Abstract
Background
The Interaction between anti-tuberculous and immunosuppressive drugs which may increase the risk of graft rejections is a major challenge in managing transplant recipients with tuberculosis (TB). Instead of rifampicin (RFM), most guidelines recommended the use of rifabutin (RFB) because of its reduced capacity to induce immunosuppressant metabolism while maintaining the same efficacy as RFM against TB. However, there has been no available data directly comparing the outcome of RFB from RFM-based anti-TB regimens in liver transplant patients with TB. This study aimed to compare the effects of RFB from RFM-based treatment in terms of the drug interaction with immunosuppressants, as well as the safety, efficacy and clinical outcomes of living donor liver transplant (LDLT) recipients with active TB.
Methods
A retrospective study was conducted on all adult LDLT recipients diagnosed with active TB from June 1994 to May 2016 that had concurrently and continuously received either RFB or RFM-based treatment and immunosuppressants.
Results
Twenty-two patients were included. Twelve (55%) patients were in the RFM group. Ten (45%) patients were in the RFB group. RFB group showed a lesser rate of immunosuppressant trough level reduction (20% vs 50%, p = 0.009) during TB treatment. There was no TB recurrence and no significant change in platelet or leukocyte count in either group. Acute cellular rejection (ACR), rate of TB-treatment completion and overall survival, rates were excellent and statistically similar in both groups.
Conclusion
The use of RFB in LDLT recipients with active TB, had a lesser drug interaction than when RFM was used. However, RFB did not significantly reduced the rate of ACR. RFB and RFM are both effective and safe to use in LDLT recipients with active TB.
Keywords: Rifabutin, Living donor liver transplant (LDLT), Liver transplant, Tuberculosis
At a glance of commentary
Scientific background on the subject
In a 2007 Cochrane Database of Systematic Review by Davies et al. [6] and a more recent 2016 study by Crabol et al. [7], the authors showed that rifampicin (RFM) and rifabutin (RFB) are equally effective against TB with no difference in effectiveness or relapse rate in both immunocompetent and HIV infected patients receiving protease inhibitors.
What this study adds to the field
Those who received RFM-based treatment in terms of their interaction between the immunosuppressants used during TB treatment, hematologic side effects, graft rejection rate, graft loss, TB-treatment completion rate and overall survival rate among living donor liver transplant (LDLT) recipients with active TB.
Tuberculosis (TB) is one of the most significant opportunistic infection affecting solid organ transplant (SOT) recipients [1]. Compared to the general population, these patients have an increased risk of developing TB and subsequently, have a higher risk of complications and death [2,3]. A recent nationwide population-based matched cohort study from Taiwan, wherein TB is endemic [4], revealed that liver transplant (LT) patients with TB had a higher mortality rate than those without TB [2]. Because of the high possibility of undesirable outcomes, effective treatment strategies that lower the risk of complications and death are essential in successfully managing LT recipients with active TB. Aside from the fact that such patients were already immunocompromised as a result of the TB infection and post-transplant medications, there were several treatment challenges present [2,5]. Among these challenges, one very important issue in the management of such patients is the pharmacokinetic interactions between immunosuppressive and anti-TB medications.
In a 2007 Cochrane Database of Systematic Review by Davies et al. [6] and a more recent 2016 study by Crabol et al. [7], the authors showed that rifampicin (RFM) and rifabutin (RFB) are equally effective against TB with no difference in effectiveness or relapse rate in both immunocompetent and HIV infected patients receiving protease inhibitors. However, despite RFM, being one of the most widely used and principal 1st-line drugs against TB, there have been concerns in its ability to decrease the immunosuppressant drug levels that prompted other authors to advise against its use in the transplant population [8,9]. Aside from the harmful effect on the liver, RFM is a strong cytochrome (CY) P3A4 inducer. RFM can significantly reduce blood levels of calcineurin inhibitors, mammalian target of rapamycin inhibitors (mTORi), steroids and even mycophenolate mofetil (MMF) by inducing CYP3A4-mediated immunosuppressant metabolism [5,[9], [10], [11], [12]]. Rifamycin (RFM, RFB and Rifapentine) induction of P-glycoprotein mediated efflux transport mediating mTORi metabolism and the induction of other enzymes such as uridine diphosphate–glucuronosyltransferases, which mediate MMF metabolism [13,14] and may also contribute to the interaction. On the other hand, in post-transplant patients with TB, other authors and guidelines only recommended the use of rifamycins (RFM, RFB or Rifapentine) for patients with severe or disseminated forms of TB or those with suspicion or resistance to isoniazid (INH) [1,15,16]. Nonetheless, even with these recommendations, with proper dose adjustment and adequate monitoring, RFM is still mostly preferred in transplant recipients with TB because of its potent sterilizing activity against TB [5,[17], [18], [19]].
In an effort to minimize the effect of this drug-to-drug interaction without compromising efficacy against TB, one recommendation is to use RFB instead of RFM. RFB is structurally similar to RFM, has the same potency against TB [15,17] and has almost the same mechanism of interaction with immunosuppressants. Although, in contrast to RFM, RFB is a weak CYP3A4 inducer and therefore, may not significantly reduce immunosuppressant trough levels [1,7,11,16,17,20]. However, there is very little available data for its use in the transplant population. Even though there have been favorable reports about using RFB in solid organ transplantation (SOT) [21,22], they were only limited to case reports and limited descriptive-retrospective data. There has been no published experience directly comparing outcomes between RFM and RFB-based treatment of LT recipients with TB. This study aimed to directly compare RFB-based anti-TB treatment to those who received RFM-based treatment in terms of their interaction between the immunosuppressants used during TB treatment, hematologic side effects, graft rejection rate, graft loss, TB-treatment completion rate and overall survival rate among living donor liver transplant (LDLT) recipients with active TB.
Patients and Methods
Study population and Data Collection
A retrospective study was conducted on all adult LDLT recipients with an active TB infection that was either diagnosed before or after LDLT from June 1994 to May 2016 and had concurrently and continuously received either RFB or RFM-based anti-TB regimen as well as immunosuppressive drugs for most of the time during TB treatment. Data were retrieved from Kaohsiung Chang Gung Memorial Hospital (KCGMH) Liver Transplantation Center's database and electronic medical records. The study population was followed-up by retrieving their respective in-patient and out-patient records until May 2017. General demographics and data related to the objectives of the study were collected which include age, sex, LDLT indication, Model for End-Staged Liver Disease (MELD) score, Child-Pugh class, TB diagnostic modalities, TB location, timing of TB diagnosis in relation to the time of LDLT, anti-TB regimen used, completeness of TB-treatment, rate of graft rejection, graft loss and mortality any time after TB diagnosis with their respective causes. As well, data on immunosuppressive drugs used, its dose and trough levels were collected. This includes platelet and white blood cell (WBC) count before TB treatment for patients who developed active TB after LDLT, during TB treatment for all patients and after TB treatment for those who developed active TB before LDLT.
This study was approved by our center's Institutional Review Board (IRB no. 201800147B0).
Active TB management in LDLT
Our pre-LDLT evaluation protocol, as well as TB diagnosis before or after LDLT, immunosuppression and surveillance protocol in LDLT recipients with active TB have been described in detail elsewhere [[23], [24], [25]]. Once diagnosed with active TB, LDLT candidates were further classified as “open TB” if patients proved to be infectious, which was demonstrated by a positive for sputum acid-fast bacilli (AFB) smear or sputum culture. Otherwise, they were defined as “non-open TB”. Patients classified as open TB are deferred to undergo LDLT until TB treatment has started and the results of sputum AFB and culture return negative, thus re-classifying the patient as non-open TB. In non-open TB patients, though it is preferred to start TB-treatment prior to transplant [26], LDLT will proceed immediately if the patient develops progressive liver failure or under any circumstance that requires urgent LT. Infectious or respiratory physicians, following World Health Organization (WHO) [27] and the Taiwan Center for Disease Control guidelines [28], are responsible for prescribing and, if needed, modifying anti-TB drugs. As first-line therapy, either 3 or 4-drug standard anti-TB regimens are used, meant to be given for at least 6 months are used. We preferred the RFB when available in our hospital since Sep 2001. As following were RFM/RFB based regimen dosage according to the 6th Taiwan Guidelines for TB Diagnosis & Treatment: [RFM 15 (10–20)mg/kg (600 mg)qd (YungShin Pharmaceuticals, Industry, Co.LTD., Taiwan), Ethambutol (EMB) 15 (15–20)mg/kg (1.6 gm)qd (PeiLi Pharm, Taiwan), and/or INH 5 (4–6)mg/kg (300 mg)qd (Shinlon Pharmaceuticals, Industry Co. LTD., Taiwan), and/or Pyrazinamide (PZA) 25 (15–30)mg/kg (2 gm)qd (PeiLi Pharm, Taiwan)]/AKURIT (INH,RFM,EMB,PZA) (Lupin LTD., India)/Rifinah (RFM,INH) (Macleods Pharmaceuticals LTD., Italy) or RFB based regimen (RFB: 5–10 mg/kg (300 mg)qd (Lupin LTD., India), INH, EMB and/or PZA) [24,26,29]. Patients who will have culture-confirmed MDR-TB are referred to the nearest government-accredited TB center dedicated to treat and monitor patients with MDR-TB [4].
Immunosuppression protocol
Our immunosuppression protocols are described in detail elsewhere [25,[30], [31], [32]]. We use a combination of multiple immunosuppressants (Tacrolimus and/or mTORi with MMF and/or prednisone) that were given at the lowest possible dose and maintained at the lowest possible trough level while closely and regularly monitoring signs and symptoms including liver function, immunosuppressive drugs serum levels, chest x-ray, liver ultrasound with doppler, etc. Immunosuppressants’ doses are increased in the case of mildly elevated aspartate aminotransferase (AST)/alanine aminotransferase (ALT). Liver biopsy is performed if rejection is suspected. Additional ancillaries (CT-scan, MRI/MRCP etc.) are likewise performed depending on the initial signs and symptoms and results of the other routine test. All of the recipients returned to our institute for lab tests, imaging studies, and check-up at clinics [[30], [31], [32]]. In post-LDLT patients with active TB, the same immunosuppression and surveillance protocols are applied. In response to any clinically significant event or complication during surveillance, especially during TB treatment, anti-TB and immunosuppressive drugs are modified accordingly. Additional intervention may also be employed if the need arises. Immunosuppressant trough level assays were done using the immunoassay method by Dimension® clinical chemistry system, (Siemens, USA) for sirolimus and tacrolimus while immunoassay method by Indiko (Thermo scientific, Germany) for everolimus trough level assay through the whole study period.
Data analysis and statistics
For all collected data, continuous variables were expressed as medians and interquartile range. Categorical variables were expressed as numbers and percentages. Patients were divided into the RFM group for those who took RFM-based anti-TB treatment and RFB group for those who took RFB-based treatment. Chi-square (X2) or Fischer's exact test was used to test the difference of categorical variables between each group, while the Mann-Whitney U test was used to test the difference of continuous variables between each group. The Wilcoxon Signed-Rank test was also used to differentiate related continuous variables within each group. Kaplan-Meier and Log-rank test were used to determine and differentiate the cumulative incidence of acute cellular rejection (ACR), TB-treatment completion and overall survival between each group. A p-value of <0.05 was considered significant. All statistical analyses were performed using SPSS ver. 20 (IBM SPSS Inc., Chicago, IL, USA).
Results
Baseline demographics and TB characteristics
Out of 1313 LDLT recipients, 26 (2%) were diagnosed with active TB. Four patients were excluded because they were not able to satisfy the inclusion criteria. Two patients received different sets of anti-TB regimens and the other 2 patients did not received concurrent and continuous anti-TB drugs and immunosuppressants, thereby limiting the interaction between them. A total of 22 patients were included in the study. Twelve patients received RFM-based anti-TB treatment and were included in the RFM group, while 10 patients received RFB-based anti-TB treatment and were included in the RFB group. There were more males than females in the RFM group, and more females in the RFB group. Most of the patients in both groups were diagnosed with hepatocellular carcinoma. Age, gender, body weight, MELD score, Child-Pugh classes of the 2 groups were not significantly different from each other (Table 1). Table 1 also shows that the timing of TB diagnosis, location of TB infection and the duration of TB treatment were not significantly different in between each group.
Table 1.
Demographics and TB characteristics.
| Total n:22 | RFM Group (n:12) | RFB Group (n:10) | p value |
| Median Age on TB Diagnosis (IQR): | 56 (8) | 58 (10) | 0.872 |
| Sex, n (%): | |||
| Male/Female | 9(75)/3(25) | 4(40)/6(60) | 0.192 |
| LDLT Indication, n (%): | |||
| HCC (+HBV/HCV/ALC) | 9 (75) | 7 (70) | 0.702 |
| HBV only | 1 (8.3) | 1 (10) | |
| HCV only | 1 (8.3) | 2 (20) | |
| HBV + ALC | 1 (8.3) | 0 | |
| Median MELD score (IQR): | 10 (5) | 14 (11) | 0.107 |
| Child-Pugh Class, n (%) | |||
| A | 6 (50) | 2 (20) | 0.104 |
| B | 4 (33) | 2 (20) | |
| C | 2 (17) | 6 (60) | |
| TB Location, n (%): | |||
| Pulmonary | 9 (75) | 7 (70) | 0.528 |
| Extra-Pulmonary | 0 | 1 (10) | |
| Disseminated | 4 (25) | 2 (20) | |
| Timing of TB Diagnosis, | |||
| n (%): | 0.391 | ||
| Pre-LDLT | 8 (67) | 4 (40) | |
| Post-LDLT | 4 (33) | 6 (60) | |
| Median Duration of TB Treatment (IQR): | 7 (3) months | 7.5 (3.5) months | 0.722 |
| Immunosuppressant used during TB Treatment,n (%): | |||
| Sirolimus based | 10 (83) | 3 (30) | 0.026 |
| Everolimus based | 0 | 3 (30) | |
| Tacrolimus based | 2 (17) | 4 (40) | |
Abbreviations: TB: tuberculosis; RFM: rifampicin; RFB: rifabutin; IQR: interquartile range; LDLT: living donor liver transplant; HCC: hepatocellular carcinoma; HBV: hepatitis B related liver cirrhosis; HCV: hepatitis C related liver cirrhosis; ALC: alcoholic liver cirrhosis.
Anti-TB and immunosuppressive drug interaction
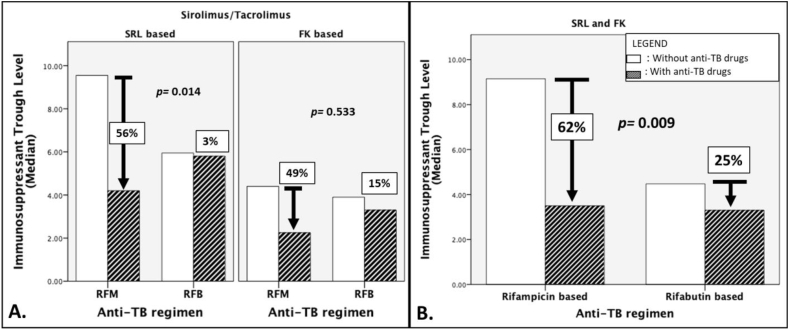
The duration of TB-treatment in both groups were statistically similar (Table 1) at around 7 months. During TB treatment, there was a 96% overall net increase in immunosuppressant dose in the RFM group and a 25% overall net increase in the RFB group. However, as Table 2 has shown, both the doses between the actual immunosuppressant dose with and without the interacting anti-TB drugs, and the doses between RFM and RFB groups were not significantly different. Despite these statistically similar doses, the trough level of tacrolimus and sirolimus during TB treatment (i.e. with the interacting anti-TB drugs) significantly dropped from 4.4 ng/ml to 2.2 ng/ml and from 9.6 ng/ml to 4.2 ng/ml respectively, in the RFM group. Meanwhile, there was no significant difference in the RFB group (Table 2). In comparing the percentage of trough level reduction during TB treatment, Fig. 1-A shows that in both sirolimus and tacrolimus, there was an actual reduction in the trough levels during TB treatment. However, even without any change of dose during TB treatment (Table 2), trough levels of patients who received sirolimus-RFB combination have a significantly lower rate of reduction than those who received the sirolimus-RFM combination. The percentages of tacrolimus trough level reduction between RFM and RFB were not significantly different. Overall, showing the percentage of trough level reduction of combined sirolimus and tacrolimus during TB treatment, Fig. 1-B shows that during TB treatment, there was an overall 62% decrease in the immunosuppressant trough level in the RFM group, which was significantly higher than the 25% overall reduction in the RFB group.
Table 2.
Comparison between RFM and RFB groups.
| Total n:22 | Rifampicin-Based Group n:12 (%) | Rifabutin-Based Group n:10 (%) | p value (Mann-Whitney U) |
| Median Dose WITHOUT anti-TB drugs, mg/day (IQR): | |||
| Tacrolimus | 3.1 (0.7) | 2 (1.3) | 0.133 |
| Sirolimus | 1 (1.1) | 1 (0) | 0.469 |
| Everolimus | – | 1 (0.75) | – |
| Median Dose WITH anti-TB drugs, mg/day (IQR): | |||
| Tacrolimus | 2.2 (0.5) | 2.5 (0.88) | 0.800 |
| Sirolimus | 2.25 (2) | 1 (0) | 0.371 |
| Everolimus | – | 0.75 (0.25) | – |
| Difference of Dose between with and without anti-TB drugs (Wilcoxon Signed Rank Test) | p 0.141 | p 0.205 | |
| Median Trough Level WITHOUT anti-TB drugs, ng/ml (IQR): | |||
| Tacrolimus | 4.4 (1.6) | 3.9 (3.7) | 0.800 |
| Sirolimus | 9.6 (8.5) | 5.9 (0.65) | 0.371 |
| Everolimus | – | 4 (2.5) | – |
| Median Trough Level WITH anti-TB drugs [ng/ml (IQR)]: | |||
| Tacrolimus | 2.2 (0.1) | 3.3 (2.3) | 0.533 |
| Sirolimus | 4.2 (3.7) | 5.8 (2.4) | 0.287 |
| Everolimus | – | 1.85 (0.5) | – |
| Difference of Trough levels between with and without anti-TB drugs (Wilcoxon Signed Rank Test) | p 0.002 | p 0.139 | |
| Median Platelet count WITHOUT anti-TB drugs, x103/uL (IQR): | 152 (91.8) | 107 (117.9) | 0.123 |
| Median Platelet count WITH anti-TB drugs, x103/uL (IQR): | 162 (35.6) | 118 (135) | 0.140 |
| Difference of Platelet count between with and without anti-TB drugs (Wilcoxon Signed Rank Test) | p 0.784 | p 0.169 | |
| Median WBC count WITHOUT anti-TB drugs, x103/uL (IQR): | 4.7 (3.4–5.2) | 4.1 (2.3–5.3) | 0.283 |
| Median WBC count WITH anti-TB drugs, x103/uL (IQR): | 4.3 (3.9–5.2) | 3.8 (3–4.4) | 0.314 |
| Difference of WBC count between with and without anti-TB drugs (Wilcoxon Signed Rank Test) | p 0.373 | p 0.767 | |
Abbreviations: TB: tuberculosis; IQR: interquartile range.
Fig. 1.
Percentage change in immunosuppressant trough level during TB treatment.Bar graphs illustrating the median percentage change in immunosuppressant trough level during TB-treatment. (A) Difference in specific percent change of sirolimus and tacrolimus between rifampicin and rifabutin-based anti-TB drugs. (B) Difference in combined sirolimus and tacrolimus percent change between rifampicin and rifabutin-based anti-TB drugs. Abbreviations used: SRL: sirolimus; FK: tacrolimus; p value: calculated via Mann-Whitney U test.
Safety, efficacy and clinical outcome
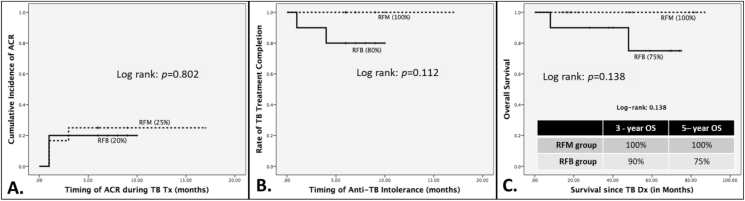
The median follow-up time of the study population was 44 months. There were no significant changes in WBC and platelet counts or the difference between the groups with or without the anti-TB drugs (Table 2). There was no TB re-infection and no graft loss in either of the groups. Though there was a trend that patients in the RFM group had developed more ACR (25%) than those in the RFB group (20%), it did not reach a statistical significance (Fig. 2-A). Fig. 2-B shows that there was a 100% TB treatment completion rate in the RFM group and 80% in the RFB group, but it was not significantly different. There was no TB-related mortality in either group. There were 2 patients who died in the RFB group. The cause of death was cervical cancer in 1 patient, who received everolimus-RFB combination and died 8 months after TB diagnosis. The other patient's cause of death was reinfection of HCV. This patient received a combination of tacrolimus-RFB and died 4 years after TB diagnosis. Both patients were not able to complete their respective TB treatment because of repeated ACR. Kaplan-Meier analysis showed a 100% and a 75% 5-year overall survival in the RFM and RFB group respectively, but it was not statistically significant (Fig. 2-C).
Fig. 2.
(A) Clinical outcomes between RFB and RFM use in LDLT recipients with active TB. ACR rate; (B) TB-treatment completion rate; (C) Overall Survival, Abbreviations used: ACR: acute cellular rejection; RFB: rifabutin; RFM: rifampicin; LDLT: living donor liver transplant; TB: tuberculosis.
Discussion
Preventing graft rejection while avoiding or at least minimizing immunosuppressant adverse drug reaction is the goal of immunosuppressive therapy in post-transplant recipients. And when transplant recipients developed diseases that would require medications that could possibly affect the trough levels of the immunosuppressive drugs, this would always present a significant challenge. This is especially true in transplant patients who have active TB. One major recommendation to decrease the risk of graft rejection in such patients is the use of RFB, instead of RFM. There have been reports demonstrating the efficacy and safety of using RFB in transplant patients. In a 2015 study by Tabarsi et al. that reported 22 post-transplant patients with TB who used RFB-based anti TB treatment, all of their patients were treated successfully, without any mortality and have concluded that RFB is an excellent alternative to RFM [22]. There were several other reports attesting to the successful result of shifting RFM to RFB once graft rejection developed [7,21,33,34]. However, all of these reported experiences in using RFB were limited to case reports and case series. In a report by Lopez-Montez et al., in 2004 [21] and by Hickey et al., in 2013 [33] demonstrating RFM and tacrolimus interaction in kidney transplant recipients, both studies showed that even after increasing the tacrolimus dosage up to 3.8-fold, Tacrolimus trough level remains to be sub-therapeutic. They were only able to increase the trough level back to the desired therapeutic level after shifting TB treatment to RFB-base regimen, while the former study had the tacrolimus dose similar to the pre-TB treatment dose and the latter study only needed a decrease of 2.8-fold in tacrolimus dose to maintain its trough level within the therapeutic range. In a 2011 report by Ngo et al. [35], it was shown that up to a 6-fold increase in sirolimus dosing was needed to maintain trough levels within therapeutic levels when using RFM in 2 post kidney transplant patients. As the studies mentioned above and recent guidelines have emphasized, increasing the immunosuppressants’ dose to at least twice the normal amount during TB treatment is essential to counteract its interaction with all rifamycin-based (RFM, RFB and rifapentine) anti-TB regimen in order to maintain a standard therapeutic trough level and subsequently prevent graft rejection. In our study, in the RFB group, there was only a median increase of 50% (i.e. less than the recommended 2-fold increase) in tacrolimus dose while there was no change in sirolimus dose. In the RFM group, the dose of tacrolimus even decreased during TB treatment while the dose of sirolimus only has a median increase of 35% (less than a 2-fold increase). These doses, however, were not significantly different between the groups with and without the interacting anti-TB drug and between the RFM and RFB groups (Table 2). Because of an on-going infection and also following our immunosuppression protocol of giving the lowest possible immunosuppressant dose, patients in the RFM group who received tacrolimus had their dose decreased during TB treatment. Even with a significant dropped in their trough level (Table 2), these patients were asymptomatic and responded normally to their liver function tests. As our study has shown, wherein the study population was all LDLT recipients with active TB may not be entirely applicable assuming the above premise of maintaining the set standard therapeutic range to prevent graft rejection.
In our study, the advantage of RFB against RFM of having less interaction with cytochrome P3A4 was further emphasized. Even with statistically the same immunosuppressant dose with and without the interacting anti-TB drugs, the rate of immunosuppressant trough level reduction is significantly lower in the RFB group than in the RFM group (Fig. 1-B). Furthermore, only in the RFM group, the actual immunosuppressant trough level during TB treatment showed to be significantly lower than the level without the interacting anti-TB drug (Table 2). Fig. 1-A suggested that LDLT recipients with active TB who are receiving sirolimus-based immunosuppressive therapy may benefit more when given RFB-based anti-TB medication. Exact determination of the possible reason and mechanism for this benefit is beyond the scope of this study, but, it may have something to do with the slight difference in metabolic pathways and a mechanism of RFB interaction with sirolimus from tacrolimus. In contrast to tacrolimus-which is extensively metabolized mainly by CYP3A4 enzymes in the liver [36]-sirolimus is extensively metabolized by the CYP3A4 isozyme in the intestinal wall and liver and undergoes counter-transport from enterocytes of the small intestine into the gut lumen by the P-glycoprotein drug efflux pump. Sirolimus is potentially recycled between enterocytes and the gut lumen to allow continued metabolism by CYP3A4 [37]. Therefore, a possible explanation for this benefit is, since sirolimus is possibly more affected by CYP3A4, a decrease in its induction by using RFB may have a significant effect compared to tacrolimus. Further studies are recommended to prove and explain this benefit. On the other hand, based on our results, patients with active TB who are receiving tacrolimus-based immunosuppressive therapy may either receive RFM or RFB-based anti-TB medication.
While our study confirms the advantage of RFB in terms of drug interaction, it did not translate to an actual reduction of ACR rates. Even while the trough levels in both groups during TB treatment were actually less or in the low-to-normal range of the supposedly desired therapeutic levels [[38], [39], [40]], ACR rates were not significantly different between the groups. The most plausible reason might be related to that we closed monitor the therapeutic level of tacrolimus then adjust the dose of immunosuppressive drugs accordingly. Besides, it also may be partly explained by the inherent capacity of the liver for immune tolerance [40,41] which was well described during liver transplantation in which regulatory T-cells may have a role [42,43], among many other proposed mechanism [44]. It can also be explained that living related liver transplantation, in which all our study population belongs, is actually associated with decrease risk of rejection [45,46]. Because of such capacity of the liver, the immunosuppressant dosing targets in liver transplantation may be lower than it was originally set, which was based on clinical trials that replicated the doses employed in kidney transplantation, where overall immunosuppression requirements are higher [47,48]. In a 2012 meta-analysis by Rodriguez-Peralvarez et al. analyzing the correlation of tacrolimus trough levels with rejection and renal impairment in LT patients, it revealed that long-term maintenance of trough level of at least 4 ng/ml does not increase the risk of graft rejection and may help reduce renal impairment. However, the lowest possible threshold of tacrolimus trough levels was not identified as it needed studies with large cohorts of LT patients with protocol biopsies to be deemed significant [49]. Though more evidence is needed to categorically state that it is possible to achieve good outcomes even for much lower trough levels, in our experience, using our immunosuppression protocol that was stated earlier, even with trough levels were kept to the minimum, ACR rates and long-term outcome for all our LDLT recipients have been remarkable [50].
Though RFB did not decrease the rate of ACR in our study, immunosuppressant trough levels were less affected by its interaction with RFB, thus making it practically easier to use and monitor, especially in the out-patient setting. With close and regular monitoring of patients, immunosuppressant dose adjustment seems to be easier when using RFB especially in patients under sirolimus-based immunosuppression. Even with this advantage, possible major side effects of RFB, like leukopenia and severe thrombocytopenia should also be part of surveillance especially in the post-transplant population. The rate of RFB-related neutropenia may reach up to 12%, but with a dose of 300 mg/day, the discontinuation rate for severe neutropenia is not different from the control arms and ranges from 0 to 2% [7]. Also, using the same dose of 300 mg/day, there was 0.5–0.7% risk of severe grade III thrombocytopenia [7] but in a study by Tabarsi et al., with the same dose, there was up to a 19% incidence of severe thrombocytopenia among solid organ transplant recipients with TB [22]. In our study, wherein our patients in the RFB group received a dose of 300 mg/day, the said hematologic side effects were not experienced by patients in both groups (Table 2).
In our previous study on the clinical outcomes of all LDLT recipients with active TB, it was shown that completing TB-treatment is associated with better overall survival rates [25]. As this present study has shown, most of the patients who completed TB-treatment and resulted in excellent and comparable overall survival. Our study likewise highlighted the efficacy of both RFM and RFB. There was no TB recurrence, no graft loss and no TB-related mortality.
Though with valuable results, this study has its limitations. Data collection is done retrospectively coming from a single institution. Data collection is likewise highly dependent on the available information which was recorded long before this study has started and in our routine practice setting. Further prospective studies with controlled immunosuppressant dosing regimens are recommended to further avoid bias and have a more conclusive finding.
Conclusion
The cornerstone in the management of LDLT recipients with active TB is choosing the right anti-TB medication that can effectively treat TB, maintain the good condition of the recipients and at the same time will not compromise liver grafts. This study clearly shows that even without a significant change in immunosuppressant dose during TB treatment, RFB has a lesser interaction with immunosuppressive drugs than RFM, but, it may not significantly reduce the rate of ACR. The results only suggested that the management and surveillance of such patients may be easier when RFB is used with sirolimus. Clinical outcomes have shown that both RFB and RFM are equally safe and efficacious in LDLT recipients with active TB.
Conflicts of interest
The authors have no financial or proprietary interest in the subject matter of this article.
Funding
This work was supported by grants from Health and welfare surcharge of tobacco products, Ministry of Health and Welfare, Taiwan (MOHW107-TDU-B-212-114022 to Chen CL).
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Aguado J.M., Torre-Cisneros J., Fortun J., Benito N., Meije Y., Doblas A., et al. Tuberculosis in solid-organ transplant recipients: consensus statement of the group for the study of infection in transplant recipients (GESITRA) of the Spanish Society of Infectious Diseases and Clinical Microbiology. Clin Infect Dis. 2009;48:1276–1284. doi: 10.1086/597590. [DOI] [PubMed] [Google Scholar]
- 2.Chen C.Y., Liu C.J., Feng J.Y., Loong C.C., Liu C., Hsia C.Y., et al. Incidence and risk factors for tuberculosis after liver transplantation in an endemic area: a nationwide population-based matched cohort study. Am J Transplant. 2015;15:2180–2187. doi: 10.1111/ajt.13235. [DOI] [PubMed] [Google Scholar]
- 3.Holty J.E., Gould M.K., Meinke L., Keeffe E.B., Ruoss S.J. Tuberculosis in liver transplant recipients: a systematic review and meta-analysis of individual patient data. Liver Transplant. 2009;15:894–906. doi: 10.1002/lt.21709. [DOI] [PubMed] [Google Scholar]
- 4.Taiwan Centers for Disease Control. Center for disease control annual report, https://www.cdc.gov.tw/InfectionReport/Info/MBykt7DHDDcSbrcfxoLRoQ?infoId=HCzh6FgAgovrYiZVrpJrbw/; 2016 [accessed 8 January 2020]
- 5.Subramanian A.K., Morris M.I., Practice ASTIDCo Mycobacterium tuberculosis infections in solid organ transplantation. Am J Transplant. 2013;13(Suppl 4):68–76. doi: 10.1111/ajt.12100. [DOI] [PubMed] [Google Scholar]
- 6.Davies G.R., Cerri S., Richeldi L. Rifabutin for treating pulmonary tuberculosis. Cochrane Database Syst Rev. 2007;2007 doi: 10.1002/14651858.CD005159.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crabol Y., Catherinot E., Veziris N., Jullien V., Lortholary O. Rifabutin: where do we stand in 2016? J Antimicrob Chemother. 2016;71:1759–1771. doi: 10.1093/jac/dkw024. [DOI] [PubMed] [Google Scholar]
- 8.Munoz L., Santin M. Prevention and management of tuberculosis in transplant recipients: from guidelines to clinical practice. Transplantation. 2016;100:1840–1852. doi: 10.1097/TP.0000000000001224. [DOI] [PubMed] [Google Scholar]
- 9.Trofe-Clark J., Lemonovich T.L., Practice ASTIDCo Interactions between anti-infective agents and immunosuppressants in solid organ transplantation. Am J Transplant. 2013;13:318–326. doi: 10.1111/ajt.12123. [DOI] [PubMed] [Google Scholar]
- 10.Niemi M., Backman J.T., Fromm M.F., Neuvonen P.J., Kivisto K.T. Pharmacokinetic interactions with rifampicin clinical relevance. Clin Pharmacokinet. 2003;42:819–850. doi: 10.2165/00003088-200342090-00003. [DOI] [PubMed] [Google Scholar]
- 11.Baciewicz A.M., Chrisman C.R., Finch C.K., Self T.H. Update on rifampin, rifabutin, and rifapentine drug interactions. Curr Med Res Opin. 2013;29:1–12. doi: 10.1185/03007995.2012.747952. [DOI] [PubMed] [Google Scholar]
- 12.Berdaguer S., Bautista J., Brunet M., Cisneros J.M. Antimicrobial and immunosuppressive drug interactions in solid organ transplant recipients. Enferm Infecc Microbiol Clín. 2012;30:86–92. doi: 10.1016/S0213-005X(12)70087-3. [DOI] [PubMed] [Google Scholar]
- 13.Kuypers D.R., Verleden G., Naesens M., Vanrenterghem Y. Drug interaction between mycophenolate mofetil and rifampin: possible induction of uridine diphosphate-glucuronosyltransferase. Clin Pharmacol Ther. 2005;78:81–88. doi: 10.1016/j.clpt.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Naesens M., Kuypers D.R., Streit F., Armstrong V.W., Oellerich M., Verbeke K., et al. Rifampin induces alterations in mycophenolic acid glucuronidation and elimination: implications for drug exposure in renal allograft recipients. Clin Pharmacol Ther. 2006;80:509–521. doi: 10.1016/j.clpt.2006.08.002. TNMI7-0005. [DOI] [PubMed] [Google Scholar]
- 15.Aguado J.M., Silva J.T., Samanta P., Singh N. Tuberculosis and transplantation. Microbiol Spectr. 2016;4 doi: 10.1128/microbiolspec.TNMI7-0005-2016. :TNMI7-0005. [DOI] [PubMed] [Google Scholar]
- 16.Meije Y., Piersimoni C., Torre-Cisneros J., Dilektasli A.G., Aguado J.M., Hosts ESGoIiC Mycobacterial infections in solid organ transplant recipients. Clin Microbiol Infect. 2014;20:89–101. doi: 10.1111/1469-0691.12641. [DOI] [PubMed] [Google Scholar]
- 17.Santoro-Lopes G., Subramanian A.K., Molina I., Aguado J.M., Rabagliatti R., Len O. Tuberculosis recommendations for solid organ transplant recipients and donors. Transplantation. 2018;102:S60–S65. doi: 10.1097/TP.0000000000002014. [DOI] [PubMed] [Google Scholar]
- 18.Sun H.Y. Treating tuberculosis in solid organ transplant recipients. Curr Opin Infect Dis. 2014;27:501–505. doi: 10.1097/QCO.0000000000000102. [DOI] [PubMed] [Google Scholar]
- 19.Sun H.Y., Munoz P., Torre-Cisneros J., Aguado J.M., Lattes R., Montejo M., et al. Tuberculosis in solid-organ transplant recipients: disease characteristics and outcomes in the current era. Prog Transplant. 2014;24:37–43. doi: 10.7182/pit2014398. [DOI] [PubMed] [Google Scholar]
- 20.Yehia B.R., Blumberg E.A. Mycobacterium tuberculosis infection in liver transplantation. Liver Transp. 2010;16:1129–1135. doi: 10.1002/lt.22133. [DOI] [PubMed] [Google Scholar]
- 21.López-Montes A., Gallego E., López E., Pérez J., Lorenzo I., Llamas F., et al. Treatment of tuberculosis with rifabutin in a renal transplant recipient. Am J Kidney Dis. 2004;44:e59–e63. [PubMed] [Google Scholar]
- 22.Tabarsi P., Farshidpour M., Marjani M., Baghaei P., Yousefzadeh A., Najafizadeh K., et al. Mycobacterial infection and the impact of rifabutin treatment in organ transplant recipients: a single-center study. Saudi J Kidney Dis Transpl. 2015;26:6–11. doi: 10.4103/1319-2442.148710. [DOI] [PubMed] [Google Scholar]
- 23.Concejero A., Chen C.L., Wang C.C., Wang S.H., Lin C.C., Liu Y.W., et al. Living donor liver transplantation for hepatocellular carcinoma: a single-center experience in Taiwan. Transplantation. 2008;85:398–406. doi: 10.1097/TP.0b013e3181622ff8. [DOI] [PubMed] [Google Scholar]
- 24.Wu Y.J., Lin C.C., Chang Y.M., Wang S.H., Lin Y.H., Lu H.I., et al. Computed tomography as primary screening for appraisal of pulmonary small nodules in liver transplant candidates. Transplant Proc. 2016;48:1036–1040. doi: 10.1016/j.transproceed.2015.08.046. [DOI] [PubMed] [Google Scholar]
- 25.Salvador N.G.A., Wee S.Y., Lin C.C., Wu C.C., Lu H.I., Lin T.L., et al. Clinical outcomes of tuberculosis in recipients after living donor liver transplantation. Ann Transplant. 2018;23:733–743. doi: 10.12659/AOT.911034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Concejero A.M., Yong C.C., Chen C.L., Lu H.I., Wang C.C., Wang S.H., et al. Solitary pulmonary nodule in the liver transplant candidate: importance of diagnosis and treatment. Liver Transpl. 2010;16:760–766. doi: 10.1002/lt.22066. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization. Guidelines for treatment of drugsusceptible tuberculosis and patient care, https://apps.who.int/iris/bitstream/handle/10665/255052/9789241550000-eng.pdf/; 2017 [accessed 8 January 2020].
- 28.Centers for Disease Control ROCT. Tuberculosis, https://www.cdc.gov.tw/english/info.aspx?treeid=E79C7A9E1E9B1CDF&nowtreeid=E02C24F0DACDD729&tid=143E4CE1C8F465E5/; 2017 [accessed 8 January 2020].
- 29.Wu Y.J., Lin C.C., Lin Y.H., Wang S.H., Lin T.L., Chen C.L., et al. Incidentally small pulmonary nodule in candidates for living donor liver transplantation. Ann Transplant. 2015;20:734–740. doi: 10.12659/aot.895450. [DOI] [PubMed] [Google Scholar]
- 30.Lin C.C., Chuang F.R., Lee C.H., Wang C.C., Chen Y.S., Liu Y.W., et al. The renal-sparing efficacy of basiliximab in adult living donor liver transplantation. Liver Transpl. 2005;11:1258–1264. doi: 10.1002/lt.20520. [DOI] [PubMed] [Google Scholar]
- 31.Lin Y.H., Lin C.C., Wang C.C., Wang S.H., Liu Y.W., Yong C.C., et al. The 4-week serum creatinine level predicts long-term renal dysfunction after adult living donor liver transplantation. Transplant Proc. 2012;44:772–775. doi: 10.1016/j.transproceed.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 32.Wu Y.J., Lin Y.H., Yong C.C., Li W.F., Wang S.H., Wang C.C., et al. Safe one-to-one dosage conversion from twice-daily to once-daily tacrolimus in long-term stable recipients after liver transplantation. Ann Transplant. 2016;21:30–34. doi: 10.12659/aot.895118. [DOI] [PubMed] [Google Scholar]
- 33.Hickey M.D., Quan D.J., Chin-Hong P.V., Roberts J.P. Use of rifabutin for the treatment of a latent tuberculosis infection in a patient after solid organ transplantation. Liver Transpl. 2013;19:457–461. doi: 10.1002/lt.23622. [DOI] [PubMed] [Google Scholar]
- 34.Lefeuvre S., Rebaudet S., Billaud E.M., Wyplosz B. Management of rifamycins-everolimus drug-drug interactions in a liver-transplant patient with pulmonary tuberculosis. Transpl Int. 2012;25:e120–e123. doi: 10.1111/j.1432-2277.2012.01561.x. [DOI] [PubMed] [Google Scholar]
- 35.Ngo B.T., Pascoe M., Kahn D. Drug interaction between rifampicin and sirolimus in transplant patients. Saudi J Kidney Dis Transpl. 2011;22:112–115. [PubMed] [Google Scholar]
- 36.Astellas Pharma. Prograf tacrolimus capsules tacrolimus injection (for intravenous infusion only), https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/050708s027,050709s021lbl.pdf/; 2009 [accessed 8 January 2020].
- 37.Wyeth Pharmaceuticals. Rapamune (sirolimus) oral solution and tablets, https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/021083s030,021110s038lbl.pdf/; 2021 [accessed 8 January 2020].
- 38.Cattaneo D., Merlini S., Pellegrino M., Carrara F., Zenoni S., Murgia S., et al. Therapeutic drug monitoring of sirolimus: effect of concomitant immunosuppressive therapy and optimization of drug dosing. Am J Transplant. 2004;4:1345–1351. doi: 10.1111/j.1600-6143.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 39.Chinnakotla S., Davis G.L., Vasani S., Kim P., Tomiyama K., Sanchez E., et al. Impact of sirolimus on the recurrence of hepatocellular carcinoma after liver transplantation. Liver Transpl. 2009;15:1834–1842. doi: 10.1002/lt.21953. [DOI] [PubMed] [Google Scholar]
- 40.Moini M., Schilsky M.L., Tichy E.M. Review on immunosuppression in liver transplantation. World J Hepatol. 2015;7:1355–1368. doi: 10.4254/wjh.v7.i10.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crispe I.N. Immune tolerance in liver disease. Hepatology. 2014;60:2109–2117. doi: 10.1002/hep.27254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ascha M.S., Ascha M.L., Hanouneh I.A. Management of immunosuppressant agents following liver transplantation: less is more. World J Hepatol. 2016;8:148–161. doi: 10.4254/wjh.v8.i3.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Demirkiran A., Kok A., Kwekkeboom J., Kusters J.G., Metselaar H.J., Tilanus H.W., et al. Low circulating regulatory T-cell levels after acute rejection in liver transplantation. Liver Transpl. 2006;12:277–284. doi: 10.1002/lt.20612. [DOI] [PubMed] [Google Scholar]
- 44.Levitsky J., Feng S. Tolerance in clinical liver transplantation. Hum Immunol. 2018;79:283–287. doi: 10.1016/j.humimm.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 45.Fisher R.A., Cotterell A.H., Maluf D.G., Stravitz R.T., Ashworth A., Nakatsuka M., et al. Adult living donor versus deceased donor liver transplantation: a 10-year prospective single center experience. Ann Hepatol. 2009;8:298–307. [PubMed] [Google Scholar]
- 46.Levitsky J., Goldberg D., Smith A.R., Mansfield S.A., Gillespie B.W., Merion R.M., et al. Acute rejection increases risk of graft failure and death in recent liver transplant recipients. Clin Gastroenterol Hepatol. 2017;15:584–593. doi: 10.1016/j.cgh.2016.07.035. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Londono M.C., Rimola A., O'Grady J., Sanchez-Fueyo A. Immunosuppression minimization vs. complete drug withdrawal in liver transplantation. J Hepatol. 2013;59:872–879. doi: 10.1016/j.jhep.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 48.Levitsky J., Mathew J.M., Abecassis M., Tambur A., Leventhal J., Chandrasekaran D., et al. Systemic immunoregulatory and proteogenomic effects of tacrolimus to sirolimus conversion in liver transplant recipients. Hepatology. 2013;57:239–248. doi: 10.1002/hep.25579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodríguez-Perálvarez M., Germani G., Darius T., Lerut J., Tsochatzis E., Burroughs A.K. Tacrolimus trough levels, rejection and renal impairment in liver transplantation: a systematic review and meta-analysis. Am J Transplant. 2012;12:2797–2814. doi: 10.1111/j.1600-6143.2012.04140.x. [DOI] [PubMed] [Google Scholar]
- 50.Pillai V.G., Chen C.L. Living donor liver transplantation in Taiwan-challenges beyond surgery. Hepatobiliary Surg Nutr. 2016;5:145–150. doi: 10.3978/j.issn.2304-3881.2015.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]