Abstract
The COVID-19 pandemic has set back progress made on antimicrobial resistance (AMR). Without urgent re-focus, we risk slowing down drug discovery and providing treatment for drug resistant Mycobacterium tuberculosis. Unique in its immune evasion, dormancy and resuscitation, the causal pathogens of tuberculosis (TB) have demonstrated resistance to antibiotics with efflux pumps and the ability to form biofilms. Repurposing drugs is a prospective avenue for finding new anti-TB drugs. There are many advantages to discovering novel targets of an existing drug, as the pharmacokinetic and pharmacodynamic properties have already been established, they are cost-efficient and can be commercially accelerated for the new development. One such group of drugs are non-steroidal anti-inflammatory drugs (NSAIDs) that are originally known for their ability to supress the host proinflammatory responses. In addition to their anti-inflammatory properties, some NSAIDs have been discovered to have antimicrobial modes of action. Of particular interest is Carprofen, identified to inhibit the efflux mechanism and disrupt biofilm formation in mycobacteria. Due to the complexities of host-pathogens interactions in the lung microbiome, inflammatory responses must carefully be controlled alongside the in vivo actions of the prospective anti-infectives. This critical review explores the potential dual role of a selection of NSAIDs, as an anti-inflammatory and anti-tubercular adjunct to reverse the tide of antimicrobial resistance in existing treatments.
Keywords: NSAIDs, Carprofen, Tuberculosis, Mycobacterium tuberculosis (Mtb), Non-tubercular mycobacteria (NTM), Antimicrobial resistance (AMR), Drug repurposing
1. Global crisis, beyond the COVID-19 pandemic: antimicrobial resistance
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causing the COVID-19 infection, has justifiably commanded global attention and has highlighted the staggering importance of communicable diseases to the wider public. Yet with infectious diseases being at the forefront of people’s minds, the hidden pandemic of antimicrobial resistance (AMR) remains one of the top ten global challenges to healthcare and a loss of focus on this significant topic could have devasting consequences (WHO, 2021a).
AMR occurs when pathogens mutate on a molecular and ultrastructural level to survive treatment and exposure to antimicrobial drugs. Furthermore, inadequate access to clean water and sanitation; inefficient and insufficient disease control; lack of patient compliance; and inappropriate use of antimicrobials in agriculture and healthcare treatments influence the development of multidrug resistance. For example, the use of antibiotics on patients with COVID-19 has been reported (Garcia-Vidal et al., 2021, Beovic et al., 2020). This was based on previously reported high numbers of influenza H1N1 and H3N2 patients being co-infected with bacterial infections (Martin-Loeches and Schultz, 2017), therefore some healthcare procedures recommended administering empiric antibiotic treatment for all COVID-19 hospitalised patients. This is an example of medical practice with limited scientific evidence, resulting in often unnecessary use of antibiotics. Additionally, in the context of COVID-19, there has been an increased demand and use of alcohol-based hand sanitisers, the improper use of which may lead to the increased mutation and survival of the most virulent pathogens.
To effectively tackle AMR, rapid testing and efficient identification of the causal pathogen(s) followed by drug treatment are crucial. There are 23,000 GeneXpert machines globally, however, the impact of missing tuberculosis (TB) diagnoses will be detrimental to the global healthcare setting. This is in addition to an estimated 1.4 million people who did not receive treatment for TB in 2020 due to the COVID-19 pandemic, a decline of 21% from 2019 (WHO, 2021b).
2. TB and its causal pathogens: M. tuberculosis complex and NTM
TB is an ancient and highly virulent disease that has affected humans for thousands of years and is causing growing concern today. Mycobacterium tuberculosis is the causative agent for TB, an aerobic, slow growing, acid-fast bacillus (Parish & Stoker, 1998). There has been some speculation around mycobacterial sporulation however Ghosh et al., (2009) found M. marinum, a slow growing non-tubercular mycobacteria (NTM), can form spores in its dormant physiological states. Approximately 2 billion people in the world are infected with the disease (WHO, 2021c). A proportion of those infected will go onto develop active TB, and people most likely to develop the disease have risk factors such as malnutrition (Feleke et al., 2019), diabetes (Gautam et al., 2021), smoking (Khan et al., 2020) and HIV (Mai et al., 2019).
M. tuberculosis is inhaled via the mouth or the nose into the lungs, infecting the alveoli. Person-to-person transmission is a result of expelling infectious droplets into the air such as by coughing or sneezing (Fennelly and Jones-López, 2015). TB most commonly affects the lungs but can be extrapulmonary, infecting sites such as bones, the larynx, kidneys and/or even brain. Tuberculosis meningitis is an infection of the central nervous system (CDC, 2013). Miliary TB (characterised by the appearance of ‘millet’ seeds) is a rare but serious dissemination of M. tuberculosis throughout the body. It travels via the bloodstream infecting multiple organs and can be fatal.
3. Alarming emergence of non-tubercular mycobacterial infection: epidemiology
There are approximately 200 species of known mycobacteria and a growing emergence of NTM. They are a group of mycobacteria outside of the M. tuberculosis complex (MTBC) that have been isolated from various environmental sources such as natural water sources, man-made water sources, soil and dust (Sharma and Upadhyay, 2020). It is thought to be less virulent than M. tuberculosis but can still cause disease in both immunodeficient and immunocompetent patients (Shah et al., 2016). They are categorised into rapid growing (<7 days) and slow growing (>7 days) cultures. NTM are mainly contracted through exposure to environmental sources and unlikely to be contracted via person-to-person transmission (Yoon et al., 2020). There are contradicting reports on the transmission of Mycobacterium abscessus – some studies suggest M. abscessus is the only species of NTM that can be transmitted person-to-person in patients with cystic fibrosis. One such study by Bryant et al., (2016) suggested due to the genetic similarity found in strands between patients that person-to-person transmission was highly likely. In contrast, a smaller study by Harris et al., (2015) demonstrated no person-to-person transmission. Both studies utilised whole genome sequencing for analysis, therefore it is clear the transmission of M. abscessus needs to be further scrutinised. NTM has increased ten-fold since 1995 not only in the UK but also is increasing globally. Countries such as Japan, Canada and Taiwan have reported increased incidence (Sharma and Upadhyay, 2020, Shah et al., 2016). NTM is not a notifiable disease in many countries, but we should be cautious of its increasing prevalence which may be due to mutations or changes in environment, host and pathogen – all can contribute to antimicrobial drug resistance (AMR).
4. Physiology (drug efflux and biofilm) – links to AMR
M. tuberculosis has many virulence determinants and resistance mechanisms, including a complex cell wall, efflux pumps and the ability to form and disperse biofilms (Smith, 2003). It evades both the innate and the adaptive host immune system by mechanisms such as interference of MHC II presentation for CD4+ T cells, phagosome-lysosome fusion and many other unknown mechanisms (Gupta et al., 2012). The mycobacterial cell wall has been subject to numerous investigations (Maitra et al., 2019) and provides a viable option for drug targets – however in this context, we discuss efflux pumps and biofilms.
5. Drug efflux mechanisms in M. tuberculosis
Efflux pumps are a non-specific, highly efficient resistance mechanism of many pathogenic bacteria including M. tuberculosis (Rodrigues et al., 2021). Efflux pumps bind and remove antibiotics from the cell back into the environment ensuring its survival. There are six families of transporter: ATP-Binding Cassette Family (ABC), Major Facilitator Superfamily (MFS), Multidrug and Toxin Extrusion (MATE), Small Multidrug Resistance (SMR), Proteobacterial Antimicrobial Compound Efflux Family (PACE) and Resistance-Nodulation-Cell Division (RND) (Laws et al., 2021). All superfamilies utilise energy from the proton motive force (PMF) except for ABC transporters which utilise ATP. RND pumps remove antibiotics out of the cell across both the inner and outer membrane via a periplasmic protein, whereas the other families pump out of the inner membrane only. Efflux pump inhibitors have two mechanisms of action which are important to note. As described by Remm et al., (2021), ‘efflux pump inhibitors’ are compounds that bind to the efflux pump itself. ‘Efflux inhibitors’ disrupt the energy source (i.e., ATP or PMF). Whilst both result in rendering the efflux pump ineffective, the mechanisms should be differentiated.
Efflux-related resistance includes not only first line drugs such as isoniazid and rifampicin, but alarmingly, M. tuberculosis developed resistance to Bedaquiline within a year, a drug approved only in 2012 (Laws et al., 2021). This was attributed to the upregulation of mycobacterial membrane protein large 5 (MmpL5) and mycobacterial membrane protein small 5 (MmpS5) – Rv0676c and Rv0677c respectively (Hartkoorn et al., 2014). M. abscessus has also been demonstrated to possess these efflux pumps rendering it resistant to Bedaquiline, Clofazimine, Thiacetazone and analogues (Johansen et al., 2020). MmpL5 and MmpS5 are multi-substrate efflux pumps and part of the RND family. They are important membrane transporters to be discussed and explored in relation to Carprofen inhibition.
6. Mycobacterial biofilms
Biofilms are a coagulative colony phenomenon, like efflux pumps, not limited to M. tuberculosis. M. tuberculosis can form biofilms in the lungs of murine models, further protecting the bacteria from the host immune system (Chakraborty et al., 2021). M. tuberculosis requires highly specific conditions in order to form mature biofilms in vitro for study, possibly due to its natural survival in human cells rather than an artificial medium (Ojha et al., 2015, Kulka et al., 2012). The formation of a biofilm starts with adhesion to a surface, followed by attachment, growth, matrix synthesis and dispersal (Esteban and García-Coca, 2018). Aside from biofilm growth inside a host and on clinical materials, NTM can form biofilms in the environment such as within water systems. In addition to colony formation and dispersal, quorum sensing is used for bacterial cell-to-cell communication. Mycobacterial biofilms require structural components such as glycopeptidolipids and short chain mycolic acids which contribute to its hydrophobic nature (Ojha et al., 2008). In vitro, pellicles form at the liquid–air interface and are phenotypically tolerant to high levels of antibiotics. The characterisation of M. abscessus biofilms by Dokic et al., (2021) were found to have an increase in free mycolic acids. Growth characteristics can differ between mycobacteria, M. abscessus and M. chelonae exhibit extensive cording, a trait similarly associated with M. tuberculosis. Biofilms can withstand substantial antibiotic attack due to insufficient penetration of drugs including isoniazid and rifampicin, making them a critical resistance mechanism to overcome.
7. Extensive drug resistance in the treatment of TB (XDR-TB).
There are four first line drugs available for killing M. tuberculosis, the two most effective are isoniazid and rifampicin, followed by ethambutol and pyrazinamide. Failure to successfully treat a patient with these drugs result in using second-line drugs with an arduous treatment regimen of up to 20 months. Without treatment mortality rates are high. There are five categories of resistant TB as defined by WHO (WHO, 2021c). Isoniazid resistant TB, rifampicin resistant (RR-TB), rifampicin and isoniazid resistant (MDR-TB), rifampicin and fluoroquinolone resistant (pre-XDR-TB) and rifampicin, fluoroquinolone and either bedaquiline or linezolid resistant (XDR-TB) tuberculosis.
Insufficient dosage, irregularity of drug treatment and incompletion of drug treatment are more likely to be the reason for resistance than bacterial mutation (Mitchison and Davies, 2012), hence the need for shortening such a taxing regimen. The Global Tuberculosis Report 2021 (WHO, 2021c) reported a decrease of 22% in people being treated for MDR/RR-TB from 2019, however provisional data also provided by WHO suggests an estimated 1.4 million fewer people received treatment for TB in 2020 than in 2019 due to the COVID-19 pandemic (WHO, 2021b). A decline in reported numbers may be misinterpreted as a positive step, as it is apparent that people may not be receiving the treatment they need.
8. Prospect of repurposing immunomodulatory drugs
The efficiency and shelf life of current antibiotics is waning, and new antibiotics are urgently needed for public use. Repurposing or “repositioning” drugs offers the potential of finding a novel drug-target interaction with an already commercially available drug - an attractive avenue for research and pharmaceutical development. Not only can they be fast-tracked, but the metabolic and safety properties are already known and approved (Maitra et al., 2020). One such group of drugs under the spotlight are immunomodulatory drugs, which work to suppress or support the inflammatory system of the host (Lee and Bhakta, 2021, Young et al., 2020). There are many categories of immunomodulatory drugs available including statins, calcium channel blockers and histone deacetylase inhibitors as have been highlighted in the review by Lee and Bhakta (2021), here we focus on non-steroidal anti-inflammatory drugs (NSAIDs) as an anti-tubercular and anti-inflammatory adjunctive treatment option.
9. Non-steroidal anti-inflammatory drugs as anti-infectives
NSAIDs are widely used and available drugs, perhaps most famously Aspirin and Ibuprofen. The NSAID mechanism of action in eukaryotes is well reported (Fatima et al., 2021, Lee and Bhakta, 2021, Maitra et al., 2016) They act by inhibiting cyclooxygenases I and II (COX-I and COX-II). Cyclooxygenases convert arachidonic acid to prostaglandin G2, which is reduced to prostaglandin H2 and in turn, to five prostaglandins including prostaglandin E2 (Chandrasekharan and Simmons, 2004). From this, isomers including thromboxane and prostacyclin are synthesised, resulting in pathophysiological symptoms such as fever and pain. COX-I is universally expressed throughout tissues and cells, whereas COX-II is mostly induced in response to stimuli such as cytokines. The complex immunobiological relationships between the host-inflammatory response, COX-I and II, and mycobacteria need to be understood to ascertain the benefits of using NSAIDs. For example, the inhibition of prostaglandin E2 revealed to reduce pneumonia and bacterial load at an advanced stage of pulmonary murine infection and improving the expression of Interferon-γ (IFN-γ), tumour necrosis factor-α (TNF-α) and nitric oxide synthase (iNOS) (Moreno et al., 2002). This demonstrates whilst inflammation has its role in tackling infection, non-productive inflammation caused can result in pulmonary impairment and post-TB lung defects including permanent lung damage. Narrowing of the airways is likely due to the proinflammatory response of the host, resulting in airway obstruction and a continued inflammatory response has been found to persist following treatment (Ravimohan et al., 2018, Malherbe et al., 2016). This indicates that adjunctive NSAID therapy may be highly beneficial at late/advanced stage pulmonary infection (Ivanyi and Zumla, 2013). Drug discovery also relies on the exploration of the mechanisms of action, chemical scaffolds, and structures in prokaryotic cells, which are yet to be uncovered. The free carboxylic acid in Ibuprofen and derivatives has been identified as essential for their anti-tubercular properties (Guzman et al., 2013). Other NSAIDs such as oxyphenbutazone have been found to have bactericidal effects unlike the similar pyrazolidinediones (e.g., phenylbutazone, suxibuzone, and sulfinpyrazone). The suggestion is the lack of phenolic hydroxyl renders it unable to form a quinonimine which depletes flavins and thiols (Gold et al., 2012). In retaliation, intracellular thiol stress has been found to induce biofilm formation in static and shaking cultures of M. tuberculosis, harbouring drug tolerant bacteria (Trivedi et al., 2016). Many studies have explored anti-biofilm activity of NSAIDs (predominantly Aspirin) on various species including Pseudomonas aeruginosa, Staphylococcus aureus and Escherichia coli (Paes Leme and da Silva, 2021).
WHO recommends using NSAIDs for joint pain relief for TB (WHO, 2010). However, if NSAIDs are proving to be effective anti-tubercular properties, then the usage and limitation of NSAIDs needs to be reviewed and understood urgently, it is not beyond the realms of possibility for M. tuberculosis or NTM to develop resistance against them. Whilst there have been previous reviews highlighting the benefits of repurposing NSAIDs on bacteria including mycobacteria (Lee and Bhakta, 2021, Paes Leme and da Silva, 2021, Maitra et al., 2016), this review discusses Carprofen and carbazole structures in greater detail providing a more focused viewpoint for tackling AMR.
10. Carbazole – a potent scaffold for antitubercular drug
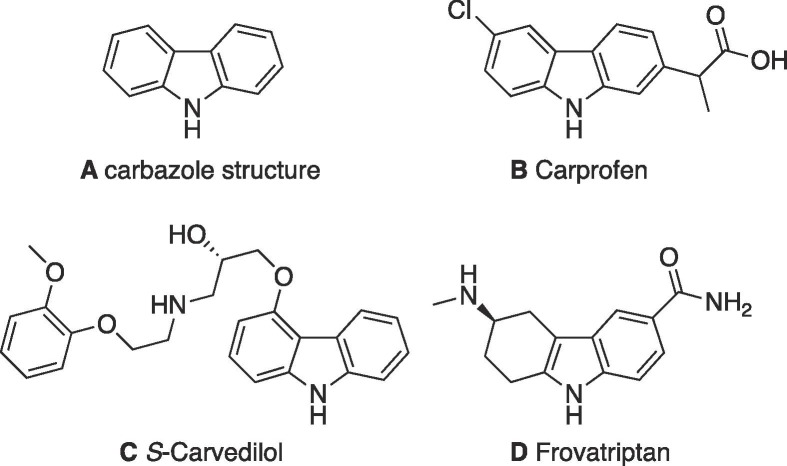
Carbazoles are tricyclic structures, two benzene rings with a pyrrole ring fused in the middle. Anti-tubercular properties have been identified in relation to naturally occurring and synthetic carbazole structures (Sellamuthu et al., 2018, Börger et al., 2017). Interestingly, one study explored the effect of N-methyl carbazole analogues on InhA inhibition, the target of isoniazid. One such structure, 4-(5-((9-Methyl-9H-carbazol-3-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)benzoic acid, a carbazolyl C-3-rhodanine conjugate, inhibited InhA by 91% (at 50 μM) (Shaikh et al., 2019). Carbazole scaffolds can be found in drugs such as Carvedilol, Carprofen and in the tetrahydrocarbazole Frovatriptan (Fig. 1 ).
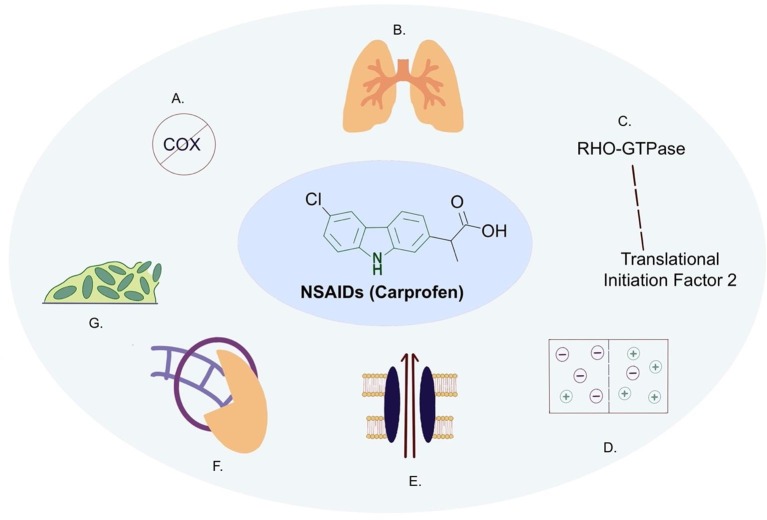
Fig. 2.
NSAIDs such as Carprofen (including the carbazole scaffold in the centre) demonstrating both anti-infective and anti-inflammatory properties. A) Cyclooxygenase inhibition in eukaryotes (Chandrasekharan and Simmons, 2004); B) mediation of non-productive inflammation in the lungs (Ravimohan et al., 2018, Malherbe et al., 2016); C) RHO-GTPase is a target of NSAIDs in eukaryotes, the homologue in M. tuberculosis is Translation initiation factor 2 protein, InfB (Rv2839c) in prokaryotes (Guzman et al., 2013); D) membrane potential disruption (Maitra et al., 2020); E) Efflux pump inhibition (Maitra et al., 2020); F) sliding clamp inhibition in E. coli (adapted from Yin et al., 2014); G) biofilm inhibition (Maitra et al., 2020).
Fig. 1.
Selected carbazole structures. A) carbazole scaffold composed of two benzene rings with a pyrrole ring in the centre; B) Carprofen, commonly used in veterinary treatment - PubChem CID, 2581 (National Centre for Biotechnology Information, 2022a); C) Carvedilol (S-isomer), a nonselective beta-adrenergic blocker used to treat cardiac disease in humans PubChem CID, 2585 (National Centre for Biotechnology Information, 2022b); D) Frovatriptan, a serotonin 1d receptor agonist – PubChem CID, 77992 (National Centre for Biotechnology Information, 2022c).
The molecular targets of carbazole scaffolds are yet to be explored. Sellamuthu et al., (2018) reported a carbazole compound effective against M. tuberculosis which inhibited ATP synthase, however docking analysis suggested ATP synthase may not be the molecular target for anti-tubercular activity. Börger et al., (2017) found promising anti-tubercular carbazole derivatives including the natural product Carbalexin-C and highlighted that the anti-tubercular activity relies on the oxygenation pattern, oxidation state of aromatic substituents and position of functional groups. This research did not investigate the effects on rapidly growing mycobacteria such as M. smegmatis or M. abscessus. In fact, there is very little research observing the effects of carbazole scaffolds on such mycobacteria, providing viable explorative opportunities.
11. Carprofen – ray of hope with its ability to reverse AMR
Carprofen is an NSAID predominantly used for animal treatment. Some veterinary research has suggested Carprofen and analogues may be a preferential COX-II inhibitor, but also inhibit both COX-I and COX-II (Deplano et al., 2020, Beretta et al., 2005). Carprofen is one of the few immunomodulatory drugs that is mycobactericidal, unlike other immunomodulatory drugs (Maitra et al., 2020). Other known efflux pump inhibitors such as Verapamil (a calcium channel blocker), or Valproic acid (a histone deacetylase inhibitor), can be developed for use as adjunctive therapy to shorten the drug duration of first line drugs, but lack mycobactericidal attributes (Chen et al., 2018, Rao et al., 2018). Carprofen has been demonstrated to inhibit M. tuberculosis efflux pumps and inhibit biofilm formation. A recent study by Maitra et al., (2020) investigated Carprofen at 0.25 × MIC (62.5 mg/L) on M. smegmatis which accumulated and retained ethidium bromide (EtBr), an efflux pump substrate. Subsequently, following the removal of Carprofen and Verapamil, the EtBr was no longer retained by the bacteria indicating reversible binding to the efflux pump. The drug at 1 × MIC (250 mg/L) demonstrated complete inhibition of biofilm formation, which is low considering some biofilm bacteria can have up to 1000-fold higher MICs than planktonic bacteria (Chakraborty et al., 2021). At a lower concentration (0.25 × MIC), Carprofen demonstrated greater effects on extra-cellular carbohydrates of the biofilm matrix, but none on lipids and proteins. Crucially, the study uncovered Carprofen, Ibuprofen and Ketoprofen all upregulated Rv0678, a transcriptional regulator for MmpL5 and MmpS5 RND efflux pumps (Rv0676c and Rv0677c) which were also upregulated (Laws et al., 2021). The crystal structure of the transcriptional regulator (Rv0678) has been solved by Radhakrishnan et al., (2014). The group demonstrated the regulator binds to MmpL5/S5, MmpL4/S4 and MmpL2/S2 promoters. This highlights an RND efflux pump as a possible endogenous drug target for M. tuberculosis. The investigation additionally demonstrated growth inhibition via disruption of membrane potential, affecting respiration although not as strongly as other membrane potential disruptors such as carbonyl cyanide m-chlorophenylhydrazone (CCCP) which disrupts the PMF, incidentally inhibiting efflux pumps in M. tuberculosis (Laws et al., 2021, Rodrigues et al., 2020). The disruption of the membrane potential is noteworthy as this raises the question of whether Carprofen disrupts the PMF, therefore disrupting the efflux pumps that require PMF for energy (‘efflux inhibitor’); or whether the efflux pumps are directly obstructed (‘efflux pump inhibitor’); or a combination of the two. The accumulation assay used Verapamil as a control, widely regarded as a direct inhibitor of both ATP and PMF powered efflux pumps, however, PMF energy disruption has also been reported with this drug in M. tuberculosis (Chen et al., 2018). This provides a good opportunity to further scrutinise the mechanism of action of Carprofen as an efflux pump disruptor. The study by Maitra et al., (2020) did not test against intracellular murine macrophages unlike Guzman et al., (2013) which found the MIC for growth inhibition of Carprofen against M. tuberculosis and M. bovis at 40 mg/L. Alongside, COX inhibition, NSAIDs additionally targets RHO-GTPase in humans, the homologue in M. tuberculosis is the translational initiation factor 2 protein, (InfB, Rv2839c) involved in protein synthesis initiation in mycobacteria and hence was proposed to be a putative endogenous target. Ibuprofen does not inhibit growth of slow growing mycobacteria as efficiently as Carprofen; however, it has been identified for adjunctive TB therapy and a clinical trial exploring the adjunctive treatment of Aspirin and Ibuprofen for TB is already underway (NCT04575519). Additionally, Ibuprofen has demonstrated inhibitory effects on M. abscessus (Kirkwood et al., 2018, Guzman et al., 2013).
Ketoprofen demonstrates no anti-tubercular property. Neither Ibuprofen nor ketoprofen are bactericidal which in part, may be attributed to their lack of carbazole scaffold, this can be further explored with docking analysis of the scaffold with the proposed endogenous targets outlined in Table 1 . Proteins involved in biofilm formation and dispersion may be an endogenous target for Carprofen as well. The characterisation of a cell wall associated protein (Rv1717) potentially involved in biofilm dispersal, including in response to stressors such as carbon starvation, was found to be upregulated by Carprofen unlike other NSAIDs (Bharti et al., 2021, Maitra et al., 2020). In addition to inhibition of efflux pumps and biofilm formation, research into the inhibitory effects of Carprofen on DNA polymerase III β subunit of Escherichia coli have been robustly demonstrated (Yin et al., 2014). Yin et al. in this paper provided the basis for sliding clamp mechanism (Table 1) as a Carprofen target in mycobacteria, an additional piece of the puzzle. Interestingly, the previously mentioned infB gene is also present in E. coli (b3168) – although this particular study did not identify InfB as a target of Carprofen.
Table 1.
Endogenous NSAID targets in mycobacteria. These prospective targets and mechanisms are discussed in further detail throughout the review.
| Putative targets for NSAIDs | Endogenous target mechanisms in mycobacteria | References |
|---|---|---|
| Translational Initiation | Ibuprofen and 2-arylpropanoic acids target traditional COX I & II in humans, additionally target RHO-GTPase. The homologue in M. tuberculosis is Translational initiation factor 2, InfB (Rv2839c) involved in protein synthesis initiation – identified as a possible target. | (Guzman et al., 2013) |
| Sliding Clamp/DNA Polymerase interaction | Inhibition of DNA polymerase III β subunit of Escherichia coli has been demonstrated using Carprofen. | (Yin et al., 2014) |
| Efflux pumps | Multiple drug efflux systems reported in M. tuberculosis and M. abscessus. Bedaquiline resistance has been attributed to the upregulation of MmpL5 and MmpS5 (Rv0676c and Rv0677c respectively). Mmp transporter proteins (MmpL5, MmpL6 and MmpS5) have been identified as a possible target as they are upregulated by Carprofen and Ibuprofen in M. smegmatis. | (Maitra et al., 2020, Hartkoorn et al., 2014) |
| Biofilm formation | M. tuberculosis can form biofilms, demonstrated by murine modelling. Complete inhibition of M. smegmatis biofilm formation has been determined at 250 mg/L (1 × MIC) of Carprofen. | (Maitra et al., 2020) |
| Membrane potential | Membrane potential of M. tuberculosis was disrupted by Carprofen, but not to the same extent as other known disruptors such as carbonyl cyanide m-chlorophenylhydrazone (CCCP), also a known efflux inhibitor disrupting the proton motive force (PMF). | (Maitra et al., 2020) |
Lsr2 is a small nucleoid associated protein, downregulated by Carprofen, conserved in mycobacteria including M. tuberculosis, M. abscessus and M. smegmatis. The necessity appears to be different depending on the species – it is essential for M. tuberculosis growth, but not for M. smegmatis or M. abscessus (Johansen et al., 2020, Le Moigne et al., 2019). In M. smegmatis, Lsr2 appears to play a critical role in colony morphology and biofilm formation (Esteban and García-Coca, 2018, Chen et al., 2006). There are two variants of M. abscessus, the smooth variant and the rough variant. The rough variant does not express glycopeptidolipids (GPL) on the cell surface and has a higher expression of lsr2 than the smooth type. Knockout of lsr2 has demonstrated lower virulence in M. abscessus (Le Moigne et al., 2019). The rough variant was demonstrated to produce serpentine cords that could not be phagocytosed by macrophages in zebrafish (Bernut et al., 2014). M. abscessus can irreversibly transition from smooth variant to rough variant during infection resulting in granuloma breakdown and a high pro-inflammatory response (Johansen et al., 2020). Lsr2 does not regulate GPL in M. abscessus, however, the rough variant has higher expression of Lsr2 than smooth variant. Lsr2 is dispensable for M. abscessus growth. Lsr2 represses ferritin, BrfB – a protein providing major iron storage for M. tuberculosis (Rodriguez et al., 2002, Kurthkoti et al., 2015). As Lsr2 was downregulated when exposed to Carprofen, this exposes the possible depletion of iron from the bacteria. These studies highlighted the importance of Lsr2 in virulence and biofilm formation in M. smegmatis and M. abscessus and creates a link between biofilm formation and the cell wall surface, but further exploration of Lsr2 in M. tuberculosis biofilms is required.
Carprofen and other carbazole analogues could be explored for their therapeutic efficacy if delivered with a nanosized formulation delivery system. Nanoparticle formulations provide benefits such as targeted delivery, appropriate circulation time, capable of only acting upon diseased tissue while leaving healthy tissues intact, biocompatibility, stability and do not induce a pro-inflammatory response (Magalhães et al., 2019). D’Souza et al., (2021) found that the nanoparticles, poly (lactic-co-glycolic acid), induced significant upregulation of tumour necrosis factor-α (TNF-α), but these were not delivered as pulmonary inhalation models. In contrast, one study found that aerosol delivered Ibuprofen and celecoxib had increased bacterial burdens (Mortensen et al., 2019). This presents the requirement for further investigations into the possibility of aerosol delivery and viability for delivery of NSAIDs in conjunction with first-line drugs.
The anti-inflammatory properties of NSAIDs presents the prospective balance between a) the use of immunomodulatory drugs that have anti-tubercular properties and b) mediating a non-productive inflammation to reduce inflammation-induced damage to the host. Although the endogenous targets for efflux pump inhibition and biofilm inhibition are yet to be uncovered, this is a positive step towards a drug offering pleiotropic properties. Most importantly, the mechanism of action of Carprofen is not the same as first line anti-tubercular drugs, reducing the risk of resistance developing if used in adjunctive therapy.
12. Challenges with complex host immunity in mycobacterial infection
M. tuberculosis encounters phagocytic cells and preferentially invades alveolar macrophages and monocytes in the host (Gupta et al, 2012). It activates the complement system and prompts an inflammatory response (Carroll et al., 2009). Pro-inflammatory cytokines are induced such as TNF-α, interleukin-1 (IL-1) and IFN. Macrophages release a host of cytokines that recruit several immunologically active cells such as natural killer cells, dendritic cells and T cells.
Bacteria are usually eliminated by the unfavourable host-cell environment in the phago-lysosome, a resultant fusion between phagosomes with lysosomes. M. tuberculosis evades the host immune system using a variety of mechanisms including interference of phagosome-lysosome fusion by manipulating host signalling pathways (Gupta et al., 2012). One such interference as described by Podinovskaia et al. (2013) is the decrease in phagosomal lipolysis in order to retain essential nutrients such as triacyl glycerol (TAG) resulting in M. tuberculosis synthesising lipids. Although further investigation is required due to differences in acidification between murine and human macrophage modelling – an important element to consider as M. tuberculosis prevents endocytic acidification (Wilson et al., 2011). M. tuberculosis produces early secreted antigenic target-6 (ESAT-6), secreted by ESX-1 (ESAT-6 secretion system 1) which induces phagosome disruption and inhibits binding with MHC I (Ferluga et al., 2020). Although ESX-1 appears to play a more crucial role in host membrane integrity than ESAT-6 (Lienard et al., 2020, Conrad et al., 2017). ESAT-6 is a mediator in macrophage differentiation and as such, a pre-requisite in granuloma formation - a classic clinical manifestation of TB infection (Refai et al., 2018). Granulomas are developed from the combination of T cells, B cells, leukocytes, macrophages, and TNF-α. It protects the host by containing the bacilli within a shell, supported by the host inflammatory response. It is at this stage the infection is classified as a latent infection which can resuscitate due to a weakened immune system. This may be due to progression of diseases such as HIV or immunosuppressive medication. TNF-α is an inflammatory mediator, regulated by the nuclear factor-κB (NF-κB) pathway. It is important in maintaining granulomas and antagonists have been found to reactivate latent M. tuberculosis infection in patients (Lee and Bhakta, 2021). However, both excessive and depleted inflammation has been found to result in the promotion of extracellular bacterial growth - one study by Tobin et al., (2012) investigated the inflammation pathway moderated by leukotriene A4 hydrolase (LT4A) which catalyses the production of leukotriene B4 (LTB4) and lipoxin A4 (LXA4), pro and anti-inflammatory pathways respectively. The study found that both excessive LTB4 and LXA4 resulted in the promotion of extracellular bacterial growth in M. marinum. However, LT4A is synthesised from arachidonic acid, catalysed by lipoxygenases rather than COX, therefore the extracellular growth of mycobacteria and its relevance to host-induced inflammation via the COX pathway needs to be further explored.
Macrophage matrix metalloproteases (MMPs) are endopeptidases upregulated by M. tuberculosis infection. They are secreted by M. tuberculosis infected macrophages as well as other host cells, playing a role in cell migration, cytokine signalling and extracellular matrix degradation (Sabir et al., 2019). MMP-1 is activated by M. tuberculosis, causing lung architecture destruction, alveolar destruction and collagen degradation (Ferluga et al., 2020, Elkington et al., 2011). MMP-9 has an important role in TB infection and similarly degrades lung architecture. Phosphatidyl-myo-inositol dimannosides (PIM2) found on the mycobacterial envelope trigger both COX-II and MMP-9. The inhibition of COX-II was found to decrease PIM2, resulting in decreased MMP-9 expression (Bansal et al., 2009). This research was conducted in peritoneal murine macrophages rather than pulmonary macrophage modelling; however, it highlights the relevance of moderating the host inflammatory system using COX inhibitors to moderate host-incurred tissue damage.
13. Conclusion: Unknowns, uncertainties, and prospects
COVID-19 has deprioritised antimicrobial resistance across the world; its true impact remains unknown. Without the promotion of research, accurate testing, informed public health messaging, globally invested surveillance, we will be forced to be reactive towards an imminent AMR outbreak. M. tuberculosis is a highly pathogenic bacteria with worrying hierarchies of drug-resistant strains. The prospect of repurposing drugs brings us hope. Whilst it is clear there are many questions surrounding carbazole-based structures and Carprofen with their anti-inflammatory and anti-tubercular properties, they offer an exciting avenue to be explored with putative endogenous targets such as efflux pump disruption and biofilm inhibition already researched. The immunobiological dynamics between host and pathogen present complex interactions and challenges, but also the option to utilise anti-inflammatory methods to moderate non-productive inflammation at late or advanced stages of pulmonary infection, aid immune mechanisms of the host, reduce inflammation following treatment and avoid host-mediated tissue damage. In addition to evidence and research available, it is important that we move out of working in silos and embrace interdisciplinary, matrix problem solving. This will take all our efforts but will save millions of lives.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We would like to thank Professor Edith Sim (Oxford University) and Professor Helen C. Hailes (University College London) for their critical review, comments, and helpful discussions on the manuscript. We would like to commemorate Professor Robert B. Sim’s noteworthy contributions and legacy in the field of Immunobiology and would like to whole-heartedly express SB’s gratitude for Bob Sim’s wisdom and valuable advice in his early developing academic career. His indomitable spirit and passion for science will live on.
Author statement
SB conceptualised the topic, outlined the literature-based research and supervised the project, CD reviewed the published literature, wrote the first draft of the manuscript, and prepared the figures and table using relevant software. CD and SB contributed to the critical review, editing and corrections of the manuscript before final submission.
References
- Bansal, K., Kapoor, N., Narayana, Y., Puzo, G., Gilleron, M. and Balaji, K. N. (2009). PIM2 Induced COX-2 and MMP-9 Expression in macrophages requires PI3K and Notch1 signaling, Sommer, P. (ed), PLoS One, 4 (3), e4911, 10.1371/journal.pone.0004911. [DOI] [PMC free article] [PubMed]
- Beovic B., Dousak M., Ferreira-Coimbra J., Nadrah K., Rubulotta F., Belliato M., Berger-Estilita J., Ayoade F., Rello J., Erdem H. Antibiotic use in patients with COVID-19: A “snapshot” infectious diseases international research initiative (ID-IRI) survey. J. Antimicrobial Chemother. 2020;75(11):3386–3390. doi: 10.1093/jac/dkaa326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beretta C., Garavaglia G., Cavalli M. COX-1 and COX-2 inhibition in horse blood by phenylbutazone, flunixin, carprofen and meloxicam: an in vitro analysis. Pharmacol. Res. 2005;52(4):302–306. doi: 10.1016/j.phrs.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Bernut A., Herrmann J.L., Kissa K., Dubremetz J.F., Gaillard J.L., Lutfalla G., Kremer L. Mycobacterium abscessus cording prevents phagocytosis and promotes abscess formation. Proc. Natl. Acad. Sci. U.S.A. 2014;111(10) doi: 10.1073/pnas.1321390111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti S., Maurya R.K., Venugopal U., Singh R., Akhtar M.S., Krishnan M.Y. Rv1717 is a cell wall-associated β-galactosidase of Mycobacterium tuberculosis that is involved in biofilm dispersion. Front. Microbiol. 2021;11(January) doi: 10.3389/fmicb.2020.611122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Börger C., Brütting C., Julich-Gruner K.K., Hesse R., Kumar V.P., Kutz S.K., Rönnefahrt M., Thomas C., Wan B., Franzblau S.G., Knölker H.-J. Anti-tuberculosis activity and structure–activity relationships of oxygenated tricyclic carbazole alkaloids and synthetic derivatives. Bioorg. Med. Chem. 2017;25(22):6167–6174. doi: 10.1016/j.bmc.2016.12.038. [DOI] [PubMed] [Google Scholar]
- Bryant J.M., Grogono D.M., Rodriguez-Rincon D., Everall I., Brown K.P., Moreno P., Verma D., Hill E., Drijkoningen J., Gilligan P., Esther C.R., Noone P.G., Giddings O., Bell S.C., Thomson R., Wainwright C.E., Coulter C., Pandey S., Wood M.E., Stockwell R.E., Ramsay K.A., Sherrard L.J., Kidd T.J., Jabbour N., Johnson G.R., Knibbs L.D., Morawska L., Sly P.D., Jones A., Bilton D., Laurenson I., Ruddy M., Bourke S., Bowler I.C.J.W., Chapman S.J., Clayton A., Cullen M., Dempsey O., Denton M., Desai M., Drew R.J., Edenborough F., Evans J., Folb J., Daniels T., Humphrey H., Isalska B., Jensen-Fangel S., Jönsson B., Jones A.M., Katzenstein T.L., Lillebaek T., MacGregor G., Mayell S., Millar M., Modha D., Nash E.F., O’Brien C., O’Brien D., Ohri C., Pao C.S., Peckham D., Perrin F., Perry A., Pressler T., Prtak L., Qvist T., Robb A., Rodgers H., Schaffer K., Shafi N., Van Ingen J., Walshaw M., Watson D., West N., Whitehouse J., Haworth C.S., Harris S.R., Ordway D., Parkhill J., Andres Floto R. Emergence and spread of a human transmissible multidrug-resistant nontuberculous mycobacterium. Science. 2016;354(6313):751–757. doi: 10.1126/science.aaf8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll M.V., Lack N., Sim E., Krarup A., Sim R.B. Multiple routes of complement activation by Mycobacterium bovis BCG. Mol. Immunol. 2009;46(16):3367–3378. doi: 10.1016/j.molimm.2009.07.015. [DOI] [PubMed] [Google Scholar]
- CDC, 2013. Chapter 2 transmission and pathogenesis of tuberculosis, Core Curriculum on Tuberculosis: What the Clinician Should Know, pp. 1–320 [Online]. Available at: https://www.cdc.gov/tb/education/corecurr/pdf/chapter2.pdf.
- Chakraborty P., Bajeli S., Kaushal D., Radotra B.D., Kumar A. Biofilm formation in the lung contributes to virulence and drug tolerance of Mycobacterium tuberculosis. Nat. Commun. 2021;12(1) doi: 10.1038/s41467-021-21748-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekharan N., Simmons D.L. The cyclooxygenases. Genome Biol. 2004;5(9):241. doi: 10.1186/gb-2004-5-9-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Gardete S., Jansen R.S., Shetty A., Dick T., Rhee K.Y., Dartoisa V. Verapamil targets membrane energetics in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2018;62(5) doi: 10.1128/AAC.02107-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.M., German G.J., Alexander D.C., Ren H., Tan T., Liu J. Roles of Lsr2 in colony morphology and biofilm formation of Mycobacterium smegmatis. J. Bacteriol. 2006;188(2):633–641. doi: 10.1128/JB.188.2.633-641.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad W.H., Osman M.M., Shanahan J.K., Chu F., Takaki K.K., Cameron J., Hopkinson-Woolley D., Brosch R., Ramakrishnan L. Mycobacterial ESX-1 secretion system mediates host cell lysis through bacterium contact-dependent gross membrane disruptions. Proc. Nat. Acad. Sci. 2017;114(6):1371–1376. doi: 10.1073/pnas.1620133114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza S., Du Plessis S.M., Egieyeh S., Bekale R.B., Maphasa R.E., Irabin A.F., Sampson S.L., Dube A. Physicochemical and biological evaluation of curdlan-poly(lactic-co-glycolic acid) nanoparticles as a host-directed therapy against mycobacterium tuberculosis. J. Pharm. Sci. 2021;1–10 doi: 10.1016/j.xphs.2021.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deplano A., Karlsson J., Fowler C.J., Onnis V. The fatty acid amide hydrolase and cyclooxygenase-inhibitory properties of novel amide derivatives of carprofen. Bioorganic Chem. 2020;101(April) doi: 10.1016/j.bioorg.2020.104034. [DOI] [PubMed] [Google Scholar]
- Dokic A., Peterson E., Arrieta-Ortiz M.L., Pan M., Di Maio A., Baliga N., Bhatt A. Mycobacterium abscessus biofilms produce an extracellular matrix and have a distinct mycolic acid profile. Cell Surface. 2021;7(April) doi: 10.1016/j.tcsw.2021.100051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkington P., Shiomi T., Breen R., Nuttall R.K., Ugarte-Gil C.A., Walker N.F., Saraiva L., Pedersen B., Mauri F., Lipman M., Edwards D.R., Robertson B.D., D’Armiento J., Friedland J.S. MMP-1 drives immunopathology in human tuberculosis and transgenic mice. J. Clin. Investig. 2011;121(5):1827–1833. doi: 10.1172/JCI45666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban J., García-Coca M. Mycobacterium biofilms. Front. Microbiol. 2018;8(JAN):1–8. doi: 10.3389/fmicb.2017.02651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatima S., Bhaskar A., Dwivedi V.P. Repurposing immunomodulatory drugs to combat tuberculosis. Front. Immunol. 2021;12(April):1–13. doi: 10.3389/fimmu.2021.645485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feleke B.E., Feleke T.E., Biadglegne F. Nutritional status of tuberculosis patients, a comparative cross-sectional study. BMC Pulmonary Med. 2019;19(1):182. doi: 10.1186/s12890-019-0953-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennelly K.P., Jones-López E.C. Quantity and quality of inhaled dose predicts immunopathology in tuberculosis. Front. Immunol. 2015;6(JUN):1–13. doi: 10.3389/fimmu.2015.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferluga J., Yasmin H., Al-Ahdal M.N., Bhakta S., Kishore U. Natural and trained innate immunity against Mycobacterium tuberculosis. Immunobiology. 2020;225(3) doi: 10.1016/j.imbio.2020.151951. [DOI] [PubMed] [Google Scholar]
- Garcia-Vidal C., Sanjuan G., Moreno-García E., Puerta-Alcalde P., Garcia-Pouton N., Chumbita M., Fernandez-Pittol M., Pitart C., Inciarte A., Bodro M., Morata L., Ambrosioni J., Grafia I., Meira F., Macaya I., Cardozo C., Casals C., Tellez A., Castro P., Marco F., García F., Mensa J., Martínez J.A., Soriano A., Rico V., Hernández-Meneses M., Agüero D., Torres B., González A., de la Mora L., Rojas J., Linares L., Fidalgo B., Rodriguez N., Nicolas D., Albiach L., Muñoz J., Almuedo A., Camprubí D., Angeles Marcos M., Cilloniz C., Fernández S., Nicolas J.M., Torres A. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin. Microbiol. Inf. 2021;27(1):83–88. doi: 10.1016/j.cmi.2020.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam S., Shrestha N., Mahato S., Nguyen T.P.A., Mishra S.R., Berg-Beckhoff G. Diabetes among tuberculosis patients and its impact on tuberculosis treatment in South Asia: a systematic review and meta-analysis. Sci. Rep. 2021;11(1):2113. doi: 10.1038/s41598-021-81057-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh J., Larsson P., Singh B., Pettersson B.M.F., Islam N.M., Sarkar S.N., Dasgupta S., Kirsebom L.A. Sporulation in mycobacteria. Proc. Natl. Acad. Sci. 2009;106(26):10781–10786. doi: 10.1073/pnas.0904104106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold B., Pingle M., Brickner S.J., Shah N., Roberts J., Rundell M., Bracken W.C., Warrier T., Somersan S., Venugopal A., Darby C., Jiang X., Warren J.D., Fernandez J., Ouerfelli O., Nuermberger E.L., Cunningham-Bussel A., Rath P., Chidawanyika T., Deng H., Realubit R., Fraser Glickman J., Nath C.F. Nonsteroidal anti-inflammatory drug sensitizes Mycobacterium tuberculosis to endogenous and exogenous antimicrobials. Proc. Nat. Acad. Sci. U.S.A. 2012;109(40):16004–16011. doi: 10.1073/pnas.1214188109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Kaul A., Tsolaki A.G., Kishore U., Bhakta S. Mycobacterium tuberculosis: immune evasion, latency and reactivation. Immunobiology. 2012;217(3):363–374. doi: 10.1016/j.imbio.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Guzman J.D., Evangelopoulos D., Gupta A., Birchall K., Mwaigwisya S., Saxty B., McHugh T.D., Gibbons S., Malkinson J., Bhakta S. Antitubercular specific activity of ibuprofen and the other 2-arylpropanoic acids using the HT-SPOTi whole-cell phenotypic assay. BMJ Open. 2013;3(6):1–13. doi: 10.1136/bmjopen-2013-002672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris K.A., Underwood A., Kenna D.T.D., Brooks A., Kavaliunaite E., Kapatai G., Tewolde R., Aurora P., Dixon G. Whole-genome sequencing and epidemiological analysis do not provide evidence for cross-transmission of mycobacterium abscessus in a cohort of pediatric cystic fibrosis patients. Clin. Inf. Dis. 2015;60(7):1007–1016. doi: 10.1093/cid/ciu967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartkoorn R.C., Uplekar S., Cole S.T. Cross-resistance between clofazimine and bedaquiline through upregulation of mmpl5 in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2014;58(5):2979–2981. doi: 10.1128/AAC.00037-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanyi J., Zumla A. Nonsteroidal anti-inflammatory drugs for adjunctive tuberculosis treatment. J. Inf. Dis. 2013;208(2):185–188. doi: 10.1093/infdis/jit153. [DOI] [PubMed] [Google Scholar]
- Johansen M.D., Herrmann J.L., Kremer L. Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat. Rev. Microbiol. 2020;18(7):392–407. doi: 10.1038/s41579-020-0331-1. [DOI] [PubMed] [Google Scholar]
- Khan A.H., Sulaiman S.A.S., Hassali M.A., Khan K.U., Ming L.C., Mateen O., Ullah M.O. Effect of smoking on treatment outcome among tuberculosis patients in Malaysia; a multicenter study. BMC Public Health. 2020;20(1):1–8. doi: 10.1186/s12889-020-08856-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood Z., Millar B., Downey D., Moore J. Antimycobacterial activity of nonantibiotics associated with the polypharmacy of cystic fibrosis (CF) against mycobacterium abscessus. Int. J. Mycobacteriol. 2018;7(4):358. doi: 10.4103/ijmy.ijmy_142_18. [DOI] [PubMed] [Google Scholar]
- Kulka K., Hatfull G., Ojha A.K. Growth of Mycobacterium tuberculosis biofilms. J. Visualized Exp. 2012;60:1–6. doi: 10.3791/3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurthkoti K., Tare P., Paitchowdhury R., Gowthami V.N., Garcia M.J., Colangeli R., Chatterji D., Nagaraja V., Rodriguez G.M. The mycobacterial iron-dependent regulator IdeR induces ferritin (bfrB) by alleviating Lsr2 repression. Mol. Microbiol. 2015;98(5):864–877. doi: 10.1111/mmi.13166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laws M., Jin P., Rahman K.M. Efflux pumps in Mycobacterium tuberculosis and their inhibition to tackle antimicrobial resistance. Trends Microbiol. 2021;1–12 doi: 10.1016/j.tim.2021.05.001. [DOI] [PubMed] [Google Scholar]
- Le Moigne V., Bernut A., Cortès M., Viljoen A., Dupont C., Pawlik A., Gaillard J.L., Misguich F., Crémazy F., Kremer L., Herrmann J.L. Lsr2 is an important determinant of intracellular growth and virulence in mycobacterium abscessus. Front. Microbiol. 2019;10(APR):1–11. doi: 10.3389/fmicb.2019.00905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C., Bhakta S. The prospect of repurposing immunomodulatory drugs for adjunctive chemotherapy against tuberculosis: a critical review. Antibiotics. 2021;10(1):1–13. doi: 10.3390/antibiotics10010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lienard J., Nobs E., Lovins V., Movert E., Valfridsson C., Carlsson F. The Mycobacterium marinum ESX-1 system mediates phagosomal permeabilization and type I interferon production via separable mechanisms. Proc. Natl. Acad. Sci. U.S.A. 2020;117(2):1160–1166. doi: 10.1073/pnas.1911646117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhães J., Pinheiro M., Drasler B., Septiadi D., Petri-Fink A., Santos S.G., Rothen-Rutishauser B., Reis S. Lipid nanoparticles biocompatibility and cellular uptake in a 3D human lung model. Nanomedicine. 2019;15(3):259–271. doi: 10.2217/nnm-2019-0256. [DOI] [PubMed] [Google Scholar]
- Mai T.Q., Martinez E., Menon R., Van Anh N.T., Hien N.T., Lan N.H., Giang D.C., Hang P.T., Thuong P.H., Van Huan H., Hoang N.P., Nhung N.V., Hoa N.B., Marais B.J., Sintchenko V. Tuberculosis risk factors and Mycobacterium tuberculosis transmission among HIV-infected patients in Vietnam. Tuberculosis. 2019;115(February):67–75. doi: 10.1016/j.tube.2019.02.001. [DOI] [PubMed] [Google Scholar]
- Maitra A., Bates S.D.S., Shaik M., Evangelopoulos Di, Abubakar I., McHugh T.D., Lipman M., Bhakta S. Repurposing drugs for treatment of tuberculosis: a role for non-steroidal anti-inflammatory drugs. Br. Med. Bull. 2016;118(1):138–148. doi: 10.1093/bmb/ldw019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra A., Munshi T., Healy J., Martin L.T., Vollmer W., Keep N.H., Bhakta S. Cell wall peptidoglycan in Mycobacterium tuberculosis: an Achilles’ heel for the TB-causing pathogen. FEMS Microbiol. Rev. 2019;43(5):548–575. doi: 10.1093/femsre/fuz016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra A., Evangelopoulos D., Chrzastek A., Martin L.T., Hanrath A., Chapman E., Hailes H.C., Lipman M., McHugh T.D., Waddell S.J., Bhakta S. Carprofen elicits pleiotropic mechanisms of bactericidal action with the potential to reverse antimicrobial drug resistance in tuberculosis. J. Antimicrobial Chemother. 2020;75(11):3194–3201. doi: 10.1093/jac/dkaa307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malherbe S.T., Shenai S., Ronacher K., Loxton A.G., Dolganov G., Kriel M., Van T., Chen R.Y., Warwick J., Via L.E., Song T., Lee M., Schoolnik G., Tromp G., Alland D., Barry C.E., Winter J., Walzl G., Lucas L., van der Spuy G., Stanley K., Thiart L., Smith B., Du Plessis N., Beltran C.G.G., Maasdorp E., Ellmann A., Choi H., Joh J., Dodd L.E., Allwood B., Koegelenberg C., Vorster M., Griffith-Richards S. Persisting positron emission tomography lesion activity and Mycobacterium tuberculosis mRNA after tuberculosis cure. Nat. Med. 2016;22(10):1094–1100. doi: 10.1038/nm.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Loeches I., Schultz J., Vincent M., Alvarez-Lerma F., Bos L.D., Solé-Violán J., Torres A., Rodriguez A. Increased incidence of co-infection in critically ill patients with influenza. Intensive Care Med. 2017;43(1):48–58. doi: 10.1007/s00134-016-4578-y. [DOI] [PubMed] [Google Scholar]
- Mitchison D., Davies G. The chemotherapy of tuberculosis: past, present and future [State of the art] Int. J. Tuberculosis Lung Dis. 2012;16(6):724–732. doi: 10.5588/ijtld.12.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno J.R., García I.E., La Luz De, García Hernández M., Leon D.A., Marquez R., Pando R.H. The role of prostaglandin E2 in the immunopathogenesis of experimental pulmonary tuberculosis. Immunology. 2002;106(2):257–266. doi: 10.1046/j.1365-2567.2002.01403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen R., Clemmensen H.S., Woodworth J.S., Therkelsen M.L., Mustafa T., Tonby K., Jenum S., Agger E.M., Dyrhol-Riise A.M., Andersen P. Cyclooxygenase inhibitors impair CD4 T cell immunity and exacerbate Mycobacterium tuberculosis infection in aerosol-challenged mice. Commun. Biol. 2019;2(1):1–10. doi: 10.1038/s42003-019-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Biotechnology Information (2022a). PubChem Compound Summary for CID 2581, Carprofen. [Online] Available at: https://pubchem.ncbi.nlm.nih.gov/compound/Carprofen.
- National Center for Biotechnology Information (2022b). PubChem Compound Summary for CID 2585, Carvedilol. [Online] Available at: https://pubchem.ncbi.nlm.nih.gov/compound/Carvedilol.
- National Center for Biotechnology Information (2022c). PubChem Compound Summary for CID 77992, Frovatriptan. [Online] Available at: https://pubchem.ncbi.nlm.nih.gov/compound/Frovatriptan.
- Ojha A.K., Baughn A.D., Sambandan D., Hsu T., Trivelli X., Guerardel Y., Alahari A., Kremer L., Jacobs W.R., Hatfull G.F. Growth of Mycobacterium tuberculosis biofilms containing free mycolic acids and harbouring drug-tolerant bacteria. Mol. Microbiol. 2008;69(1):164–174. doi: 10.1111/j.1365-2958.2008.06274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojha, A. K., Jacobs, W. R. and Hatfull, G. F. (2015) Genetic Dissection of Mycobacterial Biofilms, in Parish, T. and Roberts, D. M. (eds), Mycobacteria Protocols: third Edition, Methods in Molecular Biology, New York, NY, Springer New York, vol. 1285, pp. 215–226. Available at: www.doi.org/10.1007/978-1-4939-2450-9_12. [DOI] [PMC free article] [PubMed]
- Paes Leme R.C., da Silva R.B. Antimicrobial activity of non-steroidal anti-inflammatory drugs on biofilm: current evidence and potential for drug repurposing. Front. Microbiol. 2021;12(July):1–7. doi: 10.3389/fmicb.2021.707629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish T., Stoker N.G. In: Parish T., Stoker N.G., editors. Vol. 101. Humana Press; 1998. Mycobacteria. (Mycobacteria Protocols. Methods in Molecular Biology). [DOI] [Google Scholar]
- Podinovskaia M., Lee W., Caldwell S., Russell D.G. Infection of macrophages with Mycobacterium tuberculosis induces global modifications to phagosomal function. Cell. Microbiol. 2013;15(6):843–859. doi: 10.1111/cmi.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan A., Kumar N., Wright C.C., Chou T.H., Tringides M.L., Bolla J.R., Lei H.T., Rajashankar K.R., Su C.C., Purdy G.E., Yu E.W. Crystal structure of the transcriptional regulator Rv0678 of Mycobacterium tuberculosis. J. Biol. Chem. 2014;289(23):16526–16540. doi: 10.1074/jbc.M113.538959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao M., Valentini D., Zumla A., Maeurer M. Evaluation of the efficacy of valproic acid and suberoylanilide hydroxamic acid (vorinostat) in enhancing the effects of first-line tuberculosis drugs against intracellular Mycobacterium tuberculosis. Int. J. Inf. Dis. 2018;69:78–84. doi: 10.1016/j.ijid.2018.02.021. [DOI] [PubMed] [Google Scholar]
- Ravimohan S., Kornfeld H., Weissman D., Bisson G.P. Tuberculosis and lung damage: from epidemiology to pathophysiology. Eur. Respir. Rev. 2018;27(147) doi: 10.1183/16000617.0077-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refai A., Gritli S., Barbouche M.R., Essafi M. Mycobacterium tuberculosis virulent factor ESAT-6 drives macrophage differentiation toward the pro-inflammatory M1 phenotype and subsequently switches it to the anti-inflammatory M2 phenotype. Front. Cell. Inf. Microbiol. 2018;8(SEP):1–14. doi: 10.3389/fcimb.2018.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remm S., Earp J.C., Dick T., Dartois V., Seeger M.A. Critical discussion on drug efflux in Mycobacterium tuberculosis. FEMS Microbiol. Rev. 2021;July:1–15. doi: 10.1093/femsre/fuab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues L., Cravo P., Viveiros M. Efflux pump inhibitors as a promising adjunct therapy against drug resistant tuberculosis: a new strategy to revisit mycobacterial targets and repurpose old drugs. Expert Rev. Anti-Infect. Therapy. 2020;18(8):741–757. doi: 10.1080/14787210.2020.1760845. [DOI] [PubMed] [Google Scholar]
- Rodrigues L., Aínsa J.A., Viveiros M. (2021). Measuring Efflux and Permeability in Mycobacteria. In: Parish T., Kumar A. (eds) Mycobacteria Protocols. Methods in Molecular Biology. Vol. 2314. Humana, New York, NY. [Online] Available from: www.doi.org/10.1007/978-1-0716-1460-0_9. [DOI] [PubMed]
- Rodriguez G.M., Voskuil M.I., Gold B., Schoolnik G.K., Smith I. ideR, an essential gene in Mycobacterium tuberculosis: Role of IdeR in iron-dependent gene expression, iron metabolism, and oxidative stress response. Inf. Immun. 2002;70(7):3371–3381. doi: 10.1128/IAI.70.7.3371-3381.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabir, N., Hussain, T., Mangi, M. H., Zhao, D. and Zhou, X. (2019). Matrix metalloproteinases: expression, regulation and role in the immunopathology of tuberculosis, Cell Prolif., 52 (4), 1–14, 10.1111/cpr.12649. [DOI] [PMC free article] [PubMed]
- Sellamuthu S., Gutti G., Kumar D., Kumar Singh S. Carbazole: a potent scaffold for antitubercular drugs. Mini-Rev. Organic Chem. 2018;15(6):498–507. doi: 10.2174/1570193x15666180220141342. [DOI] [Google Scholar]
- Shah N.M., Davidson J.A., Anderson L.F., Lalor M.K., Kim J., Thomas H.L., Lipman M., Abubakar I. Pulmonary Mycobacterium avium-intracellulare is the main driver of the rise in non-tuberculous mycobacteria incidence in England, Wales and Northern Ireland, 2007–2012. BMC Inf. Dis. 2016;16(1):1–6. doi: 10.1186/s12879-016-1521-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh M.S., Kanhed A.M., Chandrasekaran B., Palkar M.B., Agrawal N., Lherbet C., Hampannavar G.A., Karpoormath R. Discovery of novel N-methyl carbazole tethered rhodanine derivatives as direct inhibitors of Mycobacterium tuberculosis InhA. Bioorg. Med. Chem. Lett. 2019;29(16):2338–2344. doi: 10.1016/j.bmcl.2019.06.015. [DOI] [PubMed] [Google Scholar]
- Sharma S.K., Upadhyay V. Epidemiology, diagnosis & treatment of non-tuberculous mycobacterial diseases, Indian. J. Med. Res. 2020;152:185–226. doi: 10.4103/ijmr.IJMR_902_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I. Mycobacterium tuberculosis pathogenesis and molecular determinants of virulence. Clin. Microbiol. Rev. 2003;16(3):463–496. doi: 10.1128/CMR.16.3.463-496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin, D. M., Roca, F. J., Oh, S. F., McFarland, R., Vickery, T. W., Ray, J. P., Ko, D. C., Zou, Y., Bang, N. D., Chau, T. T. H., Vary, J. C., Hawn, T. R., Dunstan, S. J., Farrar, J. J., Thwaites, G. E., King, M.-C., Serhan, C. N. and Ramakrishnan, L. (2012). Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections, Cell, 148 (3), 434–446, 10.1016/j.cell.2011.12.023. [DOI] [PMC free article] [PubMed]
- Trivedi A., Mavi P.S., Bhatt D., Kumar A. Thiol reductive stress induces cellulose-anchored biofilm formation in Mycobacterium tuberculosis. Nat. Commun. 2016;7(1):11392. doi: 10.1038/ncomms11392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2010. Treatment of tuberculosis guidelines [Online]. Available at https://apps.who.int/iris/bitstream/handle/10665/44165/9789241547833_eng.pdf. [PubMed]
- WHO, 2021c. Global Tuberculosis Report 2021. [Online] Available from: https://www.who.int/publications/i/item/9789240037021.
- WHO (2021a) Antimicrobial Resistance. [Online] Available at: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance.
- WHO, 2021b. Impact of COVID-19 pandemic on TB detection and mortality in 2020 [Online] Available at: https://cdn.who.int/media/docs/default-source/hq-tuberculosis/impact-of-the-covid-19-pandemic-on-tb-detection-and-mortality-in-2020.pdf?sfvrsn=3fdd251c_16.
- Wilson B.A., Salyers A.A., Whitt D.D., Walker M.E. 3rd ed. ASM Press; 2011. Bacterial Pathogenesis A Molecular Approach. [Google Scholar]
- Yin Z., Wang Y., Whittell L.R., Jergic S., Liu M., Harry E., Dixon N.E., Kelso M.J., Beck J.L., Oakley A.J. DNA replication is the target for the antibacterial effects of nonsteroidal anti-inflammatory drugs. Chem. Biol. 2014;21(4):481–487. doi: 10.1016/j.chembiol.2014.02.009. [DOI] [PubMed] [Google Scholar]
- Yoon J.K., Kim T.S., Kim J Il, Yim J.J. Whole genome sequencing of Nontuberculous Mycobacterium (NTM) isolates from sputum specimens of co-habiting patients with NTM pulmonary disease and NTM isolates from their environment. BMC Genomics. 2020;21(1):1–7. doi: 10.1186/s12864-020-6738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young C., Walzl G., Du Plessis N. Therapeutic host-directed strategies to improve outcome in tuberculosis. Mucosal Immunol. 2020;13(2):190–204. doi: 10.1038/s41385-019-0226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]