Abstract
When cells undergo large-scale senescence, organ aging ensues, resulting in irreversible organ pathology and organismal aging. The study of senescence in cells provides an important avenue to understand the factors that influence aging and can be used as one of the useful tools for examining age-related human diseases. At present, many herbal compounds have shown effects on delaying cell senescence. This review summarizes the main characteristics and mechanisms of cell senescence, age-related diseases, and the recent progress on the natural products targeting cellular senescence, with the aim of providing insights to aid the clinical management of age-related diseases.
1. Introduction
Aging is not regarded as a disease but rather as a unique and independent pathological state. It precedes the onset of many other diseases and is an inevitable biological process. Aging is a multifactor universal process that occurs at the molecular, cellular, and tissue levels. It is characterized by the loss and degeneration of constituent materials, tissue structures, and physiological functions in the body [1, 2].
Research has demonstrated the important role of cellular senescence in the aging process [3, 4]. Cellular senescence was first described as permanent cell cycle arrest when cells reach their replication limit (replication senescence). Even under suitable growth conditions, senescent cells no longer divide and the cell cycle enters an irreversible arrested state [5]. During aging, persistent DNA damage response (DDR) markers and senescence-associated secretory phenotype (SASP) are accumulated in terminally differentiated cells [6]. Cellular senescence also plays a physiological role in the normal development of the body, such as in combination with apoptosis to promote embryonic morphological development [7, 8]. In mature tissues, cellular senescence is mainly triggered by response to injury, thereby inhibiting potentially dysfunctional cells. However, over time, the abnormal accumulation of senescent cells can cause harmful effects [9]. Cellular senescence is the main mechanism that may lead to chronic diseases and age-related dysfunction [10]. In vitro experiments in cells are an important method to study cellular senescence, and these cell experiments may help provide insights into the relationship between senescence and age-related human diseases.
2. Main Indicators of Cell Senescence and the Potential Mechanism
Currently, no universal marker is available to detect cell senescence [11]. Given that biological markers expressed by senescent cells might vary with cell type, stimulation, and stimulation duration, several senescence-related markers need to be evaluated to consolidate the cell senescence phenotype [12].
2.1. Morphological and Metabolic Changes in Senescent Cells
The morphology of senescent cells is drastically different compared with that of normal cells (Figure 1). During senescence, cell density decreases and cells undergo morphological changes [ [13]]. Compared with normal cells, senescent cells typically display an enlarged although flattened shape. The intercellular boundaries of senescent cells become inconspicuous and extensive vacuolization occurs. The integrity of nuclear membranes is damaged due to the loss of lamin B1 expression. The nuclear membrane collapses and chromatin agglutination and pyknosis occur [14]. Senescent cells accumulate defective mitochondria and increased levels of reactive oxygen species (ROS). In senescent human cells, the content of lysosomes increases and lysosomal activity changes, which is manifested by an increase in β-galactosidase (β-gal) activity at pH 6 [15]. This specific β-gal activity was the first and is one of the most widely used gold standards for evaluating cellular senescence [16].
Figure 1.
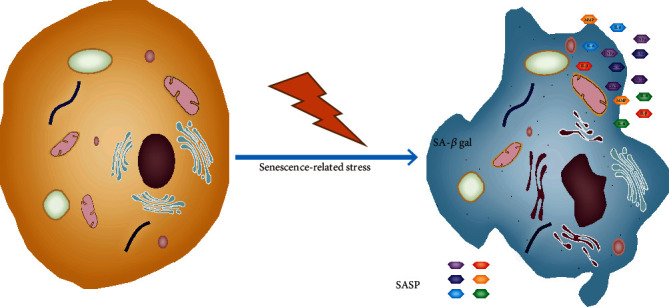
Morphological and metabolic changes in senescent cells. Markers of cell senescence include changes in β-gal activity caused by increased lysosome content and activity, the loss of lamin B1 caused by the changes in the nuclear envelope, the increase of lipofuscin labeled by Sudan black B staining, and morphological changes, such as flat cell bodies. Senescence-associated secretory phenotype- (SASP-) related factors, such as TNFα, IL-1α, IL-1β, and matrix metalloproteinase (MMP) and loss of nuclear localization of HMGB1 are also common markers of senescence.
As the first evidence for β-gal accumulation in cell senescence, Dimri et al. noted increased levels of β-gal in epidermal cells from the skin with age [15]. β-Gal is also expressed in certain nonsenescent cells, including osteoclasts and mature macrophages, under normal physiological conditions [17]. Changes in conditions, such as pH and incubation duration, can stimulate some normal cells to exhibit false-positive results [18]. β-Gal, which is rarely seen in a neutral pH environment under normal conditions, shows high enzymatic activity within 1 h in response to ionizing radiation [19]. Recently, Cai and colleagues identified a new prodrug SSK1 that is specifically cleaved by lysosomal β-gal into cytotoxic substances to stimulate apoptosis and the elimination of senescent cells. In aged mice, SSK1 eliminated senescent cells in various tissues, reduced levels of senescence-related genes such as p16 and p21, reduced mild local and systemic inflammation, and restored organismal function [20]. These findings indicate that lysosomal β-gal may represent an effective target for the selective elimination of senescent cells, providing a new strategy for the development of antisenescence drugs.
Recently, metabolomics analysis on human umbilical vein endothelial cells (HUVECs) was carried out, from the third to eighteenth population doublings, and enriched 14 overtly changed metabolic pathways in senescent cells [21]. This work provided a new perspective to understand the mechanism of cell senescence.
2.2. p16Ink4a
p16Ink4a is a cyclin-dependent kinase inhibitor that competitively binds with CDK4/6, thus inhibiting phosphorylation of the main substrate Retinoblastoma (Rb) [22]. Rb in the nonphosphorylated state binds to the transcription factor E2F, thus inhibiting the expression of genes. The expression of p16Ink4a increases with an increased number of cell divisions [23]. In response to stress factors, most cells trigger senescence through the p16Ink4a-Rb signaling cascade [24]. This process arrests the cell cycle in the G1/S phase, which leads to cell senescence [25] (Figure 2). Notably, approximately 75% of human cancer cell lines contain mutations or deletions in the p16 gene, which prevents these cells from entering the senescence process. Therefore, p16 expression is used as a cell senescence biomarker.
Figure 2.
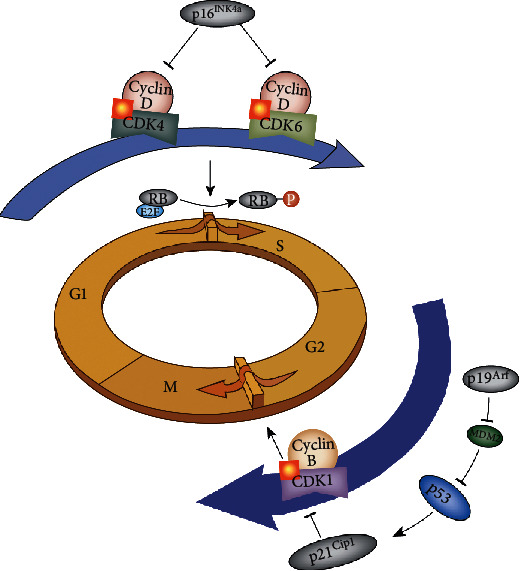
The main regulators of the cell cycle and their functions in senescence. The cyclin-dependent kinase inhibitors p16INK4A and p21Cip1 are commonly used markers of senescence. Cell cycle arrest is induced by the inhibition of cyclin-dependent kinases (CDKs) through the p53/p21Cip1 and/or Rb/p16INK4A pathways, which causes sustained DNA damage.
A recent study used p16tdTom as a reporter allele and sensitive tool to count, isolate, and identify single cells that expressed p16INK4a [26]. Grosse and colleagues developed a knock-in strategy to target p16 and monitor senescence. The authors found that p16Ink4a was rarely expressed in healthy, stress-free tissues and cells in young animals. As mice age (10–12 months old), an increasing number of cells in tissues undergoing senescence, damage, and initial tumorigenesis start to express p16Ink4a [27]. Elevated expression of p16 may be a marker to eliminate senescent cells in mice with a prolonged lifespan. However, this type of method does not appear to be particularly accurate since p16 cells are not eliminated in the colon, liver, and lymphocytes [28]. Childs and colleagues described endothelial cells from the p16-positive p16-3MR transgenic mice as senescent cells. Following ganciclovir treatment in these mice, atherosclerotic plaques were retarded [29]. While there is currently no method for targeting senescent cells that is very precise, targeting p16 may be one of the best methods.
2.3. p21Cip1
The cyclin-dependent kinase inhibitor p21Cip1, which is a transcriptional target of the p53 tumor suppressor, regulates the cell cycle by binding and inhibiting its partner cyclin, leading to the inhibited transition of cells from G1 to S phase and from G2 to M phase [30–32] (Figure 2). The p53 tumor suppressor is inactivated in most tumors, and its expression is upregulated in senescent cells. p53 protein levels are mostly regulated by ubiquitin-mediated proteosomal degradation [33]. The MDM2 ubiquitin ligase, which is highly expressed in most tumors, directly binds with p53 protein to suppress p53 transcriptional activity and promotes the degradation of p53 by ubiquitin-mediated degradation [34]. The p19Arf protein, which is encoded by the Arf gene locus that overlaps with the Ink4a gene locus, binds and inhibits MDM2 activity, subsequently activating p53 signaling [35]. Upon DNA damage (e.g., ionizing radiation and telomere dysfunction), p19Arf is upregulated to inhibit MDM2 and activate p53, which results in the induction of the p53 downstream target p21Cip1. p21Cip1 also functions in the inhibition of Rb phosphorylation; as described above, once phosphorylated, Rb cannot bind with E2F, which leads to cell cycle arrest in the G1 phase and cell senescence [36]. Chakraborty et al. reported a senescence characteristic cell phenotype in pancreatic and breast cancer cells treated with erythronol. The authors found that β-gal activity increased along with elevated expression of p21 and decreased amounts of CDK2 and cyclin D1 [37].
2.4. Telomere Shortening
Research has shown that telomeres are damaged and become shortened as cells divide [38], and the shortening or destruction of telomeres plays a critical function in influencing cell senescence [39]. Telomerase is a reverse transcriptase that is mainly responsible for telomere lengthening, completely independent of replication [40]. Telomerase activity is inhibited or lost after oxidative damage [41–44], leading to the loss of the telomeric ends in the chromosome replication process, therefore accelerating cell senescence [45]. Fouquerel et al. used a targeted combination of telomeres and photochemically generated singlet oxygen to selectively control the time and length of oxidative stress applied to telomere sites [38]. The authors repeatedly exposed cultured cancer cells to this targeted oxidation process to simulate environmental stress and inflammatory conditions. In fact, even though telomerase, which is responsible for telomere lengthening, was reactivated, the telomeres still shorten as the cells divided. Quratul and colleagues found that in mouse cortical nerve cells, the primary reason for telomere shortening with age is not due to telomerase activity (which remains almost constant) but may be from changes in the telomere reverse transcriptase protein content (rather than the RNA component) in mouse cortical nerve cell subchambers. The hTERT component of telomerase selectively increases in cytoplasmic and membrane-bound portions with age [46]. Galbiati et al. used DNA in situ bridging to detect DNA breakage sites and analyzed adjacent sites [47]. This new localization method might detect extreme telomeres that are present in cells. However, in some nonsenescent cells expressing p16Ink4a, telomere shortening and the loss of telomere function could also be detected, and some stress-induced cellular senescence was independent of the telomere shortening pathway [48–50]. Although telomere shortening is detrimental for healthy cells, targeting telomeres in tumor cells represents a method to fight cancer. Drugs that activate and regulate telomerase have been developed with the aim of designing intervention strategies to protect telomeres in healthy cells and target telomeres in cancer cells.
2.5. Senescence-Associated Secretory Phenotype- (SASP-) Related Factors
During senescence, cells secrete many active substances, such as soluble signaling messengers, proteases, and extracellular matrix proteins. Among them, soluble factors, such as cellular inflammatory factors, chemotactic cytokines, growth factors, and immunoregulatory factors, promote cell proliferation and inflammatory responses by changing the microenvironment surrounding cells and promoting the cancerous transformation of cells [51]. For example, IL-6, one of the important SASP factors that is directly regulated by DNA damage signaling, is closely associated with cellular senescence [52, 53]. SASP factors exhibit a dual regulatory role. SASP factors induce activation of the immune system to clear senescent cells and the growth stagnation of senescent cells and participate in tumor suppression. In addition, SASP factors secreted by senescent cells are involved in the destruction of normal tissue structures, induce epithelial-mesenchymal transition, and promote the proliferation of malignant tumors [54]. In some cases, however, this property of senescent cells may help protect the body in specific conditions. For example, following hemorrhagic shock in rats, liver cells immediately enter a state of senescence to prevent organ failure, preserving organismal homeostasis [55]. This could explain the distinct selection mechanisms by which immune cells eliminate senescent cells; senescent cells that promote the secretion of inflammatory substances are eliminated and cells induced to undergo senescence for protective mechanisms may not be eliminated. However, further research is required to address this possibility.
3. Diseases Related to Cellular Senescence
Senescent cells lose their ability to divide and undergo apoptosis and remain in the body [56]. Accumulation of senescent cells is associated with a series of age-related diseases [22], such as cancer, atherosclerosis, liver fibrosis, and neurodegeneration [57] (Figure 3). Therefore, better understanding of how senescent cells affect these diseases and the development of methods to eliminate accumulated senescent cells could be of significant potential for the management of many age-related pathologies.
Figure 3.
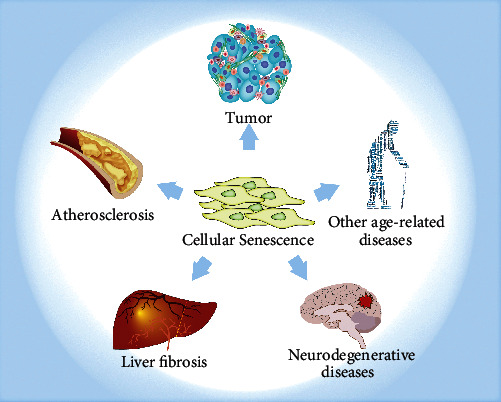
Diseases related to cellular senescence. Although cellular senescence is a normal process during development and tissue remodeling, it is related to a decline in tissue function and various disease states. These diseases include but are not limited to cancer, atherosclerosis, liver fibrosis, neurodegenerative diseases, and other diseases.
3.1. Cancer
The relationship of cellular senescence with cancer varies depending on the physiological environment [58]. Cellular senescence in the early stage of tumorigenesis can reduce the incidence of cancer. Cellular senescence involves an irreversible block of cell proliferation, which also represents a powerful mechanism for autonomously inhibiting cancer [59]. At the late tumor stage, senescent cells eventually show complex, multicomponent SASP. SASP alters the behavior of adjacent cells and the tissue microenvironment. A notable feature of SASP is the large number of proinflammatory factors, including chemokines, cytokines, and damage-associated molecular patterns (DAMPs). Chronic inflammation, as a common feature of aging tissues, is a major risk factor for cancer in later life [5, 60].
Oncogenes induce cellular senescence in the early stage of tumorigenesis. Senescent cells secrete active substances that alter the microenvironment around cells, which promotes proinflammatory responses and inhibits cell division [61]. An inflammatory response is beneficial to eliminate senescent and mutated cells and prevents tumor development and protects bodily functions [7, 62]. In senescence-related research in cancer patients, Srdic-Rajic found that low-dose doxorubicin induced cell senescence and inhibited cancer cell proliferation by promoting ROS production and DNA damage [63]. Further research revealed the appearance of proliferating cells during this process [64]. Later research found that during low-dose chemotherapy, the choice of proliferation or senescence cell fate depended on three different modalities of p21 kinetics. Drug-induced delayed or acute expression of p21 leads to cell senescence, and the intermediate p21 response often results in cell proliferation [65]. Therefore, a p21 “golden zone” was established for the continued proliferation of cells following drug treatment, which provides new guidance for the improvement in clinical chemotherapy strategies and combination medications. Chen et al. found that knockout of the Pten gene resulted in upregulated senescence markers in precancerous tissues but not in deteriorating cancer tissues in a mouse model of prostate cancer. After Pten-deficient cells enter senescence in cell culture, cell senescence is reversed by p53 inactivation [66]. A study using a mouse model with p16Ink4a luciferase labeling to observe cellular senescence and activation in real time revealed that senescent cells accumulated significantly at the site of a transplanted tumor formation in mice; this study represented the first real-time observation of senescent cells in the early stage of cancer in vivo [67]. Cellular senescence markers have been employed as early tumor markers in clinical applications.
Senescent cells accumulate in the later stages of tumorigenesis and secrete a large number of inflammatory factors, growth factors, and immunoregulatory factors, which provide an immunosuppressive microenvironment for tumor cells. This microenvironment stimulates tumor cell transformation and promotes tumor cell proliferation, migration, and invasion [68]. Although senescence therapy may be initially beneficial to inhibit the proliferation of tumor cells, it might promote the acceleration of proliferation and malignant transformation of nonsenescent tumor cells from the stimulation and accumulation of cytokines [69]. In the long term, senescent tumor cells might have certain side effects on health [70]. Therefore, combining the treatment of senescence-induced cancer with senolytics may prevent the regrowth of senescent cancer cells [71]. The survival rate of cervical cancer patients was closely related to the level of age-related proteins in the serum; a higher expression of age-related proteins was related to a lower survival rate of patients. Following radiotherapy, the number of senescent cells decreased and the survival rate of cancer patients increased [72]. Together, these studies indicate that both inducing the senescence of cancer cells and the targeted removal of senescent cells could help to fight cancer, and in-depth research into cellular senescence could be significant for cancer prevention and treatment [73].
3.2. Atherosclerosis
Vascular senescence induces the development of atherosclerosis. Senescence of vascular smooth muscle and endothelial cells promotes the formation of atherosclerotic plaques [74]. The numbers of mouse bone marrow–derived endothelial progenitor cells decreased with age, and those endothelial cells could not be replaced after peripheral damage. This was attributed to increased inflammation caused by SASP factor stimulation and a reduction in tissue homeostasis and tissue repair mediated by transforming growth factor β (TGF-β) [75]. Therefore, atherosclerotic lesions appeared in the damaged area. However, damaged blood vessel structures might be repaired better after mice received bone marrow cell transplantation from young healthy donors [76].
An independent study found that senescence foam cells led to increased numbers of macrophages by promoting an inflammatory response. This resulted in an acceleration in the initial course of atherosclerosis and released enzymes and matrix to degrade plaque in the later stages to promote the instability and rupture of fibrous caps [29].
3.3. Liver Fibrosis
Senescence of various cell types in the liver has an important role in liver fibrosis [77]. In the fibrotic areas of the liver, the telomeres of hepatocytes are significantly shortened [78]. Senescent liver cells activated surrounding stellate cells to secrete senescence-related active factors, therefore changing the microenvironment in the liver, which aggravated liver fibrosis [79, 80]. However, Krizhanovsky's group studied a mouse model of fibrosis and indicated that the first cells to undergo senescence were activated stellate cells [81]. These cells secrete cytokines to promote natural killer cells to recognize and degrade fibrous tissue and reduce the secretion of extracellular matrix, which effectively limits fibrosis in the liver. However, in p53-deficient murine hepatic fibrosis, continuous activation and proliferation of stellate cells aggravated fibrosis [82]. Therefore, liver fibrosis might be inhibited through the p53 signaling cascade to reverse liver fibrosis [81]. Notably, senescent cells have important physiological and structural functions, such as liver sinusoidal endothelial cells that exhibit important detoxification functions. The researchers used CD31 antibodies to stain the livers of mice with different genotypes and found that the removed senescent sinusoidal endothelial cells were not replaced by new cells (other CD31-positive cells) but promoted tissue fibrosis, which leads to the deterioration of health. The lack of replacement of CD31-positive cells in the liver is due to their low proliferative activity and the decline in the expression of a variety of Vegfs and their receptors due to aging [27].
3.4. Neurodegenerative Diseases
In the nervous system, cellular senescence leads to age-related neurodegenerative diseases, including Alzheimer's disease (AD), Parkinson's disease, and amyotrophic lateral sclerosis [83, 84]. Various markers for senescence have been observed in patients with neurodegenerative diseases. Previous studies showed a causal relationship between the accumulation of senescent cells and cognition-related neuronal loss [83]. However, the precise role of senescent cells in the etiology of these neurodegenerative diseases is unknown.
SASP signal activation–mediated neuroinflammation and inflammasome lead to neuron loss [85]. For example, oligodendrocyte precursor cells appear to exhibit a senescence phenotype in AD [86]. Application of senescent cell lysis therapy to AD mouse models led to significantly reduced neuroinflammation and amyloid plaques [87]. In the MAPTP301SPS19 model of tau-dependent neurodegenerative disease, the accumulation of p16INK4A-positive senescent astrocytes and microglia was observed. The elimination of these cells in INK-ATTAC transgenic mice prevented glial hyperplasia. Elimination of these cells in INK-ATTAC transgenic mice prevented gliosis, deposition of neurofibrillary tangles caused by hyperphosphorylation of soluble and insoluble tau, and degeneration of cortical and hippocampal neurons, thus maintaining cognitive function [88]. Together, these results show the vital role of senescent cells in the initiation and progression of tau-mediated diseases and the therapeutic potential of targeting senescent cells for the treatment of these comorbidities.
3.5. Other Age-Related Diseases
The senescence of insulin-secreting β cells in the pancreas is related to the progression of type I and type II diabetes and affects the autoimmunity and metabolic functions of the body, respectively [89]. Senescence reduces the proliferation capacity of β cells and the secretion of SASP components, thereby aggravating current inflammation and tissue damage [90]. The body loses its ability to keep blood sugar stable under aging, which leads to glucose toxicity [91]. This stress causes the senescence of various types of cells, such as fibroblasts, renal tubular epithelial cells, endothelial cells, and mesenchymal stem cells, which leads to other age-related diseases, such as vascular diseases and kidney diseases [92]. Similar to results in AD, the application of senescent cell lysis therapy in an animal model of diabetes showed promising effects in inhibiting the course of the disease [93].
In idiopathic pulmonary fibrosis, alveolar type II epithelial cells proliferate into new type II epithelial cells or differentiate into type I epithelial cells [94]. However, type II epithelial cells with congenital regeneration defects of short telomeres do not continue to proliferate or differentiate and cannot form normal alveolar tissue. The specific knockout of the type II epithelial cell telomere protection protein TRF2 in vitro causes DNA damage response and cell senescence [95]. The DNA damage signal from the alveolar epithelium can recruit macrophages and T cells to the alveolar tissue, and telomere shortening–mediated stem cell senescence upregulates the expression of proinflammatory cytokines and induces inflammation. Senescence increases oxidative stress, which directly leads to DNA damage [96]. Excessive oxidative stress has various adverse effects on cells, such as the activation of redox sensitive signaling pathways and the expression of cytokines and chemokines. Fibroblasts activate and secrete large amounts of collagen fibers, which leads to lung diseases such as idiopathic pulmonary fibrosis [97]. Studies have shown that senescent fibroblasts are selectively killed by dasatinib and quercetin (a senolytic) [98]. Eliminating senescent cells in INK-ATTAC transgenic mice improved lung function and physical health [94].
Osteoarthritis (OA) is a chronic disease characterized by the degradation of articular cartilage, causing pain and physical disability. Studies have found senescent chondrocytes in the cartilage cells of patients with OA and these cells have characteristics of age-related β-galactosidase positive staining, shortened telomere length, and mitochondrial degeneration [99]. In a mouse model of OA through anterior cruciate ligament transection, senescent cells are accumulated in the articular cartilage and synovium. Selective removal of these cells reduces the development of OA and relieves pain [100].
4. Common Modalities in Cell Senescence and Related Nutritional Interventions
Cellular senescence occurs through long-term culture of primary cells (replication senescence). However, in response to several stress factors (including oxidation, radiation, and toxicity), cellular senescence can be triggered prematurely [101]. In addition to the main senescent pathways, such as p16 and p53-p21, the upregulation of SIRT1, eNOS phosphorylation, SOD, GSH-Px, and E2F-1 and the downregulation of miR-34a, NF-κB, MDA content, and caveolin-1 to delay senescence have been reported in various senescent cell models.
Recent research has shown interest in drugs, such as rapamycin and metformin, and their ability to effectively prolong life and treat disease pathology. However, the antiaging ability of these drugs is not unique [102]. From >10,000 screening tests, a variety of plant extracts were identified with potent antiaging properties [103]. Many herbal compounds exhibit anticell senescence effects [104] (Figure 4). The development of new antiaging drugs from natural plants and traditional Chinese medicine has gained global attention [105]. These cell senescence interventions that are extracted from plants include carbohydrates, polyphenols, peptides, sterol compounds, and vitamins (Table 1). Our aim for Table 1 table is to highlight the cells that are suitable for the study of cell senescence, which nutritional interventions can act as positive effectors to interfere with the aging process, which markers can be detected, and whether their results can be compared with those of previous studies.
Figure 4.
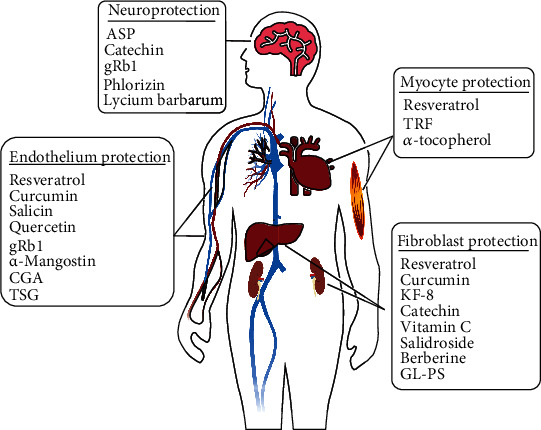
Common research areas for cellular senescence and related nutritional interventions. Many cell models are used to study cellular senescence, and the most widely used cell models are endothelial cells, fibroblasts, muscle cells, and nerve cell models. A variety of plant extracts with effective antiaging properties have been identified. Many herbal extracts exhibit antiaging effects. Natural nutritional interventions for cellular senescence mainly included carbohydrates, polyphenols, peptides, sterol compounds, and vitamins.
Table 1.
Common cellular senescence models and nutritional interventions.
| Cell type | Cell line | Induction methods | Inhibitor | Type of inhibitor | Sources (nutritional) | Molecular target(s) (major) | Read-out results | References |
|---|---|---|---|---|---|---|---|---|
| Epithelial cells | HUVEC | Ang II | TFs | Polyphenols | Carya cathayensis Sarg | SIRT1↑, miR-34a↓, p53↓, p21↓, PAI-1↓ | SA-β-GAL positive cells↓, G0/G1 cell cycle↓, S cell cycle↑ | [[152]] |
| HUVEC | D-galactose | Mulberry extract, C-3-R, C-3-G | Mixture | Mulberry | p21↓, p16↓, NAD+/NADH↑, SIRT1↑ | SA-β-GAL positive cells↓,ROS↓ | [[153]] | |
| HUVEC | Doxorubicin; replicative | bCUR; Polydatin; BCP | Polyphenol; semiterpenoids | Curcuma aromatica Salisb lemon; grapefruit | SIRT1↑, miR-146a↓, miR-21↓, p16ink4a↓, IL-6↓ | SA-β-GAL positive cells↓,SASP↓ | [[117]] | |
| HUVEC | HG | α-Mangostin | Flavonoid derivatives | Mangosteen | IL-6↓, SIRT1↑, AMPK↑, p-AMPK↑, p53↓, p21↓ | SA-β-GAL positive cells↓,ROS↓ | [[154]] | |
| HUVEC | HG | Aralia elata (Miq.) Seem | Mixture | Aralia elata | p-ERk↑,p-p38↑, cdc2↓,p53↓, cyclinB1↓, SIRT1↑,p-AKT↑, p-AMPK↑, p-eNOS↑ | SA-β-GAL positive cells↓, G0/G1 cell cycle↓ | [[155]] | |
| HUVEC | H2O2 | TSG | Glycosides | Polygonum multiflorum | SIRT1↑, p21↓, PAI-1↓ | SA-β-GAL positive cells↓, cell cycle arrest↓ | [[156]] | |
| HUVEC | H2O2 | Curcumin | Polyphenol | Curcuma aromatica Salisb., C. longa L | SIRT1↑, p21↓ | SA-β-GAL positive cells↓, Cell proliferation↑ | [[116]] | |
| HUVEC | H2O2 | CGA | Phenylpropanoids | Eucommia ulmoides Oliv. Lonicera dasytyla Rehd. | Nrf2↑, HO-1↓, SIRT1↑, PAI-1↓, p21↓, p53↓ | SA-β-GAL positive cells↓, Cell proliferation↑, DNA damage↓ | [[157]] | |
| HUVEC | H2O2 | Resveratrol | Polyphenols | Grapes, knotweed, peanuts | p-Rb↑, LC3↑, p62↑ | SA-β-GAL positive cells↓,ROS↓ | [[158]] | |
| HUVEC | Ox-LDL | gRb1 | Saponin | Panax ginseng C.A.Mey. P. quinqu efolium L. |
SIRT1↑, p62↓, LC3II/LC3I↑, PAI1↓ | SA-β-GAL positive cells↓, G0/G1 cell cycle↓ | [[159]] | |
| HUVEC | TNF-α | Salicin | Organic acid | Willow, Gaultheria, sweet birch | p21↓, PAI-1↓, Acety-p53↓, Nrf2↑ | SA-β-GAL positive cells↓, cell cycle arrest↓ | [[160]] | |
| HUVEC | Radiation | Quercetin | Flavonoids | Buckwheat, sea buckthorn, hawthorn, onion | PAI-2↓, p21↓, BCL-xL↓, p16↓ | SA-β-GAL positive cells↓ | [[115]] | |
| HAEC | Ox-LDL | Quercetin | Flavonoids | Buckwheat, sea buckthorn, hawthorn, onion | IGFBP3↓, SLC5A11↑, EIF41B↓ | SA-β-GAL positive cells↓, ROS↓ | [[161]] | |
| RAEC | HG; PA | GSC extracts | Mixture | Ginseng; San-Qi; Chuan-Xiong | Parkin↑; p21↓, p16↓, p62↓, AMPK↑ | MtROS↓, Mitosis↑ | [[118]] | |
| HaCaT | UVA | G6 | Polysaccharides | Ascophyllum nodosum | SIRT1↑, pGC1a↑, NRF1↑, NRF2↑, ERRa↑ | Respiratory chain complex activities↑, ATP content↑, NAD+/NADH ratio↑ | [[162]] | |
| HaCaT | UVB | SH extracts | Mixture | Salvia haenkei | p21↓, p27↓, IL6↓, IL18↓, SIRT1↑, MMP-2↓ | ROS↓ | [[163]] | |
| HaCaT | UVB | SS stem extracts | Mixture | Spatholobus suberectus | p-p38↓, p38↑, ERK1/2↑, p-ERK1/2↓, NF-κB↓ | ROS↓, Cell damage↓ | [[164]] | |
| Fibroblasts | HDFs | UV | Curcumin | Polyphenols | C. aromatica Salisb., C.longa L | TGF-β↑, Smad2/3↑, Bcl-2↑, MMP-1↓, MMP-3↓, caspase-3↓, NF-κB↓, GRP78↓, CHOP↓ | ROS↓, activity of antioxidant defense enzymes↑ | [[165]] |
| HDFs | UVB | Extracts | Mixture | S. aromaticum L. | MMP-1↓, p-c-jun↓, p-c-fos↓ NF-κB↓, IκB-α↑, NQO-1↑ | ROS↓, Cell viability↑ | [[166]] | |
| HDFs | H2O2 | Vitamin C | Vitamin | Tomatoes, cauliflower, citrus, Grapefruit, apples, grapes | FoxO3a↑, SIRT1↑, p-Rb↓, p53↓, p21↓, p16↓ | SA-β-GAL positive cells↓, Collagen↑, Elastic fiber↑ | [[167]] | |
| 3T3 | H2O2 | KF-8 | Peptide | Rice bran | Nrf2↑, p65↓ | ROS↓ | [[124]] | |
| Fibroblasts | UVB | GL-PS | Polysaccharides | Ganoderma lucidum | MMP-1↓, CICP↑ | SA-β-GAL positive cells↓, Cell viability↑ | [[168]] | |
| WI-38 | CuSO4 | Resveratrol | Polyphenols | Grapes, knotweed, peanuts | SIRT1↑, p21↓, TGF-β↑, ApoJ↓ | SA-β-GAL positive cells↓, Cell proliferation↑ | [[169]] | |
| 2BS | Replicative | Salidroside | Phenyl alcohol | Rhodiola rosea L | PGC-1α↑, Nrf1↑, TFAM↑, SIRT1↑, p53↓, p21↓, p16↓, Rb↓ | mitochondrial dysfunction↓, ROS↓ | [[170]] | |
| 2BS; WI-38 | Replicative; H2O2 | Berberine | Alkaloids | Coptis chinensis Franch. | p16↓, CDK4↑, cyclinD1↑, p-RB↑, E2F-1↑, SIRT1↑, p-Chk2↑ | SA-β-GAL positive cells↓, G0/G1 cell cycle↓, S/G2-M phase↑, ROS↓ | [171, 172] | |
| Myocyte | NRCMs | Hypoxia; LPS | Resveratrol | Polyphenols | Grapes, knotweed, peanuts | p53↓, SIRT1↑, p16↓, p19↓, c-Casepase3↓, Bax↓, NLRP3↓ | SA-β-GAL positive cells↓ | [[173]] |
| Neurocyte | NSCs | D-galactose | ASP | Polysaccharides | Angelica | p53↓, p21↓, TNFα↓ | SA-β-GAL positive cells↓, Cell proliferation↑, ROS↓, activity of antioxidant defense enzymes↑ | [[174]] |
| NSCs | LiCl | gRb1 | Saponin | Panax ginseng C.A.Mey. P. quinqu efolium L. |
p-Gsk-3β↓, c-myc↓, Lef↓, β-catenin↓ | SA-β-GAL positive cells↓, Cell proliferation↑ | [[175]] | |
| PC12 | D-galactose | Phlorizin | Flavonoids | Apples | Nrf2↑, HO-1↑, NQO1↑ | SA-β-GAL positive cells↓, activity of antioxidant defense enzymes↑ | [[176]] | |
| PC12 | H2O2; AAPH | Ethanol extract of P. ternata tubers | Mixture | Pinellia ternata | p53↑, RPS19BP1↓, HuR↓, SIRT1↑, Bax↓, Bcl-2↑ | SA-β-GAL positive cells↓, lipofuscin accumulation↓, cell cycle arrest at the G2/M phase↓,oxidative damage↓ | [[177]] | |
| Astrocytes | Replicative LPS/MPP+ | Astragaloside IV | Saponin | Astragalus | p16ink4a↓, CXCL1↓, IL-6↓, IL-1β↓, MMP3↓, Lamin B1↓, p62↓ | SA-β-GAL positive cells↓, accumulation of senescent astrocytes↓, MtROS↓ | [[178]] | |
| Cartilage | Chondrocytes | CCN1 | Tanshinone IIA | Ketones | Salvia miltiorrhiza Bge | CCN1↓, p16ink4a↓, p21↓, IL-1β↓, CXCL1↓, MMP3↓, IL-6↓ | SASP↓, ROS↓, | [[179]] |
4.1. Endothelial Cells
Vascular endothelial cells (VECs) are the most widely studied cell type. VEC senescence is a common pathological basis for cardiovascular diseases. Under chronic exposure to high glucose (HG) and a high-fat environment, VECs can enter an early stage of senescence. Vascular dysfunction occurs from changes in the levels of vasodilators, contractile factors, antioxidant factors, and coagulation factors [106–110]. After the senescence of HUVECs was cultured in vitro, the cells were wider with flat intercellular spaces. In addition, nuclei and nucleoli were enlarged along with reduced levels of nitric oxide (NO) and endothelial nitric oxide synthase (eNOS) activity [111]. NO is a vasodilator factor that promotes blood circulation and helps to control blood pressure. In senescent cells, the production of ROS is significantly increased, which reduces the bioavailability of NO [112]. HG promotes mitochondria to produce excessive ROS, therefore accelerating oxidative damage and cell senescence [113, 114].
Dasatinib combined with quercetin is a recently identified senolytic strategy with pronounced antiaging effects. Dasatinib is a small molecule tyrosine kinase inhibitor. Quercetin is a natural flavonoid and reduces the survival ability of senescent HUVECs to effectively trigger cell death without discernable effects on nonsenescent cells [115]. Curcumin, a natural polyphenol compound, delays endothelial cell senescence induced by hydrogen peroxide through SIRT1 signaling [116]. Recent studies found that a combination of resveratrol, curcumin, and β-caryophyllene reduced the levels of SASP factors, such as IL-1β and IL-6, in senescent HUVECs [117]. Wang and associates found that Ginseng-Sanqi-Chuanxiong extracts regulated mitosis through AMPK to prevent HG/palmitate-induced endothelial senescence and the production of mitochondrial ROS [118]. New strategic plans are required for the clinical prevention and treatment of cardiovascular-related diseases, especially those related to endothelial cell senescence.
4.2. Fibroblasts
The generation of senescent cell models using fibroblasts is a common method to explore the biological characteristics of senescence [119]. High levels of glucose have been used to induce senescence in human diploid fibroblasts [120]. An H2O2 administration method has been reported to continuously track senescence in primary nonembryonic mouse fibroblasts. After staining with SA-β-Gal, the percentage of senescent cells (positively stained for SA-β-Gal) in the H2O2-induced group was 22.23% higher than that in the normal group [121]. Another study used UV to irradiate mouse skin fibroblasts to obtain a skin photo senescence model, and rosiglitazone was found to alleviate senescence in this model [122]. However, other studies used rosiglitazone to induce senescence in bone marrow cells [28]. One possibility for these contrasting findings is that a single drug might have varying effects depending on the cell types.
The senescent fibroblast models are also used for the screening of antisenescence drugs. In a previous study in which bleomycin-induced BJ fibroblasts were used to establish a senescent cell model, the authors screened 113 plant components and obtained several drugs that effectively inhibit SASP formation [123]. Recently, we found that pretreatment with KF-8, a polypeptide extracted from rice bran, delays the H2O2-induced senescence of 3T3 cells by attenuating NF-κB/p38 signaling and Nrf2 nuclear transport [124]. Another study found that quercetin not only delays the senescence of human primary dermal fibroblasts after UV exposure but also delays the senescence of human primary dermal fibroblasts lacking HES1 (a growth control transcription factor) [ [13]]. Moreover, when quercetin and its derivative quercetin-caprylate was supplemented to senescent fibroblasts, a rejuvenating effect was observed [125]. Although quercetin has good anticellular senescence effects, its poor oral bioavailability, due to poor water solubility, cell membrane permeability, and short biological half-life, limits its clinical application [126]. Other studies have shown the benefit of resveratrol [127, 128] and fisetin [102] from vegetables and fruits in the senescence of fibroblasts.
4.3. Muscle Cells
Muscle tissue is mainly composed of highly contractile, columnar muscle cells. The contraction of muscles converts chemical energy into mechanical energy, shortening muscle fibers, which causes various body movements [129]. The senescence of myocardial cells causes a series of physiological and pathological changes in the heart, which lead to the onset of cardiovascular disease, and even mortality in severe cases. Recent evidence suggests that ellagitannins found in pomegranates are converted to Urolitin A in the gut. Urolitin A can slow the senescence of muscle cells by improving mitochondrial function [130]. In another independent study using palmitate to induce muscle cell senescence, resveratrol delayed senescence by altering autophagic flux [131]. The antisenescence ability of resveratrol has been verified in a variety of cell models. Resveratrol has been found to significantly extend lifespan in a variety of model organisms such as yeast, nematodes, fruit flies, fish, mice, and rats [ [132, 133]]. The antisenescence mechanisms of resveratrol mainly involve effects on oxidative stress, calorie restriction, and telomeres. Resveratrol is an antioxidant that ameliorates age-related diseases in mice by reducing ROS production, scavenging free radicals, and stimulating biosynthesis of endogenous antioxidants [134–137]. However, human trials are lacking.
Calorie restriction is the only known nutritional intervention that has the potential to slow down senescence. A recent study in humans has confirmed that cutting calorie intake by 15 percent over two years can slow aging and metabolism, as well as prevent age-related diseases [138]. Resveratrol has been found to have a similar effect to caloric restriction and regulates lifespan through Sir2/SIRT1, AMPK, NF-κB, and other signaling pathways [139].
4.4. Nerve Cells
Nerve tissues, composed of signal-transmitting neurons and the supporting glial cells, are basic components of the central and peripheral nervous system. The topic of cell senescence and neuronal regeneration is rapidly evolving in the neuroscience field. The decline of cognitive function and memory is closely associated with the senescence of hippocampal nerve cells and a decrease in the numbers of new neurons during aging [140, 141]. Naturally abundant compounds in plant-based foods have been found to have a wide range of health benefits and may be environmental determinants of brain structure and cognitive function. For example, resveratrol in red grape skin and epigallocatechin gallate (EGCG) in green tea have been shown to influence hippocampal neurogenesis in adults [142, 143]. Recently, quercetin, which is abundant in apple peel, was found to promote hippocampal precursor survival and neuronal differentiation in adult mice. The 3, 5-dihydroxybenzoic acid in apple pulp can significantly increase the proliferation and neurogenesis of neural precursor cells [144]. Curcumin and its analogs was found to reduce oxidative damage of senescent PC12 cells. Curcumin upregulates the level of Nrf2, inhibits ROS production, restores mitochondrial membrane potential, and reduces cell apoptosis [145]. In addition, curcumin increases the level of HO-1 and decreases the expression of Keap1 [146]. In addition to the effects of curcumin in cell models, the antioxidant and antisenescence effects of curcumin have also been verified in animal models such as nematodes and mice [147, 148]. Curcumin not only eliminates ROS and regulates the expression of SOD, catalase, and other related antioxidant enzymes [ [149]] , but it also acts as a calorie restriction mimetic to delay senescence [150]. Although a large number of experiments have shown that curcumin has antisenescence effects, the data on the long-term response to curcumin is still very limited, and clinical verification is still lacking. In addition, curcumin has low bioavailability, and an effective concentration is difficult to achieve, which is also an urgent problem to be solved. We previously reviewed the antisenescence effects of various plant-derived antioxidants on neurons and summarized the mechanism of the effects [151].
5. Conclusion and Perspectives
Cellular senescence plays an important role in a variety of pathological processes, including tumorigenesis, atherosclerosis, fibrosis, and the normal aging process. In response to telomere shortening, DNA damage, and external stimuli, senescent cells halt proliferation through various signaling pathways and secrete several factors to attract immune cells for scavenging and tissue regeneration.
Cellular senescence and biological aging are related but are distinct concepts. The study of cellular senescence is moving into a new area to determine the mechanisms of biological (organismal) senescence. Gene cloning technologies and other methods could be employed for the in-depth examination of cellular senescence–related genes to provide a more reliable base for the mechanisms of senescence and senescence-related diseases. Building animal models that mimic human aging diseases also helps further the understanding of the effects of senescent cells on diseases caused by senescence. Various models to explore senescence are currently used in research, including D-galactose induction, thymus removal, and isotope irradiation, which determine the pathological processes of senescence from different perspectives, such as energy metabolism disorders, immune disorders, and DNA damage. The thymus, spleen, serum index, and other indicators are not enough to explain the antisenescence mechanism. The mechanism of cellular senescence is determined by multiple factors, which contribute to the complexity of the modulation of cellular senescence.
Starting from the proven pathways of cellular senescence, cellular senescence modulators that are extracted from natural substances and show clinical relevance to delay cell senescence are being researched. Our group found a polypeptide from rice bran, and its antioxidant and antiaging effects have been proven in cells, nematodes, and mouse models [124]. Future studies should explore the antiaging effect of this peptide on the human body.
Despite the research in drugs targeting senescence, there are still limitations in their application. The first is the low bioavailability of natural compounds. More in-depth pharmacological and pharmacokinetic studies are required to improve the safety, purity, and bioavailability of antiaging drugs and to formulate relevant standards and specifications to ensure the applicability of antiaging drug research [180]. The second limit is that their long-term effects on human health cannot be verified in animal models; the existing animal models and technology cannot evaluate the long-term negative effects caused by clearing or regulating senescent cells. Third, the current research on the mechanism of new antiaging drugs is based on known pathways, and at present, all the mechanisms with antiaging effects remain unknown. Finally, during the research process, it was discovered that certain antiaging effects of certain drugs have sex and age restrictions or are only effective for certain types of cells [181].
Nevertheless, based on the results of current research, antiaging drug research can help identify new antiaging targets or find more effective compounds through modification. Researchers have discovered some senolytic and senomorphic pharmaceutical compounds. Senolytics are mainly effective by eliminating senescent cells, while the function of senomorphics (also called senostatics) is to regulate the characteristics of senescent cells rather than eliminating the cells [5]. The natural interventions mentioned in this article, which have the effect of delaying cell senescence, are expected to become senomorphics through in-depth research. From a long-term perspective, it is feasible to use intervention measures that affect the aging process, such as reducing the load of senescent cells, to delay the onset of age-related onset or decrease the incidence. Therefore, we need to improve the existing models to summarize the various pathological signals of senescence more prominently, to understand the cellular mechanism of senescence, and identify novel interventions that have antisenescence activity from nutrition. In-depth examination of the underlying antisenescence mechanism should help develop new antisenescence interventions such as senolytics and senomorphics for aging and age-related comorbidities.
Acknowledgments
This work was supported by funding from the Natural Science Foundation for Distinguished Young Scholars of Hunan Province (No. 2021JJ10078), the Natural Science Foundation of Hunan Province (No. 2020JJ4138), Hunan Furong Scholars Program, and Grain-oil Process and Quality Control 2011 Collaborative and Innovative Grant from Hunan Province.
Data Availability
The data used to support the findings of this study have been deposited in PubMed.
Conflicts of Interest
The authors declare that there is no financial conflict of interest.
Authors' Contributions
CZx was responsible for writing the original draft, preparation, and figures; WYx was responsible for the data curation and literature collection; LQl was responsible for the formal analysis; CJ was responsible for the figures; LX was responsible for the literature collection; LY was responsible for the conceptualization; writing, reviewing, and editing the manuscript; and investigation. All authors read and approved the final version of the manuscript.
References
- 1.Cano M., Guerrero-Castilla A., Nabavi S. M., Ayala A., Argüelles S. Targeting pro-senescence mitogen activated protein kinase (Mapk) enzymes with bioactive natural compounds. Food and Chemical Toxicology . 2019;131, article 110544 doi: 10.1016/j.fct.2019.05.052. [DOI] [PubMed] [Google Scholar]
- 2.Davan-Wetton C. S. A., Pessolano E., Perretti M., Montero-Melendez T. Senescence under appraisal: hopes and challenges revisited. Cellular and Molecular Life Sciences . 2021;78(7):3333–3354. doi: 10.1007/s00018-020-03746-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu M., Pirtskhalava T., Farr J. N., et al. Senolytics improve physical function and increase lifespan in old age. Nature Medicine . 2018;24(8):1246–1256. doi: 10.1038/s41591-018-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Deursen J. M. Senolytic therapies for healthy longevity. Science . 2019;364(6441):636–637. doi: 10.1126/science.aaw1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Micco R., Krizhanovsky V., Baker D., d'Adda di Fagagna F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nature Reviews Molecular Cell Biology . 2021;22:75–95. doi: 10.1038/s41580-020-00314-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White R. R., Vijg J. Do DNA double-strand breaks drive aging? Molecular Cell . 2016;63(5):729–738. doi: 10.1016/j.molcel.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calcinotto A., Kohli J., Zagato E., Pellegrini L., Demaria M., Alimonti A. Cellular senescence: aging, cancer, and injury. Physiological Reviews . 2019;99(2):1047–1078. doi: 10.1152/physrev.00020.2018. [DOI] [PubMed] [Google Scholar]
- 8.Muñoz-Espín D., Cañamero M., Maraver A., et al. Programmed cell senescence during mammalian embryonic development. Cell . 2013;155(5):1104–1118. doi: 10.1016/j.cell.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 9.McHugh D., Gil J. Senescence and aging: causes, consequences, and therapeutic avenues. The Journal of Cell Biology . 2018;217(1):65–77. doi: 10.1083/jcb.201708092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirkland J. L., Tchkonia T. Clinical strategies and animal models for developing senolytic agents. Experimental Gerontology . 2015;68:19–25. doi: 10.1016/j.exger.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casella G., Munk R., Kim K. M., et al. Transcriptome signature of cellular senescence. Nucleic Acids Research . 2019;47:7294–7305. doi: 10.1093/nar/gkz879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharpless N. E., Sherr C. J. Forging a signature of in vivo senescence. Nature Reviews Cancer . 2015;15:397–408. doi: 10.1038/nrc3960. [DOI] [PubMed] [Google Scholar]
- 13.Zou Z., Long X., Zhao Q., et al. A single-cell transcriptomic atlas of human skin aging. Developmental Cell . 2021;56 doi: 10.1016/j.devcel.2020.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Kosar M., Bartkova J., Hubackova S., Hodny Z., Lukas J., Bartek J. Senescence-associated heterochromatin foci are dispensable for cellular senescence, occur in a cell type- and insult-dependent manner and follow expression of p16 (ink4a) Cell Cycle . 2011;10:457–468. doi: 10.4161/cc.10.3.14707. [DOI] [PubMed] [Google Scholar]
- 15.Dimri G. P., Lee X., Basile G., et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proceedings of the National Academy of Sciences . 1995;92(20):9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernandez-Segura A., Nehme J., Demaria M. Hallmarks of cellular senescence. Trends in Cell Biology . 2018;28:436–453. doi: 10.1016/j.tcb.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Kopp H. G., Hooper A. T., Shmelkov S. V., Rafii S. Beta-galactosidase staining on bone marrow. Histology and Histopathology . 2007;22:971–976. doi: 10.14670/HH-22.971. [DOI] [PubMed] [Google Scholar]
- 18.Pati S., Jain S., Behera M., Acharya A. P., Panda S. K., Senapati S. X-gal staining of canine skin tissues: a technique with multiple possible applications. Journal of Natural Science, Biology, and Medicine . 2014;5:245–249. doi: 10.4103/0976-9668.136147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coates P. J., Lorimore S. A., Rigat B. A., Lane D. P., Wright E. G. Induction of endogenous beta-galactosidase by ionizing radiation complicates the analysis of p53-Lac Z transgenic mice. Oncogene . 2001;20:7096–7097. doi: 10.1038/sj.onc.1204904. [DOI] [PubMed] [Google Scholar]
- 20.Cai Y., Zhou H., Zhu Y., et al. Elimination of senescent cells by β-galactosidase-targeted prodrug attenuates inflammation and restores physical function in aged mice. Cell Research . 2020;30:574–589. doi: 10.1038/s41422-020-0314-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yi S., Lin K., Jiang T., et al. NMR-based metabonomic analysis of HUVEC cells during replicative senescence. Aging . 2020;12:3626–3646. doi: 10.18632/aging.102834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He S., Sharpless N. E. Senescence in health and disease. Cell . 2017;169:1000–1011. doi: 10.1016/j.cell.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serrano M., Lin A. W., McCurrach M. E., Beach D., Lowe S. W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell . 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 24.LaPak K. M., Burd C. E. The molecular balancing act of p16(INK4a) in cancer and aging. Molecular Cancer Research: MCR . 2014;12:167–183. doi: 10.1158/1541-7786.MCR-13-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim H. W., Ju J. H., Shin J. I., Seung B. J., Sur J. H. Differential and correlated expressions of p16/p21/p27/p38 in mammary gland tumors of aged dogs. Journal of Veterinary Science . 2017;18:479–485. doi: 10.4142/jvs.2017.18.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J.-Y., Souroullas G. P., Diekman B. O., et al. Cells exhibiting strong promoter activation in vivo display features of senescence. Proceedings of the National Academy of Sciences of the United States of America . 2019;116:2603–2611. doi: 10.1073/pnas.1818313116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grosse L., Wagner N., Emelyanov A., et al. Defined p16High senescent cell types are indispensable for mouse healthspan. Cell Metabolism . 2020;32:87–99.e86. doi: 10.1016/j.cmet.2020.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Baker D. J., Childs B. G., Durik M., et al. Naturally occurring p16(Ink 4a)-positive cells shorten healthy lifespan. Nature . 2016;530:184–189. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Childs B. G., Baker D. J., Wijshake T., Conover C. A., Campisi J., van Deursen J. M. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science . 2016;354:472–477. doi: 10.1126/science.aaf6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reed S. M., Quelle D. E. p53 acetylation: regulation and consequences. Cancers . 2014;7:30–69. doi: 10.3390/cancers7010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakayama Y., Yamaguchi N. Role of cyclin B1 levels in DNA damage and DNA damage-induced senescence. International Review of Cell and Molecular Biology . 2013;305:303–337. doi: 10.1016/b978-0-12-407695-2.00007-x. [DOI] [PubMed] [Google Scholar]
- 32.Malumbres M., Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nature Reviews Cancer . 2009;9(3):153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 33.Tonnessen-Murray C. A., Lozano G., Jackson J. G. The regulation of cellular functions by the p53 protein: cellular senescence. Cold Spring Harbor Perspectives in Medicine . 2017;7 doi: 10.1101/cshperspect.a026112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sherr C. J., Weber J. D. The ARF/p53 pathway. Current Opinion in Genetics & Development . 2000;10:94–99. doi: 10.1016/S0959-437X(99)00038-6. [DOI] [PubMed] [Google Scholar]
- 35.Kamijo T., Zindy F., Roussel M. F., et al. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell . 1997;91:649–659. doi: 10.1016/s0092-8674(00)80452-3. [DOI] [PubMed] [Google Scholar]
- 36.Fischer B. M., Wong J. K., Degan S., et al. Increased expression of senescence markers in cystic fibrosis airways. American Journal of Physiology-Lung Cellular and Molecular Physiology . 2013;304:L394–L400. doi: 10.1152/ajplung.00091.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chakraborty S., Rasool R. U., Kumar S., et al. Cristacarpin promotes ER stress-mediated ROS generation leading to premature senescence by activation of p21 (waf-1) Age . 2016;38:p. 62. doi: 10.1007/s11357-016-9922-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fouquerel E., Barnes R. P., Uttam S., Watkins S. C., Bruchez M. P., Opresko P. L. Targeted and persistent 8-oxoguanine base damage at telomeres promotes telomere loss and crisis. Molecular Cell . 2019;75:117–130.e6. doi: 10.1016/j.molcel.2019.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodier F., Campisi J. Four faces of cellular senescence. Journal of Cell Biology . 2011;192:547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He Y., Wang Y., Liu B., et al. Structures of telomerase at several steps of telomere repeat synthesis. Nature . 2021;593(7859):454–459. doi: 10.1038/s41586-021-03529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurz D. J., Decary S., Hong Y., Trivier E., Akhmedov A., Erusalimsky J. D. Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. Journal of Cell Science . 2004;117, Part 11:2417–2426. doi: 10.1242/jcs.01097. [DOI] [PubMed] [Google Scholar]
- 42.Buschini A., Pinelli S., Pellacani C., et al. Synthesis, characterization and deepening in the comprehension of the biological action mechanisms of a new nickel complex with antiproliferative activity. Journal of Inorganic Biochemistry . 2009;103(5):666–677. doi: 10.1016/j.jinorgbio.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 43.Haendeler J., Hoffmann J., Diehl J. F., et al. Antioxidants inhibit nuclear export of telomerase reverse transcriptase and delay replicative senescence of endothelial cells. Circulation Research . 2004;94(6):768–775. doi: 10.1161/01.res.0000121104.05977.f3. [DOI] [PubMed] [Google Scholar]
- 44.Luxton J. J., McKenna M. J., Lewis A., et al. Telomere length dynamics and DNA damage responses associated with long-duration spaceflight. Cell Reports . 2020;33(10, article 108457) doi: 10.1016/j.celrep.2020.108457. [DOI] [PubMed] [Google Scholar]
- 45.Chakravarti D., LaBella K. A., DePinho R. A. Telomeres: history, health, and hallmarks of aging. Cell . 2021;184(2):306–322. doi: 10.1016/j.cell.2020.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ain Q., Schmeer C., Penndorf D., et al. Cell cycle-dependent and -independent telomere shortening accompanies murine brain aging. Aging . 2018;10(11):3397–3420. doi: 10.18632/aging.101655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galbiati A., Beausejour C., d'Adda di Fagagna F. A novel single-cell method provides direct evidence of persistent DNA damage in senescent cells and aged mammalian tissues. Aging . 2017;16:422–427. doi: 10.1111/acel.12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lansdorp P. M. Telomeres and disease. The EMBO Journal . 2009;28(17):2532–2540. doi: 10.1038/emboj.2009.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aubert G., Hills M., Lansdorp P. M. Telomere length measurement-caveats and a critical assessment of the available technologies and tools. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis . 2012;730(1-2):59–67. doi: 10.1016/j.mrfmmm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Armanios M. Telomeres and age-related disease: how telomere biology informs clinical paradigms. Journal of Clinical Investigation . 2013;123(3):996–1002. doi: 10.1172/jci66370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coppe J. P., Desprez P. Y., Krtolica A., Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annual Review of Pathology: Mechanisms of Disease . 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rodier F., Coppe J. P., Patil C. K., et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nature Cell Biology . 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuilman T., Michaloglou C., Vredeveld L. C., et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell . 2008;133(6):1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 54.Coppe J. P., Patil C. K., Rodier F., et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biology . 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chu X., Wen J., Raju R. P. Rapid senescence-like response after acute injury. Aging Cell . 2020;19, article e13201 doi: 10.1111/acel.13201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Childs B. G., Gluscevic M., Baker D. J., et al. Senescent cells: an emerging target for diseases of ageing. Nature Reviews Drug Discovery . 2017;16:718–735. doi: 10.1038/nrd.2017.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Campisi J., Kapahi P., Lithgow G. J., Melov S., Newman J. C., Verdin E. From discoveries in ageing research to therapeutics for healthy ageing. Nature . 2019;571:183–192. doi: 10.1038/s41586-019-1365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wiley C. D., Campisi J. The metabolic roots of senescence: mechanisms and opportunities for intervention. Nature Metabolism . 2021;3(10):1290–1301. doi: 10.1038/s42255-021-00483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Collado M., Blasco M. A., Serrano M. Cellular senescence in cancer and aging. Cell . 2007;130(2):223–233. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 60.Franceschi C., Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences . 2014;69(Supplement 1):S4–S9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 61.Bottazzi B., Riboli E., Mantovani A. Aging, inflammation and cancer. Seminars in Immunology . 2018;40:74–82. doi: 10.1016/j.smim.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 62.Liu X., Mo W., Ye J., et al. Regulatory T cells trigger effector T cell DNA damage and senescence caused by metabolic competition. Nature Communications . 2018;9:p. 249. doi: 10.1038/s41467-017-02689-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Srdic-Rajic T., Santibanez J. F., Kanjer K., et al. Iscador Qu inhibits doxorubicin-induced senescence of MCF7 cells. Scientific Reports . 2017;7:p. 3763. doi: 10.1038/s41598-017-03898-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reyes J., Chen J.-Y., Stewart-Ornstein J., Karhohs K. W., Mock C. S., Lahav G. Fluctuations in p53 signaling allow escape from cell-cycle arrest. Molecular Cell . 2018;71 doi: 10.1016/j.molcel.2019.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hsu C.-H., Altschuler S. J., Wu L. F. Patterns of early p21 dynamics determine proliferation-senescence cell fate after chemotherapy. Cell . 2019;178 doi: 10.1016/j.cell.2019.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen Z., Trotman L. C., Shaffer D., et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature . 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burd C. E., Sorrentino J. A., Clark K. S., et al. Monitoring tumorigenesis and senescence in vivo with a p16(INK4a)-luciferase model. Cell . 2013;152:340–351. doi: 10.1016/j.cell.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Poropatich K., Fontanarosa J., Samant S., Sosman J. A., Zhang B. Cancer immunotherapies: are they as effective in the elderly? Drugs Aging . 2017;34:567–581. doi: 10.1007/s40266-017-0479-1. [DOI] [PubMed] [Google Scholar]
- 69.Ness K. K., Armstrong G. T., Kundu M., Wilson C. L., Tchkonia T., Kirkland J. L. Frailty in childhood cancer survivors. Cancer . 2015;121:1540–1547. doi: 10.1002/cncr.29211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Demaria M., O'Leary M. N., Chang J., et al. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discovery . 2017;7:165–176. doi: 10.1158/2159-8290.CD-16-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Borghesan M., Hoogaars W. M. H., Varela-Eirin M., Talma N., Demaria M. A Senescence-centric view of aging: implications for longevity and disease. Trends in Cell Biology . 2020;30(10):777–791. doi: 10.1016/j.tcb.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 72.Purohit S., Zhi W., Ferris D. G., et al. Senescence-associated secretory phenotype determines survival and therapeutic response in cervical cancer. Cancers (Basel) . 2020;12 doi: 10.3390/cancers12102899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fitsiou E., Soto-Gamez A., Demaria M. Biological functions of therapy-induced senescence in cancer. Seminars in Cancer Biology . 2021;81 doi: 10.1016/j.semcancer.2021.03.021. [DOI] [PubMed] [Google Scholar]
- 74.Tyrrell D. J., Goldstein D. R. Ageing and atherosclerosis: vascular intrinsic and extrinsic factors and potential role of IL-6. Nature Reviews Cardiology . 2021;18(1):58–68. doi: 10.1038/s41569-020-0431-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Uryga A. K., Bennett M. R. Ageing induced vascular smooth muscle cell senescence in atherosclerosis. The Journal of Physiology . 2016;594:2115–2124. doi: 10.1113/JP270923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rauscher F. M., Goldschmidt-Clermont P. J., Davis B. H., et al. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation . 2003;108:457–463. doi: 10.1161/01.CIR.0000082924.75945.48. [DOI] [PubMed] [Google Scholar]
- 77.Hoare M., Das T., Alexander G. Ageing, telomeres, senescence, and liver injury. Journal of Hepatology . 2010;53:950–961. doi: 10.1016/j.jhep.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 78.Sekoguchi S., Nakajima T., Moriguchi M., et al. Role of cell-cycle turnover and oxidative stress in telomere shortening and cellular senescence in patients with chronic hepatitis C. Journal of Gastroenterology and Hepatology . 2007;22(2):182–190. doi: 10.1111/j.1440-1746.2006.04454.x. [DOI] [PubMed] [Google Scholar]
- 79.Aravinthan A., Pietrosi G., Hoare M., et al. Hepatocyte expression of the senescence marker p21 is linked to fibrosis and an adverse liver-related outcome in alcohol-related liver disease. PLoS One . 2013;8(9, article e72904) doi: 10.1371/journal.pone.0072904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aravinthan A. D., Alexander G. J. M. Senescence in chronic liver disease: is the future in aging? Journal of Hepatology . 2016;65(4):825–834. doi: 10.1016/j.jhep.2016.05.030. [DOI] [PubMed] [Google Scholar]
- 81.Krizhanovsky V., Yon M., Dickins R. A., et al. Senescence of activated stellate cells limits liver fibrosis. Cell . 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nishizawa H., Iguchi G., Fukuoka H., et al. IGF-I induces senescence of hepatic stellate cells and limits fibrosis in a p53-dependent manner. Scientific Reports . 2016;6, article 34605 doi: 10.1038/srep34605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Niccoli T., Partridge L., Isaacs A. M. Ageing as a risk factor for ALS/FTD. Human Molecular Genetics . 2017;26:R105–R113. doi: 10.1093/hmg/ddx247. [DOI] [PubMed] [Google Scholar]
- 84.Xu D., Jin T., Zhu H., et al. TBK1 suppresses RIPK1-driven apoptosis and inflammation during development and in aging. Cell . 2018;174 doi: 10.1016/j.cell.2018.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang B., Wang L., Gasek N. S., et al. An inducible p21-Cre mouse model to monitor and manipulate p21-highly-expressing senescent cells in vivo. Nature Aging . 2021;1(10):962–973. doi: 10.1038/s43587-021-00107-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Segel M., Neumann B., Hill M. F. E., et al. Niche stiffness underlies the ageing of central nervous system progenitor cells. Nature . 2019;573(7772):130–134. doi: 10.1038/s41586-019-1484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang P., Kishimoto Y., Grammatikakis I., et al. Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer's disease model. Nature Neuroscience . 2019;22:719–728. doi: 10.1038/s41593-019-0372-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bussian T. J., Aziz A., Meyer C. F., Swenson B. L., van Deursen J. M., Baker D. J. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature . 2018;562:578–582. doi: 10.1038/s41586-018-0543-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Palmer A. K., Xu M., Zhu Y., et al. Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell . 2019;18(3, article e12950) doi: 10.1111/acel.12950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nieto-Vazquez I., Fernández-Veledo S., de Alvaro C., Lorenzo M. Dual role of interleukin-6 in regulating insulin sensitivity in murine skeletal muscle. Diabetes . 2008;57(12):3211–3221. doi: 10.2337/db07-1062. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 91.Gao D., Madi M., Ding C., et al. Interleukin-1β mediates macrophage-induced impairment of insulin signaling in human primary adipocytes. American Journal of Physiology-Endocrinology and Metabolism . 2014;307(3):E289–E304. doi: 10.1152/ajpendo.00430.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim Y. J., Hwang S. H., Lee S. Y., et al. miR-486-5p induces replicative senescence of human adipose tissue-derived mesenchymal stem cells and its expression is controlled by high glucose. Stem Cells and Development . 2012;21(10):1749–1760. doi: 10.1089/scd.2011.0429. [DOI] [PubMed] [Google Scholar]
- 93.Thompson P. J., Shah A., Ntranos V., Van Gool F., Atkinson M., Bhushan A. Targeted elimination of senescent beta cells prevents type 1 diabetes. Cell Metabolism . 2019;29(5) doi: 10.1016/j.cmet.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 94.Schafer M. J., White T. A., Iijima K., et al. Cellular senescence mediates fibrotic pulmonary disease. Nature Communications . 2017;8:p. 14532. doi: 10.1038/ncomms14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang L., Chen R., Li G., et al. FBW7 mediates senescence and pulmonary fibrosis through telomere uncapping. Cell Metabolism . 2020;32(5):860–877.e9. doi: 10.1016/j.cmet.2020.10.004. [DOI] [PubMed] [Google Scholar]
- 96.Numakura T., Sugiura H., Akaike T., et al. Production of reactive persulfide species in chronic obstructive pulmonary disease. Thorax . 2017;72(12):1074–1083. doi: 10.1136/thoraxjnl-2016-209359. [DOI] [PubMed] [Google Scholar]
- 97.Stanley S. E., Merck S. J., Armanios M. Telomerase and the genetics of emphysema susceptibility. Implications for pathogenesis paradigms and patient care. Annals of the American Thoracic Society . 2016;13(Supplement 5):S447–S451. doi: 10.1513/annalsats.201609-718aw. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gasek N. S., Kuchel G. A., Kirkland J. L., Xu M. Strategies for targeting senescent cells in human disease. Nature Aging . 2021;1(10):870–879. doi: 10.1038/s43587-021-00121-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McCulloch K., Litherland G. J., Rai T. S. Cellular senescence in osteoarthritis pathology. Aging Cell . 2017;16(2):210–218. doi: 10.1111/acel.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jeon O. H., Kim C., Laberge R.-M., et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nature Medicine . 2017;23(6):775–781. doi: 10.1038/nm.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Venturini W., Olate-Briones A., Valenzuela C., et al. Platelet activation is triggered by factors secreted by senescent endothelial HMEC-1 cells in vitro. International Journal of Molecular Sciences . 2020;21(9) doi: 10.3390/ijms21093287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aliper A., Jellen L., Cortese F., et al. Towards natural mimetics of metformin and rapamycin. Aging . 2017;9:2245–2268. doi: 10.18632/aging.101319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lutchman V., Dakik P., McAuley M., et al. Six plant extracts delay yeast chronological aging through different signaling pathways. Oncotarget . 2016;7:50845–50863. doi: 10.18632/oncotarget.10689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vaiserman A. M., Lushchak O. V., Koliada A. K. Anti-aging pharmacology: promises and pitfalls. Ageing Research Reviews . 2016;31 doi: 10.1016/j.arr.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 105.Zaid A. N., Al Ramahi R. Depigmentation and anti-aging treatment by natural molecules. Current Pharmaceutical Design . 2019;25:2292–2312. doi: 10.2174/1381612825666190703153730. [DOI] [PubMed] [Google Scholar]
- 106.Hayashi T., Matsui-Hirai H., Miyazaki-Akita A., et al. Endothelial cellular senescence is inhibited by nitric oxide: implications in atherosclerosis associated with menopause and diabetes. Proceedings of the National Academy of Sciences of the United States of America . 2006;103:17018–17023. doi: 10.1073/pnas.0607873103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang W., Zhang S., Yan P., et al. A single-cell transcriptomic landscape of primate arterial aging. Nature Communications . 2020;11(1):p. 2202. doi: 10.1038/s41467-020-15997-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhao L., Li Z., Vong J. S. L., et al. Pharmacologically reversible zonation-dependent endothelial cell transcriptomic changes with neurodegenerative disease associations in the aged brain. Nature Communications . 2020;11(1):p. 4413. doi: 10.1038/s41467-020-18249-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen Y., Pu Q., Ma Y., et al. Aging reprograms the hematopoietic-vascular niche to impede regeneration and promote fibrosis. Cell Metabolism . 2021;33(2):395–410.e4. doi: 10.1016/j.cmet.2020.11.019. [DOI] [PubMed] [Google Scholar]
- 110.Xu S., Ilyas I., Little P. J., et al. Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: from mechanism to pharmacotherapies. Pharmacological Reviews . 2021;73(3):924–967. doi: 10.1124/pharmrev.120.000096. [DOI] [PubMed] [Google Scholar]
- 111.Song Z., Liu Y., Hao B., et al. Ginsenoside Rb1 prevents H2O2-induced HUVEC senescence by stimulating sirtuin-1 pathway. PLoS One . 2014;9, article e112699 doi: 10.1371/journal.pone.0112699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liang Y., Li J., Lin Q., et al. Research progress on signaling pathway-associated oxidative stress in endothelial cells. Oxidative Medicine and Cellular Longevity . 2017;2017 doi: 10.1155/2017/7156941.7156941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kumar B., Kowluru A., Kowluru R. A. Lipotoxicity augments glucotoxicity-induced mitochondrial damage in the development of diabetic retinopathy. Investigative Opthalmology & Visual Science . 2015;56:2985–2992. doi: 10.1167/iovs.15-16466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Correia-Melo C., Marques F. D. M., Anderson R., et al. Mitochondria are required for pro-ageing features of the senescent phenotype. The EMBO Journal . 2016;35:724–742. doi: 10.15252/embj.201592862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhu Y., Tchkonia T., Pirtskhalava T., et al. The Achilles' heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell . 2015;14:644–658. doi: 10.1111/acel.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sun Y., Hu X., Hu G., Xu C., Jiang H. Curcumin attenuates hydrogen peroxide-induced premature senescence via the activation of SIRT1 in human umbilical vein endothelial cells. Biological & Pharmaceutical Bulletin . 2015;38:1134–1141. doi: 10.1248/bpb.b15-00012. [DOI] [PubMed] [Google Scholar]
- 117.Matacchione G., Gurău F., Silvestrini A., et al. Anti-SASP and anti-inflammatory activity of resveratrol, curcumin and β-caryophyllene association on human endothelial and monocytic cells. Biogerontology . 2021;22:297–313. doi: 10.1007/s10522-021-09915-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang X., Zhang J.-Q., Xiu C.-K., Yang J., Fang J.-Y., Lei Y. Ginseng-Sanqi-Chuanxiong (GSC) extracts ameliorate diabetes-induced endothelial cell senescence through regulating mitophagy via the AMPK pathway. Oxidative Medicine and Cellular Longevity . 2020;2020 doi: 10.1155/2020/7151946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Abbadie C., Pluquet O., Pourtier A. Epithelial cell senescence: an adaptive response to pre-carcinogenic stresses? Cellular and Molecular Life Sciences . 2017;74:4471–4509. doi: 10.1007/s00018-017-2587-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang B., Cui S., Bai X., et al. SIRT3 overexpression antagonizes high glucose accelerated cellular senescence in human diploid fibroblasts via the SIRT3-FOXO1 signaling pathway. Age . 2013;35:2237–2253. doi: 10.1007/s11357-013-9520-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lopez-Diazguerrero N. E., Lopez-Araiza H., Conde-Perezprina J. C., et al. Bcl-2 protects against oxidative stress while inducing premature senescence. Free Radical Biology and Medicine . 2006;40:1161–1169. doi: 10.1016/j.freeradbiomed.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 122.Chen L., Bi B., Zeng J., et al. Rosiglitazone ameliorates senescence-like phenotypes in a cellular photoaging model. Journal of Dermatological Science . 2015;77:173–181. doi: 10.1016/j.jdermsci.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 123.Lim H., Park B. K., Shin S. Y., Kwon Y. S., Kim H. P. Methyl caffeate and some plant constituents inhibit age-related inflammation: effects on senescence-associated secretory phenotype (SASP) formation. Archives of Pharmacal Research . 2017;40:524–535. doi: 10.1007/s12272-017-0909-y. [DOI] [PubMed] [Google Scholar]
- 124.Wang Y., Cui X., Lin Q., Cai J., Tang L., Liang Y. Active peptide KF-8 from rice bran attenuates oxidative stress in a mouse model of aging induced by d-galactose. Journal of Agricultural and Food Chemistry . 2020;68:12271–12283. doi: 10.1021/acs.jafc.0c04358. [DOI] [PubMed] [Google Scholar]
- 125.Chondrogianni N., Kapeta S., Chinou I., Vassilatou K., Papassideri I., Gonos E. S. Anti-ageing and rejuvenating effects of quercetin. Experimental Gerontology . 2010;45(10):763–771. doi: 10.1016/j.exger.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 126.Mukhopadhyay P., Prajapati A. K. Quercetin in anti-diabetic research and strategies for improved quercetin bioavailability using polymer-based carriers - a review. RSC Advances . 2015;5(118):97547–97562. doi: 10.1039/c5ra18896b. [DOI] [Google Scholar]
- 127.Birar V. C., Sheerin A. N., Ostler E. L., Faragher R. G. A. Novel resveratrol derivatives have diverse effects on the survival, proliferation and senescence of primary human fibroblasts. Biogerontology . 2020;21:817–826. doi: 10.1007/s10522-020-09896-6. [DOI] [PubMed] [Google Scholar]
- 128.Latorre E., Birar V. C., Sheerin A. N., et al. Small molecule modulation of splicing factor expression is associated with rescue from cellular senescence. BMC Cell Biology . 2017;18:p. 31. doi: 10.1186/s12860-017-0147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu M., Zhang P., Chen M., et al. Aging might increase myocardial ischemia/reperfusion-induced apoptosis in humans and rats. Age . 2012;34:621–632. doi: 10.1007/s11357-011-9259-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Andreux P. A., Blanco-Bose W., Ryu D., et al. The mitophagy activator urolithin A is safe and induces a molecular signature of improved mitochondrial and cellular health in humans. Nature Metabolism . 2019;1(6):595–603. doi: 10.1038/s42255-019-0073-4. [DOI] [PubMed] [Google Scholar]
- 131.Chang Y.-C., Liu H.-W., Chen Y.-T., Chen Y.-A., Chen Y.-J., Chang S.-J. Resveratrol protects muscle cells against palmitate-induced cellular senescence and insulin resistance through ameliorating autophagic flux. Journal of Food and Drug Analysis . 2018;26:1066–1074. doi: 10.1016/j.jfda.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Torregrosa-Muñumer R., Vara E., Fernández-Tresguerres J. Á., Gredilla R. Resveratrol supplementation at old age reverts changes associated with aging in inflammatory, oxidative and apoptotic markers in rat heart. European Journal of Nutrition . 2021;60(5):2683–2693. doi: 10.1007/s00394-020-02457-0. [DOI] [PubMed] [Google Scholar]
- 133.Li Y.-R., Li S., Lin C.-C. Effect of resveratrol and pterostilbene on aging and longevity. BioFactors . 2018;44(1):69–82. doi: 10.1002/biof.1400. [DOI] [PubMed] [Google Scholar]
- 134.Cosín-Tomàs M., Senserrich J., Arumí-Planas M., et al. Role of resveratrol and selenium on oxidative stress and expression of antioxidant and anti-aging genes in immortalized lymphocytes from Alzheimer's disease patients. Nutrients . 2019;11(8) doi: 10.3390/nu11081764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhou T., Yan Y., Zhao C., Xu Y., Wang Q., Xu N. Resveratrol improves osteogenic differentiation of senescent bone mesenchymal stem cells through inhibiting endogenous reactive oxygen species production AMPK activation. Redox Report: Communications in Free Radical Research . 2019;24(1):62–69. doi: 10.1080/13510002.2019.1658376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zou L. X., Chen C., Yan X., et al. Resveratrol attenuates pressure overload-induced cardiac fibrosis and diastolic dysfunction via PTEN/AKT/Smad 2/3 and NF-κB signaling pathways. Molecular Nutrition & Food Research . 2019;63(24, article e1900418) doi: 10.1002/mnfr.201900418. [DOI] [PubMed] [Google Scholar]
- 137.Baur J. A., Pearson K. J., Price N. L., et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature . 2006;444(7117):337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Redman L. M., Smith S. R., Burton J. H., Martin C. K., Il'yasova D., Ravussin E. Metabolic slowing and reduced oxidative damage with sustained caloric restriction support the rate of living and oxidative damage theories of aging. Cell Metabolism . 2018;27(4):805–815.e4. doi: 10.1016/j.cmet.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lam Y. Y., Peterson C. M., Ravussin E. Resveratrol vs. calorie restriction: data from rodents to humans. Experimental Gerontology . 2013;48(10):1018–1024. doi: 10.1016/j.exger.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 140.Zhang H., Li J., Ren J., et al. Single-nucleus transcriptomic landscape of primate hippocampal aging. Protein Cell . 2021;12(9):695–716. doi: 10.1007/s13238-021-00852-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Berg D. A., Su Y., Jimenez-Cyrus D., et al. A common embryonic origin of stem cells drives developmental and adult neurogenesis. Cell . 2019;177(3):654–668.e15. doi: 10.1016/j.cell.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kan Z., Wang Y., Chen Q., et al. Green tea suppresses amyloid β levels and alleviates cognitive impairment by inhibiting APP cleavage and preventing neurotoxicity in 5XFAD mice. Molecular Nutrition & Food Research . 2021;65(19, article e2100626) doi: 10.1002/mnfr.202100626. [DOI] [PubMed] [Google Scholar]
- 143.Stockinger J., Maxwell N., Shapiro D., de Cabo R., Valdez G. Caloric restriction mimetics slow aging of neuromuscular synapses and muscle fibers. The Journals of Gerontology: Series A . 2017;73(1):21–28. doi: 10.1093/gerona/glx023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ichwan M., Walker T. L., Nicola Z., et al. Apple peel and flesh contain pro-neurogenic compounds. Stem Cell Reports . 2021;16(3):548–565. doi: 10.1016/j.stemcr.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liao L., Shi J., Jiang C., et al. Activation of anti-oxidant of curcumin pyrazole derivatives through preservation of mitochondria function and Nrf2 signaling pathway. Neurochemistry International . 2019;125:82–90. doi: 10.1016/j.neuint.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 146.Xu J., Zhou L., Weng Q., Xiao L., Li Q. Curcumin analogues attenuate Aβ-induced oxidative stress in PC12 cells via Keap1/Nrf2/HO-1 signaling pathways. Chemico-Biological Interactions . 2019;305:171–179. doi: 10.1016/j.cbi.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 147.Liao V. H.-C., Yu C.-W., Chu Y.-J., Li W.-H., Hsieh Y.-C., Wang T.-T. Curcumin-mediated lifespan extension in Caenorhabditis elegans. Mechanisms of Ageing and Development . 2011;132(10):480–487. doi: 10.1016/j.mad.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 148.Azami S. H., Nazarian H., Abdollahifar M. A., Eini F., Farsani M. A., Novin M. G. The antioxidant curcumin postpones ovarian aging in young and middle-aged mice. Reproduction, Fertility and Development . 2020;32(3):292–303. doi: 10.1071/rd18472. [DOI] [PubMed] [Google Scholar]
- 149.Shen L.-R., Xiao F., Yuan P., et al. Curcumin-supplemented diets increase superoxide dismutase activity and mean lifespan in Drosophila. Age . 2013;35(4):1133–1142. doi: 10.1007/s11357-012-9438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Takano K., Tatebe J., Washizawa N., Morita T. Curcumin inhibits age-related vascular changes in aged mice fed a high-fat diet. Nutrients . 2018;10(10) doi: 10.3390/nu10101476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cui X., Lin Q., Liang Y. Plant-derived antioxidants protect the nervous system from aging by inhibiting oxidative stress. Frontiers in Aging Neuroscience . 2020;12:p. 209. doi: 10.3389/fnagi.2020.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Guo Y., Xing L., Chen N., Gao C., Ding Z., Jin B. Total flavonoids from the Carya cathayensis Sarg. leaves inhibit HUVEC senescence through the miR-34a/SIRT1 pathway. Journal of Cellular Biochemistry . 2019;120:17240–17249. doi: 10.1002/jcb.28986. [DOI] [PubMed] [Google Scholar]
- 153.Lee G.-H., Hoang T.-H., Jung E.-S., et al. Anthocyanins attenuate endothelial dysfunction through regulation of uncoupling of nitric oxide synthase in aged rats. Aging Cell . 2020;19, article e13279 doi: 10.1111/acel.13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tousian H., Razavi B. M., Hosseinzadeh H. Alpha-mangostin decreased cellular senescence in human umbilical vein endothelial cells. Daru: journal of Faculty of Pharmacy. Tehran University of Medical Sciences . 2020;28:45–55. doi: 10.1007/s40199-019-00305-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kim G. D. SIRT1-mediated protective effect of (Miq.) seem against high-glucose-induced senescence in human umbilical vein endothelial cells. Nutrients . 2019;11 doi: 10.3390/nu11112625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Guo Y., Fan W., Xie Y., Cao S., Wan H., Jin B. SIRT1 is the target gene for 2, 3, 5,4'-tetrahydroxystilbene-2-O-β-D-glucoside alleviating the HUVEC senescence. Frontiers in Pharmacology . 2020;11, article 542902 doi: 10.3389/fphar.2020.542902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hada Y., Uchida H. A., Otaka N., et al. The protective effect of chlorogenic acid on vascular senescence via the nrf2/ho-1 pathway. International Journal of Molecular Sciences . 2020;21 doi: 10.3390/ijms21124527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Du L., Chen E., Wu T., Ruan Y., Wu S. Resveratrol attenuates hydrogen peroxide-induced aging through upregulation of autophagy in human umbilical vein endothelial cells. Drug Design, Development and Therapy . 2019;13:747–755. doi: 10.2147/dddt.s179894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shi G., Liu D., Zhou B., et al. Ginsenoside Rb1 alleviates oxidative low-density lipoprotein-induced vascular endothelium senescence via the SIRT1/Beclin-1/autophagy axis. Journal of Cardiovascular Pharmacology . 2020;75:155–167. doi: 10.1097/fjc.0000000000000775. [DOI] [PubMed] [Google Scholar]
- 160.Guo F., Wu R., Xu J. Salicin prevents TNF-α-induced cellular senescence in human umbilical vein endothelial cells (HUVECs) Artificial Cells, Nanomedicine, and Biotechnology . 2019;47:2618–2623. doi: 10.1080/21691401.2019.1629949. [DOI] [PubMed] [Google Scholar]
- 161.Jiang Y.-H., Jiang L.-Y., Wang Y.-C., Ma D.-F., Li X. Quercetin attenuates atherosclerosis modulating oxidized LDL-induced endothelial cellular senescence. Frontiers in Pharmacology . 2020;11:p. 512. doi: 10.3389/fphar.2020.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Li Q., Bai D., Qin L., et al. Protective effect of L-hexaguluroic acid hexasodium salt on UVA-induced photo-aging in HaCaT cells. International Journal of Molecular Sciences . 2020;21 doi: 10.3390/ijms21041201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cocetta V., Cadau J., Saponaro M., et al. Further assessment of as an innovative strategy to counteract skin photo-aging and restore the barrier integrity. Aging . 2021;13 doi: 10.18632/aging.202464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kwon K.-R., Alam M. B., Park J.-H., Kim T.-H., Lee S.-H. Attenuation of UVB-induced photo-aging by polyphenolic-rich Spatholobus suberectus stem extract via modulation of MAPK/AP-1/MMPs signaling in human keratinocytes. Nutrients . 2019;11 doi: 10.3390/nu11061341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liu X., Zhang R., Shi H., et al. Protective effect of curcumin against ultraviolet A irradiationinduced photoaging in human dermal fibroblasts. Molecular Medicine Reports . 2018;17:7227–7237. doi: 10.3892/mmr.2018.8791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hwang E., Lin P., Ngo H. T. T., Yi T.-H. Clove attenuates UVB-induced photodamage and repairs skin barrier function in hairless mice. Food & Function . 2018;9:4936–4947. doi: 10.1039/c8fo00843d. [DOI] [PubMed] [Google Scholar]
- 167.Jeong J. H., Kim M. B., Kim C., Hwang J. K. Inhibitory effect of vitamin C on intrinsic aging in human dermal fibroblasts and hairless mice. Food Science and Biotechnology . 2018;27:555–564. doi: 10.1007/s10068-017-0252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zeng Q., Zhou F., Lei L., et al. Ganoderma lucidum polysaccharides protect fibroblasts against UVB-induced photoaging. Molecular Medicine Reports . 2017;15:111–116. doi: 10.3892/mmr.2016.6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Matos L., Gouveia A. M., Almeida H. Resveratrol attenuates copper-induced senescence by improving cellular proteostasis. Oxidative Medicine and Cellular Longevity . 2017;2017 doi: 10.1155/2017/3793817.3793817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mao G.-X., Xu X.-G., Wang S.-Y., et al. Salidroside delays cellular senescence by stimulating mitochondrial biogenesis partly through a miR-22/SIRT-1 pathway. Oxidative Medicine and Cellular Longevity . 2019;2019 doi: 10.1155/2019/5276096.5276096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dang Y., An Y., He J., et al. Berberine ameliorates cellular senescence and extends the lifespan of mice via regulating p16 and cyclin protein expression. Aging Cell . 2020;19, article e13060 doi: 10.1111/acel.13060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhu X., Yue H., Guo X., et al. The preconditioning of berberine suppresses hydrogen peroxide-induced premature senescence via regulation of sirtuin 1. Oxidative Medicine and Cellular Longevity . 2017;2017 doi: 10.1155/2017/2391820.2391820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Feng H., Mou S.-Q., Li W.-J., et al. Resveratrol inhibits ischemia-induced myocardial senescence signals and NLRP3 inflammasome activation. Oxidative Medicine and Cellular Longevity . 2020;2020 doi: 10.1155/2020/2647807.2647807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cheng X., Yao H., Xiang Y., et al. Effect of Angelica polysaccharide on brain senescence of Nestin-GFP mice induced by D-galactose. Neurochemistry International . 2019;122:149–156. doi: 10.1016/j.neuint.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 175.Xiang Y., Wang S.-H., Wang L., et al. Effects of ginsenoside Rg1 regulating Wnt/-catenin signaling on neural stem cells to delay brain senescence. Stem Cells International . 2019;2019 doi: 10.1155/2019/5010184.5010184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Liu Y., Liu Y., Guo Y., Xu L., Wang H. Phlorizin exerts potent effects against aging induced by D-galactose in mice and PC12 cells. Food & Function . 2021;12:2148–2160. doi: 10.1039/d0fo02707c. [DOI] [PubMed] [Google Scholar]
- 177.Tang D., Yan R., Sun Y., Kai G., Chen K., Li J. Material basis, effect, and mechanism of ethanol extract of Pinellia ternata tubers on oxidative stress-induced cell senescence. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology . 2020;77, article 153275 doi: 10.1016/j.phymed.2020.153275. [DOI] [PubMed] [Google Scholar]
- 178.Xia M.-L., Xie X.-H., Ding J.-H., Du R.-H., Hu G. Astragaloside IV inhibits astrocyte senescence: implication in Parkinson's disease. Journal of Neuroinflammation . 2020;17:p. 105. doi: 10.1186/s12974-020-01791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Feng M., Peng H., Yao R., et al. Inhibition of cellular communication network factor 1 (CCN1)-driven senescence slows down cartilage inflammaging and osteoarthritis. Bone . 2020;139, article 115522 doi: 10.1016/j.bone.2020.115522. [DOI] [PubMed] [Google Scholar]
- 180.Helal N. A., Eassa H. A., Amer A. M., Eltokhy M. A., Edafiogho I., Nounou M. I. Nutraceuticals' novel formulations: the good, the bad, the unknown and patents involved. Recent Patents on Drug Delivery & Formulation . 2019;13(2):105–156. doi: 10.2174/1872211313666190503112040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kirkland J. L., Tchkonia T., Zhu Y., Niedernhofer L. J., Robbins P. D. The clinical potential of senolytic drugs. Journal of the American Geriatrics Society . 2017;65(10):2297–2301. doi: 10.1111/jgs.14969. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study have been deposited in PubMed.


