Highlights
-
•
Wearable physical activity trackers may be effective tools for increasing physical activity.
-
•
The purpose of this systematic review was to evaluate the effect of these devices for improving physical activity and health-related outcomes in cancer survivors.
-
•
Findings showed that wearable trackers had significant effects on moderate-intensity physical activity, moderate-to-vigorous-intensity physical activity, total physical activity, daily steps, quality of life, aerobic fitness, physical function and reduced fatigue compared to usual care.
-
•
Identifying how these devices can be implemented for longer-term use with other intervention components remains an area for future research.
Keywords: Activity tracker, Cancer, Exercise, Physical activity, Wearable
Abstract
Purpose
This systematic review and meta-analysis aimed to evaluate the effect of wearable devices for improving physical activity and health-related outcomes in cancer survivors.
Methods
CINAHL, Cochrane, Ebscohost, MEDLINE, Pubmed, ProQuest Health and Medical Complete, ProQuest Nursing and Allied Health Source, ScienceDirect, and SPORTDiscus databases were searched for randomized controlled trials published before September 1, 2020, that evaluated interventions involving wearable devices in cancer survivors. Standardized mean differences (SMDs) were calculated to assess effects on physical activity and health-related outcomes. Subgroup analyses were conducted to assess whether the effects differed by interventions and cancer characteristics. Risk of bias was assessed using the Cochrane risk of bias tool.
Results
Thirty-five trials were included (breast cancer, n = 15, 43%). Intervention durations ranged between 4 weeks and 1 year. Most trials (n = 25, 71%) involved pedometer-based physical activity interventions. Seven (20%) involved Fitbit-based interventions, and 3 (9%) involved other wearable physical activity trackers (e.g., Polar, Garmin). Compared to usual care, wearable devices had moderate-to-large effects (SMD range 0.54−0.87, p < 0.001) on moderate-intensity physical activity, moderate-to-vigorous-intensity physical activity, total physical activity, and daily steps. Compared to usual care, those in the intervention had higher quality of life, aerobic fitness, physical function, and reduced fatigue (SMD range = 0.18−0.66, all p < 0.05).
Conclusion
Wearable physical activity trackers and pedometers are effective tools that increase physical activity and improve health-related outcomes in individuals with cancer. Identifying how these devices can be implemented for longer-term use with other intervention components remains an area for future research.
Graphical abstract

1. Introduction
Consistent evidence demonstrates that physical activity interventions, including structured exercise, improve health-related outcomes such as physical and psychosocial health and overall quality of life during and following treatment for cancer.1, 2, 3, 4 Observational studies and exploratory analyses from randomized controlled trials (RCTs) also suggests that following a cancer diagnosis, higher physical activity levels are associated with a lower risk of cancer recurrence and improved survival.5,6 These observed benefits have led international cancer7 and exercise organizations8,9 to make recommendations that promote participation in physical activity and structured exercise post-cancer diagnosis.
Despite the important benefits of exercise, many individuals with cancer are insufficiently physically active. Evidence from prospective population-based cancer cohort studies demonstrates that physical activity levels decline post-diagnosis.10,11 The proportion of patients meeting physical activity guidelines is markedly reduced between pre-diagnosis and during breast cancer treatment (from 60% to 35%)11 and between pre-diagnosis and 6 months post-diagnosis among colorectal cancer patients (from 27% to 10%).10 Thus, finding ways to safely and successfully engage cancer survivors in effective physical activity and exercise interventions remains an important focus in exercise oncology research.
Preliminary evidence suggests that wearable physical activity trackers and pedometers are effective tools that increase activity levels.12 These devices provide feedback on physical activity levels and allow users to self-monitor, set goals, seek social support, and record and review data.13, 14, 15 Previous systematic reviews have shown that interventions that incorporate wearable devices are effective for increasing physical activity in various non-clinical16 and clinical populations,17 including those with musculoskeletal diseases,18 type 2 diabetes,19 chronic obstructive pulmonary disease,20 and cardiometabolic diseases.21 Among cancer populations, multiple factors may influence the effectiveness of interventions for increasing physical activity levels, including supervision22 and the use of behavior change techniques.23 Supervised exercise interventions have been shown to be effective for increasing physical activity levels and improving health outcomes in cancer populations.8 Moreover, among women with breast cancer, larger overall effects in increasing physical activity have been shown following interventions involving extensive implementation of behavior change techniques (e.g., self-monitoring, education, goal setting, feedback), compared with trials involving sparse and moderate use of behavior change techniques (effect size = 0.76 vs. 0.28 and 0.36, respectively).23 Therefore, physical activity trackers and pedometers may be an important tool that can be implemented in addition to various intervention components such as supervision and use of behavior change counselling. Findings from a recent systematic review (n = 13) provided preliminary evidence on the efficacy of consumer-wearable devices in promoting physical activity and weight management in cancer survivors.24 However, the systematic review focused on physical activity outcomes and did not involve a meta-analysis of the data. Meta-analytic and feasibility data on the effects of wearable physical activity trackers on physical activity and health-related outcomes in individuals with cancer are lacking. Therefore, the purpose of our systematic review and meta-analysis was to evaluate the feasibility and effects of wearable physical activity tracker and pedometer-based interventions on increased physical activity and health-related outcomes in individuals with cancer.
2. Methods
2.1. Search strategy and selection criteria
The protocol for this systematic review and meta-analysis was pre-registered on the PROSPERO registry (CRD42020211813). Several changes were made to our pre-registered protocol; these are outlined in Supplementary Table 1. The Participant, Intervention, Comparator, and Outcome framework25 was used to develop the eligibility criteria as follows: (1) Participants: trials that involved participants of any age with a diagnosis of cancer at any stage of treatment (pre-treatment, undergoing treatment, or post-treatment); studies that involved samples with multiple cancer types; (2) Intervention: RCTs that evaluated a physical activity intervention involving the use of a wearable physical activity tracker (e.g., Fitbit) or pedometer; (3) Outcome and comparator: RCTs that used a comparative study design to test the feasibility or efficacy of the intervention for increasing physical activity levels or health outcomes, with random allocation of participants to either an intervention group or a usual care group. We used the following definition of a wearable physical activity tracker: “commercial wearable devices that objectively measure lifestyle physical activity and can provide feedback, beyond the display of basic activity information (i.e., steps), via the monitor display or through a partnering application to elicit continual self-monitoring of physical activity behaviour”.12 Interventions had to be designed with the aim of increasing physical activity or improving health-related outcomes (e.g., quality of life). Studies were eligible regardless of the level of supervision provided, mode of intervention delivery, or intervention duration or intensity. Studies that evaluated the validity or reliability of wearable physical activity trackers or pedometers were excluded. Studies that involved physical activity or exercise in addition to other interventions (e.g., dietary intervention) were excluded if the results of exercise could not be isolated. Studies that involved multiple intervention arms were eligible if there was at least 1 usual care or control arm.
An electronic database search was undertaken using combinations of MeSH and free-text terms for “cancer”, “physical activity”, “physical activity tracker”, “wearable”, “pedometer”, and “accelerometer” (see Supplementary Table 2 for the full search details for all databases). The following databases were searched: CINAHL, Cochrane, Ebscohost, MEDLINE, Pubmed, ProQuest Health and Medical Complete, ProQuest Nursing and Allied Health Source, ScienceDirect, and SPORTDiscus. Database searches were limited to peer-reviewed journal articles published in the English language prior to September 1, 2020.
2.2. Outcomes of interest
The primary outcome of interest was post-intervention physical activity level. Other outcomes of interest were effects on health-related outcomes and feasibility (safety and acceptability). Meta-analyses (including subgroup analyses) were undertaken to evaluate the effectiveness of wearable tracker and pedometer-based interventions on outcomes of interest that were reported in at least 3 studies. The following data/outcomes were extracted:
-
•
Physical activity. Physical activity-related domains were low-intensity physical activity, moderate-intensity physical activity, vigorous-intensity physical activity, moderate-to-vigorous-intensity physical activity (MVPA), total physical activity (assessed using either accelerometry or self-report), and daily steps (assessed using accelerometry or pedometers).
-
•
Health-related outcomes. Health-related outcomes were quality of life, fatigue, aerobic fitness, and physical function.
-
•
Safety and acceptability. Safety (associated with the physical activity intervention) was evaluated by assessing the number of reported intervention-related adverse events. Intervention-related adverse events were considered undesirable medical or health-related events that occurred during or as a result of participation in exercise or physical activity.26 One of the authors (BS) of our study graded the severity of reported adverse events using the Common Terminology Criteria for Adverse Event (Version 4.0) 27 as follows: Grade 1 (asymptomatic or mild symptoms; clinical or diagnostic observations only required), Grade 2 (moderate symptoms; minimal, local, or non-invasive intervention required that limited age-appropriate activities of daily living), Grade 3 (severe or medically significant symptoms but not immediately life-threatening; hospitalization and/or prolongation of hospitalization indicated, limits placed on self-care and activities of daily living), Grade 4 (life-threatening symptoms; urgent intervention indicated), or Grade 5 (fatal symptoms; death).2,28 Acceptability was assessed by evaluating information about the use of physical activity trackers and pedometers, including wear time, user preferences, likes and dislikes, barriers, and satisfaction with using the devices.
2.3. Data extraction
Titles and abstracts of all records identified as a result of the electronic database search were screened by one of the authors (BS) of our study. The reference lists of relevant articles were also screened manually to identify potentially eligible studies. The full texts of studies that were considered potentially eligible based on the title or abstract were then retrieved and screened against the eligibility criteria. Full-text data were extracted by BS, and data extraction accuracy of 15% of the studies was checked by a second author (EMZ). The following data were extracted from each study into tabular format: study and participant characteristics, intervention details, adverse events, and outcome results.
The quality of included RCTs was assessed by 2 authors (BS and EMZ) using the Cochrane risk of bias tool.29 This is a valid and reliable tool for assessing RCTs and consists of 7 domains (random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other potential sources of bias).29 Discrepancies in ratings were resolved by discussion with the senior author (EJH).
2.4. Statistical analyses
Physical activity and health-related outcomes were analyzed as continuous variables and involved evaluations of post-intervention means and standard deviations (SDs) between intervention and usual care groups. Standardized mean differences (SMDs) were calculated as the effect measure to allow comparison of data from different scales using RevMan software (Version 5.3; The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). R statistical software (Version 3.4.1; R Foundation for Statistical Computing, Vienna, Austria) was used to create forest plots for each meta-analysis. If means and/or SDs were not available, the authors of the study were contacted, or means and/or SDs were calculated based on reported data (e.g., using median, range, and sample size) using recommended formulas.30 If a study reported MVPA and/or total physical activity as an outcome, then data from that outcome were used for our meta-analyses. We did not compute MVPA and/or total physical activity based on other reported outcomes (e.g., by using low-, moderate-, and/or vigorous-intensity physical activity). When 2 or more methods of assessing an outcome were used in a study, either the method defined as being the gold standard or the method with demonstrated validity and reliability was used.
For each meta-analysis, data were combined at the study level. To assess publication bias, SMDs were plotted against corresponding SDs and asymmetries or missing sections within the funnel plot were assessed.31 Cochran's Q test was used to assess statistical heterogeneity, and the proportion of the outcome that was attributed to variability was computed using the I2 statistic.32,33 Heterogeneity was determined using the following values: I2 = 0%–29%, no heterogeneity; I2 = 30%−49%, moderate heterogeneity; I2 = 50%−74%, substantial heterogeneity; and I2 = 75%−100%, considerable heterogeneity.33 Planned subgroup analyses were performed to assess the effects of (1) device (Pedometer, Fitbit, or other), (2) contact or support during the intervention (in-person (with or without phone/email), phone/email/SMS only, or no intervention support), (3) intervention based on a theoretical framework or theory (yes or no), (4) intervention involving a baseline physical activity counselling or instruction session (yes or no), (5) method of physical activity assessment (self-report or objective), (6) intervention length (≤12 weeks or >12 weeks), (7) treatment status (during adjuvant treatment, post-adjuvant treatment, or mixed (studies involving mixed samples of participants undergoing and having completed adjuvant treatment)), and (8) cancer type (breast, colorectal, prostate, leukemia, or mixed (i.e., studies involving mixed cancer types)). Classifications for the magnitude of effect were: <0.20 (small effect), 0.20–0.50 (medium effect), and >0.50 (large effect).34 p < 0.05 was considered statistically significant. Safety and acceptability data were reported descriptively.
3. Results
3.1. Literature search
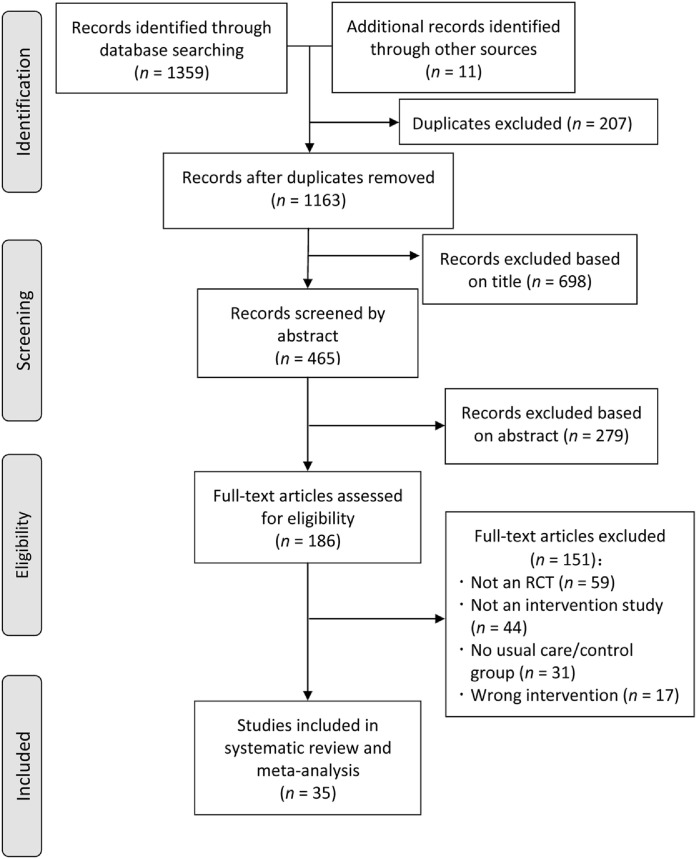
Following a search of databases, 1359 articles were identified (Fig. 1) and 1163 remained after removal of duplicates. Title and abstract screening was performed, with 186 full-text articles retrieved and screened. Of these, 151 articles were excluded because they did not meet the inclusion criteria; thus, 35 trials were included in the review. Cochrane risk of bias ratings are shown in Supplementary Fig. 1. Over one-half of the included studies were rated as low risk of bias in the domains of selection bias and attrition bias. All studies were rated as high risk of bias in the domains of performance and reporting bias. Four trials35, 36, 37, 38 had multiple intervention arms involving a wearable physical activity tracker or pedometer, in addition to a control group. Specifically, these 4 trials included a pedometer-based physical activity intervention during or following a rehabilitation program,35 a low- or high-intensity physical activity intervention involving a Polar A360® device,36 a pedometer-only or pedometer plus Nintendo Wii intervention,37 and a pedometer-only or pedometer plus print materials intervention.38 Therefore, a total of 39 intervention arms (across 35 trials) were included. An overview of included study characteristics is shown in Supplementary Table 3.
Fig. 1.
The Preferred Reporting Items for Systematic reviews and Meta-Analyses flow chart. RCT = randomized controlled trial.
3.2. Study and participant characteristics
An overview of individual study details is shown in Supplementary Table 4. Median sample size was 75 (range: 19−516), and mean participant age was 56 ± 9 years (mean ± SD). Time since diagnosis was reported in 9 trials (26%) and ranged between 11 months and 70 months (median = 24 months).39, 40, 41, 42, 43, 44, 45, 46, 47 Most trials involved participants with breast cancer (n = 15, 43%),36,38, 39, 40,42,44, 45, 46,48, 49, 50, 51, 52, 53, 54 13 trials (37%) involved patients with mixed cancer diagnoses,35,41,55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65 3 trials (9%) involved prostate cancer patients,37,66,67 3 trials (9%) involved colorectal cancer patients,47,68,69 and 1 trial (3%) involved patients with leukemia.70 One trial (3%) specifically involved patients with metastatic disease (Stage IV).64 Five trials (14%) were conducted during adjuvant chemotherapy and/or radiotherapy,48,52,53,56,60 5 trials (14%) involved mixed groups that were undergoing or had completed adjuvant chemotherapy and/or radiotherapy,46,55,64,65,70 and 25 trials (71%) were conducted post-primary treatment (i.e., following chemotherapy and/or radiotherapy).35, 36, 37, 38, 39, 40, 41, 42, 43,45,47,48,50,51,54,57, 58, 59,61, 62, 63,66,67,69,71
3.3. Intervention characteristics
Intervention durations ranged between 4 weeks41,60 and 1 year53 (median = 12 weeks). Most trials (n = 25, 71%) involved pedometer-based physical activity interventions,35,37, 38, 39, 40, 41, 42, 43, 44, 45,49, 50,52,53,55, 56, 57,60, 61, 62,64,65,67,69,70 7 trials (20%) involved Fitbit-based interventions,46,47,54,58,59,63,66 and 3 trials (9%) involved other wearable physical activity trackers (Polar36,51 and Garmin48). Intervention goals most commonly involved performing 30 min of low-to-moderate-intensity exercise (e.g., walking) at least 5 days/week38–40,42–45,52,62 or 150 min/week of moderate-intensity physical activity.36,41,46,47,49,50,54,63,67 Two trials specifically prescribed vigorous-intensity physical activity,36,66 and 4 trials involved specific resistance training recommendations.56,64,65,70 Approximately one-half of trials (49%) were based on a specific behavior change theory or model,38,40,42, 43, 44, 45, 46, 47,49,51,52,55,57, 58, 59,66,70 and two-thirds (n = 23, 66%) involved a baseline physical activity counselling or instruction session.35,39, 40, 41, 42, 43, 44, 45, 46, 47, 48,51,54,56, 57, 58,60,62, 63, 64, 65,69,72 With respect to physical activity counselling, instruction, support or contact during the intervention, 5 trials (14%) involved some level of in-person support or supervision,39,58,61,69,72 18 (51%) involved only phone or email-based support,35,36,40,42, 43, 44, 45,48,54,57,59,62, 63, 64, 65, 66, 67 and 12 trials (34%) involved no in-person, phone or email-based counselling or support during the intervention (i.e., after baseline).37,38,41,46,49, 50, 51, 52, 53,55,56,60
3.4. Physical activity outcomes
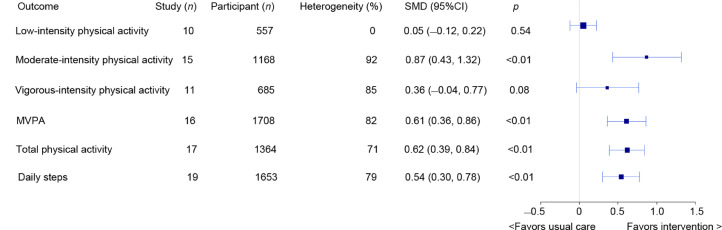
Meta-analysis results of physical activity outcomes are shown in Fig. 2. Compared to usual care, physical activity tracker and pedometer-based interventions had moderate-to-large effects (all p < 0.05) on duration of moderate-intensity physical activity (SMD = 0.87, 95% confidence interval (95%CI): 0.43−1.32), MVPA (SMD = 0.61, 95%CI: 0.36−0.86), total physical activity (SMD = 0.62, 95%CI: 0.39−0.84), and daily steps (SMD = 0.54, 95%CI: 0.30−0.78). No overall effects were observed for low-intensity physical activity (SMD = 0.05, 95%CI: −0.12 to 0.22, p = 0.54) or vigorous intensity physical activity (SMD = 0.36, 95%CI: −0.04 to 0.77, p = 0.08). An overview of all subgroup effects is shown in Supplementary Table 5. Subgroup analyses showed that interventions with baseline counselling had larger effects on moderate-intensity physical activity (yes: SMD = 1.13; no: SMD = 0.26; χ2 = 5.69, p = 0.02), MVPA (yes: SMD = 0.99; no: SMD = 0.26; χ2 = 8.48, p < 0.01), and daily steps (yes: SMD = 0.72; no: SMD = 0.09; χ2 = 11.43, p < 0.01) compared with interventions without baseline counselling. Greater increases in MVPA were observed following interventions ≥12 weeks (SMD = 0.73) in duration compared with interventions <12 weeks (SMD = 0.19; χ2 = 4.78, p = 0.03) in duration. Furthermore, theory-based interventions (SMD = 0.93) had larger effects on total physical activity than non-theory-based interventions (SMD = 0.40; χ2 = 6.05, p = 0.01). With respect to cancer variables, subgroup analyses showed that total physical activity had a large effect size increase in breast cancer trials (SMD = 0.83) compared with a moderate increase in trials involving patients with mixed cancer types (SMD = 0.29; χ2 = 7.48, p < 0.01).
Fig. 2.
Meta-analysis results of all physical activity outcomes. 95%CI = 95% confidence interval; MVPA = moderate-to-vigorous-intensity physical activity; SMD = standardized mean difference.
3.5. Health-related outcomes
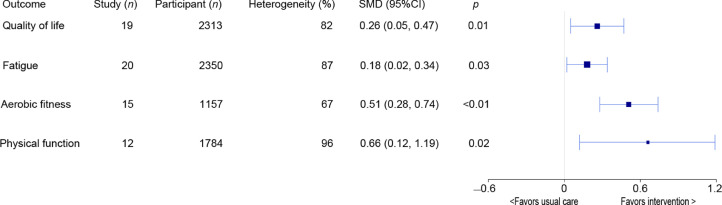
Meta-analysis results of health-related outcomes are shown in Fig. 3. Compared to usual care, wearable physical activity tracker and pedometer-based interventions had significant effects on quality of life, fatigue, aerobic fitness, and physical function (SMD range : 0.18−0.66, all p < 0.05). An overview of subgroup effects for all health-related outcomes is shown in Supplementary Table 6. Interventions with baseline counselling (SMD = 0.64) had larger effects on aerobic fitness compared with interventions without baseline counselling (SMD = 0.19; χ2 = 6.61, p < 0.01). Cancer type, treatment status, intervention length, baseline counselling, theoretical framework, and intervention contact had no other effects on quality of life, fatigue, aerobic fitness, or physical function.
Fig. 3.
Meta-analysis results of all health-related outcomes. 95%CI = 95% confidence interval; SMD = standardized mean difference.
3.6. Safety and acceptability
3.6.1. Safety (adverse events)
Fourteen trials made no comment on adverse events (whether they occurred or not),15,36,38,42,44,51,55,57,59,60,67,69,71,73 while 2 trials explicitly reported that no adverse events had occurred.46,49 Nineteen trials reported the occurrence of adverse events.35,39, 40, 41,43,44,47,48,50,52,53,56,58,61,62,64, 65, 66,70 The adverse events in 16 of these trials were reported as not exercise-related, while 3 trials reported the occurrence of 11 adverse events related to exercise or physical activity.39,53,70 These were low-severity musculoskeletal symptoms (Grade 1: n = 3 events), falls (Grade 1: n = 1 event), unspecified medical events (Grade 2: n = 5 events), and planter fasciitis (Grade 2: n = 2 events). Of these 11 adverse events, 7 events (planter fasciitis (Grade 2: n = 2 events) and unspecified medical events (Grade 2: n = 5 events)) occurred in interventions with in-person contact and 4 events (low-severity musculoskeletal symptoms (Grade 1: n = 3 events) and falls (Grade 1: n = 1 event)) occurred in an intervention with no in-person contact.
3.6.2. Acceptability outcomes related to use of physical activity trackers and pedometers
Acceptability relating to the use of the physical activity trackers was assessed in 6 studies using either a questionnaire (n = 4 Fitbit,46,47,63,66 n = 1 Polar smartwatch51) or qualitative interview (n = 1 Fitbit59). Other trials reported the time and/or frequency of wearing the activity device (n = 447,49,59,73) or the frequency of logging/reporting of activity (n = 538,57,59,67,73). A complete overview of all acceptability results is shown in Supplementary Table 7. The results suggest that participants found the devices easy to use and comfortable to wear,46 and useful for goal setting, 46,51,63 while limitations included technical issues, battery life, and the inability to monitor certain activities.46 Overall satisfaction with the Fitbit was rated at 88%−90%.46,66
4. Discussion
The use of wearable physical activity trackers is expanding rapidly and has been proposed as a tool to help increase physical activity levels in individuals with cancer.74 The key findings of our systematic review and meta-analyses were that physical activity interventions that include physical activity trackers and pedometers in addition to other intervention components (e.g., physical activity counselling) increased the weekly duration of moderate-intensity physical activity, MVPA, total physical activity, and daily steps in individuals with cancer. Improvements were also observed in quality of life, fatigue, aerobic fitness, and physical function. Findings from a small number of studies indicate that the devices are acceptable and that physical activity tracker and pedometer-based interventions are associated with low adverse event rates.
Our findings show that interventions using physical activity trackers and pedometers are effective at improving physical activity levels following a cancer diagnosis. Our findings are consistent with previous reviews, although these reviews have been limited in their ability to evaluate physical activity interventions involving wearable devices in the wider cancer population due to the evaluation of non-RCT study designs,24 a specific focus on childhood cancer75 and systematic reviews of the literature without meta-analyses.24,75,76 A small effect on MVPA (SMD = 0.21) was reported in a 2018 meta-analysis of distance-based physical activity interventions (which included the use of pedometers) for cancer survivors.77 The greater effect size in our findings (SMD = 0.61) could be attributed to greater effectiveness of newer wearable devices (e.g., Fitbits) in more recently published studies. Compared with older devices, more contemporary wearable devices are considered more engaging, provide more individualized feedback and include additional features compared with older devices such as standard pedometers.16 Our review adds to the existing literature because we conducted a comprehensive evaluation of the current literature and meta-analysis of RCTs across various cancer types, including different disease stages (Stages I−IV) and treatment phases (during adjuvant treatment and post-treatment).
While the observed increases in physical activity following the physical activity tracker and pedometer-based interventions are promising, heterogeneity across the studies, including the use of additional intervention components, limits our ability to attribute the causality of the benefits to the devices themselves rather than to the overall intervention. The physical activity tracker and pedometer-based interventions included in our review were often part of multicomponent physical activity interventions, which varied in the number and combination of components and behavior change techniques (Supplementary Table 5). Promotion of physical activity through physical activity trackers and pedometers was often accompanied by other intervention components, such as baseline counselling and in-person, telephone or email-based support during the intervention. Findings from a previous systematic review reported that physical activity tracker-based interventions were effective for increasing physical activity in healthy adults when used alone (i.e., with no additional intervention components (SMD = 0.20, 95%CI: 0.08−0.33, p < 0.05)) and when used as part of multicomponent interventions (SMD = 0.26, 95%CI: 0.12−0.41, p < 0.05).16 Previous findings have also shown that interventions involving extensive implementation of behavior change techniques are more effective at increasing physical activity compared with trials involving minimal and moderate use of behavior change techniques (effect size = 0.76 vs. 0.28 and 0.36, respectively).23 Similarly, supervised exercise interventions have been shown to be particularly effective for increasing physical activity and improving health-outcomes.78,79
We conducted subgroup analyses to explore the effects of additional intervention components. Our subgroup analyses showed that interventions that involved baseline counselling had larger effects on moderate-intensity physical activity, MVPA, and daily steps (SMD range : 0.72−1.13) compared with interventions without baseline counselling (SMD range : 0.09−0.26). Furthermore, interventions that were theory-based (SMD = 0.93) had larger effects on total physical activity than non-theory-based interventions (SMD = 0.40). Therefore, consistent with previous findings in healthy adults,16 our findings suggest that wearable devices can be effective as part of multicomponent interventions involving behavior change theory, baseline physical activity counselling and in-person or telephone support for individuals with cancer. No overall effects were observed for low- or vigorous-intensity physical activity. This was likely because most interventions targeted increases in moderate-intensity physical activity (e.g., brisk walking). Vigorous-intensity physical activity was assessed and reported in 11 trials; however, only 2 trials specifically prescribed vigorous-intensity exercise as part of the intervention. Another potential reason for the non-significant effect on low- and vigorous-intensity physical activity outcomes may include differences in adherence to using and wearing the devices across studies. Furthermore, it remains important to recognize that heterogeneity due to other intervention characteristics (e.g., intervention dose and level of intervention support or supervision) may explain the differences observed when interpreting the findings from specific subgroups.
Physical activity tracker and pedometer-based interventions were associated with significant improvements in quality of life, fatigue, aerobic fitness, and physical function. These findings are consistent with findings from previous systematic reviews and meta-analyses involving exercise interventions that did not involve physical activity trackers or pedometers in cancer populations.26,28 Our subgroup analyses indicated that certain intervention components appear to influence the effectiveness of the physical activity tracker interventions on various outcomes. For example, interventions with a baseline counselling session (SMD = 0.64) had larger effects on aerobic fitness than interventions without baseline counselling (SMD = 0.19). This suggests that certain intervention components may be implemented on an individualized basis for improvements in specific health outcomes, including the implementation of behavior change techniques and counselling and support from a qualified exercise professional. However, our subgroup analyses were exploratory; therefore, a lack of power may have reduced our ability to identity associations that are present but not represented in our findings. Furthermore, most trials that evaluated health outcomes were pedometer-based trials, and future research is required to evaluate the effectiveness of newer devices (e.g., Fitbits) for improving outcomes such as quality of life and fatigue, in addition to physical activity.
Preliminary data on acceptability and safety supports the feasibility of physical activity tracker and pedometer-based interventions as part of a multicomponent intervention to improve physical activity and health-related outcomes. Two trials assessed satisfaction with using the Fitbit, which was rated highly at 88%−90%,46,66 suggesting that individuals with cancer viewed the devices positively. Participants were highly satisfied and engaged with the devices; and themes such as self-monitoring, goal setting, feedback, motivation, and ease of use were identified as positive aspects related to device use. A common feature of the interventions was that the devices were used to self-monitor, and interventions often consisted of goal setting, which is considered an effective behavior change technique to increase physical activity levels.80 Most interventions involved individualized physical activity goals, which have been shown to be more effective in increasing physical activity compared with standard (non-individualized) goals.81 However, again there was variation in how goal setting was implemented across the included trials. Some of the interventions involved the researchers setting the goals during the baseline counselling sessions based on participants’ current physical activity, and regular support was then provided in relation reviewing and setting new goals during the intervention. In other trials, the participants set their own goals using various resources (e.g., intervention handbooks) and received individualized advice during the intervention to achieve these goals (e.g., in-person or telephone-based sessions, individual or group counselling sessions). Barriers included low battery readings or forgetting to charge the devices, inability to monitor certain activities, and technical difficulties with uploading physical activity data. Therefore, it is important for future research to identify the most effective goal-setting approaches and to identify how individuals with cancer engage with the devices, especially during different phases of treatment, to ensure that common barriers do not affect individuals’ ability to self-monitor their activities and achieve their goals. This information will provide insights into how these devices can be integrated in future interventions and how effective strategies can be developed for increasing and maintaining physical activity during and following cancer treatment. Furthermore, it is important to evaluate whether interventions designed to increase physical activity through the use of a wearable are associated with high rates of adverse events (e.g., muscle strains or overuse injuries). Our findings support the safety of these interventions. Only 3 studies (9%) reported the occurrence of adverse events related to exercise- or physical activity, all of which were of low severity (i.e., Grades 1 or 2).
4.1. Strengths and limitations
A limitation of this review was the wide range of interventions that varied in the way the devices were utilized. Interventions consisted of multiple components, and the effect of each individual component cannot be determined based on our findings. Physical activity trackers and accelerometers also often have a limited ability to monitor certain activities (e.g., resistance exercise, cycling, or water-based exercise); therefore, it is likely that participation in these activities was underestimated across the included studies. Furthermore, the bias of all studies was rated as either unclear or high risk in the domains of performance bias, reporting bias, and other bias. As with many physical activity trials, there was a high risk of recruitment and participation bias in the included studies (i.e., participants who enroll in a physical activity intervention trial are more likely to be younger, healthier, and have higher physical activity self-efficacy than those who do not enroll).82 Despite this, our findings provide evidence that physical activity trackers and pedometers can be used in addition to physical activity counselling and in-person, phone, or email-based support to increase physical activity and improve health outcomes in different cancer types (including breast, colorectal, prostate, and leukemia) across different stages of treatment (during adjuvant treatment and post-treatment). An additional limitation was that data extraction was conducted by a single author (BS). However, data extraction accuracy of 15% of the data was checked by a second author (EMZ) and no disagreements were identified. Furthermore, most trials were ≤12 weeks in duration, and only 1 trial had a 1-year duration.53 Therefore, there remains a lack of evidence for longer-term feasibility and effectiveness. Nonetheless, this systematic review and meta-analysis reflects the most comprehensive assessment of physical activity tracker and pedometer-based interventions for individuals with cancer available. Unlike previous reviews,24,75,76 our review comprehensively evaluated various cancers during different phases of treatment (during adjuvant treatment and post-treatment). Other strengths of our work include the evaluation of RCTs exclusively and the reporting of subgroup analyses that identified potential associations between treatment and intervention characteristics on the effect on health outcomes.
4.2. Future research
The findings in our analysis showed no statistically significant effects on low- or vigorous-intensity physical activity; therefore, future research should focus on investigating how interventions involving physical activity trackers can be used to improve these outcomes. In the current study, we were unable to evaluate the effectiveness of physical activity tracker and pedometer-based interventions over the long term (i.e., >1 year); thus, studies with long durations remain a priority for future research. Furthermore, most of the trials included in this review involved participants with breast cancer. Therefore, future research that evaluates less common cancer types, including during different phases of cancer treatment, is required to help improve physical inactivity rates in the wider cancer population. Future research is also required to provide further evidence on how individuals with cancer engage with physical activity trackers and pedometers over the longer term and whether there are differences in effects among age groups, genders, and/or cancer types. Such research is important for identifying how to optimally incorporate these devices into interventions and for informing best practices for increasing physical activity in cancer care.
5. Conclusion
Physical activity tracker and pedometer-based interventions offer significant promise for increasing physical activity and improving various health-related outcomes among individuals with cancer. The findings from our review suggest that these devices should be implemented as part of interventions designed to increase physical activity for individuals with cancer. The devices may also have the potential to be included as an effective tool to assist health professionals in providing ongoing monitoring and support to patients. In order to maximize the effectiveness of these devices (including newer devices) in increasing physical activity and improving health-related outcomes post-cancer diagnosis, future research should focus on identifying how they can be implemented for longer-term use in conjunction with other intervention components (e.g., in-person counselling or supervised exercise sessions).
Authors’ contributions
BS was involved in the planning and development of the study, the literature search and screening, data extraction, data analysis, and manuscript write-up; EMZ contributed to data entry, data analysis, and manuscript write-up; EJH was involved in the planning and development of the study and in manuscript write-up. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jshs.2021.07.008.
Supplementary materials
References
- 1.Segal R, Zwaal C, Green E, et al. Exercise for people with cancer: A systematic review. Curr Oncol. 2017;24:e290–e315. doi: 10.3747/co.24.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh B, Spence RR, Steele ML, Sandler CX, Peake JM, Hayes SC. A systematic review and meta-analysis of the safety, feasibility, and effect of exercise in women with Stage II+ breast cancer. Arch Phys Med Rehabil. 2018;99:2621–2636. doi: 10.1016/j.apmr.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 3.Speck RM, Courneya KS, Mâsse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: A systematic review and meta-analysis. J Cancer Surviv. 2010;4:87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 4.Cormie P, Zopf EM, Zhang X, Schmitz KH. The impact of exercise on cancer mortality, recurrence, and treatment-related adverse effects. Epidemiol Rev. 2017;39:71–92. doi: 10.1093/epirev/mxx007. [DOI] [PubMed] [Google Scholar]
- 5.Courneya KS, Segal RJ, McKenzie DC, et al. Effects of exercise during adjuvant chemotherapy on breast cancer outcomes. Med Sci Sport Exerc. 2014;46:1744–1751. doi: 10.1249/MSS.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 6.Hayes SC, Steele ML, Spence RR, et al. Exercise following breast cancer: Exploratory survival analyses of two randomised, controlled trials. Breast Cancer Res Treat. 2018;167:505–514. doi: 10.1007/s10549-017-4541-9. [DOI] [PubMed] [Google Scholar]
- 7.American Cancer Society. Physical activity and the person with cancer. Available at: https://www.cancer.org/treatment/survivorship-during-and-after-treatment/staying-active/physical-activity-and-the-cancer-patient.html. [accessed 01.04.2021].
- 8.Hayes SC, Newton RU, Spence RR, Galvão DA. The Exercise and Sports Science Australia position statement: Exercise medicine in cancer management. J Sci Med Sport. 2019;22:1175–1199. doi: 10.1016/j.jsams.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Campbell K, Winters-Stone K, Wiskemann J, et al. Exercise guidelines for cancer survivors: Consensus Statement from International Multidisciplinary Roundtable. Med Sci Sport Exerc. 2019;51:2375–2390. doi: 10.1249/MSS.0000000000002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hair BY, Hayes S, Tse CK, Bell MB, Olshan AF. Racial differences in physical activity among breast cancer survivors: Implications for breast cancer care. Cancer. 2014;120:2174–2182. doi: 10.1002/cncr.28630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung JY, Lee DH, Park JH, et al. Patterns of physical activity participation across the cancer trajectory in colorectal cancer survivors. Support Care Cancer. 2013;21:1605–1612. doi: 10.1007/s00520-012-1703-5. [DOI] [PubMed] [Google Scholar]
- 12.Lewis ZH, Lyons EJ, Jarvis JM, Baillargeon J. Using an electronic activity monitor system as an intervention modality: A systematic review. BMC Public Health. 2015;15:585. doi: 10.1186/s12889-015-1947-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson WG, Kuhle CL, Koepp GA, McCrady-Spitzer SK, Levine JA. “Go4Life” exercise counseling, accelerometer feedback, and activity levels in older people. Arch Gerontol Geriatr. 2014;58:314–319. doi: 10.1016/j.archger.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Wang JB, Cadmus-Bertram LA, Natarajan L, et al. Wearable sensor/device (Fitbit One) and SMS text-messaging prompts to increase physical activity in overweight and obese adults: A randomized controlled trial. Telemed J E Health. 2015;21:782–792. doi: 10.1089/tmj.2014.0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cadmus-Bertram LA, Marcus BH, Patterson RE, Parker BA, Morey BL. Randomized trial of a Fitbit-based physical activity intervention for women. Am J Prev Med. 2015;49:414–418. doi: 10.1016/j.amepre.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brickwood KJ, Watson G, O'Brien J, Williams AD. Consumer-based wearable activity trackers increase physical activity participation: Systematic review and meta-analysis. JMIR Mhealth Uhealth. 2019;7:e11819. doi: 10.2196/11819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.S Oliveira J, Sherrington C, RY Zheng E, Franco MR, Tiedemann A. Effect of interventions using physical activity trackers on physical activity in people aged 60 years and over: A systematic review and meta-analysis. Br J Sports Med. 2020;54:1188–1194. doi: 10.1136/bjsports-2018-100324. [DOI] [PubMed] [Google Scholar]
- 18.Mansi S, Milosavljevic S, Baxter GD, Tumilty S, Hendrick P. A systematic review of studies using pedometers as an intervention for musculoskeletal diseases. BMC Musculoskelet Disord. 2014;15:231. doi: 10.1186/1471-2474-15-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baskerville R, Ricci-Cabello I, Roberts N, Farmer A. Impact of accelerometer and pedometer use on physical activity and glycaemic control in people with type 2 diabetes: A systematic review and meta-analysis. Diabet Med. 2017;34:612–620. doi: 10.1111/dme.13331. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong M, Winnard A, Chynkiamis N, Boyle S, Burtin C, Vogiatzis I. Use of pedometers as a tool to promote daily physical activity levels in patients with COPD: A systematic review and meta-analysis. Eur Respir Rev. 2019;28 doi: 10.1183/16000617.0039-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirk MA, Amiri M, Pirbaglou M, Ritvo P. Wearable technology and physical activity behavior change in adults with chronic cardiometabolic disease: A systematic review and meta-analysis. Am J Health Promot. 2019;33:778–791. doi: 10.1177/0890117118816278. [DOI] [PubMed] [Google Scholar]
- 22.Bluethmann SM, Vernon SW, Gabriel KP, Murphy CC, Bartholomew LK. Taking the next step: A systematic review and meta-analysis of physical activity and behavior change interventions in recent post-treatment breast cancer survivors. Breast Cancer Res Treat. 2015;149:331–342. doi: 10.1007/s10549-014-3255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bluethmann SM, Bartholomew LK, Murphy CC, Vernon SW. Use of theory in behavior change interventions. Heal Educ Behav. 2017;44:245–253. doi: 10.1177/1090198116647712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coughlin SS, Caplan LS, Stone R. Use of consumer wearable devices to promote physical activity among breast, prostate, and colorectal cancer survivors: A review of health intervention studies. J Cancer Surviv. 2020;14:386–392. doi: 10.1007/s11764-020-00855-1. [DOI] [PubMed] [Google Scholar]
- 25.Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. 2007;7:16. doi: 10.1186/1472-6947-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh B, Spence R, Steele ML, Hayes S, Toohey K. Exercise for individuals with lung cancer: A systematic review and meta-analysis of adverse events, feasibility, and effectiveness. Semin Oncol Nurs. 2020;36 doi: 10.1016/j.soncn.2020.151076. [DOI] [PubMed] [Google Scholar]
- 27.National Institutes of Health; National Cancer Institute . U.S. Department of Health and Human Services; Washington, DC: 2009. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. [Google Scholar]
- 28.Singh B, Hayes SC, Spence RR, Steele ML, Millet GY, Gergele L. Exercise and colorectal cancer: A systematic review and meta-analysis of exercise safety, feasibility and effectiveness. Int J Behav Nutr Phys Act. 2020;17:122. doi: 10.1186/s12966-020-01021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higgins JPT, Deeks JJ, Altman DG. In: Cochrane handbook for systematic reviews of interventions. Higgins JPT, Green S, editors. The Nordic Cochrane Centre, The Cochrane Collaboration; Copenhagen: 2008. Special topics in statistics; pp. 32–35. [Google Scholar]
- 31.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson SG, Higgins JPT. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21:1559–1573. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- 33.Higgins JPT, Deeks JJ, Altman DG. In: Cochrane handbook for systematic reviews of interventions. Copenhagen: The Nordic Cochrane Centre, Higgins JPT, Green S, editors. The Cochrane Collaboration; 2008. Special topics in statistics; pp. 7–15. [Google Scholar]
- 34.Cohen J. 2nd ed. Routledge; New York, NY: 1988. Statistical Power analysis for the behavioral sciences. [Google Scholar]
- 35.Mayo NE, Moriello C, Scott SC, Dawes D, Auais M, Chasen M. Pedometer-facilitated walking intervention shows promising effectiveness for reducing cancer fatigue: A pilot randomized trial. Clin Rehabil. 2014;28:1198–1209. doi: 10.1177/0269215514536209. [DOI] [PubMed] [Google Scholar]
- 36.McNeil J, Brenner DR, Stone CR, et al. Activity tracker to prescribe various exercise intensities in breast cancer survivors. Med Sci Sports Exerc. 2019;51:930–940. doi: 10.1249/MSS.0000000000001890. [DOI] [PubMed] [Google Scholar]
- 37.Sajid S, Dale W, Mustian K, et al. Novel physical activity interventions for older patients with prostate cancer on hormone therapy: A pilot randomized study. J Geriatr Oncol. 2016;7:71–80. doi: 10.1016/j.jgo.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vallance JKH, Courneya KS, Plotnikoff RC, Yasui Y, Mackey JR. Randomized controlled trial of the effects of print materials and step pedometers on physical activity and quality of life in breast cancer survivors. J Clin Oncol. 2007;25:2352–2359. doi: 10.1200/JCO.2006.07.9988. [DOI] [PubMed] [Google Scholar]
- 39.Irwin ML, Varma K, Alvarez-Reeves M, et al. Randomized controlled trial of aerobic exercise on insulin and insulin-like growth factors in breast cancer survivors: The Yale Exercise and Survivorship study. Cancer Epidemiol Biomarkers Prev. 2009;18:306–313. doi: 10.1158/1055-9965.EPI-08-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matthews CE, Wilcox S, Hanby CL, et al. Evaluation of a 12-week home-based walking intervention for breast cancer survivors. Support Care Cancer. 2007;15:203–211. doi: 10.1007/s00520-006-0122-x. [DOI] [PubMed] [Google Scholar]
- 41.Park JH, Lee J, Oh M, et al. The effect of oncologists’ exercise recommendations on the level of exercise and quality of life in survivors of breast and colorectal cancer: A randomized controlled trial. Cancer. 2015;121:2740–2748. doi: 10.1002/cncr.29400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pinto BM, Frierson GM, Rabin C, Trunzo JJ, Marcus BH. Home-based physical activity intervention for breast cancer patients. J Clin Oncol. 2005;23:3577–3587. doi: 10.1200/JCO.2005.03.080. [DOI] [PubMed] [Google Scholar]
- 43.Pinto BM, Papandonatos GD, Goldstein MG, Marcus BH, Farrell N. Home-based physical activity intervention for colorectal cancer survivors. Psychooncology. 2013;22:54–64. doi: 10.1002/pon.2047. [DOI] [PubMed] [Google Scholar]
- 44.Pinto BM, Papandonatos GD, Goldstein MG. A randomized trial to promote physical activity among breast cancer patients. Heath Psychol. 2013;32:616–626. doi: 10.1037/a0029886. [DOI] [PubMed] [Google Scholar]
- 45.Pinto BM, Stein K, Dunsiger S. Peers promoting physical activity among breast cancer survivors: A randomized controlled trial. Health Psychol. 2015;34:463–472. doi: 10.1037/hea0000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh B, Spence RR, Sandler CX, Tanner J, Hayes SC. Feasibility and effect of a physical activity counselling session with or without provision of an activity tracker on maintenance of physical activity in women with breast cancer—A randomised controlled trial. J Sci Med Sport. 2020;23:283–290. doi: 10.1016/j.jsams.2019.09.019. [DOI] [PubMed] [Google Scholar]
- 47.Van Blarigan EL, Chan H, Van Loon K, et al. Self-monitoring and reminder text messages to increase physical activity in colorectal cancer survivors (Smart Pace): A pilot randomized controlled trial. BMC Cancer. 2019;19:218. doi: 10.1186/s12885-019-5427-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lynch BM, Nguyen NH, Moore MM, et al. A randomized controlled trial of a wearable technology-based intervention for increasing moderate to vigorous physical activity and reducing sedentary behavior in breast cancer survivors: The ACTIVATE Trial. Cancer. 2019;125:2846–2855. doi: 10.1002/cncr.32143. [DOI] [PubMed] [Google Scholar]
- 49.Vallance JK, Friedenreich CM, Lavallee CM, et al. Exploring the feasibility of a broad-reach physical activity behavior change intervention for women receiving chemotherapy for breast cancer: A randomized trial. Cancer Epidemiol Biomarkers Prev. 2016;25:391–398. doi: 10.1158/1055-9965.EPI-15-0812. [DOI] [PubMed] [Google Scholar]
- 50.Uhm KE, Yoo JS, Chung SH, et al. Effects of exercise intervention in breast cancer patients: Is mobile health (mHealth) with pedometer more effective than conventional program using brochure? Breast Cancer Res Treat. 2017;161:443–452. doi: 10.1007/s10549-016-4065-8. [DOI] [PubMed] [Google Scholar]
- 51.Pope ZC, Zeng N, Zhang R, Lee HY, Gao Z. Effectiveness of combined smartwatch and social media intervention on breast cancer survivor health outcomes: A 10-week pilot randomized trial. J Clin Med. 2018;7:140. doi: 10.3390/jcm7060140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gokal K, Wallis D, Ahmed S, Boiangiu I, Kancherla K, Munir F. Effects of a self-managed home-based walking intervention on psychosocial health outcomes for breast cancer patients receiving chemotherapy: A randomised controlled trial. Support Care Cancer. 2016;24:1139–1166. doi: 10.1007/s00520-015-2884-5. [DOI] [PubMed] [Google Scholar]
- 53.Haines TP, Sinnamon P, Wetzig NG, et al. Multimodal exercise improves quality of life of women being treated for breast cancer, but at what cost? Randomized trial with economic evaluation. Breast Cancer Res Treat. 2010;124:163–175. doi: 10.1007/s10549-010-1126-2. [DOI] [PubMed] [Google Scholar]
- 54.Hartman SJ, Nelson SH, Myers E, et al. Randomized controlled trial of increasing physical activity on objectively measured and self-reported cognitive functioning among breast cancer survivors: The memory & motion study. Cancer. 2018;124:192–202. doi: 10.1002/cncr.30987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Golsteijn RHJ, Bolman C, Volders E, Peels DA, de Vries H, Lechner L. Short-term efficacy of a computer-tailored physical activity intervention for prostate and colorectal cancer patients and survivors: A randomized controlled trial. Int J Behav Nutr Phys Act. 2018;15:106. doi: 10.1186/s12966-018-0734-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kleckner IR, Kamen C, Gewandter JS, et al. Effects of exercise duringchemotherapy on chemotherapy-induced peripheral neuropathy: A multicenter, randomized controlled trial. Support Care Cancer. 2018;26:1019–1028. doi: 10.1007/s00520-017-4013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ligibel JA, Meyerhardt J, Pierce JP, et al. Impact of a telephone-based physical activity intervention upon exercise behaviors and fitness in cancer survivors enrolled in a cooperative group setting. Breast Cancer Res Treat. 2012;132:205–213. doi: 10.1007/s10549-011-1882-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maxwell-Smith C, Hince D, Cohen PA, et al. A randomized controlled trial of WATAAP to promote physical activity in colorectal and endometrial cancer survivors. Psychooncology. 2019;28:1420–1429. doi: 10.1002/pon.5090. [DOI] [PubMed] [Google Scholar]
- 59.Mendoza JA, Baker KS, Moreno MA, et al. A Fitbit and Facebook mHealth intervention for promoting physical activity among adolescent and young adult childhood cancer survivors: A pilot study. Pediatr Blood Cancer. 2017;64:e26660. doi: 10.1002/pbc.26660. [DOI] [PubMed] [Google Scholar]
- 60.Mustian KM, Peppone L, Darling TV, Palesh O, Heckler CE, Morrow GR. A 4-week home-based aerobic and resistance exercise program during radiation therapy: A pilot randomized clinical trial. J Support Oncol. 2009;7:158–167. [PMC free article] [PubMed] [Google Scholar]
- 61.Backman M, Wengström Y, Johansson B, et al. A randomized pilot study with daily walking during adjuvant chemotherapy for patients with breast and colorectal cancer. Acta Oncol. 2014;53:510–520. doi: 10.3109/0284186X.2013.873820. [DOI] [PubMed] [Google Scholar]
- 62.Bennett JA, Lyons KS, Winters-Stone K, Nail LM, Scherer J. Motivational interviewing to increase physical activity in long-term cancer survivors: A randomized controlled trial. Nurs Res. 2007;56:18–27. doi: 10.1097/00006199-200701000-00003. [DOI] [PubMed] [Google Scholar]
- 63.Cadmus-Bertram L, Tevaarwerk AJ, Sesto ME, Gangnon R, Van Remortel B, Date P. Building a physical activity intervention into clinical care for breast and colorectal cancer survivors in Wisconsin: A randomized controlled pilot trial. J Cancer Surviv. 2019;13:593–602. doi: 10.1007/s11764-019-00778-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheville AL, Kollasch J, Vandenberg J, et al. A home-based exercise program to improve function, fatigue, and sleep quality in patients with Stage IV lung and colorectal cancer: A randomized controlled trial. J Pain Symptom Manage. 2013;45:811–821. doi: 10.1016/j.jpainsymman.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheville AL, Moynihan T, Herrin J, Loprinzi C, Kroenke K. Effect of collaborative telerehabilitation on functional impairment and pain among patients with advanced-stage cancer: A randomized clinical trial. JAMA Oncol. 2019;5:644–652. doi: 10.1001/jamaoncol.2019.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kenfield SA, Van Blarigan EL, Ameli N, et al. Feasibility, acceptability, and behavioral outcomes from a technology-enhanced behavioral change intervention (Prostate 8): A pilot randomized controlled trial in men with prostate cancer. Eur Urol. 2019;75:950–958. doi: 10.1016/j.eururo.2018.12.040. [DOI] [PubMed] [Google Scholar]
- 67.Lee BJ, Park YH, Lee JY, Kim SJ, Jang Y, Lee JI. Smartphone application vs. pedometer to promote physical activity in prostate cancer patients. Telemed J E Health. 2019;25:1231–1236. doi: 10.1089/tmj.2018.0233. [DOI] [PubMed] [Google Scholar]
- 68.Pinto BM, Papandonatos GD, Goldstein MG, Marcus BH, Farrell N. Home-based physical activity intervention for colorectal cancer survivors. Psychooncology. 2013;22:54–64. doi: 10.1002/pon.2047. [DOI] [PubMed] [Google Scholar]
- 69.Kim JY, Lee MK, Lee DH, et al. Effects of a 12-week home-based exercise program on quality of life, psychological health, and the level of physical activity in colorectal cancer survivors: A randomized controlled trial. Support Care Cancer. 2019;27:2933–2940. doi: 10.1007/s00520-018-4588-0. [DOI] [PubMed] [Google Scholar]
- 70.Jarden M, Møller T, Christensen KB, Kjeldsen L, Birgens HS, Adamsen L. Multimodal intervention integrated into the clinical management of acute leukemia improves physical function and quality of life during consolidation chemotherapy: A randomized trial “PACE-AL”. Haematologica. 2016;101:e316–e319. doi: 10.3324/haematol.2015.140152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee MK, Kim NK, Jeon JY. Effect of the 6-week home-based exercise program on physical activity level and physical fitness in colorectal cancer survivors: A randomized controlled pilot study. PLoS One. 2018;13 doi: 10.1371/journal.pone.0196220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jarden M, Baadsgaard MT, Hovgaard DJ, Boesen E, Adamsen L. A randomized trial on the effect of a multimodal intervention on physical capacity, functional performance and quality of life in adult patients undergoing allogeneic SCT. Bone Marrow Transplant. 2009;43:725–737. doi: 10.1038/bmt.2009.27. [DOI] [PubMed] [Google Scholar]
- 73.Hartman SJ, Nelson SH, Myers E, et al. Randomized controlled trial of increasing physical activity on objectively measured and self-reported cognitive functioning among breast cancer survivors: The memory & motion study. Cancer. 2018;124:192–202. doi: 10.1002/cncr.30987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blount DS, McDonough DJ, Gao Z. Effect of wearable technology-based physical activity interventions on breast cancer survivors’ physiological, cognitive, and emotional outcomes: A systematic review. J Clin Med. 2021;10:2015. doi: 10.3390/jcm10092015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schaffer K, Panneerselvam N, Loh KP, et al. Systematic review of randomized controlled trials of exercise interventions using digital activity trackers in patients with cancer. J Natl Compr Canc Netw. 2019;17:57–63. doi: 10.6004/jnccn.2018.7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ha L, Mizrahi D, Wakefield CE, Cohn RJ, Simar D, Signorelli C. The use of activity trackers in interventions for childhood cancer patients and survivors: A systematic review. J Adolesc Young Adult Oncol. 2021;10:1–14. doi: 10.1089/jayao.2020.0099. [DOI] [PubMed] [Google Scholar]
- 77.Groen WG, van Harten WH, Vallance JK. Systematic review and meta-analysis of distance-based physical activity interventions for cancer survivors (2013−2018): We still haven't found what we're looking for. Cancer Treat Rev. 2018;69:188–203. doi: 10.1016/j.ctrv.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 78.Hayes SC, Rye S, Disipio T, et al. Exercise for health: A randomized, controlled trial evaluating the impact of a pragmatic, translational exercise intervention on the quality of life, function and treatment-related side effects following breast cancer. Breast Cancer Res Treat. 2013;137:175–186. doi: 10.1007/s10549-012-2331-y. [DOI] [PubMed] [Google Scholar]
- 79.Cormie P, Galvão D, Spry N, et al. Can supervised exercise prevent treatment toxicity in prostate cancer patients initiating androgen deprivation therapy: A randomised controlled trial. BJU Int. 2014;115:256–266. doi: 10.1111/bju.12646. [DOI] [PubMed] [Google Scholar]
- 80.Samdal GB, Eide GE, Barth T, Williams G, Meland E. Effective behaviour change techniques for physical activity and healthy eating in overweight and obese adults: Systematic review and meta-regression analyses. Int J Behav Nutr Phys Act. 2017;14:42. doi: 10.1186/s12966-017-0494-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Conn VS, Hafdahl AR, Mehr DR. Interventions to increase physical activity among healthy adults: Meta-analysis of outcomes. Am J Public Health. 2011;101:751–758. doi: 10.2105/AJPH.2010.194381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Spence R, DiSipio T, Schmitz K, Hayes S. Is unsupervised exercise following breast cancer safe for all women? Int J Phys Med Rehabil. 2014;2:197. doi: 10.4172/2329-9096.1000197. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.