Abstract
Background
Patients with acute myeloid leukemia (AML) are at risk of hepatosplenic candidiasis (HSC). HSC is often associated with prolonged fever and difficulty in definitive clinical diagnosis. We aimed to explore the incidence, clinical features, image findings and outcomes of HSC among patients with AML in a tertiary hospital, Taiwan.
Methods
We did a chart review of patient data in our institute from 2009 to 2012. The diagnosis of HSC was based on risk factors, febrile symptoms and image findings.
Results
Two hundred and ninety-two patients with AML were analyzed. In total, 1051 chemotherapy sessions were administered. Eleven patients (4 males and 7 females) experienced HSC (incidence 3.8%, 95% conference interval 2.11–6.72%). Among those with HSC, the median age was 62. Eight patients developed HSC following induction or re-induction chemotherapies. Three developed HSC following consolidation chemotherapies. The median duration of severe neutropenia was 25 days (range 10–142). In all patients with HSC, multiple hypodense lesions were found in the involved organs by computed tomography scans. Lesions consistent with HSC could be identified by ultrasound in 5 out of 6 patients. Other than liver and spleen, lung was frequently (7 cases) and kidney occasionally (3 cases) involved. Four patients died within 90 days. Prolonged neutropenia was associated with mortality.
Conclusion
HSC occurred more often during induction or re-induction periods. Lungs are commonly involved and pleural effusion was frequently seen in CT scans. Pleural effusion may suggest more serious infections but its clinical relevance should be investigated in large-scale studies. Prolonged neutropenia is the only prognostic factor. Prophylaxis should be considered. In the absence of prophylaxis, we advise early image studies and prompt antifungal treatment in patients at risk for HSC.
Keywords: Acute myeloid leukemia, Hepatosplenic candidiasis, Antifungal, Neutropenia
At a glance of commentary
Scientific background on the subject
Hepatosplenic candidiasis may occur in patients with AML. The standard of care is not established due to insufficient clinical data and lack of prospective studies.
What this study adds to the field
Hepatosplenic candidiasis occurred more often during the induction phase. Lungs are commonly involved and effusions often found. The fungal infection was often manageable. Prolonged neutropenia is the only prognostic factor.
Patients with leukemia are subject to invasive fungal infections. Hepatosplenic candidiasis (HSC) is a rare type of invasive fungal infection which is often associated with prolonged fever and difficulty in definitive clinical diagnosis [1]. There are no prospective studies of HSC. Therefore, more cohort studies or case series may contribute to the understanding of disease characteristics and improvement of clinical management. To improve understanding of clinical and image features, as well as outcomes of HSC, we did a chart review in a tertiary hospital in Taiwan. The study aims to explore the risk and prognostic factors of HSC among patients with AML.
Methods
We did a chart review of patient data in our institute from 2009 to 2012. The diagnosis of acute myeloid leukemia (AML) was made according to the 2008 version of world health organization diagnostic criteria. Diagnosis of HSC was based on risk factors, febrile symptoms in conjugation with computed tomography (CT) findings. The diagnosis of invasive fungal disease (IFD) was categorized according to criteria of EORTC/MSD [2]. No patient had received prophylactic antifungal agents in the study period. The demographic data included age and gender. Clinical information collected in this study included anti-leukemia treatment modalities, status of leukemia (i.e. remission, refractory or relapse), duration of neutropenia and fever, timing to initiate antifungal agents, microbiology culture results, and the antibiotics used. Results of image studies (i.e. abdominal sonography and CT scans) were collected and the findings reviewed. In view of the subacute clinical course of HSC, the outcome was mainly analyzed by 90-day survival, a common endpoint used in similar studies [3,4]. In addition, a secondary outcome endpoint, overall survival was included in analysis.
Most of the analysis in this study was descriptive statistics. For comparison of numerical variables, non-parametric Mann–Whitney's U-test was used. For comparison of categorial variables, Fisher's exact test was used. In all analyses, p < 0.05 was considered significant statistically. The 95% conference interval of incidence was calculated by the Poisson process, as described in literature [5].
Results
Two hundred and ninety-two patients with AML were analyzed. In total, 1051 chemotherapy sessions were administered. Eleven patients (4 males and 7 females) experienced HSC (incidence 3.8%, 95% conference interval 2.11–6.72%). According to the EORTC/MSD definitions [2], three patients had proven (2 with candidemia and 1 diagnosis by liver biopsy) and the other 8 patients possible invasive fungal disease. All patients presented with fever. In retrospective review of medical records, patients did not report abdominal pain and physical examinations did not revealed right upper quadrant tenderness or knocking pain. Their median age was 62 (range 32–74) years old. HSC developed following induction chemotherapy in 5, re-induction chemotherapy in 3 and consolidation in 3 patients. The chemotherapy regimens included daunorubicin + cytarabine, high dose cytarabine, fludarabine + cytarabine + G-CSF and mitoxantrone + etoposide + cytarabine. The details of chemotherapies were summarized in Table 1. All patients had severe neutropenia, defined as absolute neutrophil count less than 0.5 × 109/L, prior to HSC. No other risk factors such as corticosteroids, T cell suppressants or primary immunodeficiencies were identified in any patient.
Table 1.
Demographic and clinical features of hepatosplenic candidiasis among patients with acute myeloid leukemia.
| Case No | Age (years) | Sex | Ultrasound lesion | Pleural effusion | Blood culture | AML status | Neutropenia duration (days) | Antifungal treatment results | Final outcome | OS (days) | Involved organs | Time to antifungal therapy (days) | CRP mg/L |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 51 | F | ND | (−) | CR | 13 | successful | death | 201 | H,S,L | 5 | 93.37 | |
| 2 | 67 | F | 1 cm | (+) | NR | 21 | successful | death | 58 | H | 9 | 248 | |
| 3 | 62 | M | ND | (−) | CR | 11 | successful | death | 245 | H,L | 5 | ND | |
| 4 | 32 | F | ND | (+) | NR | 142 | failure | death | 10 | H,L | 10 | 23 | |
| 5 | 34 | F | 1.6 cm | (+) | NR | 128 | successful | death | 90 | H,S | 2 | 225 | |
| 6 | 63 | M | ND | (+) | C. tropicalis | CR | 10 | successful | death | 318 | H,S,L,K | 9 | 321 |
| 7 | 74 | F | 3.9 cm | (−) | NR | 18 | successful | death | 169 | H,S | 19 | 89 | |
| 8 | 64 | M | Negative | (+) | C. tropicalis | NR | 27 | successful | death | 273 | S,K | 15 | 173 |
| 9 | 59 | F | 1.1 cm | (−) | NR | 25 | successful | death | 301 | H,S,L,K | 18 | 144 | |
| 10 | 36 | F | 1.6 cm | (−) | NR | 72 | successful | death | 72 | H,S,L | 2 | 158 | |
| 11 | 74 | M | ND | (−) | NR | 32 | successful | alive | 1470+ | H,L | 21 | 38.3 |
Abbreviations: AML: acute myeloid leukemia; ND: not done; OS: overall survival; CRP: C-reactive protein; CR: complete remission; NR: not remission.
Involved organs: H: liver; S: spleen; L: lung; K: Kidney.
The median duration of severe neutropenia was 25 days (range 10–142 days).
All patients received computed tomography (CT) scans. In all patients, multiple hypodense lesions were found in the involved organs. In addition to fungal lesions, pleural effusion was found in 5 patients (45.5%) in CT scans. The effusion was not studied in any patient. Other than liver and spleen, lung was frequently (7 cases) and kidney was occasionally (3 cases) involved. Abdominal ultrasound was done in 6 patients. Lesions which were consistent with HSC could be identified in 5 patients. However, most such lesions were interpreted as cysts or non-specific nodules and only lesions larger than 1 cm (range 1.0–3.9 cm) were identified by ultrasonography.
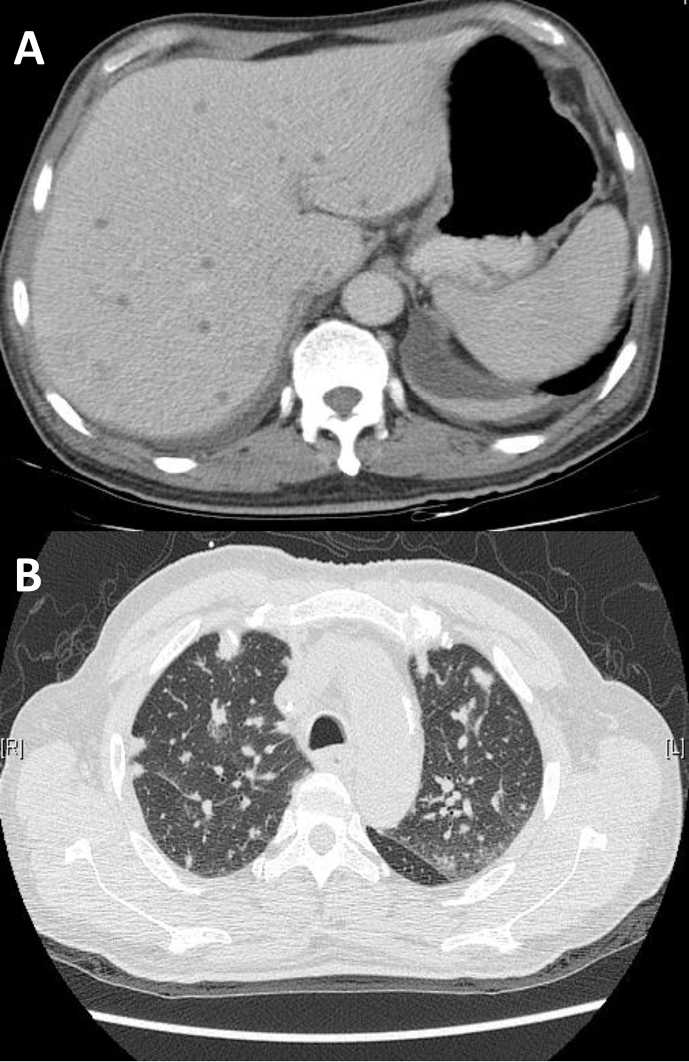
The typical CT images of hepatic and pulmonary involvement were shown [Fig. 1A]. Hepatic involvement presented with multiple hypodense microlesions without enhancement. Pulmonary involvement presented with multiple nodular lesions and scattered tree-in-bud pattens and focal consolidations [Fig. 1B].
Fig. 1.
(A) The typical CT findings of hepatic involvement of HSC are multiple hypodense microlesions without enhancement. (B) The typical CT findings of pulmonary involvement of HSC are multiple nodular lesions and scattered tree-in-bud pattens.
In blood biochemical assays, C-reactive protein (CRP) was checked in 10 patients, all of them had elevated levels and the median level was 151 mg/L. Serum AST and ALT levels were available for all 11 patients, 6 (54.5%) patients had elevated AST, and 7 (63.6%) had elevated ALT. Serum alkaline phosphatase was elevated in 4 of 6 patients (66.7%) with available data. Serum γ-GT was elevated in 3 of 5 patients (60%) with available data. Serum bilirubin was elevated in 2 of 7 patients (28.6%) with available data.
Fungus was isolated by blood cultures in two patients, both had Candida tropicalis.
The offending species of fungus was not identified in any other patient. A tissue culture obtained by a hepatic lesion biopsy revealed no growth of microorganisms in one patient but invasive fungus infection was confirmed by the pathology report (case No 2 in Table 1). Co-infections were confirmed in 4 patients. (Staphylococcus haemolyticus in case 1; Staphylococcus aureus in case 3; Acinetobacter baumanii in case 4; Staphylococcus epidermidis and Aeromonas sobria in case 6). The demographic, clinical features and image findings were summarized in Table 1.
Various antifungal agents were used for HSC treatment in the present study. Among 11 patients, 5 were initially treated with amphotericin-B and 6 with caspofungin. Antifungal treatment was changed in 9 patients. Overall, treatment was successful in 10 patients, whose fever subsided and patients were discharged. The antifungal agents were listed in Table 1.
All patients had received antibiotics before empirical antifungal treatment. The median time from fever episodes to initiation of antifungal agents was 9 days (range 2–21 days). The median survival after confirmed diagnosis of HSC (by CT scan) was 201 days. As of writing of this article, only one patient is still alive. Four patients died within 90 days of confirmed HSC. Analysis of factors contributing to 90-day mortality was summarized as Table 2. In brief, mortality was associated with longer neutropenia duration. Such associated was statistically significant (p = 0.0424) even with a small case number. Ninety-day mortality was not associated with age, gender, status of remission, progression to candidemia, presence of pleural effusion or time to initiate antifungal treatment.
Table 2.
Analysis of factors associated with survival among patients with acute myeloid leukemia.
| Death≦90 days (n = 4) | Alive>90 days (n = 7) | p value | |
|---|---|---|---|
| Median age in years (range) | 35 (32–67) | 63 (51–74) | 0.1091 |
| Gender (M/F) | 0/4 | 4/3 | 0.1939 |
| Remission | 0 | 3 | 0.2364 |
| Neutropenia duration (days) | 100 (21–142) | 18 (10–32) | 0.0424 |
| Time to antifungal treatment (days)a | 5.5 (2–10) | 15 (5–21) | 0.191 |
| With pleural effusion | 3 | 2 | 0.179 |
| Candidemia | 0 | 2 | 0.3818 |
From fever to initiation of antifungal treatment.
Discussion
HSC is an uncommon type of invasive fungal infection with an incidence of 3–29% in patients with acute leukemia [1,6]. In the two articles from Taiwan. Ma et al. reported an incidence of 9.8% among acute leukemias and Chen et al. reported an incidence of 4.3 per 100 patient-years for acute lymphoblastic leukemia and 3.6 per 100 patient-years for AML [1,3]. The incidence of 3.8% for AML in the present study appears to be comparable to other studies. HSC has been recognized since the 1980's [[7], [8], [9]], but the standard of care remains unestablished in the absence of randomized control trials [10]. However, the clinical features of HSC can be understood from studies of large cohorts or case series, including the present study [1,[9], [10], [11], [12], [13], [14], [15], [16]]. It should be noted that the present study is limited by retrospective nature and small case number. However, the results may still lay a foundation for subsequent development of optimal.
Risk factors of hepatosplenic candidiasis
The risk factors of HSC, as in other invasive fungal infections, are prolonged neutropenia, use of corticosteroids, other T cell suppressants or primary immunodeficiencies [2]. Neutropenia is common in hematology patients receiving intensive chemotherapies. As a result, HSC occurs in both AML and acute lymphoblastic leukemia [1,9,13,16]. For AML, risk for invasive fungal infection is more significant during induction and re-induction periods, in which leukemia is not in remission [17]. In the present study, much more patients (N = 8) developed HSC in the non-remission (i.e. receiving induction or re-induction chemotherapy) than patients in the remission phase (i.e. receiving consolidation chemotherapy). It demonstrated that HSC, like other invasive fungal infections, occurs more often in induction periods. Such difference in risk may result from the period of neutropenia, as neutropenia may date back to the time before initiating chemotherapy in patients with new diagnosis, refractory or relapsed AML. It is important to note that none of our patients received antifungal prophylaxis in the study period. In recent years, antifungal prophylaxis given during induction chemotherapy has significantly reduced the incidence of invasive fungal infection for patients with AML [17,18]. If prophylaxis becomes a standard practice, HSC may become a truly rare complication in AML.
Partly due to its rarity, HSC can be overlooked in clinical practice [8]. As the yield of laboratory microbiology studies is low, early diagnosis mainly relies on image studies [7,11,19,20].
Image findings of hepatosplenic candidiasis
Sonography is commonly the first image study in patients with suspected abscess or fever of unknown origins. For HSC, the sensitivity of sonography is known to be inadequate for accurate diagnosis [11]. The CT scan has been proved to be the most valuable diagnostic tool in image diagnosis of HSC [7]. The typical CT findings were multiple hypodense lesions in the liver. Such lesions were not enhanced by contrast medium in our experience. Although the sensitivity of detecting such lesion by CT scans is high, hypodense lesions may result from diverse other etiologies such as cysts, metastases, focal fat, hamartomas and abscesses [21]. It is important to correlate with clinical information for accurate diagnosis and proper treatment. In the lungs, the CT findings were more variable, ranging from consolidation, cavitation, infiltration of the tree-in-bud pattern and multiple small nodules [22]. Multiple pulmonary nodular infiltrates were the most common finding in the present study. Such findings were consistent with previously published literature [22]. Nevertheless, such pulmonary infiltrates are not specific for fungal infections. It is important to correlate with clinical information and image findings from other involved sites. In this regard, the CT scan has an additional benefit of evaluating multiple solid organs, such as liver, spleen, lung and kidney. For abdominal ultrasound, in the present study, suspicious lesions could be identified by sonography in 5 of 6 patients. As physicians performing sonographic examinations (all gastroenterologists in our hospital) were not aware of patients' clinical susceptibility of HSC, these lesions were often interpreted as non-specific cysts or nodules. If the physicians are highly alert of HSC, the sensitivity of sonography can be up to 83%. The caveat of sonographic diagnosis is the lesion size. In our experience, lesions of HSC should be at least 1 cm in order to be detected by sonography. The utility of F18-FDG PET/CT scans has been reported in recent studies and may become a useful image tool for diagnosis of HSC in the future [23,24].
Laboratory data in hepatosplenic candidiasis
No serum markers are specific in diagnosis of HSC. In some other studies, serum levels of transaminase or alkaline phosphatase are quite sensitive and therefore may serve as a screening or adjunctive diagnostic tool [1,8,11,12]. In our study, more than half patients were found with elevated hepatobiliary markers, such as AST, ALT, γ-GT, and alkaline phosphatase. These hepatobiliary markers may provide clinical clues of HSC, although none of such markers were sensitive or specific enough in supporting or excluding diagnosis of HSC. On the other hand, serum levels of CRP were elevated in all tested patients. Such result was identical to previous studies [11]. However, since all subjects were febrile patients, elevated CRP levels might not provide any additional diagnostic utility other than confirming the inflammatory response associated with fever. Based on our experience, serum parameters are not clinically relevant indicators for diagnosis of HSC. In our opinion, diagnosis of HSC relies on clinical awareness and timely image studies.
Sites of infection and outcome in hepatosplenic candidiasis
HSC may spread beyond liver and spleen. Kidney is commonly involved [7,13,15]. In chronic disseminated candidiasis, infection could spread to lung, brain and eyes [10]. Interestingly, involvement of lung is as common as spleen in the present study. Such a dominant trend of pulmonary candidiasis has not been found in previous series. In addition, HSC was associated with pleural effusion in about half patients. Although the nature of effusion was not studied in any patient, it is likely to be caused by HSC, at least in some patients. To our knowledge, pleural effusion has not been mentioned in any previous study of HSC, including the studies focused on features of CT scans [7].
In five subjects with pleural effusion, only two had pulmonary candidiasis by CT scan surveys. Therefore, pleural effusion was not directly related to pulmonary involvement of HSC in all patients. On the other hand, HSC progressed to candidemia in 2 patients (18%). Both patients were infected by C. tropicalis and both had pleural effusion. It appears to show that pleural effusion is indicative of more severe infection and potentially worse outcome. However, with our small case number, the mortality rate does not reach significance between patients with and without pleural effusion.
The long-term outcome of patients in the current study is very poor [9]. Considering that only 3 patients had remission prior to HSC, such poor outcome is more likely related to leukemia rather than HSC. The median survival in the present study was 201 days. Such a long period is not likely caused directly by HSC or any other acute infectious complications. Therefore, we had analyzed the possible prognostic factors using the cutoff duration of 90 days, which in potential was more directly related to HSC. Such a cutoff duration was also used in previous literature [3,4]. Although only 4 patients died within 90 days, some factors showed their clinical relevance. The duration of neutropenia was a statistically significant factor associated with 90-day mortality. The poor prognosis with prolonged neutropenia has been found in other studies [10,14]. None of other factors, including age, gender, status of remission, presence of candidemia or pleural effusion, was significantly associated with outcome. In supportive care, we believe prompt treatment with effective antifungal agents will be helpful in management of HSC. However, the time from febrile episodes to initiation of antifungal agents was not related to outcome in our small series. It should be noted that the median time was 9 days from fever to use of antifungal agents. Since empirical antifungal treatment is a standard practice [25,26] in management of neutropenic fever, antifungal drugs were not delayed in the practice of most hematologists, as in the cases of the present study.
Treatment of hepatosplenic candidiasis
Treatment of HSC is generally supportive. Various antifungal agents, including amphotericin-B, echinocandins, fluconazole and voriconazole, had been used with success. Invasive approaches were used only for diagnostic purposes and probably unsuitable for drainage or debridement. If possible, the duration of neutropenia should be shortened (e.g. by G-CSF). It is reasonable to speculate that in an era of antifungal prophylaxis, incidence of HSC may be significantly decreased. Therefore, antifungal prophylaxis should be administered in patients receiving induction or re-induction chemotherapies.
In summary, our study revealed the incidence was 3.8% in patients with AML. Patients were more susceptible during the induction or re-induction periods. Although a CT scan is the keystone in establishing diagnosis, abdominal sonography could be useful in case of sufficient clinical correlation. Kidney and lungs may be involved in addition to liver and spleen. Progression to candidemia is uncommon but pleural effusion was frequently seen in subjects with HSC. Pleural effusion may represent more extensive infections but its significance requires further investigations. Prolonged neutropenia is a poor prognosis factor for HSC. We advise early image studies and prompt antifungal treatment in patients at risk. In addition, antifungal prophylaxis may significantly reduce such complications in the future.
Limit of this study
This study is limited by its retrospective nature and small case number. No data from the control group (i.e. AML patients without HSC) was available for comparison. Hence, the risk factors of HSC cannot be examined.
Conflicts of interest
The author and co-authors declared they have no conflicts of interest.
Acknowledgement
This study was supportive by grants of Chang Gung Memorial Hospital (CMRPC3G1701 and CMRPG3D1221).
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Ma M.C., Wang M.C., Pei S.N., Kuo C.Y. Hepatosplenic fungal infection in adult patients with acute leukemia. Chang Gung Med J. 2008;31:74–80. [PubMed] [Google Scholar]
- 2.De Pauw B., Walsh T.J., Donnelly J.P., Stevens D.A., Edwards J.E., Calandra T., et al. Revised definitions of invasive fungal disease from the European organization for research and treatment of cancer/invasive fungal infections cooperative group and the national institute of allergy and infectious diseases mycoses study group (EORTC/MSG) consensus group. Clin Infect Dis. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen C.Y., Cheng A., Tien F.M., Lee P.C., Tien H.F., Sheng W.H., et al. Chronic disseminated candidiasis manifesting as hepatosplenic abscesses among patients with hematological malignancies. BMC Infect Dis. 2019;19:635. doi: 10.1186/s12879-019-4260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anttila V.J., Elonen E., Nordling S., Sivonen A., Ruutu T., Ruutu P. Hepatosplenic candidiasis in patients with acute leukemia: incidence and prognostic implications. Clin Infect Dis. 1997;24:375–380. doi: 10.1093/clinids/24.3.375. [DOI] [PubMed] [Google Scholar]
- 5.Rothman K.J., Greenland S. 2nd ed. Lippincott Williams & Wilkins; United States: 1998. Modern epidemiology. [Google Scholar]
- 6.Rammaert B., Desjardins A., Lortholary O. New insights into hepatosplenic candidosis, a manifestation of chronic disseminated candidosis. Mycoses. 2012;55:e74–e84. doi: 10.1111/j.1439-0507.2012.02182.x. [DOI] [PubMed] [Google Scholar]
- 7.Shirkhoda A. CT findings in hepatosplenic and renal candidiasis. J Comput Assist Tomogr. 1987;11:795–798. doi: 10.1097/00004728-198709000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Fenaux P., Lemaitre L., Ajana F., Colcher-Plantier I., Jouet J.P., Bauters F. Hepatosplenic candidiasis: an overlooked cause of prolonged fever during recovery from an episode of neutropenia. Nouv Rev Fr Hematol. 1989;31:45–49. [PubMed] [Google Scholar]
- 9.Anttila V.J., Elonen E., Nordling S., Sivonen A., Ruutu T., Ruutu P. Hepatosplenic candidiasis in patients with acute leukemia: incidence and prognostic implications. Clin Infect Dis. 1997;24:375–380. doi: 10.1093/clinids/24.3.375. [DOI] [PubMed] [Google Scholar]
- 10.Della Pepa R., Picardi M., Sora F., Stamouli M., Busca A., Candoni A., et al. Successful management of chronic disseminated candidiasis in hematologic patients treated with high-dose liposomal amphotericin B: a retrospective study of the SEIFEM registry. Support Care Canc. 2016;24:3839–3845. doi: 10.1007/s00520-016-3208-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anttila V.J., Ruutu P., Bondestam S., Jansson S.E., Nordling S., Farkkila M., et al. Hepatosplenic yeast infection in patients with acute leukemia: a diagnostic problem. Clin Infect Dis. 1994;18:979–981. doi: 10.1093/clinids/18.6.979. [DOI] [PubMed] [Google Scholar]
- 12.Blade J., Lopez-Guillermo A., Rozman C., Granena A., Bruguera M., Bordas J., et al. Chronic systemic candidiasis in acute leukemia. Ann Hematol. 1992;64:240–244. doi: 10.1007/BF01738303. [DOI] [PubMed] [Google Scholar]
- 13.Chen C.Y., Chen Y.C., Tang J.L., Yao M., Huang S.Y., Tsai W., et al. Hepatosplenic fungal infection in patients with acute leukemia in Taiwan: incidence, treatment, and prognosis. Ann Hematol. 2003;82:93–97. doi: 10.1007/s00277-002-0588-7. [DOI] [PubMed] [Google Scholar]
- 14.De Castro N., Mazoyer E., Porcher R., Raffoux E., Suarez F., Ribaud P., et al. Hepatosplenic candidiasis in the era of new antifungal drugs: a study in Paris 2000-2007. Clin Microbiol Infect. 2012;18:E185–E187. doi: 10.1111/j.1469-0691.2012.03819.x. [DOI] [PubMed] [Google Scholar]
- 15.Kauffman C.A., Bradley S.F., Ross S.C., Weber D.R. Hepatosplenic candidiasis: successful treatment with fluconazole. Am J Med. 1991;91:137–141. doi: 10.1016/0002-9343(91)90005-i. [DOI] [PubMed] [Google Scholar]
- 16.Sallah S., Semelka R.C., Wehbie R., Sallah W., Nguyen N.P., Vos P. Hepatosplenic candidiasis in patients with acute leukaemia. Br J Haematol. 1999;106:697–701. doi: 10.1046/j.1365-2141.1999.01592.x. [DOI] [PubMed] [Google Scholar]
- 17.Ruping M.J., Vehreschild J.J., Cornely O.A. Patients at high risk of invasive fungal infections: when and how to treat. Drugs. 2008;68:1941–1962. doi: 10.2165/00003495-200868140-00002. [DOI] [PubMed] [Google Scholar]
- 18.Cornely O.A., Maertens J., Winston D.J., Perfect J., Ullmann A.J., Walsh T.J., et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356:348–359. doi: 10.1056/NEJMoa061094. [DOI] [PubMed] [Google Scholar]
- 19.Gorg C., Weide R., Schwerk W.B., Koppler H., Havemann K. Ultrasound evaluation of hepatic and splenic microabscesses in the immunocompromised patient: sonographic patterns, differential diagnosis, and follow-up. J Clin Ultrasound. 1994;22:525–529. doi: 10.1002/jcu.1870220902. [DOI] [PubMed] [Google Scholar]
- 20.Albano D., Bosio G., Bertoli M., Petrilli G., Bertagna F. Hepatosplenic candidiasis detected by (18)F-FDG-PET/CT. Asia Ocean J Nucl Med Biol. 2016;4:106–108. doi: 10.7508/aojnmb.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gore R.M., Thakrar K.H., Wenzke D.R., Newmark G.M., Mehta U.K., Berlin J.W. That liver lesion on MDCT in the oncology patient: is it important? Cancer Imaging. 2012;12:373–384. doi: 10.1102/1470-7330.2012.9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franquet T., Muller N.L., Lee K.S., Oikonomou A., Flint J.D. Pulmonary candidiasis after hematopoietic stem cell transplantation: thin-section CT findings. Radiology. 2005;236:332–337. doi: 10.1148/radiol.2361031772. [DOI] [PubMed] [Google Scholar]
- 23.Teyton P., Baillet G., Hindie E., Filmont J.E., Sarandi F., Toubert M.E., et al. Hepatosplenic candidiasis imaged with F-18 FDG PET/CT. Clin Nucl Med. 2009;34:439–440. doi: 10.1097/RLU.0b013e3181a7cfba. [DOI] [PubMed] [Google Scholar]
- 24.Jennane S., Eddou H., Mahtat E.M., Konopacki J., Souleau B., Malfuson J.V., et al. Contribution of PET/CT for the management of hepatosplenic candidiasis in hematology. Med Mal Infect. 2014;44:281–283. doi: 10.1016/j.medmal.2014.03.008. French. [DOI] [PubMed] [Google Scholar]
- 25.Klastersky J., Paesmans M. Antifungal therapy in febrile neutropenic patients: review of treatment choices and strategies for aspergillar infection. Support Care Cancer. 2007;15:137–141. doi: 10.1007/s00520-006-0137-3. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg E., Gafter-Gvili A., Robenshtok E., Leibovici L., Paul M. Empirical antifungal therapy for patients with neutropenia and persistent fever: systematic review and meta-analysis. Eur J Cancer. 2008;44:2192–2203. doi: 10.1016/j.ejca.2008.06.040. [DOI] [PubMed] [Google Scholar]