Platelet transfusions are crucial for curative or prophylactic supportive treatments to lower the risk of haemorrhage. Haemorrhage is associated with oncological diseases and their treatment, myelosuppression or during transplantation of haematopoietic stem cells, and with most invasive surgical procedures. In these cases, transfusion is then the only effective treatment.
Transfusion of platelets is generally safe and largely beneficial to patients. Inactivation of pathogens in platelet concentrates (PCs) lowers the risk of bacterial transfusion-transmitted infections, and platelet-related transfusion reactions in general. Platelets that have been treated with inactivation of pathogens are clearly distinct from platelets that have not been treated. Recent randomised controlled trials, however, found that transfusing pathogen-reduced platelets did not worsen outcome when compared to transfusing regular platelet components for preventing bleeding episodes1.
Although rare, recipients of blood transfusions can experience adverse reactions, for which clinical signs can appear during the transfusion or within hours (h) to days following the procedure. The role of PCs in such serious adverse reactions (SAR) could be related to the inflammatory function of platelets. Indeed, besides their contribution to the haemostatic response, platelets are innate immunity cells that lead to pro-inflammatory events2–7. On rare occasions, SAR occur with clinical presentation of acute inflammation. Transfusion-related acute lung injury (TRALI) is among the most common cause of fatal transfusion reactions. TRALI is characterised by acute respiratory distress and non-cardiogenic lung oedema developing during, or within 6 h, of transfusion8. The pathophysiology of this transfusion complication brings complex cellular communication into play. The role played by blood platelets in TRALI pathophysiology, particularly in TRALI-related inflammatory processes, remains a subject of debate9.
The primary role of platelets is to maintain haemostasis10. However, they can also be actively involved in the inflammatory response by interacting with endothelial cells and circulating leukocytes. Platelets prepared for transfusion suffer stress-induced lesions during collection, preparation and storage4. Ex vivo processing of platelets may influence the secretion of biological response modifiers (BRM) and alter both platelet structure and function4. Recently, McVey et al. demonstrated that extracellular vesicles (EVs) derived from stored platelets cause TRALI as a consequence of their elevated ceramide and decreased sphingosine-1-phosphate (S1P) content. Their study provides new insights into helping reduce TRALI incidence and severity11 by inhibiting ceramide formation or supplementing platelet concentrates with S1P. Their identification of platelet-derived EVs as a potential therapeutic target in TRALI is an important finding11.
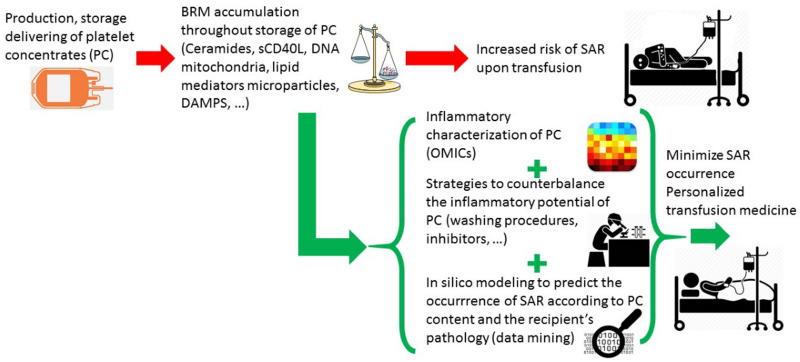
Even though platelets are typically considered to be the chief mediators of haemostasis, there is increasing evidence of a continuum between thrombosis and immunity4,6,12. Several studies support the current observations highlighting the interconnections between platelets, endothelial cells and immune cells in the context of sterile inflammation, such as transfusion13, or of infectious inflammation4,14. The various data found in the literature consolidate the hypothesis that platelet activation in concentrates may play a direct role in an inflammatory response within the recipient, depending on the storage lesion resulting in a range of BRM accumulation, including sCD40L, mitochondria, microparticle-associated molecules, lipid mediators, soluble danger associated molecular patterns (DAMPS)4,6,12. The discovery of platelet toll-like receptor molecules (TLR) expression15–17 allowed a paradigm change in the understanding of platelet contribution to pathophysiological processes, putting platelets in a continuum of innate and adaptive immunity, at the crossroad of haemostasis and thrombosis. Since 2005, functional platelet TLR4 has been the most extensively studied molecule of the TLR family18,19; platelets do express functional levels of TLR4, which contribute to the modulation of LPS-induced thrombocytopenia. One of the characteristic studies of this paradigm showed that soluble high mobility group box 1 (HMGB1) levels are also heavily associated with SAR20. It is worth noting that HMGB1 present in platelet concentrates is a receptor for advanced glycation end-products (RAGE), TLR-2, TLR-4 and TLR-9 ligands4,14,20–23; as such, HMGB1 is considered a DAMP. It therefore seems possible that the membrane or intracytoplasmic expression of platelet TLRs plays a role in the recognition of DAMPs and pathogen-associated molecular patterns. We have now provided first evidence that levels of soluble HMGB1 are also strongly associated with SAR20.
To date, consequences of the preparation, storage, and delivering processes on the inflammatory content of platelet concentrates has been underestimated, especially because of: 1) the absence of any systematic characterisation of platelet concentrates from the point of view of inflammation; 2) the problems in making any robust claims of an association between one or more inflammatory biomarkers with SAR; 3) the lack of strategies to counterbalance the inflammatory content in platelet concentrates; and 4) the variable susceptibility of recipients to transfusion, which is difficult to anticipate. However, a better understanding of the platelet storage lesions, including the loss of haemostatic function and the increase in inflammatory potential over storage time, would help optimise treatment of concentrates during storage and greatly benefit the transfusion recipients (Figure 1). Efforts should, therefore, focus on the fine characterisation of the inflammatory potential of PCs and the development of new strategies to reduce it, including inhibitors of platelet-derived inflammatory molecules, washing protocols that preserve platelet function, or additives to counterbalance the effects of inflammatory molecules secreted by platelets during storage24 (Table I). Thanks to OMICs technology and data mining, the emergence of studies into the inflammatory potential of platelet concentrates in transfusion will provide transfusion medicine experts with a variety of new toolsets to redefine the combination of donor and recipient characteristics, giving greater meaning to the concept of personalised transfusion medicine.
Figure 1.
Transfusing the right platelet concentrate to the right patient in terms of inflammation
Table I.
Strategies to reduce the occurrence of serious adverse reactions (SAR) upon transfusion
| Characterisation of the pro-inflammatory/anti-inflammatory imbalance in PC | Technical procedures to counterbalance the inflammatory potential of PC | Recipient factors to be correlated with PC features for in silico modeling of SAR occurrence | Recipient outcome upon PC transfusion |
|---|---|---|---|
Proteomics
|
Washing procedures (with additive solutions containing, or not, citrate dextrose-buffered saline) Membrane filtration Inhibitors
|
Indication for PC transfusion
Biological and clinical values Genetic polymorphisms related to platelet receptors or ligands |
Occurrence of SAR (yes/no) Type of SAR (fever, urticaria, pulmonary oedema) SAR severity (from isolated dysfunction, without clinical or biological manifestation, to death) Imputability (from excluded to certain) Transfusion efficacy (number of PC transfused, platelet yield, additional blood product used) |
Extracellular vesicles
|
Usual medication
Comorbidities |
PC: platelet concentrate; BMI: Body Mass Index.
Footnotes
The Authors declare no conflicts of interest.
REFERENCES
- 1.Schubert P, Johnson L, Marks DC, Devine DV. Ultraviolet-based pathogen inactivation systems: untangling the molecular targets activated in platelets. Front Med. 2018;5:129. doi: 10.3389/fmed.2018.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adam F, Kauskot A, Nurden P, et al. Platelet JNK1 is involved in secretion and thrombus formation. Blood. 2010;115:4083–92. doi: 10.1182/blood-2009-07-233932. [DOI] [PubMed] [Google Scholar]
- 3.Sandrock K, Nakamura L, Vraetz T, et al. Platelet secretion defect in patients with familial hemophagocytic lymphohistiocytosis type 5 (FHL-5) Blood. 2010;116:6148–50. doi: 10.1182/blood-2010-08-302943. [DOI] [PubMed] [Google Scholar]
- 4.Cognasse F, Laradi S, Berthelot P, et al. Platelet inflammatory response to stress. Front Immunol. 2019;10:1478. doi: 10.3389/fimmu.2019.01478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kapur R, Semple JW. Platelets as immune-sensing cells. Blood Adv. 2016;1:10–14. doi: 10.1182/bloodadvances.2016000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kapur R, Zufferey A, Boilard E, Semple JW. Nouvelle cuisine: platelets served with inflammation. J Immunol. 2015;194:5579–87. doi: 10.4049/jimmunol.1500259. [DOI] [PubMed] [Google Scholar]
- 7.Maouia A, Rebetz J, Kapur R, Semple JW. The immune nature of platelets revisited. Transfus Med Rev. 2020;34:209–20. doi: 10.1016/j.tmrv.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Semple JW, Rebetz J, Kapur R. Transfusion-associated circulatory overload and transfusion-related acute lung injury. Blood. 2019;133:1840–53. doi: 10.1182/blood-2018-10-860809. [DOI] [PubMed] [Google Scholar]
- 9.Semple JW, Kapur R. The contribution of recipient platelets in TRALI: has the jury reached a verdict? Transfusion. 2020;60:886–88. doi: 10.1111/trf.15814. [DOI] [PubMed] [Google Scholar]
- 10.Ho-Tin-Noé B, Boulaftali Y, Camerer E. Platelets and vascular integrity: how platelets prevent bleeding in inflammation. Blood. 2018;131:277–88. doi: 10.1182/blood-2017-06-742676. [DOI] [PubMed] [Google Scholar]
- 11.McVey JM, Maishan M, Spring C, et al. Platelet extracellular vesicles mediate transfusion-related acute lung injury by imbalancing the sphingolipid rheostat. Blood. 2021;137:690–701. doi: 10.1182/blood.2020005985. [DOI] [PubMed] [Google Scholar]
- 12.Stolla M, Refaai MA, Heal JM, et al. Platelet transfusion - the new immunology of an old therapy. Front Immunol. 2015;6:28. doi: 10.3389/fimmu.2015.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sut C, Tariket S, Aubron C, Aloui C, et al. The non-hemostatic aspects of transfused platelets. Front Med (Lausanne) 2018;5:42. doi: 10.3389/fmed.2018.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ebermeyer T, Cognasse F, Berthelot P, et al. Platelet innate immune receptors and TLRs: a double-edged sword. Int J Mol Sci. 2021;22:7894. doi: 10.3390/ijms22157894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ward JR, Bingle L, Judge HM, et al. Agonists of toll-like receptor (TLR)2 and TLR4 are unable to modulate platelet activation by adenosine diphosphate and platelet activating factor. Thromb Haemost. 2005;94:831–8. [PubMed] [Google Scholar]
- 16.Clark SR, Ma AC, Tavener SA, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–9. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 17.Cognasse F, Hamzeh H, Chavarin P, et al. Evidence of Toll-like receptor molecules on human platelets. Immunol Cell Biol. 2005;83:196–8. doi: 10.1111/j.1440-1711.2005.01314.x. [DOI] [PubMed] [Google Scholar]
- 18.Andonegui G, Kerfoot SM, McNagny K, et al. Platelets express functional Toll-like receptor-4. Blood. 2005;106:2417–23. doi: 10.1182/blood-2005-03-0916. [DOI] [PubMed] [Google Scholar]
- 19.Aslam R, Speck ER, Kim M, et al. Platelet Toll-like receptor expression modulates lipopolysaccharide-induced thrombocytopenia and tumor necrosis factor-alpha production in vivo. Blood. 2006;107:637–41. doi: 10.1182/blood-2005-06-2202. [DOI] [PubMed] [Google Scholar]
- 20.Cognasse F, Sut C, Hamzeh-Cognasse H, Garraud O. Platelet-derived HMGB1: critical mediator of SARs related to transfusion. Ann Transl Med. 2020;8:140. doi: 10.21037/atm.2019.11.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwak MS, Kim HS, Lee B, et al. Immunological Significance of HMGB1 Post-Translational Modification and Redox Biology. Front Immunol. 2020;11:1189. doi: 10.3389/fimmu.2020.01189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raucci A, Di Maggio S, Scavello F, et al. The Janus face of HMGB1 in heart disease: a necessary update. Cell Mol Life Sci. 2019;76:211–29. doi: 10.1007/s00018-018-2930-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersson U, Yang H, Harris H. High-mobility group box 1 protein (HMGB1) operates as an alarmin outside as well as inside cells. Semin Immunol. 2018;38:40–48. doi: 10.1016/j.smim.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 24.Blumberg N, Cholette JM, Schmidt AE, et al. Management of Platelet Disorders and Platelet Transfusions in ICU Patients. Transfusion medicine reviews. 2017;31:252–57. doi: 10.1016/j.tmrv.2017.04.002. [DOI] [PubMed] [Google Scholar]