Abstract
Background
The SARS-CoV-2 omicron variant (B.1.1.529) is highly transmissible, but disease severity appears to be reduced compared with previous variants such as alpha and delta. We investigated the risk of severe outcomes following infection in residents of long-term care facilities.
Methods
We did a prospective cohort study in residents of long-term care facilities in England who were tested regularly for SARS-CoV-2 between Sept 1, 2021, and Feb 1, 2022, and who were participants of the VIVALDI study. Residents were eligible for inclusion if they had a positive PCR or lateral flow device test during the study period, which could be linked to a National Health Service (NHS) number, enabling linkage to hospital admissions and mortality datasets. PCR or lateral flow device test results were linked to national hospital admission and mortality records using the NHS-number-based pseudo-identifier. We compared the risk of hospital admission (within 14 days following a positive SARS-CoV-2 test) or death (within 28 days) in residents who had tested positive for SARS-CoV-2 in the period shortly before omicron emerged (delta-dominant) and in the omicron-dominant period, adjusting for age, sex, primary vaccine course, past infection, and booster vaccination. Variants were confirmed by sequencing or spike-gene status in a subset of samples.
Results
795 233 tests were done in 333 long-term care facilities, of which 159 084 (20·0%) could not be linked to a pseudo-identifier and 138 012 (17·4%) were done in residents. Eight residents had two episodes of infection (>28 days apart) and in these cases the second episode was excluded from the analysis. 2264 residents in 259 long-term care facilities (median age 84·5 years, IQR 77·9–90·0) were diagnosed with SARS-CoV-2, of whom 253 (11·2%) had a previous infection and 1468 (64·8%) had received a booster vaccination. About a third of participants were male. Risk of hospital admissions was markedly lower in the 1864 residents infected in the omicron-period (4·51%, 95% CI 3·65–5·55) than in the 400 residents infected in the pre-omicron period (10·50%, 7·87–13·94), as was risk of death (5·48% [4·52–6·64] vs 10·75% [8·09–14·22]). Adjusted hazard ratios (aHR) also indicated a reduction in hospital admissions (0·64, 95% CI 0·41–1·00; p=0·051) and mortality (aHR 0·68, 0·44–1·04; p=0·076) in the omicron versus the pre-omicron period. Findings were similar in residents with a confirmed variant.
Interpretation
Observed reduced severity of the omicron variant compared with previous variants suggests that the wave of omicron infections is unlikely to lead to a major surge in severe disease in long-term care facility populations with high levels of vaccine coverage or natural immunity. Continued surveillance in this vulnerable population is important to protect residents from infection and monitor the public health effect of emerging variants.
Funding
UK Department of Health and Social Care.
Introduction
The novel B.1.1.529 SARS-CoV-2 variant was first detected in South Africa and was designated a variant of concern named omicron by WHO on Nov 26, 2021.1 The variant has many mutations in the spike gene, raising concerns about the effectiveness of available vaccines and antibody therapeutics.2 Since the variant emerged, a sharp increase in SARS-CoV-2 infections in all WHO regions has been observed, and as of 2022, omicron accounts for more than 98% of sequenced samples in the UK and USA,3 and more than 89% of sequenced samples globally.4 This rapid growth in infections is probably due to the variant's increased transmissibility5 and its ability to evade immunity conferred by previous infection or vaccination.2
Residents of long-term care facilities are among the frailest and most clinically vulnerable members of society and have been disproportionately affected by the pandemic. An estimated 50% of residents in long-term care facilities are aged 85 years or older,6 with high levels of comorbidity.7, 8 In the UK, despite high levels of vaccine coverage in residents (as of April 7, 2022, 89% have received a booster vaccine),9 there has been a rapid increase in the number of outbreaks in long-term care facilities since December, 2021, coinciding with the emergence of the omicron variant and a rapid increase in SARS-CoV-2 cases nationally.10 To date, mortality rates among residents have remained stable, but delays in coding for death certification means that this is a lagged indicator of disease severity.11
Research in context.
Evidence before this study
We searched MEDLINE and medRxiv for studies investigating the outcomes of infection with the omicron variant in residents of long-term care facilities or in older adults that were published between Jan 1, 2019, and Feb 2, 2022, with no language restrictions. We used two search strategies employing variations of the terms (1) “COVID-19” AND “care home” OR “older adult” AND “omicron” and (2) “omicron” AND “severity” AND “care home”. We identified three pre-print articles that reported outcomes of infection in older adults, two from the USA and one from Canada. The first study used calendar date to differentiate infections that occurred before and after the emergence of the omicron variant and reported lower risk of hospital admission in adults aged 65 years and older in the omicron-dominant period (risk ratio 0·55, 95% CI 0·44–0·68). In the second study, which used loss of S-gene detection to identify omicron infections, the hazard ratio (HR) for hospital admission in adults aged 65 years and older infected with omicron was also reduced (0·36, 95% CI 0·19–0·70). The third study from Ontario, Canada, used a combination of viral sequencing, S-gene detection, and infection onset date to differentiate between omicron and delta infections. In common with the studies from the USA, the risk of hospital admission in adults aged 60 years and older was substantially lower in those infected in the omicron-period than the pre-omicron period (HR 0·40, 95% CI 0·28–0·56). We identified no studies in frail residents of long-term care facilities. Additionally, all three studies used samples that had been obtained through symptomatic testing, and testing behaviours are likely to have differed between the pre-omicron and omicron-dominant periods.
Added value of this study
In this cohort study in 2264 residents (median age 85 years) from 259 long-term care facilities in England, we compared the risk of hospital admission and death following SARS-CoV-2 infection before and after the emergence of the omicron variant. We found a 59% reduction in the overall (unadjusted) risk of hospital admission and 51% reduction in risk of mortality in the period when omicron was dominant compared with the period before omicron emerged. On multivariable analyses we found a 36% reduction in the adjusted risk of hospital admission, and a 32% reduction in the adjusted risk of mortality, with similar findings when we restricted our analysis to samples with a confirmed variant. Although studies in the general population suggest that the severity of omicron infection is reduced by comparison to previous variants, data are sparse in older, comorbid populations who are at greatest risk of severe outcomes. These findings constitute some of the earliest real-world evidence on disease severity in frail, older adults. Additionally, regular asymptomatic screening for SARS-CoV-2 in residents in our study provides a relatively unbiased sampling frame to assess changes in disease severity over time.
Implications of all the available evidence
Overall, our findings add to accumulating evidence that the severity of infection with omicron is reduced compared with previous variants, even in frail, older populations with high rates of comorbidity. Whether these results can be extrapolated to similar populations that do not have comparable levels of vaccine coverage or high rates of natural immunity, such as fully vaccinated community-dwelling older adults with less exposure to infection, is uncertain. Finally, our findings emphasise the need for ongoing research and surveillance for SARS-CoV-2 in long-term care facilities to monitor the incidence and outcomes of emerging variants in this vulnerable population and to inform the need for re-vaccination and public health disease control measures.
Studies in the general population12, 13 suggest that the risk of severe outcomes following infection with omicron might be lower than that seen for previous variants such as delta, and this risk is attenuated further in those who have received a booster vaccination.13, 14 However, the scale of infection suggests that the total number of hospital admissions and deaths due to omicron might still be substantial, depending on the extent to which age and comorbidity influence disease severity. Data on outcomes following infection in older populations with high rates of comorbidity are scarce. Definitive conclusions about disease severity, and the ongoing need for population-wide restrictions, require studies in populations that are at the greatest risk of severe outcomes, such as residents of long-term care facilities. Therefore, in this study, we aimed to investigate the risk of severe outcomes in residents of long-term care facilities infected with the SARS-CoV-2 omicron variant.
Methods
Study design and participants
We did a prospective cohort study to investigate the risk of hospital admission and death in residents of long-term care facilities in England who tested positive for SARS-CoV-2 between Sept 1, 2021, and Feb 1, 2022, and who were participating in the VIVALDI study (ISRCTN 14447421).15 Residents in participating care homes were eligible for inclusion if they had a positive PCR or lateral flow device test during the study period which could be linked to a National Health Service (NHS) number (enabling data linkage). Participants were followed up for a maximum of 28 days following a positive test. The study accessed data collected as part of routine national surveillance; therefore, consent was not requested from participants. Research ethical approval for the study was granted by the South Central Hampshire B NHS Research Ethics Committee (ref: 20/SC/0238).
The original protocol for the VIVALDI study has previously been published.
Procedures
As part of the national testing programme, residents in long-term care facilities in England undertake monthly asymptomatic testing for SARS-CoV-2 using either PCR or lateral flow devices. They are also tested if they develop symptoms, during outbreaks, or on admission to hospital.16 Each test is linked to a unique identifier based on the individual's NHS number, which can be used to link to other routine datasets.
PCR or lateral flow device test results were linked to national hospital admission and mortality records, which include International Classification of Diseases 10th edition diagnostic codes, using the NHS-number-based pseudo-identifier. By the date of data extraction (March 2, 2022), admissions data had last been updated on March 1, 2022, and mortality data on Feb 14, 2022. Vaccine type administered in primary vaccine course, and receipt of first or second dose or booster vaccine dose were retrieved by linkage to the National Immunisation Management System. Long-term care facility size was retrieved from the Capacity Tracker dataset. Data linkage was done securely in the COVID-19 Datastore.
PCR testing was done in a network of accredited laboratories established through the national testing programme and a subset of samples selected at random were sequenced at the Wellcome Sanger Institute. We retrieved viral lineage for sequenced samples from a publicly available repository, which is established and maintained by the COVID-19 Genomics UK consortium. If sequencing was unavailable, PCR cycle threshold (Ct) values were used to identify S-Gene Target Failure (SGTF)—a reliable marker of omicron.13, 17 Samples with Ct values of more than 30 were excluded from the assessment of SGTF to reduce the risk of misclassifying samples with a low viral load (appendix p 3). Omicron cases were defined as BA.1 or BA.2 lineage or SGTF. Delta was defined as any AY lineage confirmed on sequencing or detection of S-gene on PCR testing.13 We excluded suspected Delta samples (identified by S gene on PCR) that were collected after Jan 12, 2022, and that had not been sequenced to avoid misclassification of omicron samples of the BA.2 lineage, which does not exhibit SGTF17 and which overtook delta in prevalence in England on this date.
Outcomes and covariates
We compared outcomes in residents who were infected in the pre-omicron period (Sept 1–Dec 12, 2021) when the delta variant was dominant and in residents infected in the omicron-dominant period (Dec 13, 2021–Feb 1, 2022). Because all residents of long-term care facilities in England are screened regularly for SARS-CoV-2, the risk of bias in our assessment of disease severity was relatively low.
The primary outcome was hospital admission within 14 days following a positive SARS-CoV-2 test, and the secondary outcome was mortality in the 28 days following a positive test. Our main comparison was between two exposure periods based on the date of the first omicron case in our dataset: the pre-omicron period when delta predominated and the omicron predominant period. The comparison of the risk of hospital admission and mortality was repeated in the subset of residents with confirmed or probable delta or omicron infection determined by sequencing or S-gene. Covariates included age (centred at the median for analysis), sex, previous natural infection (defined as at least one of: previous positive PCR or lateral flow device result >28 days before their positive test, previous hospital admission for SARS-CoV-2, or detection of anti-nucleocapsid IgG antibodies), primary vaccination course, and time from booster vaccination. Primary vaccination course was categorised as Pfizer BNT162b, AstraZeneca ChAdOx1, type not known (in cases for which only the booster dose was recorded), or unvaccinated. Participants were classified as boosted if they had received a third vaccination dose at least 1 week before diagnosis.
Statistical analysis
We estimated the risk of hospital admission (for any cause) in the 14 days following a positive PCR or lateral flow device test, and plotted Kaplan-Meier curves to compare the cumulative incidence of hospital admission in residents who tested positive during the pre-omicron (delta-dominant) and omicron periods. For the analysis of hospital admissions, residents entered the analysis on the date of their positive test and were censored at hospital admission (within 14 days following diagnosis) or 14 days after the date of their positive test if they were not admitted to hospital. For the analysis of deaths, residents entered the analysis on the date of their positive test, and were censored on the date of death, or at 28 days after the date of their positive test or on or Feb 14, 2022, which is the latest date in the mortality dataset (because reporting of deaths is typically more delayed than reporting of hospital admissions). Only residents from the omicron period were censored at these late dates in the dataset, because follow-up from a positive test was greater than 28 days for all pre-omicron residents. We investigated whether the comparison of the risk of hospital admission between the pre-omicron and omicron periods was modified by sex, primary vaccine course, booster status, past infection status, or age through evaluation of interaction terms in Cox models. The cumulative incidence of hospital admission was also compared between delta and omicron in the known variant cohort using Kaplan-Meier curves.
We modelled risk of hospital admission and risk of death in the main cohort using mixed-effects Cox proportional hazards regression with an added frailty term to account for clustering in long-term care facilities. Models were adjusted for age, sex, past infection, primary vaccination type, and time from booster vaccination, with exploration for evidence of an interaction with omicron period for all adjustment variables. A separate mixed-effects multivariable model was constructed in the known variant cohort for risk of hospital admission and death.
Testing for a difference in the Kaplan-Meier curves between pre-omicron and omicron periods, and between delta and omicron, was based on the log-rank test. Regression results are presented as adjusted hazard ratios (HR) with 95% CIs. A p value of less than 0·05 was considered statistically significant for effect measures. Formal sample size calculation was not undertaken.
Positive test results obtained more than 24 h following hospital admission were excluded to ensure we did not include hospital admissions for conditions unrelated to SARS-CoV-2. If duplicate samples were identified (two samples obtained from an individual within 28 days of each other), only the first sample was included in the analysis.
All statistical analyses were done with Stata (version 16.0). The legal basis for data linkage is provided by the COVID-19: notice under regulation of the 3(4) of the Health Service (Control of Patient Information) Regulations 2002 (COPI notice).18
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
A total of 795 233 tests were done in 333 long-term care facilities (staff and residents), of which 159 084 (20·0%) could not be linked to a pseudo-identifier and 138 012 (17·4%) were done in residents. We excluded seven suspected delta samples collected after Jan 12, 2022. The proportion of unlinked samples was similar in the pre-omicron and omicron periods (appendix p 4). Eight residents had two episodes of infection (>28 days apart) and in these cases the second episode was excluded from the analysis.
Overall, there were 2264 new SARS-CoV-2 diagnoses in 259 long-term care facilities (table 1 , appendix pp 3, 5). The median age of residents with infection was 84·5 years (IQR 77·9–90·0), about a third were male, 253 (11·2%) had a previous infection, and 1468 (64·8%) had received a booster vaccination more than a week before their positive test. 400 (17·7%) infections were diagnosed in the pre-omicron period and 1864 (82·3%) were diagnosed in the omicron-dominant period. There was no censoring of follow-up for the hospital admission outcome, or for the outcome of death in the pre-omicron and known delta cohorts. However, 414 (22·2%) of 1864 participants in the omicron-dominant period did not have full follow-up (median follow-up 28 days, IQR 28–28) and median follow-up in the known omicron variant cohort was 28 days (26–28).
Table 1.
Baseline characteristics for care home residents diagnosed with SARS-CoV-2
| All cases (n=2264) | Pre-omicron period (Sept 1–Dec 12, 2021; n=400) | Omicron period (Dec 13, 2021–Feb 1, 2022; n=1864) | p value | |
|---|---|---|---|---|
| Age, years | ||||
| IQR, range | 84·5 (IQR 77·9–90·0; range 53·0–105·0) | 84·5 (IQR 78·0–90·1; range 64·0–105·0) | 84·6 (IQR 77·8–90·0; range 53·0–104·7) | 0·58 |
| Sex | ||||
| Female | 1559 (68·9%) | 272 (68·0%) | 1287 (69·0%) | 0·68 |
| Male | 705 (31·1) | 128 (32·0%) | 577 (31·0%) | .. |
| Type of test | ||||
| Lateral flow device | .. | 36 (9·0%) | 162 (8·7%) | 0·84 |
| PCR | .. | 364 (91·0%) | 1702 (91·3%) | .. |
| Primary vaccine course | ||||
| AstraZeneca (ChAdOx1) | 1189 (52·5%) | 218 (54·5%) | 971 (52·1%) | 0·051 |
| Pfizer (BNT162b2) | 676 (29·9%) | 102 (25·5%) | 574 (30·8%) | .. |
| Type not known | 97 (4·3%) | 14 (3·5%) | 83 (4·5%) | .. |
| Unvaccinated | 302 (13·3%) | 66 (16·5%) | 236 (12·7%) | .. |
| Booster vaccination status* | ||||
| Booster >1 week before positive test | 1468 (64·8%) | 69 (17·3%) | 1399 (75·1%) | <0·0001 |
| Days from booster to positive test (IQR, range) | 81 (IQR 63–98; range 1–182) | 26 (IQR 6–41; range 1–83) | 83 (IQR 68–98; range 1–182) | <0·0001 |
| Infection history | ||||
| Any evidence of past infection | 253 (11·2%) | 17 (4·3%) | 236 (12·7%) | <0·0001 |
| Previous positive PCR or lateral flow device | 158 (7·0%) | 11 (2·8%) | 147 (7·9%) | <0·0001 |
| SARS-CoV-2 antibodies | 27 (1·2%) | 0 | 27 (1·4%) | NA |
| Previous COVID-19 hospital admission | 110 (4·9%) | 8 (2·0%) | 102 (5·5%) | <0·0001 |
| Other variables | ||||
| Hospital admission within 14 days of a positive test | 126 (5·6%) | 42 (10·5%) | 84 (4·5%) | <0·0001 |
| COVID-19 deaths† | 150 (6·6%) | 51 (12·8%) | 99 (5·3%) | <0·0001 |
| Number of care home beds (IQR, range) | 59 (IQR 44–80; range 17–149) | 52 (IQR 41–68; range 24–149) | 60 (IQR 46–80; range 17–149) | .. |
Eight participants had two infections more than 28 days apart in the dataset and in both cases the first positive test was included in the analysis.
Booster vaccinations shown for entire study cohort, regardless of primary vaccination status.
COVID-19 deaths defined as death within 28 days of a positive PCR test or COVID-19 recorded on the death certificate.
In total, 126 residents were admitted to hospital in the 14 days following a positive PCR or lateral flow device test. This data included 42 admissions in the 400 residents who were infected in the pre-omicron period and 84 admissions in the 1864 residents who were infected in the omicron-dominant period. Overall, three of 253 residents with a previous infection were admitted to hospital, all of whom were infected in the omicron-dominant period. There were 141 deaths in the 28 days following infection: 43 occurred in 400 residents infected in the pre-omicron period compared with 98 deaths in 1864 residents infected in the omicron-dominant period (table 1). There were 13 deaths following infection in residents with a previous infection, 12 of which occurred in residents infected in the omicron-dominant period.
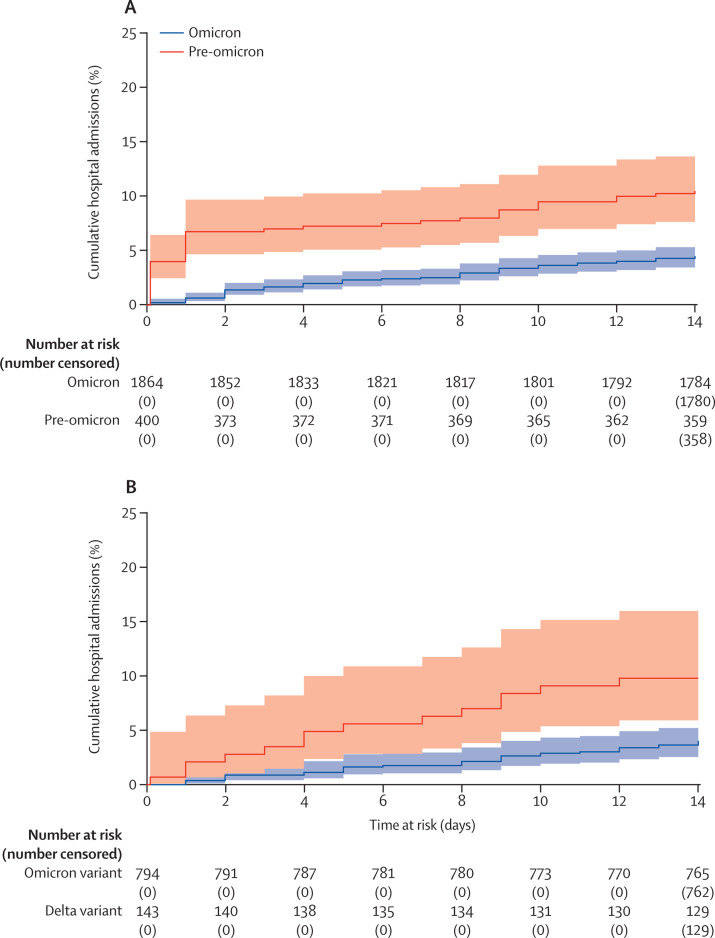
The cumulative incidence of hospital admission following infection, estimated from Kaplan-Meier curves, was lower for individuals who were infected in the omicron period (4·51%, 95% CI 3·65–5·55) admitted within 14 days of a positive test, versus the pre-omicron period (10·50%, 7·87–13·94) admitted within 14 days following a positive test (p<0·0001; figure 1 ).
Figure 1.
Kaplan-Meier curve
Cumulative incidence of hospital admission in the 14 days following a positive PCR or lateral flow test in the pre-omicron (Sept 1–Dec 12, 2021) and omicron periods (Dec 13, 2021– March 1, 2022) (A) and in residents with confirmed or probable delta infection versus those with confirmed or probable omicron infection (B) based on sequencing and S-gene target failure. Participants who were not admitted to hospital were censored at 14 days after a positive test, or on March 1, 2022.
The unadjusted HR for hospital admission following diagnosis in the omicron period compared with the pre-omicron period was 0·41 (95% CI 0·28–0·59; p<0·0001), and this effect was partly attenuated in the multivariable model (adjusted [a] HR 0·64, 95% CI 0·41–1·00; p=0·051; table 2 ). The adjusted risk of hospital admission was lower in women than in men (aHR 0·58, 95% CI 0·41–0·83; p<0·0001) and increased with each year of age (aHR 1·03, 1·00–1·05; p=0·015). Primary vaccine course was not associated with the risk of hospital admission; however, those who had received a booster vaccination more than 1 week before their positive test were at lower risk of hospital admission than were those who had been vaccinated but not boosted (aHR 0·51, 95% CI 0·32–0·82; p<0·0001). Individuals with known past infection were also at lower risk of hospital admission than were those without a previous infection (aHR 0·21, 95% CI 0·07–0·67; p<0·0001). There was evidence of an interaction between the omicron and pre-omicron period and primary vaccine course (p=0·032) with the greatest reduction in the risk of hospital admission in the omicron period compared with the pre-omicron period among Pfizer recipients (aHR 0·34, 95% CI 0·16–0·72; p=0·048; appendix p 8).
Table 2.
Mixed effects Cox proportional hazards model for hospital admission within 14 days from positive test for SARS-CoV-2 in the full cohort and the known variant cohort
|
Overall (n=2264, 259 clusters) |
Known variant cohort (n=937, 176 clusters) |
|||
|---|---|---|---|---|
| Adjusted HR (95% CI) | p value | Adjusted HR (95% CI) | p value | |
| Period or variant | ||||
| Pre-omicron (delta) | 1 (ref) | .. | 1 (ref) | .. |
| Omicron | 0·64 (0·41–1·00) | 0·051 | 0·47 (0·23–0·95) | 0·036 |
| Sex | ||||
| Male | 1 (ref) | .. | 1 (ref) | .. |
| Female | 0·58 (0·41–0·83) | <0·0001 | 0·86 (0·46–1·61) | 0·63 |
| Age | ||||
| Age (per year increase) | 1·03 (1·00–1·05) | 0·015 | 1·02 (0·98–1·06) | 0·33 |
| Primary vaccine course* | ||||
| Unvaccinated | 1 (ref) | .. | 1 (ref) | .. |
| AstraZeneca (ChAdOx1) | 0·98 (0·57–1·68) | 0·98† | 0·57 (0·24–1·37) | 0·40† |
| Pfizer (BNT162b2) | 0·94 (0·52–1·72) | .. | 0·52 (0·19–1·43) | .. |
| Type not known | 0·84 (0·30–2·30) | .. | .. | .. |
| Booster vaccine status* | ||||
| No booster | 1 (ref) | .. | 1 (ref) | .. |
| Booster more than 1 week before positive test | 0·51 (0·32–0·82) | <0·0001 | 0·73 (0·33–1·61) | 0·43 |
| Past infection status | ||||
| No past infection | 1 (ref) | .. | 1 (ref) | .. |
| Past infection | 0·21 (0·07–0·67) | <0·0001 | 0·77 (0·24–2·51) | 0·66 |
Models are adjusted for median-centred age and all other variables listed in the model (sex, primary vaccine course, time from primary booster, history of previous infection) and have frailty term for long-term care facility clustering. HR=hazard ratio.
The adjusted HRs for boosting are the additional effects of boosting in those with primary vaccination—for example, the HR for an unboosted Pfizer recipient relative to an unvaccinated individual is 0·94, but for a boosted individual with initial Pfizer vaccination relative to an unvaccinated person is 0·94 × 0·51=0·48.
p value indicates difference between unvaccinated individuals and any vaccinated individual, regardless of which vaccine was administered.
We confirmed our findings for risk of hospital admission in the subset of 794 probable or confirmed omicron infections and 143 probable or confirmed delta infections in 937 residents on the basis of lineage (268 omicron, 39 delta) or the presence of SGTF (712 omicron, 132 delta; appendix pp 3, 5). Baseline demographics of individuals with and without available S-gene or lineage data were similar (appendix pp 6–7). There were 214 (70·0%) of 307 samples with both S-gene and lineage data, of which 28 were delta and 186 omicron and all were concordant. All included omicron samples with sequencing were BA.1 lineage. In this cohort there were 14 hospital admissions and 16 deaths in 143 residents with delta versus 32 hospital admissions and 38 deaths in 794 residents with omicron. The estimated cumulative incidence of cases admitted to hospital was higher in those infected with delta (9·79%, 95% CI 5·92–15·97) than with omicron (5·22%, 3·81–7·13; p=0·0003; figure 1). The unadjusted (HR 0·40, 95% CI 0·21–0·74; p<0·0001) and adjusted (aHR 0·47, 0·23–0·95; p=0·036) risks of hospital admission for probable or confirmed omicron versus delta infections were similar to those seen in the main analysis.
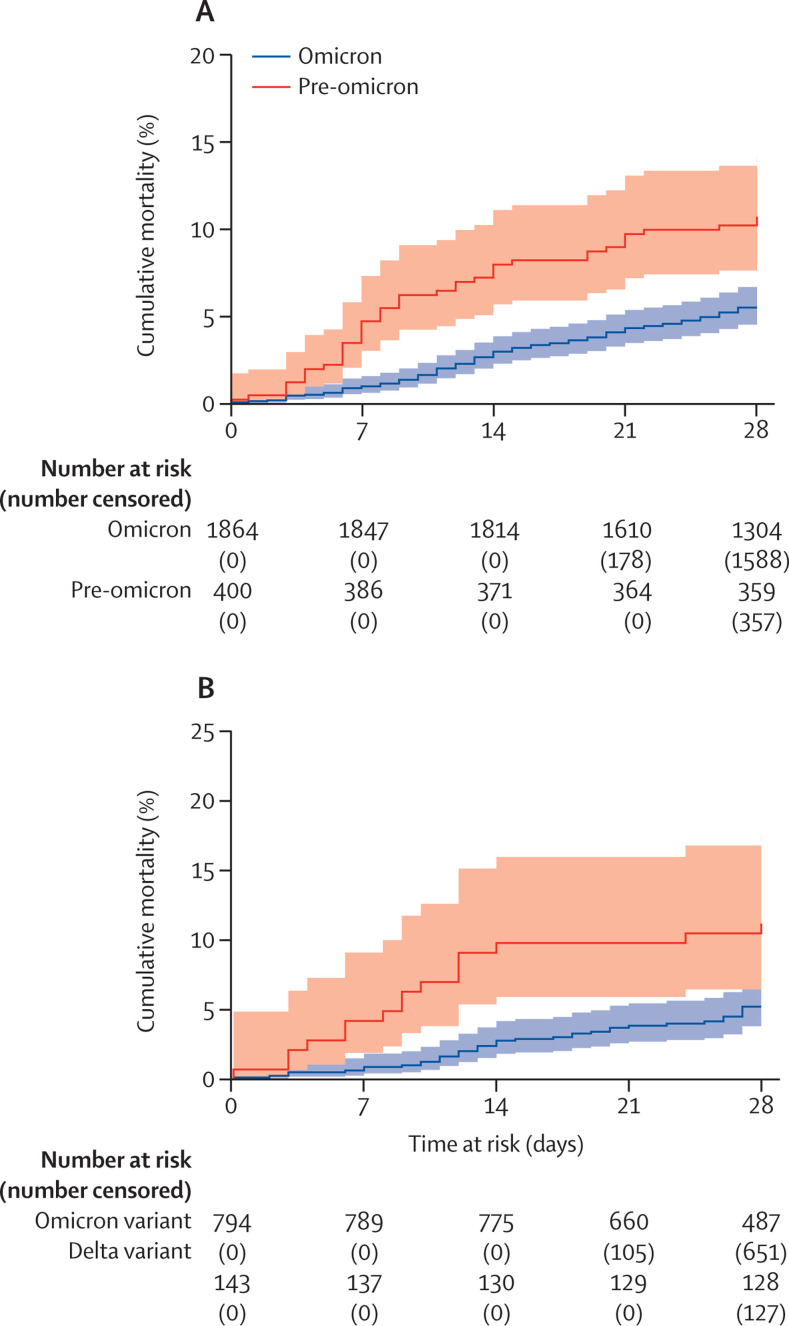
The proportion of residents that died within 28 days of a new SARS-CoV-2 diagnosis in the omicron-dominant period (5·48%, 95% CI 4·52–6·64) was lower than in the pre-omicron period (10·75%, 95% CI 8·09–14·22; p=0·0001, figure 2 ). The unadjusted HR for death within 28 days of diagnosis in the omicron versus pre-omicron period was 0·49 (95% CI 0·34–0·70; p<0·0001) and this effect was slightly reduced but no longer significant in the adjusted analysis (aHR 0·68, 95% CI 0·44–1·04; p=0·076; table 3 ). Similar to the analysis of hospital admissions, female sex (aHR 0·54, 95% CI 0·38–0·76; p<0·0001), and booster vaccination more than 1 week before diagnosis (aHR 0·60, 0·38–0·93; p=0·023) were associated with a lower risk of death, whereas each one year increase in age (aHR 1·04, 1·02–1·07; p<0·0001) was associated with increased risk of death. There was no evidence of an interaction between the omicron and pre-omicron period and any of the variables included in the adjusted model.
Figure 2.
Kaplan-Meier curve
Cumulative mortality in 28 days following SARS-CoV-2 test between Sept 1, 2021, and Feb 14, 2022 in the pre-omicron and omicron period (A) and in residents with confirmed or probable delta infection versus those with confirmed or probable omicron infection (B) based on sequencing and S-gene target failure. Participants who did not reach the outcome were censored at 28 days following the date of a positive test or on Feb 14, 2022.
Table 3.
Mixed effects Cox proportional hazards model for death within 28 days from positive test for SARS-CoV-2 in the full cohort and the known variant cohort
|
Overall (n=2264, 259 clusters) |
Known variant cohort (n=937, 176 clusters) |
|||
|---|---|---|---|---|
| Adjusted HR (95% CI) | p value | Adjusted HR (95% CI) | p value | |
| Period or variant | ||||
| Pre-omicron (delta) | 1 (ref) | .. | 1 (ref) | .. |
| Omicron | 0·68 (0·44–1·04) | 0·076 | 0·61 (0·32–1·16) | 0·13 |
| Sex | ||||
| Male | 1 (ref) | .. | 1 (ref) | .. |
| Female | 0·54 (0·38–0·76) | <0·0001 | 0·65 (0·27–1·12) | 0·099 |
| Age | ||||
| Age (per year increase) | 1·04 (1·02–1·07) | <0·0001 | 1·04 (1·01–1·08) | 0·025 |
| Primary vaccine course* | ||||
| Unvaccinated | 1 (ref) | .. | 1 (ref) | .. |
| AstraZeneca (ChAdOx1) | 0·82 (0·49–1·36) | 0·61† | 0·63 (0·29–1·36) | 0·61† |
| Pfizer (BNT162b2) | 0·70 (0·39–1·24) | .. | 0·40 (0·15–1·03) | .. |
| Type not known | 0·63 (0·50–1·60) | .. | 0·56 (0·12–2·70) | .. |
| Booster vaccine status* | ||||
| No booster | 1 (ref) | .. | 1 (ref) | .. |
| Booster more than 1 week before positive test | 0·60 (0·38–0·93) | 0·023 | 0·55 (0·27–1·12) | 0·099 |
| Past infection status | ||||
| No past infection | 1 (ref) | .. | 1 (ref) | .. |
| Past infection | 0·90 (0·50–1·60) | 0·72 | 0·45 (0·11–1·86) | 0·27 |
Models are adjusted for median-centred age and all other variables listed in the model (sex, primary vaccine course, time from primary booster, history of previous infection) and have frailty term for long-term care facility clustering. HR=hazard ratio.
The adjusted HRs for boosting are the additional effects of boosting in those with primary vaccination—for example, the HR for an unboosted Pfizer recipient relative to an unvaccinated individual is 0·70, but for a boosted individual with initial Pfizer vaccination relative to an unvaccinated person is 0·70 × 0·60=0·42.
p value indicates difference between unvaccinated individuals and any vaccinated individual, regardless of which vaccine was administered.
These findings for risk of death were confirmed in the subset of participants with a confirmed variant. There were 16 deaths within 28 days of a SARS-CoV-2 diagnosis in the 143 delta cases and 38 deaths in the 742 omicron cases. The unadjusted HR for death was lower in individuals infected with omicron than with delta (HR 0·43, 95% CI 0·24–0·77; p<0·0001), and this effect reduced and was no longer significant in the adjusted analysis (aHR 0·61, 0·32–1·16; p=0·13; table 3).
Discussion
In this study of residents of long-term care facilities with SARS-CoV-2 infection, we found that disease severity was substantially reduced following the emergence of the omicron variant, and this effect was seen for both hospital admissions and mortality. Confidence in our findings is increased by the fact that similar results were obtained when we restricted our analysis to confirmed delta or omicron infections. Given the age and high frailty of the study population, these findings strengthen the accumulating evidence that disease severity is substantially lower for omicron than for previous variants.
Most residents in our study were fully vaccinated, and 65% had received a booster vaccination (third dose) more than a week before they tested positive for SARS-CoV-2. In common with studies in the general population, our findings suggest that residents who had received a booster vaccination, and those with previous infection, were at lower risk of hospital admission.13, 14 The overall risk of hospital admission or death in those who received a primary course of the Pfizer or AstraZeneca vaccines, and for the subset of these individuals who were not boosted, was comparable to those who were unvaccinated. However, we found some evidence that the reduction in risk of hospital admission in the omicron versus the pre-omicron period was more pronounced in those who had received a primary course of the Pfizer vaccine. Disentangling the direct effect of vaccines, previous infection, or the variant on severe outcomes in this study is difficult because our analysis does not account for the overall effect of vaccination on risk of infection, and the effect of waning immunity and the community incidence of infection are likely to have varied in the pre-omicron and omicron-dominant periods.18, 19, 20, 21, 22 Additionally, long-term care facilities with outbreaks are likely to have delayed the roll out of booster vaccinations to residents, which further complicates the interpretation of findings on the protective effect of booster vaccinations. This so-called delayed vaccination effect has previously been reported in studies evaluating first-dose vaccination in health-care workers and care home residents.23, 24 We are currently investigating vaccine effectiveness in the context of the omicron variant in this cohort in a separate, linked study.
Monoclonal antibodies were first licensed in England on Aug 20, 2021, for use in the community for treatment or prevention of SARS-CoV-2 infection25 and have shown efficacy in the prevention of SARS-CoV-2 infection among residents of long-term care facilities in a randomised controlled trial in the USA.26 In the UK, most treatments are deployed to high-risk individuals in the community through the PANORAMIC trial.27 Although information on recruitment is currently unavailable, it is anticipated that the number of long-term care facility residents is small given the logistical challenges associated with administration of the drugs, and therefore the effect on our study findings is expected to be minimal.
To date, no publications have described the severity of infection with the omicron variant in residents of long-term care facilities; however, preliminary findings from community-dwelling older adults are consistent with the results that we have reported. One large study awaiting peer review that included more than 65 000 positive tests in South California in adults older than age 65 years reported a lower risk of hospital admission following symptomatic SGTF (omicron) than with non-SGTF (delta) infections (aHR 0·36, 95% CI, 0·19–0·70).28 Similarly, a matched cohort study in adults older than age 60 years in Canada, which differentiated variants using a combination of sequencing, S-gene, and onset date, reported a 60% reduction in the risk of hospital admission or death following omicron infections compared with delta infections (aHR 0·40, 95% CI 0·28–0·56).29 A further analysis of health-care records in the USA that included 2173 community-dwelling adults aged 65 years or older reported a lower risk of hospital admission in patients with the omicron variant compared with the delta variant (risk ratio 0·55, 95% CI 0·44–0·68), although this study did not include sequencing results and is awaiting peer review.30
Our study has several strengths. Regular asymptomatic testing for SARS-CoV-2 in residents of long-term care facilities in England allowed us to obtain a relatively unbiased estimate of disease severity, in contrast to most studies, which usually focus on symptomatic cases. We were able to link to routine datasets through the VIVALDI study, recorded in near-real time, which made it possible to reliably capture outcomes in participants and to rapidly assess the impact of the omicron variant. We also had access to viral lineages obtained through the UK's large-scale whole genome sequencing programme, which made it possible to confirm variant type in a third of infections. Although the presence of SGTF is an imperfect measure of the BA.1 omicron sub-lineage, this has been validated in several cohorts and is widely used to distinguish infections with the BA.1 and AY lineages. Our study also has several limitations. Because not all laboratories use assays that include the S-gene target, identifying all samples with SGTF or confirming that all samples with SGTF were cases of the omicron variant was not possible. However, all 214 samples that had been sequenced and tested for S-gene were concordant. The recent emergence of a sub-variant of omicron of the BA.2 lineage that does not exhibit SGTF4 is unlikely to have affected our analysis because we only used SGTF to identify delta samples obtained before Jan 12, 2022, when BA.2 accounted for less than 1% of omicron infections according to data from the UK's Genomic surveillance programme. Additionally, delta infections have become increasingly rare since omicron emerged, which supports our decision to use a cut-off date to define the pre-omicron and omicron-dominant periods. Our study was based on routine data and approximately 20% of tests obtained during the study period were excluded because they could not be matched to an individual. If the characteristics of residents who could not be matched differed between the pre-omicron and omicron periods, this discrepancy might have biased our results, although the proportion of matched PCR and lateral flow tests was reasonably consistent across the study period, implying that there were no major changes in testing behaviour during the study period. Furthermore, we did not have access to data on ethnicity, therefore we were unable to include this data in our analysis. Finally, we probably underestimated the prevalence of past infection in our cohort, at a value that was substantially lower than published seroprevalence estimates from the long-term care facility population,31 because only a subset of residents had been tested for antibodies to nucleocapsid. Although we defined severe outcomes in our analysis as risk of hospital admission or death following SARS-CoV-2 infection, we were not able to consider other outcomes such as long-COVID, which might be challenging to diagnose in frail residents with comorbidities. Similarly, we did not investigate the intensity or duration of hospital admission because residents might not be eligible for escalation of care (eg, non-invasive ventilation), and metrics that are commonly used to assess intensity of care (such as ICU admission or length of stay) are difficult to interpret in care home residents. Furthermore, discharge from hospital is frequently delayed in this population for non-medical reasons.
Overall, our study provides important insights into the risk of severe outcomes in residents of long-term care facilities, who are frequently excluded from research studies and who have experienced among the highest rates of morbidity and mortality related to SARS-CoV-2.Overall, the markedly decreased severity combined with high vaccination uptake and previous natural infection can be expected to limit the effect of the current wave of omicron infections on hospital admissions and deaths in residents of long-term care facilities.
Data sharing
De-identified test results and limited metadata will be made available for use by researchers in future studies, subject to appropriate research ethical approvals once the VIVALDI study cohort has been finalised. These datasets will be accessible via the Health Data Research UK Gateway (https://www.hdruk.ac.uk/).
This online publication has been corrected. The corrected version first appeared at thelancet.com/healthy-longevity on September 6, 2022
Declaration of interests
LS, TP, AC, AH, and OS report grants from the Department of Health and Social Care during the conduct of the study and LS is a member of the Social Care Working Group, which reports to the Scientific Advisory Group for Emergencies. AI-S and VB are employed by the Department of Health and Social Care, which funded the study. AH reports funding from the COVID Core Studies Programme and is a member of the New and Emerging Respiratory Virus Threats Advisory Group at the Department of Health and Environmental Modelling Group of the Scientific Advisory Group for Emergencies. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
We thank the staff and residents in the long-term care facilities who participated in this study and Mark Marshall at National Health Service (NHS) England who pseudonymised the electronic health records. This work is independent research funded by the Department of Health and Social Care (COVID-19 surveillance studies). MK is funded by a Wellcome Trust Clinical PhD Fellowship (222907/Z/21/Z). LS is funded by a National Institute for Health Research (NIHR) Clinician Scientist Award (CS-2016–007). AH is supported by Health Data Research UK (LOND1), which is funded by the UK Medical Research Council, Engineering and Physical Sciences Research Council, Economic and Social Research Council, Department of Health and Social Care (England), Chief Scientist Office of the Scottish Government Health and Social Care Directorates, Health and Social Care Research and Development Division (Welsh Government), Public Health Agency (Northern Ireland), British Heart Foundation, and Wellcome Trust. COVID-19 Genomics UK Consortium is supported by funding from the Medical Research Council (part of UK Research & Innovation), NIHR (grant code: MC_PC_19027), and Genome Research, operating as the Wellcome Sanger Institute. The views expressed in this publication are those of the authors and not necessarily those of the NHS, Public Health England, or the Department of Health and Social Care.
Contributors
LS, AC, OS, and MK conceptualised the study and developed the statistical analysis plan. MK and OS did the formal statistical analysis. MK, LS, OS, AC, PM, GT, and AH interpreted the results. MK, CF, BA, HN-L, MS, VB, TP, and AI-S were involved with project administration. LS and AH obtained research funding. MK and LS wrote the first draft of the manuscript. All authors revised and edited the manuscript. MK and OS accessed and verified the data. All authors had full access to all the data reported in the study and accept responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.WHO Update on Omicron. 2021. https://www.who.int/news/item/28-11-2021-update-on-omicron
- 2.CoVariants Variant: 21K (Omicron) 2022. https://covariants.org/variants/21K.Omicron
- 3.Centers for Disease Control and Prevention COVID Data Tracker. Variant proportions. 2022. https://covid.cdc.gov/covid-data-tracker/#variant-proportions
- 4.GISAID NextStrain. 2022. https://www.gisaid.org/phylodynamics/global/nextstrain/
- 5.Ferguson N, Ghani A, Cori A, Hogan A, Hinsley W, Volz E. Report 49: growth, population distribution and immune escape of Omicron in England. 2021. https://www.imperial.ac.uk/media/imperial-college/medicine/mrc-gida/2021-12-16-COVID19-Report-49.pdf
- 6.Office for National Statistics Care home and non-care home populations used in the deaths involving COVID-19 in the care sector article, England and Wales. 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/adhocs/12215carehomeandnoncarehomepopulationsusedinthedeathsinvolvingcovid19inthecaresectorarticleenglandandwales
- 7.Schultze A, Nightingale E, Evans D, et al. Mortality among care home residents in England during the first and second waves of the COVID-19 pandemic: an observational study of 4·3 million adults over the age of 65. Lancet Reg Health Eur. 2022;14 doi: 10.1016/j.lanepe.2021.100295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barker RO, Hanratty B, Kingston A, Ramsay SE, Matthews FE. Changes in health and functioning of care home residents over two decades: what can we learn from population-based studies? Age and Ageing. 2021;50:921–927. doi: 10.1093/ageing/afaa227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.NHS COVID-19 vaccinations. 2022. https://www.england.nhs.uk/statistics/statistical-work-areas/covid-19-vaccinations/
- 10.Department of Health and Social Care Adult social care monthly statistics, England: January 2022. 2022. https://www.gov.uk/government/statistics/adult-social-care-in-england-monthly-statistics-january-2022/adult-social-care-monthly-statistics-england-january-2022
- 11.Office for National Statistics Number of deaths in care homes notified to the Care Quality Commission, England. 2022. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/numberofdeathsincarehomesnotifiedtothecarequalitycommissionengland
- 12.Wolter N, Jassat W, Walaza S, et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet. 2022;399:437–446. doi: 10.1016/S0140-6736(22)00017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.UKHSA SARS-CoV-2 variants of concern and variants under investigation in England. 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1045619/Technical-Briefing-31-Dec-2021-Omicron_severity_update.pdf
- 14.Ferguson N, Ghani A, Hinsley W, Volz E. Report 50: hospitalisation risk for omicron cases in England. 2021. https://www.imperial.ac.uk/media/imperial-college/medicine/mrc-gida/2021-12-22-COVID19-Report-50.pdf
- 15.Krutikov M, Palmer T, Donaldson A, et al. Study protocol: understanding SARS-CoV-2 infection, immunity and its duration in care home residents and staff in England (VIVALDI) Wellcome Open Research. 2021;5:232. doi: 10.12688/wellcomeopenres.16193.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.UK Health Security Agency COVID-19 care home testing guidance for regular and outbreak testing of staff and residents. 2022. https://www.gov.uk/government/publications/coronavirus-covid-19-testing-in-adult-care-homes/covid-19-care-home-testing-guidance-for-regular-and-outbreak-testing-of-staff-and-residents#testing-policy-for-staff-and-residents
- 17.WHO Enhancing response to Omicron SARS-CoV-2 variant. 2022. https://www.who.int/publications/m/item/enhancing-readiness-for-omicron-(b.1.1.529)-technical-brief-and-priority-actions-for-member-states
- 18.NHS Digital Control of patient information (COPI) notice. 2021. https://digital.nhs.uk/coronavirus/coronavirus-covid-19-response-information-governance-hub/control-of-patient-information-copi-notice
- 19.Andrews N, Stowe J, Kirsebom F, et al. Effectiveness of COVID-19 vaccines against the Omicron (B.1.1.529) variant of concern. N Engl J Med. 2022 doi: 10.1056/NEJMoa2119451. published online March 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hogan AB, Wu SL, Doohan P, et al. Report 48: the value of vaccine booster doses to mitigate the global impact of the Omicron SARS-CoV-2 variant. 2021. https://www.imperial.ac.uk/media/imperial-college/medicine/mrc-gida/2021-12-16-COVID19-Report-48.pdf
- 21.Collie S, Champion J, Moultrie H, Bekker L-G, Gray G. Effectiveness of BNT162b2 vaccine against omicron variant in South Africa. N Engl J Med. 2021;386:494–496. doi: 10.1056/NEJMc2119270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tut G, Lancaster T, Krutikov M, et al. Booster vaccination strongly enhances SARS-CoV-2-specific antibody and cellular responses in elderly residents of care homes. SSRN. 2021 https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3990239 published online Dec 20. (preprint). [Google Scholar]
- 23.Hall VJ, Foulkes S, Saei A, et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397:1725–1735. doi: 10.1016/S0140-6736(21)00790-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shrotri M, Krutikov M, Palmer T, et al. Vaccine effectiveness of the first dose of ChAdOx1 nCoV-19 and BNT162b2 against SARS-CoV-2 infection in residents of long-term care facilities in England (VIVALDI): a prospective cohort study. Lancet Infect Dis. 2021;21:1529–1538. doi: 10.1016/S1473-3099(21)00289-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.GOV.UK; First monoclonal antibody treatment for COVID-19 approved for use in the UK. 2021. https://www.gov.uk/government/news/first-monoclonal-antibody-treatment-for-covid-19-approved-for-use-in-the-uk
- 26.Cohen MS, Nirula A, Mulligan MJ, et al. Effect of bamlanivimab vs placebo on incidence of covid-19 among residents and staff of skilled nursing and assisted living facilities: a randomized clinical trial. JAMA. 2021;326:46–55. doi: 10.1001/jama.2021.8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.GOV.UK; UK's most vulnerable people to receive life-saving COVID-19 treatments in the community. 2021. https://www.gov.uk/government/news/uks-most-vulnerable-people-to-receive-life-saving-covid-19-treatments-in-the-community
- 28.Lewnard JA, Hong VX, Patel MM, Kahn R, Lipsitch M, Tartof SY. Clinical outcomes among patients infected with Omicron (B.1.1.529) SARS-CoV-2 variant in southern California. medRxiv. 2022 doi: 10.1101/2022.01.11.22269045. published online March 7. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ulloa AC, Buchan SA, Daneman N, Brown KA. Early estimates of SARS-CoV-2 Omicron variant severity based on a matched cohort study, Ontario, Canada. medRxiv. 2022 doi: 10.1101/2021.12.24.21268382. published online Jan 2. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L, Berger NA, Kaelber DC, Davis PB, Volkow ND, Xu R. Comparison of outcomes from COVID infection in pediatric and adult patients before and after the emergence of Omicron. medRxiv. 2022 doi: 10.1101/2021.12.30.21268495. published online Jan 2. (preprint). [DOI] [Google Scholar]
- 31.Krutikov M, Palmer T, Tut G, et al. Prevalence and duration of detectable SARS-CoV-2 nucleocapsid antibodies in staff and residents of long-term care facilities over the first year of the pandemic (VIVALDI study): prospective cohort study in England. Lancet Healthy Longev. 2022;3:e13–e21. doi: 10.1016/S2666-7568(21)00282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified test results and limited metadata will be made available for use by researchers in future studies, subject to appropriate research ethical approvals once the VIVALDI study cohort has been finalised. These datasets will be accessible via the Health Data Research UK Gateway (https://www.hdruk.ac.uk/).
This online publication has been corrected. The corrected version first appeared at thelancet.com/healthy-longevity on September 6, 2022