Abstract
Purpose
The aim of this study was to evaluate the effects of intrauterine platelet-rich plasma (PRP) infusion on endometrial thickness and pregnancy outcomes in a population of patients with either recurrent implantation failure (RIF), thin endometrium (TE), or both (RIF + TE)
Methods
This retrospective study included patients attending the CReATe Fertility Centre between October 2018 and July 2021 who received intrauterine PRP infusion to prepare the endometrium for frozen embryo transfer. PRP was prepared from 21 cc of whole blood using the 2-step centrifugation method to yield 0.5–0.75 cc of concentrated platelets. Endometrial thickness was measured before infusion and within 72 h after infusion. All embryos transferred were tested for genetic abnormalities using next-generation sequencing.
Results
A total of 85 patients, 133 cycles, and 211 infusions were included. The majority of patients (56.5%) were diagnosed with RIF, some with TE (27.0%), and the remainder with both RIF and TE (16.5%). The majority of patients received one PRP infusion per cycle (55%). The endometrial thickness significantly increased across all diagnoses with a significant increase of 1.0 mm (0.5–1.7), which was also significantly greater than in previous cycles. The clinical pregnancy rate per embryo transfer after intrauterine PRP infusion was significantly greater compared to previous cycles (37% vs 20%, odds ratio 2.2) as was the live birth rate (19% vs 2%, odds ratio 11.6).
Conclusion
Our study suggests that PRP should be considered a noninvasive front-line therapy for improving endometrial thickness and implantation in patients with RIF, a TE, or both.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-022-02505-0.
Keywords: Platelet-rich plasma, Recurrent implantation failure, Thin endometrium, Endometrial insufficiency
Introduction
Recurrent implantation failure (RIF) may be caused by poor endometrial quality, low blastocyst competence, or asynchronicity between embryo and endometrium. Therefore, endometrial quality is of paramount importance for successful embryo implantation. The etiologies for endometrial insufficiency are varied; it may be damaged, non-receptive, or non-proliferative among other causes. Previous studies have demonstrated a positive correlation between endometrial thickness (EMT) and embryo implantation rates [1, 2], which has led to the general clinical practice of cancelling embryo transfer in cycles where the patient’s endometrium thickness remains below 7 mm. Intriguingly, EMT and embryo implantation may be improved by endometrial scratching [3–5]; however, it is an invasive procedure with contradictory results [6–9]. To address issues of receptivity and asynchronicity between endometrium and embryo, efforts have been made to characterize the endometrial receptivity at various stages in the reproductive cycle [10]. Despite some successes in identifying aberrant receptivity status [10, 11], recent studies have challenged the efficacy of receptivity profiling, suggesting that the technology is invasive and its applicability is limited [12, 13]). Moreover, effective treatments for poor receptivity and thin endometrium are lacking.
One novel and promising treatment is the intrauterine infusion of autologous PRP. For this therapy, blood is drawn from the patient during the proliferative phase and processed to concentrate platelets in the plasma. Red and usually white blood cells are removed, leaving a small volume (0.3–1.0 cc) of autologous PRP, which is infused into the uterine cavity 1–5 days before embryo transfer. The procedure may be repeated if the EMT remains below the desired threshold of 7 mm. Early studies have shown significant improvements in EMT, embryo implantation, and clinical pregnancy in cases of a thin endometrium (TE) [14–16] as well as in cases of RIF [17, 18]. However, these clinical reports are sparse and limited to a few centres. Additionally, none of the studies to date has investigated transfers of euploid embryos, determined by preimplantation genetic testing for aneuploidy (PGT-A), and therefore cannot rule out failed implantation or pregnancy loss due to aneuploidy. Therefore, additional studies that include PGT-A-tested euploid embryos are necessary to better assess the efficacy of intrauterine PRP treatment. The objective of this study was to investigate the efficacy of intrauterine PRP infusion to improve endometrial thickness and implantation rates. Here, we report the EMT, biochemical, and clinical pregnancy outcomes of euploid embryo transfers from 85 patients with RIF and/or TE, who received PRP infusions.
Materials and methods
Study population
This retrospective cohort study received REB approval. Patients receiving PRP treatment at the CReATe Fertility Centre in Toronto, Ontario, Canada, from October 2018 to July 2021 were considered for analysis. Patients aged 24–52, diagnosed with recurrent implantation failure and/or persistent thin endometrium who had PGT-A-tested euploid embryos and received one or more intrauterine PRP infusions were included in this study. Patients were excluded if they had an inactive endometrium, multiple embryos transferred, genetic abnormalities, hematologic disorders, or an autoimmune disease. Diploid-aneuploid mosaic embryo transfers were also excluded.
PRP preparation and infusion
Autologous PRP was prepared using the 2-step centrifugation method [19]. Briefly, 21 cc of blood was drawn into ACD-A tubes and centrifuged at 900 relative centrifugal force (rcf) and 14 °C for 10 min. The upper layer containing the plasma and platelets was transferred to a new tube; the white and red blood cell layers were discarded. The plasma was centrifuged at 1500 rcf and 14 °C for 15 min to pellet the platelets. The platelet-poor plasma was discarded, leaving 0.5–0.75 cc to resuspend the platelets. The PRP was stored at 4 °C for 2 h or less until infusion.
Endometrial preparation and embryo transfer protocol
Treatment with autologous PRP was performed in estrogen-primed FET cycles. Administration of the PRP was performed between cycle days 10–15 and patients with an EMT remaining < 7 mm received infusions until the lining reached over 7 mm in thickness. The PRP was aspirated into a Tomcat catheter and infused into the uterus under ultrasound guidance. The EMT was measured by ultrasonography every 3–4 days, prior to each PRP infusion until the lining reached the target thickness. Vaginal progesterone (100 mg qid) or intramuscular progesterone in oil (50 mg/ml × 2 cc) was prescribed for luteal phase support starting 5 days prior to ET and continued until the pregnancy was confirmed. If negative, it was discontinued, if positive it was continued until 12 weeks gestation. Biochemical pregnancy was determined by blood serum levels of β-hCG > 1 at 2 weeks post transfer, and clinical pregnancy was determined by the presence of a gestational sac with a positive foetal heartbeat at 6–7 weeks post transfer.
Data analysis
Statistical analysis was performed using GraphPad Prism v8.0 (GraphPad). Differences between EMT pre- and post-infusion were calculated based on the Wilcoxon signed-rank test, where a p-value of < 0.05 was considered statistically significant. Differences in EMT presented in text and graphs are presented as median plus (interquartile range). Changes in EMT by diagnosis were compared by the Kruskal–Wallis test with Dunn’s multiple testing correction. Nominal variables, including implantation, spontaneous abortion, and clinical pregnancy rates, were compared using Fisher’s exact test. Relative risk was calculated with the Koopman asymptotic score and reported with 95% confidence intervals. Differences in clinical pregnancy rates between the three diagnoses were tested with a Chi-square test.
Results
Patient demographics
A total of 110 patients were considered for analysis, with 85 patients meeting exclusion criteria for a total of 133 treatment cycles and 211 infusions. The mean age of patients was 38.1 (SD ± 5.3) years old (Table 1). The most common cause of infertility identified was poor ovarian reserve (54% of patients). The majority of patients receiving PRP were diagnosed with RIF due to the failed transfer of 2 or more euploid embryos (n = 48, 56.5% of patients). An additional 23 (27.1%) patients were diagnosed with TE due to an EMT of < 7.0 mm on day 10 of cycle monitoring, and the remaining 14 (16.5%) patients were treated for both RIF and TE. No adverse effects after PRP infusion were observed in any of the patients.
Table 1.
Patient demographics
| Reason for PRP treatment | TE | RIF | RIF + TE | Total | |
| Number of patients | 23 | 48 | 14 | 85 | |
| Age (years) | 38.04 ± 5.27 | 37.09 ± 5.69 | 38.77 ± 5.32 | 37.07 ± 3.77 | |
| BMI (kg/m2) | 23.49 ± 4.28 | 23.6 ± 4.76 | 23.75 ± 4.31 | 22.44 ± 2.99 | |
| Infertility duration (years) | 2.04 ± 2.01 | 2.32 ± 2.24 | 2.03 ± 1.74 | 1.64 ± 2.32 | |
| Average number of past ETs | 2.51 ± 1.61 | 1.36 ± 1.41 | 3.2 ± 1.5 | 2.19 ± 0.95 | |
| Etiology of infertility | Poor ovarian reserve (LOR/DOR/POF) | 61% (14) | 56% (27) | 36% (5) | 54% (46) |
| Repeat pregnancy loss | 26% (6) | 23% (11) | 14% (2) | 22% (19) | |
| Advanced reproductive age | 30% (7) | 42% (20) | 29% (4) | 37% (31) | |
| Asherman’s | 9% (2) | 6% (3) | 14% (2) | 8% (7) | |
| Fibroids | 9% (2) | 13% (6) | 14% (2) | 12% (10) | |
| Other diagnoses | 17% (4) | 23% (11) | 43% (6) | 24% (21) |
Values presented as mean plus SD unless otherwise indicated
Endometrial proliferation in response to PRP
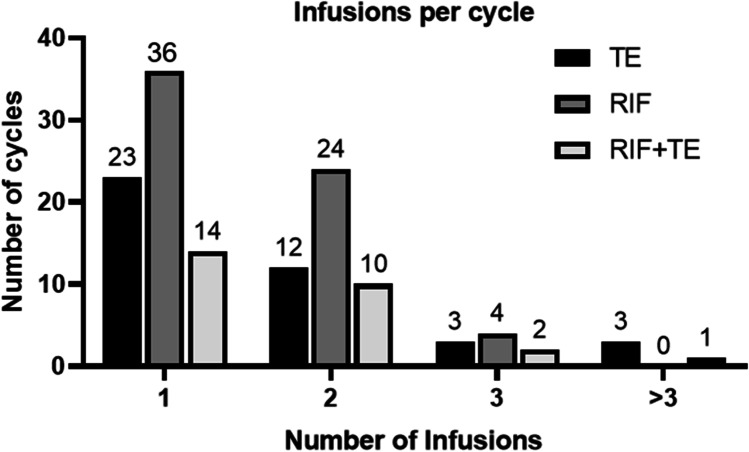
The number of PRP infusions was adjusted in response to endometrial proliferation, where patients whose EMT remained below 7 mm received multiple infusions. In the majority of cycles (n = 73, 55%), the patient received a single infusion. The number of patients and infusions is outlined in Fig. 1.
Fig. 1.
The number of cycles in which patients received one or more intrauterine PRP infusions. Patients were given multiple infusions when their EMT remained less than 7 mm
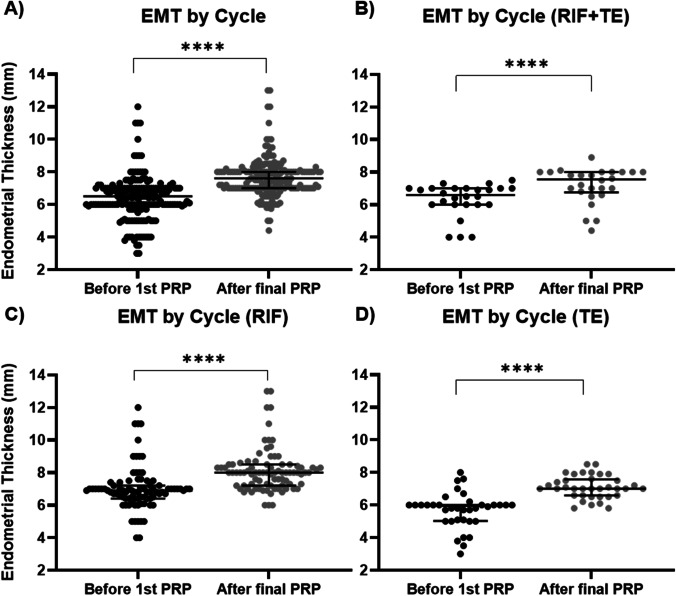
The intrauterine infusion of PRP one or more times in a cycle led to a significant increase in the median EMT from 6.7 (IQR 1.0) to 7.6 mm (IQR 1.0), regardless of diagnosis (Fig. 2A). When stratified by diagnosis, PRP significantly increased endometrial thickness in all groups (Fig. 2B-D). Furthermore, a single PRP infusion was sufficient to increase median endometrial thickness from 6.5 (IQR 1.0) to 7.3 mm (IQR 1.3) (p < 0.0001) overall, with significant increases seen in all groups (Supplementary Fig. 1).
Fig. 2.
Endometrial thickness increases in response to PRP treatment in cases of recurrent implantation failure and thin endometrium. Significant increases were seen across all patients (A) as well as when stratified for RIF + TE (B), RIF (C), and TE (D). ****p-value < 0.0001
In order to determine whether EMT changes induced by PRP were dependent on diagnosis, we compared the change in EMT by cycle between RIF, TE, and RIF + TE. We found that patients diagnosed with RIF + TE experienced a slightly smaller, but nonsignificant increase in median endometrial thickness (0.8 mm, IQR 0.6), when compared to RIF (1.0 mm, IQR 1.5, RIF + TE vs RIF p = 0.15) or TE (1.0 mm. IQR 1.2, RIF + TE vs TE p = 0.17) alone (Fig. 3). Although these data include patients receiving PRP in multiple cycles, PRP significantly improved EMT (median 6.7 to 7.8 IQR 1, p-value < 0.0001) even when analyzed by the first PRP cycle only (Supplementary Fig. 2).
Fig. 3.
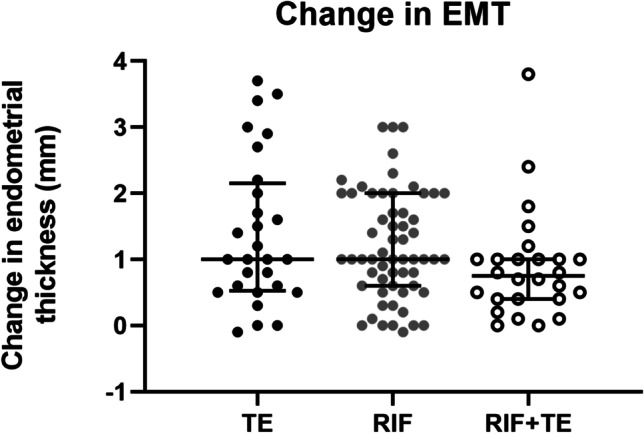
Change in EMT by cycle for each diagnosis
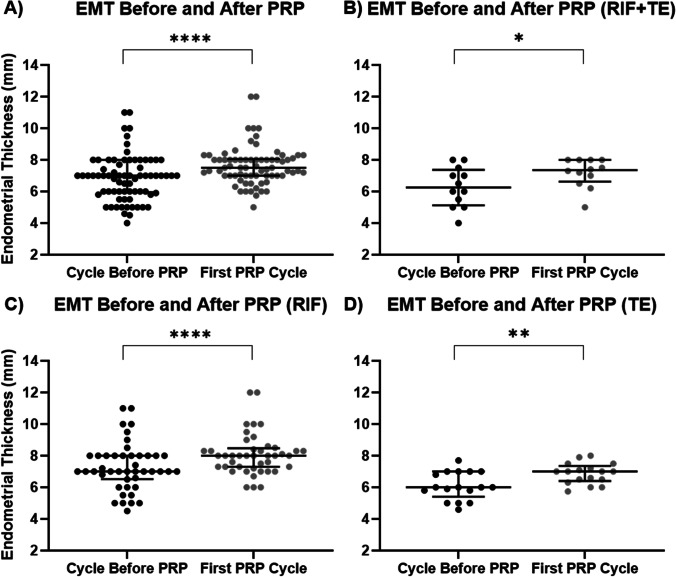
Next, we asked if the changes in EMT were mostly stimulated by the PRP treatment, instead of the routine endometrial proliferation as the cycle proceeds. Hence, we aimed to examine if there was a change in EMT in response to PRP compared to the most recent cycle at the end of hormone replacement therapy (HRT) for endometrial priming, where the patients had not been exposed to any PRP treatment. We recorded the EMT of the same patients on a day in the cycle preceding the PRP treatment, which matched the cycle day when the post-PRP measurement was taken, and found the overall EMT reached a median of 7.0 mm (IQR 2.0) in the cycle preceding PRP treatment. In contrast, after the first cycle of PRP infusion(s), the EMT increased to 7.5 mm (IQR 1; p < 0.0001) across all patients, regardless of diagnosis (Fig. 4).
Fig. 4.
EMT in comparison to previous cycles. EMT was compared on the same cycle day in the cycle immediately preceding PRP with that of the first cycle of PRP treatment. *p-value < 0.05, **p-value < 0.01, ****p-value < 0.0001
Embryo transfer outcomes
In addition to EMT, we investigated the pregnancy outcomes after the transfer of PGT-A-tested embryos in patients with a history of implantation failure and/or cancelled cycles due to a suboptimal endometrial thickness. The overall biochemical pregnancy rate in 116 cycles where patients received an intrauterine infusion of PRP prior to a single embryo transfer was 48.3% (Table 2) which was significantly higher than the 35.5% biochemical pregnancy rate for prior embryo transfers in the same patient population (RR 1.25, 95% CI 1.03–1.55). The overall clinical pregnancy rate after PRP infusions was also significantly higher at 37.1% than the 20.2% clinical pregnancy rate in previous embryo transfers (RR 1.27, 95% CI 1.10–1.50). The rate of spontaneous abortion was significantly ablated in the PRP group (RR 0.43, 95% CI 0.16–1.07), while live birth rates were higher at 19.6% compared to 2.9% in previous embryo transfers (RR 1.21, 95% CI 1.12–1.35). When stratified by diagnosis, the TE and RIF + TE groups did not show significant differences in pregnancy, spontaneous abortion, and live birth rates between PRP and prior, non-PRP cycles, possibly due to reduced sample size. In contrast, all outcomes were significantly improved in RIF patients. There were no significant differences in clinical pregnancy rate after PRP infusion when the three diagnoses were compared (Fig. 5).
Table 2.
Embryo transfer outcomes
| Outcome | PRP cycles | Previous cycles | Risk ratio (95% CI) |
p-value |
|---|---|---|---|---|
| Complete population | n = 116 | n = 187 | ||
| Biochemical pregnancy | 56 (48.3%) | 72 (35.5%) | 1.25 (1.03–1.55) | 0.03 |
| Clinical pregnancy | 43 (37.1%) | 41 (20.2%) | 1.27 (1.10–1.50) | < 0.01 |
| Spontaneous abortion | 34 (60.7%) | 68 (94.4%) | 0.43 (0.16–1.07) | 0.09 |
| Live birth | 22 (19.0%) | 4 (2.0%) | 1.21 (1.12–1.35) | < 0.01 |
| Thin endometrium | n = 31 | n = 18 | ||
| Biochemical pregnancy | 16 (51.6%) | 18 (52.9%) | 1.03 (0.61–1.72) | > 0.99 |
| Clinical pregnancy | 9 (29.0%) | 9 (26.5%) | 1.04 (0.76–1.44) | > 0.99 |
| Spontaneous abortion | 11 (68.8%) | 17 (94.4%) | 0.18 (0.03–1.00) | 0.08 |
| Live birth | 5 (16.1%) | 1 (5.6%) | 1.13 (0.89–1.42) | 0.38 |
| Recurrent implantation failure | n = 61 | n = 141 | ||
| Biochemical pregnancy | 31 (50.8%) | 48 (34.0%) | 1.34 (1.04–1.82) | 0.03 |
| Clinical pregnancy | 27 (44.3%) | 29 (20.6%) | 1.43 (1.15–1.86) | < 0.01 |
| Spontaneous abortion | 17 (54.8%) | 45 (93.8%) | 0.14 (0.05–0.41) | < 0.01 |
| Live birth | 14 (23%) | 3 (2.1%) | 1.27 (1.14–1.51) | < 0.01 |
| Recurrent implantation failure and thin endometrium | n = 24 | n = 28 | ||
| Biochemical pregnancy | 9 (37.5%) | 6 (21.4%) | 1.26 (0.88–1.90) | 0.23 |
| Clinical pregnancy | 7 (29.2%) | 3 (10.7%) | 1.26 (0.96–1.78) | 0.15 |
| Spontaneous abortion | 6 (66.7%) | 6 (100%) | 0 (0–1.47) | 0.22 |
| Live birth | 3 (19.0%) | 0 (0.0%) | ∞ (1.06–∞) | 0.09 |
n = number of embryo transfers. The total number of embryo transfers is less than the number of PRP cycles due to cancelled cycles (inadequate endometrial growth, COVID-19 exposures). Biochemical pregnancy rates are calculated as a percentage of single embryo transfers, while the other outcome rates are calculated as the percentage of biochemical pregnancies
Fig. 5.
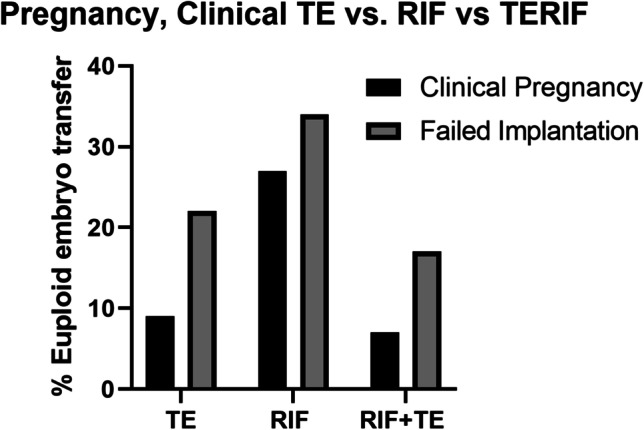
Pregnancy outcomes by diagnosis. No significant differences in the implantation or pregnancy rates were observed between diagnoses after PRP infusion
Discussion
Current treatments for RIF and TE are limited, and no single treatment has emerged as a standard of care. Autologous PRP potentially offers a noninvasive and direct method of applying concentrated growth factors and cytokines to the refractory endometrium. Studies demonstrate a milieu of stimulatory factors in PRP, including platelet-derived growth factor (PDGF), transforming growth factor (TGF), various interleukins (IL), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), and insulin-like growth factor (IGF) [20].
Autologous PRP has shown benefits in many medical contexts including alopecia, osteoarthritis, and some gynaecological disorders [21–23]. The intrauterine infusion was first reported by Chang and colleagues in 2015 but has been demonstrated to be effective in treating both RIF and TE patients in several studies since [14–16, 18]. In similar studies of sub-endometrial injection of PRP, one has shown a benefit in clinical pregnancy rates for TE patients [24], while another in RIF patients shows a nonsignificant increase in pregnancy and live birth [25]. In this study, we observed significant increases in EMT after a cycle of PRP infusions in all our patients. When compared to patients’ EMT at the end of HRT in the cycle prior to PRP therapy, their EMT post-PRP infusions were still significantly higher, suggesting the endometrial proliferation we observed in all three groups is largely contributed by the PRP therapy. Specifically, there were slightly smaller increases seen in RIF + TE patients when compared to either condition alone. It is likely that patients with RIF + TE suffer from multiple etiologies resulting in an endometrium that is particularly resistant to proliferation induced by PRP. Regardless, these increases are especially remarkable in the TE group, given that these patients have a history of cancelled embryo transfers due to persistent suboptimal endometrial thickness. This may indicate that patients diagnosed with TE only may benefit most from PRP infusions. Our data support previously published reports on the efficacy of PRP to improve endometrial thickness [26].
Although adequate endometrial thickness is key for establishing and maintaining pregnancy [27], the embryo must also be of sufficient quality for attachment and implantation. Embryo aneuploidy is highly dependent on the age of the female providing eggs, affecting 50% or more of embryos in women over 35, and typically results in failed implantation or early miscarriage [28]. To our knowledge, our study is the first to report pregnancy rates in patients utilizing intrauterine PRP prior to the transfer of PGT-A-tested euploid embryos. Analysing transfer outcomes of euploid embryos reduces the risk of confounding the data with failed pregnancies due to aneuploidies. Our overall clinical pregnancy rate of 37% in this challenging patient population is slightly below the Canadian single FET average of 39.7% [29] and is a significant improvement in the same population when compared to their previous non-PRP cycles (20.2%). The majority of the differences seen in pregnancy outcomes in this study were likely driven by the RIF group, which accounted for the majority of embryo transfers and also showed a remarkable improvement from the transfers prior. One of the limitations of this study is that a comparison with previous cycles is inherently biased, given a selection towards patients struggling to conceive through ARTs. Secondly, the previous embryo transfer data is incomplete for patients who transferred from other clinics and the records were unavailable.
Conclusions
Here, we present a retrospective analysis of patients diagnosed with RIF and/or TE, who received a novel intrauterine PRP infusion therapy prior to the transfer of PGT-A-tested euploid embryos. We observed a significant increase in endometrial thickness of 1.2 ± 0.21 mm and a clinical pregnancy rate of 37%, which is remarkable progress compared to the previous endometrial thickness measurements and embryo transfer outcomes in this set of patients. In conclusion, intrauterine PRP infusion should be considered a noninvasive front-line therapy for improving endometrial thickness and implantation.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
All authors contributed to the study’s conception and design. S. R. analyzed the data and wrote the manuscript. Y. S. K. collected and analyzed the data and reviewed the manuscript. T.T.N.N aided in the design, analysis, and revisions. C.L. aided in the writing and revisions. All authors read and approved the final manuscript.
Funding
The study was funded by the CReATe Fertility Centre through reinvestment of clinical earnings.
Data availability
De-identified patient data used for this study is available upon request.
Code availability
No code was used for the analysis of the data.
Declarations
Ethics approval
This study was approved by Veritas IRB under protocol number 16367.
Consent to participate
Patients gave informed consent for the collection and publication of their de-identified clinical data.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang X, Chen C-H, Confino E, Barnes R, Milad M, Kazer RR. Increased endometrial thickness is associated with improved treatment outcome for selected patients undergoing in vitro fertilization–embryo transfer [Internet]. Fertil Steril. 2005. 336–40. Available from: 10.1016/j.fertnstert.2004.09.020 [DOI] [PubMed]
- 2.Al-Ghamdi A, Coskun S, Al-Hassan S, Al-Rejjal R, Awartani K. The correlation between endometrial thickness and outcome of in vitro fertilization and embryo transfer (IVF-ET) outcome. Reprod Biol Endocrinol. 2008;6:37. doi: 10.1186/1477-7827-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barash A, Dekel N, Fieldust S, Segal I, Schechtman E, Granot I. Local injury to the endometrium doubles the incidence of successful pregnancies in patients undergoing in vitro fertilization. Fertil Steril. 2003;79:1317–1322. doi: 10.1016/S0015-0282(03)00345-5. [DOI] [PubMed] [Google Scholar]
- 4.Nastri CO, Ferriani RA, Raine-Fenning NJ, Martins WP. OC11.01: Endometrial injury performed in the non-transfer cycle and assisted reproduction outcomes: a randomized controlled trial [Internet]. Ultrasound Obstet Gynecol. 2013. 20–1. Available from: 10.1002/uog.12638 [DOI] [PubMed]
- 5.Raziel A, Schachter M, Strassburger D, Bern O, Ronel R, Friedler S. Favorable influence of local injury to the endometrium in intracytoplasmic sperm injection patients with high-order implantation failure [Internet]. Fertil Steril. 2007. 198–201. Available from: 10.1016/j.fertnstert.2006.05.062 [DOI] [PubMed]
- 6.Shokeir T, Ebrahim M, El-Mogy H. Hysteroscopic-guided local endometrial injury does not improve natural cycle pregnancy rate in women with unexplained infertility: randomized controlled trial [Internet]. Obstet Gynaecol Res. 2016. 1553–7. Available from: 10.1111/jog.13077 [DOI] [PubMed]
- 7.Dain L, Ojha K, Bider D, Levron J, Zinchenko V, Walster S, et al. Effect of local endometrial injury on pregnancy outcomes in ovum donation cycles [Internet]. Fertil Steril. 2014. 1048–54. Available from: 10.1016/j.fertnstert.2014.06.044 [DOI] [PubMed]
- 8.Yeung TWY, Chai J, Li RHW, Lee VCY, Ho PC, Ng EHY. The effect of endometrial injury on ongoing pregnancy rate in unselected subfertile women undergoing in vitro fertilization: a randomized controlled trial. Hum Reprod. 2014;29:2474–2481. doi: 10.1093/humrep/deu213. [DOI] [PubMed] [Google Scholar]
- 9.Lensen S, Osavlyuk D, Armstrong S, Stadelmann C, Hennes A, Napier E, et al. A randomized trial of endometrial scratching before in vitro fertilization. N Engl J Med. 2019;380:325–334. doi: 10.1056/NEJMoa1808737. [DOI] [PubMed] [Google Scholar]
- 10.Ruiz-Alonso M, Blesa D, Díaz-Gimeno P, Gómez E, Fernández-Sánchez M, Carranza F, et al. The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil Steril. 2013;100:818–824. doi: 10.1016/j.fertnstert.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Díaz-Gimeno P, Ruiz-Alonso M, Blesa D, Bosch N, Martínez-Conejero JA, Alamá P, et al. The accuracy and reproducibility of the endometrial receptivity array is superior to histology as a diagnostic method for endometrial receptivity. Fertil Steril. 2013;99:508–517. doi: 10.1016/j.fertnstert.2012.09.046. [DOI] [PubMed] [Google Scholar]
- 12.Neves AR, Devesa M, Martínez F, Garcia-Martinez S, Rodriguez I, Polyzos NP, et al. What is the clinical impact of the endometrial receptivity array in PGT-A and oocyte donation cycles? J Assist Reprod Genet. 2019;36:1901–1908. doi: 10.1007/s10815-019-01535-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan J, Kan A, Hitkari J, Taylor B, Tallon N, Warraich G, et al. The role of the endometrial receptivity array (ERA) in patients who have failed euploid embryo transfers. J Assist Reprod Genet Springer. 2018;35:683–692. doi: 10.1007/s10815-017-1112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang Y, Li J, Chen Y, Wei L, Yang X, Shi Y, et al. Autologous platelet-rich plasma promotes endometrial growth and improves pregnancy outcome during in vitro fertilization. Int J Clin Exp Med. 2015;8:1286–1290. [PMC free article] [PubMed] [Google Scholar]
- 15.Nazari L, Salehpour S, Hoseini S, Zadehmodarres S, Azargashb E. Effects of autologous platelet-rich plasma on endometrial expansion in patients undergoing frozen-thawed embryo transfer: a double-blind RCT. Int J Reprod Biomed. 2019;17:443–448. doi: 10.18502/ijrm.v17i6.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eftekhar M, Neghab N, Naghshineh E, Khani P. Can autologous platelet rich plasma expand endometrial thickness and improve pregnancy rate during frozen-thawed embryo transfer cycle? A randomized clinical trial. Taiwan J Obstet Gynecol. 2018;57:810–813. doi: 10.1016/j.tjog.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Nazari L, Salehpour S, Hosseini MS, Moghanjoughi PH. The effects of autologous platelet-rich plasma in repeated implantation failure: a randomized controlled trial [Internet]. Human Fertil. 2020. 209–13. Available from: 10.1080/14647273.2019.1569268 [DOI] [PubMed]
- 18.Dieamant F, Vagnini LD, Petersen CG, Mauri AL, Renzi A, Petersen B, et al. New therapeutic protocol for improvement of endometrial receptivity (PRIMER) for patients with recurrent implantation failure (RIF) - a pilot study. JBRA Assist Reprod. 2019;23:250–254. doi: 10.5935/1518-0557.20190035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhurat Rachita, Sukesh Ms. Principles and methods of preparation of platelet-rich plasma: a review and author’s perspective. J Cutan Aesthet Surg Wolters Kluwer -- Medknow Publ. 2014;7:189. doi: 10.4103/0974-2077.150734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lubkowska A, Dolegowska B, Banfi G. Growth factor content in PRP and their applicability in medicine. J Biol Regul Homeost Agents [Internet]. J Biol Regul Homeost Agents; 2012 [cited 2021 Mar 1];26. Available from: https://pubmed.ncbi.nlm.nih.gov/23648195/ [PubMed]
- 21.Filardo G, Previtali D, Napoli F, Candrian C, Zaffagnini S, Grassi A. PRP injections for the treatment of knee osteoarthritis: a meta-analysis of randomized controlled trials. Cartilage. 2020;1947603520931170 [DOI] [PMC free article] [PubMed]
- 22.Kramer ME, Keaney TC. Systematic review of platelet-rich plasma (PRP) preparation and composition for the treatment of androgenetic alopecia. J Cosmet Dermatol. 2018;17:666–671. doi: 10.1111/jocd.12679. [DOI] [PubMed] [Google Scholar]
- 23.Dawood AS, Salem HA. Current clinical applications of platelet-rich plasma in various gynecological disorders: an appraisal of theory and practice. Clin Exp Reprod Med. 2018;45:67–74. doi: 10.5653/cerm.2018.45.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agarwal M, Mettler L, Jain S, Meshram S, Günther V, Alkatout I. Management of a thin endometrium by hysteroscopic instillation of platelet-rich plasma into the endomyometrial junction: a pilot study. J Clin Med. 2020;9(9):2795. doi: 10.3390/jcm9092795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zargar M, Pazhouhanfar R, Najafian M, Choghakabodi P. Effects of intrauterine autologous platelet-rich plasma infusions on outcomes in women with repetitive in vitro fertilization failures: a prospective randomized study. Clin Exp Obstet Gynecol. 2020;48:179–184. [Google Scholar]
- 26.Maleki-Hajiagha A, Razavi M, Rouholamin S, Rezaeinejad M, Maroufizadeh S, Sepidarkish M. Intrauterine infusion of autologous platelet-rich plasma in women undergoing assisted reproduction: a systematic review and meta-analysis. J Reprod Immunol. 2020;137:103078. doi: 10.1016/j.jri.2019.103078. [DOI] [PubMed] [Google Scholar]
- 27.Wu Y, Gao X, Lu X, Xi J, Jiang S, Sun Y, et al. Endometrial thickness affects the outcome of in vitro fertilization and embryo transfer in normal responders after GnRH antagonist administration. Reprod Biol Endocrinol. 2014;12:96. doi: 10.1186/1477-7827-12-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, et al. The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril Elsevier Inc. 2014;101:656–63. doi: 10.1016/j.fertnstert.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Lanes A, Fell DB, Teitelbaum M, Sprague AE, Johnson M, Wang H, et al. CARTR Plus: the creation of an ART registry in Canada. Hum Reprod Open. 2020;2020:hoaa022. doi: 10.1093/hropen/hoaa022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified patient data used for this study is available upon request.
No code was used for the analysis of the data.