Abstract
Selective delivery of gold nanorods (GNRs) to sites of prostate tumor angiogenesis is potentially advantageous for localized photothermal therapy. Here, we report the cellular uptake and biodistribution of GNRs surface functionalized with the cyclic RGDfK peptide. The GNRs were synthesized to have a surface plasmon resonance (SPR) peak at 800 nm and grafted with a thiolated poly(ethylene glycol) (PEG) corona with or without RGDfK. The binding and uptake of the targeted (RGDfK) and untargeted GNRs were evaluated in DU145 prostate cancer and human umbilical vein endothelial cells (HUVEC) by high-resolution dark field microscopy, inductively coupled plasma mass spectrometry (ICP-MS), and transmission electron microscopy (TEM). The biodistribution of both GNRs was then evaluated in prostate tumor bearing mice. Targeting of the RGDfK surface-modified GNRs was confirmed in vitro due to selective binding and uptake by endothelial cells. Tumor targeting was not observed in vivo, however, due to fast clearance of the RGDfK-GNRs from the blood. Further modifications of the nanoparticle’s surface properties are needed to enhance localization of the targetable system in sites of tumor angiogenesis.
Keywords: Gold nanorods, RGD, angiogenesis targeting, prostate cancer
Introduction
The ability to precisely control material geometries with nanoscale dimension has enabled researchers with new and powerful tools not available when the same materials are in their bulk form. Examples of this include self-assembled structures from nanoparticle precursors (Li et al., 1999), precise control of reaction catalysis by modulation of nanoparticle shape and size (Narayanan & El-Sayed, 2005), as well as the fabrication of materials from nanotubes with unprecedented tensile strength and moduli (Breuer & Sundararaj, 2004). The unique optical properties of metal colloids whose dimensions are significantly smaller than the wavelength of light are a particular example as particle vibrations give rise to size and shape dependent light absorption and scattering phenomenon (Kelly et al., 2003). For this reason gold nanoparticles have received significant attention in medicine due to their utility in disease detection and therapy (Huang et al., 2007).
Gold nanoparticles in a variety of shapes, e.g. spheres, shells, and cages, can act as multifunctional platforms. In addition to their use as optical imaging-based contrast agents (Copland et al., 2004; Chen et al., 2005; Tong et al., 2009), gold nanoparticles are able to serve as delivery vehicles for drugs (Paciotti et al., 2004; Yavuz et al., 2009) and antennas for photothermal therapy (Hirsch et al., 2003; O’Neal et al., 2004; Dickerson et al., 2008; Stern et al., 2008). Current methods for tumor delivery leverage the vasculature’s enhanced permeability and retention (EPR), which occurs due to both enlarged endothelial fenestrae as well as the lack of a functioning lymphatic system (Maeda et al., 2006; Iyer et al., 2006). This technique for delivery is ubiquitous in drug delivery to solid tumors and is partially responsible for much of the success observed in that field (Duncan, 2003; O’Brien et al., 2004; Matsumura & Kataoka, 2009).
One approach to improve the delivery and tumor retention of gold nanoparticles is to employ active targeting by conjugation of targeting moieties to their surface. Examples of such targeting strategies include conjugation of anti-EGFR (El-Sayed et al., 2005) and anti-HER2 (Loo et al., 2005) antibodies, folic acid (Dixit et al., 2006), as well as nuclear localizing peptides (Tkachenko et al., 2004). In this way, selective delivery to tumor-specific markers can potentially improve the degree of tumor localization compared to passive strategies.
One set of targets that have received significant attention in the last decade are the αv β3 integrins, which regulate cell adhesion to extracellular matrix proteins. Able to bind with high affinity to the Arg-Gly-Asp (RGD) sequence of matrix proteins, such as fibronectin and vitronectin (Ruoslahti & Pierschbacher, 1987), αv β3 integrins coating the angiogenic endothelium can be used as targets for nanoconstructs with surface-modified variations of the RGD peptide (Pasqualini et al., 1997). Of particular interest in this regard is the use of the mono-cyclic RGDfK peptide which has higher affinity for αv β3 integrins and greater tumor-targeting characteristics than linear RGD (Thumshirn et al., 2003; Dijkgraaf et al., 2007). This technique for tumor targeting has been well characterized and validated using N-(2-hydroxypropyl) methacrylamide (HPMA) copolymers (Line et al., 2005; Mitra et al., 2005; Mitra et al., 2006a; 2006b; Borgman et al., 2008; Borgman et al., 2009a; 2009b; Zarabi et al., 2009) and has resulted in significant prostate tumor efficacy with both geldanamycin (Greish et al., 2011) and docetaxel (Ray et al., 2011) attached to the side chains.
In the context of using gold nanorods (GNRs) as antennas for plasmonic photothermal therapy (PPTT) (Huang et al., 2008), their active delivery to sites of tumor angiogenesis by RGDfK targeting may be advantageous. The vasculature which feeds cancerous cells has minimal organization and is devoid of smooth muscle, ultimately impeding the capacity of this tissue to respond to heat stress. Transient increases in blood flow and vascular permeability up to 43°C is typically followed by vascular collapse, hemorrhage, and circulatory stasis as temperatures rise above this threshold level (Song et al., 1980; Dudar & Jain, 1984; Horsman, 2006). During conventional hyperthermia or that induced by PPTT using passive delivery approaches, tumor mass heating ultimately causes vascular damage once the whole tumor volume has been heated above 43°C. We ultimately hypothesize, however, that by directing GNRs to the tumor’s vasculature using cyclic RGD, significantly less heat will be required to initiate vascular damage (Scheme 1). To begin testing this hypothesis, in the present paper, we first evaluated the in vitro cellular binding and uptake and in vivo biodistribution of PEGylated GNRs functionalized with RGDfK.
Scheme 1.
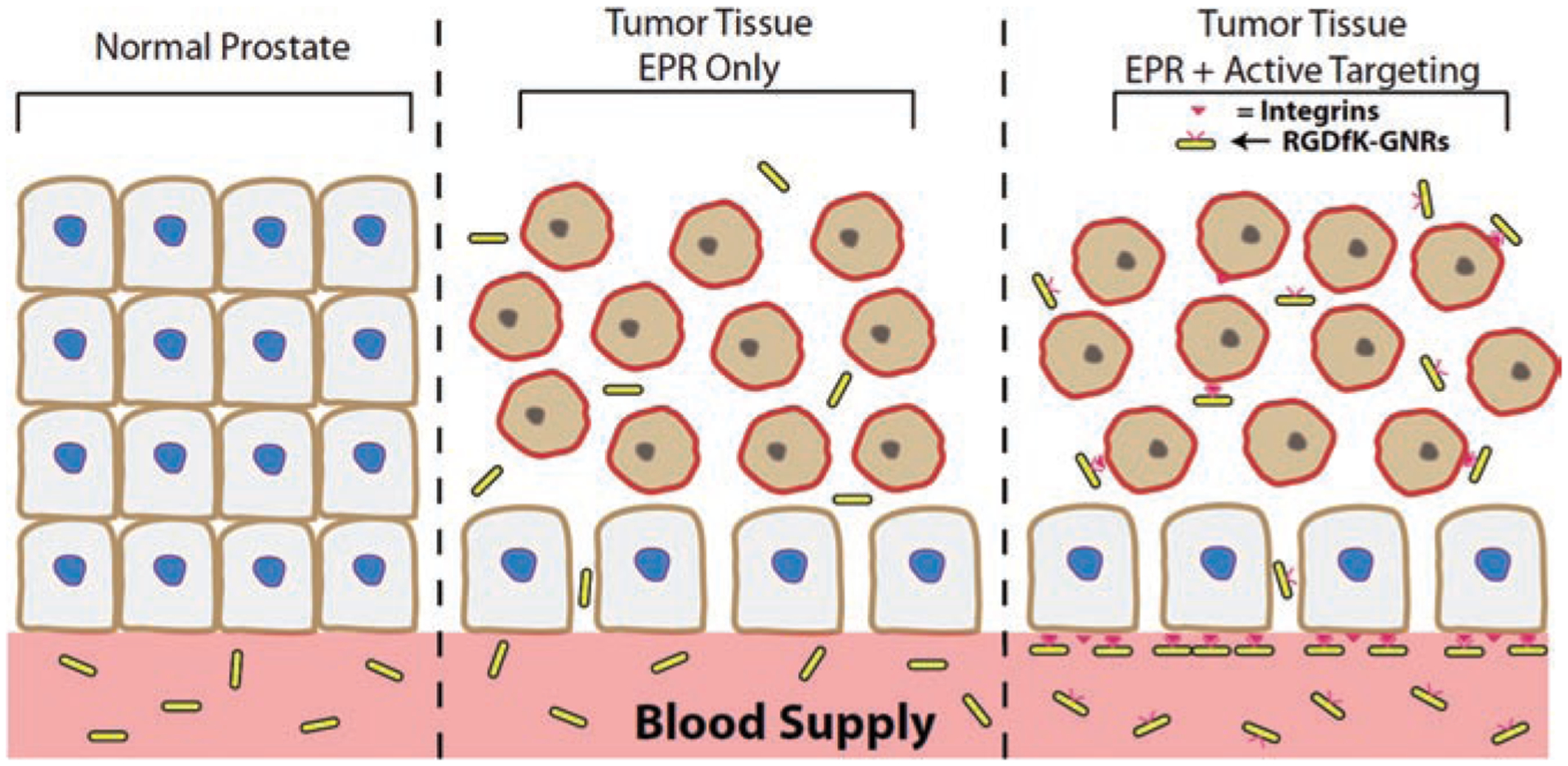
GNR delivery mechanism. (A) GNRs do not accumulate in normal tissue due to tight fenestrae in vasculature. (B) GNRs can passively diffuse in malignant tissue by EPR effect. (C) Active targeting may increase GNR concentration at the vascular bed and localize them to heat sensitive vasculature, though a balance needs to be maintained for charge, size, degree of PEGylation, and targeting moiety content.
When tested for binding and uptake with prostate cancer and endothelial cells, the system performed as intended. The targeted GNRs had significant binding to cells with an angiogenic phenotype, and this binding was found to be specific to interactions with αv β3 integrins. However, when the RGDfK-containing GNRs were compared to ones without the targeting moiety in mice bearing prostate tumors, fast blood clearance diminished the tumor accumulation of the targeted GNRs suggesting that further modifications to this system may be needed for accomplishing enhanced tumor delivery in vivo.
Methods
GNR synthesis and characterization
The GNRs were synthesized using the seed-mediated growth method (Nikoobakht & El-Sayed, 2003). Optimization of silver nitrate content and seed amount yielded GNRs with an aspect ratio such that the surface plasmon resonance (SPR) peak was between 800 and 810 nm. The GNRs were then centrifuged (6000 rcf, 30 min) and washed three times with deionized (DI) water to remove excess hexadecyltrimethylammonium bromide (CTAB). For the untargeted GNRs, poly(ethylene glycol) (PEG) (50 mg, methoxy-PEG-thiol, 5 kD, Creative PEGWorks #PLS-604) was added to the GNR suspension (100 mL, 100 μg/mL, optical density (OD) = 10) at a final PEG concentration of 100 μM and stirred for 1 h. This was done to reduce the extent of protein adsorption and improve circulation time (Niidome et al., 2006). The PEG-GNR suspension was then thoroughly dialyzed (3.5 K MWCO, Spectrum Labs #132594) and sterile filtered. Finally, the GNR suspension was centrifuged, washed three times with DI water to remove unreacted PEG and concentrated to a final concentration of 1.2 mg/mL (OD = 120). Final product was stored at 4°C for a maximum of 2 months due to polymer shedding over time before use.
Targeted GNRs were synthesized by first reacting ortho-pyridyl-disulfide-PEG-succinimidyl ester (OPSS-PEG-NHS, 5 kD, Creative PEGWorks #PHB-997, 50 mg) with RGDfK (New England Peptide, Inc., 6 mg) in anhydrous DMSO (5 mL) and three drops of diisopropylethylamine (DIPEA) while stirring for 24 h at room temperature. Dithiothreitol (DTT, 7 mg) was then added to the reaction mixture and stirred for additional 2 h to reduce the disulfide and obtain a free thiol at the end of the PEG-RGDfK polymer. The mixture was then dialyzed (3.5 K MWCO, Spectrum Labs #132594) and lyophilized to obtain the final product followed by confirmation of peptide attachment by amino acid analysis. Finally, the thiol-PEG-RGDfK polymer was grafted to the gold surface in the same way as the untargeted GNR conjugate.
The size and shape of GNR were measured by transmission electron microscopy (TEM, FEI Tecnai T12) after drop-casting the GNR suspension onto a copper grid. The GNR light absorption profile was measured before and after PEGylation using a spectrophotometer (Jasco V-650) and the stability of these conjugates was measured the same way after 30 min in 3.5% NaCl. The GNR concentration was determined by inductively coupled plasma mass spectrometry (ICP-MS, Agilent 7500ce) against a gold and internal (irradium) standard. The zeta potential of the conjugates was measured in DI water by measuring the particle’s electrophoretic mobility using laser doppler velocimetry (Malvern Instruments Zetasizer Nano-ZS). Finally, the RGDfK content on the gold was determined by amino acid analysis (University of Utah Core Research Facilities, Salt Lake City, UT, USA).
Cell culture
The binding and uptake was evaluated for targeted (RGDfK) and untargeted GNRs in two cell lines obtained from ATCC (Manassas, VA, USA): DU145 prostate cancer and human umbilical vein endothelial cells (HUVEC). The DU145 cell lines were cultured in Eagle’s Minimum Essential Medium with Earle’s Balanced Salt Solution (ATCC) supplemented with 10% (v/v) fetal bovine serum (FBS) (Thermo Scientific HyClone, Logan, UT, USA). The HUVEC cell lines were cultured in Clonetics Endothelial Cell Basal Medium-2 supplemented with 2% FBS, hydrocortisone, hFGF-B, VEGF, R3-IGF-1, ascorbic acid, hEGF, GA-1000, and heparin (Lonza EGM-2 BulletKit). Cell lines were cultured at 37°C in 100% humidity with 5% CO2. All cells were kept within logarithmic growth, and while DU145 cells were kept under 20 passages, HUVEC cells were discarded after seven.
Dark field microscopy
Cells were plated on sterile cover slips coated with fibronectin and allowed to grow until 50% confluent. The media was then replaced with either fresh media or media containing either the RGDfK or untargeted GNRs (10 μg/mL). Cells were allowed to incubate for 24 h followed by aspiration of GNR-containing media and three washing steps with phosphate buffered saline (PBS) followed by fixation for 10 min with 4% paraformaldehyde before mounting to a slide with mounting medium. To detect association (binding and uptake) of GNRs with the cells, slides were then imaged with an Olympus BX41 microscope coupled to the CytoViva 150 Ultra Resolution Imaging (URI) System (CytoViva Inc., Auburn, AL, USA) using a 100x oil objective (Vainrub et al., 2006; Skebo et al., 2007). A DAGE XLM (DAGE-MTI, Michigan City, IN, USA) digital camera and software were used to capture and store images.
ICP-MS
To quantify binding and uptake, cells were plated in 24-well plates and allowed to grow to 70% confluency. After incubation with GNRs and washing with PBS as described above, cells were lysed with 100 mM sodium hydroxide for 20 min while shaking and the protein content for each well was determined using a bicinchoninic (BCA) protein assay (Micro BCA Protein Assay Kit, Thermo Scientific Inc., Rockford, IL, USA). The lysate was then transferred to Teflon vials, digested and evaporated three times with fresh trace-metal grade aqua regia, then resuspended in 5% trace-metal grade nitric acid before being analyzed by ICP-MS for gold content quantification against a gold and internal standard. All groups were done in triplicates.
TEM
For visualization of uptake by TEM, cells were grown to 50% confluency on fibronectin-coated ACLAR® plastic films before 24 h incubation with GNRs. Cells were then washed three times with PBS and fixed with 2.5% glutaraldehyde and 1% paraformaldehyde in 0.1M sodium cacodylate with sucrose and calcium chloride. Samples were then dehydrated with washes of increasing concentrations of ethanol and embedded in an epoxy resin before sectioning with an ultramicrotome. All samples were then imaged using a FEI Tecnai T12 microscope (University of Utah Core Research Facilities, Salt Lake City, UT, USA).
Competitive inhibition of binding
Confirmation of RGDfK-GNR specificity to αv β3 integrins was performed by competitive inhibition of binding with echistatin. In brief, HUVEC cells were grown to 50% confluency on fibronectin-coated cover slips. The media was then removed and replaced with cold binding buffer (20 mmol/L Tris, pH 7.4, 150 mmol/L NaCl, 2 mmol/L CaCl2, 1 mmol/L MgCl2, 1 mmol/L MnCl2, 0.1% bovine serum albumin) containing RGDfK-GNRs (10 μg/mL), and HUVECs were co-incubated at 4°C for 2 hours with or without 50 nM echistatin (Sigma-Aldrich). Cells were then washed three times with cold binding buffer, mounted to a slide, and imaged by high-resolution dark field microscopy.
Biodistribution in prostate tumor bearing Mice
Six-week-old female athymic (nu/nu) mice were obtained from Charles River Laboratories (Davis, CA, USA) and used in accordance with the Institutional Animal Care and Use Committee (IACUC) of the University of Utah. To initiate prostate cancer xenografts, mice were anesthetized using 4% isofluorane, and 107 DU145 cells in 200 μL PBS were injected bilaterally on the flank of each animal. Tumors were then allowed to grow until average tumor volume reached 50–100 mm3 (usually 10–21 days).
The animals were separated randomly into two groups. Half received untargeted GNRs and the other half RGDfK-GNRs (9.6 mg/kg in 200 μL, OD = 120) by intravenous injection through the tail vein. The animals were allowed to rest for 6, 24, and 48 h before sacrifice by CO2 inhalation. After sacrifice, blood was collected using a heparinized needle from the inferior vena cava and the animals were perfused with at least 20 mL of saline by cardiac puncture. Blood and organs, such as the liver, spleen, lungs, heart, and kidneys, were collected and weighed as well as the tumors. Each sample was refluxed in 4 mL of fresh trace-metal grade aqua regia at 90°C for 24 h, and then dried at 130°C. Subsequently, samples were dissolved in 4 mL of 5% trace-metal grade nitric acid before quantification of gold content by ICP-MS against a gold and internal standard.
Results
GNR synthesis and characterization
The GNRs were synthesized with an SPR peak at 800 nm corresponding to a size of 60.5 × 15.0 ± 6.4 × 2.0 nm with an aspect ratio equal to 4.0 (Figure 1, Table 1). After PEGylation, with or without RGDfK, there was minimal change in absorption profile and the nanoparticles had strong stability in the presence of 3.5% NaCl. Zeta potential measures indicate that while the untargeted (methoxy terminated) GNRs had a slight negative charge (−10.0 mV), the RGDfK-GNRs had a strong negative charge (−44.1 mV). Amino acid analysis confirmed the presence of RGDfK on the targeted GNRs with a concentration equal to 5.6 × 10−11 MRGDfK/μgAu or roughly 3.0 × 103 RGDfKs per GNR.
Figure 1.
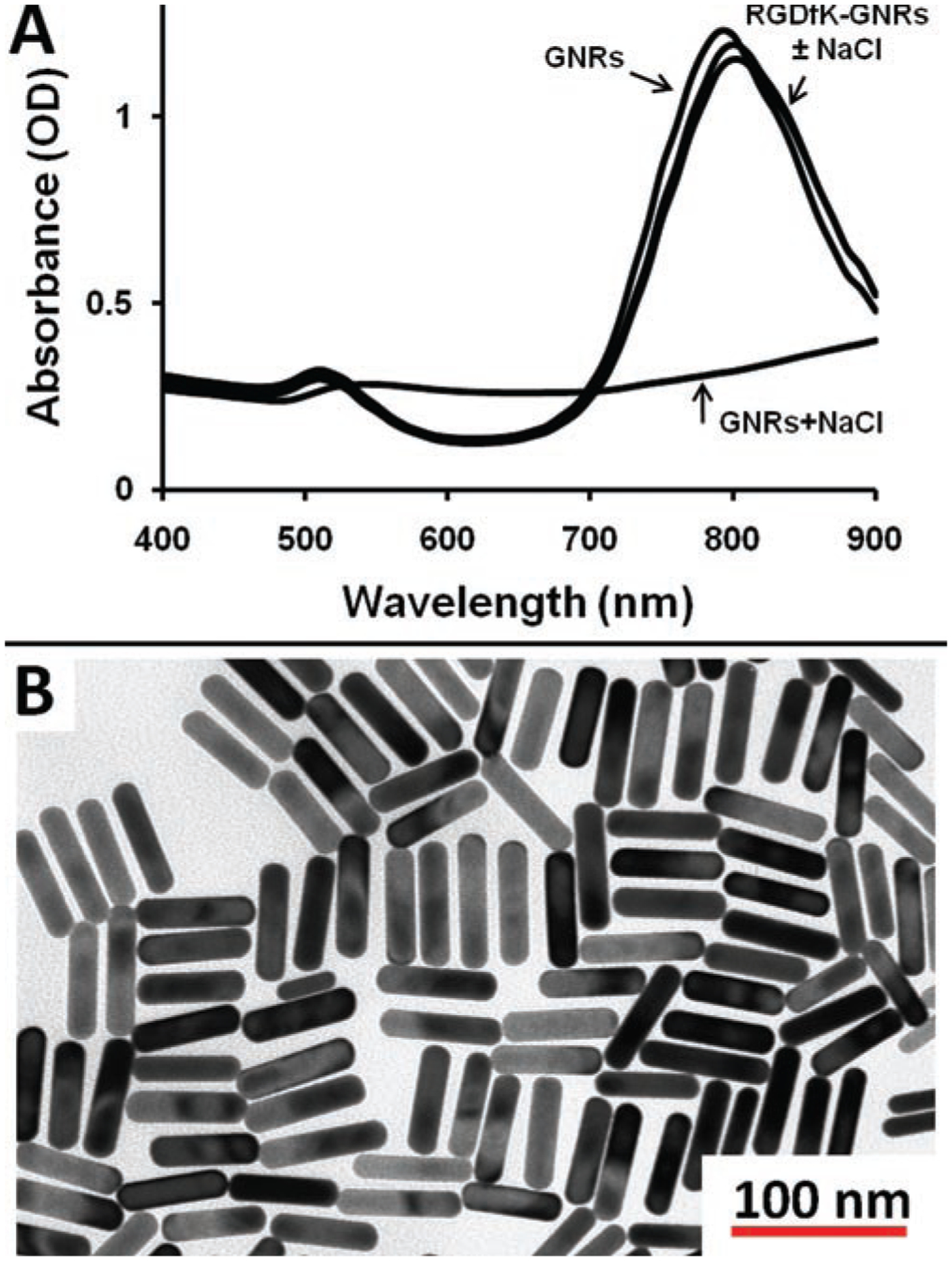
GNR characterization. (A) Light absorption profile and (B) transmission electron micrograph of GNRs. Panel A shows the absorbance profile of CTAB stabilized GNRs (GNRs), CTAB stabilized GNRs with 3.5% NaCl (GNRs + NaCl) as well as RGDfK-PEG-GNRs with and without 3.5% NaCl (RGDfK – GNRs ± NaCl). Without the polymer coating, GNRs aggregate in the presence of NaCl, whereas those stabilized with PEG-RGDfK are stable in the presence of salt.
Table 1.
Physicochemical characteristics of GNRs.
| Size (nm) | SPR | Charge | Peptide content | |
|---|---|---|---|---|
| Untargeted | −10.0 mV | NA | ||
| 60.5 × 15.0 ± 6.5 × 2.0 | 800 nm | RGDfK | −44.1 mV | 5.6 × 10−11 MRGDfK/μg (Au) |
SPR, surface plasmon resonance; NA, not available.
Binding and uptake by dark field microscopy and ICP-MS
Because GNRs scatter light to a very high extent, the binding and uptake of both the untargeted and targeted (RGDfK) GNRs were visualized by high-resolution dark field microscopy (Figure 2A). Captured images show that GNRs were associated with cultured cells to a different extent and do not affect overall cell morphology and the confluency of the culture. The untargeted GNRs showed some binding and uptake in both cell lines tested (DU145 and HUVEC). Internalized GNRs were primarily located in the perinuclear regions of the cells. Similarly, it appeared that the RGDfK-GNRs had slightly more uptake in DU145 cells than the untargeted GNRs, though this difference was not statistically significant after quantification by ICP-MS (Figure 2B). After incubation of the targeted (RGDfK) GNRs with HUVECs, however, significant binding and uptake was observed. The ICP-MS analysis revealed that these binding and uptake events were roughly 20-fold higher for the targeted GNRs than the untargeted GNRs for HUVECs (Figure 2B).
Figure 2.
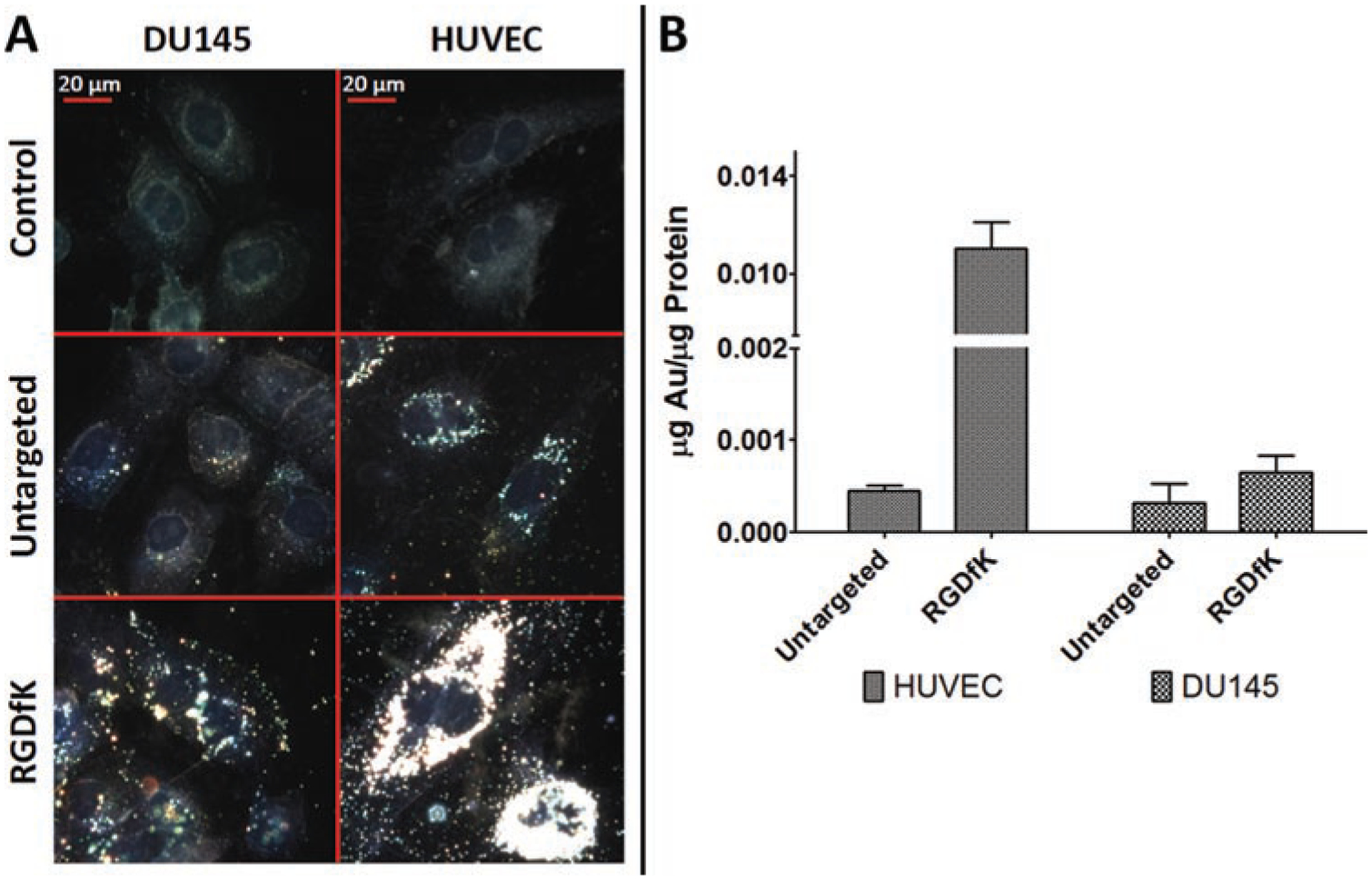
GNR binding and uptake by (A) high-resolution dark field microscopy and (B) ICP-MS after 24 h incubation with either RGDfK modified or untargeted GNRs (10 μg/mL). RGDfK-GNRs show increased binding and uptake relative to untargeted GNRs in both cell lines; however, this difference was most significant (roughly 20-fold) with HUVECs.
Binding and uptake by TEM
The GNR uptake patterns by cells were typically as agglomerates and within membrane-enclosed vacuoles (Figure 3). In some cases, the agglomerates were found in vesicles with multiple membranes suggesting possible association within the endoplasmic reticulum (ER). Despite significant uptake and GNR loading within the cells, no obvious evidence of intracellular structure and organelle damage were observed. These observations and the fact that there were no visible changes of cell culture confluence after incubation with GNRs, provide evidence related to the overall biocompatibility of the nanoparticles. Though in all cases uptake was observed by cells, the uptake of RGDfK-GNRs in HUVECs was significantly higher than that of any other cell line and particle combination.
Figure 3.
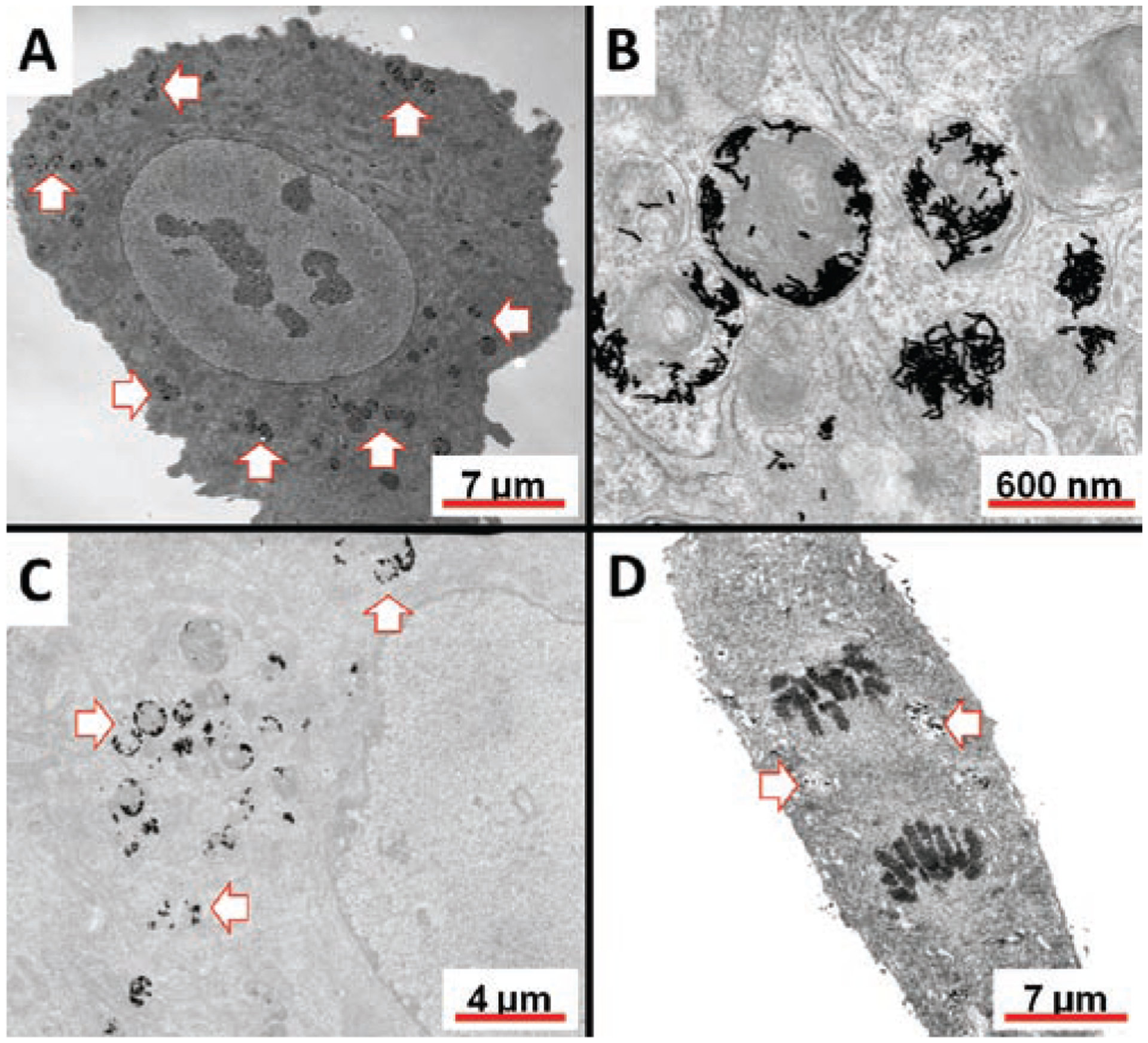
Representative TEM images of RGDfK (A–C) and untargeted (D) GNRs in HUVECs after 24 h incubation. Arrows point to location of GNRs within the cell. Some GNRs were found within multiple membranes (panel B) near the nucleus.
Competitive inhibition of binding
As echistatin is known to bind to αv β3 cell adhesion integrins with very high affinity (Kumar et al., 1997), competitive binding inhibition of the RGDfK-targeted receptors with this protein is possible. Incubation of HUVECs with RGDfK-GNRs at 4°C for 2 h in binding buffer alone resulted in some GNR binding along the cell’s surface as visualized as small green-yellow dots observable by dark field microscopy (Figure 4A). To confirm the specificity of this binding, co-incubation with echistatin (50 nM) resulted in almost complete inhibition of GNR binding to the cell’s surface (Figure 4B). In only a few cases GNRs were found on the cell’s surface which is in sharp contrast to those cells treated with RGDfK-GNRs alone where the nanoparticles were easily identifiable.
Figure 4.
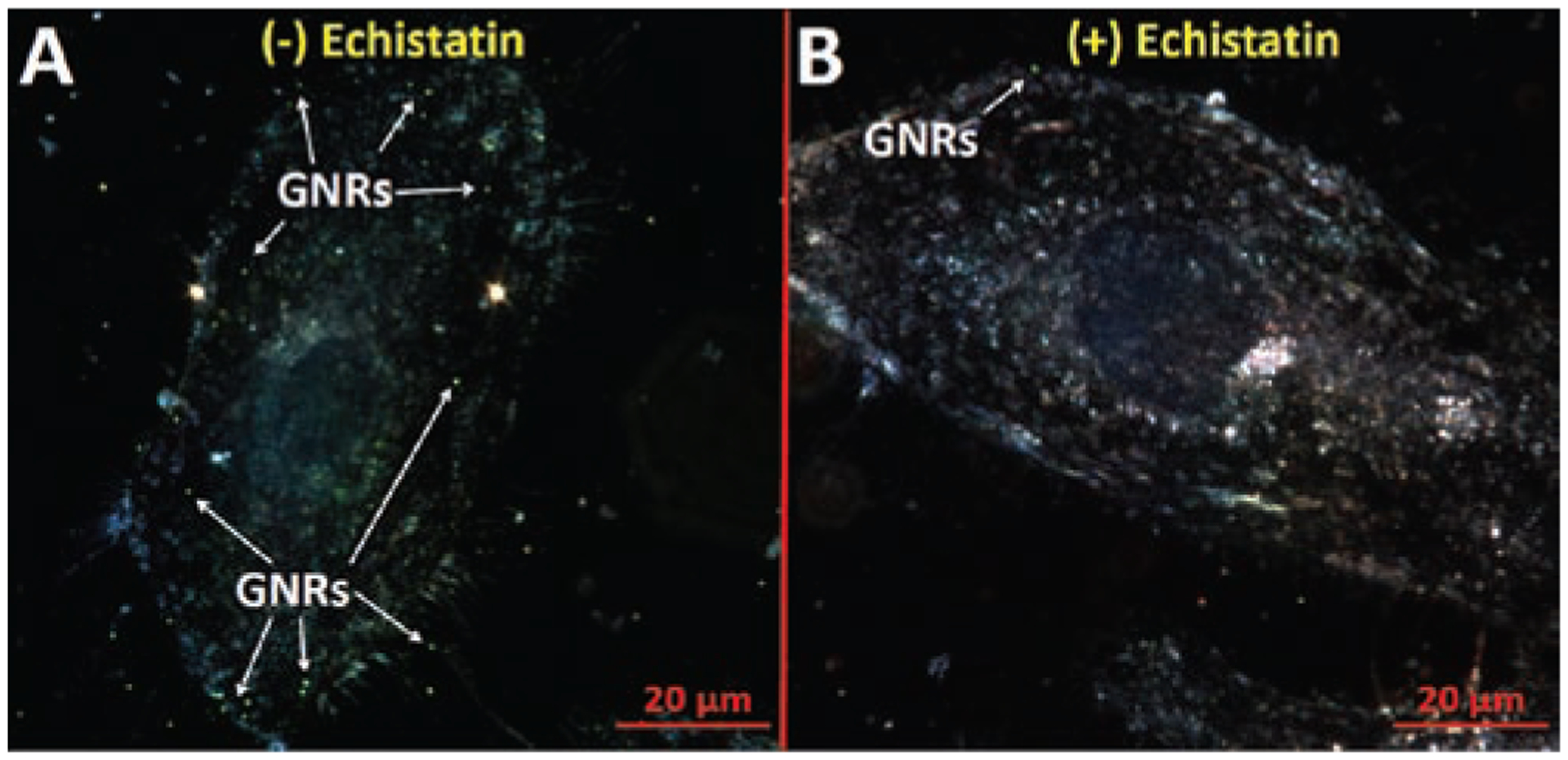
RGDfK-GNR binding to HUVECs in (A) absence and (B) presence of the αv β3 inhibitor echistatin (50 nM) at 4°C for 2 h in binding buffer. Small green-yellow dots indicate presence of GNRs on the cell surface.
Biodistribution in prostate tumor bearing mice
To test the fate of the angiogenesis-targeted GNRs compared to untargeted GNRs, their biodistribution was evaluated in prostate tumor bearing mice. Results indicate that while much of the untargeted GNRs remained in circulation after 6 h, the targeted (RGDfK) GNRs were no longer circulating (Figure 5A). When comparing the tumor accumulation of both particle types, the untargeted GNRs had 7.6-fold higher tumor localization (1.22% injected dose) compared to the RGDfK-conjugated GNRs (0.16% injected dose) at all time points (Figure 5B). Gold analysis of other organs revealed that while the untargeted GNRs showed significant uptake by the liver and spleen, this effect was more pronounced with the targeted GNRs, particularly by the spleen (Figure 6). Over time, the untargeted GNRs were increasingly found in both of these organs due to their longer circulation time, whereas the targeted GNRs had maximal organ accumulation at 6 h followed by a gradual decrease due to fast clearance from the blood. Upon dissection of the animals, the differences in hepatic and splenic uptake of the two GNR types were apparent as the liver and spleen from animals receiving RGDfK-GNRs were visibly darker than their untargeted counterparts.
Figure 5.
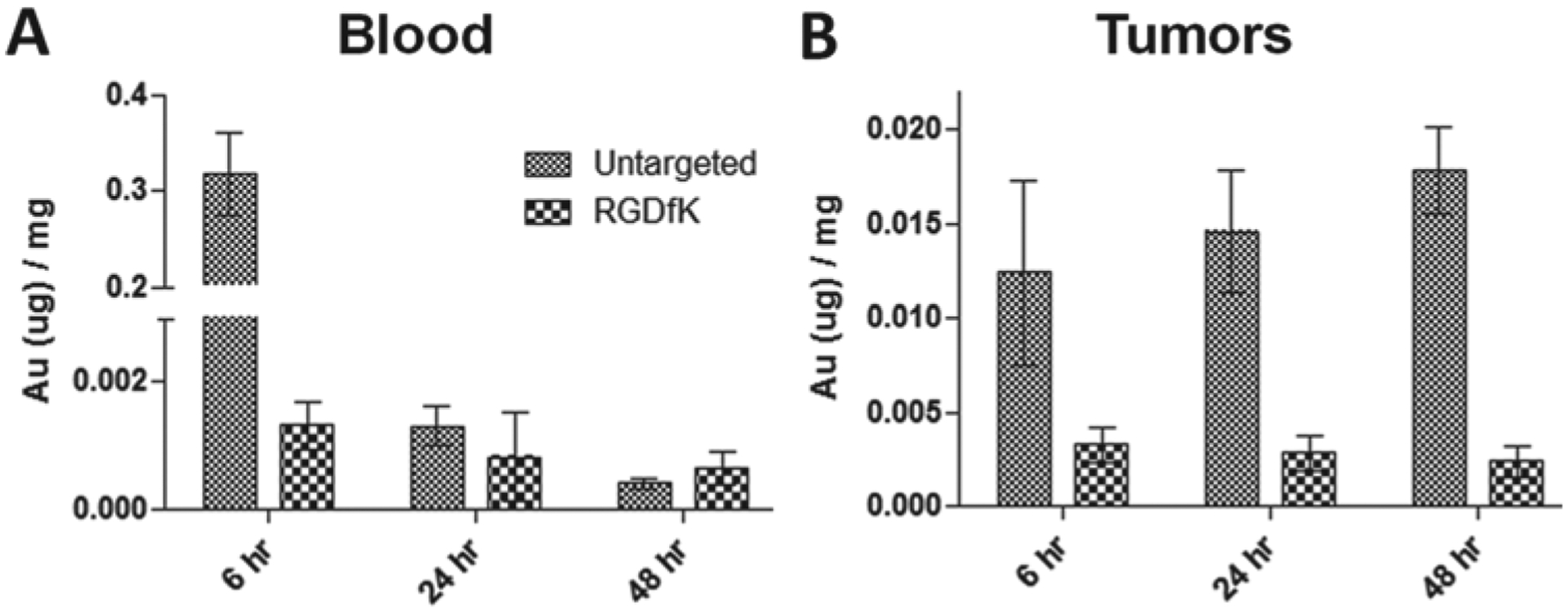
Comparison of targeted (RGDfK) and untargeted gold content by ICP-MS in (A) blood and (B) tumors of mice bearing prostate cancer xenografts at 6, 24 and 48 h post-injection. After 6 h, the RGDfK-GNRs were mostly removed from the blood, while the same was true for untargeted GNRs after 24 h.
Figure 6.
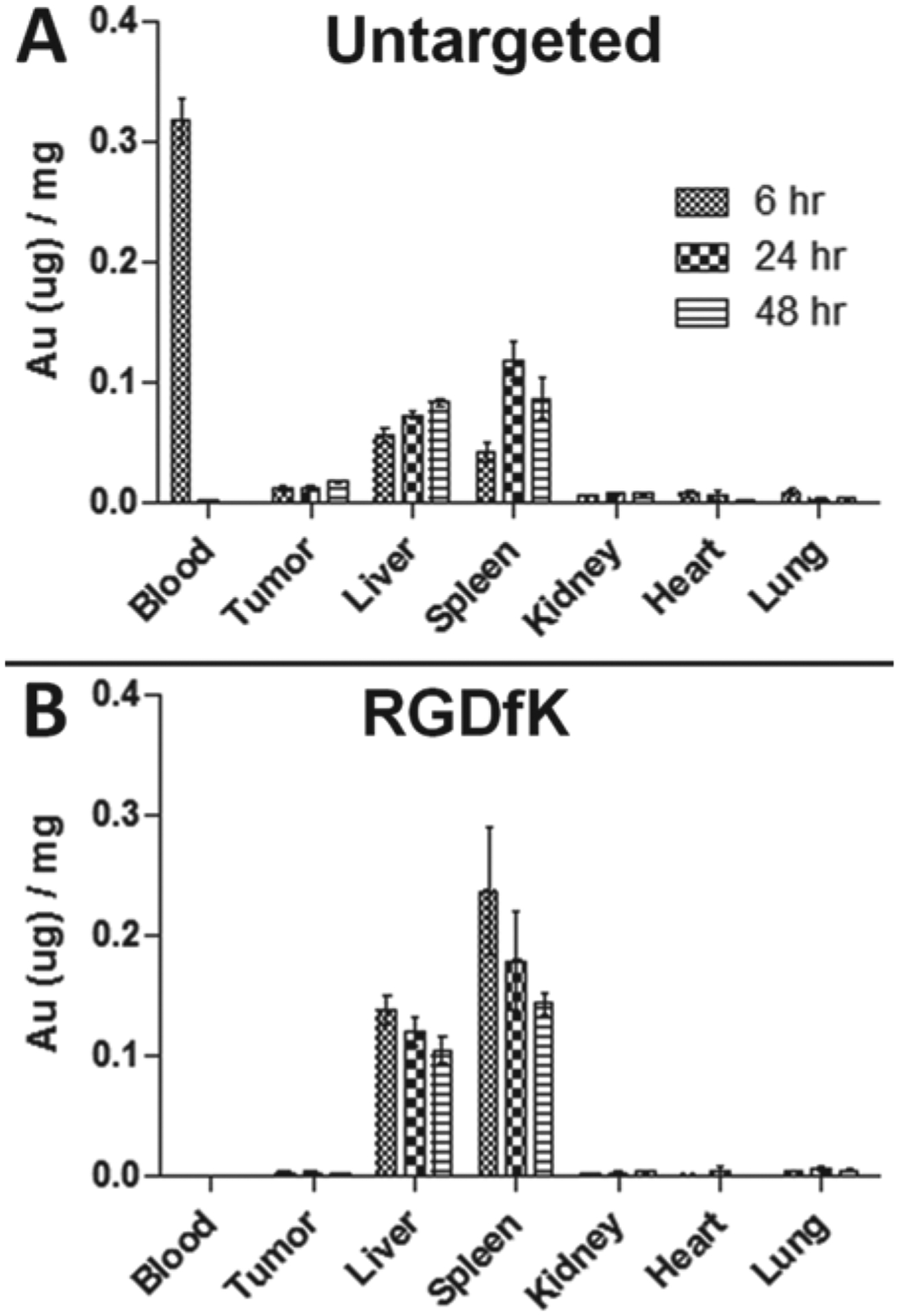
Biodistribution of gold content by ICP-MS: (A) untargeted and (B) targeted (RGDfK) GNRs in prostate cancer bearing mice.
Discussion
Among the many varieties of inorganic nanoparticles investigated for use in nanomedicine, gold nanoparticles have been applied extensively (Jain et al., 2007). Reasons for this include their ease of synthesis and surface modification with low cost materials, their stability in most environments, as well as their biocompatibility and unique optical properties. Such characteristics make these attractive nanoparticle platforms which have been used as drug delivery carriers for tumor necrosis factor-alpha (TNF-α) (Paciotti et al., 2004) and antennas for photothermal therapy (Schwartz et al., 2009).
The use of ligand-directed delivery of nanoparticles to solid tumors over passive delivery mechanisms is desired. In most cases, maximizing the tumor localized percentage injected dose represents the main value for this approach. Directed delivery to specific cells is advantageous as the imaging or therapy system may be specific to those cells. In drug delivery using water soluble polymers, such as HPMA copolymers, for example, the attached anticancer drug may be specific for the target cells (Kopecek & Kopecková, 2010). The same is true for imaging modalities, such as quantum dots, where labeling of tumor vasculature may be desired, and therefore vascular targeting approaches are used (Cai et al., 2006).
Likewise efforts aimed at directed delivery of GNRs to the tumor vasculature would be advantageous. During hyperthermia the impact of heat on the ill-formed tumor vasculature is significant and results in damage (Song et al., 1980). By localizing GNRs to the plasma membrane (by αv β3 integrin targeting) of endothelial cells lining the neovasculature, these cells are particularly susceptible to damage by light activation. This is because direct contact with the membrane limits the amount of heat needed for photothermolysis despite the heat-sink nature of the surrounding blood and interstitial fluid. Additionally, if one chooses to administer a subtherapeutic dose of heat to modulate the tumor’s vascular permeability and facilitate the passive delivery of other nanomedicines, directed PPTT to the tumor’s vasculature may dramatically reduce the light energy required for this approach (Gormley et al., 2011).
In our previous studies, we demonstrated the utility of HPMA copolymer − cyclic RGDfK conjugates for targeted delivery of drugs and imaging agents to solid tumors (Line et al., 2005; Mitra et al., 2005; Mitra et al., 2006a; Mitra et al., 2006b; Borgman et al., 2009a; Borgman et al., 2009b; Zarabi et al., 2009). Compared to non-targetable systems, HPMA copolymer-RGD4C and -RGDfK conjugates have shown increased accumulation in a variety of tumors, including prostate, lung, and breast cancer xenografts. This increased localization has been correlated with enhanced treatment efficacy and anti-angiogenic activity of both geldanamycin and docetaxel (Greish et al., 2011; Ray et al., 2011).
In addition to the presence of targeting moiety, however, other factors that influence biodistribution and tumor uptake are the size, charge, surface modification, and architecture of nanocarriers. In many cases, use of a targeting moiety does not necessarily guarantee better tumor accumulation. For example, a variety of active delivery approaches for liposomes have been tested in vivo and many of the parameters which govern their blood clearance and tumor targeting have been identified (Maruyama et al., 1999). Physicochemical properties, such as particle charge and PEG length, are known to influence the blood clearance kinetics of liposomes (Levchenko et al., 2002). Choice of targeting moiety and its structure also play a key role. For example, whole antibodies may be recognized by the mononuclear phagocyte system (MPS) to a higher extent than if antibody fragments are used (Aragnol & Leserman, 1986; Peeters et al., 1988). Finally, the architecture of the nanomedicine may play a significant role when comparing two systems. Besides the fact that carrier architecture and geometry influences biodistribution and passive tumor localization (Arnida et al., 2011 and Sadekar et al., 2011), it is likely that the bioavailability of the same peptide for its ligand on two very different platforms will be different.
In the context of angiogenesis targeting of gold nanoparticles, there exists conflicting reports. Besides examples providing evidence of targeting in vitro alone (Arosio et al., 2011), two other studies have validated this approach in vivo (Li et al., 2009; Xie et al., 2011). The first attached RGDfK to gold nanoshells and showed a slight increase in nanoshell delivery compared to the untargeted nanoshells alone (Xie et al., 2011). Also, when linear RGD functionalized dendrimer-GNRs were tested for tumor targeting, a very high (17% of the injected dose) amount of the targeted nanoparticles localized within the tumors (Li et al., 2009). Alternatively, a recent study investigating the targeting potential of GNRs with a variety of targeting peptides, including RGD, provided evidence that such functionalization does not enable better tumor delivery when compared to untargeted GNRs alone (Huang et al., 2010). While attachment of moieties, such as single-chain variable fragment (ScFv) and amino-terminal fragment (ATF) peptides, resulted in a very slight increase in tumor delivery, use of the RGD peptide had a negative impact on tumor delivery.
When the binding and uptake of the RGDfK-GNRs was evaluated with both DU145 and HUVEC cells, the system performed as intended. The GNRs with the RGDfK peptide on their surface had increased binding to cells which express αv β3 integrins. Given that the expression profile of these receptors is highly dependent on cell type, it is expected that cells which have more αv β3 integrins will have higher binding and uptake of the RGDfK modified GNRs. This was indeed the case where RGDfK-GNRs had more binding to both cell types relative to the untargeted nanoparticles, but that this difference was more pronounced with HUVECs (roughly 20-fold increase). This is related to the fact that HUVEC cells have significant expression of αv β3 integrins due to their angiogenic phenotype (Rhim et al., 1998; Romanov & Goligorsky, 1999; Sakko et al., 2003). The fact that the RGDfK-GNRs had higher binding to the DU145 cells than the untargeted nanoparticles is also not surprising considering that these cells do have some expression profile of αv β3 integrins, though not to the same extent as HUVECs.
Because there is always some concern that such binding and uptake events could be due to nonspecific interactions, such as surface charge, inhibition of binding by co-incubation with echistatin was performed and visualized by dark field microscopy. After 2 h incubation at 4°C, it is expected that minimal nonspecific binding and uptake would occur. Because RGD-ligand binding is not an energy dependent process, such conditions should still result in some cell surface binding. In this way, peptide-ligand specificity was confirmed as HUVEC cells co-incubated with echistatin showed very little GNR binding, while those cells without echistatin had GNRs decorating the cell’s surface.
Dark field microscopy in this case was used as a visualization tool as it is very efficient at detecting the presence of nanoparticles which scatter light to a high extent. However, since this imaging tool is unable to distinguish GNRs which are bound to the plasma membrane and those that are located intracellularly, it has some limitations. To address this, TEM images of the uptaken particles in HUVEC cells were acquired. The information gathered from the TEM images were not surprising based on previous findings (Salem et al., 2003; Connor et al., 2005; Shukla et al., 2005; Chithrani et al., 2006; Chithrani and Chan, 2007; Khan et al., 2007; Mao et al., 2007). Large nanoparticle agglomerates were found within single membrane vesicles indicative of endosomes and lysosomes (Figure 3A and C). However, in some cases some of these agglomerates were found within multiple membranes, which may be suggestive of their presence within the ER (Figure 3B).
Despite confirming the capacity of the RGDfK-GNRs to bind to cells undergoing angiogenesis, when the system was tested in vivo fast clearance of the targeted GNRs prevented effective targeting from occurring. Because RGD targeting of nanoparticles has been validated previously, we hypothesize that the fast clearance is related to the strong negative charge of the RGDfK-GNRs (−44.1 mV). As the presence of strong net negative charges is known to facilitate nanoparticle biorecognition (Gormley & Ghandehari, 2009), it is very possible that this physicochemical parameter alone may have initiated quick GNR uptake by the liver and spleen. Such quick MPS clearance of negatively charged conjugates has been observed with several different nanomedicines. For example, the presence of strong negative charges was previously identified as the reason for quick clearance of RGDfK-conjugated HPMA copolymers due to the presence of negatively charged 111In chelators attached to the side chains (Borgman et al., 2008). Similar observations have also been made with liposomal systems (Gabizon & Papahadjopoulos, 1992), micelles (Xiao et al., 2011), dendrimers (Sadekar et al., 2010) as well as gold nanoparticles (Arnida et al., 2011 and Sadekar et al., 2011). Therefore, for successful targeting to occur, lessons learned from nanoparticle systems with large surface charges necessitates careful consideration of particle charge in addition to other parameters, such as targeting moiety content, as well as size and shape.
While the strong negative charge of the targeted GNR is most likely due to the aspartic acid on the RGDfK peptide, there is a chance that some of the polymers were not conjugated with the peptide leaving a residual acid group after hydrolysis of the NHS ester. This possibility is, however, not as likely of a contributor as conjugation of the peptide to the polymers in a 1:1 stoichiometric ratio was confirmed by amino acid analysis. To reduce the presence of these surface charges, the number of PEG-RGDfKs on the gold surface may be decreased. However, because doing this alone has previously been shown to make little impact on the targetability of the system (Huang et al., 2010), another possibility is to change the PEG length to which the RGDfK moiety is attached. By attaching RGDfK or any other peptide to a PEG linker which is 2000 Da in size and keeping the neutrally charged PEG (methoxy terminated) at 5000 Da, a mixture of the two might hide the peptide and thus mask the surface charges. Indeed, there is a risk with this approach of not having the peptide available for binding to the ligand. In either case, an appropriate balance of enough peptide to achieve vascular targeting, but not so much that surface charges diminish blood circulation, is required to obtain a targeted system as designed.
Conclusions
In the present study, it was shown that surface modification of GNRs with the angiogenesis-targeting peptide RGDfK results in effective targeting in vitro but not in vivo. Utilization of this peptide with GNRs shows excellent biorecognition with cells and their αv β3 integrins; however, the presence of strong negative charges likely accelerated the MPS clearance of this system. In future designs, GNR surface modifications to maintain peptide-ligand bioactivity will need to be balanced with particle surface charge to increase the targetability of this system.
Acknowledgment
The authors thank Diego Fernandez for his ICP-MS support as well as Nancy Chandler for her help with the TEM images taken. The authors would also like to acknowledge Josh Gustafson, Robert Price, and Khaled Greish for their help with the animal studies.
Declaration of interest
The authors have a patent pending application relevant to this work. This research was supported by a Department of Defense Prostate Cancer Predoctoral Training Award (PC094496) as well as the National Institutes of Health (R01-DE19050 and EB-R01EB7171) and the Utah Science Technology and Research (USTAR) Initiative.
References
- Aragnol D, Leserman LD. (1986). Immune clearance of liposomes inhibited by an anti-Fc receptor antibody in vivo. Proc Natl Acad Sci USA, 83, 2699–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnida, Janát-Amsbury MM, Ray A, Peterson CM, Ghandehari H (2011). Geometry and surface characteristics of gold nanoparticles influence their biodistribution and uptake by macrophages. Eur J Pharm Biopharm, 77, 417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arosio D, Manzoni L, Araldi EM, Scolastico C. (2011). Cyclic RGD functionalized gold nanoparticles for tumor targeting. Bioconjug Chem, 22, 664–672. [DOI] [PubMed] [Google Scholar]
- Borgman MP, Aras O, Geyser-Stoops S, Sausville EA, Ghandehari H. (2009a). Biodistribution of HPMA copolymer-aminohexylgeldanamycin-RGDfK conjugates for prostate cancer drug delivery. Mol Pharm, 6, 1836–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgman MP, Coleman T, Kolhatkar RB, Geyser-Stoops S, Line BR, Ghandehari H. (2008). Tumor-targeted HPMA copolymer-(RGDfK)-(CHX-A”-DTPA) conjugates show increased kidney accumulation. J Control Release, 132, 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgman MP, Ray A, Kolhatkar RB, Sausville EA, Burger AM, Ghandehari H. (2009b). Targetable HPMA copolymer-aminohexylgeldanamycin conjugates for prostate cancer therapy. Pharm Res, 26, 1407–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuer O, Sundararaj U. (2004). Big returns from small fibers: A review of polymer/carbon nanotube composites. Polymer Composites, 25, 630–645. [Google Scholar]
- Cai W, Shin DW, Chen K, Gheysens O, Cao Q, Wang SX, Gambhir SS, Chen X. (2006). Peptide-labeled near-infrared quantum dots for imaging tumor vasculature in living subjects. Nano Lett, 6, 669–676. [DOI] [PubMed] [Google Scholar]
- Chen J, Saeki F, Wiley BJ, Cang H, Cobb MJ, Li ZY, Au L, Zhang H, Kimmey MB, Li X, Xia Y. (2005). Gold nanocages: bioconjugation and their potential use as optical imaging contrast agents. Nano Lett, 5, 473–477. [DOI] [PubMed] [Google Scholar]
- Chithrani BD, Chan WC. (2007). Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett, 7, 1542–1550. [DOI] [PubMed] [Google Scholar]
- Chithrani BD, Ghazani AA, Chan WC. (2006). Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett, 6, 662–668. [DOI] [PubMed] [Google Scholar]
- Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. (2005). Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small, 1, 325–327. [DOI] [PubMed] [Google Scholar]
- Copland JA, Eghtedari M, Popov VL, Kotov N, Mamedova N, Motamedi M, Oraevsky AA. (2004). Bioconjugated gold nanoparticles as a molecular based contrast agent: implications for imaging of deep tumors using optoacoustic tomography. Mol Imaging Biol, 6, 341–349. [DOI] [PubMed] [Google Scholar]
- Dickerson EB, Dreaden EC, Huang X, El-Sayed IH, Chu H, Pushpanketh S, McDonald JF, El-Sayed MA. (2008). Gold nanorod assisted near-infrared plasmonic photothermal therapy (PPTT) of squamous cell carcinoma in mice. Cancer Lett, 269, 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkgraaf I, Kruijtzer JA, Liu S, Soede AC, Oyen WJ, Corstens FH, Liskamp RM, Boerman OC. (2007). of RGD peptides. Eur J Nucl Med Mol Imaging, 34, 267–273. [DOI] [PubMed] [Google Scholar]
- Dixit V, Van den Bossche J, Sherman DM, Thompson DH, Andres RP. (2006). Synthesis and grafting of thioctic acid-PEG-folate conjugates onto Au nanoparticles for selective targeting of folate receptor-positive tumor cells. Bioconjug Chem, 17, 603–609. [DOI] [PubMed] [Google Scholar]
- Dudar TE, Jain RK. (1984). Differential response of normal and tumor microcirculation to hyperthermia. Cancer Res, 44, 605–612. [PubMed] [Google Scholar]
- Duncan R (2003). The dawning era of polymer therapeutics. Nat Rev Drug Discov, 2, 347–360. [DOI] [PubMed] [Google Scholar]
- El-Sayed IH, Huang X, El-Sayed MA. (2005). Surface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: applications in oral cancer. Nano Lett, 5, 829–834. [DOI] [PubMed] [Google Scholar]
- Gabizon A, Papahadjopoulos D. (1992). The role of surface charge and hydrophilic groups on liposome clearance in vivo. Biochim Biophys Acta, 1103, 94–100. [DOI] [PubMed] [Google Scholar]
- Gormley AJ, Ghandehari H. (2009). Evaluation of toxicity of nanostructures in biological systems. In: Sahu SC & Casciano DA ed. Nanotoxicity: From In Vivo and In Vitro Models to Health Risks. West Sussex: Wiley, 115–159. [Google Scholar]
- Gormley AJ, Greish K, Ray A, Robinson R, Gustafson JA, Ghandehari H. (2011). Gold nanorod mediated plasmonic photothermal therapy: a tool to enhance macromolecular delivery. Int J Pharm, 415, 315–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greish K, Ray A, Bauer H, Larson N, Malugin A, Pike D, Haider M, Ghandehari H. (2011). Anticancer and antiangiogenic activity of HPMA copolymer-aminohexylgeldanamycin-RGDfK conjugates for prostate cancer therapy. J Control Release, 151, 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch LR, Stafford RJ, Bankson JA, Sershen SR, Rivera B, Price RE, Hazle JD, Halas NJ, West JL. (2003). Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc Natl Acad Sci USA, 100, 13549–13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsman MR. (2006). Tissue physiology and the response to heat. Int J Hyperthermia, 22, 197–203. [DOI] [PubMed] [Google Scholar]
- Huang X, Jain PK, El-Sayed IH, El-Sayed MA. (2007). Gold nanoparticles: interesting optical properties and recent applications in cancer diagnostics and therapy. Nanomedicine (Lond), 2, 681–693. [DOI] [PubMed] [Google Scholar]
- Huang X, Jain PK, El-Sayed IH, El-Sayed MA. (2008). Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med Sci, 23, 217–228. [DOI] [PubMed] [Google Scholar]
- Huang X, Peng X, Wang Y, Wang Y, Shin DM, El-Sayed MA, Nie S. (2010). A reexamination of active and passive tumor targeting by using rod-shaped gold nanocrystals and covalently conjugated peptide ligands. ACS Nano, 4, 5887–5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer AK, Khaled G, Fang J, Maeda H. (2006). Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today, 11, 812–818. [DOI] [PubMed] [Google Scholar]
- Jain PK, El-Sayed IH, El-Sayed MA. (2007). Au nanoparticles target cancer. Nano Today, 2, 18–29. [Google Scholar]
- Kelly KL, Coronado E, Zhao LL, Schatz GC. (2003). The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment. J Phys Chem B, 107, 668–677. [Google Scholar]
- Khan JA, Pillai B, Das TK, Singh Y, Maiti S. (2007). Molecular effects of uptake of gold nanoparticles in HeLa cells. Chembiochem, 8, 1237–1240. [DOI] [PubMed] [Google Scholar]
- Kopecek J, Kopecková P. (2010). HPMA copolymers: origins, early developments, present, and future. Adv Drug Deliv Rev, 62, 122–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar CC, Nie H, Rogers CP, Malkowski M, Maxwell E, Catino JJ, Armstrong L. (1997). Biochemical characterization of the binding of echistatin to integrin alphavbeta3 receptor. J Pharmacol Exp Ther, 283, 843–853. [PubMed] [Google Scholar]
- Levchenko TS, Rammohan R, Lukyanov AN, Whiteman KR, Torchilin VP. (2002). Liposome clearance in mice: the effect of a separate and combined presence of surface charge and polymer coating. Int J Pharm, 240, 95–102. [DOI] [PubMed] [Google Scholar]
- Li M, Schnablegger H, Mann S. (1999). Coupled synthesis and self-assembly of nanoparticles to give structures with controlled organization. Phys Rev Lett, 82, 1345–1349. [Google Scholar]
- Li Z, Huang P, Zhang X, Lin J, Yang S, Liu B, Gao F, Xi P, Ren Q, Cui D. (2010). RGD-conjugated dendrimer-modified gold nanorods for in vivo tumor targeting and photothermal therapy. Mol Pharm, 7, 94–104. [DOI] [PubMed] [Google Scholar]
- Line BR, Mitra A, Nan A, Ghandehari H. (2005). Targeting tumor angiogenesis: comparison of peptide and polymer-peptide conjugates. J Nucl Med, 46, 1552–1560. [PubMed] [Google Scholar]
- Loo C, Lowery A, Halas N, West J, Drezek R. (2005). Immunotargeted nanoshells for integrated cancer imaging and therapy. Nano Lett, 5, 709–711. [DOI] [PubMed] [Google Scholar]
- Maeda H, Greish K, Fang J. (2006). The EPR effect and polymeric drugs: A paradigm shift for cancer chemotherapy in the 21st century. Adv Polymer Sci, 193, 103–121. [Google Scholar]
- Mao Z, Wang B, Ma L, Gao C, Shen J. (2007). The influence of polycaprolactone coating on the internalization and cytotoxicity of gold nanoparticles. Nanomedicine, 3, 215–223. [DOI] [PubMed] [Google Scholar]
- Maruyama K, Ishida O, Takizawa T, Moribe K. (1999). Possibility of active targeting to tumor tissues with liposomes. Adv Drug Deliv Rev, 40, 89–102. [DOI] [PubMed] [Google Scholar]
- Matsumura Y, Kataoka K. (2009). Preclinical and clinical studies of anticancer agent-incorporating polymer micelles. Cancer Sci, 100, 572–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra A, Coleman T, Borgman M, Nan A, Ghandehari H, Line BR. (2006a). Polymeric conjugates of mono- and bi-cyclic alphaVbeta3 binding peptides for tumor targeting. J Control Release, 114, 175–183. [DOI] [PubMed] [Google Scholar]
- Mitra A, Mulholland J, Nan A, McNeill E, Ghandehari H, Line BR. (2005). Targeting tumor angiogenic vasculature using polymer-RGD conjugates. J Control Release, 102, 191–201. [DOI] [PubMed] [Google Scholar]
- Mitra A, Nan A, Papadimitriou JC, Ghandehari H, Line BR. (2006b). Polymer-peptide conjugates for angiogenesis targeted tumor radiotherapy. Nucl Med Biol, 33, 43–52. [DOI] [PubMed] [Google Scholar]
- Narayanan R, El-Sayed MA. (2005). Catalysis with transition metal nanoparticles in colloidal solution: nanoparticle shape dependence and stability. J Phys Chem B, 109, 12663–12676. [DOI] [PubMed] [Google Scholar]
- Niidome T, Yamagata M, Okamoto Y, Akiyama Y, Takahashi H, Kawano T, Katayama Y, Niidome Y. (2006). PEG-modified gold nanorods with a stealth character for in vivo applications. J Control Release, 114, 343–347. [DOI] [PubMed] [Google Scholar]
- Nikoobakht B, El-Sayed MA. (2003). Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem Mater, 15, 1957–1962. [Google Scholar]
- O’Neal DP, Hirsch LR, Halas NJ, Payne JD, West JL. (2004). Photothermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett, 209, 171–176. [DOI] [PubMed] [Google Scholar]
- O’Brien ME, Wigler N, Inbar M, Rosso R, Grischke E, Santoro A, Catane R, Kieback DG, Tomczak P, Ackland SP, Orlandi F, Mellars L, Alland L, Tendler C; CAELYX Breast Cancer Study Group. (2004). Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol, 15, 440–449. [DOI] [PubMed] [Google Scholar]
- Paciotti GF, Myer L, Weinreich D, Goia D, Pavel N, McLaughlin RE, Tamarkin L. (2004). Colloidal gold: a novel nanoparticle vector for tumor directed drug delivery. Drug Deliv, 11, 169–183. [DOI] [PubMed] [Google Scholar]
- Pasqualini R, Koivunen E, Ruoslahti E. (1997). Alpha v integrins as receptors for tumor targeting by circulating ligands. Nat Biotechnol, 15, 542–546. [DOI] [PubMed] [Google Scholar]
- Peeters PAM, Storm G, Crommelin DJA. (1988). Immunoliposomes in vivo: state of the art. Adv Drug Deliv Rev, 1, 249–266. [Google Scholar]
- Ray A, Larson N, Pike DB, Grüner M, Naik S, Bauer H, Malugin A, Greish K, Ghandehari H. (2011). Comparison of active and passive targeting of docetaxel for prostate cancer therapy by HPMA copolymer-RGDfK conjugates. Mol Pharm, 8, 1090–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhim JS, Tsai WP, Chen ZQ, Chen Z, Van Waes C, Burger AM, Lautenberger JA. (1998). A human vascular endothelial cell model to study angiogenesis and tumorigenesis. Carcinogenesis, 19, 673–681. [DOI] [PubMed] [Google Scholar]
- Romanov VI, Goligorsky MS. (1999). RGD-recognizing integrins mediate interactions of human prostate carcinoma cells with endothelial cells in vitro. Prostate, 39, 108–118. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E, Pierschbacher MD. (1987). New perspectives in cell adhesion: RGD and integrins. Science, 238, 491–497. [DOI] [PubMed] [Google Scholar]
- Sadekar S, Ray A, Janàt-Amsbury M, Peterson CM, Ghandehari H. (2011). Comparative biodistribution of PAMAM dendrimers and HPMA copolymers in ovarian-tumor-bearing mice. Biomacromolecules, 12, 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakko AJ, Ricciardelli C, Mayne K, Suwiwat S, LeBaron RG, Marshall VR, Tilley WD, Horsfall DJ. (2003). Modulation of prostate cancer cell attachment to matrix by versican. Cancer Res, 63, 4786–4791. [PubMed] [Google Scholar]
- Salem AK, Searson PC, Leong KW. (2003). Multifunctional nanorods for gene delivery. Nat Mater, 2, 668–671. [DOI] [PubMed] [Google Scholar]
- Schwartz JA, Shetty AM, Price RE, Stafford RJ, Wang JC, Uthamanthil RK, Pham K, McNichols RJ, Coleman CL, Payne JD. (2009). Feasibility study of particle-assisted laser ablation of brain tumors in orthotopic canine model. Cancer Res, 69, 1659–1667. [DOI] [PubMed] [Google Scholar]
- Shukla R, Bansal V, Chaudhary M, Basu A, Bhonde RR, Sastry M. (2005). Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: a microscopic overview. Langmuir, 21, 10644–10654. [DOI] [PubMed] [Google Scholar]
- Skebo JE, Grabinski CM, Schrand AM, Schlager JJ, Hussain SM. (2007). Assessment of metal nanoparticle agglomeration, uptake, and interaction using high-illuminating system. Int J Toxicol, 26, 135–141. [DOI] [PubMed] [Google Scholar]
- Song CW, Kang MS, Rhee JG, Levitt SH. (1980). Effect of hyperthermia on vascular function in normal and neoplastic tissues. Ann N Y Acad Sci, 335, 35–47. [DOI] [PubMed] [Google Scholar]
- Stern JM, Stanfield J, Kabbani W, Hsieh JT, Cadeddu JA. (2008). Selective prostate cancer thermal ablation with laser activated gold nanoshells. J Urol, 179, 748–753. [DOI] [PubMed] [Google Scholar]
- Thumshirn G, Hersel U, Goodman SL, Kessler H. (2003). Multimeric cyclic RGD peptides as potential tools for tumor targeting: solid-phase peptide synthesis and chemoselective oxime ligation. Chemistry, 9, 2717–2725. [DOI] [PubMed] [Google Scholar]
- Tkachenko AG, Xie H, Liu Y, Coleman D, Ryan J, Glomm WR, Shipton MK, Franzen S, Feldheim DL. (2004). Cellular trajectories of peptide-modified gold particle complexes: comparison of nuclear localization signals and peptide transduction domains. Bioconjug Chem, 15, 482–490. [DOI] [PubMed] [Google Scholar]
- Tong L, Wei Q, Wei A, Cheng JX. (2009). Gold nanorods as contrast agents for biological imaging: optical properties, surface conjugation and photothermal effects. Photochem Photobiol, 85, 21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainrub A, Pustovyy O, Vodyanoy V. (2006). Resolution of 90 nm (lambda/5) in an optical transmission microscope with an annular condenser. Opt Lett, 31, 2855–2857. [DOI] [PubMed] [Google Scholar]
- Xiao K, Li Y, Luo J, Lee JS, Xiao W, Gonik AM, Agarwal RG, Lam KS. (2011). The effect of surface charge on in vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles. Biomaterials, 32, 3435–3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Diagaradjane P, Deorukhkar AA, Goins B, Bao A, Phillips WT, Wang Z, Schwartz J, Krishnan S. (2011). Integrin αv β3-targeted gold nanoshells augment tumor vasculature-specific imaging and therapy. Int J Nanomedicine, 6, 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavuz MS, Cheng Y, Chen J, Cobley CM, Zhang Q, Rycenga M, Xie J, Kim C, Song KH, Schwartz AG, Wang LV, Xia Y. (2009). Gold nanocages covered by smart polymers for controlled release with near-infrared light. Nat Mater, 8, 935–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarabi B, Borgman MP, Zhuo J, Gullapalli R, Ghandehari H. (2009). Noninvasive monitoring of HPMA copolymer-RGDfK conjugates by magnetic resonance imaging. Pharm Res, 26, 1121–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]


