Abstract
The onset of adolescence is associated with an increase in transgressive behaviours—from juvenile delinquency to substance use and unprotected sex—that are often attributed to increased impulsiveness. In the past, this increase was ascribed to “raging hormones”; more recently, to an imbalance in the maturation of different brain regions. However, it remains unclear how these large-scale biological changes impact specific processes that result in impulsive decisions, namely, sensitivity to immediate rewards and general discounting of future options. To gain further insight into these questions, we used an intertemporal choice task to investigate the role of testosterone in impatient decision-making in boys at the developmental transition to adolescence (N = 72, ages 11–14). Our results suggest that increased testosterone (but not age) is related to increased sensitivity to immediate rewards, whereas increased age (but not testosterone) is related to a reduction in general impatience. These results are discussed in the context of recent neurobiological models of adolescent development.
Keywords: Adolescence, Testosterone, Impulsivity, Impatience, Delay discounting, Puberty
1. Introduction
Adolescents are often characterized as impulsive decision-makers, living in the moment with little thought for the consequences of their actions. Numerous self-report and behavioural studies support this characterization (Quinn and Harden, 2013; Steinberg et al., 2009; van den Bos et al., 2015). Indeed, adolescent impulsivity is part of healthy development; it is instrumental in acquiring the new skills needed to function as an independent individual (Spear, 2013). However, increased impulsivity may also lead to various unhealthy outcomes (e.g., Nower et al., 2004). For example, adolescents make more emergency department visits because of unintentional injury or experimenting with drugs or alcohol than do either children or adults (Dahl, 2004). The challenge is therefore to develop interventions that channel adolescents’ impulsive behaviour into positive development while, at the same time, reducing its negative outcomes. A better understanding of the mechanisms underlying adolescent impulsivity is crucial to this end.
Neurodevelopmental models of adolescent brain development attribute the elevated impulsivity observed in adolescence to an imbalance in the maturation of the mostly subcortical affective brain network, the cortical cognitive control network, and the connections between the two (Casey et al., 2015; Ernst, 2014; Shulman et al., 2016). Specifically, the affective network, which is involved in the anticipation and valuation of rewards, matures earlier than the control network and its top–down connections; this incongruence is thought to result in increased impulsive behaviour. However, impulsivity is a multidimensional construct with at least three independent components: acting without thinking, impatience, and sensation seeking (Romer, 2010; but see Duckworth and Steinberg, 2015). These three components (a) have been associated with different brain regions (Robbins et al., 2012), (b) show distinct developmental trajectories across adolescence (Harden and Tucker-Drob, 2011), and (c) show distinct associations with self-reported maladaptive behaviour (Romer, 2010).
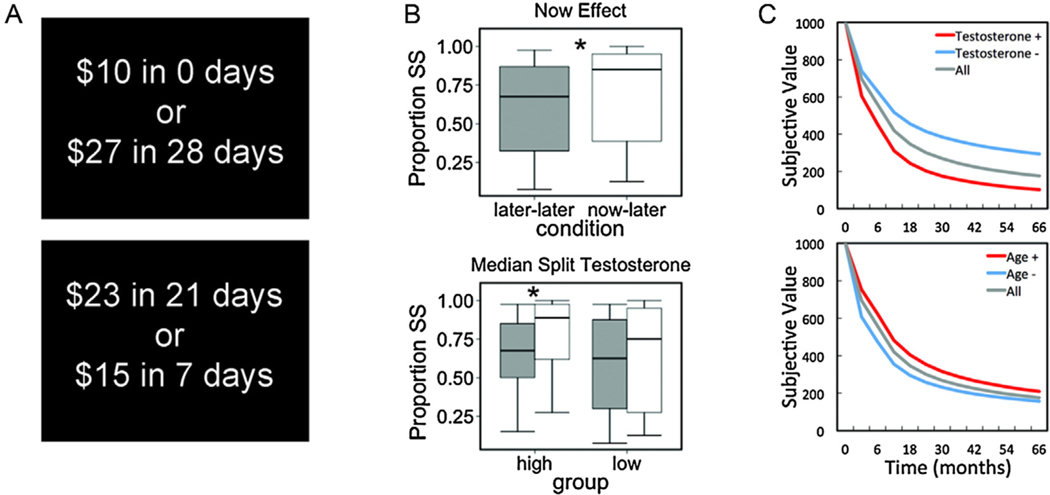
In addition, impulsivity varies considerably between genders. A meta-analysis by Cross et al. (2011) with N = 277 studies and a total of N = 149.496 participants reported significant sex differences for motivational forms of impulsive behaviour (such as sensation seeking). In line with those findings, several large-scale developmental studies reported higher sensation seeking in boys compared to girls (Steinberg et al., 2008; D’Acremont and Van Der Linden, 2005). In this study, we focus on adolescent impatience in boys, as measured by a modified intertemporal choice task (Fig. 1A). Specifically, the task was constructed in a way that made it possible to distinguish between different mechanisms underlying impatient behaviour.
Fig. 1.
(A) Screenshots of two example trials, one where the smaller sooner (SS) option is immediate, and one where both the SS and the larger later (LL) option are in the future. (B) Plots of SS choice proportions for the now/later and later/later conditions. The second panel shows the data, N = 60, when the sample was stratified into high- and low-testosterone groups by means of a median split. Post hoc testing revealed a significant effect of condition in the high-testosterone group (Wilcoxon signed rank test, Z = 4.34, p < .001) but not in the low-testosterone group (Z = 1.98, p < .07). (C) Effects of testosterone and age on the shape of the discount curve, again based on median splits (N = 60). The grey lines are identical in both graphs and show the discount function of the mean parameter estimates of all participants (for more detail on parameters, see Table 5).
Impatience can result from (a) the discounting of future outcomes, which may be a rather cognitive process and/or (b) increased sensitivity to immediate rewards, which may be more related to motivational forms of impulsivity (van den Bos et al., 2015). For instance, research suggests that the presence of an immediate reward makes people more impatient than when both options are in the future (McClure et al., 2004). However, previous developmental studies have not always been able to tease the different mechanisms apart.
Consistent with neurocognitive models of adolescent brain development, we recently found that age-related decreases in impatience between the ages 8 and 25 were associated with increasing strength of the structural and functional connections between the dorsolateral prefrontal cortex (DLPFC) and the striatum (van den Bos et al., 2015). Furthermore, a recent meta-analysis has shown that there is indeed evidence for heightened reward related activity in a wide network of regions, including the striatum (Silverman et al., 2015). However, it is not yet well understood how this heightened activity is related to increased impatient behaviour. In addition, most of the earlier studies have overlooked the role of pubertal hormones. Recently, it has been suggested that the valuation network, in particular the striatum, not only matures earlier but that its functioning is modulated by the surge in pubertal hormones in the early teen years (Crone and Dahl, 2012). Animal studies have demonstrated that testosterone significantly influences dopamine neural transmission in the adolescent brain (Allen et al., 2015). Furthermore, dopamine receptor density has been shown to correlate with motivational forms of impulsive behaviour (sensation seeking) in adult men (Gjedde et al., 2010). In addition, Braams and colleagues (2015) have shown that pubertal testosterone is associated with an increased response to rewards in the striatum. Nevertheless, the specific pathways through which pubertal changes may affect different processes underlying impatient decision-making remain unknown (for a review, see Laube and van den Bos, 2016).
One of the limitations of previous research has been that most studies selected participants based on a relatively wide age range. Given the high variability in pubertal onset, previous age-focused studies may not have been able to detect specific puberty-related changes independent of age. Data from a 5-year longitudinal study showed that puberty onset ranges from 8.0 to 14.4 years in females and from age 9.7 to 14.1 years in males (Lee, 1980). Furthermore, self-report measures of pubertal status do not entirely reflect the underlying hormonal processes (Shirtcliff et al., 2009), although this relationship seems to be stronger and more stable for boys compared to girls (Granger et al., 2004). These reasons might explain why some age-focused, and gender-mixed studies have only found a trending relationship between pubertal development and impulsive behaviour (Bromberg et al., 2015; de Water et al., 2014).
The aim of the present study was to further investigate the role of testosterone in the specific component of adolescent impulsivity – impatience. To this end, we focused specifically on the two different processes underlying adolescent impatience, sensitivity towards immediate rewards and discounting of future rewards, in a fairly large sample of males and measured both self-reported pubertal development and testosterone. In addition, we also focused on a relatively narrow age range (11–14 years) in order to investigate both age and pubertal effects. In sum, by using this age range, as well as only investigating boys, we were able to reduce the correlation between age and pubertal status, which allowed us to measure independent effects.
In order to capture sensitivity towards immediate rewards and discounting of future rewards, and investigate how they are differently impacted by age and testosterone, we used behavioural modelling in combination with a specifically designed intertemporal choice task (see Fig. 1A) that included choices with and without immediate rewards. We hypothesized that pubertal testosterone would be specifically related to sensitivity to immediate rewards, whereas increasing age would be associated with a general decline in impatience.
2. Material and methods
2.1. Participants
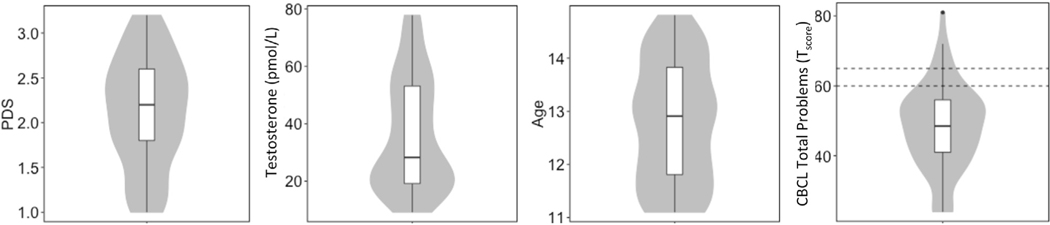
Adolescent boys (N = 72) between the ages of 11 and 14 years (M = 12.34 years, SD = 1.17) were recruited in an urban area in Northern California. Included were boys who were currently enrolled in school, medically healthy with no history of neurological or psychiatric illness, and native English speakers. In addition, the Child Behaviour Checklist (CBCL; Achenbach et al., 2001), completed by each boy’s parent, was used to assess for severe attention or thought problems that might influence task performance. Participants with elevated scores on other CBCL syndrome/problem scales were included in this study to avoid the creation of a “supernormal” sample, and the total problem score was included as covariate in all statistical models. In our sample were N = 4 participants considered as borderline clinical and N = 3 participants considered as clinical (see Fig. 3, right panel). Participants received a $50 Visa gift card in exchange for their participation in the study. The University of California, Berkeley, Institutional Review Board approved all study procedures.
Fig. 3.
Distributions of variables of interest. Boxplot represents median and confidence interval, violin plots represent actual distribution. From left to right: PDS (Pubertal Developmental Scale), testosterone, age and CBLC (Child Behaviour Checklist). Dotted lines in the fourth panel indicate the zone of scores that is considered borderline clinical, above this zone is considered clinical levels. Every panel is representing a total of N = 60 participants.
2.2. Power analyses
At the time this study started it was hard to perform an adequate power analyses given the scarcity of data on pubertal testosterone. Nevertheless, our sample size estimation was guided by several different studies investigating delay discounting or risk taking in adolescents that partly controlled for individual levels of testosterone. Based on the data collected for van den Bos et al. (2015) we could determine that for a power of .80 we only needed 33 subjects to find a significant effect of age (p < .05) on discounting using the exact same task as reported in this study. Furthermore, we were aware of the results by Bromberg et al. (2015) that indicated a strong trend between testosterone and discounting in a group of 49 adolescents (25 males) between ages 12–18. As described in the paper we hoped to increase power by restricting the age range to 11–14, and thus reduce the covariance between age and testosterone (in which we succeeded). Moreover, we were also oriented towards the findings by Peper et al. (2013), who found a significant relationship (p < .0001) between pubertal testosterone and the total number of explosions on the Balloon Analogue Risk Task (a measure of risk-taking) in a large sample of 236 subjects (115 males) between the ages of 8 and 25 years. Thus, for a similar effect with an expected medium effect size of approximately f2 =0.15, a total sample of N = 55 subjects provides adequate power (>.80) to detect a significant effect (p < .05). Due to the scarcity of studies on pubertal hormones and decision-making we could only base estimations, ranging from 33 to 55 participants, on results of studies that are only to a degree overlapping with our design. However, these studies missed some essential features, for instance they did not explicitly measure sensitivity towards immediate rewards, and had wider age ranges which may potentially be problematic when testing more complex models. It is important to point out that the authors of the original studies also reported simple correlations or regression models, thus the power estimates of the current study are only based on simple models and not on multiple regression models planned for our analyses. Consequently, because of limited information available and the differences in analyses and design we decided to aim for getting data on close to 75 participants, which was further motivated by our experience with these participants and similar studies run in our respective labs. Thus like any other novel study there are limitations on making inferences about a prior power, and thus we should be cautious when interpreting the generalizability of the current results which will have to be established by both direct and conceptual replications.
2.3. Measures
2.3.1. Testosterone
Testosterone levels were measured via two morning saliva samples provided by each participant, which is a well-validated method for assessing general circulation of testosterone (Shirtcliff et al., 2009). We used the passive drool method of saliva collection to minimize discomfort and maximize compliance. Participants were instructed to collect the two saliva samples on separate—preferably consecutive—mornings within two weeks of their initial visit to the lab, ideally 15–30 min after waking, and to immediately place the samples in the freezer. They completed a form indicating the date and time each sample was collected. When brought to the lab, the saliva samples were immediately stored in a freezer at −20°C. Subsequently, they were frozen at −80°C for long-term storage. Testosterone assays were conducted at the University of New Orleans, Louisiana, under supervision of Dr. E. A. Shirtcliff. The intra-assay correlation were very high, r(58) = .91, 95% CI [.84, .94], p < .001. Testosterone levels were therefore calculated as the average across the two samples collected by each participant. Participants who had only one sample or sampled at the wrong time of day were excluded from the analyses, which resulted in a total of N = 60 participants with reliable testosterone data.
2.3.2. Pubertal developmental scale
Because the present sample is cross-sectional, the testosterone measures reflect a combination of individual and developmental changes. Thus, developmental effects on impulsive behaviour cannot fully be isolated from individual differences.
To address this issue, we also administered the Pubertal Development Scale (PDS) self-report measure to estimate pubertal stage (Petersen et al., 1988). This measure is commonly used to assess external pubertal status and asks adolescents about hair growth, skin changes, and growth spurts, resulting in a composite puberty score. As expected, the PDS score and pubertal testosterone were positively correlated, rs = .61, bootstrapped (N = 1000) 95% CI [.40, .71], p < .001. In subsequent analyses, we analyzed to what degree the shared and non-shared variance of testosterone and pubertal development contributed to changes in behaviour. Furthermore, with regard to pubertal status, N = 4 subjects scored 1 one the PDS, but had testosterone levels >13 pmol/L. On the other hand, N = 1 subject had a testosterone level of 9.4 pmol/L, but a PDS score of 1.8. Consequently, every subject (N = 60) in the current study had reached puberty.
2.3.3. Intertemporal choice task
Participants made 80 binary choices between two hypothetical amounts of money available at different delays (see Fig. 1A). The smaller sooner (SS) option offered a small reward at a short delay; the larger later (LL) option offered a larger reward at a larger delay. For half of the trials (now/later condition), the SS option had a delay of 0; for the other half (later/later condition), the reward was 14 days in the future. The LL delays were 14, 42, 56, or 84 days (+14 days when SS was in the future). Following previous studies (McClure et al., 2004; van den Bos et al., 2015) the SS rewards were pseudo-randomly selected from a uniform distribution [between $10 and $75], and the LL rewards were determined by adding a fixed percentage to the SS value [.5%, 1%, 5%, 10%, 15%, 20%, 25%, 30%, 50%, or 75%]. Even though participants were not directly paid the actual monetary amounts used in the task, past research consistently showed that choices with hypothetical and real rewards in a delay discounting paradigm significantly correlate with each other (Bickel et al., 2009; van den Bos et al., 2015).
2.4. Statistical analysis
To investigate how age and puberty differently affected choices in the intertemporal choice task, we performed a beta regression using the betareg package for R (Bates et al., 2014). The proportion of SS choices was modelled with independent predictors for age, testosterone, condition (now/later or later/later), and the 2-way interactions with condition. The final predictors were added to test the hypothesis that higher testosterone is specifically associated with increased sensitivity to immediate rewards. In a subsequent step we have added PDS and the PDS by condition interaction to the model.
To further quantify the processes of sensitivity to near-term rewards and general discounting of future options through the intertemporal choice task, we fitted a series of models and compared them using Bayesian model comparison techniques. The basic assumption that underlies most models of discounting behaviour is that when a reward is available at a certain delay, its subjective value is discounted relative to the extent of that delay:
| (1) |
where U is the subjective utility and A represents the objective monetary amount, which is multiplied by discount function D. In the rewards domain, the subjective value can be expected to drop when the delay increases, thus 0 < D < 1. To understand impatience in the intertemporal choice task, we essentially need to understand the character of D. Here, we fit and compare three possible characterizations (with random choice as baseline). This first model is the classic hyperbolic function:
| (2) |
where t is time and κ is the discount factor (greater κ implies greater impulsivity). To better capture individual differences in sensitivity to immediate rewards as opposed to long-term rewards, we also used two well-known two-parameter discount models:
| (3) |
In this first two-parameter model (Green and Myerson, 2004), σ reflects individual differences in sensitivity to change at shorter delays relative to longer delays. A similar model that summarizes these features of behaviour is the beta-delta model (Laibson, 1997):
| (4) |
Where β is a parameter that captures the specific value placed on immediate rewards and represents the general level of exponential discounting. These two parameter functions often provide a better fit for the data, even with control for additional parameters, and are of special interest for this study because they allow us to quantify the two processes under investigation: sensitivity to near-term rewards and general discounting of future options.
We used the multivariate constrained minimization function (fmincon) of the optimization toolbox implemented in MATLAB for model fitting. To model trial-by-trial choices, we used the logistic choice rule to compute the probability (PLL) of choosing the LL option as a function of the difference in subjective value VSS and VLL:
| (5) |
where θ estimates response noise. This function assumed that each individual would choose the option with the highest subjective value with the highest probability. Individual parameter estimates for each of the models were determined as those that maximized the likelihood of the observed data. Bayesian model comparison (using the Akaike information criterion, AIC, and the Bayesian information criterion, BIC) provided an indication of the relative quality of the statistical models given the data.
3. Results
3.1. Summary statistics
As expected, the correlation with testosterone levels was strong for self-reported pubertal development, rs = .61, bootstrapped (N = 1000) 95% CI [.40, .71], p < .001, and moderate/weak for age, rs = .32, 95% CI [.12, .51], p < .02. In addition, the correlation between age and PDS was moderate to strong (r = .45, 95% CI = [.28, .56], p< .001; see also Table 1 for all zero-order correlations between all study variables). This suggests that in this sample, pubertal development is, in principle, statistically distinguishable from age (for a more detailed analyses see below). Finally, in line with our expectations, participants chose the SS option more often than the LL option (M = 65%, S.E. = 3%).
Table 1.
Zero-order correlations between all study variables.
| Mean (SD) | Variable | Age | Testosterone | PDS | CBCL | Smaller sooner choices in % |
|---|---|---|---|---|---|---|
| 12.78 (1.12) | Age | 1 | ||||
| 36.12 (19.40) | Testosterone | .32* [.12, .51] | 1 | |||
| 2.13 (.61) | PDS | .45*** [.28, .56] | .61*** [.40,.71] | 1 | ||
| 48.71 (12.60) | CBCL | .07 [−.16, .30] | .18 [−.05, .40] | .05 [−.18, .28] | 1 | |
| 69 (29) | Smaller sooner choices in % | −.32** [−.50, −.09] | .57*** [.39,.71] | .26* [.04, .48] | .04 [−.19, .27] | 1 |
Table showing the means and standard deviations for each of the study variables (left) and zero order correlations along with 95% confidence intervals (right). PDS: Pubertal Developmental Scale, CBCL: Child Behaviour Checklist. N = 60.
p <.05.
p <.01.
p <.001
3.2. Regression analyses
To test the influences of age, condition and testosterone on choice, we set up a multilevel regression model where choices were nested in conditions and conditions were nested in participants.
In our first model we tested the relationship between age, condition and choice behaviour. Consistent with previous studies, we found both a significant decrease in SS choices with age, βage = −.26, 95% CI [−.43, −.08], p < .001, and a higher proportion of SS choices in the now/later than in the later/later condition, βcondition = .55, 95% CI [.28, 82], p < .001 (see Table 2). However, we did not find a significant interaction between condition and age, βcond*age = −.15, 95% CI [−.42, .15], p = .21, suggesting that participants tendency to be more impulsive in presence of an immediate reward is not changing with age (see Table 2, Model 1).
Table 2.
Multilevel regression models.
| Model 1 |
Model 2 |
Model 3 |
||||
|---|---|---|---|---|---|---|
| β | CI | β | CI | β | CI | |
| Age | −.26** | [−.43, −.08] | −.31*** | [−.47, −14] | −.17* | [−.31, −.03] |
| Condition | .55*** | [.28, .82] | .47*** | [.21, .74] | .30** | [.09, .51] |
| Age × condition | .15 | [−.42, .15] | −.01 | −.26, .24] | −.04 | −.35, .26] |
| Testosterone | .23** | [.06, .39] | .12 | [−.14, .36] | ||
| Testosterone × condition | .39*** | [.13, .64] | .04 | [−.25, .34] | ||
| PDS | .09 | [−.12, .30] | ||||
| PDS × condition | −.06 | [−.38, .25] | ||||
| CBCL | .04 | [−.09, .17] | .001 | [−.14, .14] | −.02 | [−.13, .07] |
| R 2 | .18 | .36 | .33 | |||
| BIC | −39.25 | −68.25 | −58.74 | |||
Beta coefficients and 95% confidence intervals for variables included in the three tested models, along with model fit and BIC values for each model, respectively. Model 1 consists of four regressors: age, condition, age by condition and CBCL; Model 2 consists of six regressors: age, condition, age by condition, testosterone, testosterone by condition and CBCL; Model 3 consists of eight regressors: age, condition, age by condition, testosterone, testosterone by condition, PDS, PDS by condition and CBCL. PDS: Pubertal Developmental Scale, CBCL: Child Behaviour Checklist, Cl: confidence interval, BIC: Bayesian Information Criterion. N =60.
p <.05.
P <.01.
p <.001.
Next when we added testosterone predictors to the model we found that testosterone predicted an increase in SS choices, βtesto = .23, 95% CI [.06, .39], p = .006 (see Table 2, Model 2). Again, we found a significant effect of condition βcondition = .47, 95% CI [.21, .74], p < .001, and, importantly, the effect of immediate rewards on choice was depended on testosterone, βcond*testo = .39, 95% CI [.13, .64], p = .002. For display purposes we used a median split on testosterone levels to show the direction of interaction between condition and testosterone (see Fig. 1B). Finally, the effect of age remained significant, βage = −.31, 95% CI [−.47, − 14], p < .001, indicating that the effects of testosterone and age on choice are not only orthogonal but also statistically independent. All variance inflations factors (VIFs) were smaller than 2.05, which indicate that the shared variance (or multicollinearity) between age and testosterone regressors (see Table 1) was not problematic for fitting the model. Both the R2 and BIC clearly indicate that the model fits the data better than the age only model (for more details see Table 2).
Lastly, we added the PDS score and the PDS by condition interaction to the model. Adding the PDS regressors to the model took out the shared variance between testosterone and PDS (see zero-order correlations in Table 1) and thus allowed us to find evidence whether individual variance in testosterone independent of pubertal development is also predicting choice behaviour. Neither PDS, nor its interaction with condition had a significant effect (βPDS = .09, 95% CI [−.12, .30], p = .10; βPDS*condition = −.06, 95% CI [−.38, .25], p = .61). Interestingly, the effects of testosterone were also no longer significant (βtesto = .12, 95% CI [−.14, .36], p = .44; βcondition*testo = .04, 95% CI [−.25, .34], p = .18). However, age and condition remained significant predictors (βage = −.17, 95% CI [−.31, −.03], p < .001; βcondition = .30, 95% CI [.09, .51], p < .001, respectively, see also Table 2, Model 3). Importantly all VIFs were <2.6, which suggest that estimating the model was in principle not problematic. Based on these results we do not find evidence supporting the hypothesis that sensitivity to immediate rewards is related to individual levels in testosterone independent of pubertal development. Indeed, the results are consistent with the assumption that behavioural effects are related to pubertal related shifts in testosterone.
3.3. Modelling results
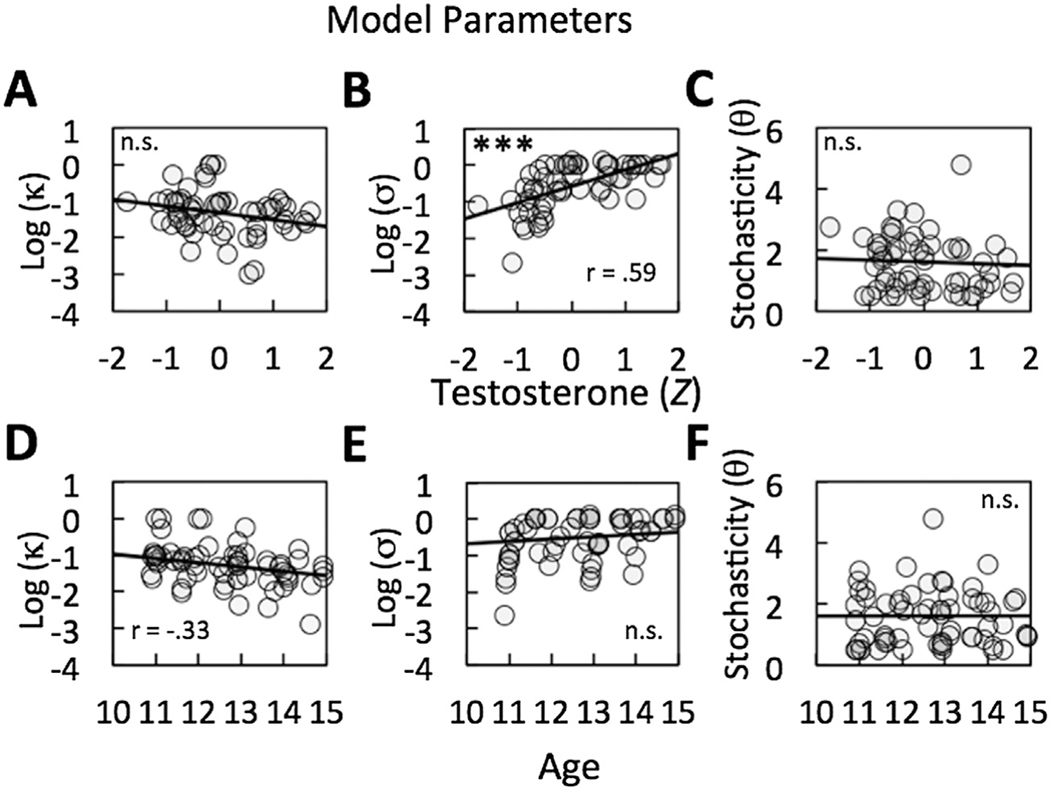
Bayesian model comparison indicated that the two-parameter hyperbolic discounting model fit the data best (see Tables 3 and 4 for the best-fitting parameters). Because the hyperbolic and two-parameter hyperbolic BIC and AIC values were moderately close, we also performed a likelihood ratio test, which showed that there was indeed a significant difference between these two models, x2(1)=5.8, p =.016, in terms of goodness of fit. Note that the parameter κ governs the rate at which subjective value decreases and that represents the relative sensitivity to more immediate rewards versus those available further in the future. Both parameters were non-normally distributed (Shapiro–Wilk’s W = 0.09, p < .001 and W = 0.91, p < .001, respectively) and were therefore log transformed for further analyses. All variables were standardized before being entered in correlation or regression analyses. A positive scaling parameter σ (significantly larger than 0, t(71) = .977, 95% CI [.45, .68], p < .001) indicates that all participants were more sensitive to rewards situated in the near future. In addition, the scaling parameter was positively correlated with testosterone (Pearson’s r(58) = .59, 95% CI = [.42, .74], p < .001, and showed a trending relationship with pubertal status, r(58) = .29, 95% CI [−.04, .50], p = .062, but no correlation with age, r(58) = .21, 95% CI [−.09, .42], p = .18 (see Fig. 2B and E). In contrast, for the discount parameter κ, we did not observe a correlation with testosterone (Fig. 2A) or PDS (both ps > .22), but we found a negative trend with age, r(58) = −.33, 95% CI [−.02, .54], p = .058 (Fig. 2D). Importantly, the relationship between the discount parameter κ and age was significant in a multiple linear regression controlling for testosterone, b = −1.01, 95% CI (−.11, −2.01), p = .039. Similarly, the relationship between the scaling parameter σ and testosterone remained significant when we controlled for age, b = 1.43, 95% CI [.21, 2.65], p = .023. As reported above, when PDS was included as a confounding variable, the effects of testosterone were no longer significant. Taken together, these results indicate that the higher levels of testosterone associated with pubertal development are specifically related to increased sensitivity to more immediate rewards, and not to the general discounting of all future rewards. At the same time, increased age is related to a reduction in the general discounting rate (see Fig. 1C and Table 5 for detail on parameters).
Table 3.
Model fits.
| Model | G 2 | BIC | AIC |
|---|---|---|---|
| Random choice | 110.9 | 110.9 | 110.9 |
| Beta-delta | 72.4(1.03) | 85.55(1.03) | 78.4(1.03) |
| Hyperbolic | 65.9(1.61) | 74.66(1.61) | 69.9(1.61) |
| Two-parameter hyperbolic | 60.1 (2.08) | 72.38(2.08) | 66.1 (2.08) |
Model fits for different discounting models. BIC: Bayesian Information Criterion, AIC: Akaike Information Criterion. N = 60.
Table 4.
Mean parameter estimates two-parameter hyperbolic.
| Two-parameter hyperbolic | |
|---|---|
| κ | .37 (.05) |
| σ | .56 (.05) |
| θ | 1.41 (.14) |
Note. None of the parameter estimates were correlated with each other (all p > .2 and all r <.22). N = 60.
Fig. 2.
Illustration of the correlations between model parameters, testosterone and age. (A) Correlation between log(κ) and testosterone. (B) Correlation between log(σ) and testosterone. (C) Correlation between θ and testosterone. (D) Correlation between log(κ) and age. (E) Correlation between log(σ) and age. (F) Correlation between θ and age. ***p < .001.
Table 5.
Median split model fits.
| Parameter | T− | T+ | diff | Age− | Age− | diff |
|---|---|---|---|---|---|---|
| κ | .42 (.05) | .27 (.06) | − | .55 (.05) | .21 (.05) | A− > A+* |
| σ | .36 (.07) | .73 (.07) | T+ >T−** | .49 (.07) | .58 (.07) | − |
| θ | 1.55 (.24) | 1.19 (.35) | − | 1.51 (.28) | 1.39 (.29) | − |
Parameter estimates based on median splits of testosterone and age, as well as their difference between the respective high and low group. T−: low testosterone group, T+: high testosterone group, Age−: younger age group, Age+: older age group, diff: difference between high and low group with respect to estimated parameter. N =60.
p <.05.
p <.01.
4. Discussion
The goal of this study was to elucidate the role of testosterone in impatient behaviour in early adolescence. To this end, we combined measures of salivary testosterone and self-reported pubertal status with detailed modelling of decisions on a curated set of intertemporal choices. Applying these techniques resulted in a more detailed understanding of the relationship between pubertal status, gonadal hormones, and temporal preferences that go beyond self-report and simple choice data. Our study revealed an interesting double dissociation: (1) consistent with previous studies, we found that age, but not testosterone, is associated with an overall decline in discounting in early adolescence, and (2) testosterone but not age is associated with increased sensitivity to immediate rewards. These findings suggest that impatient decision making is the result of at least two distinct processes that follow different developmental trajectories in early adolescence.
First, one possible assumption is that sensitivity to immediate rewards is associated with the effects of testosterone on reward-related brain regions such as the striatum. For instance, neuroanatomical studies showing that testosterone may modulate the striatal dopamine system in rodents (see Laube and van den Bos, 2016), but also reward related striatal activity in adolescents (Braams et al., 2015). Such a mechanism may have several implications for the understanding of adolescents’ reported sensitivity to arousing situations. For instance, studies on affective risk-taking behaviour in adolescence show that adolescents are more likely than children or adults to make risky choices in emotionally “hot” contexts, where feedback was immediate vs. “cold” contexts, where feedback was delayed (Figner et al., 2009). We hypothesize that these types of effect may be specifically associated with circulating testosterone impacting the striatal dopamine system. The data also suggests that the type of developmental trajectory, linear or inverted U shape, may be very much dependent on how the task taps into the specific processes involved in impulsive decision-making (e.g. are immediate rewards present or not). As a result, we should not expect that adolescents would be the most impulsive age group in every possible situation (see Defoe et al., 2015).
Second, the overall decline in discounting in early adolescents associated with age may be related to increased cognitive control. This interpretation is in line with several previous studies showing that developmental reductions in impatience across adolescence are driven primarily by increased cognitive control (Steinberg et al., 2009; van den Bos et al., 2015). More importantly, the behavioural dissociation between age and testosterone, and the modelling results, illustrate that impatient behaviour is best described as the product of multiple interacting processes (van den Bos and McClure, 2013). Recent research suggests that the striatum is one of the central regions where different valuation processes are integrated (Burton et al., 2015). Indeed, increased control across adolescence has been associated with the dorsal striatum and its connections with the prefrontal cortex (Luna et al., 2015; van den Bos et al., 2015), whereas sensitivity to immediate rewards in adults has been associated with activity in the ventral striatum (McClure et al., 2004). Investigating how these neural pathways are associated with the hormonal and age-related processes identified in this study is an exciting avenue for future research.
Our multiple-process perspective also generates interesting and testable hypotheses on how the timing of pubertal onset shapes impulsive behaviour during adolescence. For instance, Martin et al. (2001) found that retrospective report of early pubertal onset was associated with increased sensation seeking and substance use in both males and females, while controlling for gender differences in onset. Thus, early entrance into puberty may amplify the effects of testosterone on impulsivity, whereas late entrance may dampen its effects because frontal regions are, by this time, more developed.
Our results also speak to recent debates about the definition of impulsivity (Duckworth and Steinberg, 2015), which often revolve around semantic issues that are hard to resolve (van den Bos and Eppinger, 2015). We have tried to contribute to this debate by further unpacking impatience into different processes. Future research could benefit from using similar approaches to unpack related constructs (e.g., sensation seeking) by focusing on the underlying psychological processes. This approach may provide novel insights into the shared variance between related constructs. For instance, it may well be the case that sensitivity to immediate rewards is related to some aspect of sensation seeking (as suggested by Steinberg and Chein, 2015), whereas general discounting is not.
Finally, several limitations of this study should be highlighted. One is the restriction to male participants. While circulating testosterone is higher in males than in females, it also increases in females during puberty. It is therefore crucial to repeat this study in a group of girls. Furthermore, as mentioned before, within a cross-sectional design such as the present, it is difficult to distinguish pubertal maturation from non-developmental individual differences in testosterone levels. Although the relationship between PDS and testosterone lends support to our interpretation, additional longitudinal investigations are needed to disentangle individual and developmental differences. Nevertheless, a recent study with adult males showed that testosterone did not influence impulsivity (Ortner et al., 2013). Moreover, future replications, using larger sample sizes, will be needed to increase our confidence about the generalizability of the reported effects. Lastly, although the delay discounting task has a good record in predicting real-world outcomes in both adults and adolescents (Bickel et al., 1999; Duck-worth and Seligman, 2012; Petry, 2001; Reimers et al., 2009), the present data allow only indirect inferences to be drawn about the relation between real-world outcomes and pubertal testosterone.
In conclusion, by combining the assessment of pubertal testosterone with the investigation of delay discounting, this study broadens the understanding of developmental changes in impulsive behaviour, specifically related to impatience, in early adolescence. Our results highlight the importance of understanding adolescent behaviour as the endpoint of multiple interacting processes. Furthermore, they emphasize the specific impact of immediate rewards on adolescents. There are several potential ways to capitalize on this sensitivity in the development of interventions. One would be to ensure that desired behaviour has concrete short-term rewards, not only future rewards (e.g., good grades at the end of a school year). This may eventually lead to the development of commitment mechanisms that force young people to make decisions when both outcomes are in the future, thus diminishing the impact of immediate outcomes (e.g., deciding not to take your car to a party, instead of deciding not to drink once you have driven there; Bryan et al., 2010). Finally, the next steps for this research include replications with greater sample sizes and longitudinal investigation of changes in testosterone to disentangle individual and developmental differences in testosterone, mapping these processes onto real world behaviours, and investigating the associated neural mechanisms.
Acknowledgement
We thank Oisin Butler and Susannah Goss for editing the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Declaration of conflicting interests
The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
References
- Achenbach TM, Dumenci L, Rescorla LA, 2001. Ratings of relations between DSM-IV diagnostic categories and items of the CBCL/6–18, TRF, and YSR. Vermont Res., 1–9, Retrieved from http://www.childbehaviorchecklist.com/research/DSM6-18ratings.pdf. [Google Scholar]
- Allen KM, Purves-Tyson TD, Fung SJ, Shannon Weickert C, 2015. The effect of adolescent testosterone on hippocampal BDNF and TrkB mRNA expression: relationship with cell proliferation. BMC Neurosci. 16 (1), 4, 10.1186/s12868-015-0142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S, 2014. lme4: linear mixed-effects models using Eigen and S4, R package version 1.0–6, Retrieved from http://CRAN.R-project.org/package=lme4. [Google Scholar]
- Bickel WK, Odum AL, Madden GJ, 1999. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology (Berl) 146 (4), 447–454, 10.1007/PL00005490. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Pitcock JA, Yi R, Angtuaco EJC, 2009. Congruence of BOLD response across intertemporal choice conditions: fictive and real money gains and losses. J. Neurosci 29 (27), 8839–8846, 10.1523/JNEUROSCI.5319-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braams BR, van Duijvenvoorde ACK, Peper JS, Crone EA, 2015. Longitudinal changes in adolescent risk-taking: a comprehensive study of neural responses to rewards, pubertal development, and risk-taking behavior. J. Neurosci 35 (18), 7226–7238, 10.1523/JNEUROSCI.4764-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg U, Wiehler A, Peters J, 2015. Episodic future thinking is related to impulsive decision making in healthy adolescents. Child Dev. 86 (5), 1458–1468, 10.1111/cdev.12390. [DOI] [PubMed] [Google Scholar]
- Bryan G, Karlan D, Nelson S, 2010. Commitment devices. Annu. Rev. Econ 2, 671–698, 10.1146/annurev.economics.102308.124324. [DOI] [Google Scholar]
- Burton AC, Nakamura K, Roesch MR, 2015. From ventral-medial to dorsal-lateral striatum: neural correlates of reward-guided decision-making. Neurobiol. Learn. Mem 117, 51–59, 10.1016/j.nlm.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Galván A, Somerville LH, 2015. Beyond simple models of adolescence to an integrated circuit-based account: a commentary. Dev. Cogn. Neurosci 17, 128–130, 10.1016/j.dcn.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Dahl RE, 2012. Understanding adolescence as a period of social–affective engagement and goal flexibility. Nat. Rev. Neurosci 13 (9), 636–650, 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Cross CP, Copping LT, Campbell A, 2011. Sex differences in impulsivity: a meta-analysis. Psychol. Bull 137 (1), 97–130, 10.1037/a0021591. [DOI] [PubMed] [Google Scholar]
- D’Acremont M, Van Der Linden M, 2005. Adolescent impulsivity: findings from a community sample. J. Youth Adolesc 34 (5), 427–435, 10.1007/s10964-005-7260-1. [DOI] [Google Scholar]
- Dahl RE, 2004. Adolescent brain development: a period of vulnerabilities and opportunities, keynote address. Ann. N.Y. Acad. Sci 1021, 1–22, 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Defoe IN, Dubas JS, Figner B, van Aken M.a.G., 2015. A meta-analysis on age differences in risky decision making: adolescents versus children and adults. Psychol. Bull 141 (1), 48–84, 10.1037/a0038088. [DOI] [PubMed] [Google Scholar]
- de Water E, Cillessen AHN, Scheres A, 2014. Distinct age-related differences in temporal discounting and risk taking in adolescents and young adults. Child Dev. 85 (5), 1881–1897, 10.1111/cdev.12245. [DOI] [PubMed] [Google Scholar]
- Duckworth AL, Steinberg L, 2015. Unpacking self-control. Child Dev. Perspect 9 (1), 32–37, 10.1111/cdep.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, 2014. The triadic model perspective for the study of adolescent motivated behavior. Brain Cogn. 89, 104–111, 10.1016/j.bandc.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figner B, Mackinlay RJ, Wilkening F, Weber EU, 2009. Affective and deliberative processes in risky choice: age differences in risk taking in the Columbia Card Task. J. Exp. Psychol.: Learn. Mem. Cogn 35 (3), 709–730, 10.1037/a0014983. [DOI] [PubMed] [Google Scholar]
- Gjedde A, Kumakura Y, Cumming P, Linnet J, Møller A, 2010. Inverted-U-shaped correlation between dopamine receptor availability in striatum and sensation seeking. Proc. Natl. Acad. Sci 107 (8), 3870–3875, 10.1073/pnas.0912319107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DA, Shirtcliff EA, Booth A, Kivlighan KT, Schwartz EB, 2004. The “trouble” with salivary testosterone. Psychoneuroendocrinology 29 (10), 1229–1240, 10.1016/j.psyneuen.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Green L, Myerson J, 2004. A discounting framework for choice with delayed and probabilistic rewards. Psychol. Bull 130 (5), 769–792, 10.1037/0033-2909.130.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden KP, Tucker-Drob EM, 2011. Individual differences in the development of sensation seeking and impulsivity during adolescence: further evidence for a dual systems model. Dev. Psychol 47 (3), 739–746, 10.1037/a0023279. [DOI] [PubMed] [Google Scholar]
- Laibson D, 1997. Golden eggs and hyperbolic discounting. Quart. J. Econ 112 (2), 443–478, 10.1162/003355397555253. [DOI] [Google Scholar]
- Laube C, van den Bos W, 2016. Hormones and affect in adolescent decision making. In: Kim S, Reeve J, Bong M (Eds.), Recent Developments in Neuroscience Research on Human Motivation (Advances in Motivation and Achievement No. 19). Emerald Group Publishing Limited, Bingley, UK, pp. 259–281, 10.1108/S0749-742320160000019013. [DOI] [Google Scholar]
- Lee PA, 1980. Normal ages of pubertal events among American males and females. J. Adolesc. Health Care 1 (1), 26–29, 10.1016/S01970070(80)80005-2. [DOI] [PubMed] [Google Scholar]
- Luna B, Marek S, Larsen B, Tervo-Clemmens B, Chahal R, 2015. An integrative model of the maturation of cognitive control. Annu. Rev. Neurosci 38 (1), 151–170, 10.1146/annurev-neuro-071714-034054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Logan TK, Leukefeld C, Milich R, Omar H, Clayton R, 2001. Adolescent and young adult substance use: association with sensation seeking, self esteem and retrospective report of early pubertal onset. A preliminary examination. Int. J. Adolesc. Med. Health 13 (3), 211–219, 10.1515/IJAMH.2001.13.3.211. [DOI] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein GF, Cohen JD, 2004. Separate neural systems value immediate and delayed monetary rewards. Science 306 (5695), 503–507, 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Nower L, Derevensky JL, Gupta R, 2004. The relationship of impulsivity, sensation seeking, coping, and substance use in youth gamblers. Psychol. Addict. Behav 18 (1), 49–55, 10.1037/0893-164X.18.1.49. [DOI] [PubMed] [Google Scholar]
- Ortner GR, Wibral M, Becker A, Dohmen T, Klingmüller D, Falk A, Weber B, 2013. No evidence for an effect of testosterone administration on delay discounting in male university students. Psychoneuroendocrinology 38 (9), 1814–1818, 10.1016/j.psyneuen.2012.12.014. [DOI] [PubMed] [Google Scholar]
- Peper JS, Koolschijn PCMP, Crone EA, 2013. Development of risk taking: contributions from adolescent testosterone and the orbito-frontal cortex. J.Cogn. Neurosci 25 (12), 2141–2150. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A, 1988. A self-report measure of pubertal status: reliability, validity, and initial norms. J. Youth Adolesc 17 (2), 117–133, 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Petry NM, 2001. Substance abuse, pathological gambling, and impulsiveness. Drug Alcohol Depend. 63 (1), 29–38, 10.1016/S03768716(00)00188-5. [DOI] [PubMed] [Google Scholar]
- Quinn PD, Harden KP, 2013. Differential changes in impulsivity and sensation seeking and the escalation of substance use from adolescence to early adulthood. Dev. Psychopathol 25 (1), 223–239, 10.1017/S0954579412000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimers S, Maylor EA, Stewart N, Chater N, 2009. Associations between a one-shot delay discounting measure and age, income, education and real-world impulsive behavior. Personal. Individ. Diff 47 (8), 973–978, 10.1016/j.paid.2009.07.026. [DOI] [Google Scholar]
- Robbins TW, Gillan CM, Smith DG, de Wit S, Ersche KD, 2012. Neurocognitive endophenotypes of impulsivity and compulsivity: towards dimensional psychiatry. Trends Cogn. Sci 16 (1), 81–91, 10.1016/j.tics.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Romer D, 2010. Adolescent risk taking, impulsivity, and brain development: implications for prevention. Dev. Psychobiol 52 (3), 263–276, 10.1002/dev.20442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Dahl RE, Pollak SD, 2009. Pubertal development: correspondence between hormonal and physical development. Child Dev. 80 (2), 327–337, 10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman EP, Smith AR, Silva K, Icenogle G, Duell N, Chein J, Steinberg L, 2016. The dual systems model: review, reappraisal, and reaffirmation. Dev. Cogn. Neurosci 17, 103–117, 10.1016/j.dcn.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman MH, Jedd K, Luciana M, 2015. Neural networks involved in adolescent reward processing: an activation likelihood estimation meta-analysis of functional neuroimaging studies. Neuroimage 122, 427–439, 10.1016/j.neuroimage.2015.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, 2013. Adolescent neurodevelopment. J. Adolesc. Health 52 (2), S7–S13, 10.1016/j.jadohealth.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Chein JM, 2015. Multiple accounts of adolescent impulsivity. Proc. Natl. Acad. Sci. U. S. A 112 (29), 8807–8808, 10.1073/pnas.1509732112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Graham S, O’Brien L, Woolard J, Cauffman E, Banich M, 2009. Age differences in future orientation and delay discounting. Child Dev. 80 (1), 28–44, 10.1111/j.1467-8624.2008.01244.x. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J, 2008. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: evidence for a dual systems model. Dev. Psychol 44 (6), 1764, 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- van den Bos W, Eppinger B, 2015. Developing developmental cognitive neuroscience: from agenda setting to hypothesis testing. Dev. Cogn. Neurosci 17, 138–144, 10.1016/j.dcn.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos W, McClure SM, 2013. Towards a general model of temporal discounting. J. Exp. Anal. Behav 99 (1), 58–73, 10.1002/jeab.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos W, Rodriguez CA, Schweitzer JB, McClure SM, 2015. Adolescent impatience decreases with increased frontostriatal connectivity. Proc. Natl. Acad. Sci. U. S. A 112 (29), E3765–E3774, 10.1073/pnas.1423095112. [DOI] [PMC free article] [PubMed] [Google Scholar]