Abstract
Cystic fibrosis (CF) patients receive repeated courses of aminoglycoside therapy. These patients would consequently be expected to be more susceptible to cochleotoxicity, a recognized side effect with single courses of aminoglycoside therapy. The primary aim of this retrospective study was to establish the incidence and severity of auditory deficit in CF patients. Standard (0.25- to 8-kHz) and high-frequency (10- to 16-kHz) pure-tone audiometry was carried out in 70 CF patients, and the results were compared with the results from 91 control subjects. These subjects were further divided into pediatric and adult groups. Of 70 CF patients, 12 (1 pediatric) displayed hearing loss considered to be caused by repeated exposure to aminoglycosides. There was a nonlinear relationship between the courses of therapy received and the incidence of hearing loss. The severity of the loss did not appear to be related to the number of courses received. Assuming the risk of loss to be independent for each course, preliminary estimates of per course risk of hearing loss were less than 2%. Upon comparison with previous clinical studies and experimental work, these findings suggest that the incidence of cochleotoxicity in CF patients is considerably lower than would be expected, suggesting that the CF condition may confer protection against aminoglycoside cochleotoxicity.
In spite of the introduction of new classes of antibiotics, the aminoglycoside (AG) antibiotics gentamicin and tobramycin still remain primary agents of choice in treating serious gram-negative infections (3). In particular, their efficacy in the treatment of colonized pulmonary infections of Pseudomonas aeruginosa in cystic fibrosis (CF) patients is well established (42). Once colonized with Pseudomonas, CF patients almost invariably require repeated courses of AG therapy (17, 36).
It is well known that the use of AG antibiotics carries a risk of cochleotoxicity (9, 25). The potential for cochleotoxicity of both gentamicin and tobramycin has been investigated by many clinical studies, in non-CF patient groups, since the introduction of these agents over three decades ago (10, 18, 23). The majority of these studies have concerned single AG courses of 3 to 8 mg/kg/day given three times daily over 7 to 10 days, which yield peak levels in plasma of between 4 and 8 μg/ml (4, 10, 18, 23, 31). Normally, cochleotoxicity was assessed by carrying out standard pure-tone audiometry (PTA). Pure-tone thresholds were measured over a 0.25- to 8-kHz frequency range before and after a single course. Cochleotoxicity was then assessed categorically and judged to have occurred if two or more PTA thresholds had increased, typically by 15 or 20 dB, in one or both ears (10, 18, 23).
In terms of ranking the relative cochleotoxic potentials of gentamicin and tobramycin, reviews of results from clinical studies show that these two agents are generally equivalent (10, 18, 23). The range of cochleotoxic incidence was comparable for both drugs, falling between about 0 and 16%. The overall median incidences of cochleotoxicity reported for both drugs were also similar at about 7.5% (10, 18, 23).
Three primary factors identified with increased cochleotoxic risk are the total daily dose (in milligrams/per kilogram), the course length, and repeated courses of therapy (4, 18, 28). These three are common factors in the treatment of CF patients and typically increase, in comparison with non-CF patient groups, the per-course exposure to AGs by a factor of between 2 and 4. A typical course for a CF patient would be 10 mg/kg/day for 14 days (11, 34, 42). This regime and the resultant higher peak levels attained in plasma (10 to 12 μg/ml) are necessary (i) to offset the effects of the shorter half-life and higher volume of distribution (Vd) often seen in CF patients and (ii) to achieve increased penetration of the pulmonary compartment in order to reach an adequate bactericidal concentration in the sputum (14, 41).
The effects of repeated AG therapy on auditory function in CF has been reported in a number of studies (13, 24, 29, 30, 33, 39, 45). Using similar audiometric methods and criteria to those used in single-course studies, the incidence of cochleotoxicity in adult CF patients ranged from 0% (33, 39, 45) to up to 27% (30; D. W. Morgan, K. Pearman, P. M. Shenoi, and D. Stableforth, Abstr. 1987 BTS Meet., Thorax 43:236, 1988). In pediatric CF patients, who would generally be expected to have had fewer courses, this range was 0 to 6% (13, 24, 29). However, these studies did not comment on any relationship between the amount of AG received and hearing loss or return any estimates of the per-course risk of AG cochleotoxicity in this study group.
Due to the much greater exposure to AGs, CF patients are a group in which it is especially relevant to investigate cochleotoxicity. The report presented here arises from a larger retrospective study that utilized different measures of auditory performance. The overall aim of that study was to provide greater resolution of the potential sites and mechanisms of AG cochleotoxicity in humans. The specific aim of the part of the study reported here was to establish the effect of repeated courses of tobramycin and gentamicin on the auditory threshold and also to extend this to the more susceptible higher frequencies. We then intended to characterize the extent of hearing loss and the relationship between cumulative AG exposure and the risk and severity of any deficit.
MATERIALS AND METHODS
Ethical permission was obtained from the Research and Ethics committees of the four Health Trusts from which patients were recruited. Before taking part in the study, CF patients were first seen by their clinician and judged to be well enough to take part in the study. As a primary inclusion criterion, they also had to have completed at least one course of AGs previously. Auditory testing was carried out during routine visits to outpatient clinics. Recruitment was carried out randomly and sequentially, without prior knowledge of treatment history. Of 168 patients invited to participate, 74 agreed to take part in the study and gave their informed written consent. Of these, 70 fulfilled the screening criteria and went on to complete audiometric testing. In the case of children, consent was given by their parents. Patients were divided into young (10 to 18 years; n = 27; median age, 14 years) and adult (19 to 37 years; n = 43; median, age, 25 years) groups. If patients in either of these groups satisfied the criteria for cochleototoxicity defined below, they were then placed in a third CF group with hearing loss. CF was clinically confirmed by a combination of the sweat test and genotype testing. Two similarly aged control groups were drawn from a local school and university: young subjects (n = 45; median age, 16 years) and adult subjects (n = 46; median age, 27 years).
Screening by questionnaire, otoscopy, and tympanometry.
Prior to the study, patients and controls were screened by questionnaire to exclude hearing loss attributable to other causes. These causes included birth trauma; familial deafness; diseases implicated in hearing loss, e.g., meningitis; a history of chronic middle ear disease or ear surgery; concurrent or previous use of other known ototoxic agents, e.g., loop diuretics; a significant history of noise exposure; or nonorganic hearing loss (26).
Before audiometric testing, all subjects underwent otoscopy to establish a patent ear canal. Tympanometry was performed (Siemens Tympanometer Model DPU-411) to establish normal middle ear function, as determined by the presence of a normal type A tympanogram (8). One pediatric patient was excluded due to recent ear surgery to fit ventilation tubes. Two adult patients were excluded due to middle ear conductive loss. A third adult patient was withdrawn following a diagnosis of nonorganic hearing loss.
Standard and high-frequency PTA.
Standard PTA was performed over the range 0.25 to 8 kHz using a KD-29 audiometer and TDH 39P headphones fitted with audiocups (P. C. Werth, Balham, United Kingdom). Care in headphone placement was taken to reduce the effects of suboptimal positioning on subject estimation of higher-frequency thresholds (4 to 8 kHz) in the standard PTA (16). High-frequency PTA (HFPTA) was used to measure thresholds at 10, 12, 14, and 16 kHz, using ER-2 insert earphones (Etymotic Research). These were calibrated according to the manufacturer's specification with a Zwislocki coupler DB-100 (Etymotic Research). When driven with a 1-V root mean square (rms) signal, the ER-2 generated 100 ± 2 dB sound pressure level (SPL) over a range of 10 to 16 kHz within the coupler. Signals over the range of 10 to 16 kHz, to a maximum of a 1-V rms, were generated separately by a function generator (TG 230; RS Components, Corby, United Kingdom) and fed into to the external input of the KD-29. The KD-29 was then used as the attenuator for these signals, as in standard PTA.
For PTA and HFPTA, threshold values at each frequency were obtained to within 5 dB, according to a recognized protocol (5, 6). Testing was carried out in standard soundproof rooms of hospital audiology departments or in quiet rooms. These locations satisfied the criteria for ambient noise levels during audiometric testing (30, 44). In cases of marked unilateral hearing loss, narrow-band masking noise was delivered to the contralateral ear (i.e., threshold at a test frequency of ≥40 dB greater than the threshold level in the contralateral ear) (7). Prior to beginning the test, all subjects were familiarized with the test protocol.
History of AG exposure.
In CF patients, equivalent milligram/kilogram/day dosing regimes of gentamicin and tobramycin are employed to yield a comparable range of concentrations in plasma (14). These agents also have similar molecular weights (gentamicin, 477; tobramycin, 468). While gentamicin and tobramycin have not been shown to have identical cochleotoxic potential, they are considered to share a comparable categorical cochleotoxic ranking (10, 18, 23). Consequently, it was felt justifiable to treat the dosing regimes within this study with either drug as equivalent. Following audiometric testing, total lifetime exposures to tobramycin and gentamicin were calculated from an inspection of the hospital notes. Peak and trough AG levels for each course were also recorded.
Criteria employed for cochleototoxicity.
In the absence of clinically accepted normative data for high-frequency thresholds, the standard PTA alone was used in determining likely AG cochleotoxicity. The criteria applied in CF subjects were (i) the presence of two or more thresholds in the standard PTA in either ear of ≥20 dB hearing level (HL) over 2 to 8 kHz or (i) one frequency of ≥25 dB HL. These criteria were based on studies of hearing threshold distribution in young adults who had no known history of auditory insult (20, 26, 35). Over this frequency range, these criteria were typically ca. 90th-percentile threshold values for the age groups involved in this study.
Statistical analysis.
For analysis of summated thresholds versus the number of AG courses, Spearman's rank correlation was used. This nonparametric ranking test was employed since the data were not normally distributed (1, 40). For comparison of pure-tone threshold data, we used Kruskal-Wallis analysis of variance, with post hoc analysis using the Mann-Whitney U test, with P values appropriately corrected for multiple comparison (1). These tests were also employed for analysis of peak plasma level data. A summary measure of peak levels in plasma in individual patients was derived by taking the median of the peak levels for all courses recorded for that individual. This was designated the overall peak level in plasma. These values were then utilized to calculate the overall medians and percentiles in each patient group. Nonparametric testing was preferred due to the unequal numbers in the control and patient groups in the study and the requirement for fewer assumptions about data distribution (1, 40). A significance level of P < 0.05 was adopted throughout the analysis. Data were analyzed using MINITAB 10.
RESULTS
Occurrence of hearing loss in CF subjects.
Of the 70 CF patients, 12 (17%) were considered to show threshold elevation. Of the 12, one was from the pediatric group, and in 8 of 12 patients, the increases in the threshold were found to be persistent or progressive compared with previous or subsequent audiograms performed at least a year apart. The demographic details for each group are summarized in Table 1.
TABLE 1.
Demographic details of the different groups of CF subjects based on the results of standard PTA analyses
| CF subject group | Median age in yr (range) | Median no. of courses (range) | Male/female (M:F) gender ratio | n |
|---|---|---|---|---|
| Young CF: normal PTA thresholds | 14 (10–18) | 9 (1–28) | 11:15 | 26 |
| Adult CF: normal PTA thresholds | 25 (18–37) | 9 (1–53) | 16:16 | 32 |
| Young + adult CF: normal PTA thresholds | 21 (10–37) | 9 (1–53) | 27:31 | 58 |
| CF subjects with elevated PTA thresholds | 27 (16–36) | 20 (8–41) | 6:6 | 12 |
| All groups | 22 (10–37) | 11 (1–53) | 33:37 | 70 |
Gentamicin and tobramycin: trends in usage.
The possible relationship between categorical hearing loss and AG exposure was initially examined by looking for trends in use, the distribution of course durations, and the overall median levels in plasma. In total, 971 individual AG courses were administered, and the overall ratio of gentamicin to tobramycin usage (G:T use ratio) was 45 versus 55%. There were distinct divisions of use between the two (normal-hearing) age groups within the study. In the pediatric CF centres taking part in this study, gentamicin use prevailed over tobramycin. This was clearly reflected in the G:T use ratio of 96 versus 4% in the young CF group for 244 courses. In the adult CF group the trend was reversed, with a G:T use ratio of 20 versus 80% for 455 courses. For the adult CF patients, collation of their previous exposure as pediatric patients showed that they had generally been treated with gentamicin. On transfer to the adult clinic, tobramycin was favored, although the use of tobramycin was not exclusive, and gentamicin was occasionally used. Interestingly, in the group of 12 CF patients that exhibited hearing loss, the G:T use ratio was 41 versus 59% for a total of 272 courses. Two patients had received gentamicin alone, and three had received tobramycin alone. Seven patients had received these agents in combination, with the proportion of gentamicin courses ranging from 12 to 70%. The use ratios described above were also very similar when expressed in days.
Course durations.
Individual course durations ranged from 5 to 35 days, though for all CF patient groups the median course duration was 14 days with 55% of all courses, which encompassed the 25th and 75th percentiles. Moreover, the relatively tight clustering of course durations about this median was further reflected in the 10th- and 90th-percentile values of 10 and 18 days, respectively.
Peak levels of gentamicin and tobramycin in plasma.
Consideration of overall median trough levels was not feasible since the majority were simply reported as “<1.” The overall medians for peak levels could, however, be calculated for the three patient groups. These were found to be significantly different at p < 0.01 (Kruskal-Wallis test: 2 df; H = 8.9). A post hoc median comparison using the Mann-Whitney U test showed that the young CF overall peak median of 7.7 μg/ml (range, 5.2 to 9.1 μg/ml) was slightly but significantly lower than the adult overall peak median of 8.2 μg/ml (range, 5.2 to 10.1 μg/ml) at P < 0.02. The overall peak median for those with hearing loss, i.e., 8.25 μg/ml (range, 7.2 to 9.5 μg/ml), was not significantly different from the normal hearing CF adult value. Trough levels of between 2.1 and 4.2 μg/ml exceeding the normally accepted limit of 2 μg/ml for both gentamicin and tobramycin were documented 17 times (i.e., ca. 1.8% of all courses). Similarly, peak levels exceeding 12 μg/ml were seen on 15 occasions and fell between 12.2 and 19 μg/ml. These only occurred in the normal hearing adult CF group. This suggests that, at least at the start of the course measurements, there was no evidence of excessive peak or trough levels being associated with the development of hearing loss.
Concurrent administration of other potential ototoxins.
No therapy with known otototoxic potential (e.g., loop diuretics) that could independently contribute to or synergize AG cochleotoxicity was documented.
Standard PTA and HFPTA in CF subjects with hearing loss.
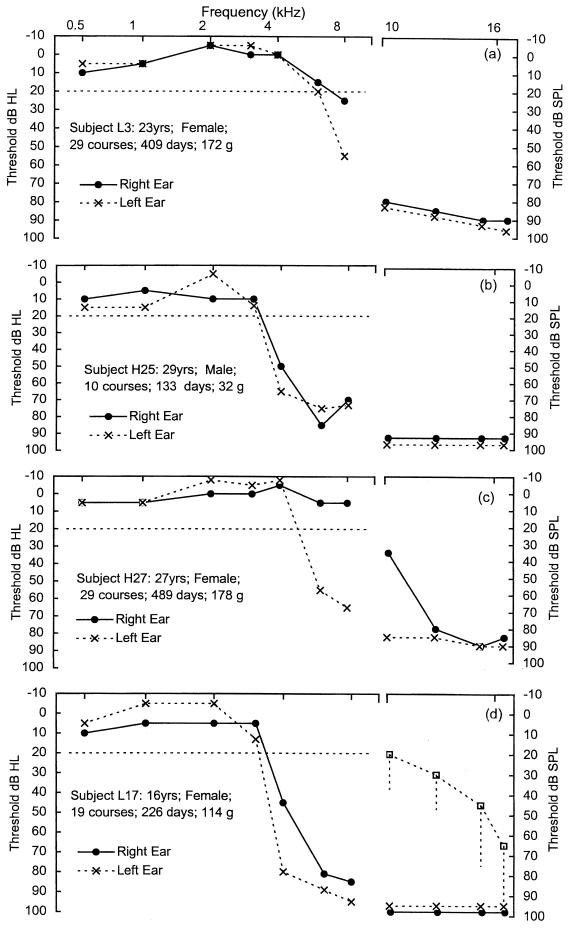
Figure 1 shows the threshold elevations in standard PTA and HFPTA analyses of four CF subjects who took part in this study. These results are illustrative of the high-frequency loss that is often reported to be associated with AG use. They also show that the severity of loss does not appear to be related to the number of courses or the total amount of AG received. Subject L3 in Fig. 1a had received a total of 29 courses, with thresholds of 25 (right ear) and 55 (left ear) dB at 8 kHz. In contrast, subject H25 in Fig. 1b had received only 10 courses, with the spread and severity of loss within the standard PTA more marked and thresholds of between 50 and 85 dB HL over a range of 4 to 8 kHz. Figure 1c shows a subject (H27) with only a unilateral loss (right ear) after receiving a total 29 courses. This loss was between 55 and 65 dB HL over a range of 6 to 8 kHz. Figure 1d shows the marked threshold elevation in the only CF subject (L17) from the pediatric group to exhibit threshold elevation, resulting from exposure to 19 courses of gentamicin. The accompanying HFPTA plots show thresholds, expressed in decibels SPL, for each subject gained for both ears. In Fig. 1d the median values with the range of the 90th-percentile values obtained from the adult control group are also included for comparison. At these frequencies, adult control median thresholds show a gradual increase from 20 to 70 dB SPL. These plots show that in these four examples, there is a very marked elevation of high-frequency thresholds well above that of the adult control group median. This would suggest that the outer hair cells in the high-frequency region of the cochlea would have lost much of their function (9).
FIG. 1.
Examples of threshold elevations in standard PTA and HFPTA analyses for four CF patients (Subjects L3 [a], H25 [b], H27 [c], and L17 [d]). These illustrate the differing severities of hearing loss with the variation in the total number of AG courses received. Note the difference in both the x and the y axis scales for the standard (0.5- to 8-kHz) and high-frequency (10- to 16-kHz) threshold plots.
When questioned, none of these patients complained of explicit hearing difficulties. They all, however, reported experiencing episodic tinnitus that often occurred during treatment.
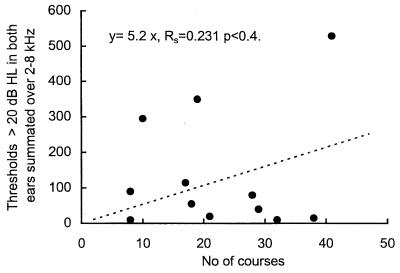
In the 12 CF patients showing threshold elevations, the relationship between the severity of the hearing loss and the AG exposure was investigated further by utilizing Spearman's nonparametric correlation ranking test. The relative degree of loss was represented by summing all threshold values over 20 dB HL between 2 and 8 kHz and plotting this against the total number of courses, as shown in Fig. 2. The number of courses was chosen as the ordinate, since it closely correlated with the total dose of AG received. In terms of clinical utility, it also serves as a more direct and accessible index of exposure than the total exposure expressed in grams or days. This plot shows that there was no clear rank relationship between the extent or severity of loss and the number of courses received at the time of testing (rs = 0.231, n = 12, P < 0.4). This figure also shows that at the time of audiometric testing, the minimum number of courses associated with threshold elevation was eight.
FIG. 2.
Summation of threshold elevations of >20 dB HL in the 12 patients with threshold elevations. The rank correlation was not significant. The figure shows the marked range of summated loss at both low (8 to 10) and at high (38 to 41) numbers of courses.
Association of hearing loss with number of AG courses received.
In both Fig. 1 and 2, it is unlikely that the observed hearing loss had simply occurred due to the previous AG course alone, and the increases in threshold are much more likely to reflect the intrasubject variability in response to repeated ototoxic challenge. Consequently, without serial and systematic audiometric measurement, it was not possible to determine the onset and the progression of hearing loss in these patients.
Notwithstanding this quantitative limitation, there was, however, a highly significant difference (P = 0.006) in the median number of courses of AGs received between the groups with and without hearing loss. At the time of testing, these were 20 (n = 12) and 9 (n = 58), respectively. Similar levels of significance were obtained when the exposure was expressed in days or grams. Along with the data shown in Fig. 2, this suggests that, as would be expected, there is likely to be an increasing risk of cochleotoxicity with the number of AG courses received. However, the relationship between risk and the number of AG courses does not appear to be a linear one.
Absence of subtle threshold elevation in CF in the “no hearing loss” category.
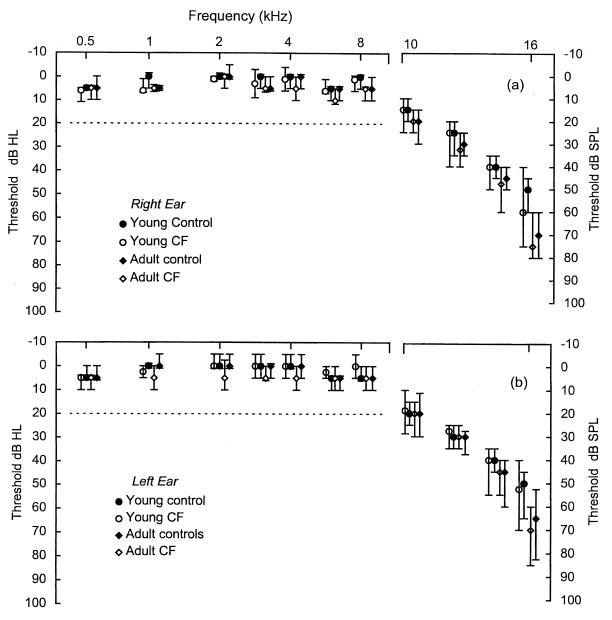
It was possible that the larger group of CF patients not exhibiting hearing loss had significant but subtle elevations in threshold. In this group, 11 had received over 20 courses and 2 had received totals of 50 and 53 courses. To test this possibility, the median thresholds in the two control groups and the two CF groups were compared for standard PTA and HFPTA frequencies. Figure 3 shows the results obtained for both left and right ears in the four groups. At each frequency, no significant differences were seen in the standard PTA, with all median thresholds falling between 0 and 10 dB HL. Similarly, no significant differences for the HFPTA median thresholds plotted over a range from 10 to 14 kHz were evident, with median thresholds over these frequencies ranging from 15 to 45 dB SPL. There was, however, evidence of a significant increase in the median thresholds seen at 16 kHz in both ears (Kruskal-Wallis test: H = 18.12, df = 3, P = 0.001 [right ear]; H = 15.13, df = 3, P = 0.002 [left ear]).
FIG. 3.
Median values with 95% confidence intervals for standard PTA and HFPTA thresholds of each group for the right (a) and left (b) ears. Note the difference in both the x and the y axis scales for standard (0.5- to 8-kHz) and high-frequency (10- to 16-kHz) threshold plots. For frequencies over the standard PTA, the numbers of subjects in this study allowed a difference of 5 dB in median values to be detected with at least 90% power at the 0.05 significance level. These power and significance values applied over the HFPTA frequencies allowed a difference of 10 dB in median values to be detected.
Further post hoc testing by Mann-Whitney pairwise comparisons utilized the young controls as the principle comparator group. No significant difference was found between this group and the young CF group. Comparison of the young control group median of 50 dB SPL with both adult control and the adult CF group medians at over 65 to 75 dB SPL was significant in both cases for P values >0.0012. Subsequent comparison of the median thresholds between the adult control and adult CF groups were, however, not significantly different from each other. This suggests that, rather than being due to AG therapy, threshold elevation at this frequency could be accounted for by aging alone, with comparable effects seen in both the adult control and the adult CF groups.
A further attempt was made in the normal-hearing young and adult CF subjects to reveal any subtle hearing loss, by performing rank correlation of summated thresholds against AG exposure, similar to that shown in Fig. 2. This was done by summating thresholds over a range of 2 to 8 kHz in both ears for the standard PTA and over a range of 10 to 16 kHz for the HFPTA. This did not reveal any correlation for either of these frequency ranges with rs of <0.1 in both cases (P > 0.4).
Generation of per-course risk estimates of hearing loss.
The results presented above suggest that the risk of developing cochleotoxicity is nonlinear. In spite of this, it would still be of be of clinical use to have some estimate of a per-course risk of developing hearing loss. The figures derived above can be used in generating a simple percentage per-course risk for the CF patients in this study.
The simplest model employs only two categories of hearing acuity, i.e., “no loss” and “loss.” It also assumes that the likelihood of an individual patient crossing this category boundary is independent of the previous history of exposure. With these criteria, the single-course risk can be derived from a single exponential equation, relating the proportion of subjects exhibiting hearing loss to the single-course risk and the number of AG courses administered: A = Bx, where A is the proportion of patients with no hearing loss after receiving x courses, B is the the proportion of patients with no hearing loss after one course, and x is the number of courses of AG therapy.
If the percentages of those with hearing loss obtained from this study are normalized, then 17% = 0.17, so A = 0.83. If the median number of courses taken by all CF subjects are used as an estimate for x then, from Table 1, x = 11. Thus, 0.83 = B11 and, by taking the 11th root of 0.83, B = 0.983. Subtracting this from 1 yields a per-course risk estimate of ca. 0.0167 or ca. 1.7%.
While this single exponential estimate has some practical utility, it is probably suboptimal. Experimental work (2, 12, 15, 19, 43) strongly suggests that AGs accumulate in cochlear sensory cells. Consequently, a better estimate would be provided by an equation including additional terms that models a cumulative component of risk. Further consideration of this modeling is outside the scope of the present study.
DISCUSSION
The results from this study provide estimates of the occurrence of cochleotoxicity in the CF patient group following repeated administration of AG therapy. They also raise broader questions about the mechanism of AG cochleotoxicity in the normal and CF genotype.
This study found that in 70 screened CF patients, the incidence of ototoxicity, judged from the degree of threshold elevation in the standard PTA, was 12 in 70 or ca. 17%. This loss was likely to be irreversible in at least eight of these patients. The range of loss in the standard PTA varied from 20 to 85 dB HL. There was also clear evidence in these 12 patients of significant threshold elevations (measured in decibels SPL) in the high-frequency (10- to 16-kHz) part of the audiogram compared to the control groups. The proportion of patients in this study identified as having sustained hearing loss by the appearance of their standard PTA is in line with two earlier reports (30; Morgan et al., Abstr. 1987 BTS Meet.). These authors found that 7 (16%) of 43 and 8 (30%) of 26 CF patients had at least one standard PTA threshold over a range of 4 to 8 kHz in excess of 30 dB HL with repeated courses of AG therapy.
Distinct differences in the G:T use ratio between the normal-hearing pediatric group and the adult group were seen, with use of tobramycin predominating in CF adults by 20 versus 80%. Since 11 of the 12 subjects in the CF hearing-loss group were adults, the G:T use ratio in this group would have perhaps been expected to be closer to 20 versus 80% rather than the actual one of 41 versus 59%. This could be taken as evidence that gentamicin use may be associated with greater cochleotoxic risk although, based on the small data set presented here, it would be premature to consider one drug to be more cochleotoxic than the other.
There was no evidence that the overall median peak levels in plasma within the CF hearing-loss group were significantly elevated above that of the adult group. Moreover, single incidences of peak or trough levels in plasma above the therapeutic range were not associated with the development of the hearing loss seen in this study.
There was an increased risk of threshold elevations in the standard audiogram related to the median number of courses of AG therapy. However, this risk did not appear to be related in a linear manner to the number of courses received. The results from Fig. 2 suggest an idiosyncratic response to repeated AG exposure. This absence of a linear relationship is likely to reflect the multifactorial contribution to the onset and progression of AG-induced hearing loss (2, 4, 12, 15, 18, 19, 28, 43). This idea is supported by a number of distinct pharmacokinetic and biochemical processes identified from experimentally induced cochleotoxicity outlined below.
Clinically, the patients in this study were asymptomatic and apparently unaware of any hearing loss, even when it had substantially progressed into the mid-frequencies of less than 6 kHz involved in speech perception. This indicates that CF patients cannot be expected to self-report hearing loss and that questioning alone is likely to miss the onset and progression of hearing loss. By the time the CF patient would be aware of any hearing loss, it would be too late to attempt to ameliorate or limit the progression by changing the antibiotic therapy. However, it is also important to note from this study that, for about the first 10 courses of AGs, the therapeutic benefit could be deemed to outweigh the risk of cochleotoxicity.
From a mechanistic point of view, the patient group who did not have any measurable elevation in standard PTA thresholds, compared with controls, are of equal interest to those who did. In this group, the absence of any threshold elevation suggests that repeated cochleotoxic challenge was consistently dealt with. This protective response appeared to extend through to the higher frequencies, which are normally considered to be particularly vulnerable to AG insult (2, 9). In fact, the success of CF patients in dealing with repeated cochleotoxic challenge is further supported by a number of previous studies (33, 45). These studies reported that no evidence of threshold elevation over the standard PTA took place in patients who had received multiple courses of AG therapy.
Taken together, the results presented here, along with those of previous reports, suggest that there is an apparent contrast in the incidence of cochleotoxicity in CF patients compared with that in non-CF patients. The estimates for cochleotoxicity in non-CF groups of 5 to 10% are typically for short single courses of AG therapy (10, 18, 23). This incidence is further contrasted by the longer courses, higher dosing, and higher peak AG concentrations in plasma required in CF patients. On these grounds alone, a doubling of the per-course risk in CF patients could be expected.
The interpretation of these results depends on whether the primary features of the AG pharmacokinetics and toxicology derived from animal models also extend to humans. The key features from this experimental work include the following: AG penetration of cochlear endolymph and perilymph in the micromolar range; selective uptake by cochlear outer and inner hair cells (OHCs and IHCs, respectively); acceleration in uptake by OHCs and IHCs with increasing length of exposure; a biphasic release from these cells, a fast phase (t1/2 measured in days) and a second slow phase (t1/2 measured in months).
Within the OHCs and IHCs, the main features determining cochleotoxicity are (i) an AG “storage” threshold, below which AG remains as a dormant “protoxin” and, when this threshold is exceeded, AG is involved in the generation of an “active toxin” species (2, 12, 15, 19, 43) and (ii) detoxicant processes involving free-radical scavengers that may ameliorate the severity of damage. This suggests that the active toxin is a free-radical species (37, 38).
Of these features, perhaps the most important in modeling repeated-course cochleotoxic risk is the prolonged half-life of AGs in the hair cells. If this also applies in humans, then repeat courses, separated by intervals shorter than this half-life, would be expected to result in the accumulation of the drug in the sensory cells.
If, however, the AG half-life in OHCs and IHCs in humans was much shorter than estimated from the experimental model (2, 15) (i.e., days instead of months), then appreciable accumulation of the drug following multiple courses would be unlikely to occur. In this case, the simplest estimation of cochleotoxic risk would be provided by a single exponential model similar to that used in the equation described above. This model is based on the assumption that in any given course, AG levels in the hair cells will exceed a simple “toxic threshold” in a probabilistic manner. If we assume there is a much shorter half-life of AGs in cochlear sensory cells, then the residual AG levels from previous exposure would be negligible. Consequently, the probability of crossing this cochleotoxic threshold in any given course would then effectively be independent of any previous exposure. If this is true, then the per-course cochleotoxic risk returned for CF patients of about 1.7% in this particular study, and this probably represents a reasonable preliminary estimate.
If, on the other hand, the characteristics of AG pharmacokinetics from the animal model do apply in humans, then these preliminary estimates of ototoxic risk would require a fundamentally different interpretation. In CF patients receiving courses of AGs every 6 to 12 months, substantial accumulation of the drug in the OHCs and IHCs would be expected to occur. If we assume that a threshold concentration of the protoxin AG has to be reached prior to the generation of an active toxin, the probability of crossing this threshold would increase with every subsequent course. This would mean that the overall per-course estimate of 1.7% returned here would not realistically reflect the repeated-course cochleototoxic risk. Instead, there would be an increase in risk with each subsequent course. The calculation of this incremental risk would lead to a much lower single-course value.
If this latter scheme is generally correct, it would have relevance to the broader understanding of the mechanisms for AG cochleotoxicity in both CF and non-CF groups. The most conservative interpretation would be to assume that cochleototoxic risk in these groups is generally similar. The results presented here, which are likely to be cases of irreversible ototoxicity, although interesting, would be clinically unremarkable. This would then suggest that the previously reviewed reports of single-course ototoxicity of between 5 and 10% (10, 18, 23) were composed of estimates of both reversible and irreversible cochleotoxicity, which were not sufficiently differentiated between by longer-term serial audiometry. The figures of ca. 1.7% reported here would then be in line with some of the more conservative reports of single-course cochleotoxicity (14, 21, 32).
Mechanistically, the extent of cochleotoxicity in any one individual would be explained by the dynamic balance between the factors outlined above, a balance between generation of the toxin and the detoxicant systems removing the toxin. For example, repeated AG exposure could cause “upregulation” of detoxicant systems in the OHCs and/or IHCs. Upregulation of these systems in response to moderate ototrauma has already been demonstrated experimentally (22).
Alternatively, these results could be explained by the possibility that the CF condition itself is responsible for significantly attenuating the progression of AG cochleotoxicity. There is clinical and experimental evidence to support this idea. It is already known that the CF condition results in a more rapid renal elimination of other drugs, including AGs (14). It has been proposed that the defective or absent CF transmembrane regulator protein (a cyclic AMP-activated chloride transporter) may underlie this accelerated elimination of some of these xenobiotics (46). This defect could also conceivably result in reduced cochleotoxicity, due to similarly enhanced outward transport of AGs from the OHC and/or IHCs. Alternatively, the CF defect may underlie an increase in the activity or efficiency of detoxicant pathways within the OHC and/or IHCs.
If these ideas are at least partly correct, then they may yield important clues as to the mediation of and protection against AG cochleotoxicity in humans. Apart from any theoretical utility, the results reported here are of direct clinical relevance, providing new estimates of cochleotoxic risk with repeated-course AG therapy in CF patients. These estimates suggest that during the first 10 courses or so, the balance is toward therapeutic benefit with a relatively low risk of cochleotoxicity.
ACKNOWLEDGMENTS
We thank N. Taub (Department of Epidemiology, University of Leicester) for statistical advice; Chris O'Callaghan and Janet Collinson (Leicester Royal Infirmary), Nick Griffin (Northampton General Hospital, Northampton, United Kingdom), and David Woolf (Peterborough District Hospital, Peterborough, United Kingdom) for their agreement to conduct the study involving their patients; and the groups of control volunteers.
This work was supported by MRC grant G78/4576.
REFERENCES
- 1.Altman D G. Practical statistics for medical research. London, England: Chapman and Hall; 1991. [Google Scholar]
- 2.Aran J M. Current perspectives on inner ear toxicity. Otolaryngol Head Neck Surg. 1995;112:133–144. doi: 10.1016/S0194-59989570313-6. [DOI] [PubMed] [Google Scholar]
- 3.Barclay M L, Begg E J. Aminoglycoside toxicity and relation to dose regimen. Adv Drug Reactions Toxicol Rev. 1994;13:207–234. [PubMed] [Google Scholar]
- 4.Bendush C L. Ototoxicity: clinical considerations and comparative information. In: Whelton A, Neu H C, editors. The aminoglycosides: microbiology, clinical use, and toxicology. New York, N.Y: Marcel Dekker; 1982. pp. 453–486. [Google Scholar]
- 5.British Journal of Audiology. Recommended procedures for pure tone audiometry using a manually operated instrument. Br J Audiol. 1981;15:213–216. doi: 10.3109/03005368109081440. [DOI] [PubMed] [Google Scholar]
- 6.British Journal of Audiology. Recommended procedures for pure tone bone conduction audiometry without masking using a manually operated instrument. Br J Audiol. 1985;19:281–282. doi: 10.3109/03005368509078985. [DOI] [PubMed] [Google Scholar]
- 7.British Journal of Audiology. Recommendations for masking in pure tone threshold audiometry. Br J Audiol. 1986;20:307–314. doi: 10.3109/03005368609079029. [DOI] [PubMed] [Google Scholar]
- 8.British Journal of Audiology. Recommended procedure for tympanometry. Br J Audiol. 1992;26:255–257. doi: 10.3109/03005369209076644. [DOI] [PubMed] [Google Scholar]
- 9.Brummett R E. Drug-induced ototoxicity. Drugs. 1980;19:412–428. doi: 10.2165/00003495-198019060-00002. [DOI] [PubMed] [Google Scholar]
- 10.Cone L A. A survey of prospective controlled clinical trials of gentamicin, tobramycin, amikacin, and netilmicin. Clin Ther. 1982;5:155–162. [PubMed] [Google Scholar]
- 11.Conway S P, Miller M G, Ramsden C, Littlewood J M. Intensive treatment of Pseudomonas chest infection in cystic fibrosis: a comparison of tobramycin and ticarcillin and netilmicin and ticarcillin. Acta Paediatr Scand. 1985;74:107–113. doi: 10.1111/j.1651-2227.1985.tb10929.x. [DOI] [PubMed] [Google Scholar]
- 12.Crann S, Schacht J. Activation of aminoglycoside antibiotics to cytotoxins. Audiol Neuro-Otol. 1996;1:80–85. doi: 10.1159/000259187. [DOI] [PubMed] [Google Scholar]
- 13.Crifo S, Antonelli M, Gagliardi N L, Marcolini P. Ototoxicity of aminoglycoside antibiotics in long-term treatment for cystic fibrosis. Int J Pediatr Otorhinolaryngol. 1980;2:251–253. doi: 10.1016/0165-5876(80)90050-6. [DOI] [PubMed] [Google Scholar]
- 14.de Groot R, Smith A L. Antibiotic pharmacokinetics in cystic fibrosis: differences and clinical significance. Clin Pharmacokinet. 1987;13:228–253. doi: 10.2165/00003088-198713040-00002. [DOI] [PubMed] [Google Scholar]
- 15.Dulon H, Hiel H, Aurousseau C, Erre J P, Aran J M. Pharmacokinetics of gentamicin in the sensory cells of organ of Corti: rapid uptake and long-term persistence. Audiology. 1993;32:78–87. [PubMed] [Google Scholar]
- 16.Flottorp G. Improving audiometric thresholds by changing the headphone position at the ear. Audiology. 1995;34:221–231. doi: 10.3109/00206099509071915. [DOI] [PubMed] [Google Scholar]
- 17.Friend P A. Pulmonary infection in cystic fibrosis. J Infect. 1986;13:55–72. doi: 10.1016/s0163-4453(86)92325-x. [DOI] [PubMed] [Google Scholar]
- 18.Govaerts P J, Claes J, Van De Heyning P H, Jorens P G, Marquet G, De Broe M E. Aminoglycoside-induced ototoxicity. Toxicol Lett. 1990;52:227–251. doi: 10.1016/0378-4274(90)90033-i. [DOI] [PubMed] [Google Scholar]
- 19.Hiel H, Erre J P, Aurousseau C, Bouali R, Dulon D, Aran J M. Gentamicin uptake by cochlear hair cells precedes hearing impairment during chronic treatment. Audiology. 1993;32:78–87. doi: 10.3109/00206099309072930. [DOI] [PubMed] [Google Scholar]
- 20.International Organization for Standardisation. Acoustics—thresholds of hearing by air conduction as a function of age and sex for otologically normal persons. ISO 7029. Geneva, Switzerland: International Organisation for Standardisation; 1984. [Google Scholar]
- 21.Jackson G G, Arcieri J J. Ototoxicity of gentamicin in man: a survey and controlled analysis of clinical experience in the United States. J Infect Dis. 1971;124(Suppl):S130–S137. doi: 10.1093/infdis/124.supplement_1.s130. [DOI] [PubMed] [Google Scholar]
- 22.Jacono A A, Hu B, Kopke R D, Henderson D, Van De Water T R, Steinman H M. Changes in cochlear antioxidant enzyme activity after sound conditioning and noise exposure in the chinchilla. Hear Res. 1998;117:31–38. doi: 10.1016/s0378-5955(97)00214-1. [DOI] [PubMed] [Google Scholar]
- 23.Kahlmeter G, Dahlager J I. Aminoglycoside ototoxicity—a review of clinical studies published between 1975 and 1982. J Antimicrob Chemother. 1982;13(Suppl. A):9–22. doi: 10.1093/jac/13.suppl_a.9. [DOI] [PubMed] [Google Scholar]
- 24.Katbamna B, Homnick D N, Marks J H. Contralateral suppression of distortion product otoacoustic emissions in children with cystic fibrosis: effects of tobramycin. J Am Acad Audiol. 1998;9:172–178. [PubMed] [Google Scholar]
- 25.Lerner S, Matz G J. Aminoglycoside ototoxicity. Am J Otol. 1980;1:169–179. doi: 10.1016/s0196-0709(80)80012-3. [DOI] [PubMed] [Google Scholar]
- 26.Lutman M E, Davis A C. The distribution of hearing thresholds in the general population aged 18–30 years. Audiology. 1994;33:327–350. doi: 10.3109/00206099409071891. [DOI] [PubMed] [Google Scholar]
- 27.McCrae W M, Raeburn J A, Harrison E J. Tobramycin therapy of infection due to Pseudomonas aeruginosa in patients with cystic fibrosis: effects of dosage and concentration in sputum. J Infect Dis. 1976;134:191–193. doi: 10.1093/infdis/134.supplement_1.s191. [DOI] [PubMed] [Google Scholar]
- 28.Moore R D, Smith C R, Lietman P S. Risk factors in the development of auditory toxicity in patients receiving aminoglycosides. J Infect Dis. 1984;149:23–30. doi: 10.1093/infdis/149.1.23. [DOI] [PubMed] [Google Scholar]
- 29.Mulheran M, Degg C. Comparison of distortion product OAE generation between a patient group requiring frequent gentamicin therapy and control subjects. Br J Audiol. 1997;31:5–9. doi: 10.3109/03005364000000004. [DOI] [PubMed] [Google Scholar]
- 30.Mulherin D, Fahy J, Grant W, Keogan B, Kavanagh M, Fitzgerald M. Aminoglycoside-induced ototoxicity in patients with cystic fibrosis. Irish J Med Sci. 1993;160:173–175. doi: 10.1007/BF02961666. [DOI] [PubMed] [Google Scholar]
- 31.Neu H C, Bendush C L. Ototoxicity of tobramycin: a clinical overview. J Infect Dis. 1976;134(Suppl. S):206–218. doi: 10.1093/infdis/134.supplement_1.s206. [DOI] [PubMed] [Google Scholar]
- 32.Nordstrom, Banck L G, Belfrage S, Juhlin I, Tjernstrom O, Toremaln N G. Prospective studies of the ototoxicity of gentamicin. Acta Pathol Microbiol Scand. 1973;81(Suppl. 241):58–61. [PubMed] [Google Scholar]
- 33.Pedersen S, Jensen T, Osterhammel D, Osterhammel P. Cumulative and acute toxicity of repeated high-dose tobramycin in cystic fibrosis. Antimicrob Agents Chemother. 1987;31:594–599. doi: 10.1128/aac.31.4.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rabin H R, Harley F L, Bryan L E. Evaluation of a high dose tobramycin and ticarcillin treatment protocol in cystic fibrosis based on improved susceptibility criteria and antibiotic pharmacokinetics. In: Sturgess J M, editor. Perspectives in cystic fibrosis. Toronto, Ontario, Canada: Canadian Cystic Fibrosis Foundation; 1980. pp. 370–375. [Google Scholar]
- 35.Robinson D W. Threshold of hearing as a function of age and sex for the typical unscreened population. Br J Audiol. 1988;22:5–20. doi: 10.3109/03005368809077793. [DOI] [PubMed] [Google Scholar]
- 36.Sammut P H, Taussig L M. Cystic fibrosis in the adolescent and adult. Curr Pulmonol. 1989;10:377–428. [Google Scholar]
- 37.Sha S H, Schacht J. Antioxidants attenuate gentamicin-induced free radical formation in vitro and ototoxicity in vivo: d-methionine is a potential protectant. Hear Res. 2000;142:34–40. doi: 10.1016/s0378-5955(00)00003-4. [DOI] [PubMed] [Google Scholar]
- 38.Sha S H, Schacht J. Stimulation of free radical formation by aminoglycoside antibiotics. Hear Res. 1999;128:112–118. doi: 10.1016/s0378-5955(98)00200-7. [DOI] [PubMed] [Google Scholar]
- 39.Shu-Li C, Bowes G, Ionnades D L. Minimisation of aminoglycoside toxicity in patients with cystic fibrosis. J Antimicrob Chemother. 1996;51:369–373. doi: 10.1136/thx.51.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siegel S A. Non-parametric statistics for the behavioural sciences. Tokyo, Japan: McGraw-Hill; 1956. [Google Scholar]
- 41.Smith C R, Lietman P S. Comparative clinical trials of aminoglycosides. In: Whelton A, Neu H C, editors. The aminoglycosides: microbiology, clinical use, and toxicology. New York, N.Y: Marcel Dekker; 1982. pp. 419–452. [Google Scholar]
- 42.Szaff M, Høiby N, Flensborg E W. Frequent antibiotic therapy improves the survival of CF patients with chronic Pseudomonas aeruginosa infection. Acta Paediatr Scand. 1983;72:651–657. doi: 10.1111/j.1651-2227.1983.tb09789.x. [DOI] [PubMed] [Google Scholar]
- 43.Tran Ba Huy P, Bernard P, Schacht J. Kinetics of gentamicin uptake and release in the rat: comparison of inner ear tissues and fluids with other organs. J Clin Investig. 1986;77:1492–1500. doi: 10.1172/JCI112463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.United Kingdom Department of Health. Health building note 12; supplement 3. ENT, audiology clinics and hearing aid centres. Leeds, England: NHS Estates; 1994. [Google Scholar]
- 45.Wood P J, Ionnades D L, Shu Li C. Minimisation of aminoglycoside toxicity in patients with cystic fibrosis. Thorax. 1996;51:369–373. doi: 10.1136/thx.51.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Woodland C, Blowey D, Ito S, Spino M, Koren G. Hypothetical framework for enhanced renal tubular secretion of drugs in cystic fibrosis. Med Hypotheses. 1998;51:489–491. doi: 10.1016/s0306-9877(98)90070-6. [DOI] [PubMed] [Google Scholar]