Highlights
-
•
Our study shows that exercize cardiac power (ECP) may provide prognostic information concerning heart failure risk prediction despite taking into account established risk factors, such as smoking, lipids, hypertension, left ventricular hypertrophy, chronic obstructive pulmonary disease, and diabetes.
-
•
This study shows that significant risk of heart failure was observed in men with the lowest level of ECP. A continuous increase in ECP (3.16 mL/mmHg) corresponds to 28% decrease in the risk for heart failure in these men.
-
•
Our study indicates that ECP may provide a valuable tool for the risk prediction of heart failure in the general population, although further studies are needed.
Keywords: Exercise cardiac power, Heart failure, Men
Abstract
Background
Little is known about exercise cardiac power (ECP), defined as the ratio of directly measured maximal oxygen uptake with peak systolic blood pressure during exercise, on heart failure (HF) risk. We examined the association of ECP and the risk of HF.
Methods
This was a population-based cohort study of 2351 men from eastern Finland. The average time to follow-up was 25 years. Participants participated at baseline in an exercise stress test. A total of 313 cases of HF occurred.
Results
Men with low ECP (<9.84 mL/mmHg, the lowest quartile) had a 2.37-fold (95% confidence interval (95%CI): 1.68−3.35, p < 0.0001) hazards ratio of HF as compared with men with high ECP (>13.92 mL/mmHg, the highest quartile), after adjusting for age. Low ECP was associated with a 1.96-fold risk (95%CI: 1.38−2.78, p < 0.001) of HF after additional adjustment for conventional risk factors. After further adjustment for left ventricular hypertrophy, the results hardly changed (hazards ratio = 1.87, 95%CI: 1.31−2.66, p < 0.001). One SD increase in ECP (3.16 mL/mmHg) was associated with a decreased risk of HF by 28% (95%CI: 17%−37%).
Conclusion
ECP provides a noninvasive and easily available measure from cardiopulmonary exercise tests in predicting HF. However, ECP did not provide additional value over maximal oxygen uptake.
Graphical abstract
1. Introduction
The aging of the population and decreased cardiac care are associated with an increasing incidence and prevalence of heart failure (HF). HF is associated with high morbidity and mortality and imposes a significant economic burden on health systems. It is, therefore, necessary to evaluate the putative risk factors that may have predictive or causal relevance to the risk for HF and, by evaluating them, help tailor preventive and therapeutic interventions. Cardiorespiratory fitness (CRF) is a relatively new measure of assessing cardiac and respiratory functioning. During recent years, the assessment of CRF has achieved significant clinical merit and is considered to be a vital part of patient risk assessment. Directly measured maximal oxygen uptake (VO2max), an objective and quantitative measure of CRF, is the gold standard for assessing the amount of oxygen consumption during exercise testing.1,2 In addition to systolic blood pressure (SBP) at rest, exercise-induced elevation of SBP has been found to be an independent predictor of hypertension,3,4 coronary heart disease,5, 6, 7 cardiovascular disease,8,9 and sudden cardiac death.10 Previous studies have shown that exercise cardiac power (ECP) predicts the risk of stroke, sudden cardiac death, and cardiovascular deaths.11, 12, 13
Although, CRF is a key marker of cardiovascular capacity, it does not take into account the detailed differences in cardiovascular resistance and cardiac afterload among the subjects, whereas ECP does take these factors into consideration.
Therefore, we hypothesized that an index measure of ECP, defined as the ratio of directly measured VO2max with peak SBP during exercise, could give prognostic information on HF risk stratification. We investigated the association of ECP during exercise with the risk of HF in a population-based sample of men from eastern Finland.
2. Methods
2.1. Subjects
Subjects were participants in the Kuopio Ischaemic Heart Disease Risk Factor Study, which was designed to investigate risk factors for cardiovascular diseases (CVD), carotid atherosclerosis, and related outcomes in a population-based, randomly selected sample of men from eastern Finland.12 Participants included 2682 men, aged 42 years, 48 years, 54 years, or 60 years, who resided in the city of Kuopio or its surrounding rural communities. Participants were examined at baseline between March 1984 and December 1989. A total of 198 participants were excluded because of death, serious disease, or migration away from the area. The Kuopio Ischaemic Heart Disease Risk Factor Study was approved by the Research Ethics Committee of the University of Kuopio, and each participant gave written informed consent. Complete data on ECP was available for 2351 subjects with no history of HF at baseline.
2.2. Assessment of ECP
A maximal symptom-limited exercise-tolerance test was performed between 8:00 a.m. and 10:00 a.m. by using an electrically braked cycle ergometer.6 The standardized testing protocol involved an increase in workload of 20 W/min, with direct analyses of respiratory gases (Medical Graphics, St. Paul, MN, USA). VO2max was defined as the highest value for, or the plateau of, oxygen uptake. Maximal exercise workload was defined as the highest workload achieved during the exercise test. Exercise workload was divided by body weight in kilograms. For safety reasons, all tests were supervised by an experienced physician with the assistance of an experienced nurse. An electrocardiogram, blood pressure, and heart rate were recorded during the exercise test.10,11 A week before the exercise test, between 8:00 a.m. and 10:00 a.m., the resting blood pressure of participants was measured after 5 min and 10 min of rest by using a Hawksley random 0 muddler sphygmomanometer (Hawksley & Sons Ltd., Lancing, UK). An experienced nurse measured the blood pressure while participants were in a seated position in a quiet room. The mean value of the 2 blood pressure readings (after 5 min and 10 min of rest) was used as resting blood pressure. Resting hypertension was defined as hypertension confirmed by current use of antihypertensive medication and/or an SBP greater than 140 mmHg and/or diastolic blood pressure greater than 90 mmHg. Immediately before the exercise phase (start), pre-exercise blood pressure was measured manually while the participant was sitting on the cycle ergometer (Tunturi EL 400; Tunturi New Fitness, Turku, Finland). Blood pressure was then measured every 2 min during and at 2 min after the exercise test. The maximal SBP was the highest value achieved during the test. ECP was defined as the ratio of directly measured VO2max with peak SBP during exercise. The most common reasons for stopping the exercise test were leg fatigue (574), exhaustion (117), breathlessness (64), and pain in the leg muscles, joints, or back (56). The test was discontinued in 86 men because of cardiorespiratory symptoms or abnormalities, such as dyspnea (48), chest pain (26), ischemic electrocardiographic change (4), arrhythmia (3), a marked change in SBP or diastolic blood pressure (2), or dizziness (3).
2.3. Assessment of other covariates
Body mass index (BMI) was computed as the ratio of weight in kilograms to the square of height in meters. Information on use of medications and diagnosis of diseases was collected at the baseline examination by an internist.11 Alcohol consumption was assessed by the use of the Nordic Alcohol Consumption Inventory. Left ventricular hypertrophy (Sokolow-Lyon index) was recorded at rest. The collection of blood specimens and the measurement of serum lipids, serum lipoproteins, and insulin, as well as the definition of type 2 diabetes, have been described elsewhere.10,11 Serum C-reactive protein was measured by an immunometric assay (Immulite High Sensitivity C-reactive protein Assay; Diagnostic Products Corp., Los Angeles, CA, USA).
2.4. Ascertainment of incident HF events
All incident HF cases that occurred from the time of study enrolment (from March 1984 to December 1989) through 2014 were included. There were no losses to follow-up.14,15 All study participants were under continuous surveillance for the development of new CVD events, including new incidences of HF cases. The sources of information on HF were based on hospital records and medicolegal reports. The diagnostic classification of HF cases was coded according to the International Classification of Diseases, 10th revision, codes I00–I99, and I50.0–I50.9, I11.0, I42.0–I42.9. The diagnosis of HF was based on the diagnostic guidelines of the European Society of Cardiology16 and included such criteria as symptoms, signs, and laboratory investigations, including the determination of N-terminal pro-brain natriuretic peptide, chest radiography results, electrocardiographic findings, and echocardiography findings. The death certificates were assessed and classified by a team of independent experts.
2.5. Statistical analysis
Descriptive data are presented as means and percentages. Risk factors for main outcomes were analyzed by a multivariate Cox model. ECP was entered into Cox proportional hazards models. Cox models were adjusted for age and other demographic and clinical factors previously reported to be predictive of HF by considering their clinical relevance. Hazards risks (HRs) with 95% confidence intervals (95%CIs), adjusted for clinical risk factors, were estimated as antilogarithms of the coefficients from multivariable models. The fit of the proportional hazards models was examined by plotting the hazard functions in different categories of risk factors over time. The proportional hazards assumption was verified for all variables by inspection of the plots of Schoenfeld residuals for covariates. The linearity assumption was satisfied for all continuous variables, and it was assessed by Martingale residuals for each continuous variable against survival time. p < 0.05 was considered statistically significant. These statistical analyses were performed with the use of SPSS (Version 21.0; IBM Corp., Armonk, NY, USA) for Windows. Different sets of covariates were used: Model 1 included age; Model 2 included Model 1 and smoking, alcohol consumption, BMI, type 2 diabetes, history of coronary heart disease, and serum high-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol. Further adjustment was made for left ventricular hypertrophy, chronic obstructive pulmonary disease (COPD), and myocardial infarction that occurred during the follow-up period. Furthermore, we adjusted for antihypertensive medication and maximal blood pressure during exercise. The cumulative survival from HF according to the presence of ECP was calculated using the Kaplan-Meier method (Fig. 1). The C-statistics index was calculated to assess the model discrimination, or the ability of the model to identify correctly subjects with respect to sudden cardiac death.17 We also computed the net reclassification index (NRI),18 which compares the shifts in reclassified categories by observed outcome.
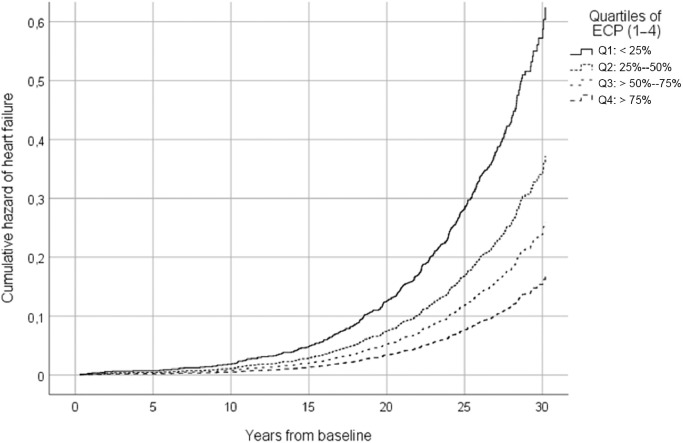
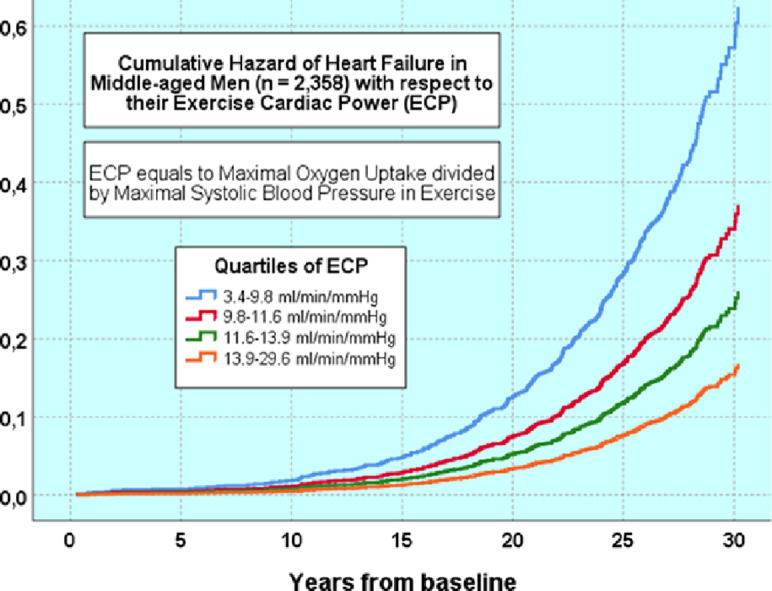
Fig. 1.
The age- and examination-adjusted survival curves of heart failure in men according to quarters of ECP. ECP = exercise cardiac power; Q = quantile.
3. Results
3.1. Baseline characteristics
At the beginning of the follow-up, the mean age of the subjects was 52.2 years (range: 42.0–61.2 years). The mean ECP was 11.9 mL/mmHg (SD = 3.16 mL/mmHg, range: 3.39–29.57 mL/mmHg). At baseline examination, men with low ECP were older; smoked more; had higher serum LDL cholesterol, SBP, and prevalence of diabetes; were less physically active; and had low serum HDL compared to those who had higher ECP (Table 1). Medications and some of the exercise parameters are shown in Table 2. Subjects were divided into quartiles on the basis of ECP.
Table 1.
Characteristics of men at baseline in the quarters of ECP.
| Overall | Q1 | Q2 | Q3 | Q4 | p | |
|---|---|---|---|---|---|---|
| Age (year) | 52.2 ± 5.2 | 55.2 ± 3.9 | 53.6 ± 4.6 | 52.5 ± 4.8 | 50.2 ± 5.3 | <0.001 |
| Cigarette smoking (pack-years)a | 7.6 ± 15.4 | 11.9 ± 19.7 | 10.4 ± 17.4 | 5.5 ± 13.1 | 5.8 ± 13.0 | <0.001 |
| Serum total cholesterol (mmol/L) | 5.90 ± 1.07 | 6.05 ± 1.12 | 6.00 ± 1.06 | 5.86 ± 1.04 | 5.70 ± 0.99 | <0.001 |
| Serum LDL cholesterol (mmol/L) | 4.04 ± 0.99 | 4.14 ± 1.00 | 4.15 ± 0.98 | 4.00 ± 0.98 | 3.84 ± 0.92 | <0.001 |
| Serum triglycerides (mmol/L) | 1.28 ± 0.81 | 1.42 ± 0.86 | 1.30 ± 0.86 | 1.22 ± 0.80 | 1.21 ± 0.72 | 0.011 |
| Systolic blood pressure (mmHg) | 134.3 ± 16.8 | 141.1 ± 19.6 | 135.2 ± 15.6 | 132.1 ± 15.3 | 128.0 ± 13.3 | <0.001 |
| Diastolic blood pressure (mmHg) | 88.1 ± 10.4 | 91.1 ± 11.2 | 89.3 ± 10.2 | 88.7 ± 10.3 | 83.4 ± 9.3 | <0.001 |
| Type 2 diabetes (% of subjects) | 5.5 | 9.8 | 5.6 | 3.3 | 3.2 | 0.001 |
| Body mass index (kg/m2) | 26.8 ± 3.5 | 26.8 ± 3.6 | 26.8 ± 3.4 | 26.7 ± 3.3 | 27.0 ± 3.4 | 0.317 |
| Leisure-time physical activity (kcal/day)b | 367.5 ± 333.9 | 335.6 ± 325.1 | 336.1 ± 295.9 | 380.6 ± 373.0 | 417.3 ± 331.0 | <0.001 |
| Serum C-reactive protein (mg/L) | 2.3 ± 3.4 | 3.2 ± 4.5 | 2.3 ± 2.9 | 1.8 ± 2.9 | 1.8 ± 2.5 | <0.001 |
| Alcohol consumption (g/week) | 74.3 ± 121.4 | 79.9 ± 157.8 | 72.6 ± 104.8 | 72.6 ± 111.4 | 72.1 ± 103.8 | 0.640 |
Notes: Data are presented as mean ± SD, expect type 2 diabetes. Q1: ECP < 9.84 mL/mmHg; Q2: ECP = 9.84–11.64 mL/mmHg; Q3: ECP = 11.65–13.92 mL/mmHg; Q4: ECP >13.92 mL/mmHg.
Abbreviations: ECP = exercise cardiac power; LDL = low-density lipoprotein; Q = quartile.
Pack-years denotes the lifelong exposure to smoking, which was estimated as a product of years smoked and the number of tobacco products smoked daily at the time of examination.
Leisure-time physical activity is defined as leisure-time physical activity using the 12-month leisure-time physical activity questionnaire.
Table 2.
Baseline exercise test characteristics and the percentage of subjects on medications according to the quarters of ECP.
| Overall | Q1 | Q2 | Q3 | Q4 | p | |
|---|---|---|---|---|---|---|
| VO2max (mL/min) | 2400 ± 636 | 1719 ± 393 | 2224 ± 319 | 2566 ± 341 | 3095 ± 491 | <0.001 |
| VO2max (mL/kg/min) | 30.2 ± 8.0 | 22.4 ± 5.5 | 28.1 ± 4.8 | 32.4 ± 5.4 | 37.7 ± 6.9 | <0.001 |
| Peak systolic blood pressure (mmHg) | 202.0 ± 28.0 | 206.0 ± 32.0 | 207.0 ± 27.6 | 201.0 ± 25.3 | 193.0 ± 24.0 | <0.001 |
| Acetylsalicylic acid (%) | 7.0 | 8.8 | 7.1 | 6.1 | 5.8 | |
| Beta-blockers (%) | 17.6 | 29.2 | 18.8 | 12.5 | 9.7 | |
| Drugs for cholesterol (%) | 0.6 | 1.5 | 0.5 | 0.2 | 0.2 |
Notes: Data are presented as mean ± SD. Q1: ECP < 9.84 mL/mmHg; Q2: ECP = 9.84–11.64 mL/mmHg; Q3: ECP = 11.65–13.92 mL/mmHg; Q4: ECP >13.92 mL/mmHg.
Abbreviations: ECP = exercise cardiac power; Q = quartile; VO2max = maximal oxygen uptake.
3.2. Risk predictors for HF
As continuous variables, the strongest statistically significant risk factors for HF were ECP (p < 0.001), smoking (p < 0.001), and type 2 diabetes (p = 0.001), after adjustment for age (Table 1). One SD increase in ECP (3.16 mL/mmHg) was associated with a decreased risk of HF by 28% (95%CI: 17%−37%).
3.3. ECP and risk for HF in men
ECP was associated with the risk of HF (Table 2). Men with low ECP (<9.84 mL/mmHg, the lowest quartile, Q1) had a 2.37-fold (95%CI: 1.68−3.35, p < 0.0001) HR of HF as compared with men with high ECP (>13.92 mL/mmHg, the highest quartile, Q4), after adjusting for age. Low ECP was associated with a 1.96-fold risk of HF after additional adjustment for conventional risk factors (smoking, alcohol consumption, BMI, type 2 diabetes, history of coronary heart disease, and serum HDL and LDL cholesterol (Table 3). After further adjustment for left ventricular hypertrophy, the results hardly changed (HR = 1.87, 95%CI: 1.31 −2.66, p < 0.001). After adjustment for antihypertensive medication, the results remained statistically significant (HR = 1.74, 95% CI: 1.22 −2.46, p = 0.002). After further adjustment for COPD and acute myocardial infarction during the follow-up period, the results remained consistent (HR = 1.75, 95%CI: 1.23 −2.49, p = 0.001).
Table 3.
The HR of heart failure (313 men) in the quartiles of ECP in 2351 men.
| ECP | n | HR (95%CI)b | p | HR (95%CI)c | p | Number of heart failures | Number of heart failures/1000 person-years |
|---|---|---|---|---|---|---|---|
| Q1 | 588 | 2.37 (1.68–3.35) | <0.0001 | 1.96 (1.38–2.78) | <0.001 | 107 | 9.9 |
| Q2 | 587 | 1.57 (1.10–2.24) | 0.012 | 1.39 (0.96–1.98) | 0.076 | 84 | 6.8 |
| Q3 | 588 | 1.22 (0.85–1.75) | 0.282 | 1.21 (0.84–2.58) | 0.300 | 71 | 5.2 |
| Q4a | 588 | 1.00 | 1.00 | 51 | 3.6 |
Note: Q1: ECP < 49.84 mL/mmHg; Q2: ECP = 9.84–11.64 mL/mmHg; Q3: ECP = 11.65–13.92 mL/mmHg; Q4: ECP >13.92 mL/mmHg.
Abbreviations: 95%CI = 95%confidence interval; ECP = exercise cardiac power; HDL = high-density lipoprotein; HR = hazards ratio; LDL = low-density lipoprotein; Q = quartile.
Q4 is treated as reference.
Adjusted for age and examination year.
Adjusted for age, examination year, cigarette smoking, alcohol consumption, body mass index, type 2 diabetes, history of coronary heart disease, HDL cholesterol, and LDL cholesterol.
When the interaction term of ECP was added in the fully adjusted model together with ECP, ECP was inversely statistically significant (HR = 0.89, 95%CI: 0.84–0.94, p < 0.001). The number of HF per 1000 person-years was 3.6 in the reference group (the highest quartile (Q4) was treated as reference), and 5.2/1000 person-years, 6.8/1000 person-years, and 9.9/1000 person-years in the quartiles, respectively (Table 3). The multivariate adjusted cumulative Kaplan-Meier curves are shown in Fig. 1. In a sensitivity analysis, we excluded 86 men who discontinued the test due to symptoms; an inverse association between ECP and HF risk remained significant.
3.4. ECP, SBP at rest, and the risk of HF
Among men with the lowest ECP (<9.84 mL/mmHg) and normal resting SBP, a markedly increased HR of HF was observed (2.37-fold, 95%CI: 1.68−3.35, p < 0.001). Men with low ECP (<9.84 mL/mmHg) and elevated resting SBP (≥140 mmHg, median) had a markedly increased HR of HF. These men had a 1.71-fold (95%CI: 1.17−2.40, p = 0.004) increased HR compared to men with high ECP and low resting SBP. The HR was not markedly different in these subgroup analyses according to resting SBP levels, and this may be related to the size of subgroups and number of outcomes.
3.5. ECP assessment and HF risk prediction
An HF risk prediction model containing established risk factors yielded a C-index of 0.708 (95%CI: 0.685–0.731). After addition of information on ECP to the model, the C-index was 0.726 (95%CI: 0.704–0.748). The continuous NRI changed 0.143% (95%CI: 0.109%–0.178%, p = 0.017) after inclusion of ECP with other risk factors.
The C-statistics index was calculated to assess the model discrimination, or the ability of the model to correctly identify subjects with respect to HF.17 We also computed the NRI,18 which compares the shifts in reclassified categories by observed outcome.
4. Discussion
ECP was associated with an increased risk of HF in a population-based study of men. The integration of afterload and preload with VO2max and peak SBP during an exercise test emphasizes the role of ergospirometry in the risk prediction of HF because it gives prognostic information in addition to that obtained by conventional methods, although it did not add additional information over VO2max. The results highlight that ECP is an important determinant of HF risk but provides negligible improvement in risk prediction. However, there was a significant improvement in reclassification.
Little is known about the association between ECP and the risk of HF. Our study shows that ECP may provide prognostic information on HF risk prediction despite taking into account established risk factors, such as smoking, lipids, hypertension, left ventricular hypertrophy, COPD, and diabetes. This study shows that significant risk of HF was observed among men with the lowest level of ECP. A continuous increase in ECP (3.16 mL/mmHg) corresponds to a 28% decrease in the risk for HF among these men. Thus, ECP may provide a valuable tool for the risk prediction for HF in the general population, although further studies are needed.
VO2max is considered to be the gold standard for measuring cardiorespiratory fitness. In addition to VO2max, SBP can be measured more reliably during cycle ergometry (as was done in our study) than with a treadmill due to the reduced upper-body movements during the exercise. It has been suggested that VO2max is a noninvasive measure of cardiac output during physical stress and reflects cardiac preload, whereas SBP is a mere indicator of afterload during exercise because cardiac output is dependent on both preload and afterload. In subjects with elevated adrenergic tone and inappropriately constricted arterial bed, cardiac output can be lowered in the presence of disproportionately elevated SBP (i.e., afterload).19,20 Consequently, VO2max may be reduced considerably over the years, and thus it may underestimate cardiac pumping capacity in subjects. Cardiac output is a noninvasive descriptor of cardiac function derived from preload, blood pressure, and left ventricular function.21 It is probable that the incidence of HF can be reduced by a combination of several approaches, thus preventing its occurrence. VO2max may be preserved in subjects with antihypertensive medications that lower afterload despite the reduced pumping capacity.
Antihypertensive medications may decrease the afterload and increase cardiac output to a higher level during progressive exercise. This was not the case in our study; we adjusted for antihypertensive medications, but the results were still significant. VO2max is determined by several physiological, environmental, and genetic factors, such as age, sex, physical activity, and prevalent diseases. Cardiorespiratory fitness can be improved by increasing physical activity, which ultimately confers long-term benefits on the cardiovascular system.
Physical inactivity leads to elevated levels of blood pressure, serum lipids, insulin resistance, and obesity, all of which may predispose an individual to the development of HF. Elevated levels of C-reactive protein have been implicated with an increased risk of HF, and there is a possibility that the association between improved CRF and reduced risk of HF might be mediated through the anti-inflammatory effects of physical activity. In addition to modifying serum cholesterol levels, exercise training may also affect the risk of nonfatal cardiovascular events through other pathways.21, 22, 23 Lower levels of physical activity are associated with endothelial dysfunction, which may contribute to preclinical atherosclerosis. In subjects with preclinical atherosclerotic changes and elevated SBP increases, the stress on the vessel wall may lead to the risk of both endothelial injury and permeability, which may result in local or multifocal oedema.20, 21, 22,24 However, additional research, including exercise-intervention trials, would help to discover the mechanistic pathways through which ECP plays a role in risk stratification. Previous studies have suggested that low cardiorespiratory fitness is comparable to other conventional risk factors for CVD.7,10,25,26 A strong, inverse, and independent association between cardiorespiratory fitness and HF risk that is consistent with a dose–response relationship has been observed in previous studies.27 Our findings suggest that ECP may be used as an additional prognostic measure for HF in addition to VO2max.
The strengths of this study are that we used a representative population-based sample of middle-aged men and had a high participation rate. There were no losses during follow-up, and reliable assessments of incident HF events were made. Other strengths include the definitive diagnoses of HF cases. The mean follow-up time of more than 25 years was sufficiently long to ascertain the risk for HF. We adjusted for a comprehensive panel of lifestyle and biochemical markers to allow adequate adjustment for potential confounding, enabling reliable assessments of the associations.
Some limitations of the present study also deserve mention. Participants were prospectively monitored using established databases for hospital admissions, which were supplemented by reliable data on health status and risk factors, thus permitting the control of potential confounders. Although we adjusted for several confounders, there may be a possibility of residual confounding due to other unmeasured confounders, which could, in part, explain the association between ECP and HF. The availability of more advanced cardiac imaging modalities to define better descriptors of cardiac function is a valuable asset in clinical studies, but cost tends to be a limiting factor in population-based studies. Not all participants had echocardiography performed at baseline; hence, there is the possibility that participants with prevalent structural heart disease may have been included in the analysis. We were unable to assess the differential impact of ECP on the risk of HF with preserved vs. reduced ejection fraction because we had no data on the ventricular function post HF development. Furthermore, using a cycle ergometer with a regular increase in the workload may not be the best way to evaluate maximal functional capacity in deconditioned people, and it could underestimate functional capacity in a subgroup of the population. It may be more difficult to measure blood pressure using a treadmill test compared to a cycle ergometer (without upper-arm movements).
Another limitation of this study is that it is based on an ethnically homogenic and middle-aged male population and cannot be extrapolated to women or other ethnicities. Differences in peak SBP are relatively small as compared to the differences in VO2max. Consistent with the previous studies, the role of VO2max still seems to be very important in the assessment of the risk of HF.
5. Conclusion
This prospective population-based study provides the first evidence that ECP is associated with an increased risk of HF.
Acknowledgments
Acknowledgments
The authors thank the staff of the Institute of Public Health and Clinical Nutrition at the University of Eastern Finland and the Kuopio Research Institute of Exercise Medicine for data collection.
Authors’ contributions
SK participated in the design of the study, data collection and analysis, and interpretation of results; SYJ, THM, and MJH participated in the design of the study; AV contributed to data analysis; JK participated in data collection; and JAL participated in data collection, study design, and interpretation of results. All authors contributed to the writing of the manuscript. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under the responsibility of Shanghai University of Sports.
Supplementary materials
References
- 1.Fletcher G.F., Balady G.J., Amsterdam E.A., et al. Exercise standards for testing and training: A statement for healthcare professionals from the American Heart Association. Circulation. 2001;104:1694–1740. doi: 10.1161/hc3901.095960. [DOI] [PubMed] [Google Scholar]
- 2.Blair S.N., Kampert J.B., Kohl H.W., III, Barlow C.E., et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276:205–210. [PubMed] [Google Scholar]
- 3.Kannel W.B., Wolf P.A., McGee D.L., Dawber T.R., McNamara P., Castelli W.P. Systolic blood pressure, arterial rigidity, and risk of stroke. The Framingham study. JAMA. 1981;245:1225–1229. [PubMed] [Google Scholar]
- 4.Singh J.P., Larson M.G., Manolio T.A., et al. Blood pressure response during treadmill testing as a risk factor for new-onset hypertension. The Framingham Heart Study. Circulation. 1999;99:1831–1836. doi: 10.1161/01.cir.99.14.1831. [DOI] [PubMed] [Google Scholar]
- 5.Laukkanen J.A., Kurl S., Salonen R., Rauramaa R., Salonen J.T. The predictive value of cardiorespiratory fitness for cardiovascular events in men with various risk profiles: A prospective population-based cohort study. Eur Heart J. 2004;25:1428–1437. doi: 10.1016/j.ehj.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Lakka T.A., Venäläinen J.M., Rauramaa R., Salonen R., Tuomilehto J., Salonen J.T. Relation of leisure-time physical activity and cardiorespiratory fitness to the risk of acute myocardial infarction. N Engl J Med. 1994;330:1549–1554. doi: 10.1056/NEJM199406023302201. [DOI] [PubMed] [Google Scholar]
- 7.Mundal R., Kjeldsen S.E., Sandvik L., Erikssen G., Thaulow E., Erikssen J. Exercise blood pressure predicts mortality from myocardial infarction. Hypertension. 1996;27:324–329. doi: 10.1161/01.hyp.27.3.324. [DOI] [PubMed] [Google Scholar]
- 8.Laukkanen J.A., Jennings J.R., Kauhanen J., Mäkikallio T.H., Ronkainen K., Kurl S. Relation of systemic blood pressure to sudden cardiac death. Am J Cardiol. 2012;110:378–382. doi: 10.1016/j.amjcard.2012.03.035. [DOI] [PubMed] [Google Scholar]
- 9.Laukkanen J.A., Kurl S., Rauramaa R., Lakka T.A., Venäläinen J.M., Salonen J.T. Systolic blood pressure response to exercise testing is related to the risk of acute myocardial infarction in middle-aged men. Eur J Cardiovasc Prev Rehabil. 2006;13:421–428. doi: 10.1097/01.hjr.0000198915.83234.59. [DOI] [PubMed] [Google Scholar]
- 10.Laukkanen J.A., Mäkikallio T.H., Rauramaa R., Kiviniemi V., Ronkainen K., Kurl S. Cardiorespiratory fitness is related to the risk of sudden cardiac death: A population-based follow-up study. J Am Coll Cardiol. 2010;56:1476–1483. doi: 10.1016/j.jacc.2010.05.043. [DOI] [PubMed] [Google Scholar]
- 11.Kurl S., Laukkanen J.A., Niskanen L., et al. Cardiac power during exercise and the risk of stroke in men. Stroke. 2005;36:820–824. doi: 10.1161/01.STR.0000157592.82198.28. [DOI] [PubMed] [Google Scholar]
- 12.Kurl S., Jae S.Y., Kauhanen J., Ronkainen K., Rauramaa R., Laukkanen J.A. Exercise cardiac power and the risk of sudden cardiac death in a long-term prospective study. Int J Cardiol. 2015;181:155–159. doi: 10.1016/j.ijcard.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 13.Kurl S., Mäkikallio T., Jae S.Y., Ronkainen K., Laukkanen J.A. Exercise cardiac power and the risk of coronary heart disease and cardiovascular mortality in men. Ann Med. 2016;48:625–630. doi: 10.1080/07853890.2016.1202444. [DOI] [PubMed] [Google Scholar]
- 14.Kurl S., Laukkanen J.A., Rauramaa R., Lakka T.A., Sivenius J., Salonen J.T. Systolic blood pressure response to exercise stress test and risk of stroke. Stroke. 2001;32:2036–2041. doi: 10.1161/hs0901.095395. [DOI] [PubMed] [Google Scholar]
- 15.Karppi J., Kurl S., Makikallio T.H., Ronkainen K., Laukkanen J.A. Serum β-carotene concentrations and the risk of congestive heart failure in men: A population-based study. Int J Cardiol. 2013;168:1841–1846. doi: 10.1016/j.ijcard.2012.12.072. [DOI] [PubMed] [Google Scholar]
- 16.McMurray J.J., Adamopoulos S., Anker S.D., et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 17.Harrell F.E., Jr, Lee K.L., Mark D.B. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 18.Pencina M.J., D'Agostino R.B., Sr, D'Agostino R.B., Jr, Vasan R.S. Evaluating the added predictive ability of a new marker: From area under ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 19.Zelis R., Flain S.F. Alterations in vasomotor tone in congestive heart failure. Prog Cardiovasc Dis. 1982;24:437–459. doi: 10.1016/0033-0620(82)90012-3. [DOI] [PubMed] [Google Scholar]
- 20.Bain R.J., Tan L.B., Murray R.G., Davies M.K., Littler W.A. The correlation of cardiac power output to exercise capacity in chronic heart failure. Eur J Appl Physiol Occup Physiol. 1990;61:112–118. doi: 10.1007/BF00236703. [DOI] [PubMed] [Google Scholar]
- 21.Scharf C., Merz T., Kiowski W., Oechslin E., Schalcher C., Brunner-La Rocca H.P. Noninvasive assessment of cardiac pumping capacity during exercise predicts prognosis in patients with congestive heart failure. Chest. 2002;122:1333–1339. doi: 10.1378/chest.122.4.1333. [DOI] [PubMed] [Google Scholar]
- 22.Fagard R.H. Exercise characteristics and the blood pressure response to dynamic physical training. Med Sci Sports Exerc. 2001;33(Suppl. 6):S484–S492. doi: 10.1097/00005768-200106001-00018. [DOI] [PubMed] [Google Scholar]
- 23.Palatini P. Exaggerated blood pressure response to exercise: Pathophysiologic mechanisms and clinical relevance. J Sports Med Phys Fitness. 1998;38:1–9. [PubMed] [Google Scholar]
- 24.Laukkanen J.A., Kurl S., Salonen J.T. Cardiorespiratory fitness and physical activity as risk predictors of future atherosclerotic cardiovascular diseases. Curr Atheroscler Rep. 2002;4:468–476. doi: 10.1007/s11883-002-0052-0. [DOI] [PubMed] [Google Scholar]
- 25.Khan H., Kunutsor S., Rauramaa R., et al. Cardiorespiratory fitness and risk of heart failure: a population-based follow-up study. Eur J Heart Fai. 2014;16:180–188. doi: 10.1111/ejhf.37. [DOI] [PubMed] [Google Scholar]
- 26.Pandey A., Garg S., Khunger M., et al. Dose-response relationship between physical activity and risk of heart failure: A meta-analysis. Circulation. 2015;132:1786–1794. doi: 10.1161/CIRCULATIONAHA.115.015853. [DOI] [PubMed] [Google Scholar]
- 27.Intwala S., Balady G.J. Physical activity in the prevention of heart failure: Another step forward. Circulation. 2015;132:1777–1779. doi: 10.1161/CIRCULATIONAHA.115.018831. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.