Abstract
Background
Acquired haemophilia A (AHA) is a rare bleeding disorder due to autoantibodies to coagulation factor VIII that may be secondary to autoimmune diseases, cancer, drugs, pregnancy, infections, or be idiopathic. Recurrent bleeding, often severe, mostly in muscles and soft tissues, and isolated prolonged activated partial thromboplastin time (aPTT), in the absence of personal and family history of bleeding, are typical features that should raise the suspicion of AHA. Poor awareness of the disease results in diagnostic delays and inappropriate treatment.
Materials and methods
The Italian Association of Haemophilia Centres (AICE) developed consensus recommendations in cooperation with the Italian Society on Thrombosis and Haemostasis (SISET). The document was shared with scientific societies of specialist physicians, laboratory professionals and pharmacists to spread knowledge about AHA and promote appropriate diagnosis/treatment.
Results
Ready availability of the aPTT mixing test is crucial, although diagnostic confirmation and optimal management require prompt referral of patients to specialised centres with rapidly available diagnostic and therapeutic facilities. If immediate referral is unfeasible, treatment must be undertaken early, under guidance of specialised centres or based on shared protocols. Recommendations about diagnosis, general management and, in bleeding patients, haemostatic therapy using bypassing agents or replacement treatment, including the recently available recombinant porcine factor VIII, are provided, considering the different clinical settings and laboratory facilities.
Discussion
This consensus document aims to improve the overall healthcare pathways for AHA, harmonise the management and therapeutic approaches to newly diagnosed patients and reduce the still relevant complications and mortality in this setting.
Keywords: acquired haemophilia A, bleeding, coagulation tests, haemostatic treatment, inhibitors
INTRODUCTION
Acquired haemophilia A (AHA) is a rare bleeding disorder due to the development of autoantibodies (inhibitors), which neutralise the activity of coagulation factor VIII (FVIII) and/or accelerate its clearance1. These autoantibodies, mainly belonging to the IgG1 and IgG4 subclasses, are polyclonal and recognise epitopes in domains A2, A3 and C2 of the FVIII molecule, interfering with its interaction with factor IX (FIX), phospholipids and von Willebrand factor (VWF)2.
As knowledge and awareness about AHA are poor among general practitioners and many specialist physicians, the diagnosis is often missed or delayed, leading to challenging management and an underestimated incidence. The recognition of patients with AHA is also complicated by the heterogeneous clinical presentation, ranging from severe, sudden-onset and potentially life-threatening bleeding to paucisymptomatic cases. Some patients with minor, often recurrent bleeding (bruising, superficial haematomas) are neglected, without appropriate diagnostic workup. Comorbidities associated with bleeding risk, particularly in older patients–those most frequently affected by AHA– and/or concomitant antithrombotic therapies can result in misdiagnosis of bleeding symptoms and delay the recognition of AHA. In addition, bleeding complications may occur during invasive procedures performed to control bleeding before AHA is diagnosed.
All the above-mentioned factors contribute to the still high mortality of subjects with AHA, 3–15% in the most recent studies and registries3–5, but even higher than 40% in the past2. Bleeding events account for about 10% of deaths of AHA patients in the largest available registries3,4, whereas the main causes of mortality are complications of the immunosuppressive treatment for inhibitor eradication (primarily infections and sepsis) and the associated diseases responsible for the autoimmune coagulopathy4. The management of AHA is therefore troublesome, with challenges from the diagnostic phase to the general therapeutic approach, the specific treatment of bleeding and patient monitoring. All these stages require a multidisciplinary approach, with close synergy between professionals with different specialist skills, as well as the referral of patients as soon as possible to centres specialised in the diagnosis and treatment of haemophilia and other coagulopathies, as recommended by expert panels and scientific societies of haemophilia specialists6–8. Nevertheless, it is crucial to spread knowledge about AHA and raise awareness among all medical professionals. Indeed, patients are frequently first seen by their general practitioners or in Emergency Departments, and evaluated by internists, geriatricians, rheumatologists, oncologists, gynaecologists and other specialists, who often lack expertise in the diagnosis and management of bleeding disorders. At the same time, multidisciplinary networks should be established and ideally institutionalised, with shared management protocols. This should enable newly diagnosed AHA patients to be referred quickly to specialised centres, or to be managed in close collaboration with such centres, to provide prompt and appropriate treatment. However, according to a recent international expert consensus, even non-expert physicians should be able to begin haemostatic treatment in bleeding patients with AHA, by referring to national recommendations or local protocols or to the literature9.
With this background, the present document aims to provide all involved professionals with key information for the rapid recognition of patients with suspected AHA, the confirmation of the laboratory diagnosis and the haemostatic treatment.
MATERIALS AND METHODS
The Italian Association of Haemophilia Centres (AICE) issued recommendations on the diagnosis and treatment of acquired coagulation inhibitors in 20147, which mainly refer to AHA. Clinical studies published in the last few years, the introduction of new therapeutic options and the recent update10 of the international recommendations for the diagnosis and treatment of AHA6, prompted the ad hoc AICE Working Group to update the 2014 document, using the same methodology as that previously described7. The most recent literature data were reviewed, with an assessment of the relevance and quality of evidence, when possible, to define the strength of recommendations according to the GRADE system11 (see Online Supplementary Content, Table SI). In the absence of sufficient evidence, indications were provided based on consensus reached among members of the Working Group and considering the Italian healthcare context.
The first version of the document was revised and agreed by the Council of the Italian Society of Haemostasis and Thrombosis (SISET). The final document was shared and approved by Scientific Societies of the specialists often involved in the identification and management of newly diagnosed AHA patients, including the professionals engaged in clinical laboratories and hospital pharmacists, with the aim to disseminate and to facilitate the implementation of multidisciplinary management of AHA.
GENERAL INFORMATION
The incidence of AHA has been estimated to be about 1.5 cases per million/year3,4, showing an increase with age, the disorder being extremely rare in children12 and significantly more frequent in people over 65 years old3,4. The two largest series documented a median age at diagnosis of 78 and 74 years. Moreover, over 80% of patients were more than 65 years old, without gender difference3,4. In women, a second, lower incidence peak is observed between 20 and 40 years, due to pregnancy-associated cases13–15.
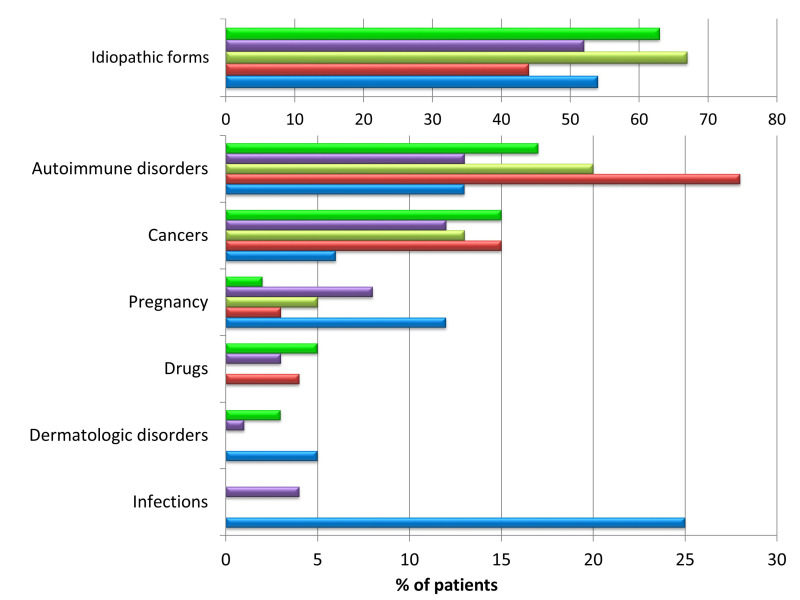
Such cases (2–15% in the largest series) are considered among “secondary” AHA, i.e. those in which a recognised condition triggers the autoimmune phenomenon leading to AHA. The most common secondary forms of AHA are summarised in Table I and include those associated with solid cancer and haematological malignancies (6–22%), autoimmune diseases (9–17%), infections (frequently reported in paediatric cases), cutaneous disorders and drugs. Recently AHA has been diagnosed in patients with coronavirus 2-associated disease 2019 (COVID-19)16 or after anti-COVID-19 vaccination17. About 50% of cases, in the lack of likely conditions associated with the development of anti-FVIII autoantibodies, are considered as idiopathic AHA. Figure 1 shows the distribution of idiopathic and secondary AHA in the largest series from studies or registries3–5,18,19.
Table I.
Clinical conditions associated with acquired haemophilia A (secondary forms)
| Autoimmune disorders | Systemic lupus erythematosus, rheumatoid arthritis, temporal arteritis, multiple sclerosis, myasthenia gravis, ulcerative colitis, autoimmune haemolytic anaemia, Sjogren’s syndrome, Goodpasture’s syndrome, autoimmune thyroid diseases |
| Malignancies | Haematological: chronic lymphocytic leukaemia, multiple myeloma, Waldenström’s macroglobulinaemia, monoclonal gammopathy of undetermined significance, non-Hodgkin’s lymphoma, myelodysplasia, myelofibrosis, other chronic myeloproliferative disorders Solid tumors: lung, prostate, pancreas, colon, stomach, melanoma, breast, kidney, head-neck |
| Cutaneous diseases | Pemphigus, psoriasis |
| Drugs | Chloramphenicol, sulphonamides, β-lactam antibiotics, phenytoin, methyldopa, non-steroidal anti-inflammatory drugs, Interferon-α, fludarabine, clopidrogrel. |
| Pregnancy | Generally, 1–4 months after delivery or abortion, although also possible pre-partum |
| Other disorders | Acute hepatitis B or C virus infection, chronic obstructive pulmonary disease, asthma |
Figure 1*. Distribution of the idiopathic forms of acquired haemophilia A and those “secondary” to associated conditions, as reported in the largest published series of patients.
Green bars: UKHCDO survey, 2007 (n=172)3; purple bars: EACH2 Registry, 2012 (n=501)4; yellow bars: GTH-AH study, 2015 (n=102)5; red bars: HTRS Registry, 2016 (n=166)18; blue bars: CARE Registry, 2019 (n=187)19. The absence of a bar indicates that the data were not reported in the study.
*The coloured figure is published online.
SYMPTOMS AND CLINICAL SUSPICION
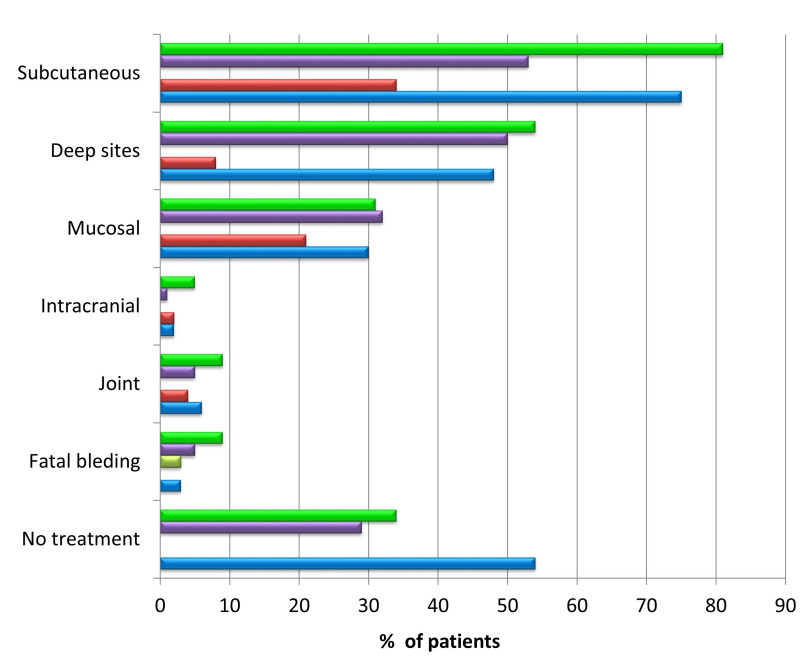
In a minority of patients, AHA is suspected in the absence of bleeding manifestations, following the detection of coagulation test abnormalities at diagnostic work-up or prior to invasive procedures or surgical interventions4. However, in more than 70% of patients, the clinical onset is characterised by bleeding events, spontaneous or induced by minor trauma or invasive procedures (positioning of venous catheters, endoscopic investigations, intramuscular injections, arterial blood sampling, etc.), in subjects with no personal or family history of bleeding1–5,20. The most common bleeds are muscle and soft tissue haematomas (about 80% of patients), which are often very extensive and potentially cause severe anaemia and/or compression of nerves and vessels, resulting in compartment syndrome. Joint bleeds, which are typical in congenital haemophilia A, are rare in AHA. Other symptoms include mucosal bleeding (epistaxis, gum bleeding, metrorrhagia, and urinary tract bleeding), but also severe up to life-threatening bleeding (gastrointestinal bleeding, retroperitoneal haematomas, intracranial haemorrhages)1,2. Figure 2 illustrates the distribution of bleeding events, including fatal haemorrhages and, when the data are available, those not receiving specific treatment3,4,18,19. In some cases, suspicion arises in bleeding patients with prolonged activated partial thromboplastin time (aPTT), who receive blood components without correction of the laboratory abnormality and/or haemostatic efficacy.
Figure 2*. Rates of main sites of bleeding and of those resulting in death or not requiring haemostatic treatment in patients with acquired haemophilia A, as reported in the largest published series of patients.
Green bars: UKHCDO survey, 2007 (n=172)3; purple bars: EACH2 Registry, 2012 (n=501)4; yellow bars: GTH-AH study, 2015 (n=102)5; red bars: HTRS Registry, 2016 (n=166)18; blue bars: CARE Registry, 2019 (n=187)19. The absence of a bar indicates that the data were not reported in the study.
Mucosal: gastrointestinal and genitourinary tract. Deep sites: muscles and retroperitoneum. No treatment: bleeding episodes in which anti-haemorragic drugs were not used. *The coloured figure is published online.
LABORATORY DIAGNOSIS
Screening and mixing tests
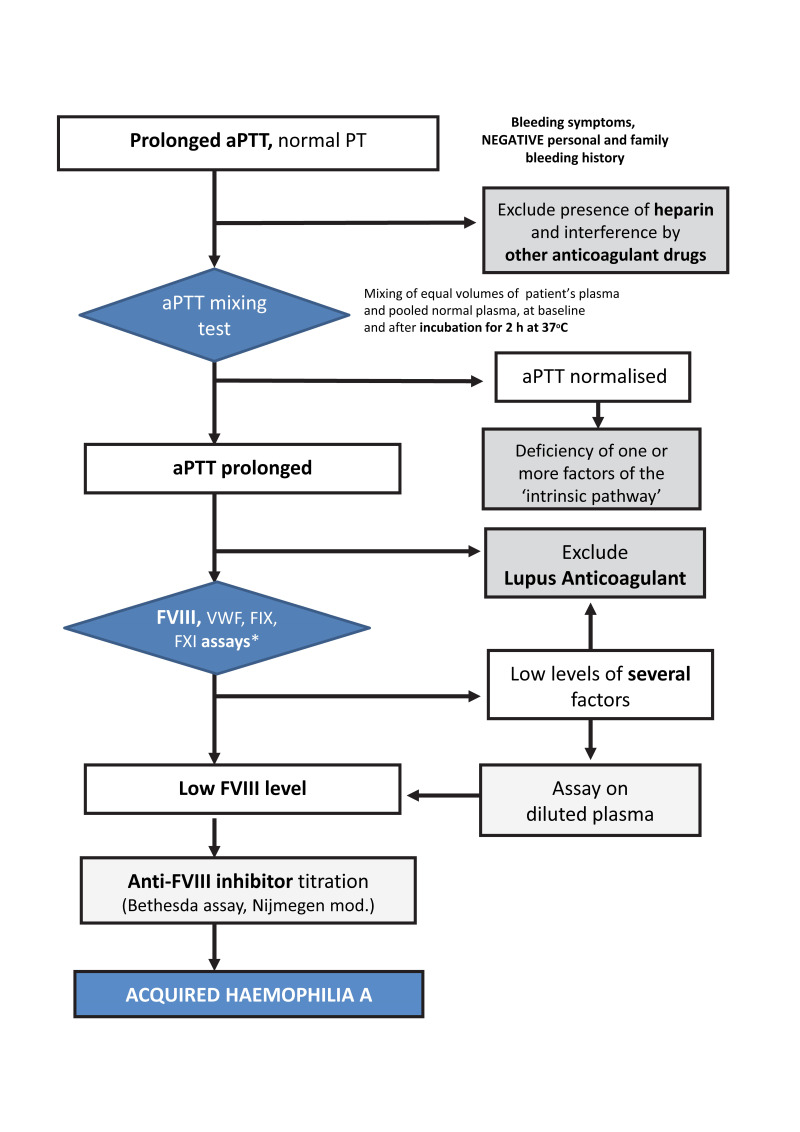
A normal prothrombin time (PT) associated with a prolonged aPTT, if uncorrected in the mixing test, is a key feature that should raise the suspicion of AHA21,22. The diagnosis should then be confirmed through the laboratory diagnostic steps shown in Figure 3. The aPTT mixing test is easy to perform, even in general clinical laboratories and should therefore be widely and quickly available, even in emergency settings. The mixing test consists of determining the aPTT in a mixture of equal volumes of a patient’s plasma and pooled normal plasma, immediately and after 2 h incubation at 37°C. In case of coagulation factor deficiencies (factor [F] XII, XI, IX and VIII, or VWF), the aPTT reverts to normal. In contrast, lack of or incomplete correction of the aPTT (especially after incubation) suggests the presence of an inhibitor. The incubation time and temperature are crucial since the inactivation of FVIII by autoantibodies is both time- and temperature-dependent. Low-titre antibodies or those with complex kinetics may be difficult to detect in the mixing test. However, the diagnostic efficacy can be improved by increasing the proportion of the patient plasma in the mixture (typically, one volume of pooled normal plasma and three of the patient’s plasma).
Figure 3. Diagnostic algorithm for acquired haemophilia A.
*Measurement of FXII levels is not indicated in the presence of bleeding symptoms, because even severe deficiencies of this factor, which causes prolongation of the aPTT, are not associated with bleeding tendency.
aPTT: activated partial thromboplastin time; PT: prothrombin time; FVIII: factor VIII; FIX: factor IX; FXI: factor XI; VWF: von Willebrand factor.
Confirmation of diagnosis and measurement of inhibitor titre
The diagnosis of the anti-FVIII inhibitor should be confirmed by measuring FVIII activity and by titrating the inhibitor with the Bethesda assay or the Nijmegen modification23. These assays are meant to assess the residual FVIII after 2 h incubation at 37°C of the patient’s plasma and pooled normal plasma. The inhibitor assays have two drawbacks. First, they were developed to titre inhibitors in subjects with congenital haemophilia, which follow type I inactivation kinetics, with residual FVIII activity proportional to the antibody concentration. In contrast, due to more complex (type II) inactivation kinetics, the correlation between residual FVIII and inhibitor titre may be poor in AHA24. Although immunological assays may overcome these problems24,25, these tests are not readily available in general laboratories. Second, because of the multiplicity of reagents/methods used to measure residual FVIII, there may be relatively poor agreement of results across laboratories. Clinicians are strongly recommended to refer to the same laboratory for monitoring AHA patients to ensure results consistency over time.
Unlike the alloantibody titre in congenital haemophilia A, the inhibitor titre in patients with AHA and the residual FVIII activity are not correlated with, nor do they reflect, the severity of the bleeding symptoms4. This is in part due to the type II inactivation kinetics of inhibitory autoantibodies. Consequently, although the assessment of the inhibitor titre and FVIII activity is useful in the patients’ follow-up, it does not allow stratification of their bleeding risk2. Compared to cases with FVIII levels >1%, patients with very low FVIII levels (<1%), as well as those with higher inhibitor titres (>20 BU/mL), have been associated with a less favourable prognosis, in terms of both success of inhibitor eradication therapy and survival5.
Differential diagnosis of anti-factor VIII inhibitors from other conditions
There are situations for which screening (and mixing) test results may mimic or obscure the presence of anti-FVIII inhibitors (Table II). Typically, these include conditions associated with a prolonged aPTT uncorrected by the mixing test, i.e. the presence of heparin, other anticoagulant drugs or lupus anticoagulants (LA)26,27. In principle, knowledge of the patient’s medical and drug history may help to distinguish the presence of FVIII inhibitors from other confounding conditions. However, information on current treatment and time elapsed from the last intake and blood sampling is not always available. While unfractionated heparin prolongs the aPTT, low molecular weight heparins and fondaparinux often do not. Furthermore, a prolonged aPTT due to unfractionated heparin is suggested by a prolonged thrombin time24. Low molecular weight heparins or fondaparinux can be detected by testing plasma for anti-factor Xa (FXa) activity24. Direct FXa-inhibiting oral anticoagulants (rivaroxaban, apixaban, edoxaban) and, to a greater extent, thrombin inhibitors (dabigatran) can also lead to a prolonged aPTT. While the presence of dabigatran can be detected by dedicated tests, such as the diluted thrombin time or the ecarin clotting (or chromogenic) time, the direct FXa inhibitors can be identified by measuring anti-FXa activity. Vitamin K antagonists variably prolong the aPTT, which, however, is normalised after discontinuation of treatment and/or administering vitamin K24. Whenever there is suspicion of interference from anticoagulants, a valid option could be discontinuation of the treatment and monitoring of the aPTT, which tends to normalise depending on the half-lives of the drug and of the inhibited coagulation factors.
Table II.
Conditions associated with prolonged activated partial thromboplastin time and differential diagnosis of acquired haemophilia A
| Clinical condition | Laboratory data in common with those of AHA | Differential diagnosis |
|---|---|---|
| Lupus anticoagulant | aPTT prolonged and not corrected by mixing test FVIII sometimes reduced |
Difficult in most patients∘. Knowledge of the clinical history helps to make differential diagnosis: AHA presents with bleeding, whereas LA presents with thromboembolic events and/or pregnancy complications |
| Unfractionated heparin | aPTT prolonged and not corrected by mixing test | Can be excluded if TT is within normal reference values |
| Low molecular weight heparin/fondaparinux | aPTT prolonged (only at therapeutic doses*) | Can be excluded by measuring anti-FXa activity^ |
| Vitamin K antagonists | aPTT prolonged | Concomitant and more marked prolongation of the PT Normalisation after administration of vitamin K FVIII within normal reference values |
| DOAC to thrombin (dabigatran) | aPTT prolonged FVIII reduced |
Possible concomitant prolongation of the PT Can be excluded by determining TT, diluted TT or ecarin clotting time |
| DOAC to FXa (apixaban, edoxaban, rivaroxaban) | aPTT prolonged FVIII reduced |
Concomitant and more marked prolongation of the PT Can be excluded by specific assays for anti-FXa |
| “Heparin-like” endogenous substances | aPTT prolonged and not corrected by mixing test | TT prolonged; FVIII within normal reference value Possible concomitant reduction of more than one coagulation factors Possible positive cancer history |
see the text for details.
at lower doses in cases of renal insufficiency with reduced heparin clearance.
the TT (prolonged) can also be evaluated.
AHA: acquired haemophilia A; aPTT: activated partial thromboplastin time; DOAC: direct oral anticoagulants; FVIII: factor VIII clotting activity; FXa: activated factor X; LA: lupus anticoagulant; PT: prothrombin time; TT: thrombin time.
The presence of LA in the test plasma may represent a typical confounding situation. LA is usually diagnosed by a three-step procedure: (i) screening, which provides evidence of a prolonged aPTT and/or dilute Russell viper venom test; (ii) a mixing test, which consists of repeating the screening procedure in a mixture of patient’s plasma and pooled normal plasma without incubation; typically, the mixture clotting time is uncorrected in the presence of LA. (iii) confirmation, which consists of repeating the abnormal screening test (aPTT and/or dilute Russell viper venom test) after increasing the concentration of phospholipids; typically, the clotting time of the confirmation procedure reverts to normal in the presence of LA. Additional investigation requires the detection of solid-phase antibodies such as anticardiolipin and anti-β2 glycoprotein 1 IgG and IgM26,27. All in all, distinguishing LA from inhibitors to FVIII cannot be easily done based on laboratory tests for at least two reasons. First, inhibitors to FVIII at high titre may be detected in the mixing test even without incubation, thus resembling LA. Second, LA and inhibitors to FVIII may yield similar results in the LA confirmatory tests, as shown by testing plasma from many patients with congenital haemophilia A and inhibitors to FVIII27. It is difficult to establish whether this is due to interference of the inhibitor to FVIII with the LA tests or whether LA and inhibitors to FVIII do coexist. The interference of LA with FVIII activity and the Bethesda assay can be eliminated or markedly reduced by using chromogenic assays that are usually insensitive to LA. Overall, knowledge of the clinical history is helpful for evaluating the patient: anti-FVIII inhibitors usually cause bleeding symptoms, while LA is asymptomatic or associated with thrombotic manifestations and/or pregnancy complications26.
Inhibitors and detection of multiple abnormalities of coagulation factors
Anti-FVIII inhibitors, especially if high-titre, can interfere with the measurement of individual coagulation factors, mimicking reduced FIX, FXI and FXII levels. Diluting test samples with a buffer may reduce the interference24. The occurrence of rare circulating inhibitors interfering with the activity of more than one coagulation factor should be considered, particularly in patients with cancer. These inhibitors are usually endogenous glycosaminoglycans (i.e., heparin-like substances)28,29.
Concluding remarks on the laboratory diagnosis of acquired haemophilia A
Overall, considering the type of assays and expertise needed, the laboratory diagnosis of suspected AHA should be carried out, or at least confirmed, in specialised laboratories working in close collaboration with centres with expertise in the diagnosis and treatment of patients with haemophilia or other coagulopathies7,8. Therefore, patients should be promptly referred to such centres with adequate laboratory facilities. As the mixing test is not always available, especially in an emergency, it is common practice in suspected cases to measure FVIII and then, in the presence of low FVIII levels, to search for the specific inhibitor. Although useful, this procedure cannot be recommended, as it does not enable the differential diagnosis in more complex situations (see above). Nevertheless, in the presence of a clear clinical suspicion of AHA in bleeding patients, it might be useful to proceed, with the measurement of FVIII and other aPTT-related coagulation factors in parallel with the mixing test after 2 hours incubation at 37°C.
TREATMENT
Speed and appropriateness of treatment and management are crucial. In the presence of haemorrhagic events, the aim of treatment is primarily to control the acute bleeding quickly but, at the same time, to eradicate the inhibitor, minimising the risk of recurrent bleeding, which persists as long as the anti-FVIII inhibitor is present7,8,10,30,31. Furthermore, the diagnosis of AHA should always be followed by appropriate investigation, aimed at identifying secondary forms with the underlying clinical conditions inducing the autoimmunity (Figure 4A). This is also important for prognosis, as the identification of associated disorders may enable therapeutic interventions aimed at removing the triggering cause, thereby contributing to the resolution of the coagulation disorder7,31. In the case of AHA related to drug intake32 (Table I), the implicated drug must be discontinued. In non-bleeding patients, this may be the only management necessary.
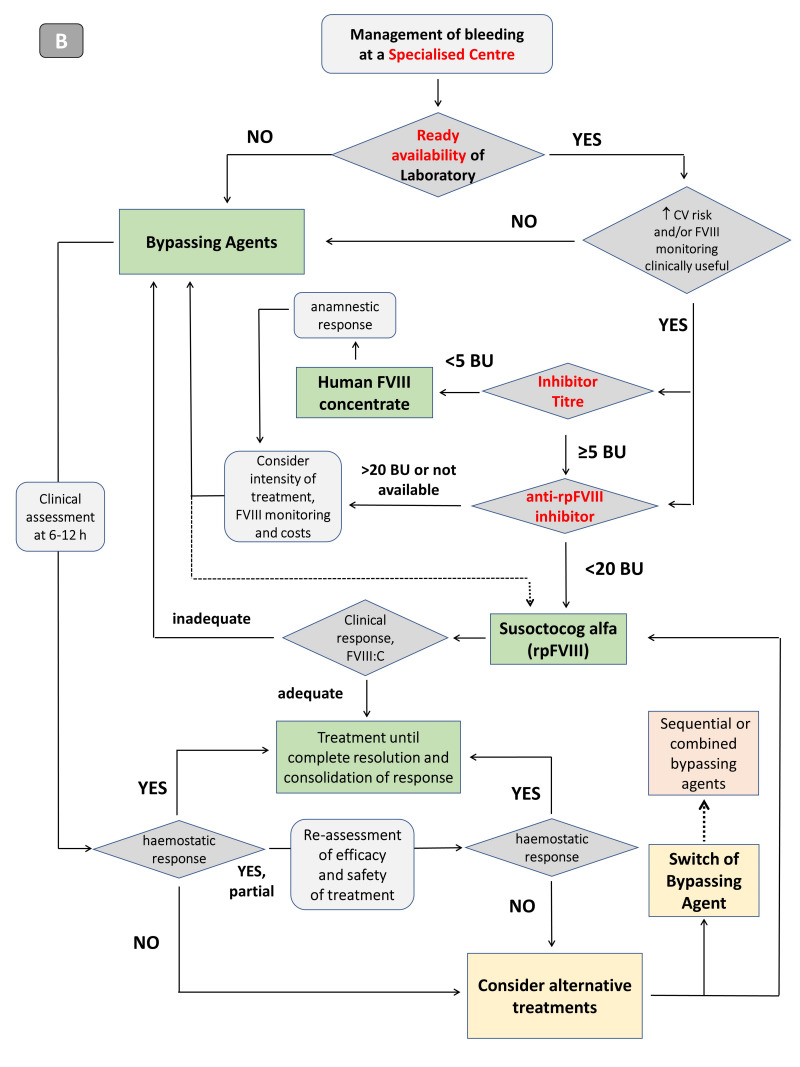
Figure 4*. Algorithm for the management of the patient with acquired haemophilia A at diagnosis, with emphasis on haemostatic treatment, depending on whether the patient is managed outside a specialised centre (A) or at a centre with clinical and laboratory expertise in the diagnosis and treatment of haemophilia and other coagulopathies (B).
AHA: acquired haemophilia A; CV: cardiovascular; FVIII: factor VIII; BU: Bethesda unit; rpFVIII: recombinant porcine factor VIII; FVIII:C: factor VIII clotting activity. *The coloured figure is published online.
Inhibitor eradication
Treatment aimed at eradicating inhibitory alloantibodies is the cornerstone of the management of AHA patients and should be undertaken immediately after diagnosis, regardless of the presence of bleeding symptoms. The only exceptions may be some forms frequently characterised by spontaneous remissions, such as AHA in children or secondary to pregnancy or the use of certain drugs, in the absence of bleeding7,9,12–15,32. However, as predictors of spontaneous remission in these forms are currently unknown, eradication should also be undertaken in these cases if a tendency to normalisation of FVIII levels and reduction of the inhibitor titres are not rapidly detected, and if even minimal bleeding symptoms appear.
Inhibitor eradication is based on immunosuppression, which is primarily achieved with the use of corticosteroids, alone or in combination with cyclophosphamide; in the case of failure or incomplete response, rituximab is added. An in-depth discussion of the types of eradication therapy and response monitoring is beyond the scope of this document. The reader should refer to previous AICE recommendations7 (Table III). In the German, Austrian and Swiss Thrombosis and Haemostasis Society (GTH) prospective registry, a homogeneous treatment protocol was adopted, which involved three successive steps of immunosuppressive therapy: steroid alone; steroid+cyclophosphamide; and steroid+rituximab.
Table III.
Summary of the Italian Association of Haemophilia Centres’ recommendations on inhibitor eradication therapy in acquired haemophilia A7
| Immunosuppressive therapy must be started as soon as possible, ideally immediately after the diagnosis of acquired haemophilia A |
| First-line treatment is prednisone (1–2 mg/kg daily) alone or in combination with cyclophosphamide* (1–2 mg/kg daily) |
| Rituximab (375 mg/m2 once weekly for a total of 4 doses) as monotherapy or in combination with other immunosuppressive drugs is the main agent for second-line therapy in patients who fail to respond to first-line therapy within 8–12 weeks |
| Rituximab may be indicated as a first-line agent in patients in whom there is a contraindication to immunosuppressive drugs |
| The combination of immunosuppressive drugs (including cyclosporine) and immunotolerance regimens are possible second-line alternatives in patients failing to respond to first-line immunosuppressive therapy. High doses of immunoglobulins are not indicated as an inhibitor eradication approach |
| Complete response to eradication therapy requires persistent negative inhibitor titres (<0.6 BU/mL) and normal factor VIII levels (>70%) |
| Patients with thromboembolic risk factors should receive mechanical and/or pharmacological thromboprophylaxis, particularly in the case of elevated factor VIII levels during or at the end of eradication treatment |
Cyclophosphamide and other alkylating agents should be avoided in women of childbearing age with acquired haemophilia A.
The transition from one step to another took place after 3 weeks, in the absence of a response to the strategy in place5. Data from this Registry showed that FVIII levels at diagnosis were the most important prognostic factor for response to eradication. Indeed, at least partial remission (FVIII >50% in the absence of bleeding and with haemostatic treatment suspended for at least 24 hours) was obtained in a significantly lower percentage and after longer times in patients with FVIII <1% than in those with higher levels (77 vs 89%, in 43 vs 24 days, respectively). Patients with high anti-FVIII inhibitor titres (>20 BU/mL) also showed a poor response to eradication with steroids only5. In the light of these data, recent international recommendations proposed a prognostic stratification of patients at diagnosis based on FVIII levels and inhibitor titre, with patients with negative prognostic factors (FVIII <1% and inhibitor titre >20 BU/mL) being candidates for combined eradication (steroid+cytotoxic agent or rituximab) already as first-line management10. However, an accurate assessment of the risk/benefit ratio of the therapeutic choices in each patient is recommended, given the extreme variability of the clinical presentations of AHA and of individual comorbidities. The sequential treatment strategy used by the GTH and the prognostic stratification suggested by international recommendations require further confirmation in larger studies; nevertheless these approaches can be useful to make inhibitor eradication and assessment of the response to treatment uniform and allow more homogeneous and comparable data collection.
Anti-haemorrhagic treatment
Table IV shows the different options for treatment of acute bleeding in patients with AHA, highlighting their advantages and limitations. There are basically two approaches: the use of bypassing agents, which activate the haemostatic process by overcoming the interference from the inhibitor, and replacement therapy, aimed at restoring haemostatic levels of FVIII.
Table IV.
Advantages and limitations of the different approaches available for treatment of bleeding in patients with acquired haemophilia A§
| Therapeutic approach | Recommended dose | Advantages | Limitations |
|---|---|---|---|
| Bypassing agents | |||
| aPCC | 50–100 U/kg every 8–12 h, until achievement of haemostasis, then at longer intervals as required Maximum 200 U/kg daily |
Efficacy demonstrated in various studies and registries Often available even in non-specialised centres |
No validated laboratory monitoring assay Potential risk of arterial or venous thromboses Large infusion volumes Possible anamnestic response# |
| rFVIIa | 90–120 μg/kg every 2–3 h until achievement of haemostasis, then at longer intervals as required | Efficacy demonstrated in various studies and registries Generally available even in non-specialised centres Flexibility of dose regimens |
No validated laboratory monitoring method Potential risk of arterial or venous thromboses Short half-life (2–3 h)^ |
| Replacement therapy ∘ | |||
| Human FVIII (plasma-derived or recombinant) | Variable depending on severity of bleeding, inhibitor titre, infusion modality (boluses or continuous infusion) | Efficacy demonstrated in some studies, in the case of low-titre inhibitors (<5 BU) Laboratory monitoring (FVIII activity) Readily available also in non-specialised centres |
Possible anamnestic response High doses of concentrate required Laboratory assays must be performed at least daily |
| rpFVIII | Starting dose 200 IU/Kg, subsequent maintenance doses in relation to the clinical response, FVIII levels (measured 30 min and 3 h after infusion) and the type/severity of bleeding; generally, infusions every 4–12 h* | Efficacy demonstrated (but studies still limited) Laboratory monitoring (one-stage FVIII activity) |
Possible development of anti-rpFVIII antibodies, with consequent reduced efficacy Not always readily available Need for round-the-clock laboratory services |
aPCC: activated prothrombin complex concentrate; rFVIIa: activated recombinant factor VII concentrate; rpFVIII: recombinant porcine factor VIII concentrate.
Specific approvals and indications in acquired haemophilia A (AHA) can be different across countries. The European Medicine Agency (EMA) licensed aPCC, rFVIIa and rpFVIII for treatment of bleeding in AHA patients, while aPCC is not specifically approved for this indication by the Food and Drug Administration (FDA). The use for prevention of bleeding in the case of surgery or invasive procedures is approved for rFVIIa by EMA and FDA.
Increase of inhibitor titre, due to the presence of small amounts of FVIII.
Limitation overcome by the possible administration in a syringe pump (polypropylene 50 mL) at timed intervals, since the drug is stable after reconstitution for 24 h at 25°C, under close clinical monitoring.
This approach also includes the administration of desmopressin (DDAVP), reported in patients with low inhibitor titres, but difficult in clinical practice because of the poor predictability of response, the possible tachyphylaxis and the risk of adverse events in the elderly and patients with comorbidities.
Dose regimen and monitoring indications from the product information leaflet, taken from the registration study, in which patients with anti-rpFVIII cross-reactivity <20 BU/mL were enrolled. Data are available from case series in which lower doses (50–120 IU/kg) were used for initial treatment, while subsequent doses were defined by monitoring clinical response and FVIII levels.
Bypassing agents
Bypassing agents, i.e., recombinant activated FVII (rFVIIa; NovoSeven®, Bagsvaerd, Denmark) and activated prothrombin complex concentrate (aPCC; FEIBA®, Baxalta Innovations, Vienna, Austria; now Takeda, Tokyo, Japan), are able to overcome the interference of anti-FVIII inhibitors on the formation of the tenase complex and induce thrombin generation with different mechanisms. aPCC supplies FII, FIX and FX, also in their activated forms33,35. However, given its plasma origin, aPCC contains traces of FVIII34 which could cause an anamnestic response with an increase in the inhibitor titre. rFVIIa acts by amplifying the initial tissue factor-dependent thrombin generation and can directly activate FX on the surface of platelets, even in the absence of FVIIIa and FIXa36. The two bypassing agents also differ in their pharmacokinetic characteristics (aPCC has a half-life of 4–7 hours34 while the half-life of rFVIIa is approximately 2–3 hours36) and infusion volumes (20 mL per 1000 U for aPCC, 1 mL per mg for rFVIIa, which is available in vials of 1, 2, 5 and 8 mg)37.
First-line treatment
Bypassing agents have generally been identified as a first-choice treatment, recommended by national and international guidelines and by groups of experts6–10. However, the rarity and heterogeneity of the disease make it difficult to obtain evidence from controlled clinical trials of good methodological quality. The available recommendations are therefore based on data from registries or case series, with consequent limitations in strength and levels of evidence. No prospective comparative studies that demonstrate the superiority of one or the other bypassing agent are available37. The choice of the first-line agent is therefore based mainly on the clinician’s experience and the availability, at the time, of one or the other product. In some cases, the recombinant origin, the flexibility of treatment doses and intervals of administration or the infusion of small volumes may lead to a preference for rFVIIa. On the other hand, the possibility of administration at longer intervals may favour the use of aPCC. However, the available studies show that both bypassing agents are highly effective in the management of acute bleeding episodes in patients with AHA38–43.
The efficacy of aPCC has been documented by numerous case reports and a retrospective study of 34 patients in whom bleeding resolved in 86% of cases35. The product was used as first-line treatment at a dose of 75 U/kg every 8–12 hours and the median number of infusions needed to control severe and moderate bleeding was 10 and 6, respectively. Furthermore, in a recent retrospective-prospective study carried out in Italy (FAIR; FEIBA® acquired haemophilia A Italian Registry) on 56 AHA patients, aPCC, used as first-line therapy in 82% of cases at a mean dose of 72.6±26.6 U/kg, was effective in 96% of bleeding episodes in a median of 8 days of treatment (interquartile range: 1–48)42.
The efficacy of rFVIIa in patients with AHA was documented by the experience in 139 patients38 in whom first-line treatment was effective in 95% of bleeding episodes at a median dose of 90 μg/kg, although there was wide variability in the dose (60–160 μg/kg), as well the number of infusions (1–33) and the duration of treatment (1–7 days). Data from the EACH2 Registry showed that rFVIIa was the most widely used haemostatic agent, with an efficacy of 92%, similar to that of aPCC (93%)40. A recent, large, Japanese post-marketing registry confirmed the haemostatic efficacy of rFVIIa in 92% of cases. The efficacy was significantly higher when treatment was started early, within hours of the onset of symptoms, and in cases treated with an initial dose ≥90 μg/kg compared with cases treated with lower doses43. Based on the published experiences, the current national and international recommendations indicate that the initial doses should be 90–120 μg/kg every 2–3 hours for rFVIIa and 50–100 U/kg every 8–12 hours for aPCC, with the maximum daily dose not exceeding 200 U/kg6,7,10.
No validated tests are available for laboratory monitoring of treatment with bypassing agents. Global haemostasis assays (rotational thromboelastometry and the thrombin generation test) have been considered. However, although providing information about changes in the haemostatic profile or thrombin generation induced by bypassing agents, the results do not correlate with clinical efficacy and resolution of bleeding, nor with the possible risk of thromboembolic complications44. Studies of relatively large numbers of patients should be undertaken to draw meaningful conclusions on the value of post-infusion laboratory testing. To date, therefore, therapeutic efficacy and safety of bypassing agents can only be assessed based on clinical criteria6,8,10.
Second-line approaches
Higher doses of bypassing agents are generally not recommended because of the possible increase in the risk of thrombosis, especially in elderly patients and/or those with comorbidities or cardiovascular/thromboembolic risk factors7,8. However, in the case of clear failure of one agent, a switch to the alternative bypassing agent is indicated and should be performed quickly to avoid potential life-threatening bleeding or disabling sequelae7,8,45. This implies that the patient should undergo assiduous clinical and laboratory assessments9, as also suggested for patients with congenital haemophilia and inhibitors46. In the case of severe bleeding, clinical assessment must be carried out within 6–12 hours after beginning the treatment in order to consider therapeutic alternatives early in the case of an inadequate response to the treatment9. The combined use of both bypassing agents can also be considered with caution, as an extreme rescue therapy7. Indeed, concerns about the safety of bypassing agents in patients with AHA do remain with regards to cardiovascular/thromboembolic risks, which have been reported for both products. The EACH2 Registry recorded 11 thrombotic episodes (7 arterial and 4 venous) in patients treated with rFVIIa or aPCC, with similar rates (2.9 vs 4.8%)40. These figures are higher than those found in patients with congenital haemophilia A and inhibitors treated with the same agents, probably because of the older age of patients with AHA and their higher burden of cardiovascular comorbidities and risk factors1. An increased incidence of thrombotic events has also been documented during sequential or combined treatment with both bypassing agents45. However, thromboembolic events were also reported in AHA patients not receiving bypassing agents (1.4%)40, demonstrating the intrinsic risk due to the clinical features of AHA patients (elderly and with comorbidities) and the intercurrent risk conditions (reduced mobility, hospitalisation, discontinuation of ongoing antithrombotic therapies, the latter in a recent Spanish study in 34% of patients at AHA diagnosis20).
Replacement therapy
Human factor VIII concentrates
With the limitations stemming from the lack of direct comparison studies, human FVIII (hFVIII) concentrates (plasma-derived or recombinant)40,47 and desmopressin (DDAVP)48 are less effective than bypassing agents in controlling bleeding in patients with AHA7,10,40. The difference in efficacy (68.3% for hFVIII and DDAVP vs 93.3% for bypassing agents) was statistically significant (p=0.003) in propensity score-matched samples from the EACH2 Registry40. However, hFVIII may only be effective in patients with low-titre inhibitors (<5 BU/mL) and if the dose administered is sufficient to saturate the inhibitor (neutralising dose) and increase circulating FVIII (increasing dose) to haemostatic levels. Moreover, the available equations for calculating the neutralising dose are limited by the inaccuracy of the laboratory methods for titrating the inhibitor23,24. Nevertheless, two therapeutic schemes are commonly used: 200–300 IU/kg administered as a bolus, followed by a continuous infusion of 4–14 IU/kg/day or 20 IU/kg for each BU of inhibitor, followed by additional doses of 40 IU/kg/day7. However, the measurement of FVIII level achieved 15–30 minutes after the infusion and the monitoring at longer times after the infusions, to determine the appropriate interval between administrations and the adequacy of the dose, are recommended. Such monitoring also allows early detection of a possible anamnestic response with an increase in inhibitor titre. In these cases, FVIII dose adjustments may still be sufficient to neutralise the inhibitor or switch to a bypassing agent could be needed. Remarkably, no thromboembolic events associated with the use of FVIII concentrates have been documented40 and hFVIII concentrates have been used successfully in some elderly patients or with high cardiovascular risk47. However, the number of cases is limited and available data need confirmation.
Potential candidates for treatment with hFVIII concentrates are patients with low-titre inhibitors for whom monitoring of FVIII levels could contribute to the efficacy and safety of treatment, such as those with severe bleeding and/or high thromboembolic risk. It is, however, essential that these cases are managed by specialised centres.
For sake of completeness, although difficult to implement in common practice, particularly in emergency situations, the use of FVIII at high doses after removal of high-titre inhibitors by therapeutic plasmapheresis or immunoadsorption on sepharose columns binding staphylococcal protein A (Immunosorba®, Excorim AB, Lund, Sweden) should be mentioned49–51. This strategy can enable temporary achievement of haemostatic levels of FVIII in patients who require urgent surgery or in the case of severe bleeding unresponsive to the usual therapeutic options7,8.
Recombinant porcine factor VIII concentrate
Since February 2017, a recombinant concentrate of B-domain deleted porcine FVIII (rpFVIII; susoctocog alfa, Obizur®, Baxalta Innovations, Vienna, Austria; now Takeda) produced in baby hamster kidney cells, has been available with indication for patients with AHA. The drug was developed to overcome the problems associated with the previous widely used plasma-derived product, demised in 2005, such as the risk of contamination by viral agents (primarily parvovirus B19), allergic reactions to porcine plasma proteins, and thrombocytopenia, due to platelet aggregation induced by the presence of porcine VWF52. The product is licensed for treatment of bleeding in adult inpatients under the supervision of physicians experienced in the treatment of haemophilia53,54. This indication limits its first-line administration as AHA patients are often initially seen in an Emergency Department or in non-specialised wards. In the pre-registration study, susoctocog alfa was successful in controlling bleeding episodes in 86% of 28 AHA patients with a median age of 70 years, being more effective when used as first-line therapy55. No thrombotic episodes or other serious adverse events were reported. The initial dose used was 200 IU/kg, regardless of anti-hFVIII inhibitor titre and rpFVIII cross-reactivity (based on the study inclusion criteria, limited to titres <20 BU/mL). In ten of the 28 patients (36%) who had initial anti-rpFVIII cross-reactivity (0.8–29 BU/mL), higher doses of susoctocog alfa were required. In four patients who did not show anti-rpFVIII reactivity prior to exposure to susoctocog alfa, anti-rpFVIII titres (18–166 BU/mL) were detected and in two of them the treatment had to be discontinued. In this study, the doses of susoctocog alfa following the first bolus were defined based on regular monitoring of FVIII levels measured with one-stage clotting assays. In the 24 patients with an effective response to treatment in the first 24 hours, a median of 3.5 infusions were given at a median interval of 7.4 hours, at lower doses than the first bolus (median reduction 65%). Overall, the median duration of treatment was 6.5 days55.
The safety and efficacy of susoctocog alfa were confirmed more recently by small case series, in which lower starting doses (100–120 IU/kg) were used56,57. Similar findings were also shown in a recent Italian study that collected the largest series available to date on the real-life use of susoctocog alfa58. In six of nine patients a mean starting dose of 100 IU/kg (range 50–120) was administered, followed by doses of 50 IU/kg, every 8–12 hours. In all patients circulating FVIII levels were measured 30–60 minutes after the infusion and in the following hours, to guide the subsequent doses and intervals of administration. Susoctocog alfa was used as first-line therapy in one third of patients, being effective in controlling bleeding in all of them. More than 70% of patients had cardiovascular disease or risk factors and some received tranexamic acid in combination. No thromboembolic complications were recorded. Overall, the available data demonstrate that susoctocog alfa is a valid option for the treatment of patients with AHA, both as first- and second-line approach, particularly in patients with comorbidities at high risk of cardiovascular and thromboembolic complications. On the basis of the pre-registration study55, the starting dose of susoctocog alfa recommended in the product information leaf let is 200 IU/kg, while the subsequent doses and frequency of administrations should be guided by the measured FVIII activity, to be maintained within the recommended limits (>50% for superficial haematomas not compromising neurovascular structures and >80% for intramuscular haematomas or retroperitoneal, gastrointestinal, or intracranial haemorrhages). At the same time, post-infusion FVIII levels exceeding 200% should be avoided. Indeed, very high FVIII levels were reported in some patients on rpFVIII treatment55,56, which might increase the risk of thromboembolic events, particularly in elderly patients and those with pre-existing cardiovascular disease59.
The available experience demonstrating the efficacy of rpFVIII in small cohorts of bleeding patients with AHA does, however, appear limited in the face of the large series and types of haemorrhagic events treated with bypassing agents reported in national and international studies and registries3,5,18,19,40. Moreover, although the final assessment of the efficacy of rpFVIII is based on purely clinical criteria, the regular monitoring of FVIII levels reached 30 minutes and 3 hours after the administration of the initial and subsequent doses, reported in the product information leaf let, limits the use of the product to centres with the close collaboration of a specialised laboratory, able to promptly measure FVIII levels around the clock. Although the implications for the haemostatic efficacy of susoctocog alfa are not fully defined, several experts suggest determining the anti-rpFVIII cross-reactivity before starting treatment and monitoring any development of anti-rpFVIII inhibitors during therapy53. A recent analysis of data from the prospective GTH Registry showed that anti-rpFVIII cross-reactivity was present in 44% of cases at baseline and was associated with higher titres of anti-hFVIII inhibitor and lower levels of FVIII. In particular, for anti-hFVIII inhibitor titres >100 BU/mL, cross-reactivity was evident in 97% of cases, while it was not detected in 90% of cases with titres <3.8 BU/mL60. A recent case series highlights the need to verify in larger studies the occurrence of treatment failure due to the development of anti-rpFVIII inhibitor activity, in some cases associated with an increase of anti-hFVIII inhibitor titre61.
In conclusion, considering the limitations highlighted above, rpFVIII is an important therapeutic option in patients with AHA, particularly in those shown to be free of or with low anti-rpFVIII cross-reactivity. Moreover, susoctocog alfa may be preferred in conditions in which monitoring of haemostatic levels of FVIII can contribute to improving the efficacy and safety of treatment, such as in the case of severe bleeding, and/or in patients with high cardiovascular or thromboembolic risk. Finally, rpFVIII may represent a valid alternative in cases of failure of first-line treatment with bypassing agents.
Desmopressin
As mentioned above, DDAVP should also be considered among the possible replacement therapy approaches. Its use has been reported in the literature, alone or in association with hFVIII concentrate, in patients with low-titre inhibitors and measurable FVIII levels48. However, the unpredictable therapeutic response, the possible phenomenon of tachyphylaxis, with lack of efficacy after several consecutive doses in a short period of time, and the risk of fluid retention with hyponatraemia, particularly in elderly patients and those being treated with drugs affecting the electrolyte-fluid balance, make the use of this drug difficult and of limited benefit. Therefore, DDAVP should be reserved to patients with the characteristics described above, only when other therapeutic approaches are unavailable.
Local measures and tranexamic acid
The management of some minor bleeds can benefit from local haemostatic measures (application of ice, prolonged compression, topical antifibrinolytics). Tranexamic acid, given systemically (10 mg/kg intravenously or 15–25 mg/kg orally, every 8 hours) or topically (in particular, as mouthwashes with 10 mL of a 5% solution for 2 minutes four times a day) may be sufficient to achieve complete control of mucosal bleeds, such as gingival bleeding, epistaxis, and metrorrhagia7,62, except urinary tract haemorrhages. The drug can be used in association with rFVIIa while its use in association with aPCC is generally contraindicated7. However, recent data support its safety even in the case of concomitant treatment with aPCC63. These data include those collected during the Italian FAIR study, in which concomitant treatment with tranexamic acid was used in about 40% of the 101 reported bleeding events. Twenty-five of the 30 patients receiving antifibrinolytics had comorbidities, in 40% cardiovascular disease. No arterial or venous thromboembolic events were observed and the aPCC plus antifibrinolytics allowed an average 16% reduction in the duration of treatment32,64.
Concluding remarks on haemostatic treatment
Given the potential severity of haemorrhagic manifestations and the use of highly specialistic, expensive, not always readily available drugs, the management of haemostatic treatment in an individual with AHA and ongoing bleeding should be the prerogative of specialised centres, able to carefully assess and monitor the risk/benefit and even cost/benefit ratios of treatment. However, pharmacoeconomic evaluations are difficult in this setting, due to the extreme heterogeneity of clinical pictures and individual patients (affecting doses and duration of treatment), as well of healthcare systems (including different costs of drugs), which make the few available data unreliable65.
Patients should be promptly referred to specialised centres, through established pathways in the various healthcare settings, when they are first seen and clinically suspected of having AHA. As mentioned above, this is crucial for the rapid confirmation of the diagnosis and implementation of the most appropriate therapeutic approach, considering the severity of bleeding symptoms, the patient’s age and clinical characteristics (in particular comorbidities and cardiovascular/thromboembolic risk), the ready availability of laboratory monitoring and of the different products for treatment of bleeding. If immediate referral to a specialised centre is unfeasible, treatment must be undertaken as soon as possible, taking into consideration the actual or prompt availability of therapeutic agents. It is therefore advisable that protocols shared with the specialised centres are already available, to offer adequate support in the early stages of treatment, pending the implementation of direct management by the specialised centre as soon as possible. Such referral is essential if second-line therapeutic approaches are needed.
Whatever the therapeutic approach, treatment should be continued until complete resolution of bleeding manifestations and consolidation of the haemostatic response7. A regimen of treatment to prevent further bleeding could be considered, especially for patients at high risk of recurrence of haemorrhagic episodes, pending response to eradication therapy33.
Figure 4 presents an algorithm for guiding the haemostatic treatment in patients with AHA, based primarily on whether the patient is managed outside (A) or at (B) a specialised centre.
Surgery and invasive procedures
Surgery and invasive procedures in patients with AHA increase the risk of bleeding complications. Therefore, non-urgent minor and major invasive procedures should be deferred, in particular in suspected cases, before the diagnosis confirmation, and possibly carried out after inhibitor eradication. Few data are available concerning the management of such procedures in this setting18. Expert panels recommend the use of bypassing agents or rpFVIII to cover surgery or invasive procedures in AHA patients10; however, the specific indication is licensed by the European and US regulatory agencies only for rFVIIa. In patients requiring emergency/urgent surgery or invasive procedures adequate haemostatic coverage should be guaranteed, starting immediately before the intervention, being more intensive in the phase of the highest post-operative bleeding risk, according to the type of intervention, and proceeding until healing has occurred. Due to the concomitant thromboembolic risk, related to the surgery itself and to the individual patient’s profile, recommended doses should not be exceeded, and laboratory monitoring, if feasible, should be performed carefully.
Monitoring factor VIII levels and antithrombotic prophylaxis
As previously mentioned, monitoring of FVIII levels is crucial during haemostatic treatment with replacement products but is needed even to assess the response to inhibitor eradication. For this purpose, FVIII activity and inhibitor titre can be measured weekly or when changes of immunosuppressive therapy are planned. In all cases, cardiovascular/thromboembolic risk of AHA patients should be considered, as this is often increased due to age, vascular comorbidities and factors such as reduced mobility and the haemostatic treatment, both in the case of bypassing agents and of replacement treatment. High FVIII levels are often reported in the follow-up of patients after successful inhibitor eradication6,7. Therefore, in patients with high thromboembolic risk, mechanical (elastic stockings) and/or pharmacological thromboprophylaxis should be considered2,6,7,66, with continuous assessment of the risk/benefit ratio. Finally, the withdrawal of ongoing antiplatelet and/or anticoagulant treatments, consequent to bleeding and the diagnosis of AHA, should be limited to the period of highest risk of bleeding, with antithrombotic drugs being re-initiated soon after the resolution of bleeding and the restoration of sufficient FVIII levels.
CONCLUSIONS
In recent years significant progress has been achieved in the understanding of the natural history, treatment, and prognosis of AHA. Nevertheless, the diagnostic and therapeutic approaches still need to be standardised and it is crucial to spread knowledge about this condition among all healthcare professionals, particularly those specialists who often first face patients suffering from this rare but potentially serious bleeding disorder.
To reduce the still very relevant complications and mortality of AHA, it is important to work synergistically with specialists of laboratories, emergency/urgent care settings and other branches of medicine and surgery, focusing on the rapid recognition of suspected cases, correct diagnosis, and appropriate and timely treatment of haemorrhagic manifestations, to be undertaken even in the absence of a specialist consultation. For these reasons an efficient network, established at an institutional level, should be available, to enable prompt contact with centres specialised in the diagnosis and treatment of haemophilia and other coagulopathies. This strategy is crucial to manage emergencies and promptly refer newly diagnosed patients for the laboratory work up and to start or continue treatment. Whenever this is unfeasible, the implementation of diagnostic-therapeutic protocols and recognised procedures should be ensured, to allow proper management of patients until they can be referred to specialised centres.
Effective strategies for controlling bleeding and eradicating inhibitors are currently available. However, they require highly specialised skills to define the indications for first-line treatment, clinical and laboratory monitoring, and alternative approaches. It is therefore extremely important to rely on principles of treatment and shared recommendations, based on the available literature and clinical experience gained across the country. The diagnostic, management and treatment issues discussed here and the resulting recommendations, with their strength according to the GRADE methodology, are summarised in Table V. It is hoped that this consensus practical guidance will help to harmonise the management and treatment of AHA and improve the overall healthcare pathway of patients with this condition.
Table V.
Summary of recommendations for the diagnosis, general management and treatment of bleeding and monitoring of patients with acquired haemophilia A
| RECOMMENDATION | STRENGTH^ |
|---|---|
| A. DIAGNOSIS | |
| The diagnosis of AHA should be considered in the event of a sudden onset of bleeding in a patient without a personal and family history of bleeding, who exhibits an isolated prolonged aPTT, not corrected in a mixing test, immediately and after incubation for 2 hours at 37 °C | 1B |
| The mixing test should be available also in non-specialised laboratories under ordinary and urgent circumstances | |
| The laboratory diagnosis of AHA should be made/confirmed by specialised laboratories that work in close collaboration with centres specialised in the diagnosis and treatment of patients with haemophilia and other coagulopathies | |
| B. GENERAL ASPECTS OF MANAGEMENT | |
| Patients with AHA should preferentially be managed in specialised centres, to which they should be immediately referred at the time of the clinical suspicion of the disease. If this referral is not readily feasible, bleeding episodes should be treated locally in close collaboration with a specialised centre or based on already shared protocols, to ensure prompt implementation of the most appropriate treatment of bleeding, as well as the best global management, in the context of a multidisciplinary approach | |
| Invasive procedures should be avoided, when possible, in patients with suspected AHA until the diagnosis has been made | 1C |
| The recognition of associated conditions triggering the inhibitor development (e.g., malignancies, drugs) is crucial from a prognostic point of view, since treatment of such condition can lead to disappearance/significant reduction of the inhibitor | 1C |
| The diagnosis of AHA should be followed by prompt initiation of immunosuppressive therapy to eradicate the inhibitor | 1B |
| In the absence of bleeding, paediatric or post-partum cases or those clearly secondary to use of drugs can be exceptions to the immediate initiation of eradication treatment, as spontaneous resolution of the autoimmune phenomenon may occur | 2B |
| If a “wait and watch” approach is adopted, immunosuppressive therapy aimed at inhibitor eradication can nevertheless be started if a rapid reduction of the inhibitor titre is not observed or bleeding symptoms occur | 2B |
| C. TREATMENT OF BLEEDING | |
| Haemostatic treatment should be initiated promptly in the event of clinically significant bleeding | 1B |
| Treatment should be managed by centres with clinical and laboratory expertise in the diagnosis and treatment of patients with haemophilia and other coagulopathies. If prompt referral to such centres is unfeasible, treatment should be undertaken as soon as possible, considering the actual or rapid local availability of therapeutic agents, according to protocols previously agreed with the specialised centres. These centres will provide adequate support in the early stages of treatment, pending their direct care of the patient | |
| Bypassing agents (activated prothrombin complex concentrate and recombinant activated FVII) are first-line options for treatment of bleeding | 1B |
| Susoctocog alfa is a first-line treatment option in patients in whom low anti-porcine FVIII cross-reactivity should previously be determined, in healthcare settings in which the necessary laboratory monitoring, in particular FVIII activity, is ensured | 1C |
| The preliminary assessment of the anti-porcine FVIII inhibitor titre is necessary to predict the effectiveness of treatment with susoctocog alfa | 1C |
| Bypassing agents should be considered as the first-line haemostatic approach if the treatment is undertaken outside of specialised centres and/or if the prompt and regular laboratory monitoring needed for the appropriate use of replacement treatment is not available | |
| The assessment of the efficacy of bypassing agents is based mainly on clinical criteria and should be carried out timely and assiduously, at intervals not exceeding 6–12 hours | |
| Susoctocog alfa should be considered for adult in-patients in whom treatment can be managed by a specialised centre, with laboratory facilities to perform coagulation tests around the clock | |
| Treatment with susoctocog alfa requires careful clinical monitoring and evaluation of FVIII levels 30 minutes and 3 hours after each administration | |
| Treatment with susoctocog alfa should be considered particularly in those conditions in which monitoring the maintenance of haemostatic levels of FVIII can improve the efficacy and safety of treatment, such as in severe bleeding, and/or in patients with high cardiovascular or thromboembolic risk | |
| If a first-line therapeutic approach is ineffective, switching to alternative treatments and/or the evaluation of further therapeutic options should be considered promptly | 1C |
| If both bypassing agents, used as single agents, are ineffective and susoctocog alfa and/or its laboratory monitoring are not available, the combined administration of the two bypassing agents can be considered as an extreme rescue therapy, taking great care with regard to the risk of thromboembolic complications, especially in the elderly and/or in patients with comorbidities increasing cardiovascular or thromboembolic risk | 2C |
| The use of human-derived FVIII concentrates can be considered in patients with low inhibitor titres (<5 BU/mL), particularly in conditions in which monitoring of FVIII levels can contribute to the efficacy and safety of treatment, such as in cases of severe bleeding, surgery, invasive procedures and/or in patients with very high cardiovascular or thromboembolic risk | 2C |
| In these cases, the initial dose of FVIII should be sufficient to neutralise the inhibitor and ensure adequate haemostatic levels of the coagulation factor. Treatment should be monitored by measuring FVIII levels after administration of the initial dose and, thereafter, at least daily or more frequently, based on the haemostatic response assessed clinically at least every 6–12 h. This is also useful to detect possible anamnestic responses | |
| Due to poor response predictability and possible adverse events, DDAVP is not usually recommended, unless other therapeutic options are unavailable and the patient has a low-titre inhibitor | 1C |
| Tranexamic acid can be useful, alone or in association with other haemostatic agents, especially in the case of mucosal bleeding | 2C |
| In the presence of haematuria, tranexamic acid should be avoided. Caution should be adopted in the elderly and in those with comorbidities increasing cardiovascular and thromboembolic risk | |
When possible (grey rows), depending on the data available in the literature, a recommendation was assigned a strength, based on the GRADE methodology11. In the remaining cases (white rows) general principles of management and treatment are reported, based on consensus and common clinical practice.
AHA: acquired haemophilia A; aPTT: activated partial thromboplastin time; BU: Bethesda unit; DDAVP: desmopressin; FVIII: factor VIII.
Supplementary Information
ACKNOWLEDGEMENTS
The Authors, on behalf of the Italian Association of Haemophilia Centres (AICE) and of the Italian Society on Thrombosis and Haemostasis (SISET), thank the Councils of the Scientific Societies which shared this consensus document for their efforts dedicated to improving knowledge and awareness about AHA: Federazione Centri per la Sorveglianza della trombosi e delle terapie Antitrombotiche (FCSA); Associazione Italiana Ematologia e Oncologia Pediatrica (AIEOP); Associazione Italiana di Ostetricia (AIO); Associazione Italiana Oncologia Medica (AIOM); Associazione Ostetrici Ginecologi Ospedalieri Italiani (AOGOI); Federazione delle Associazioni Dirigenti Ospedalieri Internisti (FADOI); Società Italiana di Allergologia, Asma ed Immunologia Clinica (SIAAIC); Società Italiana di Biochimica Clinica (SIBioC); Società Italiana di Chirurgia Oncologica (SICO); Società Italiana di Ematologia (SIE): Società Italiana Ematologia Sperimentale (SIES); Società Italiana di Farmacologia (SIF); Società Italiana di Farmacia Clinica (SIFAC); Società Italiana di Farmacia Clinica e Terapia (SIFACT); Società Italiana di Gerontologia e Geriatria (SIGG); Società Italiana di Ginecologia e Ostetricia (SIGO); Società Italiana di Medicina d’Emergenza-Urgenza (SIMEU); Società Italiana di Medicina di Emergenza ed Urgenza Pediatrica (SIMEUP); Società Italiana di Medicina Generale e delle cure primarie (SIMG); Società Italiana di Medicina Trasfusionale e Immunoematologia (SIMTI); Società Italiana di Patologia clinica e Medicina di Laboratorio (SIPMeL); Società Italiana di Reumatologia (SIR); Società Italiana di Scienze Ostetrico-Ginecologico-Neonatali (SISOGN).
Appendix 1.
Members of the ad hoc Working Group are listed in alphabetical order (affiliation in parenthesis)
Raffaele Antonelli Incalzi (Società Italiana di Gerontologia e Geriatria [SIGG], Florence, Italy)
Giordano Beretta (Associazione Italiana di Oncologia Medica [AIOM] Milan, Italy)
Pier Luigi Berti (Società Italiana di Medicina Trasfusionale e Immunoematologia [SIMTI], Rome, Italy),
Chiara Biasoli (Unit of Transfusion Medicine and Centre for Inherited Bleeding Disorders, “M. Bufalini” Hospital, Cesena, Italy),
Alessandra Borchiellini (Regional Reference Center of Haemostasis and Thrombosis, Haematology Unit, “Città della Salute e della Scienza” University Hospital, Turin, Italy),
Antonio Chiantera (Società Italiana di Ginecologia e Ostetricia - Federazione Italiana [SIGO], Rome, Italy),
Claudio Cricelli (Società Italiana di Medicina Generale e delle Cure Primarie [SIMG], Florence, Italy),
Dorina Cultrera (Haemophilia Regional Reference Center, “Vittorio Emanuele” University Hospital, Catania, Italy),
Giovanni Di Minno (Department of Clinical Medicine and Surgery, University of Naples “Federico II”, Naples, Italy),
Gabriella Gamba (Associazione Italiana dei Centri Emofilia [AICE] Scientific Committee, Milan, Italy),
Adele Giampaolo (Istituto Superiore di Sanità [ISS], Rome, Italy),
Paola Giordano (Department of Biomedical Science and Human Oncology-Pediatric Unit, University of Bari “Aldo Moro”, Bari, Italy),
Maria Golato (Società Italiana di Patologia Clinica e Medicina di Laboratorio [SIPMeL], Castel Franco Veneto, Italy),
Corrado Giua Marassi (Società Italiana di Farmacia Clinica [SIFAC], Cagliari, Italy),
Anna Chiara Giuffrida (Haemophilia Centre, Transfusion Medicine Unit, Integrated University Hospital of Verona, Verona, Italy),
Alessandro Gronchi (Società Italiana di Chirurgia Oncologica [SICO], Naples, Italy),
Hamisa Jane Hassan (Associazione Italiana dei Centri Emofilia [AICE] Scientific Committee, Milan, Italy),
Giuseppe Lassandro (Department of Biomedical Science and Human Oncology-Pediatric Unit, University of Bari “Aldo Moro”, Bari, Italy),
Riccardo Lubrano (Società Italiana di Medicina di Emergenza ed Urgenza Pediatrica [SIMEUP], Milan, Italy),
Salvatore Manca (Società Italiana della medicina di emergenza-urgenza [SIMEU], Turin, Italy),
Maria Elisa Mancuso (Center for Thrombosis and Hemorrhagic Diseases, IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy),
Dario Manfellotto (Federazione delle Associazioni dei Dirigenti Ospedalieri Internisti [FADOI], Milan, Italy),
Antonella Marchi (Associazione Italiana Odontoiatri Sede Nazionale [AIO], Turin, Italy),
Maria Gabriella Mazzucconi (Associazione Italiana dei Centri Emofilia [AICE] Scientific Committee, Milan, Italy),
Massimo Morfini (Chair, Associazione Italiana dei Centri Emofilia [AICE] Scientific Committee, Milan, Italy),
Pellegrino Musto (Società Italiana di Medicina e Chirurgia Estetica [SIES], Florence, Italy),
Antonella Nespoli (Società Italiana di Scienze Ostetrico-Ginecologico Neonatali [SISOGN], Salerno, Italy),
Lucia Dora Notarangelo (Direzione Mediche, ASS Spedali Civili di Brescia, Brescia, Italy),
Angelo Palozzo (Società Italiana di Farmacia Clinica e Terapia [SIFACT], Milan, Italy),
Giorgio Racagni (Società Italiana di Farmacologia [SIF], Milan, Italy),
Elena Santagostino (Angelo Bianchi Bonomi Haemophilia and Thrombosis Centre, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy; currently Swedish Orphan Biovitrum, Stockholm, Sweden),
Laura Sciacovelli (Società Italiana di Biochimica Clinica e Biologia Molecolare Clinica - Medicina di Laboratorio [SIBioC], Milan, Italy),
Gianenrico Senna (Società Italiana di Allergologia, Asma ed Immunologia Clinica [SIAAIC], Milan, Italy),
Luigi Sinigaglia (Società Italiana di Reumatologia [SIR], Milan, Italy)
Sergio Siragusa (Società Italiana di Ematologia [SIE], Bologna, Italy),
Sophie Testa (Federazione dei Centri Sorveglianza Anticoagulati [FCSA], Rome, Italy),
Alberto Tosetto (Hematology Department, Ospedale San Bortolo, Vicenza, Italy),
Elsa Viora (Associazione degli Ostetrici e Ginecologi Ospedalieri Italiani [AOGOI], Milan, Italy),
Marco Zecca (Associazione Italiana di Ematologia e Oncologia Pediatrica [AIEOP], Bologna, Italy).
Footnotes
CONFLICT OF INTEREST DISCLOSURE
AC has acted as a paid consultant or speaker for Bayer, Kedrion, Novo Nordisk, Roche and Werfen. MF has acted as a paid consultant or speaker for Bayer and Novo Nordisk. AT has received speaker fees from Roche, Stago, Sobi and Werfen. GC has participated as a speaker or in advisory boards for Alexion, CSL Behring, Kedrion, Novo Nordisk, Pfizer, Sanofi, Sobi, Takeda, Uniqure and Werfen, acted as a consultant to Roche, and received research funding directly to his Institution from CSL Behring, Pfizer and Sobi. CS has acted as a paid consultant/advisor/speaker for Amgen, Bayer, Biomarin, CSL Behring, Novo Nordisk, Roche, Sobi and Takeda. LC has received speaker fees from Bayer and Novo Nordisk. RDC has received speaker fees from Bayer, Sanofi, Roche, Takeda, and Sobi and participated in scientific advisory boards for Bayer, Sanofi, Pfizer, Werfen, Sobi and Kedrion. ACM has acted as an advisor for Bayer, CSL Behring, Roche, SOBI and Takeda and has received speaker fees from Bayer, CSL Behring, Kedrion, Novo Nordisk, Pfizer, Roche, SOBI and Takeda. PG has received speaker fees from Sanofi. AR has acted as a paid consultant/advisor/speaker for Bayer, CSL Behring, Shire/Takeda, Novo Nordisk, Kedrion, Pfizer, Roche and Sobi. RCS, RM, EZ and GFR declare that they have no interests which might be perceived as conflicts or bias.
REFERENCES
- 1.Franchini M, Vaglio S, Marano G, et al. Acquired hemophilia A: a review of recent data and new therapeutic options. Hematology. 2017;25:1–7. doi: 10.1080/10245332.2017.1319115. [DOI] [PubMed] [Google Scholar]
- 2.Coppola A, Favaloro EJ, Tufano A, et al. Acquired inhibitors of coagulation factors: part I - acquired hemophilia A. Semin Thromb Hemost. 2012;38:433–46. doi: 10.1055/s-0032-1315757. [DOI] [PubMed] [Google Scholar]
- 3.Collins PW, Hirsch S, Baglin TP, et al. for the UK Haemophilia Centre Doctors’ Organisation. Acquired haemophilia A in the United Kingdom: a 2-year national surveillance study by the United Kingdom Haemophilia Centre Doctors’ Organisation. Blood. 2007;109:1870–7. doi: 10.1182/blood-2006-06-029850. [DOI] [PubMed] [Google Scholar]
- 4.Knoebl P, Marco P, Baudo F, et al. on behalf of the EACH2 Registry Contributors. Demographic and clinical data in acquired hemophilia A: results from the European Acquired Haemophilia Registry (EACH2) J Thromb Haemost. 2012;10:622–31. doi: 10.1111/j.1538-7836.2012.04654.x. [DOI] [PubMed] [Google Scholar]
- 5.Tiede A, Klamroth R, Scharf RE, et al. Prognostic factors for remission of and survival in acquired hemophilia A (AHA): results from the GTH-AH 01/2010 study. Blood. 2015;125:1091–7. doi: 10.1182/blood-2014-07-587089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huth-Kühne A, Baudo F, Collins P, et al. International recommendations on the diagnosis and treatment of patients with acquired hemophilia A. Haematologica. 2009;94:566–75. doi: 10.3324/haematol.2008.001743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franchini M, Castaman G, Coppola A, et al. AICE Working Group. Acquired inhibitors of clotting factors: AICE recommendations for diagnosis and management. Blood Transfus. 2015;13:498–513. doi: 10.2450/2015.0141-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins PW, Chalmers E, Hart D, et al. Diagnosis and management of acquired coagulation inhibitors: a guideline from UKHCDO. Br J Haematol. 2013;162:758–73. doi: 10.1111/bjh.12463. [DOI] [PubMed] [Google Scholar]
- 9.Tiede A, Giangrande P, Teitel J, et al. Clinical evaluation of bleeds and response to haemostatic treatment in patients with acquired haemophilia: a global expert consensus statement. Haemophilia. 2019;25:969–78. doi: 10.1111/hae.13844. [DOI] [PubMed] [Google Scholar]
- 10.Tiede A, Collins P, Knoebl P, et al. International recommendations on the diagnosis and treatment of acquired hemophilia A. Haematologica. 2020;105:1791–801. doi: 10.3324/haematol.2019.230771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schunemann H, Brozek J, Guyatt G, Oxman A, editors. GRADE Handbook. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. [Accessed 15/11/2021.]. Updated October 2013. Available at http://gdt.guidelinedevelopment.org/central_prod/design/client/handbook/handbook.html.
- 12.Franchini M, Zaffanello M, Lippi G. Acquired hemophilia in pediatrics: a systematic review. Pediatr Blood Cancer. 2010;55:606–11. doi: 10.1002/pbc.22657. [DOI] [PubMed] [Google Scholar]
- 13.Solymoss S. Postpartum acquired factor VIII inhibitors: results of a survey. Am J Haematol. 1998;59:1–4. doi: 10.1002/(sici)1096-8652(199809)59:1<1::aid-ajh1>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 14.Franchini M. Postpartum acquired factor VIII inhibitors. Am J Hematol. 2006;81:768–73. doi: 10.1002/ajh.20702. [DOI] [PubMed] [Google Scholar]
- 15.Tengborn L, Baudo F, Huth-Kühne A, et al. on behalf of the EACH2 Registry Contributors. Pregnancy-associated acquired haemophilia A: results from the European Acquired Haemophilia (EACH2) registry. BJOG. 2012;119:1529–37. doi: 10.1111/j.1471-0528.2012.03469.x. [DOI] [PubMed] [Google Scholar]
- 16.Franchini M, Glingani C, De Donno G, et al. The first case of acquired hemophilia A associated with SARS-CoV-2 infection. Am J Hematol. 2020;95:E197–8. doi: 10.1002/ajh.25865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radwi M, Farsi SA. Case report of acquired haemophilia following COVID-19 vaccine. J Thromb Haemost. 2021;19:1515–8. doi: 10.1111/jth.15291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kessler CM, Ma AD, Al-Mondhiry HA, Gut RZ, Cooper DL. Assessment of acquired hemophilia patient demographics in the United States: the Hemostasis and Thrombosis Research Society Registry. Blood Coagul Fibrinolysis. 2016;27:761–9. doi: 10.1097/MBC.0000000000000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun B, Xue F, Feng Y, et al. Outcome of CARE: a 6-year national registry of acquired haemophilia A in China. Br J Haematol. 2019;187:653–65. doi: 10.1111/bjh.16128. [DOI] [PubMed] [Google Scholar]
- 20.Mingot-Castellano M-E, Pardos Gea J, et al. Management of acquired hemophilia A: results from the Spanish registry. Blood Adv. 2021;5:3821–9. doi: 10.1182/bloodadvances.2021004626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kershaw G, Jayakodi D, Dunkley S. Laboratory identification of factor inhibitors: the perspective of a large tertiary hemophilia center. Semin Thromb Hemost. 2009;35:760–8. doi: 10.1055/s-0029-1245108. [DOI] [PubMed] [Google Scholar]
- 22.Kershaw G, Orellana D. Mixing tests: diagnostic aides in the investigation of prolonged prothrombin times and activated partial thromboplastin times. Semin Thromb Hemost. 2013;39:283–90. doi: 10.1055/s-0033-1336832. [DOI] [PubMed] [Google Scholar]
- 23.Verbruggen B, Novakova I, Wessels H, et al. The Nijmegen modification of the Bethesda assay for factor VIII:C inhibitors: improved specificity and reliability. Thromb Haemost. 1995;73:247–51. [PubMed] [Google Scholar]
- 24.Tiede A, Werwitzke S, Scharf RE. Laboratory diagnosis of a cquired hemophilia A: limitations, consequences and challenges. Semin Thromb Hemost. 2014;4:803–11. doi: 10.1055/s-0034-1390004. [DOI] [PubMed] [Google Scholar]
- 25.Werwitzke S, Geisen U, Nowak-Goettl U, et al. Diagnostic and prognostic value of factor VIII binding antibodies in acquired hemophilia A: data from the GTH-AH 01/2010 study. J Thromb Haemost. 2016;14:940–7. doi: 10.1111/jth.13304. [DOI] [PubMed] [Google Scholar]
- 26.Devreese KMJ, de Groot PG, de Laat B, et al. Guidance from the Scientific and Standardization Committee for lupus anticoagulant/antiphospholipid antibodies of the International Society on Thrombosis and Haemostasis Update of the guidelines for lupus anticoagulant detection and interpretation. J Thromb Haemost. 2020;18:2828–39. doi: 10.1111/jth.15047. [DOI] [PubMed] [Google Scholar]
- 27.Tripodi A, Mancuso ME, Chantarangkul V, et al. Lupus anticoagulants and their relationship with the inhibitors against coagulation factor VIII: considerations on the differentiation between the 2 circulating anticoagulants. Clin Chem. 2005;51:1883–5. doi: 10.1373/clinchem.2005.054312. [DOI] [PubMed] [Google Scholar]
- 28.Willner CA, Chisti MM. Treatment of bleeding diathesis associated with a heparin-like anticoagulant in plasma cell neoplasia using protamine. Case Rep Hematol. 2018;2018:4342301. doi: 10.1155/2018/4342301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nacoti M, Cantù D, Bonacina D, et al. Heparin-like effect resistant to protamine in a child with haemorrhagic shock. Do we need heparinase? Blood Transfus. 2018;16:394–6. doi: 10.2450/2017.0088-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kruse-Jarres R, Kempton CL, Baudo F, et al. Acquired hemophilia A: updated review of evidence and treatment guidance. Am J Hematol. 2017;92:695–705. doi: 10.1002/ajh.24777. [DOI] [PubMed] [Google Scholar]
- 31.Windyga J, Baran B, Odnoczko E, et al. Guidelines for acquired hemophilia A. Ginekol Pol. 2019;90:353–64. doi: 10.5603/GP.2019.0063. [DOI] [PubMed] [Google Scholar]
- 32.Franchini M, Capra F, Nicolini N, et al. Drug-induced anti-factor VIII antibodies: a systematic review. Med Sci Monit. 2007;13:RA55–61. [PubMed] [Google Scholar]
- 33.Zanon E, Pasca S, Siragusa S, et al. FAIR Study Group. Low dose of aPCC after the initial treatment in acquired haemophilia A is useful to reduce bleeding relapses: data from the FAIR registry. Thromb Res. 2019;174:24–6. doi: 10.1016/j.thromres.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 34.Turecek PL, Váradi K, Gritsch H, Schwarz HP. FEIBA: mode of action. Haemophilia. 2004;10(Suppl 2):3–9. doi: 10.1111/j.1365-2516.2004.00934.x. [DOI] [PubMed] [Google Scholar]
- 35.Sallah S. Treatment of acquired haemophilia with factor eight inhibitor bypassing activity. Haemophilia. 2004;10:169–73. doi: 10.1046/j.1365-2516.2003.00856.x. [DOI] [PubMed] [Google Scholar]
- 36.Franchini M, Lippi G. Recombinant activated factor VII: mechanisms of action and current indications. Semin Thromb Hemost. 2010;36:485–92. doi: 10.1055/s-0030-1255442. [DOI] [PubMed] [Google Scholar]
- 37.Franchini M, Coppola A, Tagliaferri A, Lippi G. FEIBA versus NovoSeven in hemophilia patients with inhibitors. Semin Thromb Hemost. 2013;39:772–8. doi: 10.1055/s-0033-1354425. [DOI] [PubMed] [Google Scholar]
- 38.Hay CRM, Negrier C, Ludlam CA. The treatment of bleeding in acquired hemophilia with recombinant factor VIIa: a multicenter study. Thromb Haemost. 1997;78:3–7. [PubMed] [Google Scholar]
- 39.Sumner MJ, Geldziler BD, Pedersen M, et al. Treatment of acquired haemophilia with recombinant activated FVII: a critical appraisal. Haemophilia. 2007;13:451–61. doi: 10.1111/j.1365-2516.2007.01474.x. [DOI] [PubMed] [Google Scholar]
- 40.Baudo F, Collins P, Huth-Kühne A, et al. on behalf of the EACH2 registry contributors. Management of bleeding in acquired hemophilia A: results from the European Acquired Haemophilia (EACH2) Registry. Blood. 2012;120:39–46. doi: 10.1182/blood-2012-02-408930. [DOI] [PubMed] [Google Scholar]
- 41.Ehrlich HJ, Henzl MJ, Gomperts ED. Safety of factor VIII inhibitor bypass activity (FEIBA): 10-year compilation of thrombotic adverse events. Haemophilia. 2002;8:83–90. doi: 10.1046/j.1365-2516.2002.00532.x. [DOI] [PubMed] [Google Scholar]
- 42.Zanon E, Pasca S, Santoro C, et al. Activated prothrombin complex concentrate (FEIBA®) in acquired haemophilia A: a large multicentre Italian study - the FAIR Registry. Br J Haematol. 2019;184:853–5. doi: 10.1111/bjh.15175. [DOI] [PubMed] [Google Scholar]
- 43.Amano K, Seita I, Higasa S, Sawada A, Kuwahara M, Shima M. Treatment of acute bleeding in acquired haemophilia A with recombinant activated factor VII: analysis of 10-year Japanese postmarketing surveillance data. Haemophilia. 2017;23:50–8. doi: 10.1111/hae.13033. [DOI] [PubMed] [Google Scholar]
- 44.Nogami K. The utility of thromboelastography in inherited and acquired bleeding disorders. Br J Haematol. 2016;174:503–14. doi: 10.1111/bjh.14148. [DOI] [PubMed] [Google Scholar]
- 45.Ingerslev J, Sørensen B. Parallel use of by-passing agents in haemophilia with inhibitors: a critical review. Br J Haematol. 2011;155:256–62. doi: 10.1111/j.1365-2141.2011.08854.x. [DOI] [PubMed] [Google Scholar]
- 46.Teitel J, Berntorp E, Collins P, et al. A systematic approach to controlling problem bleeds in patients with severe congenital haemophilia A and high-titre inhibitors. Haemophilia. 2007;13:256–63. doi: 10.1111/j.1365-2516.2007.01449.x. [DOI] [PubMed] [Google Scholar]
- 47.Zanon E, Milan M, Brandolin B, et al. High dose of human plasma-derived FVIII-VWF as first-line therapy in patients affected by acquired haemophilia A and concomitant cardiovascular disease: four case reports and a literature review. Haemophilia. 2013;19:e50–3. doi: 10.1111/hae.12033. [DOI] [PubMed] [Google Scholar]
- 48.Franchini M, Lippi G. The use of desmopressin in acquired haemophilia A: a systematic review. Blood Transfus. 2011;9:377–82. doi: 10.2450/2011.0113-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Freedman J, Rand ML, Russell O, et al. Immunoadsorption may provide a cost-effective approach to management of patients with inhibitors to FVIII. Transfusion. 2003;43:1508–13. doi: 10.1046/j.1537-2995.2003.00559.x. [DOI] [PubMed] [Google Scholar]
- 50.Zeitler H, Ulrich-Merzenich G, Panek D, et al. Extracorporeal treatment for the acute und long-term outcome of patients with life-threatening acquired hemophilia. Transfus Med Hemother. 2012;39:264–70. doi: 10.1159/000341913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grahammer F, Fischer KG. Successful immunoadsorption of life-threatening bleeding in factor VIII inhibitor disease, but no long-term remission with anti-CD20 treatment. BMJ Case Rep. 2015;2015:bcr2015210034. doi: 10.1136/bcr-2015-210034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morrison AE, Ludlam CA, Kessler C. Use of porcine factor VIII in the treatment of patients with acquired hemophilia. Blood. 1993;81:1513–20. [PubMed] [Google Scholar]
- 53.Mannucci PM, Franchini M. Porcine recombinant factor VIII: an additional weapon to handle anti-factor VIII antibodies. Blood Transfus. 2017;15:365–8. doi: 10.2450/2016.0030-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.EMA [internet] Obizur: Summary of product characteristics; [Accessed on 29/08/2021.]. https://www.ema.europa.eu/en/documents/product-information/obizur-epar-product-information_en.pdf . [Google Scholar]
- 55.Kruse-Jarres R, St-Louis J, Greist A, et al. Efficacy and safety of OBI-1, an antihaemophilic factor VIII (recombinant), porcine sequence, in subjects with acquired haemophilia A. Haemophilia. 2015;21:162–70. doi: 10.1111/hae.12627. [DOI] [PubMed] [Google Scholar]
- 56.Tarantino MD, Cuker A, Hardesty B, et al. Recombinant porcine sequence factor VIII (rpFVIII) for acquired haemophilia A: practical clinical experience of its use in seven patients. Haemophilia. 2017;23:25–32. doi: 10.1111/hae.13040. [DOI] [PubMed] [Google Scholar]
- 57.Campbell S, Mason J, Prasad R, Amboise H, Hunt S, Tran H. Acquired haemophilia and haemostatic control with recombinant porcine FVIII: case series. Intern Med J. 2021;51:215–9. doi: 10.1111/imj.14773. [DOI] [PubMed] [Google Scholar]
- 58.Zanon E, Pasca S, Borchiellini A, et al. Susoctocog-alfa (Obizur®) in the treatment of nine elderly patients with acquired haemophilia A: an Italian multicentre real-life experience. Blood Transfus. 2020;18:312–21. doi: 10.2450/2020.00006-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koster T, Blann AD, Briët E, Vandenbroucke JP, Rosendaal FR. Role of clotting factor VIII in effect of von Willebrand factor on occurrence of deep-vein thrombosis. Lancet. 1995;345:152–5. doi: 10.1016/s0140-6736(95)90166-3. [DOI] [PubMed] [Google Scholar]
- 60.Türkantoz H, Königs C, Knöbl P, et al. Cross-reacting inhibitors against recombinant porcine factor VIII in acquired hemophilia A: data from the GTH-AH 01/2010 Study. J Thromb Haemost. 2020;18:36–43. doi: 10.1111/jth.14618. [DOI] [PubMed] [Google Scholar]
- 61.Abou-Ismail MY, Vuyyala S, Prunty J, Schmaier AH, Nayak L. Short-term efficacy of recombinant porcine factor VIII in patients with factor VIII inhibitors. Haemophilia. 2020;26:601–6. doi: 10.1111/hae.14014. [DOI] [PubMed] [Google Scholar]
- 62.Mannucci PM. Hemostatic drugs. N Engl J Med. 1998;339:245–53. doi: 10.1056/NEJM199807233390407. [DOI] [PubMed] [Google Scholar]
- 63.Holmström M, Tran HT, Holme PA. Combined treatment with APCC (FEIBA®) and tranexamic acid in patients with haemophilia A with inhibitors and in patients with acquired haemophilia A – a two-centre experience. Haemophilia. 2012;18:544–9. doi: 10.1111/j.1365-2516.2012.02748.x. [DOI] [PubMed] [Google Scholar]
- 64.Pasca S, Ambaglio C, Rocino A, et al. FAIR Study Group. Combined use of antifibrinolytics and activated prothrombin complex concentrate (aPCC) is not related to thromboembolic events in patients with acquired haemophilia A: data from FAIR Registry. J Thromb Thrombolysis. 2019;47:129–33. doi: 10.1007/s11239-018-1750-y. [DOI] [PubMed] [Google Scholar]
- 65.Kim CH, Simmons SC, Wang D, Najafzadeh P, Azad A, Pham HP. An economic analysis of different treatments for bleeding in patients with acquired haemophilia. Vox Sang. 2020;115:192–9. doi: 10.1111/vox.12877. [DOI] [PubMed] [Google Scholar]
- 66.Tufano A, Coppola A, Guida A, et al. Acquired haemophilia A in the elderly: case reports. Curr Gerontol Geriatr. 2010;2010:927503. doi: 10.1155/2010/927503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.