Abstract
mRNA vaccines have gained popularity over the last decade as a versatile tool for developing novel therapeutics. The recent success of coronavirus disease (COVID-19) mRNA vaccine has unlocked the potential of mRNA technology as a powerful therapeutic platform. In this review, we apprise the literature on the various types of cancer vaccines, the novel platforms available for delivery of the vaccines, the recent progress in the RNA-based therapies and the evolving role of mRNA vaccines for various cancer indications, along with a future strategy to treat the patients. Literature reveals that despite multifaceted challenges in the development of mRNA vaccines, the promising and durable efficacy of the RNA in pre-clinical and clinical studies deserves consideration. The introduction of mRNA-transfected DC vaccine is an approach that has gained interest for cancer vaccine development due to its ability to circumvent the necessity of DC isolation, ex vivo cultivation and re-infusion. The selection of appropriate antigen of interest remains one of the major challenges for cancer vaccine development. The rapid development and large-scale production of mRNA platform has enabled for the development of both personalized vaccines (mRNA 4157, mRNA 4650 and RO7198457) and tetravalent vaccines (BNT111 and mRNA-5671). In addition, mRNA vaccines combined with checkpoint modulators and other novel medications that reverse immunosuppression show promise, however further research is needed to discover which combinations are most successful and the best dosing schedule for each component. Each delivery route (intradermal, subcutaneous, intra tumoral, intranodal, intranasal, intravenous) has its own set of challenges to overcome, and these challenges will decide the best delivery method. In other words, while developing a vaccine design, the underlying motivation should be a reasonable combination of delivery route and format. Exploring various administration routes and delivery route systems has boosted the development of mRNA vaccines.
Keywords: mRNA, Cancer vaccine, Covid-19, Oncology, Optimization
Introduction
Vaccinations play a vital role in reducing disease, disability, and mortality from a variety of infectious diseases [1]. The use of conventional vaccines such as live attenuated vaccines, inactivated pathogens, subunit vaccines or toxoid vaccines provides durable efficacy against various infectious diseases [2]. Nucleic acid vaccines mainly, plasmid DNA(pDNA) and messenger RNA (mRNA), came to existence in 1900s due to their innate ability to stimulate inoculation with live organism-based vaccines, notably for cell-mediated immune stimulation [3]. For several decades later, pDNA-based approaches dominated the field, since mRNA-based approach was considered unstable due to inefficient in-vivo delivery and excessive stimulation of inflammatory responses [4], [5]. Eventually in late 2000s, a series of improvement in manufacture, modification and stabilization of mRNA led to its recognition as a resourceful platform for developing novel therapy [4], [5]. mRNA vaccines thus hold a lot of promise and confer several advantages over traditional vaccines.
The recent outbreak of SARS-CoV-2 and coronavirus disease (Covid-19) has demonstrated an urgent need of rapid vaccine development. Two mRNA vaccines, BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna TX), have acquired authorization from FDA that are currently being used to prevent COVID-19 [6]. Both vaccines have good efficacy as demonstrated in the various phase III trials and real world studies [7], [8], [9], [10], [11]. Knowledge gained from these trials and versatile therapeutic potential of the mRNA can be applied for the development of vaccine for the infectious diseases and cancer. In this review, we focus on the therapeutic aspect of mRNA vaccines as a cancer therapy. In addition, we would apprise the literature on the various types of cancer vaccines, the novel platforms available for delivery of the vaccines, the recent progress in the RNA-based therapies and the evolving role of mRNA vaccines for various cancer indications, the available clinical and preclinical studies with the future chapter in treatment of patients.
The available platforms for development of anti-cancer vaccines
Cancer vaccines are a promising new immunotherapeutic strategy for both prevention and treatment. Vaccines targeting tumor associated or tumor-specific antigens (TAAs or TSAs) can destroy malignant cells that overexpress the antigens due to immunologic memory, resulting in a durable therapeutic response. Compared to other immunotherapies, cancer vaccines provide a precise, safe, and acceptable treatment. Currently, 2 prophylactic vaccines have been approved by the U.S. Food and Drug Administration (FDA) for routine use in clinical practice. Gardasil-9 is approved for prevention of HPV infection that is the cause of most HPV cancers. The other one is hepatitis B (HBV) vaccine, for example HEPLISAV-B, to prevent HBV infection that is known to cause hepatocellular carcinoma [12], [13].
In 2010, PROVENGE (sipuleucel-T), an immune-cell based therapeutic cancer vaccine was granted approval for the treatment of individuals with asymptomatic or mild symptomatic metastatic castration-resistant prostate cancer (mCRPC) [14]. Besides, therapeutic vaccines are available for the treatment of early-stage bladder cancer (TheraCys® and TICE® Bacillus Calmette-Guerin (BCG)) [15] and melanoma [IMLYGIC® (talimogene laherparepvec/T-VEC)] [16]. Despite significant attempts to produce cancer vaccines, clinical translation of cancer vaccines into effective therapeutics has remained difficult for decades due to the wide range of tumor antigens and low immune response [17], originating the need to develop more potent vaccine approaches. Furthermore, there is a growing demand for vaccine development, large-scale manufacture, and dissemination, particularly in the case of non-viral diseases such as cancer [18], [19].
In general, cancer vaccine platforms are classified into tumor cell, peptide, viral vector, dendritic cell (DC), DNA and RNA types (Fig. 1 ). Allogenic or autologous patient-derived tumor cells are used to make cellular vaccines [20]. This approach is beneficial in that target antigens does not have to be determined in advance [21]. The whole cell cancer vaccine approach using granulocyte–macrophage colony-stimulating factor (GM-CSF) has been studied in several types of cancer both in animals as well as human trials. The phase I and II studies with allogeneic GM-CSF–transduced vaccine post-radiation (derived from two pancreatic tumor lines) demonstrated durable efficacy and prolonged survival in patients with pancreatic cancer [22], [23].
Fig. 1.
The commonly available platforms and mechanisms for cancer vaccine development. (a) Whole cell-based vaccines (an autologous tumor cell vaccine using a patient’s own cancer cells is injected as vaccine). (b) Viral vector-based vaccines (the genome of viral particles is modified to contain one or more genes encoding for the antigens of interest). (c) Dendritic cell-based vaccines (the dendritic cells efficiently capture the antigens, internalize, and process into peptides that are then presented in the context of MHC I and II molecules. These complexes are later recognized by the T-cell receptor (TCR) of CD8+ and CD4 + T cells) (d) DNA based vaccines (DNA plasmids are designed to deliver genes encoding TAs, eliciting or augmenting the adaptive immune response towards TA-bearing tumor cells. It induces the innate immune response, stimulates several DNA-sensing pathways in the cytosol of transfected cells due to the presence of CpG motifs and the double stranded structure itself) (e) Peptide-based vaccines (the peptides bind with the restricted MHC molecule expressed in APC. The peptide/MHC complex is then transported to the cell surface after intracellular processing and later recognized by the TCR on the surface of T cells, leading to activation of T lymphocytes) (f) RNA based vaccines (conventional non-replicating mRNA consists of 5 structural elements such as cap structures, a 5′ untranslated region (5′-UTR), an open reading frame encoding antigens of interest, a 3′-UTR; and an adenine repeating nucleotide sequence that forms a polyadenine (poly(A) tail. The non-replicating mRNA encodes antigen of interest, while self-amplifying mRNA encodes antigen of interest and a replication machinery, a self-replicating single-stranded RNA virus).
Peptide vaccines are made up of amino acid sequences that contain an epitope which can cause an immune response. Due to the difficulties of small peptides to attach directly to major histocompatibility complexes (MHC) I molecules, long peptides (containing of between 25 and 35 amino acids) are frequently favored over short peptides (consisting of approximately 10 amino acids). Short peptides also fail to activate CD4 helper T cells, which are required for full cytotoxic T lymphocyte activation (CTLs). These shortcomings can be overcome by using a long-peptide vaccine, that forces dendritic cells (DCs) to phagocytose the long-peptide before it is exposed on MHC I and attached to T cells. Long peptide vaccines also increase the HLA-related compatibility that exist with short-peptide vaccine. Furthermore, using a long peptide vaccine permits APCs to be presented via MHC II, which stimulates CD4+ lymphocytes, allowing for a more efficient immune response against tumor cells. However, because peptides are not self-immunogenic, administering an adjuvant at the same time is required for producing maximum efficiency [24]. So far, the peptide-based vaccines tested in laboratory has been able to elicit limited tumor-targeting immune responses, mostly because of intrinsic changes in cancer cells that reduce antigenicity and/or changes immunosuppressive alterations in the tumor microenvironment [25]. Therefore, other approaches are being developed including its combination with other immunotherapies, targeting antigenic epitopes arising from tumor cells and identifying target population [25].
Genetically modified viruses are also used for mRNA delivery. Application of positive strand RNA viruses via translation with host ribosomal machinery. However, challenges with host genome integration and the likelihood of host rejection, as well as cytotoxicity and immunogenicity, remains the major challenges. The MHC allows cancer cells to create peptide antigens that are present on their membrane surface. T cell receptors (TCRs) on cytotoxic T lymphocytes (CTLs) identify these antigens, resulting in cancer cell lysis. The antiviral immune response neutralizes viral vectors, limiting the number of vaccines that can be given [21].
Finally, to boost the adaptive immune system against tumor antigens, DNA cancer vaccines are created from bacterial plasmids (naked DNA) expressing one or more tumor antigens. The capacity of DNA vaccines lies in its ability to combine many genes expressing numerous tumor-antigens to establish a precise and broader adaptive immune response at the same time. However, these vaccines are poorly immunogenic [24]. To improve the immunological response of DNA vaccines, researchers have looked into encoding xenogeneic versions of antigens, fusing antigens with compounds that activate T cells or trigger associative recognition, DNA vector priming followed by viral vector boosting, and immunomodulatory molecules [26]. In contrast, RNA cancer vaccines are superior to DNA vaccines. While RNA is more susceptible to RNase breakdown, this can be minimized through chemical changes and the insertion of modified nucleosides such as pseudo uridine. Furthermore, unlike DNA, which must overcome the second barrier, the nuclear membrane, to reach the nucleus, RNA just needs to enter the cytoplasm [21]. The encoded proteins are converted into peptides that are present on MHC I and II to excite CD8+ and CD4+ T cells, respectively, after RNA translation. The fundamental pharmacology of mRNA vaccines is presented in Fig. 2 .
Fig. 2.
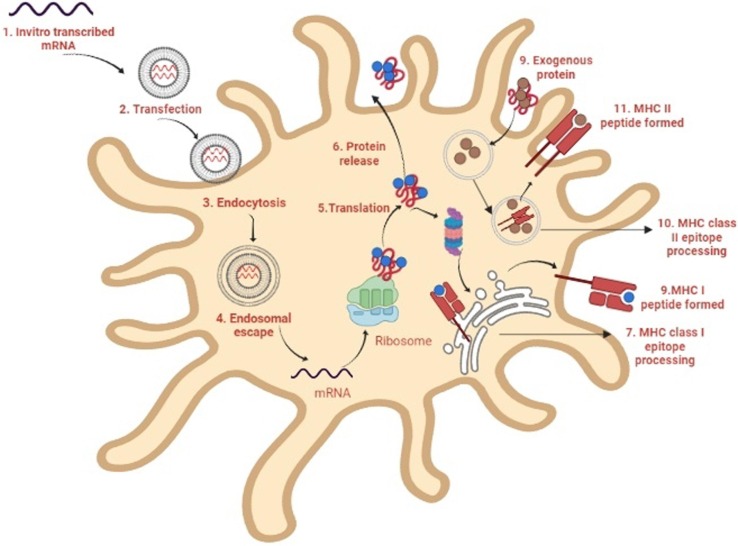
Mechanism of action of mRNA vaccines. 1. In a cell-free system, mRNA is in vitro transcribed (IVT) from a DNA template. 2. IVT mRNA is then transfected into dendritic cells (DCs) by the process of (3) endocytosis. 4. Endosomal escape allows entrapped mRNA to be released into the cytoplasm. 5. The mRNA is translated into antigenic proteins using the ribosome translational mechanism. After post-translational modification, the translated antigenic protein is ready to act in the cell where it was produced. 6. The protein gets secreted by the host cell. 7. Antigen proteins are digested in the cytoplasm by the proteasome and transferred to the endoplasmic reticulum, where they are loaded onto MHC class I molecules (MHC I). 8. MHC I-peptide epitope complexes with loaded MHC I-peptide epitopes produced, resulting in induction. 9. Exogenous proteins are taken up DCs. 10. They are degraded in endosomes and delivered via the MHC II pathway. Furthermore, to obtain cognate T-cell help in antigen-presenting cells, the protein should be routed through the MHC II pathway. 11. The generated antigenic peptide epitopes are subsequently loaded onto MHC II molecules.
Given the importance of DCs in initiating adaptive immunity in vitro and in vivo through generating CTLs, mRNA-transfected DC vaccine is an approach gaining interest for cancer vaccine development [24]. DC-based mRNA cancer vaccines have shown promising effects in various phases of clinical trials. Boczkowski and colleagues in 1996 first demonstrated that electroporation of DCs with mRNA could elicit potent immune responses against tumor in mice [27]. Since then, several human trials with electroporation of DCs have been conducted [28], [29]. Bulk mRNA isolated from autologous tumors is another method for pulsing DCs with tumor antigen-loaded mRNA [30], [31]. Direct injection of mRNA can be used instead of DC vaccines since it eliminates the need for DC isolation, ex vivo cultivation, and re-infusion [32]. Directly injecting the mRNA into secondary lymphoid tissue aids in delivering antigen to APCs at the T cell activation site, circumventing the need for DC movement [33].
Unlocking the potential of mRNA cancer vaccines
The cancer vaccines have the ability to elicit immune response to tumor antigens. The selection of a suitable target antigen is pivotal in the development of a vaccine design. Currently, the majority of vaccinations are TAAs, which are self-proteins that are improperly expressed by cancerous cells [21]. Developing vaccines against TAAs is challenging, as B- and T-cells might be subjected to removal by central and peripheral tolerance [34]. Besides, along with overexpression on tumor cells, TAAs might also be expressed in normal healthy cells leading to collateral damage [21]. In contrast, TSAs, which consists of neoantigens and viral oncoproteins are expressed only in cancerous cells. The prophylactic viral oncoproteins work by inducing the production of powerful neutralizing antibodies that block viral entrance into host cells and neoplasia caused by viruses [21]. However, these vaccines were ineffective in curing cancer as humoral immunity cannot effectively eliminate larger number of virus-infected cancer cells [21]. Neoantigens, like viral oncoproteins, are specific to tumor cells and are recognized by the immune system as foreign substances. Lately, neoantigens are being considered as a potential target in the progress of anti-cancer vaccine development. Numerous pre-clinical trials and early phase clinical trials have shown the ability of neoantigen based vaccines to minimize the potential induction of central and peripheral tolerance as well as the risk of autoimmunity [35], [36].
TAAs with shared expression across cancer types, such as melanoma-associated antigen (MAGE1) and NY-ESO-1.37, has encouraged studies to target TAAs that are habitually overexpressed in a certain type of cancer, along with the prospect of generating a common vaccine per tumor type [37]. Empirical clinical experience has also suggested that vaccines targeting specific tumor antigens are ineffective in tackling tumor heterogeneity, as well as in dealing with the challenges of clonal evolution and immune evasion by the tumor [38]. As a result, with the increasing importance of therapeutic cancer vaccines, the rapid development and large-scale production using mRNA platform introduces the potential for the development of both personalized vaccines and off-shelf cocktail vaccines.
Personalized cancer vaccines (PCV)
The neoantigens remain unique for each individual, with their numbers varying on the type of cancer. This necessitates for a tailored approach in which the tumor genome is sequenced and mutations are detected, neoantigens are predicted using computerized algorithms and a vaccine is then created and delivered to the patient. Mice vaccinated with a computationally engineered synthetic mRNA comprising numerous MHC class II neoepitopes showed 100% tumor rejection in preclinical studies, demonstrating antigen distribution [39]. The safety and efficacy of this approach was established in a first-in-human clinical study involving 13 patients with metastatic melanoma. Each patient was given a vaccine that contained 10 neoepitopes specific to their tumor. In certain patients, antitumor responses were discovered in metastases removed after immunization, where T-cell infiltration and neoepitope-specific apoptosis of autologous tumor cells were discovered after vaccination. All patients exhibited CD4+ and CD8+ T-cell responses [40]. Since then, therapeutic cancer treatment with tailored mRNA vaccines has received a lot of interest, and several clinical trials are presently underway, according to the US National Library of Medicine. A recent study with mRNA-4650, a KRAS personalized vaccine, developed by Moderna and Merck, in combination with or without pembrolizumab was conducted to treat patients with pancreatic carcinoma. The lipid nanoparticles (LNPs) approach for delivery of mRNA-4650 showed well-tolerated anti-tumoral immune response [41]. Another personalized vaccine, mRNA-4157, targeting 20 TAAs and useful in treating various types of tumors, in single or in combination with pembrolizumab demonstrated acceptable safety profile with cytotoxic T-lymphocyte (CTL)- and memory T-cell-dependent immune responses [42]. Based on the ability of mRNA-4157 to elicit clinical response, a phase II trial is currently undergoing to evaluate the efficacy of the postoperative adjuvant therapy with mRNA-4157 and pembrolizumab in comparison with pembrolizumab monotherapy in high-risk recurrent individuals with complete resection of tumor (NCT03897881). A first-in-human phase Ib study of RO7198457, a combination of systemically administered RNA-Lipoplex iNeST with the PD-L1 antibody atezolizumab is presently conducted in patients with locally advanced or metastatic solid tumors. The preliminary results of this study suggest significant level of neoantigen immune-tumor response. A randomized phase II study of RO7198457 in first-line for patients with melanoma in combination with pembrolizumab is currently ongoing, and 2 randomized clinical trials are planned for the adjuvant treatment of individuals with non-small cell lung cancer (NSCLC) and colorectal cancer (CRC) [43].
Tetravalent vaccine and combination therapies
A tetravalent RNA-lipoplex cancer vaccine, BNT111, contains 4 types of naked RNA such as RBL001.1, RBL002.2, RBL003.1, and RBL004.1 encoding 4 melanoma-associated antigens (MAAs), the cancer-testis antigen NY-ESO-1, the human MAGE- A3, tyrosinase, and putative tyrosine-protein phosphatase (TPTE), encapsulated in liposomes. The vaccine upon intravenous administration is taken up by the APCs, and after being translocated to the cytoplasm, is translated into the 4 tumor-associated proteins. As a result, CD8+ and CD4+ T-cell responses against 4 selected antigens are produced [44]. A phase I trial showed that this vaccine alone and in combination with immune checkpoint inhibitors (ICIs) induced durable objective responses and exhibited a favorable safety profile among patients with advanced melanoma [45]. A phase II trial is ongoing to evaluate the vaccine candidate in combination with the anti-PD-1 antibody cemiplimab for patients with unresectable stage III or stage IV melanoma who are refractory to or relapsed after anti-PD-1 therapy [46].
mRNA-5671, another tetravalent vaccine, is an LNP-formulated mRNA-based vaccine that targets 4 of the most frequent KRAS mutations (G12D, G13D, G12C and G12V). APCs take up and translate mRNA-5671 after immunization. Following translation, the MHCs displays the epitopes on the surface of APCs, resulting in the development of both cytotoxic T-lymphocyte- and memory T-cell-dependent immune responses directed at tumor cells with KRAS mutations [47]. CD8 T cell responses to KRAS antigens were considerably improved in preclinical investigations after immunization with mRNA encoding KRAS mutations [48]. Patients with advanced or metastatic NSCLC, colorectal cancer, or pancreatic adenocarcinoma and KRAS mutations are being enrolled in a phase I research using mRNA-5671 with or without pembrolizumab (NCT03948763).
Due to heterogenous and ever evolving nature of cancer mechanisms, the clinical benefit of monotherapy regimen in patients with advanced cancer is not adequate. Tumor-specific T lymphocytes produced by vaccines do not operate efficiently against the tumor due to their lack of motility and/or gradual depletion. As a result, combining procedure that prevent immune escape pathways is critical [49]. For instance, a phase II clinical trial in chemotherapy treated patients with metastatic castration-resistant prostate cancer (mCRPC) showed similar and durable tumor immune responses on addition of DC vaccines [50]. Monoclonal antibodies (mAbs) targeting CTLA-4 and the PD-1/PD-L1 expression have revolutionized the treatment paradigm for several types of cancers, including renal cancer, melanoma, bladder cancer, lung cancer and Hodgkin's lymphoma [51]. CureVac GmbH systemic mRNA immunotherapy and local irradiation therapy can eradicate established macroscopic E.G7-OVA and LLC cancers in a synergistic manner. Moreover, this combination boosted CD4+, NKT and CD8+cell infiltration in tumor infected mouse [52]. CV9202, vaccine encoding 6 NSCLC-associated antigens (NY-ESO-1, MUC-1, MAGE-C2, MAGE-C1, 5T4 and survivin) have been proven to induce targeted immune responses. The combination of this vaccine with radiotherapy in a phase Ib clinical trial in 26 stage IV NSCLC patients revealed elevated CV9202 antigen-specific immune responses in 84% of patients, with 80% increased antigen-specific antibody levels, 40% patients with functional T cells and about 52% of patients had multiple antigen specificities [53]. In another study, researchers used an mRNA vaccine expressing the TAA MUC1 in combination with an anti-CTLA-4 monoclonal antibody to boost the vaccine’s immune response against triple-negative breast cancer (TNBC) by improving T cell activity [54].
Recent advancement of mRNA vaccines in various types of cancer
Preclinical and clinical evidence have shown that using mRNA for prophylaxis and therapy can help prevent infectious disease and treat cancers, and that mRNA vaccines are safe and well tolerated in both animal models and humans. Further enhancements might also boost antigen-specific immune responses as well as B and T cells immune responses [55]. As of 21st December 2021, 23 RNA vaccines are currently under phase I/II/III clinical trials, while 24 vaccines are at pre-clinical stage.
Breast cancer
Breast cancer remains a cause of mortality for women globally [56]. More often, 81% women suffer from invasive breast cancer, which comprises of at least 21 distinct histological subtypes and 4 molecular subgroups (luminal A, luminal B, triple-negative and HER2-enriched) that differ in risk factors, presentation, response to treatment, and outcomes [57]. Invasive breast cancer can spread to adjacent lymph nodes or other organs over time. It is because of widespread metastasis that a woman dies from breast cancer [58]. Using modern methodologies for mRNA sequencing, such as The Cancer Genome Atlas (TCGA) data, it has been established that increased expression of T- and B-cell predicts higher overall survival (OS) in a variety of tumor types, including breast cancer [59]. The current treatment approach for breast cancer includes radiation therapy, surgery, chemotherapy, as well as hormonal and targeted therapies. Lately, the development of medications that can prevent breast cancer from developing in the first place, as well as their recurrence, has gathered attention. The overexpression of high-affinity transmembrane receptors such as HER3, HER2, c-MET, EGFR, and the transmembrane protein epithelial mucin-1 (MUC-1) are the key oncogenic drivers for breast cancer [60]. Treatment of breast cancer, especially, TNBC is gaining importance, since lack of therapeutic targets makes such type of cancer unresponsive to typical endocrine therapies and HER2-targeted therapy. In such a case, cancer vaccines which aid in activation and amplification of TAA-specific immunity combined with a sustained memory T cell immune response may be an effective therapy for preventing breast cancer recurrence in patients [61]. Previous vaccination strategies in adjuvant settings, against HER2+ self-antigens have shown substantial efficacy in patients with breast cancer [62], [63], [64]. However, such an approach is usually weak as immune response as T-lymphocytes have affinity to HER2+ and thus are subject to central tolerance [65]. An ongoing phase I/II trial is being conducted in patients with TNBC and who completed standard of care chemotherapy, where patients are allocated to receive either 8 vaccination cycles of mRNA WAREHOUSE vaccine (containing pre-formulated, shared tumor antigens, non-mutated) or mRNA MUTANOME vaccine (containing individual mutations). The preliminary data of this trial showed mRNA WAREHOUSE is feasible approach for treatment of TNBC [66]. Another phase I trial from the Schmidt and colleagues was conducted with the addition of a third arm where patients were injected with IVAC_M_uID [Individualized NeoAntigen Specific Immunotherapy (iNeST)] which encodes 20 cancer mutations neoepitopes derived from NGS. The initial results reported promising results of iNeST IVAC_M_uID in inducing strong polyepitope T-cell responses in patients with TNBC in the post-(neo)adjuvant phase or post-surgery. All the patients reported CD4+ and/or CD8+ T-cell responses against 1 to 10 of the vaccine neoepitopes [67]. Theoretically, this treatment regimen will lead to a transition from an individualized therapy targeting a single biomarker (e.g., HER2) to a fully specialized treatment targeting specific mutations in each patient. The ongoing trials related to mRNA vaccine in breast cancer is listed in Table 1 .
Table 1.
Clinical trials for breast cancer.
| Conditions | NCT number | Study design | Interventions | Status |
|---|---|---|---|---|
| Triple negative Breast Cancer | NCT02316457 | Phase I | IVAC_W_bre1_uID/IVAC_M_uID | Active, not recruiting |
| Breast Cancer | NCT00003432 | Phase I/II | carcinoembryonic antigen RNA-pulsed DC cancer vaccine | Terminated |
| Breast Cancer | NCT03788083 | Phase I | Trimix mRNA | Recruiting |
| Breast Cancer | NCT03739931 | Phase I | mRNA-2752/Durvalumab | Recruiting |
Non-small cell lung cancer
Lung cancer remains a major cause of cancer worldwide after breast cancer. Despite recent therapeutic advancements, the overall 5-year survival rate for LC is still less than 20%. Because most cancers exhibit mutational variability, conventional cancer treatment techniques, such as surgery and chemotherapy, are far from optimum, especially for advanced stage malignancies. Currently, the information related to mRNA-based approach in treatment for NSCLC is limited. CV9201 is a cancer immunotherapy based on RNActive® that encodes 5 NSCLC antigens: melanoma antigen family C1/C2, NY esophageal squamous cell carcinoma-1, trophoblast glycoprotein and survivin. About 46 patients with locally advanced (n = 7) or metastatic (n = 39) NSCLC received 5 intradermal CV9201 injections (400–1600 g of mRNA) in a phase I/IIa dose-escalation experiment. After initial dose administration, the median progression-free survival and OS were 5.0 months (95 percent CI 1.8–6.3) and 10.8 months (8.1–16.7), respectively. In addition, 60% of patients reported an increased frequency of >2 fold followed by activation of IgD+ CD38hi B cells. This showed that CV9201 was well tolerated, and immunological responses could be observed following therapy, indicating that further clinical research is warranted [68]. The ongoing trials related to mRNA vaccine in breast cancer is listed in Table 2 .
Table 2.
Clinical trials for non-small cell lung cancer.
| Conditions | NCT Number | Study type | Interventions | Status |
|---|---|---|---|---|
| Non-Small Cell Lung Cancer | NCT03908671 | Observational study | Personalized mRNA Tumor Vaccine | Not yet recruiting |
| Metastatic Non-small Cell Lung Cancer | NCT03164772 | Phase II | Durvalumab/Tremelimumab/BI 1361849 | Completed |
| Non-Small Cell Lung Cancer | NCT03948763 | Phase I | V94/Pembrolizumab | Active, not recruiting |
| Non-Small Cell Lung Cancer | NCT04998474 | Phase II | FRAME-001 personalized vaccine | Not yet recruiting |
| Non-Small-Cell Lung Cancer With Bone Metastases | NCT02688686 | Phase I/II | Genetically modified dendritic cells + cytokine-induced killer | Unknown |
| Non-Small Cell Lung Cancer | NCT03166254 | Phase 1 | Pembrolizumab/NEO-PV-01 vaccine/Poly ICLC | Withdrawn |
| Stage IIIB/IV Non-Small Cell Lung Cancer | NCT00923312 | Phase I/II | CV9201 | Completed |
| Non-Small Cell Lung Cancer | NCT04355806 | Prospective | PD-1/PD-L1 inhibitors/Inactivated trivalent influenza vaccine | Not yet recruiting |
| Stage II-III Non-Small Cell Lung Cancer | NCT04267237 | Phase II | Atezolizumab/RO7198457 | Withdrawn |
Prostate cancer
The standard treatment for prostate cancer includes androgen deprivation and chemotherapy. However, patients become resistant after prolonged treatment with these agents. Relapse or progression of disease occur even after complete androgen blockage and when plasma concentrations of testosterone are reduced to <50 ng/dL by castration or gonadotropin-releasing hormone analogs, and the effects of the remaining androgens are suppressed by androgen receptor antagonists [69]. With the advent of Sipuleucel-T, a dendritic-cell based vaccine, for treatment of advanced stages of prostate cancer, immunotherapy for prostate cancer has come into limelight. However, besides sipuleucel-T, there have been disappointing results in prostate cancer. In patients with mCRPC, large phase III studies of the CTLA-4 inhibitor, ipilimumab did not show significant benefit in OS compared to placebo before or after chemotherapy treatment. In addition, nivolumab, a single-agent PD-1 antibody, was found to have little effect in men with mCRPC. However, administering both CTLA-4 and PD-1 inhibitors combination has resulted in some PSA and objective responses, showing that a minority of patients may benefit. Pembrolizumab was given to mCRPC patients who were advancing on enzalutamide in a recent study, and a significant number of men had remarkable PSA and objective responses. PSA and objective responses appeared to be more common in another small trial combining ipilimumab and nivolumab than with either treatment alone, and it was suggested that patients with DNA repair gene mutations benefited the most. Finally, in a limited fraction of individuals with prostate cancer, pembrolizumab, which was recently licensed for mismatch repair-deficient or microsatellite-unstable tumors, might be beneficial. The use of synthetic nucleotide-based DNA or RNA vaccines is an alternative route for in vivo cancer vaccine design. The use of plasmid DNA expressing TAAs to stimulate humoral and cellular immune responses has been shown earlier. However, in contrast to the features of mRNA, the potential of DNA-based anti-cancer vaccines integrating into the host genome and resulting in malignant transformation is a major barrier. Due to the instability of natural mRNA molecules, Cure Vac (Tubingen, Germany) had developed RNActive® vaccines, which are mRNA molecules optimized to elicit powerful, well-balanced immunological responses including humoral and cellular responses, effector and memory responses, and Th1 and Th2 immune cell activation. These molecules stimulate the immune system by interreacting with toll-like receptor 7 and do not modify the primary amino acid sequences [70]. Initial assessment of immune response in compounds encoding prostate specific membrane antigen (PSMA) or oval albumin demonstrated strong humoral immune response with Th2 and Th1 cells, with repeated immunization increasing the frequency of IFN-γ-secreting CD8+ T cells while maintaining CD4+ regulatory T cells frequency [70].
Early intervention in patients with hormone-refractory prostate cancer with CV9103 elicited significant cytotoxic T-cell response against all tested PSAs. A phase I/IIa clinical study with CV9103, a prostate cancer vaccine containing 4 antigens, mainly, tumor associated antigens PSA, PSMA, prostate stem cell antigen and six transmembrane epithelial antigen of the prostate 1, displayed a high level of immunogenicity in patients with mCRPC, where, 58% responders reacted against multiple antigens. About 74% patients had antigen-unspecific B-cells, while 79% of responders had antigen-specific T-cells. One patient displayed >85% drop in his PSA-level [71]. Though the initial responses in these trials were encouraging, the subsequent trial with CV9104 for prostate cancer were terminated due to no significant effect on OS [72]. These findings indicate that selection of antigen is crucial for activating APCs and immune response. Several studies have highlighted the efficacy of mRNA vaccine in other therapeutic areas [73], [74]; when MS2 delivery platform is used. Using recombinant protein technology, the MS2 capsid can interact with specific 19-nucleotide stem-loop, can pack the target RNA, thereby preventing degradation by nucleases [75]. Li et al. observed that MS2 virus-like particles (VLPs)-based hPAP–GM–CSF mRNA vaccine might decrease prostatic-tumor growth in C57BL/6 mice, implying that this vaccine could elicit an effective immune response in a short period of time and is a viable treatment for prostate tumors [76]. The recent advancement in prostate cancer is the delivery of mRNA as nanoparticle. The co-delivery of C16-R848 adjuvant-pulsed mRNA vaccination with OVA RNA increased TAA presentation while simultaneously stimulating CD8+ T cell expression into the tumor and improved the overall anti-tumor response, demonstrating effective adaptive immune. The vaccine significantly reduced 80% of tumor growth when given before tumor engraftment and suppressed tumor growth by 60% when given post tumor engraftment in syngeneic allograft mouse models of lymphoma and prostate cancer. These data imply that adding C16-R848 adjuvant pulsation to mRNA vaccine NP is a rational design strategy for improving the efficacy of synthetic mRNA vaccines [77]. Further, clinical trials related to mRNA vaccine in prostate cancer is listed in Table 3 .
Table 3.
Clinical trials for prostate cancer.
| Study population | NCT number | Study design | Intervention | Status |
|---|---|---|---|---|
| Hormonal Refractory Prostate Cancer |
NCT00831467 | Phase I/II | CV9103 | Completed |
| mCRPC | NCT01153113 | Phase I/II | hTERT mRNA DC |
Withdrawn |
| Prostate Cancer | NCT01197625 | Phase I/II | Dendritic cell vaccine | Active, not recruiting |
| Prostate cancer | NCT01278914 | Phase I/II | Dendritic Cells (DC) prostate | Completed |
| mCRPC | NCT01817738 | Phase I/II | CV9104 | Terminated |
| Prostate cancer | NCT01446731 | Phase II | mRNA transfected dendritic cell/Docetaxel | Completed |
| Prostate Cancer | NCT00006430 | Phase I | Autologous dendritic cells transfected with amplified tumor RNA | Unknown |
| Prostate Cancer | NCT02140138 | Phase II | CV9104 | Terminated |
| Prostate Cancer | NCT02692976 | Phase II | mDC vaccine/pDC vaccine/mDC and pDC vaccine | Completed |
| Prostate cancer | NCT00108264 | Phase I | Tumor RNA transfected dendritic cells | Completed |
| Prostate cancer | NCT00004211 | Phase I/II | PSA RNA-pulsed dendritic cell vaccine | Completed |
| Prostate cancer | NCT00010127 | Phase I | Therapeutic autologous dendritic cells | Terminated |
| Prostate cancer | NCT04382898 | Phase I/II | BNT112 with Cemiplimab | Recruiting |
| Hormonal Refractory Prostate Cancer | NCT00906243 | Phase I/II | CV9103 | Terminated |
| Prostate cancer | NCT01784913 | Phase I/II | UV1 synthetic peptide vaccine and GMCSF | Active, not recruiting |
Lymphoma
Lymphomas are a biologically and clinically heterogeneous group of carcinomas that develop in secondary lymphoid organs from mature B- or T-lymphocytes [78]. Global statistics reveal Hodgkin lymphoma occurs in 0.4% of total cancer population, while, non-Hodgkin lymphoma (NHL) is more frequent and accounts for 2.8% of all types of cancer [56]. With the increase in the incidence of lymphoma and no known effective treatment, there is an urgent need to develop novel therapies [79]. Patients with lymphoma have been benefitted from monoclonal antibodies such as rituximab, however, majority of patients remain incurable or die of the disease [79]. The identification of B-cell receptor variable regions as B-NHL unique antigens aided the development of tailored made vaccines to protect patients against their own tumors. Despite promising early results, this technique has yet to demonstrate enough clinical value to gain regulatory approval [80]. Use of personalized and standardized approach have been tested earlier, but have experienced drawbacks, with slim chance of success in clinical trials. Further, tumor-induced immunosuppressive factors and immune regulatory mechanism might limit the ability of immune system to generate antitumor immune response. Currently, the mRNA vaccine approaches are mostly in the nascent stage with preclinical studies demonstrating promising efficacy in reducing tumor growth. As mentioned earlier in the paragraph for prostate cancer, the co-delivery of C16-R848 adjuvant-pulsed mRNA vaccination with OVA RNA significantly reduced tumor growth in syngeneic allograft mouse models of lymphoma [77]. Similarly, in another study, 6 female C57BL mice were subcutaneously injected with E.G7 OVA expressing lymphoma cells to test the therapeutic efficacy of mRNA Galsomes over NPs containing unmodified mRNA. After intravenous administration, mRNA Galsomes transmits nucleoside-modified antigen-encoding mRNA as well as the glycolipid antigen and immunopotentiator α-galactosyl ceramide to APCs. Both the treatments showed significant tumor reduction in 40% of animals and prolonged OS. Further combination of mRNA Galsomes with PD-L1 checkpoint inhibitor indicated a synergistic behavior in tumor reduction [81]. The clinical trials related to mRNA vaccine in lymphoma is listed in Table 4 .
Table 4.
Clinical trials for lymphoma.
| Conditions | NCT Number | Phases | Interventions | Status |
|---|---|---|---|---|
| Primary/Relapsed Acute Lymphoblastic Leukemia | NCT03559413 | Phase I/II | Individual peptide vaccination with adjuvant GM-CSF and Imiquimod | Active, not recruiting |
| Acute Leukemia/Acute Lymphoblastic Leukemia/Acute Myeloid Leukemia | NCT04969601 | Phase I/II | Vaccine COMIRNATY® (BNT162b2) | Recruiting |
| Relapsed/Refractory Solid Tumor Malignancies or Lymphoma | NCT03739931 | Phase I | mRNA-2752/Durvalumab | Recruiting |
| Relapsed/Refractory Solid Tumor Malignancies or Lymphoma | NCT03323398 | Phase I/II | mRNA-2752/Durvalumab | Active, not recruiting |
| Lymphoma | NCT04847050 | Phase II | mRNA-1273 | Recruiting |
Pancreatic cancer
Pancreatic cancer is a fatal malignancy with survival rate of 10.8% over 5 years [82]. The pancreatic cancer cells are also distinguished by several germline or genetic mutations including KRAS (90%), TP53 (75%–90%), CDK2NA (90%), SMAD4/DPC4 (50%). Surgical resection is a possible treatment for this type of cancer, however, primarily, the cancer goes undetected at an earlier stage and those who opt for surgery show signs of recurrence within 2 years after operation. The other treatment strategies include combination chemotherapy, immune checkpoint inhibitors and targeted therapies. The aggressive nature of the tumor cells along with the hostile tumor microenvironment nature has resulted in the chemoresistance [83]. Hence, the target of recent clinical investigations have been shifted to newer therapies such as macrophage and cytotoxic T-lymphocyte targeted therapies, adoptive T-cell therapy and cancer vaccines [84]. Designing a pancreatic cancer vaccine based on peptide, tumor cell, dendritic cell or DNA based system has several disadvantages leading to poor therapeutic efficacy [85], [86], [87], [88]. On the contrary, mRNA cannot incorporate into the genome and hence does not pose any risk of insertional mutagenesis, with a superior safety profile. Nonetheless, mRNA vaccine against pancreatic cancer antigens has remained underdeveloped so far, and no suitable patient population has been identified. A recent study by Huang et al, identified 6 potential antigens, namely, WNT7A, ADAM9, MET, EFNB2, TPX2 and TMOD3 for mRNA vaccine development [89]. Patients with immune subtypes 4 and 5 considered as immunological “cold” phenotypes were found to be suitable for vaccination [89]. A list of clinical trials for the development of pancreatic cancer vaccine is reported in Table 5 .
Table 5.
Clinical trials for pancreatic cancer.
| Conditions | NCT Number | Study design | Interventions | Status |
|---|---|---|---|---|
| Pancreatic Cancer | NCT04157127 | Phase I | Autologous DC vaccine | Recruiting |
| Pancreatic Cancer | NCT05116917 | Phase II | Nivolumab/Ipilimumab/Influenza vaccine/Stereotactic body radiation therapy | Recruiting |
| Pancreatic Cancer | NCT04627246 | Phase I | Autologous Dendritic Cell Vaccine Loaded with Personalized Peptides (PEP-DC vaccine) | Recruiting |
| Pancreatic Cancer | NCT03948763 | Phase I | V941/Pembrolizumab | Active, not recruiting |
| Pancreatic Cancer | NCT04161755 | Phase I | Atezolizumab/RO7198457/mFOLFIRINOX | Active, not recruiting |
Melanoma
Melanoma is a malignant tumor that originates from melanocytes, with a 5-year survival rate of 10% in patients with end-stage melanoma [90]. Nowadays, several therapies are available, including chemotherapy, radiation therapy, immunotherapy, and surgery. Of these, immunotherapy with ipilimumab, nivolumab and pembrolizumab have been approved as standard therapy for cutaneous melanoma. Chemotherapeutic treatment regimens damage the normally dividing cells along with tumor-infected cells [91]. Hence, the process of development of further treatment strategies which suppress tumor growth is being explored. mRNA based vaccines are the latest development for treatment of melanoma. An initial phase I/II trial in 21 metastatic melanoma patients co-injected with protamine-protected mRNA induced antitumor immune response. Especially in patients injected with keyhole limpet hemocyanin (KLH) along with vaccine, the frequency of Foxp3+/CD4+ regulatory T cells decreased upon mRNA vaccination in their peripheral blood, whereas myeloid suppressor cells (CD11b + HLA-DRlo monocytes) were reduced in the patients not receiving KLH [92]. A recent application of personalized RNA mutanomes in 5 humans demonstrated prolonged progression-free survival. Two of the five patients experienced vaccine-related objective responses, while 1 patient had a late relapse suggesting acquired resistance mechanism. The third patient develop complete response to vaccination in combination with PD-1 blockade therapy [40]. Another preclinical study in C57BL/6 mouse model of B16F10 melanoma reported the promising immune response of lipid encapsulated mRNA vaccine encoding TRP2. In addition, co-delivery of mRNA vaccine and PD-L1 siRNA downregulated PD-L1 in the dendritic cells promoting T cell activation and proliferation, in turn inhibiting tumor growth and metastasis [93]. Various combinations of mRNA with ICIs are currently being explored in clinical trials. For instance, Wilgenhof et al. assessed the anti-tumor activity of TriMixDC-MEL (an autologous monocyte-derived dendritic cell electroporated with synthetic mRNA encoding CD40 ligand) in patients with pretreated advanced melanoma, either as a monotherapy (NCT01066390) or combined with ipilimumab (NCT01302496) and in disease free melanoma patients following local treatment of macro metastases. The median progression-free survival and overall survival was substantially improved in both the groups with more durable increase in patients with combination therapy [94]. An interim analysis by Sahin et al. showed that BNT111 alone or in combination with PD1 inhibitors, mediates durable objective responses in checkpoint-inhibitor experienced patients with unresectable melanoma. These responses were accompanied by strong induction of CD4+ and CD8 + T cell immunity against the vaccine antigens [45]. A list of clinical trials for the development of melanoma vaccine is reported in Table 6 .
Table 6.
Clinical trials for melanoma.
| Conditions | NCT Number | Study design | Interventions | Status |
|---|---|---|---|---|
| Melanoma | NCT02410733 | Phase I | Tetravalent RNA-lipoplex cancer vaccine targeting 4 TAAs (RBL001.1, RBL002.2, RBL003.1, and RBL004.1 |
Active, not recruiting |
| Metastatic Melanoma | NCT00672542 | Phase I | Proteasome siRNA and tumor antigen RNA-transfected dendritic cells | Completed |
| Melanoma | NCT01684241 | Phase I | RBL001/RBL002 | Completed |
| Melanoma | NCT04526899 | Phase II | BNT111/Cemiplimab | Recruiting |
| Melanoma | NCT00126685 | Phase I/II | autologous tumor cell vaccine/therapeutic autologous dendritic cells | Unknown |
| Melanoma | NCT01456104 | Phase I | Langerhans-type dendritic cells | Active, not recruiting |
| Melanoma | NCT05264974 | Phase I | Autologous total tumor mRNA loaded DOTAP liposome vaccine | Not yet recruiting |
| Melanoma | NCT02035956 | Phase I | Ivac mutanome, rbl001/rbl002 | Completed |
| Metastatic Melanoma | NCT01216436 | Phase I | RNA-transfected mature autologous DC | Terminated |
| Melanoma | NCT00074230 | Phase I/II | Autologous Dendritic Cells loaded with MAGE-A3, MelanA and Survivin | Completed |
| Melanoma | NCT01676779 | Phase I/II | mRNA Electroporated Autologous Dendritic Cells | Completed |
| Resected melanoma | NCT03394937 | Phase I | ECI-006 | Terminated |
| Stage III/IV Malignant Melanoma |
NCT01066390 | Phase I | TriMixDC | Completed |
| Stage III/IV Malignant Melanoma |
NCT01302496 | Phase II | TriMix-DC and ipilimumab | Completed |
| Malignant Melanoma | NCT00204516 | Phase I/II | mRNA coding for melanoma associated antigens | Completed |
| Advanced Malignant Melanoma | NCT01278940 | Phase I/II | Dendritic Cells loaded RNA | Completed |
| Advanced Melanoma | NCT03815058 | Phase II | RO719845/Pembrolizumab | Active, not recruiting |
| High-Risk Melanoma | NCT03897881 | Phase II | mRNA-4157/pembrolizumab | Active, not recruiting |
| Melanoma | NCT02285413 | Phase II | DC based mRNA/cisplatin | Completed |
| Melanoma | NCT00204607 | Phase I/II | mRNA | Completed |
| Metastatic melanoma | NCT00961844 | Phase I/II | Tumor-derived mRNA/Temolomide | Terminated |
| Melanoma | NCT01530698 | Phase I/II | Autologous dendritric mRNA | Completed |
| Melanoma Stage III or IV | NCT00243529 | Phase I/II | Autologous dendritric mRNA | Completed |
| Breast Cancer or Malignant Melanoma | NCT00978913 | Phase I | DC mRNA | Completed |
| Melanoma | NCT00940004 | Phase I/II | DC mRNA | Completed |
| Melanoma | NCT03480152 | Phase I/II | NCI-4650 | Terminated |
| Stage III/IV malignant melanoma | NCT01973322 | Phase II | DC mRNA | Recruiting |
Several clinical investigations for mRNA vaccines in various type of cancer have reported promising preliminary results. A list of clinical trials along with their results are presented in Table 7 .
Table 7.
Summary of clinical trials for mRNA vaccine and their results in various cancers.
| Interventions | Conditions | Results | NCT Number | Sponsor | Study design |
|---|---|---|---|---|---|
| VAC_W_bre1_uID/IVAC_M_uID | Breast Cancer | iNeST IVAC_M_uID is highly efficient in inducing strong poly-epitopic T-cell responses in patients with TNBC in the post-(neo) adjuvant setting | NCT02316457 | BioNTech SE | Phase I |
| mRNA-2752/Durvalumab | Relapsed/Refractory Solid Tumor Malignancies or Lymphoma/ Triple Negative Breast Cancer, HNSCC, Non-Hodgkin’s, Urothelial Cancer, Immune Checkpoint Refractory Melanoma, and NSCLC Lymphoma | Tumor regressions was observed in approximately 50% of patients with head and neck cancer with mRNA 2752 and durvalumab | NCT03739931 | ModernaTX, Inc. | Phase I |
| CV9103 | Hormonal Refractory Prostate Cancer |
The two-component mRNA vaccine mediates a strong antitumor response against OVA-expressing tumor cells, not only in a prophylactic but also in a therapeutic setting | NCT00831467/NCT00923312 | CureVac AG | Phase I/II |
| Dendritic cell vaccine | Prostate Cancer | Adjuvant DCV mitigates the time to biochemical progression | NCT01197625 | Oslo University Hospital | Phase I/II |
| CV9104 | mCRPC | CV9104 exhibited antigen-specific immune responses post vaccination | NCT01817738 | CureVac AG | Phase I/II |
| Autologous dendritic cell | mCRPC | Adjuvant therapy with autologous dendritic cell vaccine provided longer median PFS and DSS | NCT01446731 | Inge Marie Svane | Phase II |
| mDC and pDC vaccination | mCRPC | Blood-derived CD1c+ myeloid dendritic cells induced functional antigen-specific T cells which in turn is correlated with an improved clinical outcome. | NCT02692976 | Radboud University Medical Center | Phase II |
| BNT112 and cemiplimab | Prostate cancer | BNT112 induces immune and PSA responses in patients with advanced prostate cancer. | NCT04382898 | BioNTech SE | Phase I/II |
| PSA RNA-pulsed dendritic cell vaccine |
Prostate cancer | Escalating doses of PSA mRNA-transfected DCs were administered with no evidence of dose-limiting toxicity or adverse effects, including autoimmunity. | NCT00004211 | Duke University, National Cancer Institute | Phase I/II |
| Lipo-MERIT | Melanoma | Lipo-MERIT vaccine is a potent immunotherapy in patients with CPI-experienced melanoma, and induced strong CD4+ and CD8 + T cell immunity against the vaccine antigens | NCT02410733 | BioNTech SE | Phase I |
| Proteasome siRNA and tumor antigen RNA-transfected dendritic cells | Metastatic melanoma | Tumor antigen-loaded DCs provided partial clinical response, exhibited diffuse dermal and soft tissue metastases, had a complete response. | NCT00672542 | Scott Pruitt | Phase I |
| Langerhans-type dendritic cells electroporated with TRP-2 mRNA | Melanoma | TRP2 mRNA-electroporated LC vaccines produced antigen-specific responses especially in terms of cytokine secretion, cytolytic degranulation, and increased TCR clonality leading to clinical outcomes. | NCT01456104 | Memorial Sloan Kettering Cancer Center, Rockefeller University | Phase I |
| IVAC MUTANOME, RBL001/RBL002 | Melanoma | 60% of the 125 selected neo-epitopes elicited a T-cell response. No severe adverse drug reactions were reported Vaccination with IVAC® MUTANOME was very well tolerated. | NCT02035956 | BioNTech RNA Pharmaceuticals GmbH | Phase I |
| Autologous Dendritic Cells loaded with MAGE-A3, MelanA and Survivin | Stage IV melanoma | Few patients achieved full remission and/or survived for >10 years, while 2 patients developed asymptomatic sarcoidosis after treatment with autologous dendritic cells | NCT00074230 | University Hospital Erlangen | Phase I/II |
| TriMixDC-MEL | Stage III/IV melanoma | TriMixDC-MEL is tolerable and results in a high rate of durable tumor responses | NCT01676779 | Universitair Ziekenhuis Brussel, RIZIV | Phase II |
| ECI-006 | Melanoma | ECI-006 was generally well tolerated and demonstrated immunogenic response | NCT03394937 | eTheRNA immunotherapies | Phase I |
| TriMix-DC | Melanoma | TriMixDC-MEL was safe and produced immunogenic response. Durable antitumor activity was observed across the investigated iv dose levels | NCT01066390 | Bart Neyns | Phase I |
| TriMix-DC and ipilimumab | Stage III/IV melanoma | TriMixDC provided robust CD8 + T-cell responses in melanoma patients, and in patients with a clinical response | NCT01302496 | Bart Neyns, Vrije Universiteit Brussel | Phase II |
| Dendritic cells with or without cisplation | Stage III/IV melanoma | Combination therapy of DC vaccine and cisplatin is safe and produces immune response but the clinical response is similar to DC vaccine monotherapy | NCT02285413 | Radboud University Medical Center | Phase II |
| mRNA with GM-CSF | Malignant melanoma | Direct injection of protamine-protected mRNA is feasible and safe. | NCT00204607 | University Hospital Tuebingen | Phase I/II |
| mRNA-2416 | Relapsed/Refractory Solid Tumor Malignancies or Lymphoma,Ovarian Cancer | mRNA-2416 was well-tolerated at all dose levels. Analyses of tumor post-treatment demonstrate increased OX40L protein expression, elevated PD-L1 levels and pro-inflammatory activity. | NCT03323398 | ModernaTX, Inc. | Phase I/II |
| Dendritic vaccine | Breast cancer and malignant melanoma | Treatment with autologous DCs mRNA was feasible and safe and did not alter the percentage of Tregs in patients | NCT00978913 | Inge Marie Svane | Phase I |
| NCI-4650 | Melanoma, Colon Cancer, Gastrointestinal Cancer, Genitourinary Cancer, Hepatocellular Cancer | NCI-4650 was found to be safe and elicited mutation-specific T cell responses | NCT03480152 | National Cancer Institute | Phase I/II |
Optimization of the mRNA vaccine pharmaceutical features
Vaccine design and modification
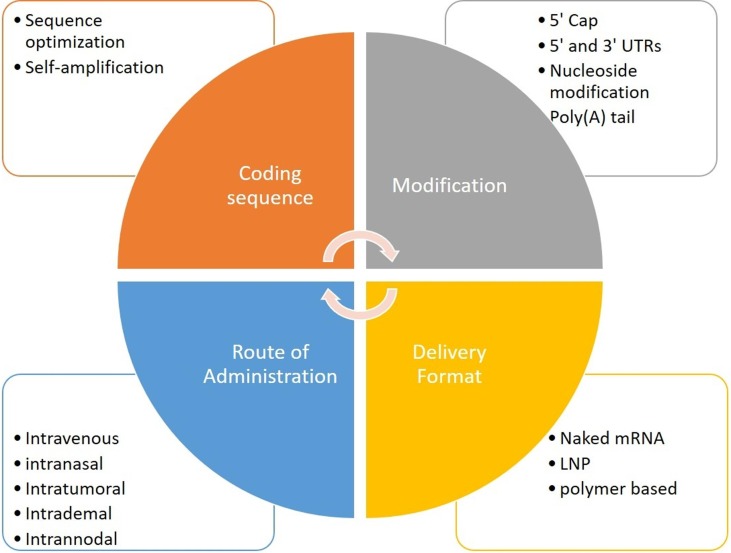
The development of cancer vaccine depends on type of cancer, vaccine design and its modification and route of delivery (Fig. 3 ). The mRNA vaccine is presented under 2 categories: self-amplifying RNA (saRNA) and nonreplicating mRNA. The typical non-replicating mRNA comprises of a cap, flanked by 5′-untranslated regions (UTR) and 3′-UTRs, open reading frame (ORF) encoding vaccine antigens and poly(A) tail. Similar to conventional mRNA vaccine, mRNA is prepared synthetically by in vitro transcription of a linearized plasmid DNA or PCR construct containing the targeted gene and a promoter region when bacteriophage polymerase binds and initiates synthesis [95]. While the saRNA is more complex and comprises of the coding sequences of a viral replicase complex, a genomic and a sub genomic promoter, along with the basic elements of a conventional mRNA molecule. The change of mRNA's non-coding regions (5′ cap structure and capping efficiency, 5′- and 3′ UTRs), 3′ poly(A) tail), and nucleoside base modifications are all part of the optimization process.
Fig. 3.
Key elements that affect mRNA vaccine stability and translation efficacy. LNP – lipid nanoparticles, RNA – ribonucleic acid, UTR – untranslated region.
Codon optimization
Translation efficiency is known to be influenced by codon composition. The rate of protein production and the time spent in the ribosome repository can be affected by mRNA sequence codon optimization [96]. It was discovered that replacing a nucleotide with N1-methyl-pseudouridine (N1mΨ) improves base pair stability, resulting in a complex secondary structure and better mRNA translation [96]. Substituting rare codons with regular identical codons that contain plenty of similar tRNA in the cytosol is a common practice to alleviate mRNA production [97]. Although a high GC sequence may cause problems with mRNA secondary structure, it translates 100-fold higher than a low GC sequence [98].
Noncoding region optimization
The 5ʹ and 3ʹ UTR elements bordering the coding sequence have significant impact on the stability and translation of mRNA, both of which are crucial considerations in the optimal vaccine design. These optimization increases the efficiency and half-life of mRNA [99], [100]. For effective mRNA protein synthesis, a 5ʹ cap structure is essential [101]. This can be achieved by applying 5ʹ cap in multiple versions during or after the transcription process by using a vaccinia virus capping enzyme [102] or by incorporating synthetic cap or anti-reverse cap analogues [103]. An appropriate length of poly(A) tail also plays a critical role in regulation of mRNA translation and stability, thus it must be inserted directly from the encoding DNA template or with poly(A) polymerase [104]. A recent study suggested that mRNAs with phosphorothioate groups within the poly(A) tail were less sensitive to 3′-deadenylase degradation than unmodified mRNA and were more efficiently produced in cultured cells, paving the way for future progress of mRNA-based therapeutics [105].
Modifications of untranslated regions of mRNA also represents one of the approaches to enhance both mRNA efficiency and stability. Warren et al. used a synthetic 5′ UTR with a strong Kozak translation signal and the alpha globin 3′UTR to increase protein synthesis during fibroblast conversion to induced pluripotent stem cells [106]. Elsewhere, the globin 3′UTR has been used to increase mRNA stability since globin mRNAs generate large amount of protein with longer half-life [99]. Recently, in a review article by Miao et al. suggested 3 steps for modification of UTR as: “avoid the presence of start codon (AUG), and non-canonical start codons (CUG) in the 5′ UTR, second, avoid the presence of highly stable secondary structures, which can prevent ribosome recruitment and codon recognition. Thirdly, shorter 5’UTR may be introduced as previous studies have shown that this type of 5’UTR is more conducive to mRNA translation process” [17]. A screening method using a diverse set of 5′UTR and 3′UTR combinations for better expression of the Arginase 1 protein highlighted 5′ UTR as an essential driver in protein expression for exogenously delivered mRNA [107].
Elimination of pathogen-associated molecular patterns in mRNA via incorporation of modified nucleosides, such as pseudouridine [108] and 1-methylpseudouridine (m1Ψ) [109], and fast protein liquid chromatography purification to remove double-stranded RNA contaminants [110] is another approach to improve mRNA therapeutic efficiency. An advantage of such optimization is that the vaccine is able to bypass the transcription process directly starting the translation phase to produce the immunogenic protein inside the human cells [33].
Self-amplification vaccine
Self-amplifying mRNA (SAM) vaccines are derived from an α-virus genome, which enables the intact RNA replication but the structural protein genes substituted with the antigen of interest. Due to intracellular replication of the antigen-encoding RNA, the SAM can produce a significant amount of antigen from a very little vaccination dose [33]. SAM vaccines are capable of creating their own complements of dsRNA structures, replicate intermediates and other features which could contribute to their high effectiveness. However, due to the inherent nature of these RNAs, modulating the inflammatory profile or reactogenicity of SAM vaccines may be difficult [33]. The applications of SAM in cancer vaccine development are however limited to animal models, and 2 clinical trials against colorectal cancers (NCT01890213 and NCT00529984).
Delivery format
Due to negatively charged structure of naked RNA and large molecular size, mRNA is prone to nuclease degradation and cannot cross the cell membrane. Thus to overcome this obstacle, several mRNA vaccine delivery strategies have been employed such as, naked mRNA delivery, mRNA delivery through viral vectors, polymer-based vectors, lipid-based vectors, lipid-polymer hybrid nanoparticles, and peptide-based vectors [4], [111], Subcutaneous administration has been found very efficient for translation of encoded protein for mRNA, with the ability to induce both cellular and immune response through this route. However, the outermost layer of skin represents a tough barrier for drugs absorption and hence various approaches have been adopted to overcome this barrier, including microneedles, microporation, and jet injection, electroporation, iontophoresis, sonophoresis, formulation as NPs and liposomes [112].
Lipid-based vectors/nanoparticles
The LNPs are derived from cationic lipids containing tertiary or quaternary amines to encapsulate polyanionic mRNA. A study reported antigen-specific CTL activity and suppressed the OVA-suppressing tumors in mice injected with OVA-encoding mRNA in 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) and/or DOPE liposomes [113]. Coadministration of the mRNA for GM-CSF increased OVA-specific cytolytic responses in the same research. Another study found that subcutaneous distribution of LNP-formulated mRNA expressing two melanoma-associated antigens inhibited tumor growth in mice, and that co-delivery of lipopolysaccharide (LPS) in LNPs boosted CTL and antitumor activity [114]. A study by Kranz et al. [115] reported that mRNA-lipoplexes encoded with DOTMA/DOPE lipids were able to protect antigen-encoding mRNA against extracellular ribonucleases, which accumulated in the spleen and successfully delivered the mRNA into DCs following systemic treatment, leading in the development of an antigen-specific immune response. A preclinical study in mice injected with antibody-encoding mRNA delivery showed promising response against cancer [116]. Similarly, another study on mice inoculated with luciferase expressing Raji lymphoma cells and treated with mRNA-LNP encoding rituximab revealed diminished tumor growth, underscoring the importance of mRNA coated antibodies as a viable therapeutic option for treatment of cancer [117]. In general, mRNA cancer vaccines have shown to be immunogenic in people, but further improvement of vaccination methods based on fundamental immunological studies will almost certainly be required to gain higher clinical effects.
Polymer-based vectors
Polymeric materials though are less clinically investigated than ionizable lipids, they coat mRNA without the hassles of self-degradation and also promote protein expression. The drawback of polymeric materials however are polydispersity and the clearance of large molecules [91]. To improve the stability of the polymeric platforms, structural modifications such as lipid chains, expansion of branch structures and construction of biodegradation-promoting domains is considered [118]. A polyethyleneimine-polyplex nanoparticle carrying mRNA expressing the influenza virus hemagglutinin and nucleocapsid was employed in a research of mRNA vaccinations. mRNA was successfully transported to dendritic cells, transferred to the cytosol, and translated into proteins in this study, resulting in both humoral and cellular immunological responses [119]. However, because extremely positively charged polyethylene-based formulations attach to negatively charged serum proteins, they are more hazardous; as a result, new cationic polymers, such as poly(2-dimethylaminoethyl methacrylate) have been created [120]. Polymer-based delivery system research is still in the early stages of development.
Route of delivery
Researchers have investigated various methods for delivery of mRNA vaccines. For instance, mRNA vaccines can be delivered via lipid- or polymer-based system. Dendritic cells can be delivered ex-vivo and transferred to the hosts. Targeting of mRNA efficiently into DCs via in vivo route remains a major issue. When it comes to solving the delivery problem, there are two key variables to consider: delivery route (the route/portals of entry into the body) and delivery format (stabilized, naked, encapsulated, complexed, adsorbed, etc.). Each delivery route (intradermal, intra tumoral, intranodal, intravenous, subcutaneous, intranasal) has its own set of challenges to overcome, and these challenges will decide the best delivery method. In other words, while developing a vaccine design, the underlying motivation should be a reasonable combination of delivery route and format. Obtaining adequate immunological responses with a certain format and distribution route does not necessarily imply that particular delivery route is better or it is the best route [4]. In short, the route of administration is significant in determining the efficacy of mRNA vaccine [111].
Both naked and lipid-formulated mRNA administered subcutaneously cause cell transfection, with naked mRNA surpassing lipid-formulated mRNA in terms of translational efficiency. Both formats have demonstrated to induce antigen-specific T cells, but neither has been shown to transfect nodal cells [121], [122]. In contrast, a study using lipid nano formulations (approx.70–100 nm) found high and long-lasting translation at the injection site, as well as in CD11c+ cells in draining lymph nodes, leading to delayed tumor growth [114]. Kreiter et al. found that intranodal delivery of adjusted naked antigen-encoding mRNA elicited effective antitumor immunity and mRNA was internalized and translated via micropinocytosis by lymph node resident conventional and cross-presenting CD8a + DCs [121]. Another study by Thielemans et al. validated the potency of intranodal delivery and format in additional tumor models [123]. Thielemans et al. pioneered intertumoral administration to DCs. When in vivo transfected with TriMix, their findings show that naked mRNA is mostly picked up by cross-presenting CD8a + DCs, and that these cells can reawaken T cells at the tumor site as well as move to the draining lymph. The mRNA-encoded secreted proteins might relieve part of the load on immune cells by lowering MDSC repression, boosting DCs, and activating T cell lysis, which improved tumor growth delay when paired with PD-1 inhibition [123].
Future perspectives and conclusion
With the development and global approval of mRNA vaccines against SARS-CoV-2 virus in the last year have outscored the potential of mRNA technology. Most patients with cancer are non-responsive to current immunotherapies, frequently patients experience relapse and subsequently toxicities to therapies. In this context, therapeutic cancer vaccines is an appealing option to immunotherapy setting because of their potential for safety, specificity, and long-term response due to immunological memory stimulation [21]. The favorable features of potency, fast and relative low-cost production of mRNA vaccine provide an attractive platform for cancer therapy. The mRNA cancer vaccine can be a preferred combination agent with currently available therapies for long-term cancer treatment considering the favorable safety profile observed to date.
Apart from recent progress in the lipid-based delivery system for mRNA vaccines, chimeric antigen receptor (CAR)-T cell immunotherapy is emerging as an encouraging treatment approach for treating malignancies. CAR-T therapy is a personalized form of cell therapy where patient-T cells are genetically engineered to express receptors allowing them to recognize tumor antigens. The adoptive transfer of genetically modified T cells for expressing a CAR have shown encouraging response against hematological tumors. Given this approach, mRNA electroporation has been utilized to generate T cells with CAR expression in preclinical trials [124], [125] and subsequently in human trials [126], [127]. The preclinical data showed that Descartes-08, an autologous CD8 + CAR T therapy inhibits development of BCMA CAR-specific myeloma and substantially prolongs host survival. Furthermore, an ongoing clinical trial of Descartes-08 reported a favorable therapeutic index with durable responses upon preliminary analysis in patients with relapsed/refractory myeloma (NCT03448978), thus providing a framework for another study using Descartes-11 which is an optimized or humanized version of Descartes-08 in patients with newly diagnosed myeloma patients and having residual disease after induction therapy [128].
Future research should concentrate on deciphering the immunological pathways triggered by different mRNA vaccine platforms and attempt to improve current techniques based on these mechanisms. Utilizing immune-gene therapy with the transfection of autologous mRNA vaccine is another upcoming approach which deserves exploration. For instance, a phase I/II study is currently underway to determine the efficacy and safety of ELI-002, a lipid-conjugated immune-stimulatory oligonucleotide (Amph-CpG-7909) as an adjuvant therapy with a mixture of lipid- conjugated peptide-based antigens (Amph-Peptides) for minimally residual disease in patients with KRAS/neuroblastoma Ras viral oncogene homolog mutated pancreatic cancer or other solid tumors (NCT04853017).
To conclude, despite multifaceted challenges remain in the development of mRNA vaccines, such as extremely large size, susceptibility to enzymatic degradation and instability, the durable efficacy of the RNA in various stages of clinical trials deserves consideration. The work of mRNA on other types of cancer such as ovarian cancer, gastrointestinal cancer remains to be explored. However, delivering mRNA to specific organs, tissues, or cell types remains a significant challenge in the area. It is thus necessary to identify the validated biomarkers that can predict mRNA vaccine efficacy and be utilized for further optimization of the vaccine. Additionally, combination therapies of mRNA with immune checkpoint inhibitors and other immunosuppressing drugs are showing promise [129], nevertheless, more research is needed to determine the most effective combinations and the optimal drug dose for each component. mRNA vaccines will become a significant class of medicine as delivery technologies and vaccine formulations improve, allowing them to effectively combat a variety of health conditions such as infectious diseases and malignancies.
Author contributions
All authors contributed to manuscript conception, preparation, and approved the final version of the manuscript for submission.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors acknowledge Anwesha Mandal and Dr. Amit Bhat of Indegene Pvt Ltd. For their medical writing and editorial support.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Medical writing and editorial support were funded by Shanghai Fosun Pharmaceutical Industrial Development, Co., Ltd.
References
- 1.Orenstein W.A., Ahmed R. Simply put: vaccination saves lives. Proc Natl Acad Sci USA. 2017;114:4031–4033. doi: 10.1073/pnas.1704507114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pollard A.J., Bijker E.M. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol. 2021;21:83–100. doi: 10.1038/s41577-020-00479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chandler M., Johnson M.B., Panigaj M., Afonin K.A. Innate immune responses triggered by nucleic acids inspire the design of immunomodulatory nucleic acid nanoparticles (NANPs) Curr Opin Biotechnol. 2020;63:8–15. doi: 10.1016/j.copbio.2019.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grunwitz C., Kranz L.M. mRNA cancer vaccines-messages that prevail. Curr Top Microbiol Immunol. 2017;405:145–164. doi: 10.1007/82_2017_509. [DOI] [PubMed] [Google Scholar]
- 5.Suschak J.J., Williams J.A., Schmaljohn C.S. Advancements in DNA vaccine vectors, non-mechanical delivery methods, and molecular adjuvants to increase immunogenicity. Hum Vaccin Immunother. 2017;13:2837–2848. doi: 10.1080/21645515.2017.1330236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Commissioner O of the COVID-19 Vaccines. FDA; 2021.
- 7.Baden Lindsey R., El Sahly Hana M., Essink Brandon, Kotloff Karen, Frey Sharon, Novak Rick, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu Laurence, McPhee Roderick, Huang Wenmei, Bennett Hamilton, Pajon Rolando, Nestorova Biliana, et al. A preliminary report of a randomized controlled phase 2 trial of the safety and immunogenicity of mRNA-1273 SARS-CoV-2 vaccine. Vaccine. 2021;39(20):2791–2799. doi: 10.1016/j.vaccine.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ali Kashif, Berman Gary, Zhou Honghong, Deng Weiping, Faughnan Veronica, Coronado-Voges Maria, et al. Evaluation of mRNA-1273 SARS-CoV-2 vaccine in adolescents. N Engl J Med. 2021;385(24):2241–2251. doi: 10.1056/NEJMoa2109522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polack Fernando P., Thomas Stephen J., Kitchin Nicholas, Absalon Judith, Gurtman Alejandra, Lockhart Stephen, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olson Samantha M., Newhams Margaret M., Halasa Natasha B., Price Ashley M., Boom Julie A., Sahni Leila C., et al. Effectiveness of BNT162b2 vaccine against critical Covid-19 in adolescents. N Engl J Med. 2022;386(8):713–723. doi: 10.1056/NEJMoa2117995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai H.-J. Clinical cancer chemoprevention: from the hepatitis B virus (HBV) vaccine to the human papillomavirus (HPV) vaccine. Taiwanese J Obstet Gynecol. 2015;54:112–115. doi: 10.1016/j.tjog.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Cancer Vaccines: Preventive, Therapeutic, Personalized. Cancer Research Institute n.d. <https://www.cancerresearch.org/en-us/immunotherapy/treatment-types/cancer-vaccines> [accessed December 29, 2021].
- 14.Cheever M.A., Higano C.S. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin Cancer Res. 2011;17:3520–3526. doi: 10.1158/1078-0432.CCR-10-3126. [DOI] [PubMed] [Google Scholar]
- 15.Morales A. BCG: a throwback from the stone age of vaccines opened the path for bladder cancer immunotherapy. Can J Urol. 2017;24:8788–8793. [PubMed] [Google Scholar]
- 16.FDA approves first oncolytic virus therapy: imlygic for melanoma. Oncol Times 2015;37:36. <https://doi.org/10.1097/01.COT.0000475724.97729.9e>.
- 17.Miao L., Zhang Y., Huang L. mRNA vaccine for cancer immunotherapy. Mol Cancer. 2021;20:41. doi: 10.1186/s12943-021-01335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y., Zhang Z., Luo J., Han X., Wei Y., Wei X. mRNA vaccine: a potential therapeutic strategy. Mol Cancer. 2021;20:33. doi: 10.1186/s12943-021-01311-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maruggi G., Zhang C., Li J., Ulmer J.B., Yu D. mRNA as a transformative technology for vaccine development to control infectious diseases. Mol Ther. 2019;27:757–772. doi: 10.1016/j.ymthe.2019.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le D.T., Pardoll D.M., Jaffee E.M. Cellular vaccine approaches. Cancer J. 2010;16:304–310. doi: 10.1097/PPO.0b013e3181eb33d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hollingsworth R.E., Jansen K. Turning the corner on therapeutic cancer vaccines. NPJ Vacc. 2019;4:7. doi: 10.1038/s41541-019-0103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eric Lutz, Yeo Charles J., Lillemoe Keith D., Biedrzycki Barbara, Kobrin Barry, Herman Joseph, et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann Surg. 2011;253(2):328–335. doi: 10.1097/SLA.0b013e3181fd271c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaffee Elizabeth M., Hruban Ralph H., Biedrzycki Barbara, Laheru Daniel, Schepers Karen, Sauter Patricia R., et al. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol. 2001;19(1):145–156. doi: 10.1200/JCO.2001.19.1.145. [DOI] [PubMed] [Google Scholar]
- 24.Beyaert S., Machiels J.-P., Schmitz S. Vaccine-based immunotherapy for head and neck cancers. Cancers (Basel) 2021;13:6041. doi: 10.3390/cancers13236041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bezu Lucillia, Kepp Oliver, Cerrato Giulia, Pol Jonathan, Fucikova Jitka, Spisek Radek, et al. Trial watch: peptide-based vaccines in anticancer therapy. Oncoimmunology. 2018;7(12):e1511506. doi: 10.1080/2162402X.2018.1511506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang B., Jeang J., Yang A., Wu T.C., Hung C.-F. DNA vaccine for cancer immunotherapy. Hum Vaccin Immunother. 2015;10:3153–3164. doi: 10.4161/21645515.2014.980686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boczkowski D., Nair S.K., Snyder D., Gilboa E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med. 1996;184:465–472. doi: 10.1084/jem.184.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aarntzen Erik H.J.G., Schreibelt Gerty, Bol Kalijn, Lesterhuis W. Joost, Croockewit Alexandra J., de Wilt Johannes H.W., et al. Vaccination with mRNA-electroporated dendritic cells induces robust tumor antigen-specific CD4+ and CD8+ T cells responses in stage III and IV melanoma patients. Clin Cancer Res. 2012;18(19):5460–5470. doi: 10.1158/1078-0432.CCR-11-3368. [DOI] [PubMed] [Google Scholar]
- 29.Wilgenhof Sofie, Van Nuffel An M.T., Corthals Jurgen, Heirman Carlo, Tuyaerts Sandra, Benteyn Daphné, et al. Therapeutic vaccination with an autologous mRNA electroporated dendritic cell vaccine in patients with advanced melanoma. J Immunother. 2011;34(5):448–456. doi: 10.1097/CJI.0b013e31821dcb31. [DOI] [PubMed] [Google Scholar]
- 30.Coosemans A., Vanderstraeten A., Tuyaerts S., Verschuere T., Moerman P., Berneman Z., et al. Immunological response after WT1 mRNA-loaded dendritic cell immunotherapy in ovarian carcinoma and carcinosarcoma. Anticancer Res. 2013;33:3855–3859. [PubMed] [Google Scholar]
- 31.Vik-Mo Einar Osland, Nyakas Marta, Mikkelsen Birthe Viftrup, Moe Morten Carstens, Due-Tønnesen Paulina, Suso Else Marit Inderberg, et al. Therapeutic vaccination against autologous cancer stem cells with mRNA-transfected dendritic cells in patients with glioblastoma. Cancer Immunol Immunother. 2013;62(9):1499–1509. doi: 10.1007/s00262-013-1453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beck J.D., Reidenbach D., Salomon N., Sahin U., Türeci Ö., Vormehr M., et al. mRNA therapeutics in cancer immunotherapy. Mol Cancer. 2021;20:69. doi: 10.1186/s12943-021-01348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines — a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pedersen S.R., Sørensen M.R., Buus S., Christensen J.P., Thomsen A.R. Comparison of vaccine-induced effector CD8 T cell responses directed against self- and non-self-tumor antigens: implications for cancer immunotherapy. J Immunol. 2013;191:3955–3967. doi: 10.4049/jimmunol.1300555. [DOI] [PubMed] [Google Scholar]
- 35.Carreno B.M., Magrini V., Becker-Hapak M., Kaabinejadian S., Hundal J., Petti A.A., et al. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science. 2015;348(6236):803–808. doi: 10.1126/science.aaa3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ott Patrick A., Hu Zhuting, Keskin Derin B., Shukla Sachet A., Sun Jing, Bozym David J., et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547(7662):217–221. doi: 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gjerstorff Morten F., Andersen Mads H., Ditzel Henrik J. Oncogenic cancer/testis antigens: prime candidates for immunotherapy. Oncotarget. 2015;6(18):15772–15787. doi: 10.18632/oncotarget.4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenberg S.A., Yang J.C., Restifo N.P. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kreiter Sebastian, Vormehr Mathias, van de Roemer Niels, Diken Mustafa, Löwer Martin, Diekmann Jan, et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature. 2015;520(7549):692–696. doi: 10.1038/nature14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sahin Ugur, Derhovanessian Evelyna, Miller Matthias, Kloke Björn-Philipp, Simon Petra, Löwer Martin, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547(7662):222–226. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- 41.Cafri Gal, Gartner Jared J., Zaks Tal, Hopson Kristen, Levin Noam, Paria Biman C., et al. mRNA vaccine-induced neoantigen-specific T cell immunity in patients with gastrointestinal cancer. J Clin Invest. 2020;130(11):5976–5988. doi: 10.1172/JCI134915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burris Howard A., Patel Manish R., Cho Daniel C., Clarke Jeffrey Melson, Gutierrez Martin, Zaks Tal Z., et al. A phase I multicenter study to assess the safety, tolerability, and immunogenicity of mRNA-4157 alone in patients with resected solid tumors and in combination with pembrolizumab in patients with unresectable solid tumors. JCO. 2019;37(15_suppl):2523. [Google Scholar]
- 43.Lopez JS, Camidge R, Iafolla M, Rottey S, Schuler M, Hellmann M, et al. Abstract CT301: a phase Ib study to evaluate RO7198457, an individualized Neoantigen Specific immunoTherapy (iNeST), in combination with atezolizumab in patients with locally advanced or metastatic solid tumors. Cancer Res 2020;80:CT301–CT301. <https://doi.org/10.1158/1538-7445.AM2020-CT301>.
- 44.Definition of tetravalent RNA-lipoplex cancer vaccine BNT111 – NCI Drug Dictionary – National Cancer Institute; 2011. <https://www.cancer.gov/publications/dictionaries/cancer-drug/def/tetravalent-rna-lipoplex-cancer-vaccine-bnt111> [accessed January 5, 2022].
- 45.Sahin Ugur, Oehm Petra, Derhovanessian Evelyna, Jabulowsky Robert A., Vormehr Mathias, Gold Maike, et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature. 2020;585(7823):107–112. doi: 10.1038/s41586-020-2537-9. [DOI] [PubMed] [Google Scholar]
- 46.BioNTech SE. Open-label, Randomized Phase II Trial With BNT111 and Cemiplimab in Combination or as Single Agents in Patients With Anti-PD-1-refractory/Relapsed, Unresectable Stage III or IV Melanoma. clinicaltrials.gov; 2021.
- 47.Definition of mRNA-derived KRAS-targeted vaccine V941 – NCI Drug Dictionary – National Cancer Institute; 2011. <https://www.cancer.gov/publications/dictionaries/cancer-drug/def/mrna-derived-kras-targeted-vaccine-v941> [accessed January 13, 2022].
- 48.Moderna, Inc. – SEC.gov n.d. <https://www.sec.gov/Archives/edgar/data/1682852/000168285219000009/moderna10-k12312018.htm> [accessed January 13, 2022].
- 49.Mougel A., Terme M., Tanchot C. Therapeutic cancer vaccine and combinations with antiangiogenic therapies and immune checkpoint blockade. Front Immunol. 2019;10:467. doi: 10.3389/fimmu.2019.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kongsted Per, Borch Troels Holz, Ellebaek Eva, Iversen Trine Zeeberg, Andersen Rikke, Met Özcan, et al. Dendritic cell vaccination in combination with docetaxel for patients with metastatic castration-resistant prostate cancer: A randomized phase II study. Cytotherapy. 2017;19(4):500–513. doi: 10.1016/j.jcyt.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 51.Sharma P., Allison J.P. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 52.Fotin-Mleczek M., Zanzinger K., Heidenreich R., Lorenz C., Kowalczyk A., Kallen K.-J., et al. mRNA-based vaccines synergize with radiation therapy to eradicate established tumors. Radiat Oncol. 2014;9:180. doi: 10.1186/1748-717X-9-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Papachristofilou Alexandros, Hipp Madeleine M., Klinkhardt Ute, Früh Martin, Sebastian Martin, Weiss Christian, et al. Phase Ib evaluation of a self-adjuvanted protamine formulated mRNA-based active cancer immunotherapy, BI1361849 (CV9202), combined with local radiation treatment in patients with stage IV non-small cell lung cancer. J Immunother Cancer. 2019;7(1) doi: 10.1186/s40425-019-0520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Lina, Wang Yuhua, Miao Lei, Liu Qi, Musetti Sara, Li Jun, et al. Combination immunotherapy of MUC1 mRNA nano-vaccine and CTLA-4 blockade effectively inhibits growth of triple negative breast cancer. Mol Ther. 2018;26(1):45–55. doi: 10.1016/j.ymthe.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang C., Maruggi G., Shan H., Li J. Advances in mRNA vaccines for infectious diseases. Front Immunol. 2019;10:594. doi: 10.3389/fimmu.2019.00594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sung Hyuna, Ferlay Jacques, Siegel Rebecca L., Laversanne Mathieu, Soerjomataram Isabelle, Jemal Ahmedin, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 57.Street W. Breast Cancer Facts & Figures 2019–2020 n.d.:44.
- 58.Breast cancer n.d. <https://www.who.int/news-room/fact-sheets/detail/breast-cancer> [accessed December 31, 2021].
- 59.Iglesia Michael D., Parker Joel S., Hoadley Katherine A., Serody Jonathan S., Perou Charles M., Vincent Benjamin G. Genomic analysis of immune cell infiltrates across 11 tumor types. J Natl Cancer Inst. 2016;108(11):djw144. doi: 10.1093/jnci/djw144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shah D., Osipo C. Cancer stem cells and HER2 positive breast cancer: the story so far. Genes Dis. 2016;3:114–123. doi: 10.1016/j.gendis.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burke E.E., Kodumudi K., Ramamoorthi G., Czerniecki B.J. Vaccine therapies for breast cancer. Surg Oncol Clin N Am. 2019;28:353–367. doi: 10.1016/j.soc.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 62.Mittendorf E.A., Ardavanis A., Symanowski J., Murray J.L., Shumway N.M., Litton J.K., et al. Primary analysis of a prospective, randomized, single-blinded phase II trial evaluating the HER2 peptide AE37 vaccine in breast cancer patients to prevent recurrence. Ann Oncol. 2016;27(7):1241–1248. doi: 10.1093/annonc/mdw150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peoples George E., Gurney Jennifer M., Hueman Matthew T., Woll Mike M., Ryan Gayle B., Storrer Catherine E., et al. Clinical trial results of a HER2/neu (E75) vaccine to prevent recurrence in high-risk breast cancer patients. J Clin Oncol. 2005;23(30):7536–7545. doi: 10.1200/JCO.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 64.Disis Mary L., Wallace Danelle R., Gooley Theodore A., Dang Yushe, Slota Meredith, Lu Hailing, et al. Concurrent trastuzumab and HER2/neu-specific vaccination in patients with metastatic breast cancer. J Clin Oncol. 2009;27(28):4685–4692. doi: 10.1200/JCO.2008.20.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schmidt M., Heimes A.-S. Immunomodulating therapies in breast cancer—from prognosis to clinical practice. Cancers (Basel) 2021;13:4883. doi: 10.3390/cancers13194883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schmidt M, Bolte S, Frenzel K, Heesen L, Derhovanessian E, Bukur V, et al. Abstract OT2-06-01: Highly innovative personalized RNA-immunotherapy for patients with triple negative breast cancer. Cancer Res 2019;79:OT2-06. <https://doi.org/10.1158/1538-7445.SABCS18-OT2-06-01>.
- 67.Schmidt M., Vogler I., Derhovanessian E., Omokoko T., Godehardt E., Attig S., et al. 88MO T-cell responses induced by an individualized neoantigen specific immune therapy in post (neo)adjuvant patients with triple negative breast cancer. Ann Oncol. 2020;31:S276. [Google Scholar]
- 68.Sebastian Martin, Schröder Andreas, Scheel Birgit, Hong Henoch S., Muth Anke, von Boehmer Lotta, et al. A phase I/IIa study of the mRNA-based cancer immunotherapy CV9201 in patients with stage IIIB/IV non-small cell lung cancer. Cancer Immunol Immunother. 2019;68(5):799–812. doi: 10.1007/s00262-019-02315-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rausch S., Schwentner C., Stenzl A., Bedke J. mRNA vaccine CV9103 and CV9104 for the treatment of prostate cancer. Hum Vaccin Immunother. 2014;10:3146–3152. doi: 10.4161/hv.29553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kallen Karl-Josef, Heidenreich Regina, Schnee Margit, Petsch Benjamin, Schlake Thomas, Thess Andreas, et al. A novel, disruptive vaccination technology: self-adjuvanted RNActive(®) vaccines. Hum Vaccin Immunother. 2013;9(10):2263–2276. doi: 10.4161/hv.25181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kübler H., Maurer T., Stenzl A., Feyerabend S., Steiner U., Schostak M., et al. Final analysis of a phase I/IIa study with CV9103, an intradermally administered prostate cancer immunotherapy based on self-adjuvanted mRNA. JCO. 2011;29(15_suppl):4535. [Google Scholar]
- 72.Stenzl A., Feyerabend S., Syndikus I., Sarosiek T., Kübler H., Heidenreich A., et al. Results of the randomized, placebo-controlled phase I/IIB trial of CV9104, an mRNA based cancer immunotherapy, in patients with metastatic castration-resistant prostate cancer (mCRPC) Ann Oncol. 2017;28:v408–v409. [Google Scholar]
- 73.Zhou W.-Z., Hoon D.S.B., Huang S.K.S., Fujii S., Hashimoto K., Morishita R., et al. RNA melanoma vaccine: induction of antitumor immunity by human glycoprotein 100 mRNA immunization. Hum Gene Ther. 1999;10(16):2719–2724. doi: 10.1089/10430349950016762. [DOI] [PubMed] [Google Scholar]
- 74.Mockey M., Bourseau E., Chandrashekhar V., Chaudhuri A., Lafosse S., Le Cam E., et al. mRNA-based cancer vaccine: prevention of B16 melanoma progression and metastasis by systemic injection of MART1 mRNA histidylated lipopolyplexes. Cancer Gene Ther. 2007;14(9):802–814. doi: 10.1038/sj.cgt.7701072. [DOI] [PubMed] [Google Scholar]
- 75.Fu Y., Li J. A novel delivery platform based on Bacteriophage MS2 virus-like particles. Virus Res. 2016;211:9–16. doi: 10.1016/j.virusres.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li J., Sun Y., Jia T., Zhang R., Zhang K., Wang L. Messenger RNA vaccine based on recombinant MS2 virus-like particles against prostate cancer. Int J Cancer. 2014;134:1683–1694. doi: 10.1002/ijc.28482. [DOI] [PubMed] [Google Scholar]
- 77.Islam Mohammad Ariful, Rice Jamie, Reesor Emma, Zope Harshal, Tao Wei, Lim Michael, et al. Adjuvant-pulsed mRNA vaccine nanoparticle for immunoprophylactic and therapeutic tumor suppression in mice. Biomaterials. 2021;266:120431. doi: 10.1016/j.biomaterials.2020.120431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zappasodi R., de Braud F., Di Nicola M. Lymphoma immunotherapy: current status. Front Immunol. 2015;6:448. doi: 10.3389/fimmu.2015.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pierpont T.M., Limper C.B., Richards K.L. Past, present, and future of rituximab-the world’s first oncology monoclonal antibody therapy. Front Oncol. 2018;8:163. doi: 10.3389/fonc.2018.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brody J., Levy R. Lymphoma immunotherapy: vaccines, adoptive cell transfer and immunotransplant. Immunotherapy. 2009;1:809–824. doi: 10.2217/imt.09.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Verbeke R., Lentacker I., Breckpot K., Janssens J., Van Calenbergh S., De Smedt S.C., et al. Broadening the message: a nanovaccine co-loaded with messenger RNA and α-GalCer induces antitumor immunity through conventional and natural killer T cells. ACS Nano. 2019;13:1655–1669. doi: 10.1021/acsnano.8b07660. [DOI] [PubMed] [Google Scholar]
- 82.Cancer of the Pancreas – Cancer Stat Facts. SEER n.d. <https://seer.cancer.gov/statfacts/html/pancreas.html> [accessed January 3, 2022].
- 83.Feig C., Gopinathan A., Neesse A., Chan D.S., Cook N., Tuveson D.A. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18:4266–4276. doi: 10.1158/1078-0432.CCR-11-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chiorean E.G., Coveler A.L. Pancreatic cancer: optimizing treatment options, new, and emerging targeted therapies. Drug Des Devel Ther. 2015;9:3529–3545. doi: 10.2147/DDDT.S60328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gjertsen Marianne K., Buanes Trond, Rosseland Arne R., Bakka Arne, Gladhaug Ivar, Soreide Odd, et al. Intradermal ras peptide vaccination with granulocyte-macrophage colony-stimulating factor as adjuvant: Clinical and immunological responses in patients with pancreatic adenocarcinoma. Int J Cancer. 2001;92(3):441–450. doi: 10.1002/ijc.1205. [DOI] [PubMed] [Google Scholar]
- 86.Bernhardt S.L., Gjertsen M.K., Trachsel S., Møller M., Eriksen J.A., Meo M., et al. Telomerase peptide vaccination of patients with non-resectable pancreatic cancer: a dose escalating phase I/II study. Br J Cancer. 2006;95(11):1474–1482. doi: 10.1038/sj.bjc.6603437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Le Dung T., Lutz Eric, Uram Jennifer N., Sugar Elizabeth A., Onners Beth, Solt Sara, et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother. 2013;36(7):382–389. doi: 10.1097/CJI.0b013e31829fb7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Le Dung T., Wang-Gillam Andrea, Picozzi Vincent, Greten Tim F., Crocenzi Todd, Springett Gregory, et al. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol. 2015;33(12):1325–1333. doi: 10.1200/JCO.2014.57.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang X., Zhang G., Tang T., Liang T. Identification of tumor antigens and immune subtypes of pancreatic adenocarcinoma for mRNA vaccine development. Mol Cancer. 2021;20:44. doi: 10.1186/s12943-021-01310-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Heistein JB, Acharya U. Malignant Melanoma. StatPearls Publishing; 2021. [PubMed]
- 91.Bidram Maryam, Zhao Yue, Shebardina Natalia G., Baldin Alexey V., Bazhin Alexandr V., Ganjalikhany Mohamad Reza, et al. mRNA-based cancer vaccines: a therapeutic strategy for the treatment of melanoma patients. Vaccines (Basel) 2021;9(10):1060. doi: 10.3390/vaccines9101060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Weide Benjamin, Pascolo Steve, Scheel Birgit, Derhovanessian Evelyna, Pflugfelder Annette, Eigentler Thomas K., et al. Direct injection of protamine-protected mRNA: results of a phase 1/2 vaccination trial in metastatic melanoma patients. J Immunother. 2009;32(5):498–507. doi: 10.1097/CJI.0b013e3181a00068. [DOI] [PubMed] [Google Scholar]
- 93.Wang Y., Zhang L., Xu Z., Miao L., Huang L. mRNA vaccine with antigen-specific checkpoint blockade induces an enhanced immune response against established melanoma. Mol Ther. 2018;26:420–434. doi: 10.1016/j.ymthe.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wilgenhof S., Corthals J., Heirman C., Neyns B., Thielemans K. Clinical trials with MRNA electroporated dendritic cells for stage III/IV melanoma patients. J Immunother Cancer. 2015;3:P211. doi: 10.1186/2051-1426-3-S2-P211. [DOI] [Google Scholar]
- 95.Minnaert An-Katrien, Vanluchene Helena, Verbeke Rein, Lentacker Ine, De Smedt Stefaan C., Raemdonck Koen, et al. Strategies for controlling the innate immune activity of conventional and self-amplifying mRNA therapeutics: Getting the message across. Adv Drug Deliv Rev. 2021;176:113900. doi: 10.1016/j.addr.2021.113900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mauger David M., Cabral B. Joseph, Presnyak Vladimir, Su Stephen V., Reid David W., Goodman Brooke, et al. mRNA structure regulates protein expression through changes in functional half-life. PNAS. 2019;116(48):24075–24083. doi: 10.1073/pnas.1908052116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cannarozzi Gina, Schraudolph Nicol N., Faty Mahamadou, von Rohr Peter, Friberg Markus T., Roth Alexander C., et al. A role for codon order in translation dynamics. Cell. 2010;141(2):355–367. doi: 10.1016/j.cell.2010.02.036. [DOI] [PubMed] [Google Scholar]
- 98.Kudla Grzegorz, Lipinski Leszek, Caffin Fanny, Helwak Aleksandra, Zylicz Maciej, Hurst Laurence D. High guanine and cytosine content increases mRNA levels in mammalian cells. PLoS Biol. 2006;4(6):e180. doi: 10.1371/journal.pbio.0040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ross J., Sullivan T.D. Half-lives of beta and gamma globin messenger RNAs and of protein synthetic capacity in cultured human reticulocytes. Blood. 1985;66:1149–1154. [PubMed] [Google Scholar]
- 100.Holtkamp Silke, Kreiter Sebastian, Selmi Abderraouf, Simon Petra, Koslowski Michael, Huber Christoph, et al. Modification of antigen-encoding RNA increases stability, translational efficacy, and T-cell stimulatory capacity of dendritic cells. Blood. 2006;108(13):4009–4017. doi: 10.1182/blood-2006-04-015024. [DOI] [PubMed] [Google Scholar]
- 101.Gallie D.R. The cap and poly(A) tail function synergistically to regulate mRNA translational efficiency. Genes Dev. 1991;5:2108–2116. doi: 10.1101/gad.5.11.2108. [DOI] [PubMed] [Google Scholar]
- 102.Muttach F., Muthmann N., Rentmeister A. Synthetic mRNA capping. Beilstein J Org Chem. 2017;13:2819–2832. doi: 10.3762/bjoc.13.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kocmik Ilona, Piecyk Karolina, Rudzinska Magdalena, Niedzwiecka Anna, Darzynkiewicz Edward, Grzela Renata, et al. Modified ARCA analogs providing enhanced translational properties of capped mRNAs. Cell Cycle. 2018;17(13):1624–1636. doi: 10.1080/15384101.2018.1486164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jalkanen A.L., Coleman S.J., Wilusz J. Determinants and implications of mRNA Poly(A) tail size – does this protein make my tail look big? Semin Cell Dev Biol. 2014:24–32. doi: 10.1016/j.semcdb.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Strzelecka D., Smietanski M., Sikorski P.J., Warminski M., Kowalska J., Jemielity J. Phosphodiester modifications in mRNA poly(A) tail prevent deadenylation without compromising protein expression. RNA. 2020;26:1815–1837. doi: 10.1261/rna.077099.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Warren Luigi, Manos Philip D., Ahfeldt Tim, Loh Yuin-Han, Li Hu, Lau Frank, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7(5):618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Asrani K.H., Farelli J.D., Stahley M.R., Miller R.L., Cheng C.J., Subramanian R.R., et al. Optimization of mRNA untranslated regions for improved expression of therapeutic mRNA. RNA Biol. 2018;15:756–762. doi: 10.1080/15476286.2018.1450054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Karikó Katalin, Muramatsu Hiromi, Welsh Frank A, Ludwig János, Kato Hiroki, Akira Shizuo, et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol Ther. 2008;16(11):1833–1840. doi: 10.1038/mt.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Andries O., Mc Cafferty S., De Smedt S.C., Weiss R., Sanders N.N., Kitada T. N(1)-methylpseudouridine-incorporated mRNA outperforms pseudouridine-incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice. J Control Release. 2015;217:337–344. doi: 10.1016/j.jconrel.2015.08.051. [DOI] [PubMed] [Google Scholar]
- 110.Karikó Katalin, Muramatsu Hiromi, Ludwig János, Weissman Drew. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucl Acids Res. 2011;39(21):e142. doi: 10.1093/nar/gkr695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zeng C, Zhang C, Walker PG, Dong Y. Formulation and Delivery Technologies for mRNA Vaccines, Berlin, Heidelberg: Springer; n.d., p. 1–40. <https://doi.org/10.1007/82_2020_217>.
- 112.Wadhwa A., Aljabbari A., Lokras A., Foged C., Thakur A. Opportunities and challenges in the delivery of mRNA-based vaccines. Pharmaceutics. 2020;12:102. doi: 10.3390/pharmaceutics12020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hess P.R., Boczkowski D., Nair S.K., Snyder D., Gilboa E. Vaccination with mRNAs encoding tumor-associated antigens and granulocyte-macrophage colony-stimulating factor efficiently primes CTL responses, but is insufficient to overcome tolerance to a model tumor/self antigen. Cancer Immunol Immunother. 2006;55:672–683. doi: 10.1007/s00262-005-0064-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Oberli Matthias A., Reichmuth Andreas M., Dorkin J. Robert, Mitchell Michael J., Fenton Owen S., Jaklenec Ana, et al. Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Lett. 2017;17(3):1326–1335. doi: 10.1021/acs.nanolett.6b03329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kranz Lena M., Diken Mustafa, Haas Heinrich, Kreiter Sebastian, Loquai Carmen, Reuter Kerstin C., et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534(7607):396–401. doi: 10.1038/nature18300. [DOI] [PubMed] [Google Scholar]
- 116.Stadler Christiane R, Bähr-Mahmud Hayat, Celik Leyla, Hebich Bernhard, Roth Alexandra S, Roth René P, et al. Elimination of large tumors in mice by mRNA-encoded bispecific antibodies. Nat Med. 2017;23(7):815–817. doi: 10.1038/nm.4356. [DOI] [PubMed] [Google Scholar]
- 117.Thran Moritz, Mukherjee Jean, Pönisch Marion, Fiedler Katja, Thess Andreas, Mui Barbara L, et al. mRNA mediates passive vaccination against infectious agents, toxins, and tumors. EMBO Mol Med. 2017;9(10):1434–1447. doi: 10.15252/emmm.201707678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kaczmarek James C., Patel Asha K., Kauffman Kevin J., Fenton Owen S., Webber Matthew J., Heartlein Michael W., et al. Polymer-lipid nanoparticles for systemic delivery of mRNA to the lungs. Angew Chem Int Ed Engl. 2016;55(44):13808–13812. doi: 10.1002/anie.201608450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Démoulins Thomas, Milona Panagiota, Englezou Pavlos C., Ebensen Thomas, Schulze Kai, Suter Rolf, et al. Polyethylenimine-based polyplex delivery of self-replicating RNA vaccines. Nanomedicine. 2016;12(3):711–722. doi: 10.1016/j.nano.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 120.Üzgün Senta, Nica Gabriela, Pfeifer Corinna, Bosinco Michele, Michaelis Kai, Lutz Jean-François, et al. PEGylation improves nanoparticle formation and transfection efficiency of messenger RNA. Pharm Res. 2011;28(9):2223–2232. doi: 10.1007/s11095-011-0464-z. [DOI] [PubMed] [Google Scholar]
- 121.Kreiter Sebastian, Selmi Abderraouf, Diken Mustafa, Koslowski Michael, Britten Cedrik M., Huber Christoph, et al. Intranodal vaccination with naked antigen-encoding RNA elicits potent prophylactic and therapeutic antitumoral immunity. Cancer Res. 2010;70(22):9031–9040. doi: 10.1158/0008-5472.CAN-10-0699. [DOI] [PubMed] [Google Scholar]
- 122.Phua K.K.L., Leong K.W., Nair S.K. Transfection efficiency and transgene expression kinetics of mRNA delivered in naked and nanoparticle format. J Control Release. 2013;166:227–233. doi: 10.1016/j.jconrel.2012.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Van Lint Sandra, Goyvaerts Cleo, Maenhout Sarah, Goethals Lode, Disy Aurélie, Benteyn Daphné, et al. Preclinical evaluation of TriMix and antigen mRNA-based antitumor therapy. Cancer Res. 2012;72(7):1661–1671. doi: 10.1158/0008-5472.CAN-11-2957. [DOI] [PubMed] [Google Scholar]
- 124.Zhao Yangbing, Moon Edmund, Carpenito Carmine, Paulos Chrystal M., Liu Xiaojun, Brennan Andrea L., et al. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res. 2010;70(22):9053–9061. doi: 10.1158/0008-5472.CAN-10-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Barrett D.M., Liu X., Jiang S., June C.H., Grupp S.A., Zhao Y. Regimen-specific effects of RNA-modified chimeric antigen receptor T cells in mice with advanced leukemia. Hum Gene Ther. 2013;24:717–727. doi: 10.1089/hum.2013.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Beatty Gregory L., Haas Andrew R., Maus Marcela V., Torigian Drew A., Soulen Michael C., Plesa Gabriela, et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res. 2014;2(2):112–120. doi: 10.1158/2326-6066.CIR-13-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bai Yun, Kan Shifeng, Zhou Shixin, Wang Yuting, Xu Jun, Cooke John P, et al. Enhancement of the in vivo persistence and antitumor efficacy of CD19 chimeric antigen receptor T cells through the delivery of modified TERT mRNA. Cell Discov. 2015;1(1) doi: 10.1038/celldisc.2015.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lin Liang, Cho Shih-Feng, Xing Lijie, Wen Kenneth, Li Yuyin, Yu Tengteng, et al. Preclinical evaluation of CD8+ anti-BCMA mRNA CAR T-cells for treatment of multiple myeloma. Leukemia. 2021;35(3):752–763. doi: 10.1038/s41375-020-0951-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.De Keersmaecker Brenda, Claerhout Sofie, Carrasco Javier, Bar Isabelle, Corthals Jurgen, Wilgenhof Sofie, et al. TriMix and tumor antigen mRNA electroporated dendritic cell vaccination plus ipilimumab: link between T-cell activation and clinical responses in advanced melanoma. J Immunother Cancer. 2020;8(1):e000329. doi: 10.1136/jitc-2019-000329. [DOI] [PMC free article] [PubMed] [Google Scholar]