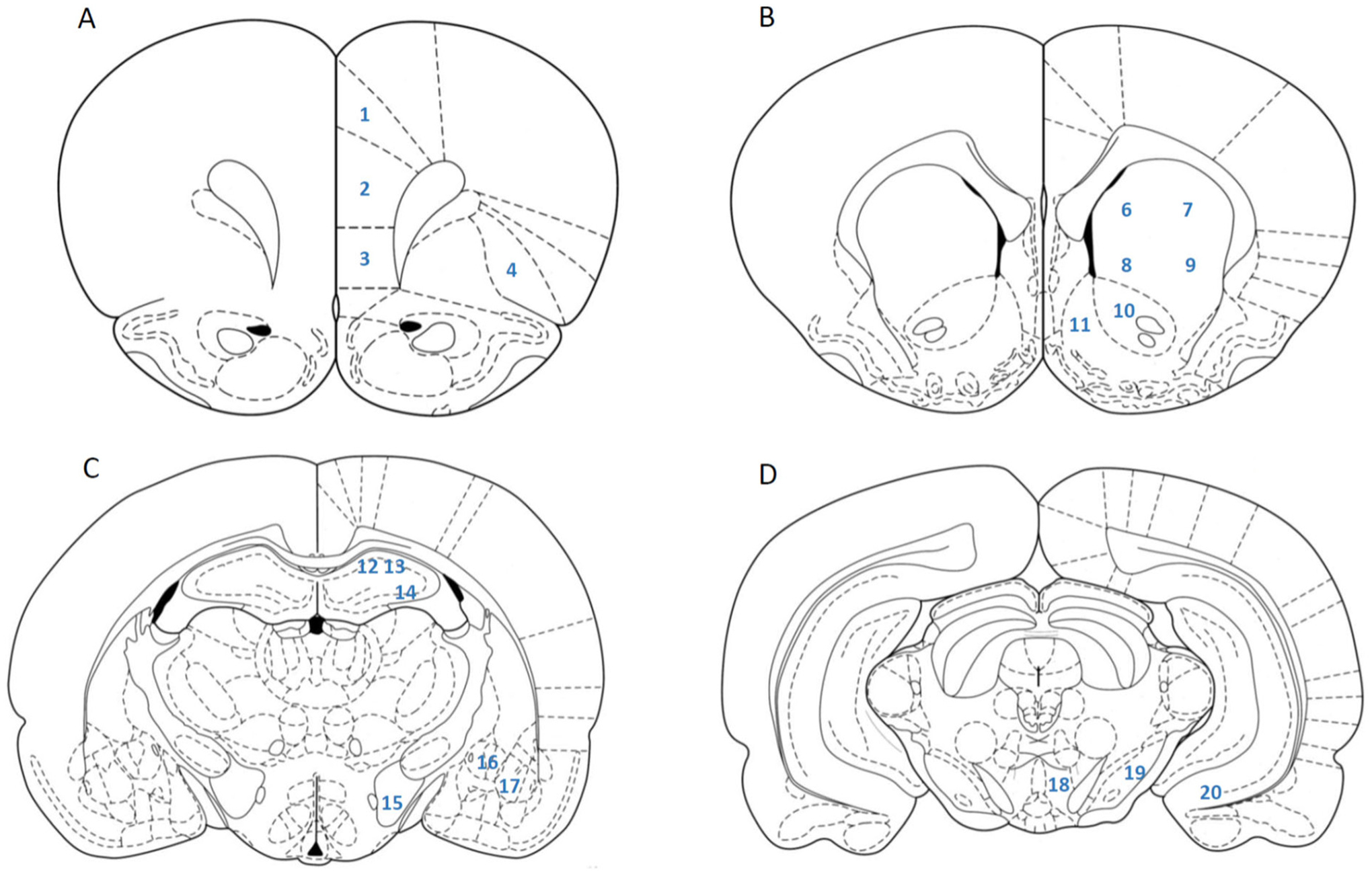
Brain regions identified by the atlas of Paxinos and Watson (2007) and examined for c-Fos expression. A: Bregma 3.24; 1-Cg1, 2-PrL, 3-IL, 4-LO. B: Bregma 1.80; 5-Cg2, 6-dmCPu, 7-dlCPu, 8-vmCPu, 9-vlCPu, 10-NAcC, 11-NAcSh. C: Bregma −2.64; 12-CA1, 13-CA2, 14-CA3, 15-LH, 16-CEA, 17-BLA. D: Bregma −5.88; 18-VTA, 19-SNR, 20-VS.
2.12. c-Fos immunohistochemistry
Following sectioning, brain sections were stored for no >48 h in a 0.02 M phosphate buffered saline (PBS): 0.1% sodium azide solution (Zhao and Li, 2010). For c-Fos immunohistochemistry, brain sections incubated on ice for 1 h in blocking solution [10% normal goat serum (NGS): 0.3% Triton X-100: 0.02 M PBS]. Sections were then washed 3 times in wash buffer (0.3% Triton X-100: 0.05% NGS: 0.02 M PBS) for 10 min per wash. Washing was proceeded by incubation in 1.5% hydrogen peroxide: 50% methanol for 30 min on ice. This was followed by another round of 3 washes with wash buffer (10 min per wash). Sections were then incubated with c-Fos primary antibody [c-Fos Antibody (4): sc-52 (discontinued), Santa Cruz Biotechnology, Dallas, TX, USA; 1:3000 dilution] in 0.3% Triton X-100:1% NGS: 1% blocking reagent:0.02 M PBS for 48 h at 4 °C. Following incubation with the primary antibody, sections were washed 3 times for 10 min per wash with wash buffer. Then sections were incubated with biotinylated goat anti-rabbit secondary antibody (Vector Labs, Burlingame, CA, USA; 1:200 dilution) in 1% NGS:0.02 M PBS for 2 h on ice. This was followed by 3 washes (10 min per wash) with 0.02 M PBS. Sections were then incubated with horseradish peroxide avidin-biotin complex (Vectastain Elite ABC Kit, Vector Labs, Burlingame, CA, USA: 1:200 dilution) in 0.02 M sodium azide- free PBS. This was followed by 3 washes with 0.05 Tris-HCl. Sections were then incubated for 5 min in diaminobenzidine-based peroxide substrate (DAB Substrate Kit, Vector Labs, Burlingame, CA, USA) to aid in protein visualization. Brain sections were then mounted on gelatin-coated slides and allowed to air-dry at room temperature. They were then dehydrated in ascending alcohol concentrations, cleared in xylene and cover slipped with mounting medium (Permount, Fisher Scientific, Suwanee GA, USA).
2.13. c-Fos imaging and quantification
A digital image (20× objective lens magnification; 490 μm2) was captured from each region of interest from anatomically matched sections (1 image from each tissue section × 3 tissue sections per area for each rat) using a light microscope (Olympus CX41RF, Tokyo, Japan) fitted with a digital camera (Infinity Lite, Ottawa, ON, Canada). For each image, c-Fos immunoreactivity was automatically identified and counted using NIH ImageJ software (Abramoff et al., 2004; Charntikov et al., 2012). Sample photomicrographs of c-Fos expression are provided in Fig. 2. Imaging and immunoreactivity quantification using ImageJ were performed blind to the treatment status of the sections.
Fig. 2.
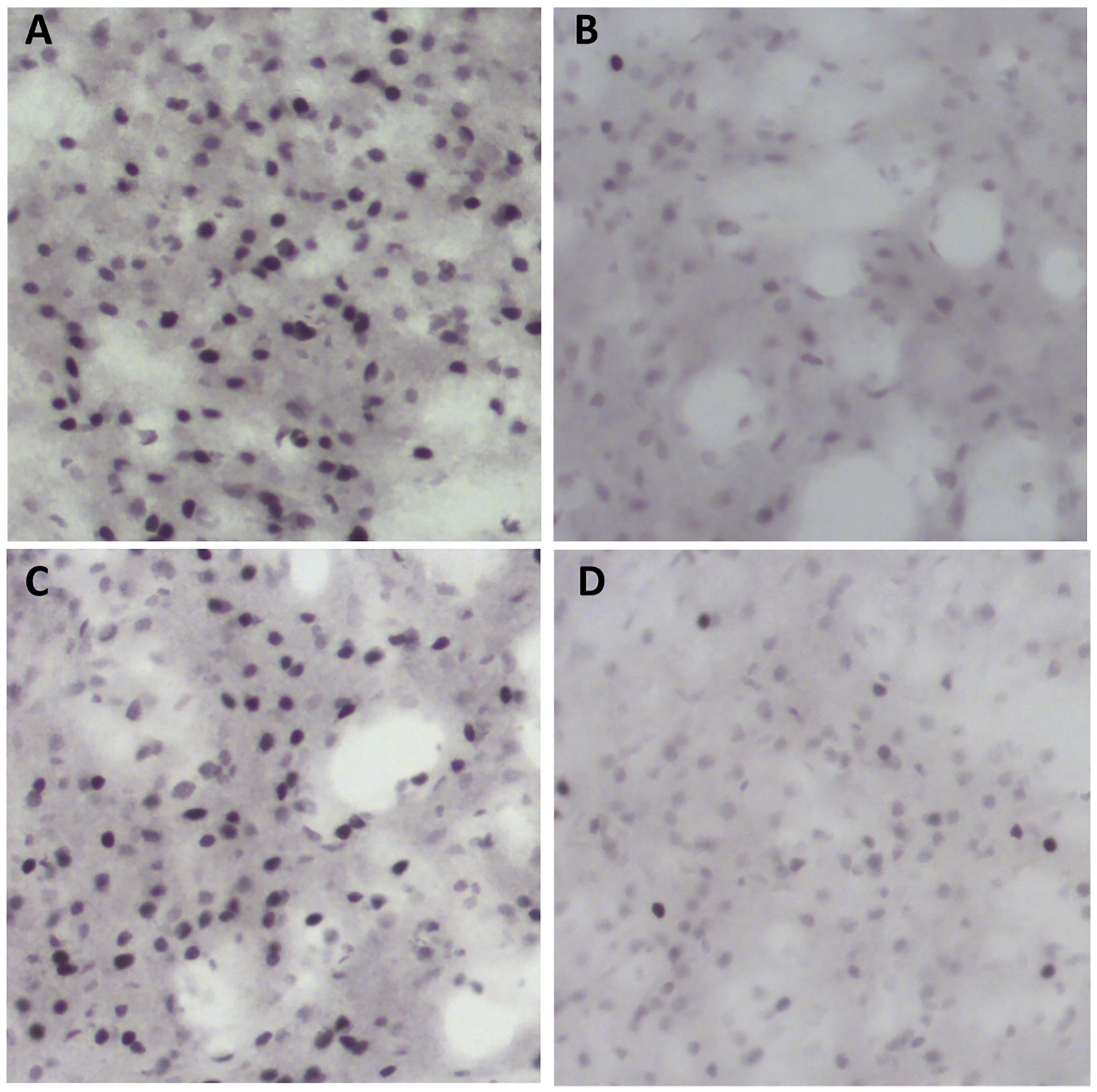
Photomicrographs of c-Fos expression in the CEA. A: Subject 199; Female MethSA/MethT. B: Subject 205; Female MethSA/SalineT. C: Subject 184: Female SalineSA/MethT. D: Subject 182: Female SalineSA/SalineT.
2.14. Dependent measures
Lever-pressing was the primary dependent measure during the behavioral phases of the experiment. To show inactive lever responding relative to active lever responding during self-administration, a discrimination index was calculated using the following formula: Discrimination Index = [Active Lever Presses/ (Inactive Lever Presses + Active Lever Presses)]. A Discrimination Index value of 0.5 indicates equal responding on the active and inactive lever (i.e., no discrimination between levers); a value >0.5 indicates more pressing on the active lever. Lever pressing on the inactive lever was near zero following early training and remained there for the rest of the experiment (data not displayed). Positively identified c-Fos cells in each brain region was the primary dependent measure used for neuronal activation. For each rat, the number of positively labeled nuclei was averaged between the 3 tissue sections in each region and used as a unit of measurement (cf. Charntikov et al., 2012; Zhao and Li, 2010; Shram et al., 2007).
2.15. Statistical analyses
Active lever responding in the self-administration and extinction phase were analyzed by separate 3-way mixed measures analysis of variance (ANOVA; Type III Sum of Squares) with Sex (Female vs Male) and Group (MethSA vs SalineSA) as between-subjects factors and Session as a within-subjects factor. This same ANOVA was also utilized to examine the discrimination index. Active lever responding in reinstatement was analyzed by a 3-way ANOVA with Sex (Female vs Male) and Self-Administration Drug (Meth vs Saline) and Reinstatement Drug (Meth vs Saline as between-subjects factors). Three-way ANOVAs with Sex, Self-Administration Drug, and Reinstatement Drug as between-subjects factors were also used to analyze regional c-Fos activation following reinstatement testing. Post-hoc analyses were conducted on significant interactions and on planned comparisons in reinstatement behavior and c-Fos activation. The complete list of a priori comparisons can be found in Table 1. To adjust for multiple comparisons, Tukey HSDs were utilized for post-hoc analysis of behavioral data. Statistical significance was declared at p < 0.05.
Table 1.
List of a priori comparisons examined by post-hoc analysis.
| Planned comparisons | |
|---|---|
| Within females | |
| 1 | MethSA/MethT vs MethSA/SalineT |
| 2 | MethSA/MethT vs SalineSA/MethT |
| 3 | SalineSA/SalineT vs SalineSA/MethT |
| 4 | SalineSA/SalineT vs MethSA/SalineT |
| Within males | |
| 5 | MethSA/MethT vs MethSA/SalineT |
| 6 | MethSA/MethT vs SalineSA/MethT |
| 7 | SalineSA/SalineT vs SalineSA/MethT |
| 8 | SalineSA/SalineT vs MethSA/SalineT |
| Between sex | |
| 9 | Female MethSA/MethT vs male MethSA/MethT |
| 10 | Female MethSA/SalineT vs male MethSA/SalineT |
| 11 | Female SalineSA/MethT vs male SalineSA/MethT |
| 12 | Females SalineSA/SalineT vs male SalineSA/SalineT |
3. Results
3.1. Self-administration
Rats in the meth groups demonstrated robust active lever pressing (Fig. 3). Analysis of active lever pressing revealed significant main effects of Group [F(1, 108) = 240.34; p < 0.001], Session [F(20, 2160) = 10.66; p < 0.001], and a Group × Session interaction [F(20, 2160) = 14.47; p < 0.001]. Rats responded significantly more for meth compared to saline in all 21 self-administration sessions. Whereas lever pressing among saline groups sharply decreased from the beginning (sessions 1–9) to the end (sessions 15–21), there was a slight increase in lever pressing among the meth groups (Fig. 3; top versus bottom panel). This finding was not surprising given the preliminary training with food. Females and males responded similarly on the active lever as neither the main effect of Sex (F < 1; p = 0.982), the Sex × Group interaction [F(1, 108) = 2.84; p = 0.095], the Sex × Session interaction [F(20, 2160) = 1.01; p = 0.443], nor the Sex × Group × Session interaction [F(20, 2160) = 1.33; p = 0.159] was significant.
Fig. 3.
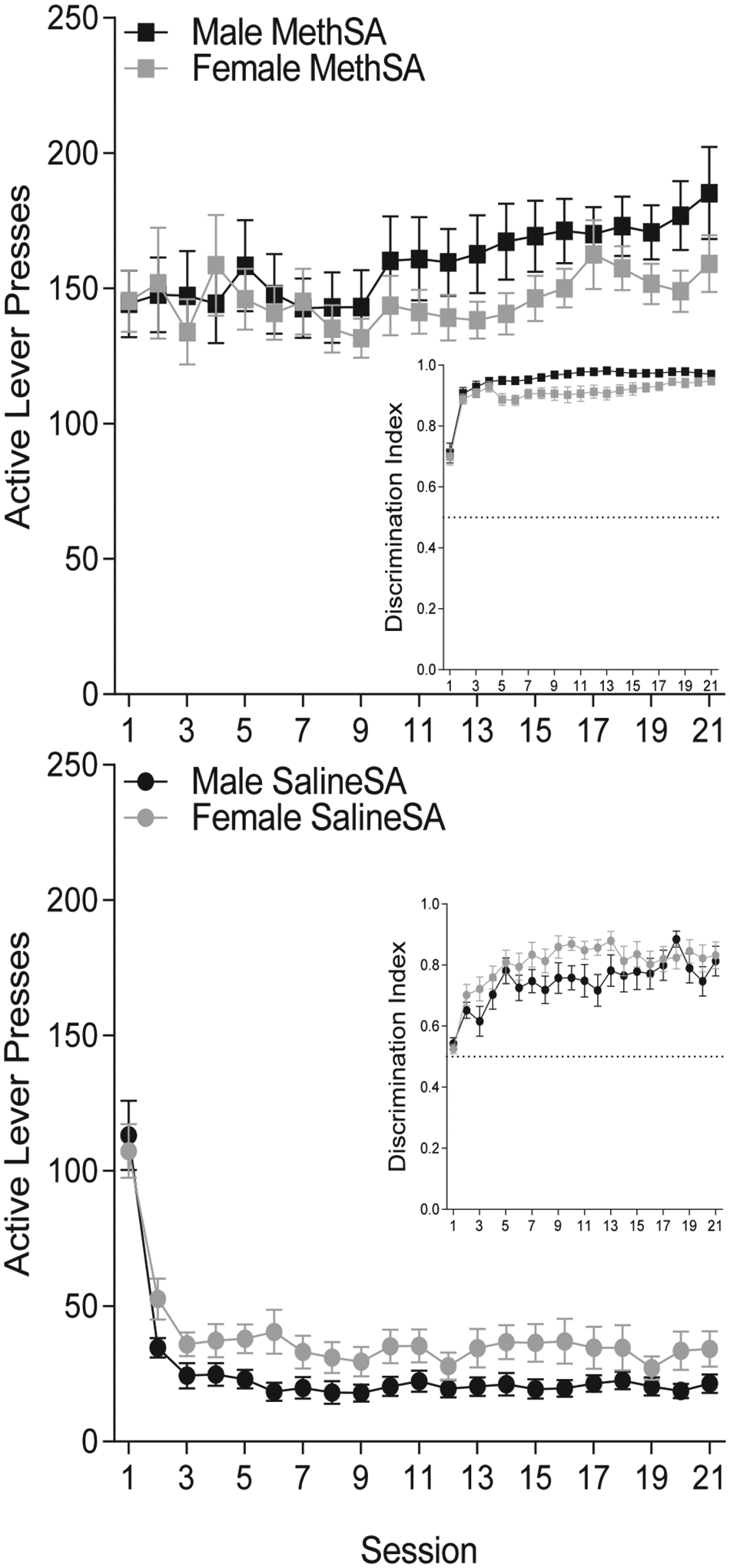
Lever pressing (± SEM) during self-administration sessions for males (black) and females (grey) in the meth (square) and saline (circle) conditions. Inset: Discrimination Index (± SEM) during self-administration sessions for male and female rats in the meth and saline conditions.
Males and females displayed clear discrimination between the active and inactive lever in the meth and the saline conditions (Inset graphs, Fig. 3; Discrimination Index well above 0.5). Analysis of the discrimination index did reveal significant main effects of Group [F(1, 108) = 74.73, p < 0.001] and Session [F(20, 2160) = 20.75, p < 0.001], as well as significant Sex × Group [F(1, 108) = 8.71, p = 0.004] and Group × Session [F(20, 2160) = 1.76, p = 0.020] interactions. Rats displayed better discrimination when receiving meth vs saline infusions. Lever discrimination increased in the meth and saline conditions, however it increased more quickly in the meth condition. Notably, in the saline condition, females showed statistically better discrimination compared to the males. In the meth condition, however, this effect was reversed, with males tending to show better discrimination than their female counterparts, although this effect did not reach significance (p = 0.074). The Sex × Group × Session [F(20, 2160) = 1.26, p = 0.198] and Sex × Session [F < 1, p = 0.926] interactions were not significant.
3.2. Extinction
Active lever pressing in the meth condition was attenuated during the extinction phase of this experiment (Fig. 4). Analysis of active lever pressing during extinction revealed significant main effects of Group [F (1, 108) = 64.89; p < 0.001] and Session [F(11, 1188) = 45.43; p < 0.001], as well as a significant Group × Session interaction [F(11, 1188) = 53.32; p < 0.001]. Responding in the meth condition was elevated compared to saline responding for the first 11 sessions, but was reduced to saline levels by session 12 (p = 0.080). Responding in the meth condition was higher during initial extinction sessions compared to the later extinction sessions, while responding in the saline condition remained stable throughout extinction. This outcome was expected, given that extinction sessions contained the timeout cues that were presumably maintaining a modest level of responding in the saline condition (cf. Pittenger et al., 2017). Responding was again similar between males and females. The main effect of Sex (F(1,108) = 1.255 p = 0.265), the Sex × Group interaction (F < 1; p = 0.506), the Sex × Session interaction (F < 1; p = 0.5), and the Sex × Group × Session interaction (F < 1; p = 0.957) were not significant.
Fig. 4.
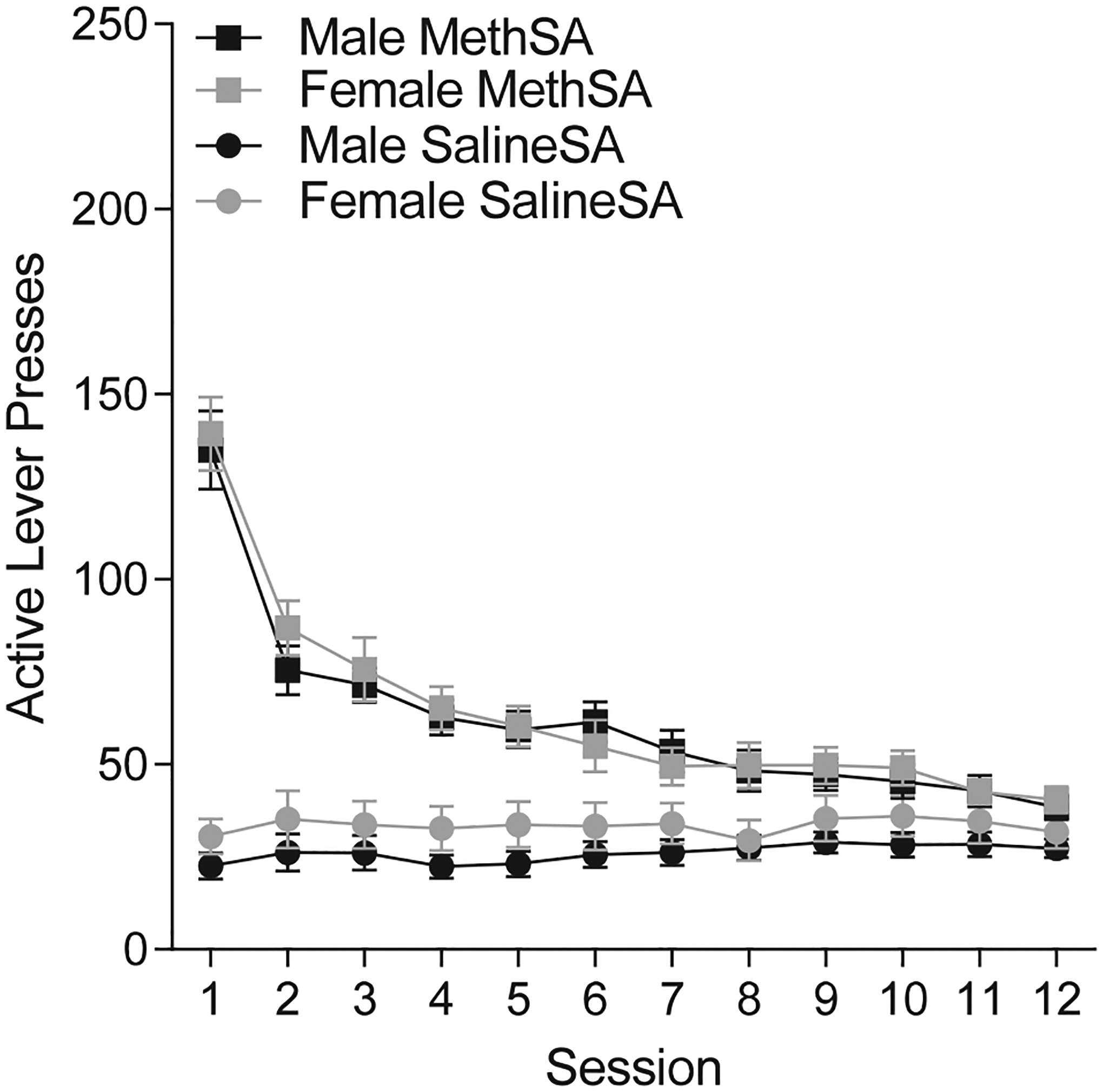
Active lever presses (± SEM) in extinction sessions for male (black) and female (grey) rats in the meth (square) and saline (circle) conditions.
3.3. Reinstatement
Analysis of active lever pressing during the reinstatement test (Fig. 5) revealed significant main effects of Sex [F(1, 104) = 12.57; p < 0.001], Self-Administration Drug [F(1, 104) = 57.93; p < 0.001], and Reinstatement Drug [F(1, 104) = 59.43; p < 0.001]. The Sex × Reinstatement Drug interaction [F(1, 104) = 6.67; p = 0.012] and the Self Administration Drug × Reinstatement Drug interaction [F(1, 104) = 25.297; p < 0.001] were both significant. The overall 3-way Sex × Self-Administration Drug × Reinstatement Drug interaction just missed the cutoff for significance [F(1, 104) = 3.879; p = 0.051]. The Sex × Self-Administration Drug interaction was not significant [F(1, 104) = 1.35; p = 0.248].
Fig. 5.
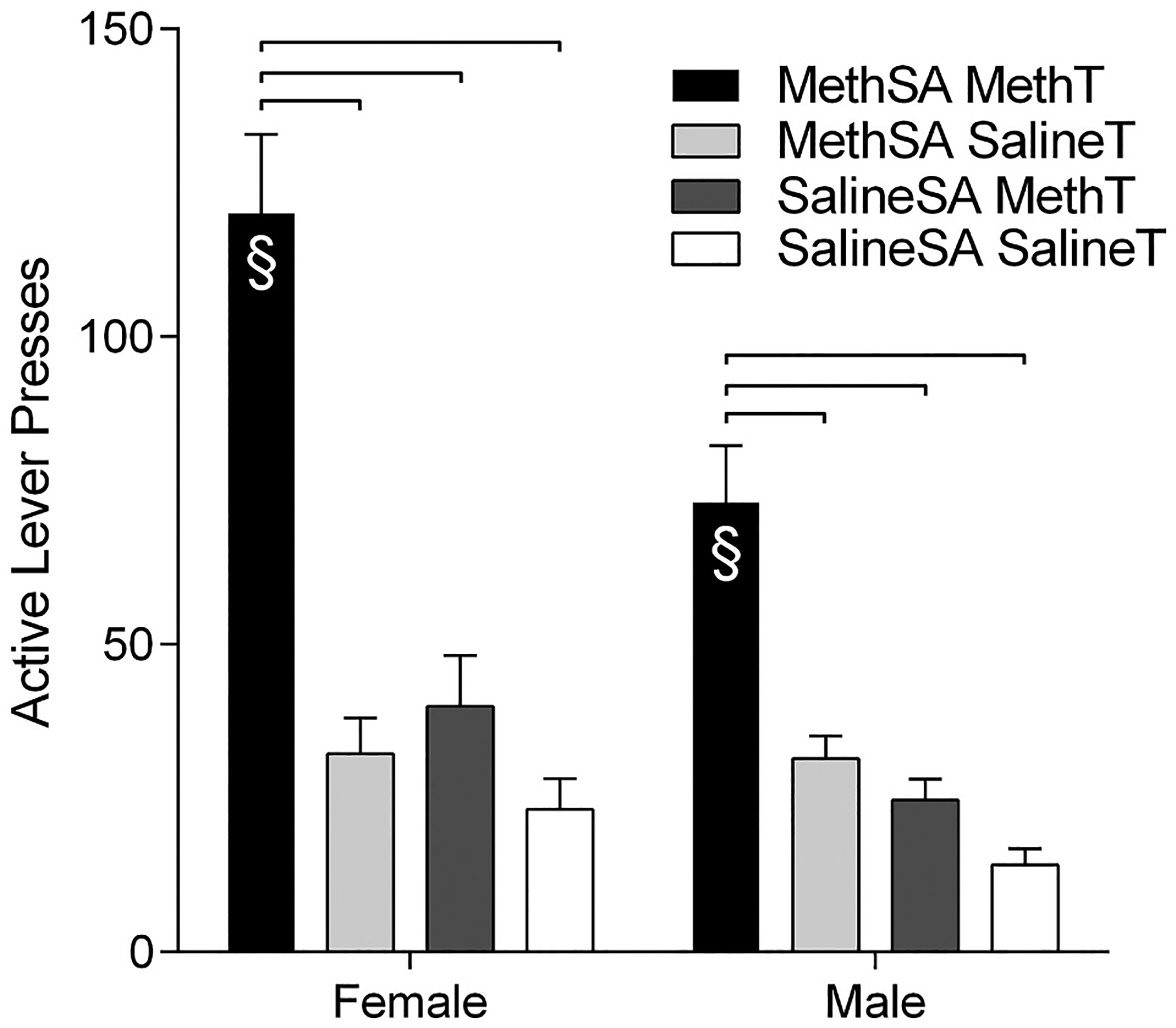
Active lever presses (±SEM) for female (left side) and male (right side) rats during the reinstatement session. Groups are delineated by color. Significant differences between groups are marked by hanging bars. Significant sex differences within conditions of Self-Administration Drug and Reinstatement Drug are indicated with §.
Post-hoc tests on the planned comparisons revealed several interesting findings. In both sexes, the groups that received meth during self-administration and received meth as a trigger (MethSA/MethT) showed more reinstatement than the groups receiving meth in self-administration and not receiving a drug prime (MethSA/SalineT), receiving a meth trigger without prior meth experience (SalineSA/MethT), or the groups that never had meth at any point in the study (SalineSA/SalineT). The females and males responded similarly in the MethSA/SalineT, SalineSA/MethT, and SalineSA/SalineSA groups. Notably, the only difference between the males and females was in the groups that received meth-primed reinstatement following meth self-administration. Females in this MethSA/MethT group responded significantly more than their male counterparts. This difference in reinstatement behavior between the sexes was not a result of general differences following long-term meth self-administration (MethSA/SalineT), acute meth administration (SalineSA/MethT), nor basal behavioral differences (SalineSA/SalineT). Females only responded more during meth-primed reinstatement of meth-seeking (MethSA/MethT).
3.4. c-Fos immunohistochemistry
Investigation of c-Fos activation in the brain following reinstatement also detected notable findings. Statistical analysis of main effects and interactions for each area are reported in Table 2. Analysis of the planned comparisons (refer to Table 1) revealed in the female rats, the MethSA/MethT group showed more activation than the MethSA/SalineT group in the Cg1, LO, PrL, dlCPu, dmCPu, vmCPu, NacC, NAcSh, and CEA (Fig. 6). The group that received acute meth exposure (SalineSA/MethT) also had higher c-Fos activation levels compared to the long-term self-administration with no prime group in several regions. These regions were the Cg1, vlCPu, NAcC, and CEA (Fig. 6). With the exception of the vlCPu, the activation following an acute meth injection was less robust than the activation in the group that received a meth injection following a self-administration history with meth. This data pattern suggests that the learning history with meth self-administration further potentiated neural activity associated with meth administration, except in the vlCPu which shows more activation following acute meth administration. Differences in the females between the baseline group (SalineSA/SalineT) and the reinstatement group (MethSA/MethT) were found in the Cg1, LO, dmCPu, vmCPu, NAcC, NAcS, and CEA (Fig. 6), while differences between the baseline group and acute meth injection group were only detected in the Cg1, vlCPu, NAcC, and CEA (Fig. 6).
Table 2.
Statistical analysis of main effects and interactions for each region of interest.
| Area | Main effects | Interactions | |||||
|---|---|---|---|---|---|---|---|
| Sex | Reinstatement drug | Self-administration drug | Sex × reinstatement drug | Sex × self-administration | Self-administration drug × reinstatement drug | Sex × Self-Administration Drug × Reinstatement Drug | |
| Cg1 | F(1,104)=11.199, p=0.001 |
F(1,104)=17.923, p<0.001 |
F<1, p=0.341 | F(1,104)=5.061, p=0.027 |
F(1,104)=1.531, p=0.219 |
F(1,104)=1.428, p=0.235 |
F<1, p=0.433 |
| Cg2 | F(1,104)=2.230, P=0.138 |
F(1,104)=3.232, p=0.075 |
F<1, p=0.618 | F(1,104)=1.591, p<0.210 |
F<1, p=0.52l | F<1, p=0.579 | F<1, p=0.832 |
| IL | F<1, p=0.423 | F(1,104)=2.156, P=0.145 |
F(1,104), p=0.302 | F<1, p=0.962 | F<1, p=0.357 | F(1,104)=1.613, p=0.207 |
F<1, p=0.826 |
| LO | F(1,104)=17.256, p=0.001 |
F(1,104)=7.231, p=0.008 |
F<1, p=0.361 | F(1,104)=4.415, p=0.038 |
F<1, p=0.337 | F(1,104)=1.250, p=0.266 |
F<1, p=0.352 |
| PrL | F(1,104)=1.456, P=0.230 |
F(1,104)=8.392, p=0.004 |
F(1,104)=2.558, P=0.112 |
F<1, p=0.716 | F<1, p=0.543 | F(1,104)=4.729, p=0.0319 |
F(1,104)=2.108, p=0.150 |
| dlCPu | F(104)=6.001, p=0.016 |
[F(104)=9.377, p=0.002 |
F<1, p=0.946 | F(1,104)=2.042, p=0.l56 |
F<1, p=0.603 | F(1,104)=1.978, p=0.163 |
F<1, p=0.527 |
| draCPu | F(1,104)=1.359, P=0.246 |
F(1,1o4)=16.082, p<0.001 |
F<1, p=0.377 | F<1, p=0.343 | F<1, p=0.677 | F(1,104)=2.748 | F<l, p=0.662 |
| vlCPu | F(1,104)=5.605, p=0.020 |
F(1,104)=8.816, p=0.005 |
F(1,104)=2.647, p=0.107 |
F(1,104)=3.511, P=0.064 |
F(1,104)=2.095, p=0.151 |
F(1,104)=1.088, p=0.299 |
F(1,104)=1.088, p=0.299 |
| vmCPu | F(1,104)=5.408, p=0.022 |
F(1,104)=10.978, p=0.001 |
F<1, p=0.734 | F(1,104)=4.185, p=0.043 |
F<1, p=0.878 | F(1,104)=1.764; P=0.187 |
F<1, p=0.974 |
| NAcC | F(1,104)=7.917, p=0.005 |
F(1,104)=15.737, p<0.001 |
F<1, p=0.541 | F(1,104)=9.755, p=0.002 |
F<1, p=0.894 | F<1, p=0.562 | F<1, p=0.996 |
| NAcSh | F(1,104)=2.752, P=0.100 |
F(1,104)=6.787, p=0.011 |
F=1.901, p=0.170 | F(1,104)=5.189, p=0.025 |
F<1, p=0.559 | F<1, p=0.362 | F<1, p=0.985 |
| CA1 | F(1,104)=3.476 p=0.065 |
F(1, 104)=6.156, p=0.015 |
F<1, p=0.353 | F<1, p=0.355 | F<1, p=0.535 | F(1,104)=1.537, p=0.217 |
F<1, p=0.355 |
| CA2 | F<1, p=0.450 | F(1,104)=1.49, p=0.225 |
F(1,104)=2.970, p=0.088 |
F<1, p=0.811 | F<1, p=0.460 | F(1,104)=4.85, p=0.030 |
F<1, p=0.811 |
| CA3 | F(1,104)=1.932, p=0.168 |
F(1,104)=2.861, p=0.094 |
F<1, p=0.883 | F(1,104)=1.211, p=0.273 |
F<1, p=0.902 | F(1,104)=1.851, p=0.177 |
F(1,104)=1.109, p=0.295 |
| VS | F(1,104)=1.492, p=0.224 |
F<1, p=0.383 | F<1, p=0.639 | F(1,104)=1.069, p=0.304 |
F<1, p=0.612 | F(1,104)=2.911, p=0.091 |
F<1, p=0.894 |
| BLA | F<1, p=0.600 | F(1,102)=4.478, p=0.036 |
F(1,102)=2.621, p=0.109 |
F<1, p=0.814 | F(1,102)=1.360, p=0.246 |
F(1,102)=2.045, P=0.156 |
F<1, p=0.605 |
| CEA | F(1,102)=1.751, p=0.189 |
F(1,102)=32.584, p<0.001 |
F(1,104)=1.417, P=0.237 |
F(1,102)=1.689, p=0.197 |
F(1,102)=2.589, p=0.111 |
F<1, p=0.975 | F<1, p=0.451 |
| LH | F(1,104)=3.417, p=0.067 |
F(1,104)=6.378, p=0.013 |
F(1,104)=1.024, P=0.314 |
F<1, p=0.754 | F<1, p=0.570 | F<1 p=0.334 | F<1, p=0.489 |
| SNR | F(1,103)=2.696, p=0.104 |
F<1 p=0.648 | F<1, p=0.888 | F<1, p=0.757 | F<1, p=0.727 | F(1,103)=1.099, p=0.297 |
F<1, p=0.998 |
| VTA | F(1,103)=1.890, p=0.172 |
F<1, p=0.601 | F<1, p=0.676 | F<1, p=0.780 | F<1, p=0.500 | F<1, p=0.371 | F<1, p=0.732 |
Fig. 6.
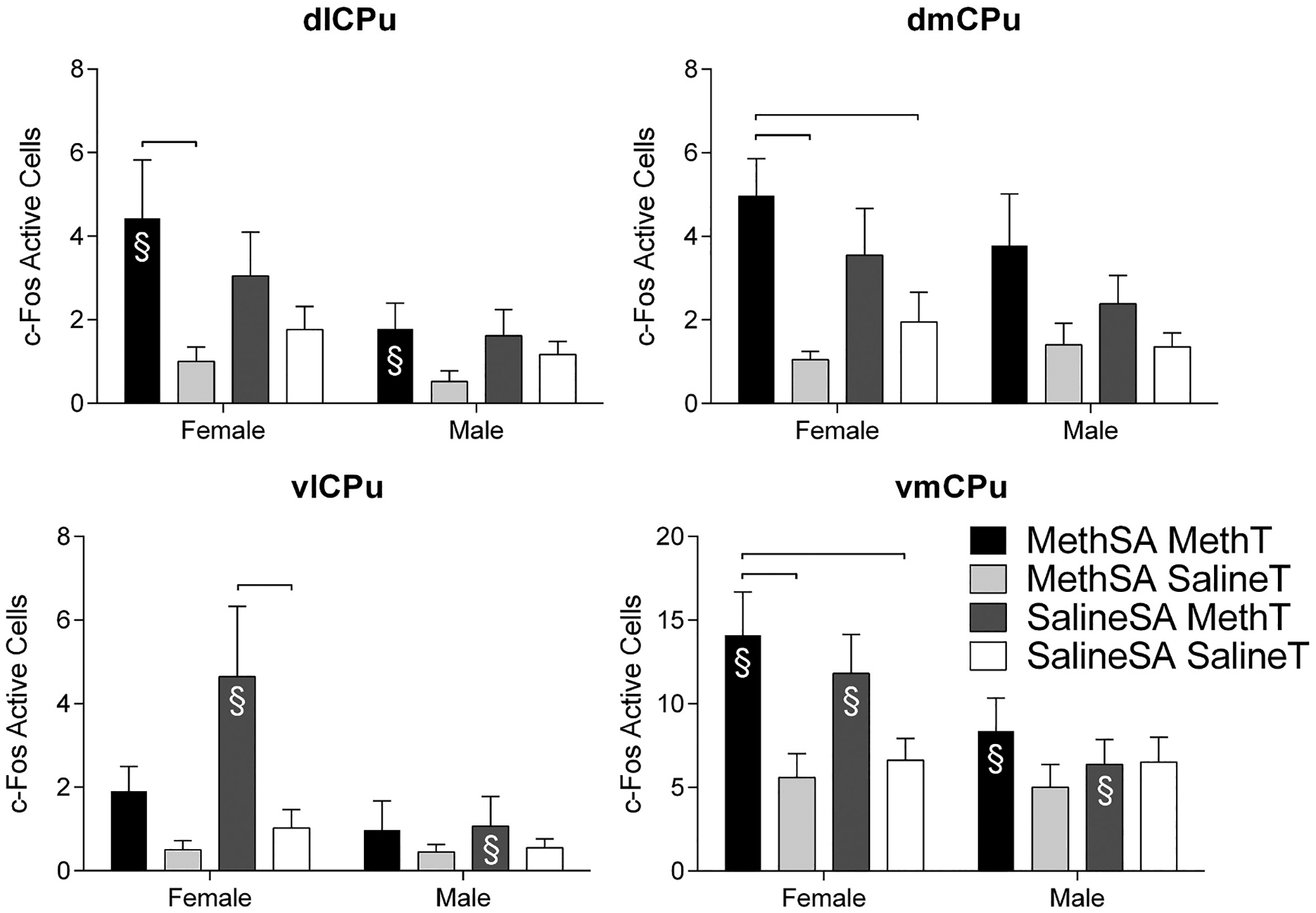
Number of c-Fos active cells (± SEM) during reinstatement in females (left) and males (right) in regions of the caudate putamen. Groups are delineated by color. Significant differences between groups within sex are marked by hanging bars. Significant sex differences within conditions of Self-Administration Drug and Reinstatement Drug are indicated with §.
Examination of the planned comparisons within the male groups detected substantially fewer differences. In fact, the only significant difference in the males was found in the CEA with the group that received acute meth injection showing higher c-Fos activation than the group that was drug free following long-term self-administration (SalineSA/MethT>MethSA/SalineT; Fig. 7).
Fig. 7.
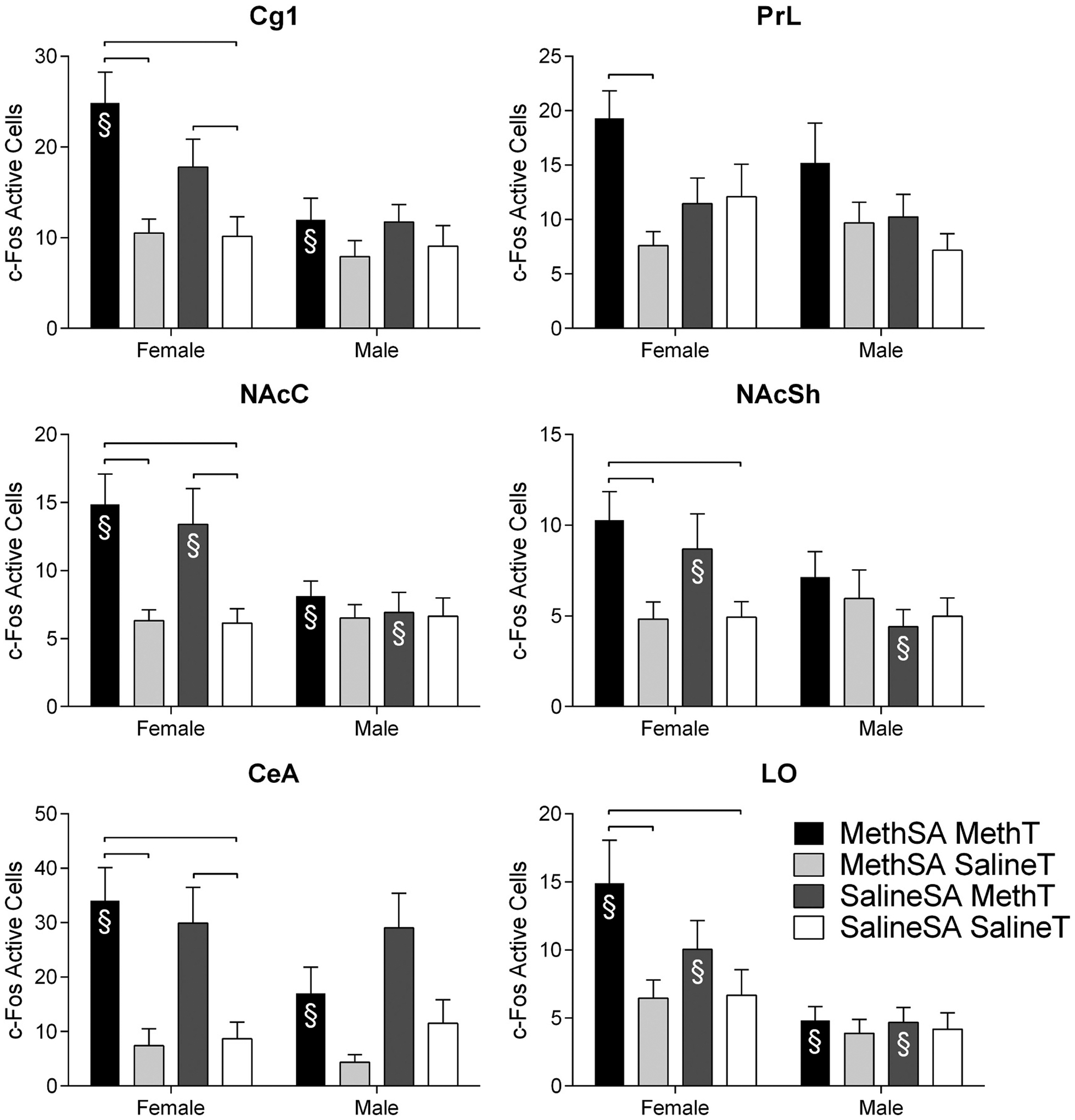
Number of c-Fos active cells (± SEM) during reinstatement in females (left) and males (right) in additional regions of interest with significant group differences (regions without group differences are not shown). Groups are delineated by color. Significant sex differences within conditions of Self-Administration Drug and Reinstatement Drug are indicated with §.
There were numerous significant differences detected in the planned comparisons between sexes (Fig. 6 and 7). Females in the reinstatement group (MethSA/MethT) had higher c-Fos activation than their male counterparts in the Cg1, LO, dlCPu, vmCPu, NAcC, and CEA (Fig. 6 and 7). There were also differences between males and females following acute meth administration (SalineSA/MethT). Females had more c-Fos immunoreactivity following acute meth in the LO, vlCPu, vmCPU, NAcC, NAcSh, and CEA (Fig. 6 and 7). No differences were detected between males and females in the baseline group (SalineSA/SalineT) or the meth self-administration group that did not receive a meth-prime (MethSA/SalineT) suggesting that differences between the sexes were not a result of sexually dimorphic basal neuronal activation levels. There were no significant differences in the planned group comparisons in the Cg2, IL, CA1, CA2, CA3, VS, BLA, LH, SNR, or VTA (data not shown).
4. Discussion
Using the standard meth self-administration procedures in our lab, we found that female and male rats readily self-administered meth. Robust meth self-administration is concordant with previous work in our lab (Charntikov et al., 2015; Pittenger et al., 2016; Reichel et al., 2008, 2009) and the findings of other labs (Beardsley et al., 2010; Coordie and McFadden, 2019; Cornish et al., 2012; Cox et al., 2013; Holtz et al., 2012; Hofford et al., 2014; Reichel et al., 2012; Roth and Carroll, 2004; Rubio et al., 2015; Shepard et al., 2004; Sobieraj et al., 2016). In the current study, females and males did not differ in active lever responding in the self-administration phase. This lack of sex difference during self-administration is common (e.g., Pena-Bravo et al., 2019; Pittenger et al., 2017), but not ubiquitous. Differences in sex, with females self-administering more than males, are often established when self-administration sessions are longer in duration than the 2-h protocol used in this study (Roth and Carroll, 2004; Reichel et al., 2012). The lack of difference in our procedures can be viewed as a strength. Given that meth intake was similar during the self-administration phase, we are not concerned with differential intake complicating interpretation of the sex differences found in the later reinstatement phase.
The inclusion of the saline self-administration conditions (i.e., Saline SA/SalineT and SalineSA/MethT) provided the critical controls in which to interpret the outcome of the meth self-administration conditions (i.e., MethSA/SalineT and MethSA/MethT). In fact, their inclusion also served as a methodological strength. Recall that the only difference between the meth condition and the saline condition is the type of infusion (meth or saline); pre-training, infusion/timeout cues, progression through the study, handling, transport, etc. were similar. Thus, this saline benchmark allowed for the detection of c-Fos differences specific to drug type in self-administration and for a careful analysis of the behavior controlled by meth compared to that controlled by the weak reinforcing effects of infusion/timeout cues (Caggiula et al., 2009; Chaudhri et al., 2006; Palmatier et al., 2006; Pittenger et al., 2017). Indeed, the groups that received saline during self-administration differentially responded on the active vs inactive lever. However, this responding was significantly lower than responding for meth. Note that our protocol initially trains lever pressing with sucrose. While this approach certainty provided the instrumental response, this training cannot easily explain lever discrimination or persistence of low yet stable active lever presses for 21 sessions. Recall that both levers had a similar history of reinforcement and that the active lever was randomly selected at the start of the meth self-administration phase. Rather, we suggest that this finding supports the notion that the timeout cues (i.e., light illumination and lever extraction for 20 s) have weak reinforcing value that maintains modest levels of responding (Barrett et al., 2020; Caggiula et al., 2009; Chaudhri et al., 2006; Palmatier et al., 2006).
In extinction, responding in the meth groups was attenuated to levels comparable to the saline benchmarks, an expected outcome, as extinction sessions included the timeout cues that were presumably maintaining the low levels of responding in the saline groups. Responding during extinction was similar between females and males. While some work has found females may be more resistant to extinction of meth self-administration (Cox et al., 2013), the current finding matches others that do not report sex differences in extinction rates (e.g., Reichel et al., 2012; Holtz et al., 2012; Pittenger et al., 2017). The cause of this variation remains unknown; however, the lack of sex differences in extinction reported herein allowed the assessment of possible sex differences in subsequent reinstatement without the concern of baseline differences in lever pressing.
Males and females showed significant meth-seeking behavior following a meth-prime injection. Responding was higher in the male and female groups that had a learning history with meth and received a meth trigger (MethSA/MethT) than the group that had a learning history with meth and did not receive a meth prime (MethSA/SalineT), the group that received an acute injection of meth (SalineSA/MethT), and the group that never received meth. Notably, this meth-primed reinstatement effect was potentiated in females compared to males. Responding in the female MethSA/MethT group was significantly higher than responding in the male MethSA/MethT group; this was the only behavioral group that differed between males and females. The difference in these groups alone suggests that differences in reinstatement behavior between the sexes were not a result of general differences following long-term meth self-administration, acute meth administration, nor basal behavioral differences. Females only responded more during meth-primed reinstatement of meth-seeking, which is concordant with previous work that also found amplified reinstatement behavior in females (Cox et al., 2013; Holtz et al., 2012; Reichel et al., 2012).
There were several notable findings in the examination of c-Fos as a marker of neuronal activation. Generally speaking, c-Fos immunoreactivity showed a similar pattern to the behavioral reinstatement data, particularly in females. c-Fos was higher in females with a learning history with meth and received a meth-prime compared to females that received long-term meth self-administration and no prime in the Cg1, LO, PrL, dlCPu, dmCPu, vmCPu, NacC, NAcSh, and CEA (recall Fig. 6). These differences were a result of both the high activation levels following a prime and lower levels following long-term meth self-administration (i.e., levels in the MethSA/SalineT group were marginally lower than even the baseline SalineSA/SalineT group). These results suggest that hyperactivation was prevalent after a meth prime in females and, notably, there may be hypofunctioning in the female rat brain following long-term meth self-administration and extinction. As extinction and reinstatement in the MethSA/SalineT groups were conducted drug free, this could be conceptualized as a withdrawal period. Accordantly, past work has shown hypofunction in critical areas associated with drug addiction during withdrawal (e.g., Parsegian and See, 2014) and suggests females show heightened sensitivity to withdrawal symptoms (for a review see O’Dell and Torres, 2014).
The increased c-Fos expression in the females in cortical, striatal, and amygdala regions during reinstatement is concordant with several studies investigating c-Fos expression in male rats following reinstatement (Bossert et al., 2012; Ciccocioppo et al., 2001; Cornish et al., 2012; Hamlin et al., 2008a, 2008b; Kufahl et al., 2009; Miller and Marshall, 2005; Recinto et al., 2012; Zavala et al., 2007). These increases in c-Fos during drug-seeking paradigms are not universal (Sobieraj et al., 2016; Zahm et al., 2010; Zhou et al., 2014). In fact, the only other study to examine possible sex dependent neural correlates in reinstatement actually found c-Fos expression in the NAcC and NAcSh lower in reinstating male and female rats compared to control rats that did not receive reinstatement (Zhou et al., 2014). These discrepancies in neuronal activation have been explained by inhibitory GABAergic and dopaminergic neurotransmission in these regions, as well as significant differences between the studies in self-administration drug and reinstatement trigger (Bossert et al., 2012; Ciccocioppo et al., 2001; Cornish et al., 2012; Hamlin et al., 2008a, 2008b; Kufahl et al., 2009; Miller and Marshall, 2005; Neisewander et al., 2012; Recinto et al., 2012; Sobieraj et al., 2016; Zahm et al., 2010; Zhou et al., 2014).
While the research on sex differences and neural processes associated with meth-triggered reinstatement is quite limited, the female-specific increase in c-Fos labeling in the prelimbic cortex for the MethSA/MethT group versus its controls is consistent with the observation that inhibition of the PrL attenuates meth-triggered reinstatement (Coordie and McFadden, 2019). While this finding by Cordie and McFadden was not sex-specific, Pena-Bravo et al. (2019) found that the amplitude of evoked excitatory post-synaptic current in layer V and VI of the PrL was enhanced relative to a saline control only in female rats that had self-administered meth. Male rats, in contrast, did not differ from saline controls in the amplitude of the evoked excitatory post-synaptic current, but its decay was reduced in male rats that had self-administered meth. Pena-Bravo et al. (2019) further reported that this current appears to be mediated in part by N-Methyl-d-aspartate (NMDA) receptor containing the GluN2B subunit only for male rats. The current study did not utilize double-staining techniques to identify the specificity of neuronal activation. As such, future research examining cell-specific neuronal activation in female and male meth reinstatement will be of interest.
Females in the meth-triggered reinstatement group (MethSA/MethT) had higher c-Fos activation than their male counterparts in the Cg1, LO, dlCPu, vmCPu, NAcC, and CEA. There were also differences between males and females following acute meth administration (SalineSA/MethT). Females had greater c-Fos immunoreactivity following acute meth in the LO, vlCPu, vmCPu, NAcC, NAcSh, and CEA. This significant overlap in neural activation when meth was administered, regardless of self-administration drug type, suggests that many of the differences between the sexes may be a result of initial meth administration and not necessarily in differences in meth as a drug trigger for reinstatement of meth-seeking. The two exceptions to this notion were the Cg1 and dlCPu which only showed differences between the sexes in the group that received meth following a learning history with meth (MethSA/MethT). The Cg1 is particularly significant as previous work suggests this region is of particular importance in multiple forms of reinstatement (Ciccocioppo et al., 2001; Neisewander et al., 2000; Thomas and Everitt, 2001; Wexler et al., 2001). In fact, Neisewander et al. (2000) determined that the cingulate cortex was the sole region that was activated by cocaine-primed reinstatement. Recinto et al. (2012) extended this work to a meth-prime model, also determining the cingulate cortex was integral in reinstatement. The work presented here further solidified that notion and places the cingulate cortex as a region associated with behavioral sex differences detected during meth reinstatement.
Rats in the present study were food restricted to 90% of free-feeding weight. Under certain conditions, the extent and pattern of food restriction, can increase self-administration and/or affect reinstatement (e.g., Comer et al., 1995; Glick et al., 1987). Whether food restriction contributed to the pattern of reinstatement, c-Fos activation, or sex-dependent effects will require more research. Along these lines, the experiment reported herein did not examine gonadal hormone levels, however, previous work does show they likely play a role in the amplified vulnerability to drug addiction found in females. In general, estrogen enhances and progesterone inhibits acquisition and escalation of self-administration, resistance to extinction, and reinstatement of drug-seeking [for a review see Carroll and Anker (2010) and Becker and Koob (2016)]. Specific to drug-primed reinstatement, multiple studies have shown that estrogen treatment enhanced cocaine-primed reinstatement in ovariectomized rats (Anker et al., 2007; Larson and Carroll, 2007; Larson et al., 2005). Preclinical work specifically with meth reinstatement is not nearly as extensive as that with cocaine. With meth, studies have not detected differences in reinstatement based on phase of estrous cycle (Ruda-Kucerova et al., 2015; Cox et al., 2013). However, allopregnanolone does reduce meth-primed reinstatement in female, but not male rats, suggesting gonadal hormones may be a factor (Holtz et al., 2012). Future work further elucidating the precise brain areas involved in these hormonal effects on meth-primed reinstatement will be important.
Acknowledgments
This research was supported in part by NIH research grant DA034389. RAB was partially supported by DA046109 and GM130461 while preparing this manuscript for publication.
References
- Abramoff MD, Magelhaes PJ, Ram SJ, 2004. Image processing with imageJ. Biophoton. Int 11, 36–42. [Google Scholar]
- Anker JJ, Larson EB, Gliddon LA, Carroll ME, 2007. Effects of progesterone on the reinstatement of cocaine-seeking behavior in female rats. Exp. Clin. Psychopharmacol 15, 472–480. [DOI] [PubMed] [Google Scholar]
- Baicy K, London ED, 2007. Corticolimbic dysregulation and chronic methamphetamine abuse. Addiction 102, 5–15. [DOI] [PubMed] [Google Scholar]
- Bamford NS, Zhang H, Joyce JA, Scarlis CA, Hanan W, Wu NP, Andre VM, Cohon R, Cepeda C, Levine MS, Harleton E, Sulzer D, 2008. Repeated methamphetamine causes long-lasting presynaptic corticostriatal depression that is renormalized with drug readministration. Neuron 58 (1), 89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett ST, Thompson BM, Emory JR, Larsen CE, Pittenger ST, Harris EN, Bevins RA (2020). Sex differences in the reward enhancing effects of nicotine on ethanol reinforcement: a reinforcer demand analysis. Nicotine and Tobacco Research 22(2): 238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM, Shelton KL, Hendrick E, Johnson KW, 2010. The glial cell modulator and phosphodiesterase inhibitor, AV411 (ibudilast), attenuates prime- and stress-induced methamphetamine relapse. Eur. J. Pharmacol 637 (1–3), 102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Koob GF, 2016. Sex differences in animal models: focus on addiction. Pharmacol. Rev 68 (2), 242–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacktop JM, Sorg BA, 2018. Perineuronal nets in the lateral hypothalamus area regulate cue-induced reinstatement of cocaine-seeking behavior. Neuropsychopharmacology 44 (5), 850–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Chen TJH, Downs BW, Bowirrat A, Waite RL, Braverman ER, Madigan M, Oscar-Berman M, DiNublie N, Gold M, 2009. Neurogentics of dopaminergic receptor super-sensitivity in activation of brain reward circuitry and relapse: proposing “Deprivation-Amplification Relapse Therapy” (DART). Postgrad. Med 121 (6), 176–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortz DM, Grace AA, 2018. Medial septum differentially regulates dopamine neuron activity in the rat ventral tegmental area and the substantia nigra via distinct pathways. Neuropsychopharmacology 43 (10), 2093–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Stern AL, Theberge FRM, Marchant NJ, Wang HL, Morales M, Shaham Y, 2012. Role of projections from ventral medial prefrontal cortex to nucleus accumbens shell in context-induced reinstatement of heroin seeking. J. Neurosci 32 (14), 4982–4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht ML, O’Brien A, Von Mayrhauser C, Anglin MD, 2004. Methamphetamine use behaviors and gender differences. Addict. Behav 29 (1), 89–106. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF, 2009. The role of nicotine in smoking: a dual-reinforcement model. Neb. Symp. Motiv 55, 91–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ, 2010. Sex differences and ovarian hormones in animal models of drug dependence. Horm. Behav 58 (1), 44–56. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST, 1999. Meta-analysis of cue-reactivity in addiction research. Addiction 94 (3), 327–340. [PubMed] [Google Scholar]
- Charntikov S, Tracy ME, Zhao C, Li M, Bevins RA, 2012. Conditioned response evoked by nicotine conditioned stimulus preferentially induces c-Fos expression in medial regions of caudate-putamen. Neuropsychopharmacology 37 (4), 876–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charntikov S, Swalve N, Pittenger S, Fink K, Schepers S, Hadlock GC, et al. , 2013. Iptakalim attenuates self-administration and acquired goal-tracking behavior controlled by nicotine. Neuropharmacology 75C, 138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charntikov S, Pittenger ST, Thapa I, Bastola DR, Bevins RA, Pendyala G, 2015. Ibudilast reverses the decrease in the synaptic signaling protein phosphatidylethanolamine-binding protein 1 (PEBP1) produced by chronic methamphetamine intake in rats. Drug Alcohol Depend 152, 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF, 2006. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology 184 (3–4), 353–366. [DOI] [PubMed] [Google Scholar]
- Chornock WM, Stitizer ML, Gross J, Leischow S, 1992. Experimental model of smoking re-exposure: effects on relapse. Psychopharmocology (Berl) 108 (4), 495–500. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F, 2001. Cocaine-predictive stimulus induces drug seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D1 antagonists. Proc. Natl. Acad. Sci. U. S. A 98 (4), 1976–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Lac ST, Wyvell CL, et al. , 1995. Food deprivation affects extinction and reinstatement of responding in rats. Psychopharmacology 121, 150–157. [DOI] [PubMed] [Google Scholar]
- Coordie R, McFadden LM, 2019. Optogenetic inhibition of the medial prefrontal cortex reduces methamphetamine-primed reinstatement in male and female rats. Behavioral Pharmacology 30 (6), 506–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Hunt GE, Robins L, McGregor, 2012. Regional c-Fos and FosB/ΔFosB expression associated with chronic methamphetamine self-administration and methamphetamine-seeking behavior in rats. Neuroscience 206, 100–114. [DOI] [PubMed] [Google Scholar]
- Cox BM, Young AB, See RE, Reichel CM, 2013. Sex differences in methamphetamine seeking in rats: impact of oxytocin. Psychoneuroendocrinology 38 (10), 2343–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T, Morgan JI, 1985a. Superinduction of c-fos by nerve growth factor in the presence of peripherally active benzodiazepines. Science 229 (4719), 1265–1268. [DOI] [PubMed] [Google Scholar]
- Curran T, Morgan JI, 1985b. Fos: an immediate-early transcription factor in neurons. J Neurobio 26 (3), 403–412. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ, 2001. Dissociable effects of antagonism of NMDA and AMPA/KA receptors in the nucleus accumbens core and shell on cocaine-seeking behavior. Neuropsychopharmacology 25 (3), 341–360. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ, 2004. Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J. Neurosci 24 (32), 7167–7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dluzen DE, Liu B, 2008. Gender differences in methamphetamine use and responses: a review. Gend Med 5 (1), 24–35. [DOI] [PubMed] [Google Scholar]
- Duryee MJ, Bevins RA, Reichel CM, Murray JE, Dong Y, Thiele GM, Sanderson SD, 2009. Immune responses to methamphetamine by active immunization with peptide-based, molecular adjuvant-containing vaccines. Vaccine 27, 2981–2988. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE, 2005. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology 30, 296–309. [DOI] [PubMed] [Google Scholar]
- Glick SD, Hinds PA, Carlson JN, 1987. Food deprivation and stimulant self-administration in rats: differences between cocaine and D-amphetamine. Psychopharmacology 91, 372–374. [DOI] [PubMed] [Google Scholar]
- Grant KM, LeBan T, Wells S, Li M, Stoltenberg S, Gendelman HE, Carlo G, Bevins RA, 2012. Methamphetamine-associated psychosis. J. NeuroImmune Pharmacol 7, 113–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg ME, Ziff EB (1984). Stimulation of 3T3 cells induces transcription of the c-Fos proto-oncogene. Nature 311(5985): 433–438. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, McNally GP (2008a). Renewal of extinguished cocaine-seeking. Neuroscience 151: 659–670. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, McNally GP, 2008b. Renewal of extinguished cocaine-seeking. Neuroscience 151, 659–670. [DOI] [PubMed] [Google Scholar]
- Hofford RS, Darna M, Wilmouth CE, Dwoskin LP, Bardo MT, 2014. Environmental enrichment reduces methamphetamine cu-induced reinstatement but does not alter methamphetamine reward or VMAT2 function. Behav. Brain Res 270, 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtz NA, Lozama A, Prisinzano TE, Carroll ME, 2012. Reinstatement of methamphetamine seeking in male and female rats treated with modafinil and allopregnanolone. Drug Alcohol Depend 120 (1–3), 233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser YI, Evans E, Huang YC, 2005. Treatment outcomes among women and men methamphetamine abusers in California. J. Subst. Abus. Treat 28 (1), 77–85. [DOI] [PubMed] [Google Scholar]
- Ilango A, Kesner AJ, Keller KL, Stuber GD, Bonci A, Ikemoto S, 2014. Similar roles of substantia nigra and ventral tegmental dopamine neurons in reward and aversion. J. Neurosci 34 (3), 817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan RF, Cooney NL, Baker LH, Gillespie RA, Meyer RE, Pomerleau OF (1985). Reactivity to alcohol-related cues: physiological and subjective responses in alcoholics and nonproblem drinkers. J. Stud Alcohol 46(4): 267–272. [DOI] [PubMed] [Google Scholar]
- Katz JL, Higgins ST, 2003. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology 168, 21–30. [DOI] [PubMed] [Google Scholar]
- Kovacs K, 1998. c-Fos as a transcription factor: a stressful (re)view from a functional map. Neurochem. Int 33, 287–297. [DOI] [PubMed] [Google Scholar]
- Kufahl PR, Zavala AR, Singh A, Thiel Kj, Dickey ED, Joyce JN, Neisewander JL, 2009. c-Fos expression associated with reinstatement of cocaine-seeking behavior by response-contingent conditioned cues. Synapse 63 (10), 823–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson EB, Carroll ME, 2007. Estrogen receptor beta, but not alpha, mediates estrogen’s effecto on cocaine-induced reinstatement of extinguished cocaine-seeking behavior in ovariectomized female rats. Neuropsychopharmacology 32 (6), 1334–1345. [DOI] [PubMed] [Google Scholar]
- Larson EB, Roth ME, Anker JJ, Carroll ME, 2005. Effect of short- vs long-term estrogen on reinstatement of cocaine-seeking behavior in female rats. Pharmacol. Biochem. Behav 82 (1), 98–108. [DOI] [PubMed] [Google Scholar]
- Lin SK, Ball D, Hsiao CC, Chiang YL, Ree SC, Chen CK, 2004. Psychiatric comorbidity and gender differences of persons incarcerated for methamphetamine abuse in Taiwan. Psychiatry Clin. Neurosci 58 (2), 206–212. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Brodnik ZD, Cox BM, Buchta WC, Bentzley BS, Quintanilla J, et al. (2019). Chemogenetic manipulations of ventral tegmental area dopamine neurons reveal multifaceted roles in cocaine abuse. Journal of Neurosci 39(3): 503–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW, 2001. The circuitry mediating cocaine-induced reinstatement of drug seeking behavior. J. Neurosci 21 (21), 8655–8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J, See RE, 2003. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology 168, 57–65. [DOI] [PubMed] [Google Scholar]
- Miller CA, Marshall JF, 2005. Altered Fos expression in neural pathways underlying cue-elicited drug seeking in the rat. Eur. J. Neurosci 21, 1385–1393. [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse, 2013. NIDA Research Report Series Methamphetamine: Abuse and Addiction. NIH Pub Number 13–4210. [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LTL, Palmer A, Marshall FJ, 2000. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J. Neurosci 20 (2), 798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Torres OV (2014). A mechanistic hypothesis of the factors that enhance vulnerability to nicotine use in females. Neuropharmacology 76 Pt B: 566–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, et al. , 2006. Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology 184 (3–4), 391–400. [DOI] [PubMed] [Google Scholar]
- Parsegian A, See RE, 2014. Dysregulation of dopamine and glutamate release in the prefrontal cortex and nucleus accumbens following methamphetamine self-administration and during reinstatement in rats. Neuropsychopharmacology 39, 811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Stewart JL, 2020. Neurobiology, clinical presentation, and treatment of methamphetamine use disorder: a review. JAMA Psychiatry 10.1001/jamapsychiatry.2020.0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C, 2007. The Rat Brain in Stereotaxic Coordinates, 6th edn. Academic Press, San Diego, CA, USA. [Google Scholar]
- Pena-Bravo JI, Penrod R, Reichel CM, Lavin A, 2019. Methamphetamine self-administration elicits sex-related changes in postsynaptic glutamate transmission in the prefrontal cortex. eNeuro 6. 10.1523/ENEURO.0401-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger ST, Barrett ST, Chou S, Bevins RA, 2016. The effects of varenicline on methamphetamine self-administration and drug-primed reinstatement in female rats. Behav. Brain Res 300, 150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger ST, Barrett ST, Chou S, Bevins RA, 2017. The effects of varenicline on methamphetamine self-administration and drug-primed reinstatement in male rats. Behav. Brain Res 320, 195–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KL, Sullivan JT, Strain EC, Bigelow GE, 1992. Effects of cocaine alone and in combination with bromocriptine in human cocaine abusers. J Pharmocol Exp Ther 262 (1), 279–291. [PubMed] [Google Scholar]
- Quinn RK, James MH, Hawkins GE, Brown AL, Heathcote A, Cairns MJ, Dayas CV, 2018. Temporally specific miRNA expression patterns in the dorsal and ventral striatum of addiction-prone rats. Addict. Biol 23 (2), 631–642. [DOI] [PubMed] [Google Scholar]
- Rawson RA, Gonzales R, Obert JL, McCann MJ, Brethen P, 2005. Methamphetamine use among treatment-seeking adolescents in Southern California: participant characteristics and treatment response. J. Subst. Abus. Treat 29 (2), 67–74. [DOI] [PubMed] [Google Scholar]
- Recinto P, Samant ARH, Chavez G, Kim A, Yuan CJ, Soleiman M, Grant Y, Edwards S, Wee S, Koob GF, George O, Mandyam CD, 2012. Levels of neural progenitors in the hippocampus predict memory impairment and relapse to drug seeking as a function of excessive methamphetamine self-administration. Neuropsychopharmacology 37, 1275–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Linkugel JD, Bevins RA, 2008. Bupropion differentially impacts acquisition of methamphetamine self-administration and sucrose-maintained behavior. Pharmacol. Biochem. Behav 89 (3), 463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Murray JE, Grant KM, Bevins RA, 2009. Bupropion attenuates methamphetamine self-administration in adult male rats. Drug Alcohol Depend 100 (1–2), 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Chan CH, Ghee SM, See RE, 2012. Sex differences in escalation of methamphetamine self-administration: cognitive and motivational consequences in rats. Psychopharmacology 223 (4), 371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich MT, Huang YH, Torregrossa MM, 2019. Plasticity at thalamo-amygdala synapses regulates cocaine-cue memory formation and extinction. Cell Rep 26 (4), 1010–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha A, Kalivas PW, 2010. Role of the prefrontal cortex and nucleus accumbens in reinstating methamphetamine seeking. Eur. J. Neurosci 31 (5), 903–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi MA, Sukharnikova T, Hayrapetyan VY, Yang L, Yin HH, 2013. Operant self-administration of dopamine neurons in the substantia nigra. PLOS 8 (6), e65799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth ME, Carroll ME, 2004. Sex differences in the acquisition of IV methamphetamine self-administration and subsequent maintenance under a progressive ratio schedule in rats. Psychopharmacology 172 (4), 443–449. [DOI] [PubMed] [Google Scholar]
- Rubio FJ, Liu QR, Li X, Cruz FC, Leão RM, Warren BL, Kambhampati S, Babin KR, McPherson KB, Cimbro R, Bossert JM, Shaham Y, Hope BT, 2015. Context-induced reinstatement of methamphetamine seeking is associated with unique molecular alterations in Fos-expressing dorsolateral striatum neurons. J. Neurosci 35 (14), 5625–5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruda-Kucerova J, Amchova P, Babinska Z, Dusek L, Micale V, Sulcuva A, 2015. Sex differences in the reinstatement of methamphetamine seeking after forced abstinence in Sprague-Dawley rats. Front Psychiatry 6, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE, Kruzich PJ, Grimm JW (2001). Dopamine, but not glutamate, receptor blockade in the basolateral amygdala attenuates conditioned reward in a rat model of relapse to cocaine-seeking behavior. Psychopharmacology (Berl) 301–310. [DOI] [PubMed] [Google Scholar]
- Self D, Nestler EJ, 1998. Relapse to drug-seeking: neural and molecular mechanisms. Drug Alcohol Depend 51, 49–60. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y, 2004. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol. Psychiatry 55 (11), 1082–1089. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Lê AD, 2007. Acute nicotine enhances c-Fos mxexpression differentially in reward-related substrates of adolescent and adult rat brain. Neurosci. Lett 418, 286–291. [DOI] [PubMed] [Google Scholar]
- Simpson JL, Grant KM, Daly P, Kelley SG, Carlo G, Bevins RA, 2016. Psychological burden in men and women methamphetamine-dependent patients in treatment. J. Psychoactive Drugs 48, 261–269. [DOI] [PubMed] [Google Scholar]
- Sobieraj JC, Kim A, Fannon MJ, Mandyam CD, 2016. Chronic wheel running-induced reduction of extinction and reinstatement of methamphetamine seeking in methamphetamine dependent rats is associated with reduced number of periaqueductal gray dopamine neurons. Brain Struct. Funct 221, 261–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell TR, Hodgson HJ, Taylor RC, 1982. Alcohol dependence, beliefs and the priming effect. Behav. Res. Ther 20 (5), 513–522. [DOI] [PubMed] [Google Scholar]
- Sun W, Rebec GV, 2003. Lidocaine inactivation of ventral subiculum attenuates cocaine-seeking behavior in rats. J. Neurosci 23 (32), 10258–10264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taepavarapruk P, Phillips AG, 2003. Neurochemical correlates of relapse to d-amphetamine self-administration by rats induced by stimulation of the ventral subiculum. Psychopharmacology 168, 99–108. [DOI] [PubMed] [Google Scholar]
- Takashima Y, Fannon MJ, Galinato M, Steiner N, An M, Zemljic-Harpf AE, Somkuwar SS, et al. , 2018. Neuroadaptations in the denate gyrus following contextual cued reinstatement of methamphetamine seeking. Brain Struct. Funct 233 (5), 2197–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KL, Everitt BJ, 2001. Limbic-cortical-ventral striatal activation during retrieval of a discrete cocaine-associated stimulus: a cellular imaging study with protein kinase C expression. J. Neurosci 21 (7), 2526–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL, 2001. Relapse to cociane-seeking after hippocampal theta burst stimulation. Science 292, 1175–1178. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Haberny KA, Bigelow GE, 2000. Modulation of intravenous cocaine effects by chronic oral cocaine in humans. Psychopharmacology 150, 361–373. [DOI] [PubMed] [Google Scholar]
- Westermeyer J, Boedicker AE, 2000. Course, severity, and treatment of substance abuse among women versus men. Am J Drug Alcohol Abuse 26 (4), 523–535. [DOI] [PubMed] [Google Scholar]
- Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, Rounsaville BJ, Gore JC, 2001. Functional magnetic resonance imaging of cocaine craving. Am. J. Psychiatry 158, 86–95. [DOI] [PubMed] [Google Scholar]
- Whitelaw RB, Robbins TW, Everitt BJ, Markou EA, 1996. Excitotoxic lesions of the basolateral amygdala impair the acquisition of cocaine-seeking behaviour under a second-order schedule of reinforcement. Psychopharmacology 127 (3), 213–224. [PubMed] [Google Scholar]
- Wu LT, Pilowsky DJ, Schlenger WE, Galvin DM, 2007. Misuse of methamphetamine and prescription stimulants among youths and young adults in the community. Drug Alcohol Depend 89 (2–3), 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS, Becker ML, Frieman AJ, Strauch S, DeGarmo B, Geisler S, Meredith GE, Marinelli M, 2010. Fos after single and repeated self-administration of cocaine and saline in the rat: emphasis on the basal forebrain and recalibration of expression. Neuropsychopharmacology 35, 445–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala AR, Biswas S, Harlan RE, Neisewander JL, 2007. Fos and glutamate AMPA receptor subunit coexpression associated with cue-elicited cocaine-seeking behavior in abstinent rats. Neuroscience 16 145 (2), 438–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Li M, 2010. c-Fos identification of neuroanatomical sites associated with haloperidol and clozapine disruption of maternal behavior in the rat. Neuroscience 166, 1043–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Pruitt C, Shin CB, Garcia AD, Zavala AR, See RE, 2014. Fos expression induced by cocaine-conditioned cues in male and female rats. Brain Struct. Funct 219, 1831–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]


