Abstract
Little is known to date about the in vitro activity of fluoroquinolones against Borrelia species. Our study aimed at determining the in vitro activities of 15 quinolones against nine isolates of the Borrelia burgdorferi sensu lato complex in addition to one Borrelia valaisiana and one Borrelia bissettii tick isolate. For the determination of MICs, a standardized colorimetric microdilution method was applied. Determination of minimal borreliacidal concentrations providing 100% killing of the final inoculum (MBCs) after 72 h and time-kill experiments were performed by conventional culture in Barbour-Stoenner-Kelly medium in combination with dark-field microscopy. The rank order of potency on a microgram-per-milliliter basis for the substances with in vitro activity against B. burgdorferi was gemifloxacin (MIC at which 90% of the isolates tested are inhibited [MIC90], 0.12 μg/ml) > sitafloxacin (MIC90, 0.5 μg/ml), grepafloxacin (MIC90, 0.5 μg/ml) > gatifloxacin (MIC90, 1 μg/ml), sparfloxacin (MIC90, 1 μg/ml), trovafloxacin (MIC90, 1 μg/ml) > moxifloxacin (MIC90, 2 μg/ml), ciprofloxacin (MIC90, 2 μg/ml) > levofloxacin (MIC90, 4 μg/ml) > ofloxacin (MIC90, 8 μg/ml), norfloxacin (MIC90, 8 μg/ml) > fleroxacin (MIC90, >16 μg/ml), and pefloxacin (MIC90, 32 μg/ml) > nalidixic acid (MIC90, 256 μg/ml). After 72 h of exposure, gemifloxacin was borreliacidal (100% killing) against the isolates investigated at a median MBC of 4 μg/ml. In the other compounds tested, median MBCs were higher (≥8 μg/ml). Results of electron microscopy and time-kill studies clearly support an in vitro activity of some fluoroquinolones against borreliae. Our study demonstrates for the first time the enhanced in vitro effectiveness of some of the recently introduced 4-quinolones against B. burgdorferi.
Although the spectrum of antimicrobial agents active against Borrelia burgdorferi in vitro has been enlarged, therapeutic failures and a protracted course of the disease continue to be problems for clinicians in the management of patients suffering from chronic Lyme disease (12). A major consideration in selecting appropriate agents for the treatment of Lyme borreliosis remains the knowledge of the in vitro antimicrobial susceptibility of B. burgdorferi (2). Generally speaking, β-lactams, macrolides, and tetracyclines are recommended for stage-dependent therapy of Lyme borreliosis, whereas borreliae are resistant to aminoglycosides and primary quinolones, such as nalidixic acid and pefloxacin (11, 16). To date, there is no classification scheme for fluoroquinolones that has been generally accepted, but there have been attempts to group the quinolones into “generations” or classes according to their spectrum of antimicrobial activity. For the fluorinated quinolones, Naber and Adam (21) recently proposed for descriptive purposes that the primary 6-fluoro-7-piperazinyl derivatives, such as pefloxacin and norfloxacin, which preferably inhibit gram-negative bacteria, as well as ofloxacin and ciprofloxacin, which also show some activity against Staphylococcus aureus, be classified as class I and II fluoroquinolones, respectively. Novel fluoroquinolones with additional substitutions at C-5 or C-8 and new substituents at C-7 or at C-1, e.g., sparfloxacin (class III) and moxifloxacin, clinafloxacin, sitafloxacin, gatifloxacin, and gemifloxacin (class IV), show enhanced activity against gram-positive organisms and—to a variable extent—also against anaerobic bacterial species. During the last decade, the modern fluoroquinolones have become widely used as alternatives to the β-lactam agents in the treatment of a variety of infections in adults (18). Some of the recently introduced broad-spectrum fluoroquinolones, if effective in vitro and in vivo, also may prove to be useful agents in the therapy of certain manifestations of Lyme disease, e.g., erythema migrans and Lyme arthritis, on account of their oral availability, favorable pharmacodynamic profiles, and high levels in tissue. Nonetheless, almost nothing is known to date about the pharmacodynamic interactions of fluoroquinolones with Borrelia species. As with all antimicrobial agents, initial investigations involving B. burgdorferi commence with the determination of MICs and minimal borreliacidal concentrations (MBCs). Our study aimed at determining the in vitro activities of 15 quinolones against 11 isolates of the B. burgdorferi sensu lato complex, including all three genospecies pathogenic for humans, in addition to Borrelia valaisiana and Borrelia bissettii tick isolates.
MATERIALS AND METHODS
Spirochetes.
The B. burgdorferi sensu lato isolates studied included B31 (B. burgdorferi sensu stricto, tick isolate, United States, ATCC 35210), LW2 (B. burgdorferi sensu stricto, skin isolate, Germany), PKa-I (B. burgdorferi sensu stricto, cerebrospinal fluid isolate, Germany), EB1 (Borrelia afzelii, skin isolate, Germany), FEM1 (B. afzelii, skin isolate, Germany), PKo (B. afzelii, skin isolate, Germany), G1 (Borrelia garinii, cerebrospinal fluid isolate, Germany), PSth (B. garinii, skin isolate, Germany), PTrob (B. garinii, skin isolate, Germany), VS116 (B. valaisiana, tick isolate, Switzerland), and 25015 (B. bissettii, tick isolate, United States). For MIC determination in all cases except B. burgdorferi strain B31, low-passage-number isolates (10 to 20 passages) were employed in the test system. Genospecies were identified by investigating the restriction fragment length polymorphism patterns of DNA of all isolates after digestion with endonuclease MluI and by application of plasmid analysis as described previously (1, 3). Reference strains tested for methodological and control purposes were S. aureus ATCC 29213, Enterococcus faecalis ATCC 29212, Escherichia coli ATCC 25922, and Pseudomonas aeruginosa ATCC 27853.
Antimicrobial agents.
In the present study, the 15 tested fluoroquinolones were grouped for descriptive purposes only in classes I to IV of quinolones (Table 1) as proposed recently (21). In addition, we included ceftriaxone as a control substance with known activity against borreliae. The antimicrobial agents were supplied in lyophilized form ready for use by Merlin-Diagnostika GmbH, Bornheim-Hersel, Germany, and are summarized in Table 1 together with the respective ranges of concentrations used for susceptibility testing.
TABLE 1.
Overall antimicrobial activities of 15 quinolones and ceftriaxone against 11 B. burgdorferi isolates as determined by colorimetric microdilution technique and conventional subcultures
| Antimicrobial | Concn (μg/ml)
|
|||||
|---|---|---|---|---|---|---|
| Test range | MIC range | MIC50 | MIC90 | MBC range | Median MBC | |
| Ceftriaxone | 0.015–32 | ≤0.015–0.06 | 0.03 | 0.03 | 0.125–4 | 1 |
| Nalidixic acid | 0.5–512 | 128–>512 | 256 | 256 | ≥512 | >512 |
| Class I | ||||||
| Norfloxacin | 0.03–64 | 1–16 | 4 | 8 | 8–>64 | 64 |
| Pefloxacin | 0.03–64 | 4–64 | 16 | 32 | 16–>64 | >64 |
| Class II | ||||||
| Fleroxacin | 0.008–16 | 4–>16 | >16 | >16 | ≥16 | >16 |
| Ofloxacin | 0.008–16 | 1–16 | 4 | 8 | 8–>16 | >16 |
| Ciprofloxacin | 0.008–16 | 0.25–8 | 1 | 2 | 4–>16 | 16 |
| Class III | ||||||
| Levofloxacin | 0.008–16 | 0.5–8 | 2 | 4 | 4–>16 | 16 |
| Sparfloxacin | 0.008–16 | 0.06–8 | 0.50 | 1 | 2–>16 | 8 |
| Grepafloxacin | 0.008–16 | 0.06–2 | 0.25 | 0.50 | 2–>16 | 8 |
| Class IV | ||||||
| Gatifloxacin | 0.008–16 | 0.25–2 | 0.50 | 1 | 4–16 | 16 |
| Trovafloxacin | 0.008–16 | 0.25–2 | 0.50 | 1 | 1–16 | 16 |
| Moxifloxacin | 0.008–16 | 0.25–2 | 1 | 2 | 4–16 | 16 |
| Clinafloxacin | 0.008–16 | 0.25–4 | 0.50 | 1 | 1–16 | 8 |
| Sitafloxacin | 0.008–16 | 0.06–4 | 0.25 | 0.50 | 1–16 | 8 |
| Gemifloxacin | 0.008–16 | 0.03–0.25 | 0.06 | 0.12 | 0.25–16 | 4 |
Broth microdilution susceptibility testing.
For susceptibility testing, a colorimetric assay was performed as previously described (9, 10). Briefly, borrelial stock cultures were thawed, cultured in modified Barbour-Stoenner-Kelly medium (BSK) at 33°C until the log phase of growth, and adjusted to 2.5 × 107 borreliae/ml as determined by enumeration with a Kova counting chamber (Hycor, Garden Grove, Calif.) in combination with dark-field microscopy. Final concentrations of the lyophilized antibiotics were reconstituted by adding 200 μl of the final inoculum suspension (5 × 106 cells/well) in BSK containing phenol red (25 μg/ml) as a growth indicator. Cells were cultured at 33°C in 5% CO2. Kinetic measurement of indicator color shift at 562 and 630 nm with a commercially available enzyme-linked immunosorbent assay reader (PowerWave 200; Bio-Tek Instruments, Winooski, Vt.) in combination with a software-assisted calculation program (Microwin 3.0; Microtek, Overath, Germany) was applied to detect bacterial growth after 0, 24, 48, and 72 h.
Determination of MIC.
We recently demonstrated the reliability and reproducibility of our colorimetric microdilution MIC method for the in vitro susceptibility testing of borreliae with a variety of antimicrobial agents (9, 10). Colorimetric MICs of drugs for isolates were measured in triplicate by the quantification of growth utilizing a software-assisted (Microwin 3.0) calculation of growth curves. Growth of samples and controls finally was determined for each well based on the decrease of absorbance after 72 h (Et72) in comparison to the initial absorbance values (Et0). In mathematical terms, the well was reported to be negative for borrelial growth if Et72 > Et0 − 10%. Comparison of the growth characteristics between test and growth control wells was made in determining the endpoints for each isolate tested. A 10% threshold value was chosen in this study to compensate for residual metabolic activity of spirochetes after 24 to 48 h of incubation prior to the onset of the bacteriostatic and borreliacidal effects of the quinolones (Fig. 1 and 2).
FIG. 1.
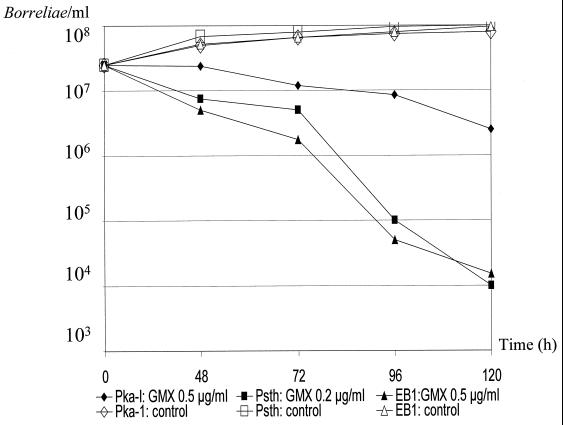
Time-kill curves and control experiments for B. burgdorferi sensu lato isolates PKa-I, PSth, and EB1 with concentrations of gemifloxacin (GMX) four times the respective MICs. Experiments were performed on different days by investigation of growth using conventional cell counts, and data were reported as the means of two experiments.
FIG. 2.
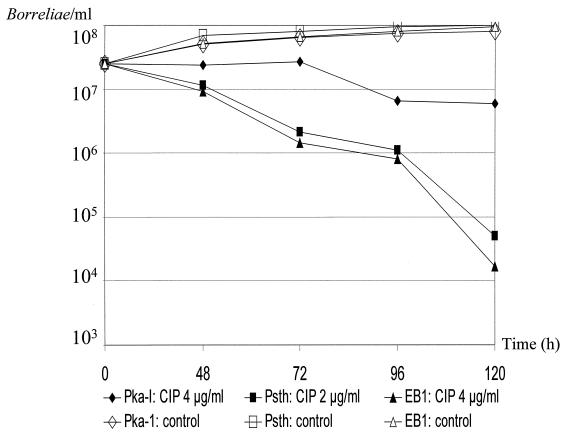
Time-kill curves and control experiments for B. burgdorferi sensu lato isolates PKa-I, PSth, and EB1 with concentrations of ciprofloxacin (CIP) four times the respective MICs. Experiments were performed on different days by investigation of growth using conventional cell counts, and data were reported as the means of two experiments.
As a control for antibacterial activity and in order to detect significant MIC variations due to obvious antibiotic-medium interactions, MICs of drugs for ATCC reference strains S. aureus ATCC 29213, E. faecalis ATCC 29212, E. coli ATCC 25922, and P. aeruginosa ATCC 27853 also were determined in triplicate in the same medium under the same test conditions after 24 h of incubation in accordance with NCCLS standard protocols (22).
Determination of MBCs.
Lyme borreliosis represents a disorder of potentially chronic proportions, and the survival of small numbers of bacteria may result in a relapse clinically. We thus determined the drug concentrations providing 100% killing of the borrelial inoculum under more stringent conditions. MBCs were determined on three different days. All isolates were investigated after 72 h of incubation with the antibiotic, and MBCs were reported as the medians of three experiments. To enhance the sensitivity of MBC determination, after 72 h aliquots (20 μl) were taken from all vials lacking detectable growth and diluted 1:75 with fresh BSK to achieve a sample dilution below the MIC. Incubation was continued at 33°C in 5% CO2 for an additional 3 weeks (10). After gentle agitation of the subcultures, 5 to 10 high-power fields were examined by dark-field microscopy for the presence or absence of borreliae. The MBC was defined as the lowest concentration of the antimicrobial at which no spirochetes could be detected after 3 weeks of subculture (10).
Time-kill studies.
Time-kill studies were performed with borrelial isolates PKa-I, PSth, and EB1 with ciprofloxacin and gemifloxacin. This was done to compare the activity of one of the recently introduced compounds with enhanced effectiveness against borreliae with that of ciprofloxacin, which is regarded as a marker substance among the commonly used fluoroquinolones. Isolates PKa-I (B. burgdorferi sensu stricto), PSth (B. garinii), and EB1 (B. afzelii) were used to determine generation times in BSK and rates of killing on the part of both antimicrobial agents at four times the respective MICs. Experiments were performed on different days, and cell counts were reported as the means of two experiments. Growth control experiments were undertaken with all isolates under the same test conditions except for the addition of antibiotics. Inasmuch as motility strongly corresponds to the viability of spirochetes (4, 24), samples and controls (200 μl) of the final inoculum suspension (5 × 106 borreliae/well) for each isolate were investigated for morphologically unaffected motile borreliae (24) by conventional cell counts (see above). Cell counts were performed on two different days for each of the isolates tested after 0, 48, 72, 96, and 120 h of incubation by applying identical test conditions in the presence and absence of antibiotics.
Electron microscopy.
Electron microscopy was performed as previously described (14). BSK cultures (10 ml) of PSth (B. garinii; inoculum, 2.5 × 107 borreliae/ml) in the log phase of growth were treated with 0.5, 2, and 8 μg of ciprofloxacin/ml and 0.06, 0.25, and 2 μg of gemifloxacin/ml for 72 h. Cells exposed to the antimicrobials and antibiotic-free controls then were harvested by centrifugation at 5,000 × g for 30 min at 37°C. Cells were resuspended in 200 μl of veronal-buffered saline. Fixation was performed by adding an equal volume of 4% glutaraldehyde in 0.2 M sodium cacodylate buffer (pH 7.4) followed by incubation at room temperature for 1 h. For thin-section preparation, glutaraldehyde-fixed cells were fixed further in OsO4 and then embedded in Spurr's resin. Thin sections were contrasted with 2% (wt/vol) aqueous uranyl acetate (pH 4.8) and lead citrate. All specimens were examined with a model EM 109 microscope (Zeiss, Oberkochen, Germany).
Statistics.
There is evidence for a heterogeneity of the borrelial genospecies with regard to their antimicrobial susceptibility patterns (9, 10). To investigate possible differences in the susceptibility patterns of the borrelial genospecies tested, the Kruskal-Wallis test was performed for all MICs and MBCs of the antibiotics as determined by our experiments with the different borrelial isolates.
RESULTS
MIC determination.
The overall antimicrobial activities and the individual MICs of ceftriaxone and the 15 fluoroquinolones for the 11 borrelial strains are summarized in Tables 1 and 2. The following rank order of in vitro activity against B. burgdorferi for the tested substances on a microgram-per-milliliter basis emerged: ceftriaxone (MIC at which 90% of the isolates tested are inhibited [MIC90], 0.03 μg/ml), gemifloxacin (MIC90, 0.12 μg/ml) > sitafloxacin (MIC90, 0.5 μg/ml), grepafloxacin (MIC90, 0.5 μg/ml) > gatifloxacin (MIC90, 1 μg/ml), sparfloxacin (MIC90, 1 μg/ml), trovafloxacin (MIC90, 1 μg/ml) > moxifloxacin (MIC90, 2 μg/ml), ciprofloxacin (MIC90, 2 μg/ml) > levofloxacin (MIC90, 4 μg/ml) > ofloxacin (MIC90, 8 μg/ml), norfloxacin (MIC90, 8 μg/ml) > fleroxacin (MIC90, >16 μg/ml), and pefloxacin (MIC90, 32 μg/ml) > nalidixic acid (MIC90, 256 μg/ml). Gemifloxacin proved to be the most potent fluoroquinolone, exhibiting low MICs ranging between 0.03 and 0.25 μg/ml against the isolates tested. For all fluoroquinolones except gemifloxacin, grepafloxacin, and sitafloxacin, MIC50 and MIC90 were found to be >0.25 and >0.5 μg/ml, respectively (Table 1).
Statistical analysis, however, performed on all measured MICs (n = 495) to investigate possible heterogeneity of borrelial susceptibility patterns for the quinolones showed no significant differences with respect to the MICs for the different genospecies tested.
To ensure that significant MIC deviations attributable to interactions of the fluoroquinolones with components of the BSK did not hamper our MIC determinations, MICs of the drugs for NCCLS reference strains S. aureus ATCC 29213, E. faecalis ATCC 29212, E. coli ATCC 25922, and P. aeruginosa ATCC 27853 were determined by using BSK under identical test conditions. For all four strains, the MIC ranges of the drugs tested (except trovafloxacin and sitafloxacin) were within the ranges specified in the NCCLS guidelines (22), when read in triplicate after 24 h. For trovafloxacin, the measured MICs were 1 to 2 log2 unit dilutions above the upper limit as published for this agent by the NCCLS. For sitafloxacin, no such NCCLS ranges are currently available.
MBCs.
When determined on three different days, the MBCs of the antibiotics tested for the same isolate spanned a range of only 2 to 3 log2 unit dilutions, thereby indicating high reproducibility. The common MBC definition (99.9% killing) means survival of bacteria at several log2 unit dilutions above the MIC, depending on the final inoculum and the substance tested. Our findings concerning the MBC ranges and the median MBCs as determined by subculture experiments under more stringent conditions (100% killing after 72 h) are summarized in Table 1. Ceftriaxone, which was included as a control substance, under these conditions was borreliacidal at a median MBC of 1 μg/ml. Against the isolates investigated, only the class III antibiotic agents sparfloxacin and grepafloxacin (median MBCs, 8 μg/ml) and the class IV antibiotic agents clinafloxacin and sitafloxacin (median MBCs, 8 μg/ml) and gemifloxacin (median MBC, 4 μg/ml) revealed borreliacidal activity after 72 h of exposition, displaying MBCs (100% killing) at 2 to 6 log2 unit dilutions above the MIC90. For the other class III and IV compounds tested, median MBCs were even higher. In addition, a genospecies-based statistical analysis of all measured MBCs (n = 495) was performed. As revealed by our subculture experiments, the MBCs of grepafloxacin, clinafloxacin, sitafloxacin, and gemifloxacin for B. burgdorferi sensu stricto isolates appeared to be significantly higher (range of MBCs: grepafloxacin, 8 to ≥16 μg/ml; clinafloxacin, 8 to ≥16 μg/ml; sitafloxacin, 8 to ≥16 μg/ml; and gemifloxacin, 2 to 16 μg/ml) than those for B. afzelii, B garinii, B. valaisiana, and B. bissettii isolates (range of MBCs: grepafloxacin, 0.5 to 16 μg/ml; clinafloxacin, 0.5 to 16 μg/ml; sitafloxacin, 0.5 to 8 μg/ml; and gemifloxacin, 0.25 to 8 μg/ml; P <0.05). As anticipated, class I and II quinolones did not have any borreliacidal effect at concentrations of ≤16 μg/ml.
Time-kill studies.
For strains PSth and EB1, exposure to gemifloxacin, the most potent compound on a microgram-per-milliliter basis, at four times the respective MICs of the drug for the strains led to a >3-log10-unit (99.9%) reduction of morphologically intact motile cells of the final inoculum after 96 to 120 h (Fig. 1). Ciprofloxacin was almost as effective as was gemifloxacin against isolate EB1 but was less effective against isolates PSth and PKa-I (Fig. 2). For PKa-I, however, the number of intact motile spirochetes clearly decreased more slowly and showed only a ∼1-log10-unit reduction of the final inoculum upon application of both antimicrobials after 120 h (Fig. 1 and 2). The initial borrelial inoculum, in contrast, tripled in the presence of both antimicrobials, provided that only one-half of the respective MICs was tested (data not shown), and in control experiments without the addition of antibiotics (Fig. 1 and 2). As revealed by our in vitro experiments for PKa-I, PSth, and EB1, the generation time is approximately 24 to 48 h.
Electron microscopy.
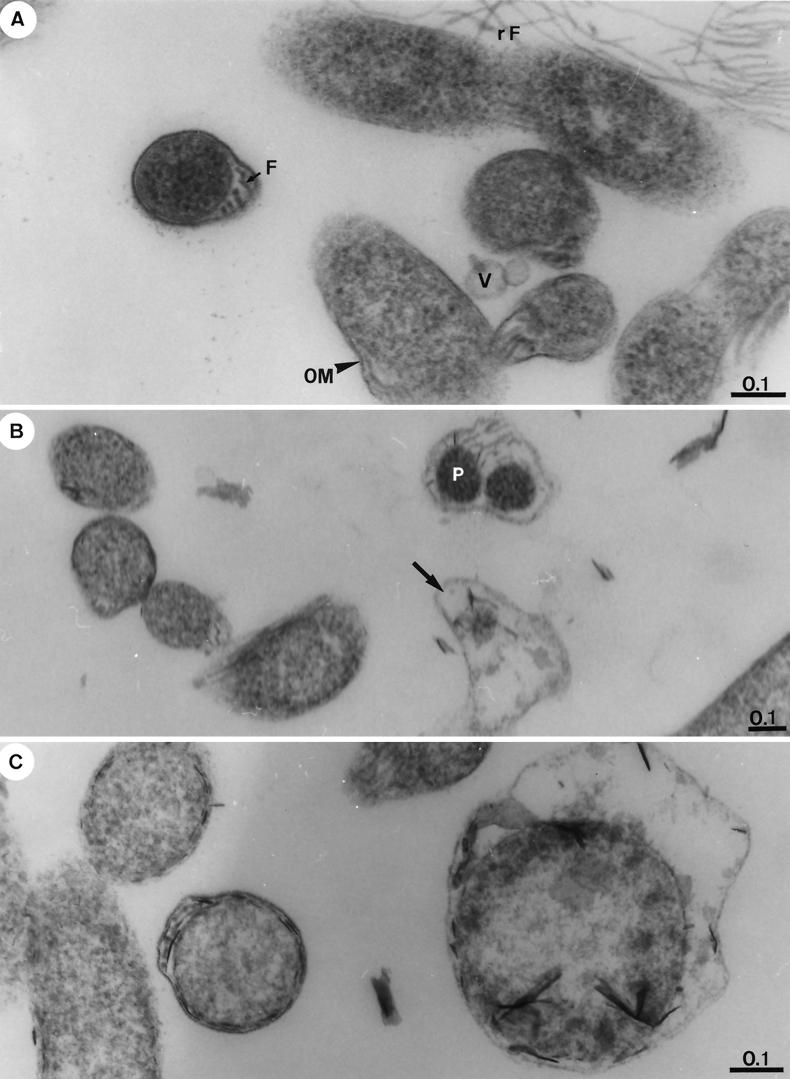
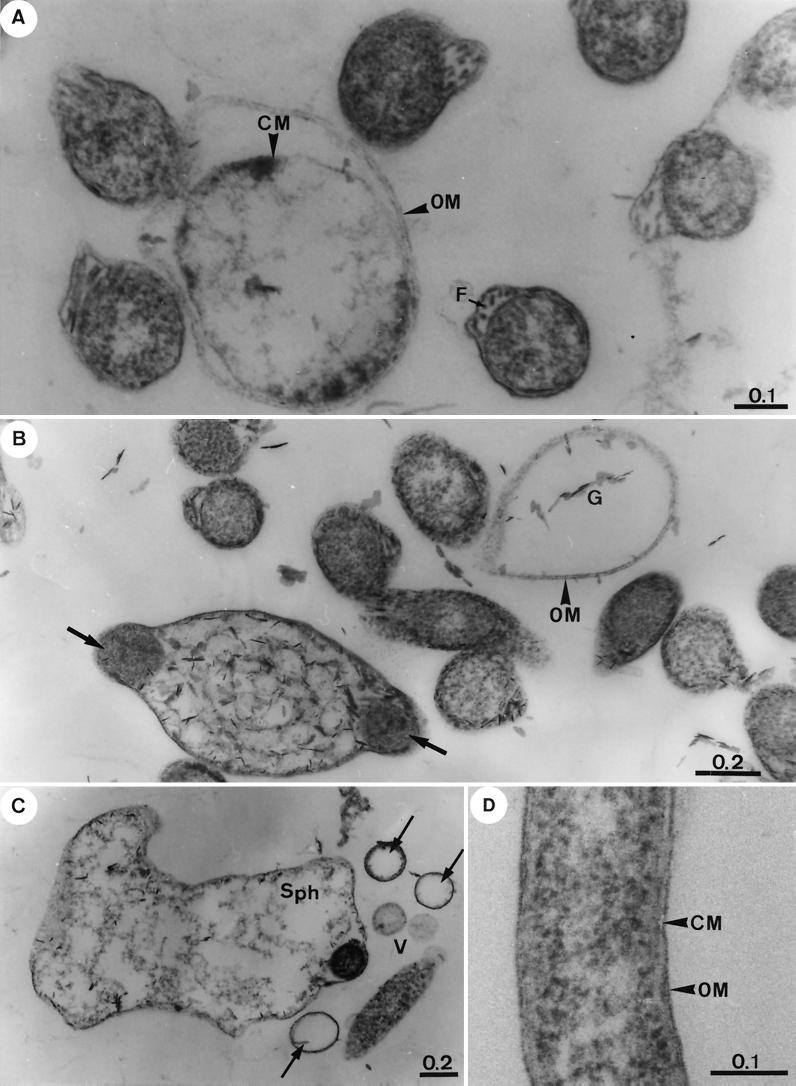
Electron microscopy was performed after exposure of strain PSth to increasing concentrations of ciprofloxacin and gemifloxacin for 72 h (Fig. 3 and 4). Exposure of borreliae to ciprofloxacin at the MIC (0.5 μg/ml) showed that significant numbers of the spirochetes remained ultrastructurally intact (Fig. 3A). Other borrelial cells, however, showed a beginning lysis, as indicated by outer membrane lesions, the occurrence of vesicles, and the abundant flagella released in the cell vicinity. In the presence of 2 and 8 μg of ciprofloxacin/ml, increasing signs of degeneration or an advanced stage of lysis of borreliae were evident (Fig. 3B and C). In the presence of gemifloxacin at the MIC (0.06 μg/ml), the toxic alterations in the fine structure of borrelial cells were even more obvious than those seen in samples treated with 0.5 μg of ciprofloxacin/ml (Fig. 3A, 4A, and 4B). After exposure to gemifloxacin at 0.25 μg/ml (four times the MIC), the cytoplasm of the majority of cells appeared severely damaged and highly vacuolated, and protoplasmic cylinders were fragmented (Fig. 4B). Cytotoxic effects were even more pronounced in the presence of 2 μg of gemifloxacin/ml, and many cells were transformed into large spheroplasts filled with the breakdown products of the protoplasmic cylinder (Fig. 4C). Our observations clearly indicate a stronger cytotoxic effect on borreliae of gemifloxacin than of ciprofloxacin as a result of enhanced in vitro activity for gemifloxacin even at lower drug concentrations. Control sections of identically treated antibiotic-free samples revealed morphologically intact borrelial cells (Fig. 4D) similar to those described elsewhere in greater detail recently (15). In cross sections of control cells, the endoflagellae were located in the periplasmic space, similar to those shown in Fig. 4A (cell on the left).
FIG. 3.
Representative transmission electron thin-section micrographs of B. garinii isolate PSth after exposure for 72 h to increasing concentrations of ciprofloxacin. (A) Borreliae exposed to 0.5 μg of ciprofloxacin/ml (MIC). An essential part of cells remains ultrastructurally intact (e.g., cells on the left with endoflagella [F]), whereas others show lesions of the outer membrane (OM), the occurrence of vesicles (V), and abundant flagella (rF) released in the cell vicinity. (B) Borreliae exposed to 2 μg of ciprofloxacin/ml (four times the MIC). Cell lysis (arrow) is apparent; two fragments of the protoplasmic cylinders (P) are surrounded by degenerated cell wall layers. (C) Borreliae exposed to 8 μg of ciprofloxacin/ml (16 times the MIC). Borrelial cells become substantially increased in volume and show an advanced stage of lysis. Bar lengths are in micrometers in all micrographs.
FIG. 4.
Representative transmission electron thin-section micrographs of B. garinii isolate PSth after exposure for 72 h to increasing concentrations of gemifloxacin and of the untreated control. (A) Borreliae exposed to 0.06 μg of gemifloxacin/ml (MIC). Significant ultrastructural alterations are evident in comparison with the untreated control (see below). OM, outer membrane; F, flagella; CM, cytoplasmic membrane. (B) Borreliae exposed to 0.25 μg of gemifloxacin/ml (four times the MIC). The outer membrane (OM) often appeared altered or deteriorated. The cytoplasm of all cells appears severely damaged and often highly vacuolated, and protoplasmic cylinders become fragmented (arrows). Emergence of cellular ghosts (G) with increasing cell diameter is visible. (C) Borreliae exposed to 2 μg of gemofloxacin/ml. Borreliae were transformed into large spheroplasts (Sph) filled with breakdown products of the protoplasmic cylinder. Others show a highly vacuolated cytoplasm, vesicles (V), or nearly empty cells (arrows). (D) Control section of cells treated identically except for the addition of antibiotics. OM, outer membrane; CM, cytoplasmic membrane.
DISCUSSION
Up to now, the sparse reports on the effectiveness of 4-quinolones against borreliae have been somewhat contradictory. Whereas most authors report borreliae to be resistant to the fluoroquinolones (11, 16), others observe some in vitro activity (5, 20, 25). Owing to the limited in vitro activity of the initial compounds against borreliae, in particular nalidixic acid and pefloxacin (11, 16), the fluoroquinolones have not been recommended drugs of choice to date for the treatment of Lyme disease. Molecular biological studies, however, indicate the presence of a target structure for the quinolones, i.e., a functional DNA gyrase consisting of full-length GyrA and GyrB subunits, which is required for growth of borreliae (25). The present study, therefore, was specifically designed to systematically investigate the activities of 15 fluoroquinolones against 11 isolates of the B. burgdorferi sensu lato complex pathogenic for humans and against other previously designated genospecies (Table 2). Here, direct evidence is provided for the first time for enhanced in vitro activity of some of the newly developed antimicrobial agents against borreliae. Moreover, the range of MICs is clearly class dependent, insofar as class I and II compounds mostly revealed higher MIC50s and MIC90s than did class III and IV compounds (Table 1). In our study, the rank order of potency on a microgram-per-milliliter basis for the quinolones with enhanced in vitro activity against B. burgdorferi was gemifloxacin > sitafloxacin, grepafloxacin > gatifloxacin, clinafloxacin, trovafloxacin, sparfloxacin (Table 1). Our observations concerning increased in vitro effectiveness of some of the recently introduced 4-quinolones against borreliae are in agreement with those of previous investigators who demonstrated the activity of sparfloxacin and the DNA-gyrase inhibitor coumermycin A1 against B. burgdorferi (5, 25). According to our data, gemifloxacin proved to be the most potent fluoroquinolone against borreliae, thereby exhibiting MICs that ranged between 0.03 and 0.25 μg/ml against the borrelial isolates tested. In fact, the activity of gemifloxacin, sitafloxacin, and grepafloxacin is approximately 10 to 100 times higher than that of the older 4-quinolones but is clearly lower than that of ceftriaxone, which we included as a control substance with known activity against borreliae.
TABLE 2.
Individual antibiotic susceptibilities of 11 B. burgdorferi isolates to 15 quinolones and ceftriaxonea
| Isolate | MIC (μg/ml) for antibiotic:
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nalidixic acid | Norfloxacin | Pefloxacin | Fleroxacin | Ofloxacin | Ciprofloxacin | Levofloxacin | Sparfloxacin | Grepafloxacin | Gatifloxacin | Trovafloxacin | Moxifloxacin | Clinafloxacin | Sitafloxacin | Gemifloxacin | Ceftriaxone | |
| B. burgdorferi sensu stricto | ||||||||||||||||
| B31 | 256 | 8 | 16 | >16 | 4 | 1 | 2 | 0.50 | 0.25 | 0.50 | 0.50 | 1 | 0.50 | 0.25 | 0.06 | 0.03 |
| LW2 | 256 | 8 | 16 | >16 | 8 | 2 | 4 | 1 | 1 | 2 | 0.12 | 2 | 1 | 0.50 | 0.12 | 0.03 |
| PKa-I | 256 | 8 | 16 | 16 | 8 | 1 | 2 | 1 | 0.25 | 0.50 | 0.50 | 1 | 0.50 | 0.25 | 0.12 | 0.015 |
| B. garinii | ||||||||||||||||
| PTrob | 256 | 4 | 32 | >16 | 8 | 1 | 4 | 4 | 0.25 | 0.50 | 0.50 | 1 | 0.50 | 0.50 | 0.06 | 0.03 |
| G1 | 256 | 4 | 16 | >16 | 4 | 1 | 2 | 0.25 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.25 | 0.06 | <0.015 |
| PSth | 256 | 2 | 8 | 8 | 2 | 0.50 | 1 | 0.50 | 0.12 | 0.25 | 0.50 | 0.50 | 0.50 | 0.12 | 0.06 | 0.015 |
| B. afzelii | ||||||||||||||||
| EB1 | 256 | 4 | 16 | >16 | 4 | 1 | 2 | 0.50 | 0.50 | 0.50 | 1 | 1 | 1 | 0.25 | 0.12 | 0.03 |
| FEM1 | 128 | 2 | 8 | 8 | 2 | 0.50 | 1 | 0.50 | 0.25 | 0.25 | 0.25 | 1 | 0.25 | 0.50 | 0.06 | 0.03 |
| PKo | 128 | 2 | 4 | 16 | 2 | 0.50 | 2 | 0.25 | 0.25 | 0.50 | 0.50 | 0.50 | 0.50 | 0.25 | 0.06 | 0.03 |
| B. valaisiana VS116 | 512 | 8 | 32 | >16 | 8 | 2 | 4 | 0.50 | 0.50 | 1 | 0.50 | 2 | 1 | 0.25 | 0.12 | 0.03 |
| B. bissettii 25015 | 256 | 8 | 32 | 16 | 8 | 1 | 2 | 0.50 | 0.25 | 0.50 | 1 | 1 | 0.50 | 0.50 | 0.06 | 0.03 |
Antimicrobial susceptibilities were determined on three different days, and MICs for each isolate were reported as the median of three experiments.
Previous investigators report the occurrence of test medium side effects on almost every antimicrobial agent tested (2, 5). We performed experiments for quality control purposes according to NCCLS protocols to investigate possible antibiotic-medium interactions. For all four ATCC strains, the MICs of the fluoroquinolones tested with the exception of trovafloxacin were all within the NCCLS ranges (22).
With regard to the functions and chemical structures of the borrelial topoisomerases, little is known at present. For the borrelial DNA gyrase, it must be kept in mind, however, that both the naturally occurring protein from B. burgdorferi and the homologous C-terminal region from E. coli GyrA are biochemically distinct, sharing only 24% identity at the amino acid level (13). Moreover, the GyrA C-terminal domain from E. coli is acidic, with a predicted isoelectric point of 4.0, whereas in contrast, the naturally occurring 34-kDa protein from B. burgdorferi is basic, with a predicted isoelectric point of 9.1 (13). These differences may explain the lower activity of class I and II derivatives against borreliae than against common gram-negative bacteria like E. coli.
Available data on gemifloxacin show a high potency of the antimicrobial against gram-positive anaerobic species but only moderate activity against gram-negative anaerobes (7). The finding of higher susceptibilities of B. burgdorferi to class III and IV quinolones—derivatives exhibiting enhanced activity against gram-positive species and anaerobes—points to the fact that the in vitro susceptibility of borreliae probably does not resemble that of common gram-negative bacteria (6). Similarly, vancomycin, which usually does not possess in vitro activity against common gram-negative bacteria, displays a significant antibiotic effect against B. burgdorferi (6).
Gemifloxacin carries a new 3-aminomethyl-4syn-methoxyimino-1-pyrrolidinyl substituent at the C-7 position of the 6-fluoro-1,8-naphthyridone core (23). With nearly equal effectiveness against gram-negative and gram-positive organisms, gemifloxacin also remains active against resistant mutants carrying multiple quinolone resistance mutations in gyrA and parC (A. Schulte and P. Heisig, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 819, 1999) and is the most active fluoroquinolone against borreliae. For Streptococcus pneumoniae, previous investigations revealed that, in addition to gemifloxacin's activity against the pneumococcal DNA gyrase, the improved effectiveness of the drug probably is related to its remarkably high affinity for topoisomerase IV (19). Accordingly, it can be speculated that a higher affinity for one of the borrelial topoisomerases may be the cause for enhanced in vitro activity of gemifloxacin against borreliae as well. With regard to the results of our time-kill studies (Fig. 1 and 2) and the electron microscope analysis of quinolone-exposed borreliae (Fig. 3 and 4), our findings clearly support the existence of in vitro activity on the part of the quinolones against borreliae, which results in a reduction of intact motile cells and in increasing ultrastructural damage of spirochetes in the presence of higher concentrations of ciprofloxacin and gemifloxacin. Furthermore, our observations indicate the stronger cytotoxic effect of gemifloxacin than of ciprofloxacin on borreliae. As demonstrated in Fig. 1 and 2, little or no bactericidal activity of ciprofloxacin and gemifloxacin occurred in the isolates during the first 24 to 72 h of exposure, but killing rates increased markedly after longer incubation periods. An optimum reduction of the initial inoculum was reached after 96 to 120 h. Correspondingly, our conventional subculture experiments performed under very restrictive conditions also demonstrated that higher drug concentrations are required for the fluoroquinolones for 100% killing (MBC) of borreliae after 72 h of incubation. These observations are similar to those obtained previously with E. faecalis: much slower antibiotic killing is observed in comparison to S. aureus after exposure to ciprofloxacin and ofloxacin (17). For borreliae, this finding may result from the longer generation time required for the replication cycle and cell division. As such, detection of impaired plasmid relaxation and supercoiling in B. burgdorferi strain B31 due to coumermycin A1 requires at least 2 h, compared to only about 20 min in E. coli (25).
Careful evaluation of the MBCs determined in our study revealed that the MBCs of grepafloxacin, clinafloxacin, sitafloxacin, and gemifloxacin for B. burgdorferi sensu stricto isolates were significantly higher than those for B. afzelii, B garinii, B. valaisiana, and B. bissettii isolates (P < 0.05) after 72 h of incubation. The finding of lower killing activities of these substances for B. burgdorferi sensu stricto isolates than for the other borrelial isolates investigated was confirmed by the results of time-kill experiments (Fig. 1 and 2), once again pointing to slow killing of borreliae by the quinolones and to possible differences in the drug interactions with regard to the tested genospecies of the B. burgdorferi complex.
Regarding the discrepancies reported to date on the part of the in vitro and in vivo effectiveness of antimicrobials against borreliae (8), fluoroquinolones cannot be recommended for the treatment of Lyme disease on account of limited in vivo experience. Our study, however, is one of the first to supply evidence for in vitro effectiveness of some of the recently introduced 4-quinolones against B. burgdorferi. Consequently, we wish to emphasize the importance of ongoing tests involving more isolates together with the performance of in vivo experiments to determine the definitive value of modern fluoroquinolones in the therapy of Lyme disease. Such investigations are under way in our institution to address these questions.
ACKNOWLEDGMENTS
We thank V. Preac-Mursic, H. Karch, G. R. Burmester, and A. van Dam for kindly providing some of the tested isolates. We also thank Rita Grotjahn, Christa Hanssen-Hübner, and Angelika Sames for their excellent technical assistance.
REFERENCES
- 1.Belfaiza J, Postic D, Bellenger E, Baranton G, Saint-Girons I. Genomic fingerprinting of Borrelia burgdorferi sensu lato by pulsed-field gel electrophoresis. J Clin Microbiol. 1993;31:2873–2877. doi: 10.1128/jcm.31.11.2873-2877.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boerner J, Failing K, Wittenbrink M M. In vitro antimicrobial susceptibility testing of B. burgdorferi: influence of test conditions on minimal inhibitory concentration (MIC) values. Zentbl Bakteriol. 1995;283:49–60. doi: 10.1016/s0934-8840(11)80890-x. [DOI] [PubMed] [Google Scholar]
- 3.Busch U, Hizo-Teufel C, Boehmer R, Wilske B, Preac-Mursic V. Molecular characterization of Borrelia burgdorferi sensu lato strains by pulsed-field gel electrophoresis. Electrophoresis. 1995;16:744–747. doi: 10.1002/elps.11501601122. [DOI] [PubMed] [Google Scholar]
- 4.Callister S M, Schell R F, Lovrich S D. Lyme disease assay which detects killed Borrelia burgdorferi. J Clin Microbiol. 1991;29:1773–1776. doi: 10.1128/jcm.29.9.1773-1776.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dever L L, Jorgensen J H, Barbour A G. In vitro antimicrobial susceptibility testing of Borrelia burgdorferi: a microdilution MIC method and time-kill studies: J. Clin Microbiol. 1992;30:2692–2697. doi: 10.1128/jcm.30.10.2692-2697.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dever L L, Jorgensen J H, Barbour A G. In vitro activity of vancomycin against the spirochete Borrelia burgdorferi. Antimicrob Agents Chemother. 1993;37:1115–1121. doi: 10.1128/aac.37.5.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstein E J C. Review of the in vitro activity for gemifloxacin against Gram-positive and Gram-negative anaerobic pathogens. J Antimicrob Chemother. 2000;45(Suppl. S1):55–65. doi: 10.1093/jac/45.suppl_3.55. [DOI] [PubMed] [Google Scholar]
- 8.Hansen K, Hovmark A, Lebech A M, Lebech K, Olsson I, Halkier-Sorensen L, Olsson E, Asbrink F. Roxithromycin in Lyme borreliosis: discrepant results of an in vitro and in vivo animal susceptibility study and a clinical trial in patients with erythema migrans. Acta Dermato-Venereol. 1992;72:297–300. [PubMed] [Google Scholar]
- 9.Hunfeld K P, Kraiczy P, Wichelhaus T A, Schäfer V, Brade V. New colorimetric microdilution method for in vitro susceptibility testing of Borrelia burgdorferi against antimicrobial substances. Eur J Clin Microbiol Infect Dis. 2000;19:27–32. doi: 10.1007/s100960050005. [DOI] [PubMed] [Google Scholar]
- 10.Hunfeld K-P, Kraiczy P, Wichelhaus T A, Schäfer V, Brade V. Colorimetric in vitro susceptibility testing of penicillins, cephalosporines, macrolides, streptogramins, tetracyclines and aminoglycosides against Borrelia burgdorferi isolates. Int J Antimicrob Agents. 2000;15:11–17. doi: 10.1016/s0924-8579(00)00116-3. [DOI] [PubMed] [Google Scholar]
- 11.Johnson R C. Isolation techniques for spirochetes and their sensitivity to antibiotics in vivo and in vitro. Rev Infect Dis. 1989;11(Suppl. 6):S1505–S1510. doi: 10.1093/clinids/11.supplement_6.s1505. [DOI] [PubMed] [Google Scholar]
- 12.Kersten A, Poitschek C, Rauch S, Arberer E. Effects of penicillin, ceftriaxone, and doxycycline on morphology of Borrelia burgdorferi. Antimicrob Agents Chemother. 1995;39:1127–1133. doi: 10.1128/aac.39.5.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knight S W, Samuels D S. Natural synthesis of a DNA-binding protein from the C-terminal domain of DNA gyrase A in Borrelia burgdorferi. EMBO J. 1999;18:4875–4881. doi: 10.1093/emboj/18.17.4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kraiczy P, Hunfeld K-P, Würzner R, Acker G, Brade V. Comparison of two laboratory methods for the determination of serum resistance in Borrelia burgdorferi isolates. Immunobiology. 2000;201:406–419. doi: 10.1016/S0171-2985(00)80094-7. [DOI] [PubMed] [Google Scholar]
- 15.Kraiczy P, Acker G, Brade V. Characteristics of the pathogen. In: Oschmann P, Kraiczy P, Halperin J, Brade V, editors. Lyme borreliosis and tick-borne encephalitis. Bremen, Germany: UNI-Med Verlag AG, International Medical Publishers; 1999. pp. 20–24. [Google Scholar]
- 16.Lakos A, Nagy G. Effect of an antibiotic combination on the propagation of Borrelia burgdorferi, causative agent of Lyme disease. Orv Hetil. 1999;140:1529–1532. [PubMed] [Google Scholar]
- 17.Lewin C S, Morrissey I, Smith J T. The bactericidal activity of sparfloxacin. J Antimicrob Chemother. 1992;30:625–632. doi: 10.1093/jac/30.5.625. [DOI] [PubMed] [Google Scholar]
- 18.Moellering R C., Jr The place of quinolones in everyday clinical practice. Chemotherapy. 1996;42(Suppl. 1.):54–61. doi: 10.1159/000239492. [DOI] [PubMed] [Google Scholar]
- 19.Morrissey I, George J T. Purification of pneumococcal type II topoisomerases and inhibition by gemifloxacin and other quinolones. J Antimicrob Chemother. 2000;45(Suppl. S1):101–106. doi: 10.1093/jac/45.suppl_3.101. [DOI] [PubMed] [Google Scholar]
- 20.Murgia R, Marchetti F, Cinco M. Comparative bacteriostatic and bactericidal activities of cefodizime against Borrelia burgdorferi sensu lato. J Antimicrob Chemother. 1999;43:3030–3032. doi: 10.1128/aac.43.12.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naber K G, Adam D. Einteilung der Fluorochinolone. Chemother J. 1998;7:66–68. [Google Scholar]
- 22.NCCLS. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard. 5th ed. Wayne, Pa: NCCLS; 2000. pp. M7–A5. [Google Scholar]
- 23.Oh J-I, Paek K-S, Ahn M-J, Kim M-Y, Hong C Y, Kim I-C, Kwak J-H. In vitro and in vivo evaluation of LB203004, a new fluoronaphthyridone. Antimicrob Agents Chemother. 1996;40:1564–1568. doi: 10.1128/aac.40.6.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Preac-Mursic V, Marget W, Busch U, Pleterski-Rigler D, Hagel S. Kill kinetics of Borrelia burgdorferi and bacterial findings in relation to the treatment of Lyme borreliosis. Infection. 1996;24:9–16. doi: 10.1007/BF01780643. [DOI] [PubMed] [Google Scholar]
- 25.Samuels D S, Garon C F. Coumermycin A1 inhibits growth and induces relaxation of supercoiled plasmids in Borrelia burgdorferi, the Lyme disease agent. Antimicrob Agents Chemother. 1993;37:46–50. doi: 10.1128/aac.37.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]