Abstract
Background
Non-bismuth containing quadruple therapy (concomitant therapy) is an alternative treatment for Helicobacter pylori (H. pylori) eradication with increasing clarithromycin-resistant strains over times. This study compared the efficacies of non-bismuth containing quadruple therapy (concomitant therapy) in the treatment of first-line anti-Helicobacter Pylori between two time intervals (January 2013 to June 2014 and June 2016 to December 2017).
Methods
H. pylori-infected patients were recruited in the intention-to-treat (ITT analysis) and divided into EACM-A group (enrolled from January 2013 to June 2014, N = 98) and EACM-B group (enrolled from June 2016 to December 2017, N = 99). Patients were prescribed with 7-day esomeprazole 40 mg bid., clarithromycin 500 mg bid., amoxicillin 1 g bid. and metronidazole 500 mg bid. Ninety patients and 93 patients were analyzed in the per protocol (PP) analysis (8 and 6 patients lost follow-up in each group). Urea breath tests were performed 4–8 weeks thereafter.
Results
The eradication rates for EACM-A and EACM-B groups were 87.8% (95% confidence interval [CI] = 79.7%–93.5%) and 84.8% (95% CI = 76.2%–91.2%) (p = 0.55) in intention-to-treat (ITT) analysis; 95.6% (95% CI = 89.1%–98.8%) and 90.3% (95% CI = 82.4%–95.5%) (p = 0.17) in per protocol (PP) analysis. The adverse event rates were 16.7% vs. 10.8% in the 2 groups (p = 0. 0.24). The antibiotic resistance rates between the 2 groups were amoxicillin (0%), tetracycline (0%); clarithromycin (11.8% vs. 17.8%, p = 0.46); metronidazole (32.4% vs. 33.3%, p = 0.93) and levofloxacin (14.7% vs. 37.8%, p = 0.02).
Conclusion
The success rate of 7-days concomitant therapy encountered an approximately 5% decrease across 4-year time interval (2013–2017) with the changes of clarithromycin resistance from 11.8% to 17.8% in Taiwan.
Keywords: Helicobacter pylori eradication, Concomitant therapy, Antibiotic resistances
At a glance of commentary
Scientific background on the subject
Non-bismuth containing quadruple therapy (concomitant therapy) is an alternative treatment for Helicobacter pylori (H. pylori) eradication with increasing clarithromycin-resistant strains over times.
What this study adds to the field
This study reported the success rate of 7-days concomitant therapy encountered an approximately 5% decrease across 4-year time interval (2013-2017) with the changes of clarithromycin resistance from 11.8% to 17.8% in Taiwan. Provided that the local clarithromycin resistance rate has exceeded the bound of 15% and may have further increased up to now, prolongation of concomitant therapy to 10-14 days appears to be a considerable option. A change of treatment regimens such as bismuth quadruple therapy, high dose dual therapy, hybrid or reverse hybrid therapy should be also considered.
Helicobacter pylori infection is linked to peptic ulcer disease and gastric malignancies, and choosing an efficient treatment is vital for successful H. pylori eradication [[1], [2], [3], [4]]. Standard triple therapy, consisting of a proton pump inhibitor (PPI), amoxicillin, plus clarithromycin, has been abandoned as the first-line treatment in many other countries due to the increasing clarithromycin-resistant strains due to the decline of success rates to less than 80% [[5], [6], [7], [8]]. Consensus and guidelines recommends abandoning triple therapy when the clarithromycin resistance rate in the region is more than 15% [1,9,10].
Fortunately, many alternative therapies are available which provide eradication rates of over or near 90%, including non-bismuth containing quadruple therapy (concomitant therapy), high dose dual therapy, and hybrid or reverse hybrid therapy [8,[11], [12], [13], [14], [15], [16]]. The success rate of concomitant therapy was much better than standard triple therapy but was affected by the dual clarithromycin and metronidazole resistances [[8], [9], [10], [11], [12], [13], [14], [15], [16], [17]]. The reported primary resistance rates of clarithromycin and metronidazole in Taiwan up to 2012 were 11.2% and 25.7% respectively, and dual clarithromycin and metronidazole resistance was 4.3%, but also with considerable variation among different districts reported [18,19]. With the reported increase of antibiotic resistance rates from studies from all areas of the world, it is rational assuming the increasing antibiotic resistances can affect the success rates effect of H. pylori eradication over times. In this study, we aimed to compare the efficacies of concomitant therapy in the treatment of first-line anti-Helicobacter Pylori between two time intervals (January 2013 to June 2014 and June 2016 to December 2017) and the clinical factors affecting the treatment success.
Material and methods
Ethics statements
The data collection in this study is based on reviewing computerized medical charts. This study was approved by both the Institutional Review Board and Ethics Committee of Chang Gung Memorial Hospital, Taiwan Permitted number 202001193B0. The Ethics Committee waived the requirement for informed consent, and each patient's medical records were anonymized and deidentified prior to access. All patients provided their written informed consent for endoscopic examinations before the procedure. None of our patients belonged to the minors/children age groups. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution's human research committee.
Patients
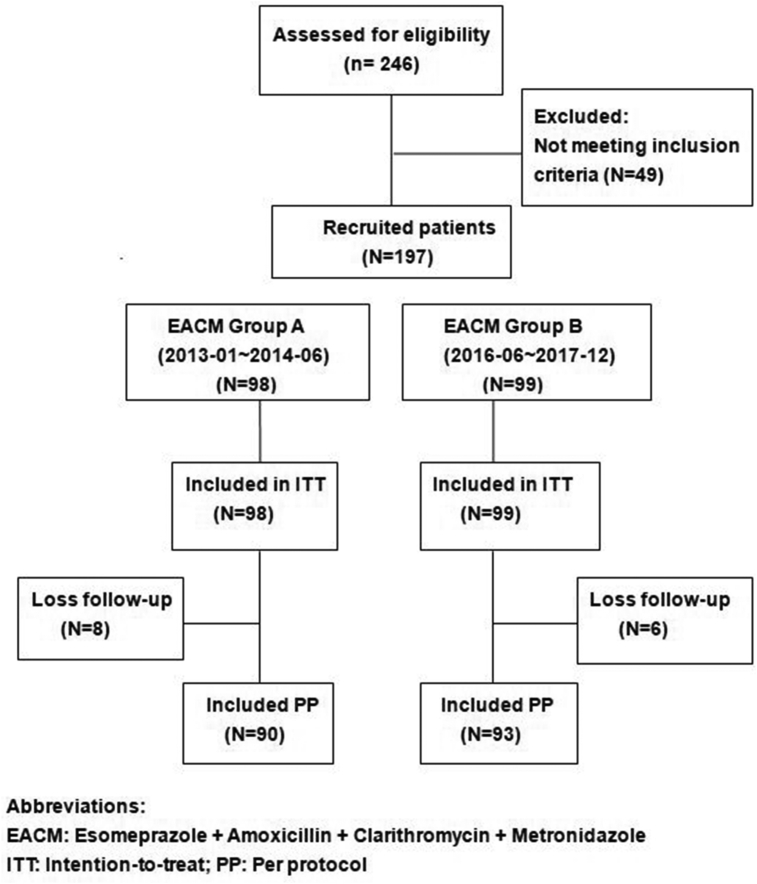
A total of 246 patients were treated with concomitant therapy as first line H. pylori eradication regimen in naive infected subjects aged eighteen or older from January 2013 to December 2017 at Chang Gung Memorial Hospital in Kaohsiung. After excluding 46 patients who were prescribed lansoprazole, pantoprazole as the proton-pump inhibitors (PPI) among the four concomitant drugs, we enrolled a total of 197 [Fig. 1]. The reason was to standardize PPI to only esomeprazole to avoid potential effect of different potencies of PPIs. The patients all underwent endoscopy examinations, and biopsies of gastric mucosa were taken from the antrum and gastric body for rapid urease test and culture. Patients with any two positive results following rapid urease test, histology, and/or culture confirmed the presence of H. pylori. Those who had taken antibiotics, bismuth, PPI within four weeks, were allergic to the medications used, had history of previous gastric surgery or serious concomitant illness, currently pregnant, or those who refused to participate were excluded from study.
Fig. 1.
Disposition of patients.
All 197 eligible patients included from January 2013 to December 2017 were treated with 7-day non-bismuth quadruple therapy (Esomeprazole 40 mg BID, amoxicillin 1 gm BID, clarithromycin 500 mg BID, and metronidazole 500 mg BID; EACM). They were divided into two groups, 1.5 years' duration in each group as EACM-A (enrolled from January 2013 to June 2014, N = 98) and EACM-B (enrolled from June 2016 to December 2017, N = 99).
Definition
Drug compliance was assessed by follow-up on the 8th day of treatment by reviewing any remaining medications. When the patients failed to finish at least 80% of the medications, they were defined as poor compliance [20,21]. Successful eradication of H. pylori was defined by a negative urea breath test followed-up four to eight weeks after treatment [22].
Outcomes
The primary endpoint of our study was the successful eradication of H. pylori. We also conducted additional analyses on adverse events during the therapies.
Diagnosis of H. pylori infection
Rapid urease test
The rapid urease test by using previous methodology reported in our studies.8,11, 13 One biopsy specimen was taken from the antrum and corpus and placed straightaway in 1 mL of a10% solution of urea in deionized water (pH 6.8) and added two drops of 1% phenol red solution. After this, it was incubated at 37 °C up to 2 h. If the yellowish color around the area of the specimen changed to pink color in the following 2 h, this was considered as a positive test.
Urea breath test
The urea breath test was also performed according to our previous studies with the cut-off value was set at 4.8% of d13CO2 [8,11,13].
Culture and antimicrobial resistance
All stock cultures were preserved at −80 °C in Brucella broth (Becton, Dickinson and Company, Franklin Lakes, NI, USA) augmented with 20% glycerol (Sigma Chem. Co., St. Louis, MO, USA) in our laboratory. The susceptibility was tested by using the E-test (AB Biodisck, Solna, Sweden). EUCAST breakpoints defined resistance were as follows: minimal inhibitory concentration (MIC) value of ≥0.5 for amoxicillin, ≥1 for clarithromycin, ≥1 for levofloxacin, ≥4 for tetracycline, and ≥8 mg/L for metronidazole, respectively.
Statistical analysis
The eradication rates, adverse events, patient compliance, and antibiotic resistance between the two groups were compared by using the χ2 test with or without Yates correction for stability and Fisher's exact test when appropriate. Information on eradication rates in both ITT and PP studies were assessed, and patients with unknown outcome were counted as treatment failures. A p-value less than 0.05 was considered statistically significant. Finally, factors influencing treatment response were analyzed by logistic regressing analysis.
Results
The demographic data
Figure 1 shows the patient flowchart. A total of 197 patients, ninety-eight in EACM-A and ninety-nine in EACM-B, were included in the intention-to-treat (ITT) analysis. Fourteen patients were lost during follow-up (eight in EACM-A and six in EACM-B), resulting in 90 for EACM-A group and 93 for EACM-B group in the per protocol (PP) study. The demographic data and endoscopic appearance of the two patient groups are presented in [Table 1].
Table 1.
Demographic data and endoscopic appearance of two patient groups.
| Characteristics | Group A (n = 90) | Group B (n = 93) | p-value |
|---|---|---|---|
| Age (year) (mean ± SD) | 48.1 ± 11.5 | 54.8 ± 13.4 | 0.26 |
| Gender (male/female) | 44/46 | 50/43 | 0.51 |
| Smoking | 14 | 14 | 0.93 |
| Alcohol drinking | 19 | 23 | 0.56 |
| Previous history of peptic ulcer | 14 (15.6) | 4 (4.3) | 0.01 |
| Endoscopic Findings | 0.05 | ||
| Gastritis | 32 | 47 | |
| Gastric ulcer | 21 | 24 | |
| Duodenal ulcer | 30 | 25 | |
| Gastric and duodenal ulcer | 7 | 7 | |
Outcome
The eradication rates in groups EACM-A and EACM-B are shown in [Table 2]. In the ITT analysis, the eradication rates were 87.8% (95% CI = 79.6%–93.5%) vs. 84.8% (95% CI = 76.2%–91.2%) (p = 0.553) in groups A and B, and in the PP analysis they were 95.6% (95% CI = 89.1%–98.8%) vs. 90.3% (95% CI = 82.4%–95.5%) (p = 0.168).
Table 2.
The major outcomes of two period's groups.
| Eradication rate |
|||
|---|---|---|---|
| Group A (n = 90) | Group B (n = 93) | p-value | |
| Intention-to-treata | 87.8% (86/98) | 84.8% (84/99) | 0.55 |
| Per-protocol | 95.6% (86/90) | 90.3% (84/93) | 0.17 |
| Adverse events | 16.7% (15/90) | 10.8% (10/93) | 0.24 |
| Compliance | 100% (90/90) | 100% (93/93) | – |
The eradication rates for Group A and Group B were 87.8% (95% confidence interval [CI] = 79.64%–93.54%) and 84.8% (95% CI = 76.19%–91.23%) (p = 0.553) in intention-to-treat (ITT) analysis; 95.6% (95% CI = 89.07%–98.80%) and 90.3%(95% CI = 82.40%–95.46%) (p = 0.168) in per protocol (PP) analysis.
In this analysis, patients with unknown outcome are counted as treatment failures.
Adverse events and complications
The adverse event rates were similar in the two groups (16.7% in EACM-A and 10.8% in EACM-B, p = 0.244) [Table 2] The details of all adverse events were listed in [Table 3] which were mild and tolerated by the patients. Both groups had good drug compliances (100%).
Table 3.
Adverse events of two groups.
| Adverse event | Group A (n = 90) | Group B (n = 93) | p-value |
|---|---|---|---|
| Abdominal pain | 4 (4.4) | 4 (4.3) | 0.96 |
| Constipation | 0 | 0 | – |
| Diarrhea | 8 (8.9) | 1 (1.1) | 0.02 |
| Dizziness | 3 (3.3) | 1 (1.1) | 0.30 |
| Headache | 4 (4.4) | 2 (2.2) | 0.38 |
| Nausea/vomiting | 8 (8.9) | 3 (3.2) | 0.11 |
| Skin rash | 0 | 0 | – |
Antibiotic resistance and H. pylori eradication
The culture and susceptibility data was available in 79 patients (34 in EACM-A and 45 in EACM-B). The antibiotic resistance rates between the 2 groups were amoxicillin (0%), tetracycline (0%); clarithromycin (11.8% vs. 17.8%, p = 0.46); metronidazole (32.4% vs. 33.3%, p = 0.93) and levofloxacin (14.7% vs. 37.8%, p = 0.02). The changes in antibiotic resistance between the two groups were shown in [Table 4]. Levofloxacin resistance had increased from 14.7% to 37.8% (p = 0.02) in group EACM-B. On the other hand, clarithromycin resistance also changed from 11.8% to 17.8% (p = 0.46) but metronidazole resistances were 32.4% and 33.3% between the two groups (p = 0.93) [Table 4].
Table 4.
Antibiotics resistance between two groups.
| Group A (n = 34) | Group B (n = 45) | p-value | |
|---|---|---|---|
| Clarithromycin | 4 (11.8) | 8 (17.8) | 0.46 |
| Metronidazole | 11 (32.4) | 15 (33.3) | 0.93 |
| Levofloxacin | 5 (14.7) | 17 (37.8) | 0.02 |
| Amoxicillin | 0 | 0 | – |
| Tetracycline | 0 | 0 | – |
Multiple logistic regression analysis showed that antibiotic resistances to single clarithromycin, single metronidazole resistance and dual resistance to clarithromycin and metronidazole were the factors influencing the efficacy of H. pylori eradication therapy in the univariate analysis [Table 5]. When strains with single resistance to clarithromycin were present, the H. pylori eradication rates was 75% compared to 92.5% in those with susceptible strains (p = 0.05). The H. pylori eradication rates was only 76.9% in those metronidazole compared to 96.2% in those with susceptible strains (p = 0.008). When dual resistance strains to clarithromycin and metronidazole were present, the eradication rate dropped to only 50% compared to 93.2% in those with susceptible strains (p < 0.001).
Table 5.
Univariate analysis of the clinical factors influencing the efficacy of H. pylori eradication therapy in per protocol analyses.
| Principle parameter | Case no. | Eradication Rate (%) | p-value |
|---|---|---|---|
| Age | |||
| <60 years | 123/131 | 93.9 | 0.41 |
| ≥60 years | 47/52 | 90.4 | |
| Sex | |||
| Female | 80/89 | 89.9 | 0.12 |
| Male | 90/94 | 95.7 | |
| Previous history of peptic ulcer | |||
| (−) | 152/165 | 92.1 | 0.22 |
| (+) | 18/18 | 100 | |
| HP eradication timing (per-protocol) | |||
| Group A | 86/90 | 95.6 | 0.17 |
| Group B | 84/93 | 90.3 | |
| Compliance | |||
| Good | 183/183 | 100.0 | – |
| Poor | 0 | ||
| H. pylori culture (n = 79) | |||
| Single amoxicillin resistance | 0 | – | – |
| Single clarithromycin resistance (+) | 9/12 | 75.0 | 0.05 |
| Single clarithromycin resistance (−) | 62/67 | 92.5 | |
| Single metronidazole resistance (+) | 20/26 | 76.9 | 0.008 |
| Single metronidazole resistance (−) | 51/53 | 96.2 | |
| Dual clarithromycin and metronidazole resistance (+) | 3/6 | 50.0 | <0.001 |
| Dual clarithromycin and metronidazole resistance (−) | 68/73 | 93.2 | |
Discussion
The increasing rates of antibiotic resistance, especially clarithromycin or dual resistance to clarithromycin and metronidazole, is a major cause of first-line H. pylori eradication failure worldwide [7,12,23]. We observed an increase of clarithromycin resistance from 11.8% to 17.8% over times in Taiwan. The ITT and PP success rates for concomitant therapy as first line H. pylori eradication has decreased over the two study period in this study. Antibiotics resistances were the clinical factors affecting the failure of eradication.
Anti-microbial resistance always plays a crucial role in the success of H. pylori eradication and is relevant to the point mutations in the antimicrobial resistance of H. pylori as the primary role. The increase of primary clarithromycin resistance over the study period was linked to an increasing consumption of macrolides over times because clarithromycin had been widely used in many infectious diseases such as standard triple therapy for H. pylori eradication compared to metronidazole. Moreover, clarithromycin resistance in H. pylori is easily caused by point mutations in the rrl gene encoding two 23S rRNA nucleotides (2142 and 2143) or associated with the efflux pump system [[24], [25], [26]]. Clarithromycin resistance has a large impact on the decrease in efficacy of standard triple therapy, with increasing resistance rates reported in most parts of the world. Therefore, standard triple therapy had been abandoned in many countries. However, in Taiwanese consensus it was recommended that clarithromycin-based triple therapy is the first-line regimen for H. pylori infection in geographic regions with the prevalence of primary clarithromycin resistance below 15% [19,27]. Even up to now, 7-day standard triple therapy is still the recommended first-line regimen according to the Taiwanese National Health Insurance Administration currently allowing only 7-days of clarithromycin 500 mg BID in each treatment course.
Nevertheless, there were more and more reports on the drops of standard triple therapies in Taiwan because of the increase of clarithromycin resistance in Taiwan [6,11,14,15,18]. Concomitant therapy was one of the treatment options recommended which was superior to clarithromycin-triple therapy of the same duration for the eradication of H. pylori and has been recommended as the first line therapy in areas of high (>15%) clarithromycin resistance [1,24]. In our hospital, most physicians’ usually prescribed concomitant therapy for naïve H. pylori infected patients since then. In current study, a change of clarithromycin resistance from 11.8% to 17.8% was observed between the two time intervals. The concern was that whether the success rates of the concomitant therapy was affected by the changes of antibiotics resistances. It was not surprise that both the ITT and PP success rates for concomitant therapy as first line H. pylori eradication has decreased over the two study period in this study. Although the both groups still maintained >90% PP analysis, an approximately 5% drop was observed. Antibiotic resistances to clarithromycin, metronidazole and dual antibiotic resistance (clarithromycin and metronidazole) were the factors influencing the efficacy of H. pylori eradication therapy in the logistic regression analysis. The eradication rate was 75.0% in the clarithromycin resistance subgroup and 76.9% among those with metronidazole resistance and dropped to 50% with dual clarithromycin and metronidazole resistance. This could imply a further decrease in the eradication rates as times go by because it is rational to speculate a further increase in the resistances as concomitant involves multiple antibiotics. Further investigation is warranted to determine the appropriate empiric regimen in Taiwan, as the rates of successful eradication is likely to decrease with the gradual increase of antibiotic resistance. Provided that the local clarithromycin resistance rate has exceeded the bound of 15% and may have further increased up to now, prolongation of concomitant therapy to 10–14 days appears to be a considerable option.
Metronidazole resistance in H. pylori is relevant to mutational inactivation of the redox-related gene (frxA, rdxA) [28]. There was no significant increase of metronidazole resistant strains in this study (32.4% and 33.3% between the two groups, p = 0.93) but resistance to metronidazole was still one of the clinical factor for treatment failure. Metronidazole was prescribed to patients 1000 mg per day in concomitant therapy which might be insufficient to kill the bacteria when resistant strains existed. However, a higher dose of metronidazole (1500 mg −2000mg per day) had been reported to be able to overcome the resistance to some degree and achieved a higher eradication success [21,29].
A significant increase in levels of levofloxacin resistance (from 14.7% to 37.8%) was highlighted in this study, which may decisively affect the performance of levofloxacin-based regimens especially in the second line rescue treatment. The Maastricht V/Florence Consensus Report recommended fluoroquinolone-amoxicillin triple or quadruple therapy as a second-line therapy for H. pylori infection [1]. Realistically, Tai et al. reported that H. pylori was eradicated among all patients treated by prescribing 14 days’ levofloxacin-amoxicillin triple therapy in levofloxacin-susceptible strains, but only half of patients with levofloxacin-resistant strains were successfully eradicated [7]. A systemic review and meta-analysis revealed that levofloxacin-amoxicillin triple therapy achieved an overall eradication rate of 78% after failure of the first line non-bismuth quadruple therapy and concomitant therapies [30]. Obviously, it is mandatory to continuing searching for other options for secondary treatment regimens with high eradication rates. Hsu et al. reported that ten-day PPI-bismuth-tetracycline-levofloxacin quadruple therapy is a good option for rescue treatment of H. pylori infection following failure of standard triple or non-bismuth quadruple therapy with a 98% of successful eradication [31].
The reasons for eradication failure do not count on antibiotics resistance only. Patient adherence to the prescribed medication and tolerances are also very important factors for treatment success. Side-effects may be the main reasons for poor compliance causing treatment failure and the subsequent development of resistant bacterial strain. The bottom line is, the adverse event rates were 16.7% and 10.8% in both groups (p = 0.24%) and the compliance was 100% in all patients. In our hospital, every patient who received treatment was well explained about the potential side effects of the treatment to improve drug compliance. Importantly, all the adverse events in this study were reported to be mild and well compliance.
There are some strength and limitations to this study. First, the availability of pretreatment antibiotic susceptibility, with assessment of its impact on eradication rates, is a major strength. However, results may be biased due to small sample size (n = 79 patients; 12 and 26 with resistance to clarithromycin and metronidazole, respectively). Second, the follow-up of H. pylori eradication status was performed at 4–8 weeks after treatment. Some of these patients who received urease breath test at 4 weeks may be too short. Lastly, the interval time between the two groups may be too short because the differences between them could be more significant assuming the antibiotic resistances could increase more over times. On the other hands, the eradication rates did not achieve a specific difference (EACM-A vs EACM-B = 87.8% vs84.8% in ITT, and 95.6% vs 90.3% in PP analysis), and the case numbers were relative small and therefore bias could exist.
In conclusion, the success rate of 7-days concomitant therapy encountered an approximately 5% decrease across 4-year time interval (2013–2017) with increasing clarithromycin resistance from 11.8% to 17.8% in Taiwan. Provided that the local clarithromycin resistance rate has exceeded the bound of 15% and may have further increased up to now, prolongation of concomitant therapy to 10–14 days appears to be a considerable option. A change of treatment regimens such as bismuth quadruple therapy, high dose dual therapy, hybrid or reverse hybrid therapy should be also considered.
Conflicts of interest
All the authors have no conflicts of interest related to the manuscript.
Acknowledgments
The authors would like to acknowledge Miss Ching-Yi Lin for her assistance in this study.
Footnotes
Peer review under responsibility of Chang Gung University.
Contributor Information
Shih-Cheng Yang, Email: d5637700@cgmh.org.tw2.
Wei-Chen Tai, Email: luketai1019@gmail.com.
References
- 1.Malfertheiner P., Megraud F., O'Morain C.A., Gisbert J.P., Kuipers E.J., Axon A.T., et al. Management of Helicobacter pylori infection-the Maastricht V/Florence consensus report. Gut. 2017;66:6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 2.Pellicano R., Ribaldone D.G., Caviglia G.P. Strategies for Helicobacter pylori eradication in the year 2020. Saudi J Gastroenterol. 2020;26:63–65. doi: 10.4103/sjg.SJG_95_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan M.C., Graham D.Y. Gastric cancer risk stratification and surveillance after Helicobacter pylori eradication: 2020. Gastrointest Endosc. 2019;90:457–460. doi: 10.1016/j.gie.2019.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar S., Metz D.C., Ellenberg S., Kaplan D.E., Goldberg D.S. Risk factors and Incidence of gastric cancer after detection of Helicobacter pylori infection: a large cohort study. Gastroenterology. 2020;158:527–536. doi: 10.1053/j.gastro.2019.10.019. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graham D.Y., Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143–1153. doi: 10.1136/gut.2009.192757. [DOI] [PubMed] [Google Scholar]
- 6.Liang C.M., Cheng J.W., Kuo C.M., Chang K.C., Wu K.L., Tai W.C., et al. Levofloxacin-containing second-line anti-Helicobacter pylori eradication in Taiwanese real-world practice. Biomed J. 2014;37:326–330. doi: 10.4103/2319-4170.125650. [DOI] [PubMed] [Google Scholar]
- 7.Tai W.C., Lee C.H., Chiou S.S., Kuo C.M., Kuo C.H., Liang C.M., et al. The clinical and bacteriological factors for optimal levofloxacin-containing triple therapy in second-line Helicobacter pylori eradication. PloS One. 2014;9 doi: 10.1371/journal.pone.0105822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tai W.C., Liang C.M., Lee C.H., Chiu C.H., Hu M.L., Lu L.S., et al. Seven-day non-bismuth containing quadruple therapy could achieve a grade “A” success rate for first-line Helicobacter pylori eradication. BioMed Res Int. 2015;2015:623732. doi: 10.1155/2015/623732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chey W.D., Leontiadis G.I., Howden C.W., Moss S.F. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112:212–239. doi: 10.1038/ajg.2016.563. [DOI] [PubMed] [Google Scholar]
- 10.Sugano K., Tack J., Kuipers E.J., Graham D.Y., El-Omar E.M., Miura S., et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353–1367. doi: 10.1136/gutjnl-2015-309252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tai W.C., Liang C.M., Bi K.W., Kuo C.M., Lu L.S., Wu C.K., et al. A comparison between dexlansoprazole modified release-based and lansoprazole-based nonbismuth quadruple (concomitant) therapy for first-line Helicobacter pylori eradication: a prospective randomized trial. Infect Drug Resist. 2019;12:2923–2931. doi: 10.2147/IDR.S213998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu D.C., Hsu P.I., Wu J.Y., Opekun A.R., Kuo C.H., Wu I.C., et al. Sequential and concomitant therapy with four drugs is equally effective for eradication of H pylori infection. Clin Gastroenterol Hepatol. 2010;8:36–41. doi: 10.1016/j.cgh.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tai W.C., Liang C.M., Kuo C.M., Huang P.Y., Wu C.K., Yang S.C., et al. A 14-day esomeprazole- and amoxicillin-containing high dose dual therapy achieves high eradication rate in the first line anti-helicobacter pylori treatment in Taiwan: a Prospective Randomized Trial. J Antimicrob Chemother. 2019;74:1718–1724. doi: 10.1093/jac/dkz046. [DOI] [PubMed] [Google Scholar]
- 14.Tsay F.W., Wu D.C., Yu H.C., Kao S.S., Lin K.H., Cheng J.S., et al. Randomized controlled trial shows that both 14-day hybrid and bismuth quadruple therapies cure most patients with Helicobacter pylori infection in populations with moderate antibiotic resistance. Antimicrob Agents Chemother. 2017;61 doi: 10.1128/AAC.00140-17. e00140-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu P.I., Tsay F.W., Graham D.Y., Tsai T.J., Tsai K.W., Kao J.Y., et al. Equivalent efficacies of reverse hybrid and bismuth quadruple therapies in eradication of Helicobacter pylori infection in a randomized controlled trial. Clin Gastroenterol Hepatol. 2018;16:1427–1433. doi: 10.1016/j.cgh.2018.03.031. [DOI] [PubMed] [Google Scholar]
- 16.Hsu P.I., Tsay F.W., Kao J.Y., Peng N.J., Tsai K.W., Tsai T.J., et al. Equivalent efficacies of reverse hybrid and concomitant therapies in first-line treatment of Helicobacter pylori infection. J Gastroenterol Hepatol. 2020;35:1731–1737. doi: 10.1111/jgh.15034. [DOI] [PubMed] [Google Scholar]
- 17.Graham D.Y., Lee Y.C., Wu M.S. Rational Helicobacter pylori therapy: evidence-based medicine rather than medicine-based evidence. Clin Gastroenterol Hepatol. 2014;12:177–186e3. doi: 10.1016/j.cgh.2013.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen M.C., Lei W.Y., Lin J.S., Yi C.H., Wu D.C., Hu C.T. Levofloxacin-amoxicillin/clavulanate-rabeprazole versus a standard seven-day triple therapy for eradication of Helicobacter pylori infection. BioMed Res Int. 2014;2014:158520. doi: 10.1155/2014/158520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liou J.M., Chang C.Y., Chen M.J., Chen C.C., Fang Y.J., Lee J.Y., et al. The primary resistance of Helicobacter pylori in Taiwan after the national policy to restrict antibiotic consumption and its relation to virulence factors-A nationwide study. PloS One. 2015;10 doi: 10.1371/journal.pone.0124199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chuah S.K., Hsu P.I., Chang K.C., Chiu Y.C., Wu K.L., Chou Y.P., et al. Randomized comparison of two non-Bismuth-containing second-line rescue therapies for Helicobacter pylori. Helicobacter. 2012;17:374–381. doi: 10.1111/j.1523-5378.2012.00937.x. [DOI] [PubMed] [Google Scholar]
- 21.Chuah S.K., Liang C.M., Lee C.H., Chiou S.S., Chiu Y.C., Hu M.L., et al. A randomized control trial comparing two levofloxacin-containing second-line therapies for Helicobacter pylori eradication. Medicine (Baltim) 2016;95 doi: 10.1097/MD.0000000000003586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chuah S.K., Tai W.C., Hsu P.I., Wu D.C., Wu K.L., Kuo C.M., et al. The efficacy of second-line anti-Helicobacter pylori therapy using an extended 14-days levofloxacin/amoxicillin/protonpump inhibitors -A pilot study. Helicobacter. 2012;17:374–381. doi: 10.1111/j.1523-5378.2012.00960.x. [DOI] [PubMed] [Google Scholar]
- 23.Hsu P.I., Wu D.C., Chen W.C., Tseng H.H., Yu H.C., Wang H.M., et al. Randomized controlled trial comparing 7-day triple, 10-day sequential, and 7-day concomitant therapies for Helicobacter pylori infection. Antimicrob Agents Chemother. 2014;58:5936–5942. doi: 10.1128/AAC.02922-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arslan N., Yılmaz Ö., Demiray-Gürbüz E. Importance of antimicrobial susceptibility testing for the management of eradication in Helicobacter pylori infection. World J Gastroenterol. 2017;23:2854–2869. doi: 10.3748/wjg.v23.i16.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bina J.E., Alm R.A., Uria-Nickelsen M., Thomas S.R., Trust T.J., Hancock R.E. Helicobacter pylori uptake and efflux: basis for intrinsic susceptibility to antibiotics in vitro. Antimicrob Agents Chemother. 2000;44:248–254. doi: 10.1128/aac.44.2.248-254.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirata K., Suzuki H., Nishizawa T., Tsugawa H., Muraoka H., Saito Y., et al. Contribution of efflux pumps to clarithromycin resistance in Helicobacter pylori. J Gastroenterol Hepatol. 2010;25 Suppl 1:S75–S79. doi: 10.1111/j.1440-1746.2009.06220.x. [DOI] [PubMed] [Google Scholar]
- 27.Sheu B.S., Wu M.S., Chiu C.T., Lo J.C., Wu D.C., Liou J.M., et al. Consensus on the clinical management, screening-to-treat, and surveillance of Helicobacter pylori infection to improve gastric cancer control on a nationwide scale. Helicobacter. 2017;22 doi: 10.1111/hel.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsugawa H., Suzuki H., Satoh K., Hirata K., Matsuzaki J., Saito Y., et al. Two amino acids mutation of ferric uptake regulator determines Helicobacter pylori resistance to metronidazole. Antioxidants Redox Signal. 2011;14:15–23. doi: 10.1089/ars.2010.3146. [DOI] [PubMed] [Google Scholar]
- 29.Moon J.Y., Kim G.H., You H.S., Lee B.E., Ryu D.Y., Cheong J.H., et al. Levofloxacin, metronidazole, and lansoprazole triple therapy compared to quadruple therapy as a second-line treatment of Helicobacter pylori infection in Korea. Gut Liver. 2013;7:406–410. doi: 10.5009/gnl.2013.7.4.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen P.Y., Wu M.S., Chen C.Y., Bair M.J., Chou C.K., Lin J.T., et al. Systematic review with meta-analysis: the efficacy of levofloxacin triple therapy as the first- or second-line treatments of Helicobacter pylori infection. Aliment Pharmacol Ther. 2016;44:427–437. doi: 10.1111/apt.13712. [DOI] [PubMed] [Google Scholar]
- 31.Hsu P.I., Tsai F.W., Kao S.S., Hsu W.H., Cheng J.S., Peng N.J., et al. Ten-day quadruple therapy comprising proton pump inhibitor, bismuth, tetracycline, and levofloxacin is more effective than standard levofloxacin triple therapy in the second-line treatment of Helicobacter pylori infection: a randomized controlled trial. Am J Gastroenterol. 2017;112:1374–1381. doi: 10.1038/ajg.2017.195. [DOI] [PubMed] [Google Scholar]