Abstract
Background
COVID-19 convalescent plasma (CCP) is an experimental treatment against SARS-CoV-2. Although there has so far been no evidence of transmission through transfusion, pathogen reduction technologies (PRT) have been applied to CCP to mitigate risk of infectious disease. This study aims to assess the impact of methylene blue (MB) plus visible light PRT on the virus-neutralising activity of the specific antibodies against SARS-CoV-2.
Material and methods
Thirty-five plasma doses collected by plasmapheresis from COVID-19 convalescent donors were subjected to MB plus visible light PRT. Anti-SARS-CoV-2 RBD S1 epitope IgGs antibodies were quantified by ELISA. Titres of SARS-CoV-2 neutralising antibodies (NtAbs) were measured before and after the PRT process. A Spearman’s correlation was run to determine the relationship between antibody neutralisation ability and SARS-CoV-2 IgG ELISA ratio. Pre- and post-inactivation neutralising antibody titres were evaluated using a Wilcoxon test.
Results
The plasma pathogen reduction procedure did not diminish NtAbS titres and so did not cause a change in the viral neutralisation capacity of CCP. There was a strong correlation between pre-and post-PRT NtAbs and anti-SARS-CoV-2 IgGs titres.
Discussion
Our results showed PRT with MB did not impair the CCP passive immunity preserving its potential therapeutic potency. Therefore, PRT of CCP should be recommended to mitigate the risk for transmission of transfusion-associated infectious disease. There is a good correlation between SARS-CoV-2 IgG titres determined by ELISA and the neutralising capacity. This allows blood centres to select CCP donors based on IgG ELISA titres avoiding the much more labour-intensive laboratory processes for determining neutralising antibodies.
Keywords: fresh frozen plasma, COVID19, methylene blue, neutralising antibodies
INTRODUCTION
The coronavirus disease 2019 (COVID-19) pandemic has spread globally and caused high morbidity and mortality. The disease consists of a severe acute respiratory syndrome caused by the highly transmissible coronavirus 2 (SARS-CoV-2), first identified in Wuhan, People’s Republic of China, in 2019. Despite aggressive efforts and research, none of the therapies adopted so far have been proven to be effective1,2.
Coronaviruses are enveloped positive-sense single-stranded RNA viruses. Previous studies indicate that they are generally susceptible to acid, alkaline media, and heat3. The risk of transmission by transfusion was a concern for blood banks from the very beginning of the crisis4. After more than one year of pandemic evolution, the SARS-CoV-2 is not currently considered a high or moderate priority for blood transfusion safety.
Diagnosis of infection has largely been based on RT-PCR amplification of viral nucleic acid from upper respiratory tract swab tests. However, detection of viral RNA (vRNA) has also been reported in blood, serum, and plasma samples in a limited number of clinical cases5. The frequency and quantification of SARS-CoV-2 RNA in blood fractions, and the significance of blood transfusion as a route of transmission, remain unknown. Furthermore, there is an urgent need to consider whether the detection of viral RNA in blood samples reflects the presence of infectious virions, and its implications for transfusion safety5. On the other hand, it should be considered that, for SARS-Cov-2, virions have never been isolated in reactive RNA blood donor samples. The study of Chang et al. reported 3 samples out of 7,425 healthy blood donors (0.04%) containing viral RNA6. Though emerging viruses may pose a potential threat to transfusion safety, there is no evidence to date that SARS-CoV-2 has been transmitted through the transfusion of blood products. However, the fact that the mortality rate is high (infection fatality rates range from 0.00 to 1.63%7) and that there is a universal transmission requires an increase in awareness of the potential threat for blood safety.
Few treatment options are available when a novel virus first emerges, but convalescent plasma (CP) may be the only therapeutic approach until other treatments are developed. It consists of the infusion of a virus-specific-antibody-rich plasma obtained from patients who have recovered from the disease, aiming to provide passive immunity. Passive antibody transfer dates back to the 1890s with varying degrees of success as a treatment for severe infectious diseases8–11. Convalescent plasma was used effectively during the 1918 influenza pandemic10. More recently, it has been adopted as an initial approach for several emerging infectious disease (EID) outbreaks, such as severe acute respiratory syndrome (SARS)12, the H1N1 influenza virus pandemic (in 2009–2010)13, the Middle East respiratory syndrome (MERS)14, the H5N1 and H7N9 avian fluoutbreaks15, the West African Ebola epidemic (in 2013), and other viral haemorrhagic fevers (Bolivian haemorrhagic fever, Lassa fever, and the Argentine haemorrhagic fever)16,17. In 2014, a meta-analysis concluded that CP may reduce mortality and should be studied as a treatment for MERS coronavirus infection18. COVID-19 convalescent plasma (CCP) therapy has been demonstrated to be a safe option with minor side effects, although controlled clinical efficacy data are only just beginning to come in and some of them are controversial. The transmission of different infectious diseases through the transfusion of plasma represents a real risk, particularly in regions with a high prevalence of transmissible diseases; pathogen reduction technologies can mitigate this kind of risk8.
Currently, there are three different pathogen reduction technologies (PRT) available that may be effective in reducing the infectious pathogen load of bacteria, viruses, and parasites of plasma components. The amotosalen and UVA based technology (Intercept® Blood System, Cerus, Concord, CA, USA)19, the riboflavin plus UV (e.g., Mirasol® PRT, Terumo BCT Europe N.V., Leuven, Belgium)20, and methylene blue (MB)/visible light21–23.
Recently a PRT system based on methylene blue plus visible light has been demonstrated to be effective in inactivating SARS-CoV-224–26, with a reduction in virus titre of up to 4.5 log10 TCID50/mL24.
The THERAFLEX MB (TMB)-Plasma (Macopharma, Mouvaux, France) is a photodynamic pathogen reduction system for the treatment of plasma. Plasma units derived from single blood donations are illuminated with visible light in the presence of the phenothiazine dye methylene blue (MB). When plasma is MB/light-treated, singlet oxygen is generated, which leads to the destruction of viral nucleic acids. The MB/light-based method has been in routine use in Europe for more than 20 years27. For CCP, it is essential to preserve the antibody function after pathogen reduction procedures. It is known that MB/light treatment can damage some labile plasma proteins (e.g., plasma factor VIII and fibrinogen) while maintaining the functionality and life span of other proteins in fresh frozen plasma22,26,28,29. Taking this effect into account, it may be possible to observe a deleterious effect of the pathogen reduction techniques on the effectiveness of CCP after inactivation. However, data regarding the effect of pathogen-inactivation methods on the functionality of SARS-CoV-2 neutralising antibodies are scarce.
The objective of this study was to evaluate the effect of methylene blue and light pathogen reduction technology on the functional properties of CCP, investigating if antibody-binding affinity in human plasma is affected by the TMB plasma treatment. For this purpose, the neutralising ability of anti-SARS-CoV-2 antibodies was tested before and after MB/light treatment. We also checked the correlation between SARS-CoV-2 IgGs recognising the spike (S) protein receptor-binding domain (RBD), and NtAb to use an ELISA test as a surrogate marker for NtAb.
MATERIALS AND METHODS
COVID-19 convalescent plasma collection
Each donor had a documented history of laboratory-confirmed SARS-CoV-2 infection based on a positive RT-PCR test result. All plasma was donated by recovered and healthy COVID-19 patients who had been asymptomatic for >28 days. Donors were between 18 and 65 years old. All donors provided written informed consent and had tested negative for SARS-CoV-2 by RT-PCR. If eligible according to standard blood donor criteria, donors were enrolled in an intensive plasmapheresis programme. Donors were male without previous transfusion history and were negative for hepatitis B virus, hepatitis C virus, HIV, Chagas disease, and syphilis, as per standard blood bank practices.
CCP was obtained by apheresis using the Aurora Plasmapheresis System (Fresenius Kabi Bad Homburg, Germany/Fresenius, Lake Zurich, IL, USA). Plasma (650 mL) was collected from each donor and divided into two 325 mL units.
Anti-SARS-CoV-2 ELISA
Donors’ peripheral venous blood was also screened before plasmapheresis for anti-SARS-CoV-2 IgG antibodies. Anti-SARS-CoV-2 RBD S1 epitope IgGs antibodies were quantified by ELISA (Euroimmun, Lübeck, Germany). This is a semi-quantitative method where results are expressed as a ratio, calculated by dividing the optical densities of the sample by the cut-off (S/CO). The cut-off for samples to be considered positive was ≥1.1 and borderline positive from 0.8 and 1.09 the S/CO ratio was considered as a titre.
Anti-SARS-CoV-2 neutralising antibody assay
The neutralisation capacity of circulating antibodies against the spike protein of SARS-CoV-2 was assessed using a vesicular stomatitis virus pseudotyped with the SARS-CoV-2 spike protein (VSV-S). Experiments were performed as previously described30 with the exception that the spike sequence carried the D614G mutation, and the assay was performed in A549 ACE2 TMPRSS2 cells (InvivoGen catalog code a549-hace2tps). All tests were carried out in duplicate using 5-fold serum dilutions ranging from 1:20 to 1:12,500. The reciprocal of the antibody dilution resulting in 50% virus neutralisation was calculated using the DRC package (version 3.0-1) in R via a two-parameter log-logistic regression model (LL.2 model) and considered as the NtAbs’ titre. Pre- and post-TMB treatment samples were tested for neutralising antibody (NtAb) titre retention.
Methylene blue and visible light pathogen reduction treatment
CCP units were treated using the THERAFLEX MB-Plasma MB + visible light PRT system as previously described29. Briefly, CCP units were transferred to two illumination bags (THERAFLEX MB-Plasma bags [Ref. SDV0001XU]) utilising a sterile connection device (Terumo TSCD II). The THERAFLEX MB-Plasma system uses a 0.65 μm membrane filter (Plasmaflex PLAS4; MacoPharma) which removes residual leukocytes, red cells, platelets, and aggregates. The filtered plasma then flows through a dry pill of 85 μg anhydrous MB chloride which is integrated into the bag system providing an approximate final concentration of 1 μmol/L for a volume of plasma between 235 and 315 mL. The illumination is achieved by a microprocessor-controlled device under Good Manufacturer Practice (GMP) controlled conditions where illumination dose, intensity, and temperature are monitored and exposed to the required light dose of 180 J/cm2 of energy. After treatment, over 90% of the residual MB combined with its photo-activated products are removed by a specially designed filter (Blue-flex; MacoPharma). Thus, plasma is filtered twice, resulting in virtually cell-free plasma29.
Samples for analysis were taken before connection to the PRT system (Pre-Treat), and after MB removal (Post-Treat). Sample aliquots were stored frozen (≤ −79°C) in cryovials until testing.
Statistical analysis
CCP units were analysed for anti-SARS-CoV-2 IgG antibodies, and SARS-CoV-2 antibody neutralising activity (Pre-Treat and Post-Treat). Descriptive statistics including the mean and standard deviation were calculated for all continuous parameters.
To analyse the normality of continuous variables, we used the Shapiro-Wilk test. Data sets exhibiting a non-normal distribution were evaluated non-parametrically using a Wilcoxon matched-pairs signed-rank test. The Spearman rank-order correlation coefficient was used to assess the relationship between continuous variables using the entire dataset. p<0.05 was considered statistically significant. Statistical analysis was performed using SPSS v.20.0 software (SPSS, Chicago, IL, USA).
RESULTS
Twenty-nine donors provided 35 CCP units. Five donors gave plasma on multiple occasions (4 of them on two and 1 of them on three occasions). The donors ranged in age from 20 to 65 years with a median age of 49.44 years, and all of them were males. ABO blood type distribution is shown in Table I.
Table I.
Blood type distribution
| Blood type | N | % |
|---|---|---|
| O | 10 | 34.5 |
| A | 16 | 55.2 |
| B | 1 | 3.4 |
| AB | 2 | 6.9 |
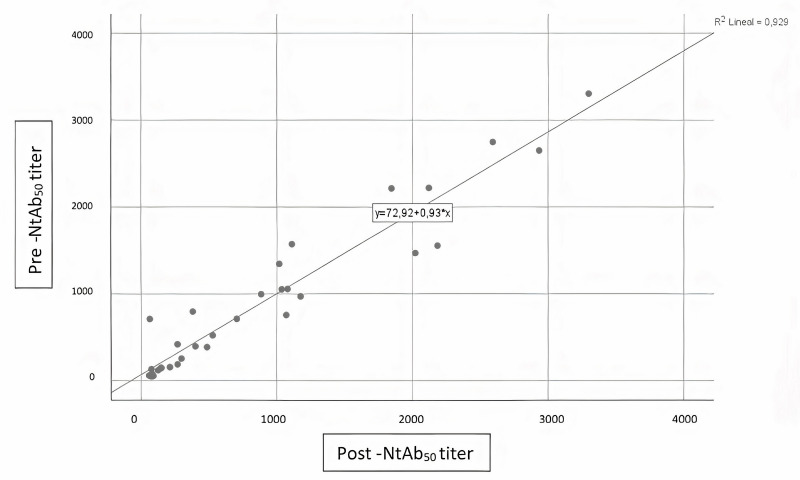
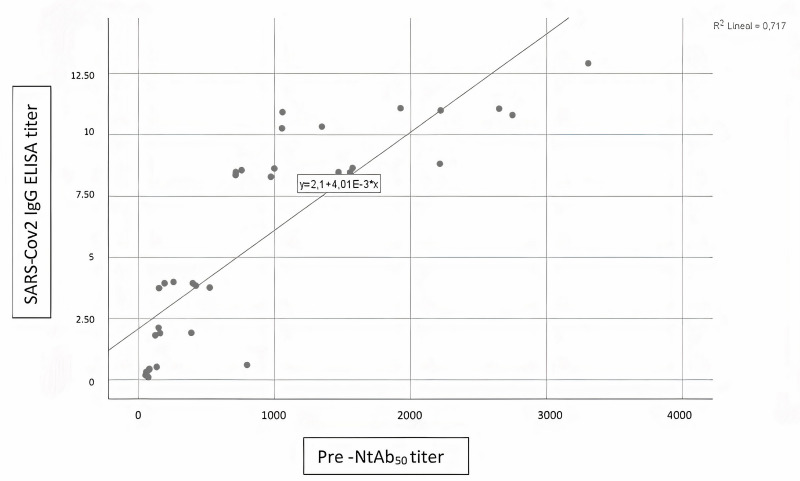
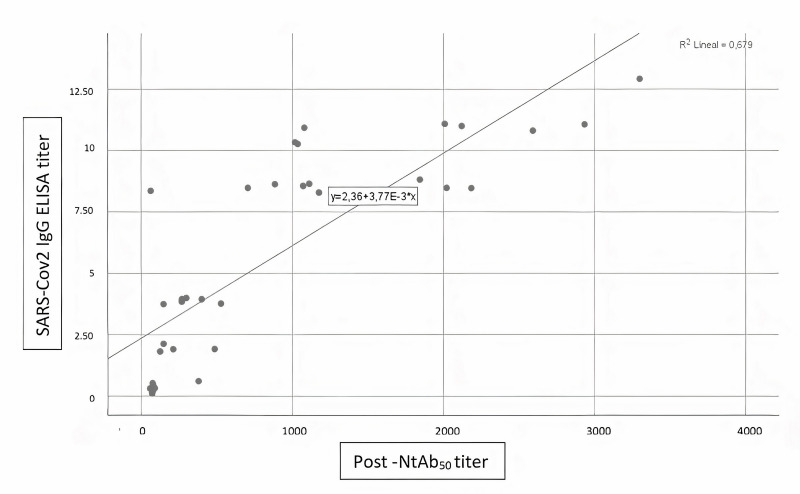
No influence of THERAFLEX MB-Plasma treatment on reciprocal antibody dilution resulting in 50% virus neutralisation (NtAb50) was found; titres before and after TMB treatment did not change (Table II). A Wilcoxon signed-rank test showed that plasma pathogen reduction procedure with TMB did not elicit a statistically significant change in neutralisation capacity of circulating antibodies against the spike protein of SARS-CoV-2 (Z= −0.16, p=0.987). A Spearman’s rank-order correlation was run to determine the relationship between antibody neutralisation ability and SARS-CoV-2 IgG ELISA ratio. There was a strong, positive correlation between neutralisation ability Pre-Treat and SARS-CoV-2 IgG ELISA ratio (rs[8]=0.929, p=0.000), between neutralisation ability Post-Treat and SARS-CoV-2 IgG ELISA ratio (rs[8]=0.867, p=0.000), and between neutralisation ability Pre-Treat and neutralisation ability Post-Treat (rs[8]=0.927, p=0.000); all these relationships were statistically significant (see Figures 1–3).
Table II.
Influence of treatment with the THERAFLEX MB-Plasma system on results
| N | Mean ± SD | Min. | Max. | Median | |
|---|---|---|---|---|---|
| Pre-Treat | 35 | 895.4 ± 894.8 | 50.5 | 3305.4 | 712.0 |
| Post-Treat | 35 | 883.2 ± 926.1 | 57.3 | 3297.7 | 485.5 |
Figure 1.
Correlation between pre- and post-inactivation antibody neutralisation ability
Figure 2.
Correlation between SARS-CoV-2 IgG ELISA ratio and pre-inactivation antibody neutralisation ability
Figure 3.
Correlation between SARS-CoV-2 IgG ELISA ratio and postinactivation antibody neutralisation ability
DISCUSSION
Convalescent plasma therapy has been used as a rapid and effective treatment of severe EID for more than 100 years. In 1890, CP was shown to neutralise the bacillus toxicity of tetanus injected into the body, and the earliest CP therapy allowed humans to overcome diphtheria31. In modern times, it has been used during several Ebola virus disease outbreaks17,32, SARS coronavirus12, and many other infectious diseases33–37. Given this long history of serum/plasma-based treatment, and its established safety and efficacy, convalescent plasma transfusion was considered as a first-line therapeutic approach for COVID-19 patients.
Transfusion safety remains a top priority and studies are needed to determine whether SARS-CoV-2 can be transmitted through transfusion. One of the reasons for using pathogen reduction technologies of blood components is to be proactive by providing general protection against emerging and re-emerging infectious agents which constitute a continuous challenge to the safety of the blood supply. Conversely, the conventional reactive approach, which is to wait until screening programmes are implemented, takes time and cannot therefore provide the much needed rapid response. Manufacturers of pathogen reduction methods are required to continuously test the inactivation capacity of their systems for new infectious agents27. In this sense, the efficacy of the TMB pathogen inactivation system for coronaviruses was previously demonstrated with SARS-CoV and MERS-CoV27,38. According to Jin et al., methylene blue treatment plus light can reduce the 4.5 log10 TCID50/mL in 2 minutes for the new coronavirus SARS-CoV-224.
In case SARS-CoV-2 is transfusion transmissible, its threshold concentration to elicit disease must be determined in order to assess the PRT capacity in preventing transmission. Nevertheless, the log reduction factor achieved by the THERAFLEX MB-Plasma may effectively reduce the potential risk of transmission of SARS-CoV-224. In the study of Raster et al., it was shown that pathogen reduction of plasma with MB treatment did not affect the IgM and IgG binding to their cognitive epitopes, or IgG binding to Fc receptors. This could be due to the low affinity of MB to neutral macromolecules like immunoglobulins. The authors concluded that preservation of the immunoglobulin function is key for the use of MB-treated CP for the treatment of infections, such as COVID-1926. However, the latter study did not test the preservation of the neutralising capacity of anti-SARS-CoV-2 antibodies, so they could not make definite assumptions on an eventual change in the effectiveness of pathogen-reduced CCP.
Our study evaluates the effect of MB treatment on the functional properties of CCP by measuring neutralising antibody activity pre- and post-PRT. Our results show good preservation of neutralising antibody titres, which remain the same after PRT. With these data, we can assume that CCP-TMB treatment would be as effective as non-PRT CCP. The stability of the antibodies demonstrated in our study is consistent with previous assessments of antibody function in PRT-treated plasma39. Our results are in line with the conclusions of Kostin et al. This study highlights that, of the three currently available PRTs, MB is that which best preserves the neutralising function, with 81% of the units remaining unchanged in terms of SARS-CoV-2 neutralising antibodies titres40.
On the other hand, we observed a good correlation between SARS-CoV-2 IgG titres determined by ELISA and the neutralising capacity. This observation has already been reported with other commercial immunoassays and the plaque reduction neutralisation test, concluding it may constitute a surrogate method to evaluate NtAb titres41. This correlation is important because it allows blood centres to select CCP donors based on IgG ELISA titre, thus avoiding the much more labour-intensive laboratory processes for determining neutralising antibodies. However, it must be said, that the level of anti-SARS-CoV-2 IgG antibodies could have been evaluated with other methods capable of detecting and quantifying them, and then converted to international standard NIBSC code 20/136 to allow comparison between different analytical methods.
CONCLUSIONS
Our results showed that PRT with MB did not impair the CCP passive immunity preserving its potential therapeutic potency. Therefore, PRT of CCP should be recommended to mitigate the risk for transmission of transfusion-associated infectious disease.
ACKNOWLEDGEMENTS
The Authors thank the medical and laboratory staff of the Transfusion Center of the Valencian Community (Centro de Transfusión de la Comunidad Valenciana) without whose assistance the study would not have been possible.
Footnotes
AUTHORSHIP CONTRIBUTIONS
LL wrote the initial draft, contributed to the preparation of the manuscript, and wrote and submitted the manuscript; EC reviewed the initial draft, contributed to the manuscript preparation and wrote the manuscript; LN and BV collected data and contributed to the preparation of the manuscript; CF and BS carried out the experimental work in the laboratory; AG, EC and MC collected the data; MV and VM contributed to the preparation of the manuscript; VC, MIO and RR reviewed the initial draft and contributed to the preparation of the manuscript; RG supervised the experimental work in laboratory and contributed to the preparation of the manuscript; CA reviewed the initial draft, contributed to the preparation of the manuscript, and wrote the manuscript.
The Authors declare no conflicts of interest.
REFERENCES
- 1.Salazar E, Perez KK, Ashraf M, et al. Treatment of Coronavirus Disease 2019 (COVID-19) patients with convalescent plasma. Am J Pathol. 2020;190:1680–90. doi: 10.1016/j.ajpath.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for Coronavirus Disease 2019 (COVID-19): a review. JAMA. 2020;323:1824–36. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 3.Rabenau HF, Cinatl J, Morgenstern B, et al. Stability and inactivation of SARS coronavirus. Med Microbiol Immunol. 2005;194:1–6. doi: 10.1007/s00430-004-0219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eickmann M, Gravemann U, Handke W, et al. Inactivation of three emerging viruses - severe acute respiratory syndrome coronavirus, Crimean–Congo haemorrhagic fever virus and Nipah virus - in platelet concentrates by ultraviolet C light and in plasma by methylene blue plus visible light. Vox Sang. 2020;115:146–51. doi: 10.1111/vox.12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersson MI, Arancibia-Carcamo Cv, Auckland K, et al. SARS-CoV-2 RNA detected in blood products from patients with COVID-19 is not associated with infectious virus. Wellcome Open Res. 2020;5:181. doi: 10.12688/wellcomeopenres.16002.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang L, Zhao L, Gong H, et al. Severe acute respiratory syndrome coronavirus 2 RNA detected in blood donations. Emerg Infect Dis. 2020;26:1631–3. doi: 10.3201/eid2607.200839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ioannidis JPA. Infection fatality rate of COVID-19 inferred from seroprevalence data. Bull World Health Org. 2021;99:19–33F. doi: 10.2471/BLT.20.265892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yonemura S, Hartson L, Dutt T, et al. Preservation of neutralizing antibody function in COVID-19 convalescent plasma treated using a riboflavin and ultraviolet light-based pathogen reduction technology. Vox Sang. 2021;116:1076–83. doi: 10.1111/vox.13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zingher A, Mortimer P. Convalescent whole blood, plasma and serum in the prophylaxis of measles: JAMA, 12 April, 1926; 1180–1187. Rev Med Virol. 2005;15:407–18. doi: 10.1002/rmv.480. discussion 418–21. [DOI] [PubMed] [Google Scholar]
- 10.Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med. 2006;145:599–609. doi: 10.7326/0003-4819-145-8-200610170-00139. [DOI] [PubMed] [Google Scholar]
- 11.Degkwitz R. Uber Masern-Rekonvaleszentenserum. Z Kinder-Heilk. 1920;27:171–94. [In German.] [Google Scholar]
- 12.Cheng Y, Wong R, Soo YOY, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24:44–6. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hung IFN, To KKW, Lee CK, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52:447–56. doi: 10.1093/cid/ciq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arabi YM, Hajeer AH, Luke T, et al. Feasibility of using convalescent plasma immunotherapy for MERS-CoV infection, Saudi Arabia. Emerg Infect Dis. 2016;22:1554–61. doi: 10.3201/eid2209.151164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J, Zhu H, Horby PW, et al. Specificity, kinetics and longevity of antibody responses to avian influenza A(H7N9) virus infection in humans. J Infect. 2020;80:310–9. doi: 10.1016/j.jinf.2019.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown BL, McCullough J. Treatment for emerging viruses: convalescent plasma and COVID-19. Transfus Apher Sci. 2020;59:102790. doi: 10.1016/j.transci.2020.102790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burnouf T, Seghatchian J. Ebola virus convalescent blood products: Where we are now and where we may need to go. Transfus Apher Sci. 2014;51:120–5. doi: 10.1016/j.transci.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mair-Jenkins J, Saavedra-Campos M, Baillie JK, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bubinski M, Gronowska A, Szykula P, et al. Plasma pooling in combination with amotosalen/UVA pathogen inactivation to increase standardisation and safety of therapeutic plasma units. Transfus Med. 2021;31:136–41. doi: 10.1111/tme.12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corbin Frank., 3rd Pathogen Inactivation of Blood Components: Current Status and Introduction of an Approach Using Riboflavin as a Photosensitizer. Int J Hematol. 2002;76(Suppl 2):253–7. doi: 10.1007/BF03165125. [DOI] [PubMed] [Google Scholar]
- 21.Lozano M, Cid J, Müller TH. Plasma treated with methylene blue and light: Clinical efficacy and safety profile. Transfus Med Rev. 2013;27:235–40. doi: 10.1016/j.tmrv.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Larrea L, Ortiz-de-Salazar M-I, Martínez P, Roig R. Quantitative analysis of plasma proteins in whole blood-derived fresh frozen plasma prepared with three pathogen reduction technologies. Transfus Apher Sci. 2015;52:305–310. doi: 10.1016/j.transci.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Gironés N, Bueno JL, Carrión J, et al. The efficacy of photochemical treatment with methylene blue and light for the reduction of Trypanosoma cruzi in infected plasma. Vox Sang. 2006;91:285–91. doi: 10.1111/j.1423-0410.2006.00840.x. [DOI] [PubMed] [Google Scholar]
- 24.Jin C, Yu B, Zhang J, et al. Methylene blue photochemical treatment as a reliable SARS-CoV-2 plasma virus inactivation method for blood safety and convalescent plasma therapy for the COVID-19 outbreak. BMC Infect Dis. 2021;21:357–65. doi: 10.1186/s12879-021-05993-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Strakhovskaya MG, Meerovich GA, Kuskov AN, et al. Photoinactivation of coronaviruses: going along the optical spectrum. Laser Phys Lett. 2020;17:093001. [Google Scholar]
- 26.Raster J, Zimmermann K, Wesche J, et al. Effect of methylene blue pathogen inactivation on the integrity of immunoglobulin M and G. Transfus Med Hemother. 2021;48:148–52. doi: 10.1159/000514485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eickmann M, Gravemann U, Handke W, et al. Inactivation of Ebola virus and Middle East respiratory syndrome coronavirus in platelet concentrates and plasma by ultraviolet C light and methylene blue plus visible light, respectively. Transfusion. 2018;58:2202–7. doi: 10.1111/trf.14652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Handke W, Gravemann U, Sumian C, et al. Theraflex MB-plasma treatment does not interfere with the antibody integrity in human plasma. Vox Sang. 2015;109:194–5. [Google Scholar]
- 29.Seghatchian J, Struff WG, Reichenberg S. Main properties of the THERAFLEX MB-plasma system for pathogen reduction. Transfus Med Hemother. 2011;38:55–64. doi: 10.1159/000323786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gozalbo-Rovira R, Gimenez E, Latorre V, et al. SARS-CoV-2 antibodies, serum inflammatory biomarkers and clinical severity of hospitalized COVID-19 patients. J Clin Virol. 2020;132:104635. doi: 10.1016/j.jcv.2020.104611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hifumi T, Yamamoto A, Ato M, et al. Clinical serum therapy: Benefits, cautions, and potential applications. Keio J Med. 2017;66:57–64. doi: 10.2302/kjm.2016-0017-IR. [DOI] [PubMed] [Google Scholar]
- 32.van Griensven J, Edwards T, de Lamballerie X, et al. Evaluation of convalescent plasma for Ebola virus disease in Guinea. N Engl J Med. 2016;374:33–42. doi: 10.1056/NEJMoa1511812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marson P, Cozza A, de Silvestro G. The true historical origin of convalescent plasma therapy. Transfus Apher Sci. 2020;59:102847. doi: 10.1016/j.transci.2020.102847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marano G, Vaglio S, Pupella S, et al. Convalescent plasma: new evidence for an old therapeutic tool? Blood Transfus. 2016;14:152–7. doi: 10.2450/2015.0131-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roback JD, Guarner J. Convalescent plasma to treat COVID-19: possibilities and challenges. JAMA. 2020;323:1561–2. doi: 10.1001/jama.2020.4940. [DOI] [PubMed] [Google Scholar]
- 36.Xu Z, Zhou J, Huang Y, et al. Efficacy of convalescent plasma for the treatment of severe influenza. Crit Care. 2020;24:469. doi: 10.1186/s13054-020-03189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong H, Lee C. Pivotal role of convalescent plasma in managing emerging infectious diseases. Vox Sang. 2020;115:545–7. doi: 10.1111/vox.12927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eickmann M, Gravemann U, Handke W, et al. Inactivation of three emerging viruses-severe acute respiratory syndrome coronavirus, Crimean-Congo haemorrhagic fever virus and Nipah virus-in platelet concentrates by ultraviolet C light and in plasma by methylene blue plus visible light. Vox Sang. 2020;115:146–51. doi: 10.1111/vox.12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tonn T, Corman VM, Johnsen M, et al. Stability and neutralising capacity of SARS-CoV-2-specific antibodies in convalescent plasma. Lancet Microbe. 2020;1:e63–e63. doi: 10.1016/S2666-5247(20)30037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kostin AI, Lundgren MN, Bulanov AY, et al. Impact of pathogen reduction methods on immunological properties of the COVID-19 convalescent plasma. Vox Sang. 2021;116:665–72. doi: 10.1111/vox.13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Padoan A, Bonfante F, Cosma C, et al. Analytical and clinical performances of a SARS-CoV-2 S-RBD IgG assay: comparison with neutralisation titers. Clin Chem Lab Med. 2021;59:1444–52. doi: 10.1515/cclm-2021-0313. [DOI] [PubMed] [Google Scholar]