Abstract
The Port Delivery System with ranibizumab (PDS) is an innovative intraocular drug delivery system designed for the continuous delivery of ranibizumab into the vitreous for 6 months and beyond. The PDS includes an ocular implant, a customized formulation of ranibizumab, and four dedicated ancillary devices for initial fill, surgical implantation, refill-exchange, and explantation, if clinically indicated. Ranibizumab is an ideal candidate for the PDS on account of its unique physicochemical stability and high solubility. Controlled release is achieved via passive diffusion through the porous release control element, which is tuned to specific drug characteristics to accomplish a therapeutic level of ranibizumab in the vitreous. To characterize drug release from the implant, release rate was measured in vitro with starting concentrations of ranibizumab 10, 40, and 100 mg/mL, with release of ranibizumab 40 and 100 mg/mL found to remain quantifiable after 6 months. Using a starting concentration of 100 mg/mL, active release rate at approximately 6 months was consistent after the initial fill and first, second, and third refills, demonstrating reproducibility between implants and between multiple refill-exchanges of the same implant. A refill-exchange performed with a single 100-µL stroke using the refill needle was shown to replace over 95% of the implant contents with fresh drug. In vitro data support the use of the PDS with fixed refill-exchange intervals of at least 6 months in clinical trials.
Keywords: Ranibizumab, Port Delivery System, continuous drug delivery, nAMD, ocular implant, retina
Introduction
Neovascular age-related macular degeneration (nAMD) is a chronic, progressive retinal disease and a leading cause of irreversible vision loss in adults aged 50 years and older, despite widespread availability and use of anti-vascular endothelial growth factor (VEGF) therapy (Flaxman et al., 2017; Wykoff et al., 2018; Flaxel et al., 2020a). Diabetic retinopathy (DR) and associated diabetic macular edema (DME) are also major contributors to vision loss globally, with DR a principal cause of preventable blindness in working-age adults in the United States (Mohamed et al., 2007; Cheung et al., 2010; Flaxman et al., 2017). Anti-VEGF therapy is the current first-line standard of care for treating nAMD and DME, and an effective treatment option for proliferative DR (Flaxel et al., 2020a, 2020b). Anti-VEGF drugs currently approved and used in ophthalmology are ranibizumab, aflibercept, and brolucizumab (Lucentis [package insert], 2018; Eylea [package insert], 2019; Beovu [package insert], 2020). However, optimal vision outcomes are only achieved with frequent injections and monitoring (Campochiaro et al., 2019). This high frequency of treatment and monitoring places a considerable burden on patients, caregivers, and clinicians; regular clinic visits (as often as monthly) are a challenge to maintain (Prenner et al., 2015; Maguire et al., 2016) and a frequent source of anxiety for patients (Senra et al., 2017). In the real world, undertreatment results in visual outcomes that fall short of clinical trial results (Maguire et al., 2016; Ciulla et al., 2020). There is significant unmet need for consistent treatment to the retina to achieve and maintain vision gains while reducing treatment frequency (Campochiaro et al., 2019).
The requirement for frequent injections of anti-VEGF therapy reflects the short intraocular half-life of these biologic agents, and the development of long-acting ocular drug delivery strategies has proved difficult (reviewed in Kim and Woo (2021)). Despite advances in the field, only a few technologies have been assessed in clinical trials, and only one, the Port Delivery System with ranibizumab (PDS; commercially available as Susvimo, Genentech, Inc., South San Francisco, CA), has progressed to late-stage investigation and subsequent approval from the US Food and Drug Administration (FDA; Kim and Woo, 2021; Clinicaltrials.gov, 2021b; Holekamp et al., 2022; Susvimo [package insert], 2021).
The PDS is an innovative drug delivery technology that enables continuous delivery of a customized formulation of ranibizumab into the vitreous over extended periods of time. The PDS is the first permanent, refillable, ocular implant for the treatment of chronic retinal disease, recently FDA-approved for the treatment of nAMD in adults who have previously responded to at least two anti-VEGF injections. By reducing the need for monthly injections, and through controlled and sustained intravitreal VEGF suppression, it has the potential to reduce the treatment and monitoring burden in patients with retinovascular degeneration and retinovascular diseases and reduce the negative impact of undertreatment in clinical practice (Campochiaro et al., 2019; Khanani et al., 2020, 2021; Holekamp et al., 2022).
A phase 1 study (NCT01186432; Clinicaltrials.gov, 2021e) conducted with a PDS prototype filled with ranibizumab 10 mg/mL (commercially available as Lucentis, Genentech, Inc., South San Francisco, CA) in patients with nAMD demonstrated vision and implant functionality outcomes supportive of further investigation (Loewenstein et al., 2020). The phase 2 Ladder trial (NCT02510794; Clinicaltrials.gov, 2021g) compared the PDS implant filled with 1 of 3 ranibizumab dose concentrations (10, 40, 100 mg/mL) with pro re nata refills vs monthly intravitreal ranibizumab 0.5 mg injections in patients with nAMD. Visual and anatomic outcomes in the PDS 100 mg/mL arm were comparable with monthly intravitreal ranibizumab 0.5 mg injections and approximately 80% of patients went at least 6 months without meeting refill criteria (Campochiaro et al., 2019; Khanani et al., 2020). Archway (NCT03677934) was a phase 3 randomized open-label trial of the PDS in patients with nAMD (Clinicaltrials.gov, 2021b). Archway met its primary endpoint, demonstrating that PDS 100 mg/mL with fixed refill-exchanges every 6 months was both clinically equivalent and noninferior to monthly intravitreal ranibizumab 0.5 mg injections in terms of vision at the average of weeks 36 and 40 (Holekamp et al., 2022).
This manuscript provides an overview of the development of the Port Delivery Platform (PD-P). It describes findings from in vitro studies performed before the phase 2 and phase 3 clinical trials of the PDS; characterization of ranibizumab release from the PDS implant, reproducibility of ranibizumab release after refill-exchange, and refill-exchange efficiency of the PDS implant and refill needle.
Materials and methods
Experimental setup
Implants were filled with ranibizumab 10, 40, or 100 mg/mL before being individually suspended on custom-designed hangers and placed in 1.5-mL polypropylene tubes containing 300 µL of phosphate buffered saline (PBS). PBS was used to model vitreous humor because it approximately matches the expected pH (7.0–7.4; Sobolewska et al., 2017) and osmolality (286.6–292.5 mOsmol/kg; Kokavec et al., 2016); it also contained 0.01% (w/v) polysorbate 20 to reduce protein loss due to adsorption and 0.02% (w/v) sodium azide to avoid bacterial contamination during the drug release study. Experimental setup is shown in Figure 1.
Figure 1.
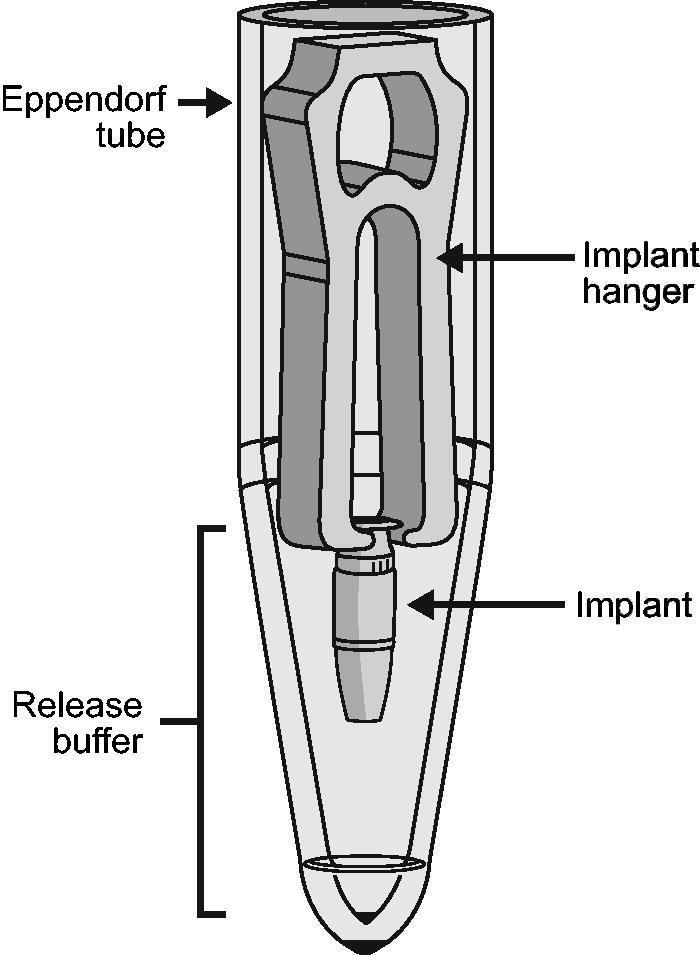
Schematic of experimental setup for in vitro evaluation of ranibizumab release from the PDS implant. To enable in vitro evaluation of drug release, PDS implants were filled with ranibizumab solution and individually suspended on custom-designed hangers, placed in 1.5-mL polypropylene tubes containing 300 µL of release buffer. Implants were transferred to new polypropylene tubes with fresh buffer at regular intervals to maintain sink conditions for ranibizumab release. Abbreviation: PDS: Port Delivery System with ranibizumab. Yohe S, Maass KF, Horvath J, Rea J, Barteselli G, Ranade SV. J Control Release. 2022;345:101–107. Licensed under CC-BY-NC-ND 4.0 (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Implants were incubated at physiological temperature (34 °C; Landers et al., 2012) during drug release. The implants were transferred to new polypropylene tubes with fresh buffer weekly during the first month, and every other week thereafter, to represent ocular ranibizumab clearance and sustain release kinetics similar to that expected in vivo. Notably, ranibizumab release from the implant is slow compared with ocular and systemic elimination (half-lives are ≥100 days, ∼7 days, and ∼2 h in the implant, vitreous, and serum, respectively; Yohe et al., 2022). Due to drug clearance, there is no drug accumulation in the vitreous; thus, drug release from the PDS will proceed under sink conditions. Experiments were designed to simulate rather than duplicate in vivo conditions, with sink conditions maintained to avoid any impact of release volume on release rates. Using 300 µL of PBS resulted in drug concentrations lower than 500 µg/mL in the release buffer, corresponding to at least a 150-fold lower drug concentration in the buffer than in the implant. The collected release buffer was kept at 2–8 °C until sample analysis.
Measurement of ranibizumab release and activity
Ranibizumab concentrations in release buffer were measured using the Coomassie-Plus (Bradford) Assay kit (Thermo Fisher Scientific Inc., Waltham, MA) using automated sample preparation. Drug concentration and volume were used to calculate a mean daily ranibizumab release rate at each timepoint. The VEGF-binding activity (potency) of ranibizumab released from implants was measured using a Surface Plasmon Resonance assay (Biacore, Cytiva, Marlborough, MA), with results used to determine percentage and release rate of active ranibizumab. Release rate data were fit using an exponential model to mimic the expected first-order release kinetics of diffusion and were compared with predicted release rate kinetics.
Determining the efficiency of refill-exchange
The PDS implant was first filled with PBS buffer using an initial fill needle, then refilled using a primed refill needle attached to a 1-mL syringe containing ranibizumab drug product. To assess efficiency of refill-exchange across a range of refill volumes, 20, 40, 60, 80, or 100 µL of ranibizumab 100 mg/mL was delivered accurately by a high-precision syringe pump (Pump 11 Elite Programmable Syringe Pump, Harvard Apparatus, Holliston, MA). After refill, the implant content was collected using an empty 3/10-mL insulin syringe with a 29-gauge (G) needle inserted into the implant septum. The collected sample was analyzed for ranibizumab content using ultraviolet-visible spectrophotometry (UV-VIS spectrophotometer, Model 8453, Agilent Technologies, Santa Clara, CA). Refill efficiency was calculated as the ratio of the ranibizumab concentration measured in the implant after refill vs the concentration of the fill solution.
The PDS implant and ancillary devices
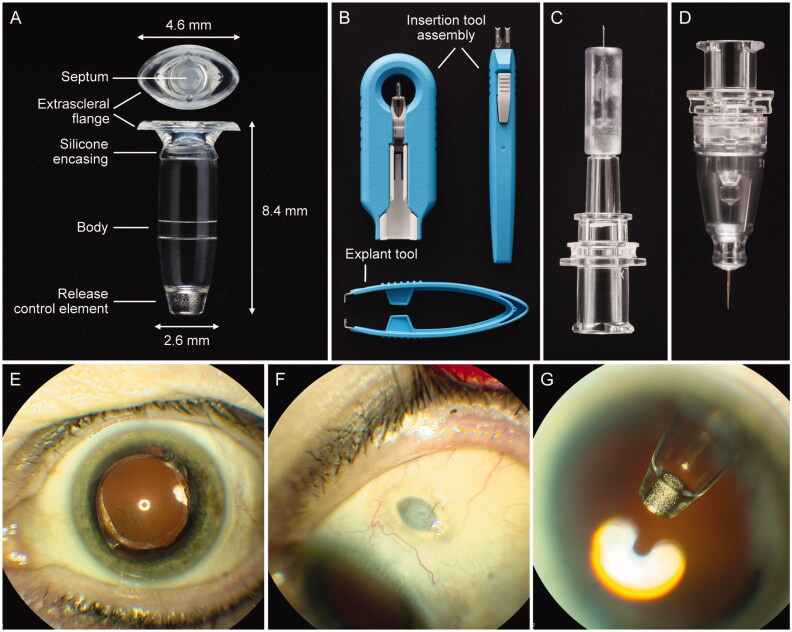
The PDS includes a customized formulation of ranibizumab, an ocular implant, and four dedicated ancillary devices that are used for the initial fill with ranibizumab, surgical implantation, refill-exchange, and if clinically necessary, explantation (Figure 2(A–D)).
Figure 2.
The PDS ocular implant, ancillary devices, and position in situ. (A) PDS implant showing four key features: an extrascleral flange that anchors the implant in the sclera, a self-sealing septum that allows for implant refills, an implant body that contains the drug reservoir for the customized formulation of ranibizumab, and the porous titanium RCE that controls the rate of ranibizumab diffusion into the vitreous. (B) Insertion tool assembly to facilitate handling of the implant during the initial filling and insertion procedures, and explant tool (lower image) to grasp and securely hold the implant flange during the explant procedure (if explantation is clinically indicated). (C) Initial fill needle to fill the implant with ranibizumab before insertion. (D) Refill needle to refill-exchange, in situ, the implant with ranibizumab. (E–G) show the PDS implant in situ. Images from a PDS-implanted patient showing (E) eye in primary position (implant not visible), (F) eye looking down with the PDS implant septum visible, and (G) eye looking up with implant visible through dilated pupil. Copyright 2021 F. Hoffmann-La Roche Ltd., all rights reserved. Used with permission. Abbreviations: PDS: Port Delivery System with ranibizumab; RCE: release control element.
The PDS implant (Figure 2(A)) has four key features: an extrascleral flange that anchors the implant in the sclera, a self-sealing septum that allows for implant refills, a hollow implant body that forms the drug reservoir for the customized ranibizumab formulation, and the porous titanium release control element (RCE) that controls the rate of ranibizumab diffusion into the vitreous. The implant has a capacity of approximately 20 µL and is transparent to allow visualization of ranibizumab solution within the reservoir during initial fill. The septum is made of a self-sealing silicone, and is bonded to the implant body. The PDS implant is surgically placed at the pars plana and is intentionally designed to be biocompatible, permanent, and durable. The implant and extracts from the implant and ancillary devices have been determined to be nongenotoxic, noncytotoxic, nonsensitizing, and nonirritating (Bantseev et al., 2021). The drug reservoir and RCE reside within the vitreous, whereas the septum remains accessible under the conjunctiva and Tenon’s capsule for minimally invasive in-clinic refill-exchanges. The implant has been designed to be noninterfering with the patient’s field of vision and is covered by the eyelid; thus, it is not visible with the eye in primary gaze (Figure 2(E–G)).
The insertion tool assembly (Figure (2B)) has a carrier design engineered to protect the implant during storage and handling, allowing simple, error-free, and ergonomic filling. The removable guide sleeve enables the implant to be filled with ranibizumab using the initial fill needle before insertion and, further, allows the attachment of the insertion tool handle to gripper tips to facilitate handling of the implant during the implant insertion procedure. The initial fill needle (Figure 2(C)) has a thin gauge (34 G), with a custom length that precludes contact with the RCE while filling. The custom-designed refill needle (Figure 2(D)) allows for implant contents to be extracted simultaneously as the implant is filled with fresh ranibizumab during an in-office refill-exchange procedure (eVideo in the Supplement). The refill needle is a 34 G double cannula, with a vented, custom-length needle and fluid collection reservoir. The explant tool (Figure 2(B); lower image) is used to remove the implant in the rare situation when explantation is clinically indicated. The tips of the explant tool are designed specifically to grasp the implant neck and flange securely without causing damage to the implant during removal.
The PDS implant and ancillary devices have evolved throughout the course of clinical development to optimize their performance and safety, with improvements made to both the materials and manufacturing procedures. Table 1 lists design changes that have been incorporated into the PDS commercial devices and procedures.
Table 1.
Evolution of the PDS implant and ancillary devices during clinical development.
| Device | Changes | Purpose/Rationale |
|---|---|---|
| Implant |
|
|
| Insertion tool |
|
|
| Refill needle |
|
|
| Explant tool |
|
|
Abbreviations: MRI: magnetic resonance imaging; PDS: Port Delivery System with ranibizumab; PD: pharmacodynamic; PK: pharmacokinetic; RCE: release control element.
Drug compatibility with the Port Delivery System: suitability of ranibizumab
Specific drug characteristics are required for compatibility with the Port Delivery System implant and ancillary devices. The biologic drug must remain relatively stable within the implant to maintain potency and clinical safety between refills; isotonicity and high protein concentration are also critical. Ranibizumab is an ideal candidate for the Port Delivery System on account of its unique physicochemical stability in the vitreous, and its photostability and thermal stability in the implant under physiological conditions (34 °C), allowing it to stay in the implant for many months while remaining safe and effective (Chang et al., 2021; Rajagopal et al., 2021). Ranibizumab is highly water soluble and stable, and high-concentration solutions (up to 150 mg/mL) can be prepared with moderate to low viscosity for ease of implant filling and refilling.
Controlled release of ranibizumab is achieved via passive diffusion through the porous RCE. The drug diffusion follows Fick’s law, wherein ranibizumab molecules move from an area of high concentration in the implant to low concentration in the vitreous cavity along a concentration gradient. The rate of diffusion is dependent on the ranibizumab concentration in the implant, which decreases over time. Diffusion, and thus drug release rate, is also controlled by the implant volume and RCE characteristics such as porosity and dimensions. The implant and ranibizumab formulation are designed to maintain drug concentration within therapeutic levels for the entire treatment cycle, with the RCE specifically tailored to the molecular weight and concentration of ranibizumab. The RCE must be tuned to specific drug characteristics, such as size, stability, and clearance, to accomplish a therapeutic level of drug in the vitreous; this is achieved via changes to the RCE porosity, tortuosity, and dimensions.
In vitro characterization of ranibizumab release and PDS refill-exchange
In vitro studies were performed to investigate the kinetics of ranibizumab release from the PDS implant, the reproducibility of ranibizumab release after refill-exchange, and the refill-exchange efficiency of the PDS implant and the refill needle. The aim of these studies was to provide further data to support the PDS implant as a feasible and effective means of administering ranibizumab intravitreally across multiple refill-exchanges.
Ranibizumab release from the PDS implant at different starting concentrations
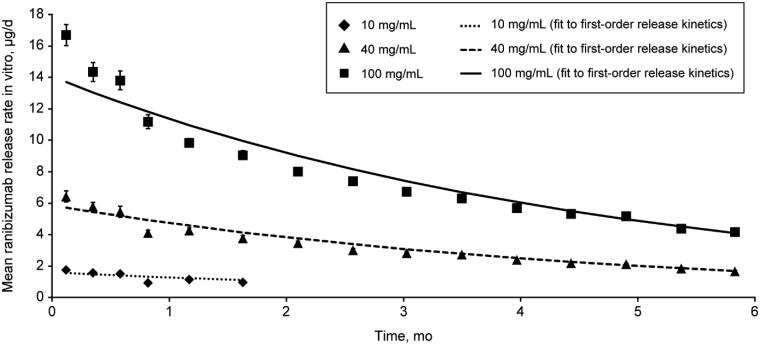
The effect of initial ranibizumab concentration in the PDS implant was evaluated at 10, 40, and 100 mg/mL, showing increased starting mean (SD) drug release rates from 1.75 (0.07) to 16.69 (0.67) µg/day (Figure 3). A starting concentration of 10 mg/mL resulted in ranibizumab concentrations below the lower limit of quantification within 2 months of filling. Starting concentrations of 40 and 100 mg/mL resulted in quantifiable drug release for longer than 6 months, with mean (SD) release rates at 6 months of 1.68 (0.05) and 4.16 (0.05) µg/day, respectively. These results demonstrate that drug release from the PDS implant is tunable by means of the starting concentration of the drug product. Ultimately, the phase 2 Ladder trial determined the ranibizumab concentration (100 mg/mL) and duration (time between refills) for subsequent clinical trials (Campochiaro et al., 2019; Khanani et al., 2020).
Figure 3.
Mean ranibizumab release rate in vitro with the PDS 10, 40, or 100 mg/mL. Graph shows mean ranibizumab release rates in vitro in µg/day over a period of 6 months, from PDS implants filled with ranibizumab at 10, 40, or 100 mg/mL. Dashed lines show theoretical drug release curves, calculated based on first-order drug release kinetics. Error bars show SD. Abbreviation: PDS, Port Delivery System with ranibizumab.
Active release rate after refill-exchange
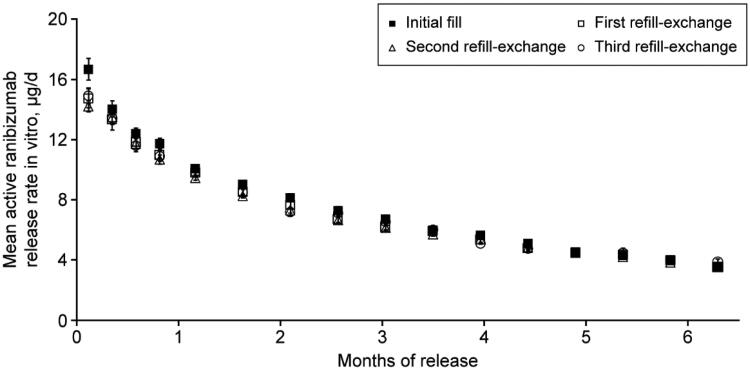
The active release rate of ranibizumab 100 mg/mL in vitro was shown to be consistent across treatment cycles (Figure 4). The mean (SD) active release rate at 6 months was 3.95 (0.17), 3.99 (0.13), 3.85 (0.15), and 4.00 (0.17) µg/day after initial fill, first, second, and third refill-exchanges, respectively, demonstrating reproducibility from implant to implant and between multiple refill-exchanges of the same implant.
Figure 4.
Mean ranibizumab release rate in vitro from the PDS 100 mg/mL after refill-exchange. Graph shows the mean active ranibizumab release rate in vitro in µg/day from the PDS 100 mg/mL over a period of 6 months, after the initial fill, first, second, and third refill-exchange. Error bars show SD. Abbreviation: PDS: Port Delivery System with ranibizumab.
Efficiency of refill-exchange
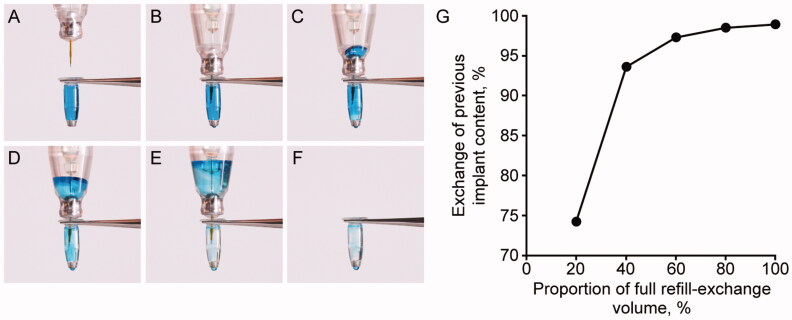
During the refill-exchange procedure (eVideo in the Supplement), previous implant contents were exchanged for fresh ranibizumab in a single injection stroke, with no significant bolus through the RCE during the procedure (Figure 5(A–F)). A single 100-µL refill-exchange procedure removed over 95% of the implant contents and replaced it with fresh drug (Figure 5(G)).
Figure 5.
Refill-exchange of the PDS implant using the refill needle: procedure and efficiency. Images (A) to (F) show the sequential refill-exchange of the PDS implant using the refill needle, using a colored blue liquid as a proxy for previous implant contents. (G) shows the exchange of previous implant content when using 20–100% of the full refill-exchange volume. Copyright 2021 F. Hoffmann-La Roche Ltd., all rights reserved. Used with permission. Abbreviation: PDS: Port Delivery System with ranibizumab.
Discussion
Achieving and maintaining a therapeutic level of drug, whether biologic or small molecule, with long-term intraocular stability remains the biggest challenge in ocular drug delivery. Several strategies have been explored to enhance delivery efficiency by sustaining drug release, improving biocompatibility, and reducing potential for drug degradation, including molecular conjugation and formulation-based approaches (Kim and Woo, 2021).
A number of sustained release intravitreal steroid and small-molecule implants have been developed and approved for use in ocular disease indications, including Ozurdex (Allergan USA Inc., Madison, NJ; dexamethasone; biodegradable) for DME, retinal vein occlusion, and noninfectious uveitis; Iluvien (Alimera Sciences, Alpharetta, GA, USA) and Retisert (Bausch + Lomb, Bridgewater, NJ)/Yutiq (EyePoint Pharmaceuticals US Inc., Watertown, MA; fluocinolone acetonide; nonbiodegradable) for DME and chronic noninfectious uveitis, respectively; and Vitrasert (Bausch + Lomb; ganciclovir; nonbiodegradable) for cytomegalovirus retinitis (Edelhauser et al., 2010; Iluvien [package insert], 2016; Ozurdex [package insert], 2020; Kim and Woo, 2021; US EP, 2021; Retisert [package insert], 2021). For sustained anti-VEGF therapy, implant-based systems tested in humans include the Ophthalmic MicroPump System (Replenish, Inc., Pasadena, CA; Humayun et al., 2014) and the encapsulated cell technology implant NT-503 (Neurotech Pharmaceuticals Inc., Cumberland, RI; Clinicaltrials.gov, 2021h), both of which did not progress beyond phase 1 clinical trials (Kiss, 2017; Seah et al., 2020).
The PDS is the first ocular implant to effectively deliver a biologic agent to the retina long term and offers the advantages of being permanent and refillable in situ. To date, the PDS has demonstrated longevity, with the first patients enrolled in the Ladder trial now successfully retaining the implant since 2015, with no evidence of changing drug release characteristics of the RCE (Campochiaro et al., 2019; Khanani et al., 2020). Patient preference was evaluated during the Archway trial, with 93.2% of patients preferring ranibizumab as delivered via the PDS implant compared with intravitreal injection (Holekamp et al., 2022).
The in vitro analyses described here show that ranibizumab release from the PDS implant continues over 6 months and beyond, with a gradually decreasing rate over time, and that release rate can be tuned based on the starting concentration of the drug, with higher concentrations increasing the rate of release. Ranibizumab release rates are shown to be predictable and consistent after multiple refill-exchanges, and further, the refill-exchange procedure was found to be highly efficient, with successful replacement of over 95% of the implant contents.
Both the in vitro findings described here, and in vivo data from clinical trials, demonstrate that ranibizumab release from the PDS is continuous and reproducible across multiple refill-exchanges. In the Ladder trial, PDS 40 mg/mL and 100 mg/mL resulted in serum ranibizumab levels above the trough concentration observed with monthly injections at month 9 (Campochiaro et al., 2020). Further, pharmacokinetic data from the Archway trial show that serum ranibizumab levels in PDS 100 mg/mL patients are maintained within the range experienced with monthly injections over the 6-month refill-exchange interval. PDS 100 mg/mL also maintained aqueous humor ranibizumab levels above the trough concentration of monthly injections (Campochiaro et al., 2021). In vitro ranibizumab release rate and ranibizumab vitreal elimination rate can be used to predict vitreous ranibizumab concentration over time with the PDS (Yohe et al., 2022). Using this model, PDS 100 mg/mL would result in vitreous ranibizumab levels that remain within the levels expected with monthly intravitreal ranibizumab injections. Together, these results support the clinical efficacy of the PDS in Archway, with continuous ranibizumab delivery from the PDS 100 mg/mL every 24 weeks shown to provide equivalent and noninferior visual acuity outcomes compared with monthly injections (Holekamp et al., 2022).
Several large-scale clinical trials are currently investigating the PDS with ranibizumab 100 mg/mL. The phase 3 b Velodrome trial (NCT04657289; Clinicaltrials.gov., 2021c) is evaluating the efficacy, safety, and pharmacokinetics of the PDS with refill-exchange every 9 months compared with every 6 months in patients with nAMD, with the extension study Portal (NCT03683251; Clinicaltrials.gov., 2021d) evaluating the long-term safety and tolerability of the PDS in patients from Ladder, Archway, and Velodrome. Beyond nAMD, the phase 3 Pavilion trial (NCT04503551) is investigating the efficacy, safety, and pharmacokinetics of the PDS in patients with DR without DME (Clinicaltrials.gov., 2021a), and the phase 3 Pagoda trial (NCT04108156) is evaluating the PDS compared with monthly intravitreal ranibizumab injections in patients with DME (Clinicaltrials.gov., 2021i). These studies will include measurements of ranibizumab concentration in serum (and aqueous humor in Portal, Pagoda, and Pavilion) to further understand the relationship between ranibizumab concentration and efficacy. Data collection is still ongoing, but it is anticipated that these data will mirror the in vivo and in vitro findings from the Archway trial, with PDS 100 mg/mL found to continuously release ranibizumab over the 24-week refill interval, delivering drug concentrations within the range experienced with monthly ranibizumab 0.5 mg injections (Campochiaro et al., 2021).
Despite its advantages, the PDS may not be suitable for all patients, and patients and clinicians will make the informed choice between the PDS and intravitreal injections based on a number of variables. Patients must have demonstrated response to anti-VEGF therapies and be suitable candidates for surgery; factors such as ocular allergies, scleral disorders, and thin conjunctiva may mean that PDS implantation is not recommended. Importantly, ocular surgery carries some potential risks. For example, the FDA has issued a boxed warning for the PDS due to association with a 3-fold higher rate of endophthalmitis compared with monthly intravitreal injections of ranibizumab (Susvimo [package insert], 2021). However, any ocular adverse events related to the PDS procedures are well understood, manageable, and are continually being analyzed to optimize patient outcomes (Holekamp et al., 2022). PDS surgical procedures have evolved based on trial learnings (Campochiaro et al., 2019; Bantseev et al., 2020), with an emphasis on surgeon training to standardize surgical techniques, supported by a state-of-the-art virtual reality training platform (Heimann et al., 2021).
The PD-P technology offers the potential to use other drugs, which should be carefully evaluated to ensure that they are suitable for continuous delivery (e.g. with adequate physicochemical characteristics) and are compatible with the implant and its RCE. A comparison of ranibizumab, bevacizumab, and aflibercept in PBS showed that although ranibizumab demonstrated long-term stability, bevacizumab and aflibercept were increasingly prone to aggregation over time, with loss of potency, suggesting that they are unlikely to be compatible with the PD-P (Chang et al., 2021; Rajagopal et al., 2021). In addition to stability and potency of a drug candidate, diffusion properties and solubility also play critical roles in achieving efficacious drug delivery rates from the PD-P. An ongoing phase 1 trial (NCT04567303) is currently investigating the safety, tolerability, and pharmacokinetics of RO7250284, a bispecific human antigen-binding antibody fragment that inhibits both angiopoetin-2 and VEGF, after intravitreal administration and via continuous delivery from the PD-P in patients with nAMD (Kirkner, 2021; Clinicaltrials.gov., 2021f). Further, there is the potential to alter the design of the RCE to enable the use of other drugs with the PD-P in the future, which may lead to novel options for the management of various ocular diseases.
Conclusions
The PDS is an innovative ocular drug delivery system, engineered to continuously and reproducibly deliver ranibizumab to the retina over an unprecedented period of at least 6 months; and is the first long-acting ocular biologic drug delivery system with demonstrated clinical safety and efficacy. Drug delivery of ranibizumab from the PDS implant is highly reproducible during in vitro studies, and has been demonstrated to be clinically effective in providing visual and anatomic outcomes comparable to monthly injections in nAMD with refill-exchange intervals of at least 6 months (Holekamp et al., 2022). These findings support the potential for the PDS to reduce the overall treatment and monitoring burden in clinical practice, with PDS regimens currently under investigation for patients with nAMD, DME, and DR. The Port Delivery System is a recently FDA-approved technology that delivers a biologic therapeutic to the eye over an extended period of time, with the potential to fundamentally change treatment paradigms in retinal diseases in the future.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Josh Horvath for discussions and help with aspects of device development.
Funding Statement
Genentech, Inc., a member of the Roche Group, provided financial support for the study and participated in the study design; conducting the study; data collection, management, analysis, and interpretation; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication. Third-party writing assistance was provided by Dionne Turnbull, PhD, of Envision Pharma Group, funded by Genentech Inc., a member of the Roche Group.
Author contributions
SVR had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Ranade, Tam, Rea, Horvath, Hieb.
Acquisition, analysis, or interpretation of data: Ranade, Wieland, Tam, Rea, Horvath, Hieb, Jia, Barteselli, Stewart.
Drafting and critical revision of the manuscript for important intellectual content: Ranade, Wieland, Tam, Rea, Horvath, Hieb, Jia, Grace, Barteselli, Stewart.
Supervision: Ranade
Meeting presentation
Parts of these data were presented at the Association for Research in Vision and Ophthalmology Virtual Meeting, May 1–7, 2021; the Controlled Release Society Virtual Annual Meeting, July 25–29, 2021; the American Association of Pharmaceutical Scientists, PharmSci 360, Oct 17–20, 2021; and the American Academy of Ophthalmology Annual Meeting, Nov 12–15, 2021.
Disclosure statement
Dr Ranade, Ms Tam, Dr Rea, Dr Horvath, Dr Hieb, Dr Jia, Ms Grace, and Dr Barteselli reported being employees of Genentech, Inc. Dr Wieland reported financial support from and being a consultant for Genentech, Inc.; and performing research for Genentech, Inc., Kodiak, Norlase, and Regeneron. Dr Stewart reported being a consultant for Genentech, Inc., Merck, Surrozen, and Valitor; and a personal financial interest in Roche.
References
- Bantseev V, Horvath J, Barteselli G, et al. (2021). Nonclinical toxicology and biocompatibility program supporting clinical development and registration of the Port Delivery System with ranibizumab for neovascular age-related macular degeneration. Toxicol Pathol 49:663–72. [DOI] [PubMed] [Google Scholar]
- Bantseev V, Schuetz C, Booler HS, et al. (2020). Evaluation of surgical factors affecting vitreous hemorrhage following Port Delivery System with ranibizumab implant insertion in a minipig model. Retina 40:1520–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beovu [package insert]. ( 2020). East Hanover, NJ: Novartis Pharmaceuticals Corporation. https://www.novartis.us/sites/www.novartis.us/files/beovu.pdf.
- Campochiaro PA, Gune S, Maia M, et al. (2021). Pharmacokinetic profile of the Port Delivery System with ranibizumab (PDS) in the phase 3 Archway trial. Invest Ophthalmol Visual Sci 62:350. [Google Scholar]
- Campochiaro PA, Gune S, Maia M, Maass K. (2020). Pharmacokinetic (PK) profile of the Port Delivery System with ranibizumab (PDS) in the phase 2 ladder trial. Invest Ophthalmol Visual Sci 61:1157. [Google Scholar]
- Campochiaro PA, Marcus DM, Awh CC, et al. (2019). The Port Delivery System with ranibizumab for neovascular age-related macular degeneration: results from the randomized phase 2 ladder clinical trial. Ophthalmology 126:1141–54. [DOI] [PubMed] [Google Scholar]
- Chang DP, Burra S, Day ES, et al. (2021). Long-term stability of anti-vascular endothelial growth factor (a-VEGF) biologics under physiologically relevant conditions and its impact on the development of long-acting delivery systems. J Pharm Sci 110:860–70. [DOI] [PubMed] [Google Scholar]
- Cheung N, Mitchell P, Wong TY. (2010). Diabetic retinopathy. Lancet 376:124–36. [DOI] [PubMed] [Google Scholar]
- Ciulla TA, Hussain RM, Pollack JS, Williams DF. (2020). Visual acuity outcomes and anti-vascular endothelial growth factor therapy intensity in neovascular age-related macular degeneration patients: a real-world analysis of 49 485 eyes. Ophthalmol Retina 4:19–30. [DOI] [PubMed] [Google Scholar]
- Clinicaltrials.gov . (2021a). A multicenter, randomized study in participants with diabetic retinopathy without center-involved diabetic macular edema to evaluate the efficacy, safety, and pharmacokinetics of ranibizumab delivered via the Port Delivery System relative to the comparator arm (PAVILION). Available at: https://clinicaltrials.gov/ct2/show/NCT04503551 [last accessed 12 July 2021].
- Clinicaltrials.gov . (2021b). A phase III study to evaluate the Port Delivery System with ranibizumab compared with monthly ranibizumab injections in participants with wet age-related macular degeneration (Archway). Available at: https://clinicaltrials.gov/ct2/show/NCT03677934 [last accessed 25 Oct 2021].
- Clinicaltrials.gov . (2021c). A study of the efficacy, safety, and pharmacokinetics of a 36-week refill regimen for the Port Delivery System with ranibizumab in patients with neovascular age-related macular degeneration (Velodrome). Available at: https://www.clinicaltrials.gov/ct2/show/NCT04657289. [last accessed 12 July 2021.]
- Clinicaltrials.gov . (2021d). Extension study for the Port Delivery System with ranibizumab (Portal). https://clinicaltrials.gov/ct2/show/NCT03683251 [last accessed 12 July 2021].
- Clinicaltrials.gov . (2021e). Preliminary safety and efficacy of the PDS-1.0 in patients with neovascular age related macular degeneration (AMD) (NCT01186432).
- Clinicaltrials.gov . (2021f). Study of RO7250284 in participants with neovascular age-related macular degeneration. Available at: https://clinicaltrials.gov/ct2/show/NCT04567303 [last accessed 12 July 2021].
- Clinicaltrials.gov . (2021g). Study of the efficacy and safety of the ranibizumab port delivery system for sustained delivery of ranibizumab in patients with subfoveal neovascular age-related macular degeneration (LADDER). Available at: https://clinicaltrials.gov/ct2/show/NCT02510794 [last accessed 25 Oct 2021].
- Clinicaltrials.gov . (2021h). Study of the intravitreal implantation of NT-503-3 encapsulated cell technology (ECT) for the treatment of recurrent choroidal neovascularization (CNV) secondary to age-related macular degeneration (AMD). Available at: https://clinicaltrials.gov/ct2/show/NCT02228304 [last accessed 19 July 2021].
- Clinicaltrials.gov . (2021i). This study will evaluate the efficacy, safety, and pharmacokinetics of the port delivery system with ranibizumab in participants with diabetic macular edema compared with intravitreal ranibizumab (Pagoda). Available at: https://clinicaltrials.gov/ct2/show/NCT04108156 [last accessed 12 July 2021].
- Edelhauser HF, Rowe-Rendleman CL, Robinson MR, et al. (2010). Ophthalmic drug delivery systems for the treatment of retinal diseases: basic research to clinical applications. Invest Ophthalmol Vis Sci 51:5403–5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eylea [package insert]. ( 2019). Tarrytown, NY: Regeneron Pharmaceuticals, Inc. https://www.regeneron.com/downloads/eylea_fpi.pdf.
- Flaxel CJ, Adelman RA, Bailey ST, et al. (2020a). Age-related macular degeneration preferred practice pattern®. Ophthalmology 127:P1–P65. [DOI] [PubMed] [Google Scholar]
- Flaxel CJ, Adelman RA, Bailey ST, et al. (2020b). Diabetic retinopathy preferred practice pattern®. Ophthalmology 127:P66–P145. [DOI] [PubMed] [Google Scholar]
- Flaxman SR, Bourne RRA, Resnikoff S, et al. (2017). Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health 5:e1221–34. [DOI] [PubMed] [Google Scholar]
- Heimann F, Barteselli G, Brand A, et al. (2021). A custom virtual reality training solution for ophthalmologic surgical clinical trials. Adv Simul 6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holekamp NM, Campochiaro PA, Chang M, et al. (2022). Archway randomized phase 3 trial of the Port Delivery System with ranibizumab for neovascular age-related macular degeneration. Ophthalmology 129:295–307. [DOI] [PubMed] [Google Scholar]
- Humayun M, Santos A, Altamirano JC, et al. (2014). Implantable micropump for drug delivery in patients with diabetic macular edema. Transl Vis Sci Technol 3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iluvien [package insert]. ( 2016). Alpharetta, GA: Alimera Sciences. https://iluvien.com/wp-content/uploads/2015/03/Prescribing-Information.pdf.
- Khanani AM, Aziz AA, Weng CY, et al. (2021). Port Delivery System: a novel drug delivery platform to treat retinal diseases. Expert Opin Drug Deliv 18:1571–6. [DOI] [PubMed] [Google Scholar]
- Khanani AM, Callanan D, Dreyer R, et al. (2021). End-of-study results for the ladder phase 2 trial of the Port Delivery System with ranibizumab for neovascular age-related macular degeneration. Ophthalmol Retina. 5:775–87. [DOI] [PubMed] [Google Scholar]
- Kim HM, Woo SJ. (2021). Ocular drug delivery to the retina: current innovations and future perspectives. Pharmaceutics 13:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkner RM. (2021). Many new entries, but no impactful exits. Fourth Annual Pipeline Report. Available at: https://www.retina-specialist.com/article/many-new-entries-but-no-impactful-exits [last Accessed 12 July 2021].
- Kiss S. (2017). Failed cell therapy study offers positives, raises new questions. Modern Retina. Published online 24 March 2017. [Google Scholar]
- Kokavec J, Min SH, Tan MH, et al. (2016). Biochemical analysis of the living human vitreous. Clin Exp Ophthalmol 44:597–609. [DOI] [PubMed] [Google Scholar]
- Landers MB, 3rd, Watson JS, Ulrich JN, et al. (2012). Determination of retinal and vitreous temperature in vitrectomy. Retina 32:172–6. [DOI] [PubMed] [Google Scholar]
- Loewenstein A, Laganovska G, Bressler NM, et al. (2020). Phase 1 clinical study of the Port Delivery System with ranibizumab for continuous treatment of neovascular age-related macular degeneration. Invest Ophthalmol Visual Sci 61:4201. [Google Scholar]
- Lucentis [package insert]. ( 2018). South San Francisco, CA: Genentech, Inc. https://www.gene.com/download/pdf/lucentis_prescribing.pdf.
- Maguire MG, Martin DF, Ying GS, et al. (2016). Five-year outcomes with anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration: the comparison of age-related macular degeneration treatments trials. Ophthalmology 123:1751–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed Q, Gillies MC, Wong TY. (2007). Management of diabetic retinopathy: a systematic review. JAMA 298:902–16. [DOI] [PubMed] [Google Scholar]
- Ozurdex [package insert]. ( 2020). Madison, NJ: Allergan USA Inc. https://www.rxabbvie.com/pdf/ozurdex_pi.pdf.
- Prenner JL, Halperin LS, Rycroft C, et al. (2015). Disease burden in the treatment of age-related macular degeneration: findings from a time-and-motion study. Am J Ophthalmol 160:725–31.e1. [DOI] [PubMed] [Google Scholar]
- Rajagopal K, Yen C, Ranade S, et al. (2021). Sustained delivery of ocular protein therapeutics. In: Srivastava V, ed. Implantable technologies: peptides and small molecules drug delivery. UK: Royal Society of Chemistry. [Google Scholar]
- Retisert [package insert]. ( 2021). Bridgewater, NJ: Bausch + Lomb. https://pi.bausch.com/globalassets/pdf/PackageInserts/Pharma/retisert-prescribing-information.pdf?ver=2018-04-23-125740-133.
- Seah I, Zhao X, Lin Q, et al. (2020). Use of biomaterials for sustained delivery of anti-VEGF to treat retinal diseases. Eye 34:1341–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senra H, Balaskas K, Mahmoodi N, Aslam T. (2017). Experience of anti-VEGF treatment and clinical levels of depression and anxiety in patients with wet age-related macular degeneration. Am J Ophthalmol 177:213–24. [DOI] [PubMed] [Google Scholar]
- Sobolewska B, Heiduschka P, Bartz-Schmidt K-U, Ziemssen F. (2017). pH of anti-VEGF agents in the human vitreous: low impact of very different formulations. Int J Retina Vitreous 3:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susvimo [package insert]. ( 2021). South San Francisco, CA: Genentech, Inc. https://www.gene.com/download/pdf/susvimo_prescribing.pdf.
- Yutiq [package insert] . (2021). Watertown, MA: EyePoint Pharmaceuticals US, Inc. https://yutiq.com/downloads/US-YUT-2100035%20YUTIQ%20Prescribing%20Information-2021.pdf.
- Wykoff CC, Clark WL, Nielsen JS, Brill JV, et al. (2018). Optimizing anti-VEGF treatment outcomes for patients with neovascular age-related macular degeneration. J Manag Care Spec Pharm 24:S3–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohe S, Maass KF, Horvath J, et al. (2022). In-vitro characterization of ranibizumab release from the Port Delivery System. J Control Release 345:101–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.