Abstract
Candida albicans is implicated in many biomaterial-related infections. Typically, these infections are associated with biofilm formation. Cells in biofilms display phenotypic traits that are dramatically different from those of their free-floating planktonic counterparts and are notoriously resistant to antimicrobial agents. Consequently, biofilm-related infections are inherently difficult to treat and to fully eradicate with normal treatment regimens. Here, we report a rapid and highly reproducible microtiter-based colorimetric assay for the susceptibility testing of fungal biofilms, based on the measurement of metabolic activities of the sessile cells by using a formazan salt reduction assay. The assay was used for in vitro antifungal susceptibility testing of several C. albicans strains grown as biofilms against amphotericin B and fluconazole and the increased resistance of C. albicans biofilms against these antifungal agents was demonstrated. Because of its simplicity, compatibility with a widely available 96-well microplate platform, high throughput, and automation potential, we believe this assay represents a promising tool for the standardization of in vitro antifungal susceptibility testing of fungal biofilms.
Candida spp. are increasingly associated with biomaterial-related infections (11). Indeed, the majority of manifestations of candidiasis are associated in one way or another with the formation of Candida biofilms on the surface of inert or biological surfaces, and this phenotype is associated with infections at both the mucosal and systemic sites (6, 8, 9, 11, 13). One of the main consequences of the biofilm mode of growth is the increased resistance to antimicrobial therapy, which is the main reason why biofilm-associated infections are frequently refractory to conventional antibiotic therapy (2, 10, 22, 27).
Antifungal susceptibility testing represents a means of predicting therapeutic concentrations of antifungal drugs used to treat a variety of Candida infections (14, 19, 20). It was not until recently that the National Committee for Clinical Laboratory Standards (NCCLS) published its guidelines for a standardized broth macro- and microdilution assay for in vitro testing of antifungal susceptibilities (35). Although these tests, to some extent, have been shown to exhibit good in vitro-in vivo correlation, mainly in the setting of oropharyngeal candidiasis in human immunodeficiency virus-infected individuals (19, 36), occasionally the antifungal susceptibility data do not correlate with the desired clinical outcome. A variety of host factors could account for the lack of good correlations, particularly in disseminated infections in individuals with various degrees of immunosupression. Also, the lack of correlation is likely to be related to the fact that Candida infections can be chronic and are most commonly associated with microbial biofilms. Discrepancies in correlating susceptibility data are perhaps related to the testing strategies that do not account for this alternative mode of growth. NCCLS guidelines use free suspended planktonic cells for in vitro susceptibility testing. However, sessile cells from biofilms are phenotypically distinct from their planktonic counterparts and are associated with an increased-resistance phenotype (2–4, 22, 26, 27). Consequently, for suspected biofilm-related infections, NCCLS standardized testing does not provide an accurate in vitro-in vivo correlation. As a result, an alternative testing strategy should be sought.
Decreased susceptibility of sessile cells to antimicrobial agents compared to that of planktonic cells has been reported extensively over the past decade (1, 2, 15–17, 22, 24, 30, 37). However, the comparatively new field of biofilm research has progressed at such a rate that the development of assays to measure sessile antimicrobial data, often ingenious, has resulted in a plethora of different antimicrobial testing strategies. Moreover, biofilms can be quantified by a variety of techniques, such as direct microscopic enumeration, total viable plate counts, metabolically active dyes, radiochemistry, and luminometry (1, 2, 4, 7, 12, 15, 16, 18, 23, 25, 29, 37). Consequently, there are a myriad of potential techniques to measure biofilm antimicrobial susceptibilities. It is therefore imperative that a standardized antimicrobial susceptibility testing protocol for biofilms be implemented, from both a clinical and research standpoint.
Here we report on a rapid, inexpensive, easy to use, accurate, and reproducible methodology for antifungal susceptibility testing of Candida biofilms that benefits from the use of conventional 96-well microtiter plates coupled to a colorimetric method to assess the effects of antifungal agents against biofilm cells.
MATERIALS AND METHODS
Isolates.
Candida albicans isolates SC5314, 3153A, ATCC 64550, ATCC 64558, ATCC 76615, ATCC 90028, and ATCC 90029 were used in the course of this study. They were stored on Sabouraud dextrose slopes (BBL, Cockeysville, Md.) at −70°C.
Antifungal susceptibility testing.
Fluconazole (Pfizer, Inc., New York, N.Y.) and amphotericin B (Bristol-Myers Squibb, Princeton, N.J.) were used in the course of this study.
Antifungal testing to determine the MICs of planktonic cells was performed by the NCCLS M-27A broth microdilution method (35). We used the spectrophotometric method of inoculum preparation corresponding to a concentration of 0.5 × 103 to 2.5 × 103 cells per ml for each of the isolates prepared in the test medium. Yeast inocula (100 μl) were added to each well of microdilution trays containing 100 μl of antifungal drug solution (prepared at a 2× final concentration). Antibiotic-free controls were also included. The microtiter plates were then incubated at 35°C, and the endpoints were read visually at 48 h. Testing of these isolates was performed in quadruplicate.
For antifungal susceptibility testing of sessile cells, isolates were propagated in yeast peptone dextrose (YPD) medium (1% [wt/vol] yeast extract, 2% [wt/vol] peptone, 2% [wt/vol] dextrose [U.S. Biological, Swampscott, Mass.]). Flasks containing liquid medium (20 ml) were inoculated with a loopful of cells from YPD agar plates containing freshly grown isolates and incubated overnight in an orbital shaker (100 rpm) at 30°C. All strains grew in the budding yeast phase under these conditions. Cells were harvested and washed in sterile phosphate-buffered saline (PBS; 10 mM phosphate buffer, 2.7 mM potassium chloride, 137 mM sodium chloride [pH 7.4] [Sigma, St. Louis, Mo.]). Cells were resuspended in RPMI 1640 supplemented with l-glutamine and buffered with morpholinepropanesulfonic acid (Angus Buffers and Chemicals, Niagara Falls, N.Y.) to a cellular density equivalent to 1.0 × 106 cells per ml with a Bright Line hemocytometer (Hausser Scientific, Horsham, Pa). This density of cells was selected because previous experiments in our laboratory demonstrated that optimal biofilm formation occurs at this particular density (not shown). Biofilms were formed on commercially available presterilized, polystyrene, flat-bottom 96-well microtiter plates (Corning Inc., Corning, N.Y.). Biofilms were formed by pipetting standardized cell suspensions (100 μl of the 106 cells/ml) into selected wells of the microtiter plate and incubating them for 48 h at 37°C as described above. After biofilm formation, the medium was aspirated, and nonadherent cells were removed by thoroughly washing the biofilms three times in sterile PBS. Residual PBS was removed by blotting with paper towels before the addition of antifungal agents. Fluconazole and amphotericin B were then added to the biofilms in serially double-diluted concentrations (1,024 to 1 μg/ml and 32 to 0.125 μg/ml, respectively, from stock [concentrated] solutions of each antifungal agent prepared in RPMI medium directly) and incubated for a further 48 h at 35°C. A series of antifungal agent-free wells and biofilm-free wells were also included to serve as positive and negative controls, respectively. Sessile MICs (SMICs) were determined at 50 and 80% inhibition SMIC50 and SMIC80, respectively) by using the XTT reduction assay described below. Testing of these isolates was performed in quadruplicate.
XTT-reduction assay.
A semiquantitative measure of biofilm formation was calculated by using an XTT [2,3-bis(2-methoxy-4-nitro-5-sulfo-phenyl)-2H-tetrazolium-5-carboxanilide]-reduction assay, adapted from previous reports (28, 39). Briefly, XTT (Sigma) was prepared in a saturated solution at 0.5 g/liter in Ringer's lactate. The solution was filter sterilized through a 0.22-μm-pore-size filter, aliquoted, and stored at −70°C. Prior to each assay, an aliquot of stock XTT was thawed, and menadione (Sigma; 10 mM prepared in acetone) was added to a final concentration of 1 μM. A 100-μl aliquot of the XTT-menadione solution was then added to each prewashed biofilm and to control wells (for the measurement of background XTT-reduction levels). The plates were then incubated in the dark for up to 2 h at 37°C. A colorimetric change in the XTT-reduction assay, a direct correlation of the metabolic activity of the biofilm, was then measured in a microtiter plate reader (Benchmark Microplate Reader; Bio-Rad, Hercules, Calif.) at 490 nm.
Kinetics of biofilm formation on microtiter plates.
C. albicans 3153A biofilm formation was initiated in microtiter plates as described above. Biofilms were formed over a series of time intervals (2, 4, 6, 8, 24 and 48 h). At each time interval, biofilm formation was measured with the XTT assay and concurrently assessed by light microscopy. For each time interval, 11 biofilm replicates were formed.
Total viable cell counts in control and fluconazole-treated biofilms.
Biofilms were preformed on 15-mm-diameter polystyrene discs within 24-well tissue culture trays and challenged with antifungal agents, as described above. Following antifungal challenge and subsequent washing, sessile cells were removed from the surface of the disc by scraping with a sterile scalpel. The sessile cells were added to sterile PBS, sonicated for 5 min to disaggregate clumps, and then vortexed for 30 s. Total viable counts were then estimated by the method described by Miles and Misra (as cited in reference 5). Briefly, serial 10-fold dilutions in sterile PBS were performed on each sample. Measured volumes of each dilution were dispensed onto YPD plates and then incubated for 18 to 24 h at 37°C. Colonies were counted the following day to estimate the total viable cell counts from each disc. SMICs were then assessed relative to those of the nondrug controls. XTT analysis of duplicate biofilms was performed in parallel to the total viable counts assay to demonstrate correlation between these two techniques.
Statistical analysis.
The optical density (OD) values from individual biofilms were compared by one-way analysis of variance and by using the Bartlett's test for homogeneity of variances and the Bonferroni's multiple comparison post-test. P < 0.05 was considered significant. The analyses were performed with GraphPad Prism version 3.00 for Windows (GraphPad Software, San Diego, Calif.).
RESULTS
C. albicans biofilm formation in wells of microtiter plates.
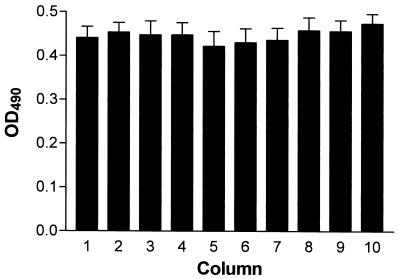
We performed a series of preliminary experiments to assess the variability between C. albicans 3153A biofilms formed in independent wells of the same microtiter plate. All biofilms formed on the microtiter plates over a 24-h time period displayed consistent XTT readings when the intensity of the colorimetric product was measured in a microtiter plate reader at 490 nm. As seen in Fig. 1, no statistically significant difference was noted between C. albicans 3153A biofilms formed on multiple wells in each of 10 columns of the same microtiter plate (P > 0.05), a requisite for valid comparisons for susceptibility testing. Furthermore, there were no significant differences in the XTT absorbance readings from 70 independent biofilms formed on the same microtiter plate. Table 1 shows a description of statistical measurements of multiple biofilms to demonstrate the validity of 70 independent biofilms. The remaining 26 wells served as negative controls. The results validate the equivalency between each biofilm formed in multiple independent wells.
FIG. 1.
Colorimetric readings (OD490s) of biofilms formed in wells of microtiter plates. Values represent the mean and standard deviations of multiple independent biofilms formed in wells of each of 10 different columns of the same microtiter plate. The variability between the colorimetric readings was analyzed by statistical methods as described in Materials and Methods.
TABLE 1.
C. albicans biofilm formation on 70 independent wells of polystyrene microtiter plates grown for 24 h
| Parameter | OD490a |
|---|---|
| Mean | 0.4466 |
| Median | 0.4435 |
| Standard deviation | 0.0297 |
| Standard error | 0.00355 |
| Lower 95% confidence limit | 0.4395 |
| Upper 95% confidence limit | 0.4536 |
| Maximum | 0.5150 |
| Minimum | 0.3860 |
The variability between the colorimetric readings was analyzed by statistical methods as described in Materials and Methods.
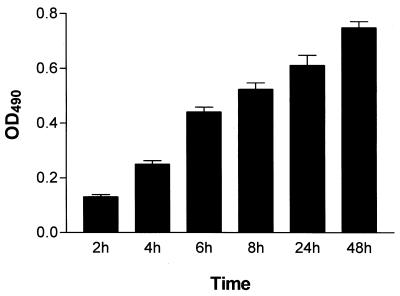
The biofilm growth curve (Fig. 2) demonstrates how the increased colorimetric reading obtained by the XTT-reduction assay correlates with increased cellular density in the biofilms, as assessed by microscopy techniques. The biofilms were highly metabolically active in the first 8 h. However, as the biofilm matured and the complexity increased (24 to 48 h), the metabolic activity reached a plateau, but remained high, reflecting the increased number of cells that constituted the mature biofilm. Light microscopy observations demonstrated how the biofilm began with small microcolonies comprised mainly of yeast cells (2 h). After 2 to 4 h, the yeast cells budded and started to filament, forming pseudohypha and eventually true hyphae. At 8 h, microcolonies then merged into an intricate network of spatially dispersed filamentous forms that intertwined to form a coherent woven-like structure (24 to 48 h), with yeast cells forming aggregates along the hyphae.
FIG. 2.
Kinetics of C. albicans biofilm formation in wells of microtiter plates as determined by the colorimetric XTT-reduction assay.
Susceptibility of C. albicans biofilms to clinically used fluconazole and amphotericin B.
The antifungals tested in this study showed decreased activity against sessile cells of all C. albicans strains tested (Table 2). The planktonic MICs reported for strain ATCC 90028, used for quality control purposes, fell within the breakpoints quoted by experts and NCCLS guidelines (35). Biofilms from all C. albicans strains tested were intrinsically resistant to fluconazole. The resistance to amphotericin B was less pronounced and more variable between the isolates tested. Amphotericin B was up to 1 to 32 times less active against sessile cells than planktonic MICs. This polyene antifungal still demonstrated some activity against C. albicans biofilms, as indicated by SMIC50s. However, all SMIC80s already fell within the resistant range for this antifungal agent (>1 μg/ml). Of note, even at the highest concentration of amphotericin B used against biofilms (16 μg/ml) complete killing was never achieved. Quadruplicate antifungal susceptibility testing of biofilms from the same strain displayed identical MICs for both antifungal agents tested.
TABLE 2.
Antifungal susceptibilities of different C. albicans strains under planktonic or biofilm (SMIC data) growth conditions
| Strain | MIC (μg/ml) of:
|
|||||
|---|---|---|---|---|---|---|
| Fluconazole
|
Amphotericin B
|
|||||
| Planktonic | SMIC50 | SMIC80 | Planktonic | SMIC50 | SMIC80 | |
| SC5314 | 4 | >1,024 | >1,024 | 0.25 | 0.25 | 1.0 |
| 3153A | 4 | >1,024 | >1,024 | 0.5 | 0.5 | 1.0 |
| ATCC 64550 | 16 | >1,024 | >1,024 | 0.25 | 0.25 | 8.0 |
| ATCC 64558 | 0.5 | >1,024 | >1,024 | 0.25 | 0.5 | 1.0 |
| ATCC 76615 | 0.25 | >1,024 | >1,024 | 0.25 | 0.5 | 2.0 |
| ATCC 90028 | 0.5 | >1,024 | >1,024 | 0.5 | 0.5 | 1.0 |
| ATCC 90029 | 0.5 | >1,024 | >1,024 | 0.5 | 0.5 | 2.0 |
Correlation between colorimetric readings with the XTT assay and viable cell counts after exposure to fluconazole.
C. albicans 3153A biofilms were formed on plastic discs in a similar manner to those in microtiter plates. XTT absorbance measurements and total viable cell counts were performed with two triplicate sets of discs. XTT absorbance measurements of fluconazole-treated discs (up to 1,024 μg/ml) did not deviate significantly from the positive non-drug-treated control. Likewise, the total viable cell counts from fluconazole-treated discs (mean = 2.1 × 108 cells/ml) were only slightly reduced compared to that of the drug-free control (mean = 2.7 × 108 cells/ml). These data confirmed the resistance of C. albicans biofilm cells to this azole derivative and the validity of the XTT-reduction assay as an indicator of the efficacy of antibiotic treatment.
DISCUSSION
In this study, we present a method for antifungal susceptibility testing of sessile organisms. In this era of widespread increased antimicrobial resistance and increased use of indwelling devices, it is crucial that we establish standard methodologies that allow evaluation of current and new antifungal agents against cells in biofilms (11). This painstaking work has been previously developed for many planktonic organisms; however, the consideration of a sessile microbial lifestyle appears to have been so far neglected. The increased resistance phenotype of sessile organisms emphasizes the need for a standardized assay to test biofilm antimicrobial susceptibilities (10, 17, 22, 30, 38). The microplate method described here is fast, efficient, reliable, and reproducible, with high throughput potential. For semiquantitative analysis of the preformed biofilms exposed to antifungal drugs, a colorimetric assay was developed, based on the studies by Tellier et al. and Hawser (26, 28, 39). A semiquantitative colorimetric technique was chosen in preference to classical total viable cell counts primarily because of the inherent problems associated with enumerating highly variable morphological forms of C. albicans. Colorimetric evaluation shows no bias in this respect. This assay was reliant upon the mitochondrial dehydrogenases of the live cells to convert an XTT tetrazolium salt into a reduced formazan-colored product that could be measured spectrophotometrically (26, 28, 39). The colometric XTT metabolic assay was shown to produce color changes that upon spectrophotometric determination of absorbance exhibited no statistically significant differences between 70 independent biofilms formed on 96-well microtiter plates. We clearly showed by using the growth curve that the XTT assay absorbance readings were proportional to the cellular density of the biofilm (Fig. 2). These phenomena have been previously demonstrated by Tellier et al. and Hawser (26, 28, 39), which lends validity to this alternative method of biofilm quantification. Because of its water solubility, the XTT-reduction assay can be easily quantified without performing additional steps such as centrifugation, addition of lysis buffer, solubilization, removal of medium, and sonication. It was our hypothesis that sessile cells that resisted the actions of antifungal agents would continue to be metabolically active and therefore initiate color changes of XTT, whereas dead cells would not.
We have also successfully used this model to evaluate antifungal susceptibility testing against biofilms formed by a number of C. albicans clinical isolates and by other Candida spp., such as C. glabrata and C. dubliniensis (unpublished observations). Other authors have described the ability of C. krusei, C. tropicalis, C. guilliermondii, and C. parapsilosis to bind to the wells of microtiter plates (25). Overall, by using this methodology, multiple parameters can be easily investigated with relative ease, which is in contrast to other proposed techniques for the examination of antibiotic susceptibilities of biofilm cells. For example, Domingue et al. (12) proposed the use of the Modified Robbin's Device (MRD) technology to produce multiple biofilms for antimicrobial testing. While this technique is a well-recognized model, it requires expert handling, relatively few equivalent biofilms can be produced, requires longer processing times, and is more open to contamination than our method. Formation of biofilms by using other technologies such as the perfused biofilm fermenter models or membrane-associated biofilm models (3, 18) are not amenable to high-throughput screening and require the use of specialized equipment not generally available in a clinical laboratory. Our model, like that proposed by Ceri and coworkers (7), minimizes sample handling, is rapid and reproducible, and allows the testing of multiple factors within a single trial (different antimicrobials, biofilm ages, growth media, etc.). However, our assay is nondestructive and does not require subsequent culture of cells following antimicrobial challenge.
We used the XTT assay to measure the metabolic activity of 48-h C. albicans biofilms treated for 48 h with the antifungal agents fluconazole and amphotericin B. By this method, it was possible to estimate the SMIC50 and SMIC80 for the biofilm organisms from the absorbance readings determined by the microtiter plate reader. For fluconazole, the absorbance readings of the XTT-reduction assay in biofilms at concentrations as high as 1,024 μg/ml were similar to those of the control biofilms (no drug). Total viable cell counts were also shown to be similar between treated and untreated discs; however, this took a considerably longer time to demonstrate. Our results corroborate previous observations indicating the increased resistance of adherent populations of Candida cells to clinically used antifungal agents (2–4, 26, 27). As with bacteria, the general trend we report is an increased resistance phenotype of sessile Candida cells. Multiple explanations have been proposed to account for this resistance. These include (i) the effects of the glycocalyx causing decreased diffusion or sequestration of antimicrobials (24, 29, 31, 32), (ii) sessile microbial populations metabolic quiescence in comparison to their planktonic counterparts, because they exhibit low rates of growth (3, 21), which has inferences for antimicrobial therapy; (iii) different patterns of gene expression in sessile and planktonic cells that may influence resistance (34), and (iv) the presence of a few persisters that are actually preserved by antibiotic pressure (33). It has to be noted that the very nature of an in vitro preformed biofilm with high cellular densities may partially explain the increased resistance of sessile cells compared to their planktonic counterparts (SMICs MICs). However, this is also the situation that antifungal agents encounter in vivo against biofilms formed within the host. Overall, the complexity of resistance associated with sessile cells is in reality most likely to be multifactorial.
In summary, we have developed a methodology that allows simple, inexpensive, rapid and accurate testing of the in vitro susceptibility of Candida biofilms to antifungal agents. Because of its compatibility with the 96-well microtiter platform and high throughput potential, this technique should prove important in the standardization of in vitro antifungal susceptibility testing of fungal biofilms, both as a research tool and in the clinical laboratory. Use of this technology should be helpful for the selection of antifungal agents active against biofilms and for the screening of new effective antifungal agents to combat biofilm-associated infections.
ACKNOWLEDGMENTS
This work was supported by grant ATP 3659-0080 from the Texas Higher Education Coordinating Board (Advance Technology Program, Biomedicine). J.L.L.-R. is the recipient of a New Investigator Award in Molecular Pathogenic Mycology from the Burroughs Wellcome Fund.
We thank W. Fonzi for C. albicans strain SC5314.
REFERENCES
- 1.Amorena B, Gracia E, Monzon M, Leiva J, Oteiza C, Perez M, Alabart J L, Hernandez-Yago J. Antibiotic susceptibility assay for Staphylococcus aureus in biofilms developed in vitro. J Antimicrob Chemother. 1999;44:43–55. doi: 10.1093/jac/44.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Baillie G S, Douglas L J. Candida biofilms and their susceptibility to antifungal agents. Methods Enzymol. 1999;310:644–656. doi: 10.1016/s0076-6879(99)10050-8. [DOI] [PubMed] [Google Scholar]
- 3.Baillie G S, Douglas L J. Effect of growth rate on resistance of Candida albicans biofilms to antifungal agents. Antimicrob Agents Chemother. 1998;42:1900–1905. doi: 10.1128/aac.42.8.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baillie G S, Douglas L J. Iron-limited biofilms of Candida albicans and their susceptibility to amphotericin B. Antimicrob Agents Chemother. 1998;42:2146–2149. doi: 10.1128/aac.42.8.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown R, Poxton I R, Wilkinson J F. Centrifuges, colorimeters and bacterial counts. In: Collee J G, Duguid J P, Fraser A G, Marmion B P, editors. Practical medical microbiology. 13th ed. Edinburgh, United Kingdom: Churchill Livingstone; 1989. pp. 240–247. [Google Scholar]
- 6.Cannon R D, Chaffin W L. Oral colonization by Candida albicans. Crit Rev Oral Biol Med. 1999;10:359–383. doi: 10.1177/10454411990100030701. [DOI] [PubMed] [Google Scholar]
- 7.Ceri H, Olson M E, Stremick C, Read R R, Morck D, Buret A. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J Clin Microbiol. 1999;37:1771–1776. doi: 10.1128/jcm.37.6.1771-1776.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaffin W L, López-Ribot J L, Casanova M, Gozalbo D, Martinez J P. Cell wall and secreted proteins of Candida albicans: identification, function, and expression. Microbiol Mol Biol Rev. 1998;62:130–180. doi: 10.1128/mmbr.62.1.130-180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Challacombe S J. Immunologic aspects of oral candidiasis. Oral Surg Oral Med Oral Pathol. 1994;78:202–210. doi: 10.1016/0030-4220(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 10.Costerton J W, Stewart P S, Greenberg E P. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 11.Crump J A, Colllgnon P J. Intravascular catheter-associated infections. Eur J Clin Microbiol Infect Dis. 2000;19:1–8. doi: 10.1007/s100960050001. [DOI] [PubMed] [Google Scholar]
- 12.Domingue G, Ellis B, Dasgupta M, Costerton J W. Testing antimicrobial susceptibilities of adherent bacteria by a method that incorporates guidelines of the National Committee for Clinical Laboratory Standards. J Clin Microbiol. 1994;32:2564–2568. doi: 10.1128/jcm.32.10.2564-2568.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ell S R. Candida ‘the cancer of silastic.’. J Laryngol Otol. 1996;110:240–242. [PubMed] [Google Scholar]
- 14.Espinel-Ingroff A, Barchiesi F, Hazen K C, Martinez-Suarez J V, Scalise G. Standardization of antifungal susceptibility testing and clinical relevance. Med Mycol. 1998;36(Suppl. 1):68–78. [PubMed] [Google Scholar]
- 15.Evans D J, Allison D G, Brown M R, Gilbert P. Susceptibility of Pseudomonas aeruginosa and Escherichia coli biofilms towards ciprofloxacin: effect of specific growth rate. J Antimicrob Chemother. 1991;27:177–184. doi: 10.1093/jac/27.2.177. [DOI] [PubMed] [Google Scholar]
- 16.Evans D J, Brown M R, Allison D G, Gilbert P. Susceptibility of bacterial biofilms to tobramycin: role of specific growth rate and phase in the division cycle. J Antimicrob Chemother. 1990;25:585–591. doi: 10.1093/jac/25.4.585. [DOI] [PubMed] [Google Scholar]
- 17.Gander S. Bacterial biofilms: resistance to antimicrobial agents. J Antimicrob Chemother. 1996;37:1047–1050. doi: 10.1093/jac/37.6.1047. [DOI] [PubMed] [Google Scholar]
- 18.Gander S, Gilbert P. The development of a small-scale biofilm model suitable for studying the effects of antibiotics on biofilms of gram-negative bacteria. J Antimicrob Chemother. 1997;40:329–334. doi: 10.1093/jac/40.3.329. [DOI] [PubMed] [Google Scholar]
- 19.Ghannoum M A. Susceptibility testing of fungi and correlation with clinical outcome. J Chemother. 1997;9(Suppl. 1):19–24. [PubMed] [Google Scholar]
- 20.Ghannoum M A, Rex J H, Galgiani J N. Susceptibility testing of fungi: current status of correlation of in vitro data with clinical outcome. J Clin Microbiol. 1996;34:489–495. doi: 10.1128/jcm.34.3.489-495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilbert P, Collier P J, Brown M R W. Influence of growth rate on susceptibility to antimicrobial agents: biofilms, cell cycle, dormancy, and stringent response. Antimicrob Agents Chemother. 1990;34:1865–1868. doi: 10.1128/aac.34.10.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilbert P, Das J, Foley I. Biofilm susceptibility to antimicrobials. Adv Dent Res. 1997;11:160–167. doi: 10.1177/08959374970110010701. [DOI] [PubMed] [Google Scholar]
- 23.Gracia E, Fernandez A, Conchello P, Alabart J L, Perez M, Amorena B. In vitro development of Staphylococcus aureus biofilms using slime-producing variants and ATP-bioluminescence for automated bacterial quantification. Luminescence. 1999;14:23–31. doi: 10.1002/(SICI)1522-7243(199901/02)14:1<23::AID-BIO513>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 24.Gristina A G, Hobgood C D, Webb L X, Myrvik Q N. Adhesive colonization of biomaterials and antibiotic resistance. Biomaterials. 1987;8:423–426. doi: 10.1016/0142-9612(87)90077-9. [DOI] [PubMed] [Google Scholar]
- 25.Hawser S. Adhesion of different Candida spp. to plastic: XTT formazan determinations. J Med Vet Mycol. 1996;34:407–410. [PubMed] [Google Scholar]
- 26.Hawser S. Comparisons of the susceptibilities of planktonic and adherent Candida albicans to antifungal agents: a modified XTT tetrazolium assay using synchronised C. albicans cells. J Med Vet Mycol. 1996;34:149–152. [PubMed] [Google Scholar]
- 27.Hawser S P, Douglas L J. Resistance of Candida albicans biofilms to antifungal agents in vitro. Antimicrob Agents Chemother. 1995;39:2128–2131. doi: 10.1128/aac.39.9.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hawser S P, Norris H, Jessup C J, Ghannoum M A. Comparison of a 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) colorimetric method with the standardized National Committee for Clinical Laboratory Standards method of testing clinical yeast isolates for susceptibility to antifungal agents. J Clin Microbiol. 1998;36:1450–1452. doi: 10.1128/jcm.36.5.1450-1452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoyle B D, Alcantara J, Costerton J W. Pseudomonas aeruginosa biofilm as a diffusion barrier to piperacillin. Antimicrob Agents Chemother. 1992;36:2054–2056. doi: 10.1128/aac.36.9.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoyle B D, Costerton J W. Bacterial resistance to antibiotics: the role of biofilms. Prog Drug Res. 1991;37:91–105. doi: 10.1007/978-3-0348-7139-6_2. [DOI] [PubMed] [Google Scholar]
- 31.Hoyle B D, Jass J, Costerton J W. The biofilm glycocalyx as a resistance factor. J Antimicrob Chemother. 1990;26:1–5. doi: 10.1093/jac/26.1.1. [DOI] [PubMed] [Google Scholar]
- 32.Hoyle B D, Williams L J, Costerton J W. Production of mucoid exopolysaccharide during development of Pseudomonas aeruginosa biofilms. Infect Immun. 1993;61:777–780. doi: 10.1128/iai.61.2.777-780.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis K. Riddle of biofilm resistance. Antimicrob Agents Chemother. 2001;45:999–1007. doi: 10.1128/AAC.45.4.999-1007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maira-Litran T, Allison D G, Gilbert P. Expression of the multiple antibiotic resistance operon (mar) during growth of Escherichia coli as a biofilm. J Appl Microbiol. 2000;88:243–247. doi: 10.1046/j.1365-2672.2000.00963.x. [DOI] [PubMed] [Google Scholar]
- 35.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts: approved standard. NCCLS document M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 36.Rex J H, Pfaller M A, Galgiani J N, Bartlett M S, Espinel-Ingroff A, Ghannoum M A, Lancaster M, Odds F C, Rinaldi M G, Walsh T J, Barry A L. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and Candida infections. Subcommittee on Antifungal Susceptibility Testing of the National Committee for Clinical Laboratory Standards. Clin Infect Dis. 1997;24:235–247. doi: 10.1093/clinids/24.2.235. [DOI] [PubMed] [Google Scholar]
- 37.Schierholz J M, Beuth J, Konig D, Nurnberger A, Pulverer G. Antimicrobial substances and effects on sessile bacteria. Zentbl Bakteriol. 1999;289:165–177. doi: 10.1016/s0934-8840(99)80101-7. [DOI] [PubMed] [Google Scholar]
- 38.Stickler D. Biofilms. Curr Opin Microbiol. 1999;2:270–275. doi: 10.1016/S1369-5274(99)80047-2. [DOI] [PubMed] [Google Scholar]
- 39.Tellier R, Krajden M, Grigoriew G A, Campbell I. Innovative endpoint determination system for antifungal susceptibility testing of yeasts. Antimicrob Agents Chemother. 1992;36:1619–1625. doi: 10.1128/aac.36.8.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]