Abstract
As photoreceptor cells die during retinal degeneration, the surrounding microenvironment undergoes significant changes that are increasingly recognized to play a prominent role in determining the efficacy of therapeutic interventions. Chondroitin Sulphate Proteoglycans (CSPGs) are a major component of the extracellular matrix that have been shown to inhibit neuronal regrowth and regeneration in the brain and spinal cord, but comparatively little is known about their expression in retinal degeneration. Here we provide a comprehensive atlas of the expression patterns of four individual CSPGs in three models of inherited retinal degeneration and wildtype mice. In wildtype mice, Aggrecan presented a biphasic expression, while Neurocan and Phosphacan expression declined dramatically with time and Versican expression remained broadly constant. In degeneration, Aggrecan expression increased markedly in Aipl1-/- and Pde6brd1/rd1, while Versican showed regional increases in the periphery of Rho-/- mice. Conversely, Neurocan and Phosphacan broadly decrease with time in all models. Our data reveal significant heterogeneity in the expression of individual CSPGs. Moreover, there are striking differences in the expression patterns of specific CSPGs in the diseased retina, compared with those reported following injury elsewhere in the CNS. Better understanding of the distinct distributions of individual CSPGs will contribute to creating more permissive microenvironments for neuro-regeneration and repair.
Subject terms: Cell death in the nervous system, Retina, Macular degeneration
Introduction
Chondroitin sulphate proteoglycans (CSPGs) are major components of the extracellular matrix (ECM) in the central nervous system (CNS) and can exert effects on cell adhesion, motility, axonal outgrowth, synaptic plasticity and neural regeneration1–3. They are typically upregulated in response to injury and in a wide range of neurodegenerative diseases. Importantly, they have been shown to reduce the efficacy of various therapeutic approaches4–6. CSPGs consist of a core protein covalently attached to long glycosaminoglycan (GAG) carbohydrate chains7. There are 16 different types of CSPGs, of which Neurocan, Brevican, Phosphacan, Aggrecan and Versican are most common within the nervous system7. CSPGs exert much of their inhibitory function via their GAG chain composition3, however the core proteins themselves also inhibit neurite outgrowth8, an effect that persists after the removal of GAGs9. Understanding the specific changes in expression of individual CSPGs in response to injury or disease is crucial for creating extracellular environments that are permissive for regenerative therapies4.
Aggrecan is one of the major CSPGs expressed in the nervous system and is a constituent of perineuronal nets (PNNs)3,10,11. It has been studied extensively in spinal cord injury where it prevents Schwan cell migration following transplantation by inhibiting integrin-mediated cell adhesion12, as well as inhibiting endogenous axonal growth in injured spinal cord in vivo13. Neurocan is similarly elevated around acute lesions in the CNS and active multiple sclerosis plaque edges14–17 as well as in many experimental models of acute injury in the brain and is commonly associated with inhibition of neurite outgrowth14,18,19.
Little is known about CSPG expression in the retina, although there are indications of unique roles of individual CSPGs: In the rodent retina, Phosphacan protein levels increase from late embryonic to late postnatal stages but are markedly reduced in the adult retina20. Conversely, Aggrecan increases with age21,22. In the normal rat retina, Neurocan, Aggrecan and Versican are differentially distributed at embryonic, postnatal and adult stages21. With respect to the human eye, a recent study using human embryonic stem cell-derived retinal organoids indicated that BREVICAN and VERSICAN were found throughout the retina across much of development, although a downregulation of VCAN, and a concomitant upregulation of BCAN expression was observed in late developmental stages23. In the human adult eye, VERSICAN and AGGRECAN are found throughout the neural retina, choroid and sclera22.
Much less is known about CSPG expression in the diseased retina. Using the pan-CSPG marker CS-56, we have previously shown that CSPG deposition varies markedly both across degeneration and between different murine models of photoreceptor degeneration24. However, the changes in expression of specific CSPGs following different types ocular injury, and particularly inherited disease, are poorly characterised. Aggrecan was shown to be upregulated in rat models of retinal dystrophy25. Conversely, Aggrecan expression decreased, while Brevican and Phosphacan mRNA levels were unchanged in the neural retina following induced injury (ischaemia) in the rat retina, but all three were significantly increased in the optic nerve26.
To explore the potential heterogeneity in the microenvironment between different degenerative disease models, we examined four individual CSPGs, Neurocan, Aggrecan, Phosphacan and Versican. We present a comprehensive assessment of protein and mRNA expression both across time and with degeneration in three murine models of retinal degeneration of differing severity. Our findings revealed a marked heterogeneity in the expression of different CSPG core proteins and this can arise even between models with similar rates of photoreceptor loss. This data provides a valuable resource and reference for future studies but also shows the vital importance of understanding the specific microenvironment changes associated with a given disease type when assessing the suitability and timings of therapeutic interventions.
Methods
Animals
Mouse lines used in the study include wildtype C57BL/6 J (Harlan, UK), Rho-/- (on a C57BL/6 J background; kind gift from P. Humphries, Trinity College Dublin, Republic of Ireland), Aipl1-/- (kind gift from T. Li, National Eye Institute, USA; formerly Harvard Medical School at the Massachusetts Eye and Ear Infirmary, USA), and Pde6brd1/rd1 (line originally on C3H/HeJ background [Harlan, UK], but backcrossed with C57BL/6 J to remove confounding Gpr179 (Bipolar cell) mutant alleles; see Nishiguchi et al. 201527. All experiments have been conducted in accordance with the Policies on the Use of Animals and Humans in Neuroscience Research, revised and approved by the ARVO Statement for Use of Animals in the Ophthalmic Research, under the regulation of the UK Home Office Animals (Scientific Procedures) Act 1986. Mice were used according to the NC3R ARRIVE guidelines. Mice were maintained in the animal facility at University College London or King’s College London in individually ventilated cages and given access to nesting material and food and water ad libitum. They were kept on a standard 12/12 h light/ dark cycle and at the same light levels throughout the study and used at the ages specified in the text. Animals of both sexes were used in this study without discrimination.
Time points and degeneration models
We used the Aipl1-/- model of Lebers congenital amourosis, and the Pde6brd1/rd1 and Rho-/- models of Retinitis Pigmentosa, all of which have mutations in genes expressed by photoreceptors. We examined each model at ‘early’ (defined as ONL > 70% of the thickness of the wild-type retinae), ‘mid’ (ONL thickness of 30–70% of wildtype), and ‘advanced’ stage degeneration (ONL < 30%), as previously defined1,24 and summarised in Table 1. For Aipl1-/- mice, which exhibit a very rapid degeneration that starts almost as soon as the retina is formed, we used P10 for early (no degeneration, 10 rows of nuclei in photoreceptor layer), P14 for mid (photoreceptors layer is reduced by half) and P19-P21 for advanced (a single layer of photoreceptor nuclei remains) stages, in accordance with previously published data28,29. Pde6brd1/rd1 is a model of fairly fast degeneration and was examined at P10 weeks for early (no degeneration, 10 rows of nuclei in photoreceptor layer), 3 weeks for mid (photoreceptors layer is reduced by half) and 6 weeks for advanced (a single layer of photoreceptor nuclei remains) stages. Rho-/- degenerates more slowly and was examined at early (3–4 weeks, 9 rows of nuclei in photoreceptor layer), 6 weeks for mid (photoreceptors layer is reduced by half) and 10–12 wks for advanced (2–3 rows of nuclei in the photoreceptor layer) stages. C57BL/6 J wildtype mice were used as background- and age-matched controls. We used 6 time points for wildtype mice to match the different time points used for the degenerative models: Postnatal day (P)10, when neurogenesis is largely completed but the synaptic layers are forming; P14, when synaptogenesis is nearly complete; 3 weeks; 6 weeks, 3 months and 6 months. Of note, in order to compare the expression levels in the postnatal period, real-time qPCR was performed for P10 Rho-/- mice in addition to the later degeneration stages.
Table 1.
Summary of timepoints used in retinal degeneration models.
| Model | Degeneration (progression rate) |
Early (timepoint) | Mid (timepoint) | Advanced (timepoint) |
|---|---|---|---|---|
| Aipl1-/- | Very fast | P10 | P14 | 3 weeks |
| Pde6brd1/rd1 | Moderate | P10 | 3 weeks | 6 weeks |
| Rho-/- | Moderate to slow | 3–4 weeks | 6 weeks | 10–12 weeks |
Immunohistochemistry and histology
Animals were euthanized and a small burn was administered to the sclera overlying the orbit to provide a landmark for the superior retina. Eyes were enucleated and eye cups prepared in phosphate-buffered saline (PBS) and then carefully orientated and embedded in a standardized fashion in OCT (TissueTek) without fixation. Embedded eyecups were frozen and left at − 20 °C at least overnight before being cut as transverse sections, 18 μm thick. In order to avoid oblique cuts, all images shown are from the central most region of the eye, immediately adjacent to the optic nerve (Fig. 1f). Specific details for each antibody and protocols used for immunohistochemistry can be found in Supplementary Table S1. Briefly, cryosections were air-dried for 15–30 min and washed in PBS. Sections were pre-blocked for 1–1.5 h at room temperature (RT) in a blocking solution before being incubated with appropriate primary antibody overnight (o/n) at 4 °C and at RT for 1–1.5 h. After rinsing with PBS, sections were incubated with secondary antibody (1:400) for 2–2.5 h at RT, rinsed and counter-stained with DAPI (0.5 μg/ml). Negative controls omitted the primary antibody. Qualitative assessments made using immunohistochemistry are based on N > 3 independent eyes per time point, per model, respectively.
Figure 1.
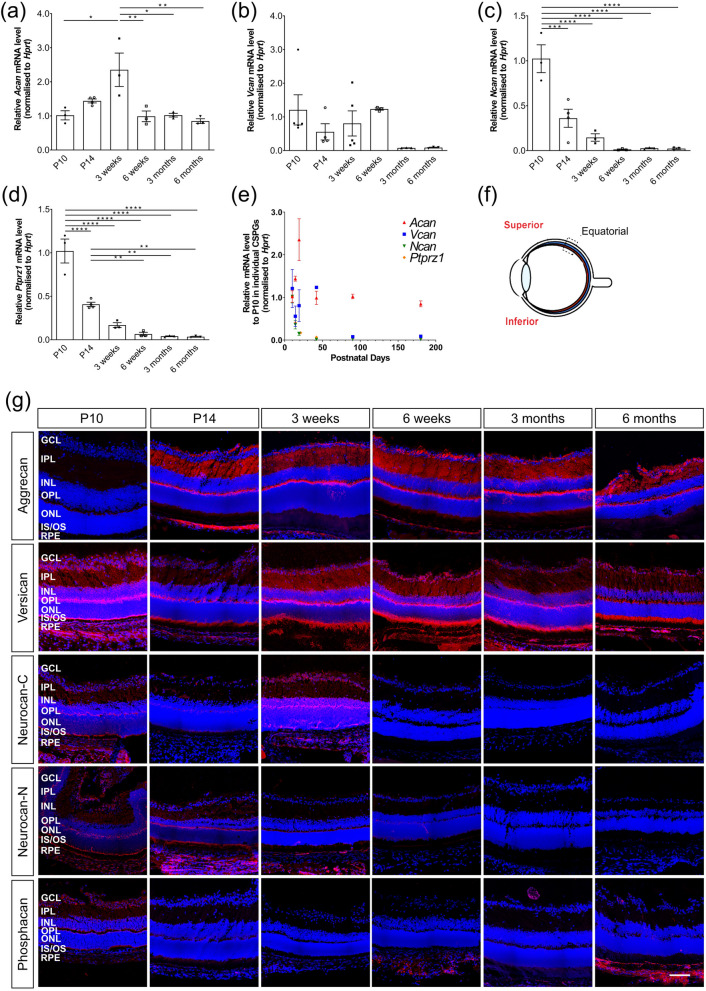
Expression patterns of Aggrecan, Versican, Neurocan and Phosphacan in wildtype mice over time. (a)–(d) histograms showing mean (+ /− SEM) mRNA levels of Acan (a), Vcan (b), Ncan (c) and Ptprz1 (d) in whole retinae. (e) best-fit curves summarizing the changes in expression of all four CSPGs over time. (Lognormal for Acan and Vcan, one phase decay for Ncan and Ptprz1.) (f) a schematic of the regions where immunostaining images were taken. (g) Immunostaining for Aggrecan, Versican, Neurocan and Phosphacan (red) over time. (a) Relative expression of Acan mRNA increased between P10 and 3 weeks, decreasing thereafter and remained at a similar level throughout adulthood. (b) Vcan expression was fairly constant across the time points examined, reducing at 3 and 6 months, although inter-sample variation was high. (c) (d) Ncan and Ptprz1 mRNA expression decreased significantly with time. (e) Neurocan-C and -N fractions and Versican were sparsely distributed throughout all the layers of retina when they are expressed. Phosphacan and Aggrecan were restricted mostly to the GCL, IPL and OPL. Images show confocal maximum projection images (MIPs) of the superior retina in the equatorial region. Scale bar, 100 µm. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 (one-way ANOVA test with Bonferroni’s correction). ONL—outer nuclear layer; OPL—outer plexiform layer; INL—inner nuclear layer; IPL—inner plexiform layer; GCL—ganglion cell layer. Nuclei are counter stained with Dapi -blue; CSPGs -red.
Chondroitinase ABC treatment
The lyophilized ChABC enzyme (Sigma, C2905) was reconstituted in 0.01% BSA in dH2O solution to a concentration [1U/ml] and stored at − 80 °C. Prior staining the enzyme was further diluted in an activation buffer TrisHCL [100 mM], (pH = 8) & Sodium Acetate [120 mM] as per Table S1, in a final volume of 100 μl/slide. The slides were covered with parafilm, to avoid evaporation and then incubated in a humidity chamber in a HybEZ™ II Oven (ACD Bio) at 37 °C for 3 h.
Confocal microscopy
Retinal sections were viewed on a confocal microscope (Leica TCS SPE, Leica Microsystems, Milton Keynes, UK). Unless otherwise stated, images show merged maximum intensity projection (MIP) images of xyz confocal stacks through retinal sections. Images are montages acquired using the tile scan function except co-staining. Individual xy images were acquired using a 2-frame average (1 frame for DAPI) at 1 μm intervals, and all taken at × 40 magnification, unless otherwise stated. Images were taken in both the superior (presented in Main Figs. 2, 3, 4, 5, 6) and inferior (presented in Supplementary Figs. S1–S4, S6, S7) retina at standardized regions in the equatorial region, and the superior retina in anterior margin (denoted as ‘peripheral’ in the images) immediately adjacent to the optic nerve (see schematics in figures). Images were acquired within 7 days of completing immunostaining and the same laser intensity, gain and offset settings were used across all sections for any given marker. Note that in order to allow direct visual comparison between wildtype data sets and those from the different degenerating models in different figures, images from wildtype retinae are duplicated in a number of figures. Schematics denoting regions imaged are adapted from our previous publication24.
Figure 2.
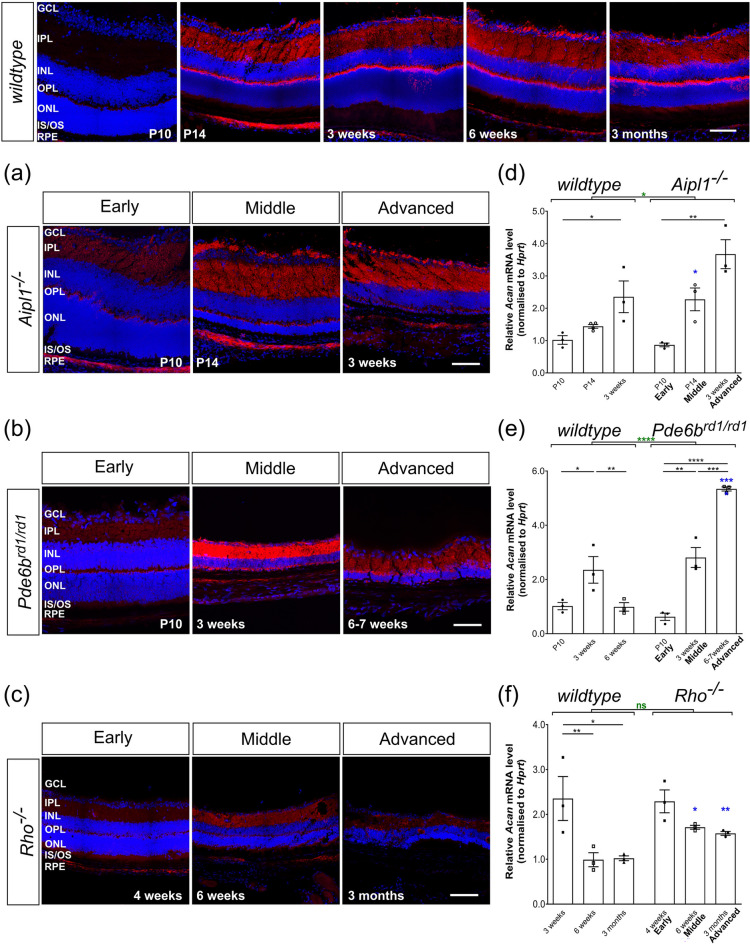
Aggrecan expression is increased in the Aipl1-/- and Pde6brd1/rd1 but not Rho-/- models of retinal degeneration. (a)–(c), Immunostaining for Aggrecan (red) was mainly seen in the OPL, IPL, and GCL with relatively little staining in ONL in all models. Increasing expression was most notable in the IPL, compared to early time-points, in wildtype, Aipl1-/- and Pde6brd1/rd1 retinae. (d)–(f), Acan mRNA levels were dramatically elevated with disease progression, showing increased expression in (d) P14 (mid-stage) Aipl1-/- and (e) 6 week (advanced-stage) Pde6brd1/rd1 retinae compared to age-matched wildtype mice. (f) Acan mRNA levels decreased in adulthood in Rho-/- retinae but remained higher than age-matched wildtype retinae. N.B. mRNA levels shown for wildtype are the same as in Fig. 1b. Scale bar, 100 µm. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 (one-way ANOVA test with Bonferroni’s correction, black; unpaired t-test for age-matched comparisons between wildtype and disease model, blue; two-way ANOVA test applied for assessments of change over time, red). ONL—outer nuclear layer; OPL—outer plexiform layer; INL—inner nuclear layer; IPL—inner plexiform layer; GCL—ganglion cell layer. Nuclei are counter stained with Dapi (blue).
Figure 3.
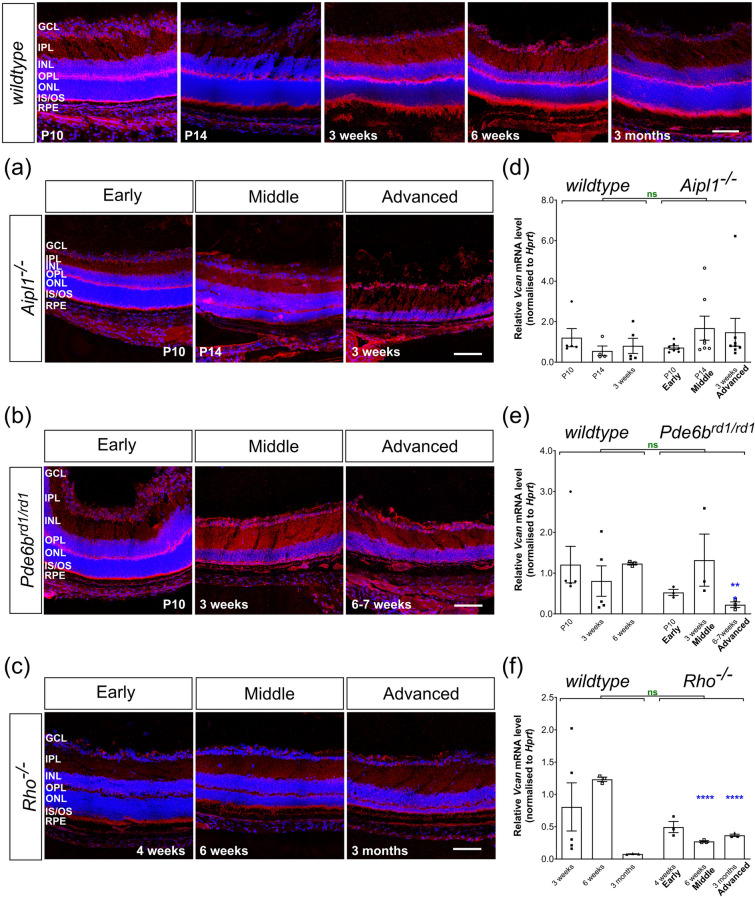
Versican expression remains similar to wildtype during degeneration. (a)–(c), Immunolabelling for Versican (red) was detected in all the layers of the retina in wildtype, Aipl1-/- and Pde6brd1/rd1 with relatively strong signals in the INL and IPL, particularly in mid and advanced stage Pde6brd1/rd1 and advanced-stage Rho-/- retinae. (d)–(f) Vcan mRNA levels were largely unchanged in Aipl1-/- and Pde6brd1/rd1 retinae (with the exception of a reduction at 6 weeks age of Pde6brd1/rd1 mice). (f) In Rho-/-, Vcan mRNA expression remained similar between P10 and 3 months of age, decreasing slightly, but remained significantly higher than age-matched wildtype mice. N.B. mRNA levels shown for wildtype are the same as in Fig. 1c. Scale bar, 100 µm. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 (one-way ANOVA test with Bonferroni’s correction, black; unpaired t-test for age-matched comparisons between wildtype and disease model, blue; two-way ANOVA test applied for assessments of change over time, red). ONL—outer nuclear layer; OPL—outer plexiform layer; INL—inner nuclear layer; IPL—inner plexiform layer; GCL—ganglion cell layer. Nuclei are counter stained with Dapi (blue).
Figure 4.
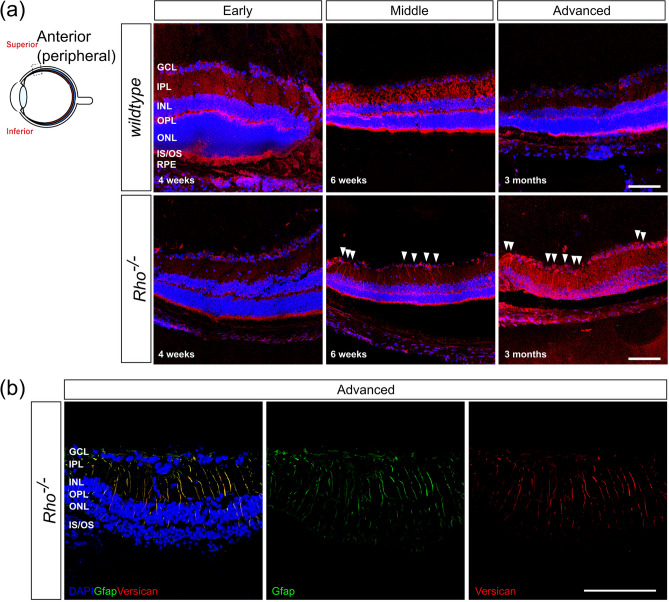
Versican colocalizes with Müller glia in the peripheral retina in advanced stage degeneration of Rho-/- mice. (a) Versican + radial processes (red) were observed in mid- to advanced-stage degeneration of Rho-/- mice in the peripheral retina (white arrows) but not in age-matched wildtype mice, or in any other model examined. (b) Versican + processes (red) co-labelled for the reactive glial marker, Glial Fibrillary Acidic Protein (GFAP; green). Image was acquired at × 63 magnification and a single optical section is shown. Scale bar, 100 µm. Nuclei are counter stained with Dapi (blue).
Figure 5.
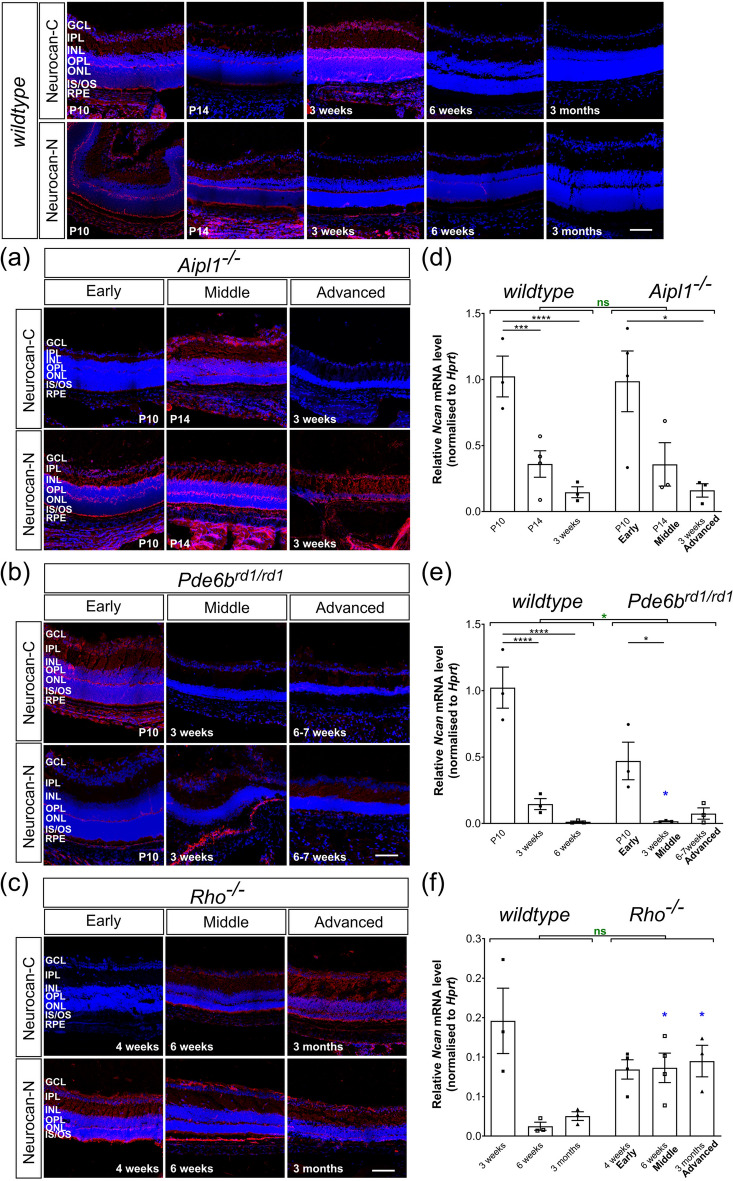
Neurocan expression decreases with time and is unaffected by degeneration. Neurocan immunolabelling (red) was distributed throughout all the layers of the retina and low levels of mRNA transcripts were observed in adult retinae in all models. mRNA levels shown for wildtype are the same as in Fig. 1d. (a) (b) Immunolabelling of the Neurocan C-terminal fraction typically decreased in intensity in Aipl1-/- and Pde6brd1/rd1 mice with time, while that of the Neurocan N-terminal fraction remained constant in these models. (d) (e) mRNA levels of Ncan decreased with time in Aipl1-/-, Pde6brd1/rd1 and wildtype mice. (f) Ncan mRNA and (c) immunolabelling for both Neurocan-C and -N were largely unchanged across degeneration in Rho-/- mice. Scale bar, 100 µm. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 (one-way ANOVA test with Bonferroni’s correction, black; unpaired t-test for age-matched comparisons between wildtype and disease model, blue; two-way ANOVA test applied for assessments of change over time, red). ONL—outer nuclear layer; OPL—outer plexiform layer; INL—inner nuclear layer; IPL—inner plexiform layer; GCL—ganglion cell layer. Nuclei are counter stained with Dapi (blue).
Figure 6.
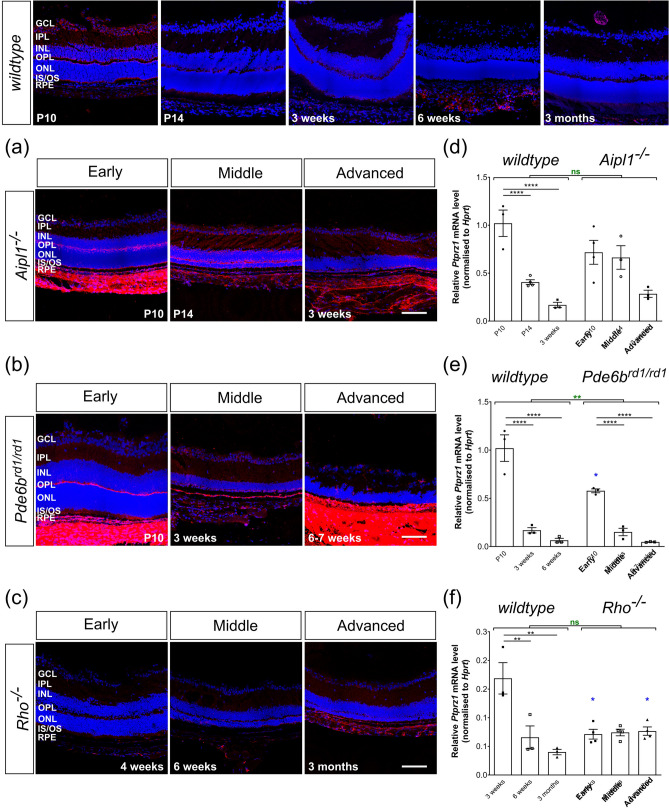
Phosphacan expression decreases with time and is unaffected by degeneration. (a)–(c) Immunolabelling for Phosphacan (red) was mostly restricted to GCL, IPL and OPL. In P10 wildtype, labelling was particularly evident at the outer margin of the OPL and the edge of the ONL itself. In (a) Aipl1-/- and (b) Pde6brd1/rd1 mice, labelling was most prominent in the OPL and RPE, compared to age-matched wildtype. (c) In Rho-/- retinae, labelling was weak at all time-points. (d)–(f) Ptprz1 mRNA expression was significantly decreased with time in all models including wildtype retinae. N.B. mRNA levels shown for wildtype are the same as in Fig. 1e. Scale bar, 100 µm. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 (one-way ANOVA test with Bonferroni’s correction, black; parametric t-test for age-matched comparisons between wildtype and disease model, blue; two-way ANOVA test applied for assessments of change over time, red). ONL—outer nuclear layer; OPL—outer plexiform layer; INL—inner nuclear layer; IPL—inner plexiform layer; GCL—ganglion cell layer. Nuclei are counter stained with Dapi (blue).
RNA extraction and reverse transcription
Retinal tissues were carefully dissected, snap frozen in liquid nitrogen and kept in − 80 °C until extracted. Total RNA was isolated using DNA/RNA mini kit (Qiagen), following manufacturer’s instructions. Total RNA (700 µg) was reverse transcribed to obtain cDNA using QuantiTect Reverse Transcription kit, following manufacturer’s instructions. Briefly, total RNA was incubated for 15 min at 42 °C with gDNA Wipeout Buffer to eliminate genomic DNA, and the RNA samples was reverse transcribed using Quantiscript Reverse Transcriptase, Quantiscript RT Buffer, and RT Primer Mix at 42 °C for 1 h. The reaction was inactivated at 95 °C for 3 min.
Real-time qPCR
TaqMan analysis was used to measure mRNA levels of the target genes. To measure mRNA levels of Neurocan (Ncan) and Phosphacan (Ptprz1), the following primers and probes (TaqMan Universal ProbeLibrary; Roche) were used at final concentration of 200 nM and 100 nM, respectively: Hprt; Fw TCCTCCTCAGACCGCTTTT, Rev CCTGGTTCATCATCGCTAATC, Probe number 95. Ncan; Fw GCACAGAGCCAATGCTACC, Rev GCCCGATAATGGAACACG, Probe number 3. Ptprz1; Fw GGCACTCAGGAGTATCCAACA, Rev GACCAATACGAGACTCATGGCTA, Probe number 34. For Aggrecan (Acan) and Versican (Vcan), TaqMan Gene Expression Assays (Thermo Fisher Scientific) were used with the following probes (1 µl per reaction): Hprt; Mm01318741_m1, Acan; Mm01317794_m1, Vcan; Mm01283060_g1. The probes, primers and cDNA were mixed with PerfeCTa® qPCR FastMix II® with ROX (VWR International Ltd) at the total volume of 20 µl per reaction. Amplification of the selected genes from each sample was performed with in three parallel runs on a 96-well reaction plate. The reactions were performed using QuantStudio 6 Flex System (Thermo Fisher Scientific) with the following protocol: 2 min at 50 °C, 10 min polymerase activation at 95 °C and 40 cycles of 15 s denaturation at 95 °C, 1 min annealing and extension at 60 °C.
Statistics
Relative mRNA expression of the target gene at each time point was calculated using the comparative 2ΔΔCT method (Livak and Schmittgen, 2001), normalised to Hprt and relative to the levels at P10 in wildtype for each target gene. Specifically, the relative expression levels at each time point were calculated using the equation, where ΔΔCT = (CT,Target − CHprt)Time x − (CT,Target − CHprt)Time P10(wildtype).This time point was chosen as one representing a point where development is complete and before degeneration begins in the fast-degenerating models, like Aipl1-/- and Pde6brd1/rd1. Mean, standard error of the mean (SEM) and statistics were calculated from the 2-ΔΔCT. All means are stated ± SEM.
Data were analysed based on N > 3 individual retinae taken from different litters. Specifically, this included N = 4 for wildtype P14, all time-points in Rho-/- for Ncan and Ptprz1, and Aipl1-/- P10 for Ptprz1; N = 5 for wildtype P10 and 3 wks for Vcan; N = 7 for Aipl1-/- P10 and P14 for Vcan; N = 8 for Aipl1-/- 3 weeks for Vcan; and N = 3 for all other measurements. Statistical tests were performed using Graph Pad Prism v9 software. Two-way ANOVA was used to assess the differences across time within models (red asterisk in the graphs), one-way ANOVA with Bonferroni’s multiple comparison test for inter-group comparisons (black asterisk), and parametric t-tests (unpaired) for comparisons against age-matched wildtype controls, blue asterisk). Statistical significance is presented in the figures as follows: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns; non-significant.
Results
Changes in expression of four CSPGs commonly expressed in the CNS, Neurocan, Aggrecan, Phosphacan and Versican, were assessed across three different murine models of inherited blindness and in age-matched wildtype mice. In order to qualitatively assess the regional changes protein distribution in each model we used immunohistochemistry (IHC) and examined both the superior and inferior retina and equatorial regions, adjacent to the optic nerve (see schematics in each figure) in early, mid and advanced stages of degeneration. For quantitative analysis, real-time (RT-)qPCR was used to examine the levels of mRNA expression in whole neural retina, without RPE, over time. We first describe the patterns of expression of all four CSPGs in the normal wildtype mouse across time, from P10 through to 6 months of age, and then how these expression patterns are altered by retinal degeneration.
Aggrecan, Versican, Neurocan and Phosphacan show distinct changes in expression in later stages of maturation and across adulthood in wildtype retinae
RT-qPCR revealed striking differences in the pattern of expression of Acan, Vcan, Ncan and Ptprz1 mRNA across the later stages of postnatal development and adulthood. These trends are summarised in Fig. 1e and Table 2. Specifically, Acan showed a biphasic pattern of expression in wildtype mice (Fig. 1a); expression increased significantly between P10 and 3 weeks of age (fold change, 2.35 ± 0.49; Table 2), which was followed by a significant decrease between 3 and 6 weeks. Expression levels remained consistently low thereafter, up to 6 months of age (latest timepoint examined). While comparisons of expression levels by IHC should be assessed cautiously, a similar trend was seen in the IHC, with immunolabelling being low at P10, increasing substantially between P10 and P14 and remaining fairly constant throughout adulthood. With respect to its distribution within the retina, Aggrecan immunolabelling was particularly evident in the outer plexiform layers (OPL), inner plexiform layer (IPL) and ganglion cell layer (GCL), with relatively little staining in the outer nuclear layers (ONL) (Fig. 1g; Supplementary Figs. S1, S2). Note that for clarity of presentation, main figures show representative images of the central, superior retina (Fig. 1f); equivalent panels for the central, inferior retina for all CSPG immunostainings are shown in Supplementary Information (Supplementary Figs. Fig. S1–S4, S6, S7).
Table 2.
Relative values of Acan, Vcan, Ncan and Ptprz1 mRNA expression across time and models.
| Model/time point | Acan | Vcan | Ncan | Ptprz1 |
|---|---|---|---|---|
| wildtype/P10 | 1.01 ± 0.13 | 1.21 ± 0.45 | 1.02 ± 0.15 | 1.02 ± 0.14 |
| wildtype/P14 | 1.44 ± 0.06 | 0.56 ± 0.24 | 0.36 ± 0.10 | 0.41 ± 0.02 |
| wildtype/3 weeks | 2.35 ± 0.49 | 0.81 ± 0.37 | 0.15 ± 0.04 | 0.17 ± 0.03 |
| wildtype/6 weeks | 0.99 ± 0.16 | 1.23 ± 0.03 | 0.01 ± 0.01 | 0.07 ± 0.02 |
| wildtype/3 months | 1.02 ± 0.05 | 0.08 ± 0.00 | 0.03 ± 0.01 | 0.04 ± 0.00 |
| wildtype/6 months | 0.85 ± 0.07 | 0.09 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.01 |
| Aipl1-/-/P10 | 0.87 ± 0.06 | 0.72 ± 0.37 | 0.99 ± 0.23 | 0.72 ± 0.12 |
| Aipl1-/-/P14 | 2.27 ± 0.35 | 1.68 ± 0.59 | 0.36 ± 0.16 | 0.66 ± 0.12 |
| Aipl1-/-/3 weeks | 3.67 ± 0.45 | 1.47 ± 0.68 | 0.16 ± 0.05 | 0.28 ± 0.04 |
| Pde6brd1/rd1/P10 | 0.62 ± 0.13 | 0.53 ± 0.08 | 0.47 ± 0.14 | 0.58 ± 0.02 |
| Pde6brd1/rd1/3 weeks | 2.81 ± 0.37 | 1.32 ± 0.64 | 0.02 ± 0.00 | 0.15 ± 0.04 |
| Pde6brd1/rd1/6–7 weeks | 5.34 ± 0.08 | 0.23 ± 0.07 | 0.07 ± 0.04 | 0.05 ± 0.00 |
| Rho-/-/P10 | 1.75 ± 0.30 | 0.60 ± 0.01 | 0.23 ± 0.13 | 0.55 ± 0.10 |
| Rho-/-/3–4 weeks | 2.29 ± 0.25 | 0.49 ± 0.09 | 0.08 ± 0.01 | 0.07 ± 0.01 |
| Rho-/-/6 weeks | 1.71 ± 0.04 | 0.27 ± 0.01 | 0.09 ± 0.02 | 0.07 ± 0.01 |
| Rho-/-/3 months | 1.58 ± 0.04 | 0.37 ± 0.02 | 0.10 ± 0.02 | 0.08 ± 0.01 |
Data shown is fold change ± SEM, relative to wildtype at P10.
Levels of Vcan mRNA showed greater inter-sample variability than that of the other CSPGs. However, expression was broadly similar between P10 and 6 weeks of age, and lower at 3 and 6 months of age, although this was not a statistically significant reduction (Figs. 1b, e, g; Table 2), due to inter-sample variations. IHC showed that Versican is present in all the layers of the retina, with particularly strong labelling in the photoreceptor inner/outer segment region (IS/OS) region and the OPL (Fig. 1g; Supplementary Figs. S1, S2). Staining intensity did not change markedly over time in wildtype mice, either across the later stages of maturation or during adulthood, although did appear weaker at 3 and 6 months (Fig. 1g, Supplementary Figs. S1, S2), consistent with the PCR data.
Ncan mRNA was already present at P10, but expression reduced dramatically within the first three weeks of life in wildtype mice, with a relative value (compared to P10 wildtype) of 0.15 (± 0.04) by 3 weeks and 0.01 (± 0.01) at 6 weeks of age, a level maintained up until at least 6 months of age (Fig. 1c, e; Table 2). Neurocan can undergo proteolytic cleavage of the intact molecule, resulting in smaller isoforms; the C-terminal fraction, known as Neurocan-C, and two variants of the N-terminal end, termed Neurocan-N. We therefore used two antibodies, one that detects Neurocan-C and another that detects both forms of Neurocan-N (Fig. 1g, Supplementary Figs. S1, S2). In the P10 wildtype retina, both Neurocan-C and Neurocan-N were expressed throughout all layers of the retina (Fig. 1g, Supplementary Figs. S1, S2). Both forms were particularly highly expressed in the OPL and at the apical side of the retina, particularly in the region of the photoreceptor inner segments, and at the basal side of the retina, in the GCL. This distribution pattern and level of expression was maintained until 3 weeks, but staining decreased dramatically between 3 and 6 weeks and remained low thereafter (Fig. 1g, Supplementary Figs. S1, S2).
Next, we investigated the expression levels and distribution of Phosphacan. Phosphacan is a splice variant of the receptor protein tyrosine phosphatase (also known as PTP-zeta, Ptprz1). Consistent with other reports (Inatani et al. 2000; Leung et al. 2004), we found that Ptprz1 expression was highest at P10 and declined thereafter, with a relative value of 0.17 (± 0.03) by 3 weeks and 0.03 (± 0.01) at 6 months of age (Fig. 1d, e, Table 2). IHC showed that Phosphacan immunolabelling was most evident in the GCL, at the apical margin of the OPL and in the photoreceptor inner segment region, with weak staining in the INL and ONL (Fig. 1g). Similar to Neurocan, immunolabelling for Phosphacan decreased markedly after the first 3 weeks, with very little staining evident from 6 weeks of age in wildtype retinae (Fig. 1g, Supplementary Figs. S1, S2).
Together, these data show that the four CSPG core proteins show very different and distinct patterns of expression both with respect to their distribution in the retina and the amount of each core protein expressed over time in the normal wildtype adult retina. We next examined how expression of these four core proteins changes in three different models of inherited retinal degeneration. Each model was examined at early, mid and advanced stages of degeneration and compared against the relevant age-matched wildtype controls.
Aggrecan expression is markedly increased in degeneration in Pde6brd1/rd1 mice
Among the CSPGs we examined, Aggrecan expression showed the most distinct changes in expression both compared to wildtype and between models. In Aipl1-/- and Pde6brd1/rd1 mice, Acan mRNA increased between P10 and 3 weeks, following a similar pattern to wildtype. Notably, at middle stages of degeneration in Aipl1-/- and advanced stage in Pde6brd1/rd1 mice, Acan expression was significantly higher when compared to age-matched wildtype (Fig. 2d, e). The increase in Acan expression was most evident in Pde6brd1/rd1 mice, with a relative value of 5.34 (± 0.08) at advanced stage (6 weeks) compared to 0.87 (± 0.06) at early stage (P10) degeneration in the same model (Fig. 2e, Table 2). This also yielded a highly significant increase in expression compared to age-matched (6 week) wildtype controls (5.34 ± 0.08 and 0.99 ± 0.16, respectively; Fig. 2e, Table 2). The overall distribution patterns of Aggrecan immunolabelling in Aipl1-/- and Pde6brd1/rd1 were similar to that of wildtype, being most evident in the OPL, inner nuclear layer (INL), IPL and GCL, although increases in labelling was most noticeable in the IPL (Fig. 2a, b, Supplementary Fig. S3).
Surprisingly, Rho-/- mice, which exhibit a slower rate of degeneration compared to either Aipl1-/- or Pde6brd1/rd1, presented a different pattern of Aggrecan expression. Acan mRNA levels followed the same pattern as seen in development, reducing between 4 and 6 weeks of age, and lower at 6 months of age (Figs. 1b, 2f, Supplementary Fig. S8a). However, similar to Aipl1-/- and Pde6brd1/rd1 mice, expression was elevated at mid and advanced stages of degeneration, compared to age-matched wildtype mice (Figs. 1b, 2f). IHC revealed a pattern of immunolabelling very similar to that of wildtype and no degeneration-associated changes in the localisation of expression were observed (Fig. 2c, Supplementary Fig. S3).
Versican expression in degeneration is similar to the pattern of expression in wildtype retina across time but demarcates Müller glial processes in Rho-/- mice
In the disease models, Vcan mRNA expression remained largely unchanged across the respective time points examined for each model (Fig. 3d–f, Supplementary Fig. S8b). There were weak trends for an increase in Vcan expression between P10 and 3 weeks in Aipl1-/- (Fig. 3d) and a peak in expression at 3 weeks (compared to P10 and 6 weeks) in Pde6brd1/rd1 (Fig. 3e), reflecting the relevant IHC data, but these were not statistically significant due to large inter-sample variation. In Rho-/- retinae, Vcan mRNA expression showed a trend for reducing expression with time (Fig. 3f). Notably, Vcan expression was significantly higher in more advanced stages of degeneration in Rho-/-, but was similar to, or even lower than, age-matched wildtype between P10 and 6 weeks. Versican maintained a similar distribution as seen in wildtype across time, even in the later stages of degeneration in both Aipl1-/- and Pde6brd1/rd1, with expression predominantly located in the two plexiform layers, INL and GCL (Fig. 3a, b, Supplementary Fig. S4). Conversely, in Rho-/- the immunolabelling for Versican was stronger in 3 months old Rho-/- retinae, compared both to earlier time points and to age-matched wildtype controls; labelling was most evident in the ONL and IS/OS at this stage (Fig. 3c, Supplementary Fig. S4). Versican was also more noticeable in the INL and IPL at middle time-points in Pde6brd1/rd1 mice and at later time-points in Rho-/- mice, something not seen in age-matched wildtype mice (Fig. 3b, c, Supplementary Fig. S4).
As noted above, main figures show representative images of the central, superior retina only. However, we also examined the entire retina in all cases for other spatially restricted changes. In the main, there were no obvious region-specific differences, but we did observe a disease- and region-specific change in Versican expression. Versican was found associated with Müller glial-like processes in the peripheral retina of Rho-/- mice from 6 weeks onwards (Fig. 4a). Co-staining with Müller specific markers confirmed the identity of the Versican-positive processes (Fig. 4b). This observation was specific to Versican and Rho-/- and was not observed for any other model or CPSG we examined (see Supplementary Figs. S1–S7). Given this apparently glia-specific pattern of Versican expression, we also examined whether Versican was expressed by Iba1-posiitive microglia cells (Supplementary Fig. S5). Co-staining was not possible due to incompatible labelling protocols; however, comparison of the patterns of Iba1 and Versican immuno-labelling does not indicate colocalization, at least within the ONL, OPL, INL and IPL. However, since some microglia somata may be located in the GCL and nerve fiber layer, we cannot rule out the possibility that these may express Versican in the later stages of degeneration in the periphery of Rho-/- mice.
Neurocan expression in degeneration is similar to the pattern of expression in wildtype retina across time
Perhaps surprisingly in view of the reported upregulation of Neurocan in other regions of the brain and the optic nerve following injury14,18,19,30, qRT-PCR analysis revealed no indication of an increase in Ncan mRNA levels in any of the three models of progressive degeneration, compared to age-matched wildtype retinae. Both Aipl1-/- and Pde6brd1/rd1 mice showed the same profile of expression across age, with highest levels seen at P10 and decreasing with age, in line with wildtype mice (Fig. 5d, e; Table 2). However, the relative levels of Ncan at P10 were lower than that of age-matched wildtype mice in both Pde6brd1/rd1 and Rho-/- mice and expression dropped further after P10 (Fig. 5e, f, Supplementary Fig. S8c). This initial reduction was most apparent in Rho-/- (Fig. 5f) although Ncan mRNA levels then remained stable between 3–4 weeks and 3 months of age in Rho-/- mice. This differs from wildtype mice where the levels continued to drop after 6 weeks of age, yielding a significant difference between Rho-/- and age-matched wildtype mice (Fig. 5f).
The spatial distribution of both Neurocan-C and Neurocan-N is similar in the different models of degeneration as in the wildtype eye, with expression predominantly locating to the OPL and outer margin of the retina in the segment region (Fig. 5a, b, c, Supplementary Figs. S1, S6). Neurocan-N levels were broadly similar in all disease models, although immunolabelling was consistently stronger in the Aipl1-/- mice than that observed in age-matched wildtype mice (Fig. 5a, Supplementary Figs. S6). Conversely, immunolabelling for Neurocan-C was typically weaker in mid to advanced stages of degeneration in both Aipl1-/- and Pde6brd1/rd1 eyes, compared to wildtype (Fig. 5a, b, Supplementary Figs. S1, S6). In Rho-/-, Neurocan-N remained fairly consistent between 3–4 weeks and 3 months of age (earlier time points not examined), while immunostaining intensity increased over the same period for Neurocan-C (Fig. 5c, Supplementary Fig. S6). Both Neurocan-C and Neurocan-N were distributed in a pattern similar to that seen in the wildtype retina, throughout all the layers of retina, with increased deposition of Neurocan-C at mid to advanced stages in GCL, IPL, INL and OPL (Fig. 5c, Supplementary Fig. S6). No consistent differences between the superior or inferior retina in the patterns of Neurocan (C or N) staining were observed in any of the models (Figs. 5a, b, c, Supplementary Figs. S1, S6).
Phosphacan expression in degeneration is similar to the pattern of expression in wildtype retina across time
In all disease models, Ptprz1 mRNA levels followed the same expression profile as wildtype (Fig. 1d), with expression of Ptprz1 decreasing significantly over time (Fig. 6d, e, f). Of note, and similar to Ncan, mRNA levels of Ptprz1 in Pde6brd1/rd1 at P10 was statistically lower than that of the same age in wildtype mice (Fig. 6e). The same tendency was also observed in Aipl1-/- and Rho-/- mice but was not statistically significant (Fig. 6d, f, Supplementary Fig. S8d). In adult Rho-/- mice, Ptprz1 expression remained low and unchanged over time and degeneration, like age-matched adult wildtype retina (Fig. 6f). Aipl1-/- and Pde6brd1/rd1 mice showed a similar distribution of Phosphacan protein as that seen in wildtype mice, although labelling was more intense in the OPL and RPE, and comparatively low in the GCL (Fig. 6a, b, Supplementary Fig. S7). Of note, Rho-/- mice showed little or no change in immunolabelling for Phosphacan and the distribution remained similar to age-matched wildtype retinae throughout (Fig. 6c, Supplementary Fig. S7).
Discussion
CSPGs are widely reported to be upregulated following neuronal injury. This study demonstrates that this is an oversimplification and that there is significant heterogeneity in response to progressive neuronal degeneration, with different CSPG core proteins exhibiting very distinct patterns of expression, even between models with similar rates of neurodegeneration. Previously, we examined global changes in CSPG protein deposition using the CS-56 antibody24 and found that deposition broadly increased over time in wildtype and Rho-/- and decreased in Pde6brd1/rd1. However, such an approach may mask different changes in individual CSPGs. Our present data examining mRNA levels for four CSPG core proteins showed decreasing expression of Ncan and Ptprz1 in all models, contrasted by a significant increase of Acan in Pde6brd1/rd1 and Aipl1-/- retinae, and no significant change of Vcan in any model, including wildtype. These differences highlight the importance of studying specific CSPGs, as well as global changes. This study is the first comprehensive assessment of specific CSPGs in murine models of progressive retinal degeneration. It also provides the first comparative assessment of multiple CSPGs across time in the late postnatal and adult wildtype retina.
Many factors affect the relative levels of a given CSPG core protein, including synthesis and degradation. CSPG-degrading enzymes such as MMP (matrix metalloproteinases) and ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin Motifs)31–33 degrade different CSPGs with different efficiencies. The expression of these enzymes can change significantly, both during development and in ocular diseases, such as diabetic retinopathy, myopic chorioretinal atrophy, cataract and retinitis pigmentosa34–38, and alongside Müller glia proliferation39. Other CSPGs that were not assessed in the current study may also contribute to the global changes in CSPG deposition, as assessed with CS-56.
Understanding how CSPGs exert their actions on neurite outgrowth is essential if we are to manipulate the diseased microenvironment in order to make it more permissive for regeneration. In our present work, Aggrecan and Versican were most prevalent, particularly in degeneration, and with aging, while Neurocan and Phosphacan were much less evident. Aggrecan and Versican each contain more GAG chains (~ 100 and 5–23, respectively) than either Neurocan or Phosphacan (< 5)40, suggesting that these CSPGs may present an inhibitory chemical barrier to synaptic plasticity during degeneration and/or regeneration. Enzymatic digestion of GAG chains can promote axonal regeneration and plasticity following CNS damage7. Consistent with this, treatment of recipient retinas with Chondroitinase ABC improved donor-host interactions following cell transplantation1,41–44, while viral diffusion and transduction efficiency were improved by co-application of ChABC with viral vector delivery45–47. Enzymatic degradation is not without its limitations, however48,49 and significant inhibitory function is retained by the core protein, even after GAG digestion9.
Of the CSPGs examined, Aggrecan demonstrated the greatest heterogeneity across disease models. In the normal adult eye, it is thought to play a role in the maintenance of the laminar structure of the retina50–52. In mice, refinement of synaptic sub-laminae is not complete until P2153. Aggrecan expression is seen from embryonic stages and increases with age; it is widespread, even in the normal adult retina, particularly in the synaptic layers21,22. Consistent with this, we found Acan expression to increase significantly between P10 and 3 weeks of age and remain at a similar level thereafter in the wildtype retina; immunolablling for Aggrecan increased markedly between P10 and P14 and was most evident in the inner and outer plexiform layers. Together, these changes indicate a role for Aggrecan in the establishment and maintainance of laminar structure. Previous reports examining the developing rat and mouse retina have reported the expression of both Neurocan and Phosphacan from E13-17, peaking around P720,21,54,55. Given that the primary focus of this study was to examine expression across progressive degeneration, we did not look earlier than P10. However, consistent with these reports, we found that the expression of Neurocan and Phosphacan was highest in all models at P10 and declined thereafter.
Aggrecan’s upregulation following acute injuries, such as spinal cord crush13,37, is well reported. It impedes the regeneration of both endogenous and transplanted neurons12,13. Its expression in progressive degeneration is less well characterised but it is upregulated in rats with retinal dystrophy52 and induced retinal ischemia26. Similarly, we found that Aggrecan expression was dramatically upregulated in the Aipl1-/- and Pde6brd1/rd1 models of rapid photoreceptor degeneration, but, surprisingly, not in the more slowly degenerating Rho-/-, which undergoes a slower rate of degeneration. These differences are intriguing and further exploration of Aggrecan in other models of slow-to-moderate degeneration would be of interest to establish whether rate of degeneration is a determining factor. Some ADAMTS genes, including the aggrecanase ADAMTS5 (also known as ADAMTS11), are reported to be upregulated in AMD and are thought to influence retinal pathology by proteolytic modification of the retinal extracellular matrix56. Of note, studies in the barrel cortex have shown that of PNNs are lost during sensory deprivation and that this is mediated by a reduction in the expression of Aggrecan57,58. Hence, targeted reduction of Aggrecan may be beneficial for therapeutic strategies that require the formation of new synapses.
Although Versican expression was broadly similar in wildtype, Aipl1-/- and Pde6brd1/rd1 retinae, we found notable region-specific changes in expression in the Rho-/- mouse. In our study, Versican + /Gfap + Müller glial processes were observed, but only in the peripheral retina of Rho-/- mice and at later stages of degeneration. Iba1 + microglia in the same region did not appear to co-label with Versican. The differential distribution of Versican, both with respect to a sub-population of Müller glia within a given retina and also between models is worthy of further investigation. Note that the rod photoreceptors in the Rho-/- model are non-functional from their genesis onwards so it is conceivable that some of the model-specific changes observed in this study may reflect a developmental alteration in response to the absence of rod function. Cone function is, however, normal in these mice. In order to assess all isoforms of Versican, we used an antibody detecting the G3 domain to assess regional changes and chose PCR primers corresponding to the same sequence used to raise the antibody (exon 14–15). The G3 domain has been reported to play a key role in the secretion of Versican from the cell59 and binds to several molecules including the EGF receptor, tenascin, fibronectin and integrin β160,61. The G3 domain has been shown to promote neurite outgrowth and cell attachment in hippocampal neurons via an EGF receptor-mediated pathway62, potentially indicating a localised pro-regenerative impact on the environment in the periphery. The specific association of G3 domain with peripheral Müller glial processes, which in lower vertebrates retain a more immature, progenitor/stem cell-like phenotype, indicates local microenvironmental differences within the degenerating retina.
Neurocan is upregulated in many experimental models of acute injury in the brain14,18,19, optic nerve crush30 and in the ischaemic rat retina63. In contrast, we did not observe any upregulation of Neurocan with progressive retinal degeneration. Indeed, mRNA levels of Ncan and Ptprz1 at P10 were actually significantly lower in Pde6brd1/rd1 and Rho-/-, compared to age-matched wildtype mice. There was an accompanying reduction in Neurocan-C staining at the OPL/ONL interface as degeneration progresses in Aipl1-/- and Pde6brd1/rd1, while labelling for the Neurocan-N fraction remained fairly constant in all disease models. Neurocan, and its cleavage, demonstrates dynamic changes during development, injury and remodelling in the CNS; full length Neurocan was expressed in juvenile rat brain65 and following acute brain injury14,65. In contrast, only cleaved Neurocan was present in rat adult brain, and more interestingly, only N- and not C-terminal fragments were observed as a component of PNNs in adult rat cerebellum64. A potential role for cleaved Neurocan was indicated in a recent study showing Neurocan-N fractions are essential in Semaphorin 3F-induced dendric spine remodelling66. In our data, the labelling of both C- and N-terminal fractions were observed in early stages, indicating that full length fractions are likely present in the juvenile retina. Conversely, neither of the fractions were seen in significant amounts from 3 weeks of age in wildtype mice. Both changes are similar to those described for the brain64. In contrast, Neurocan-N and -C fractions predominated in Aipl1-/- and Pde6brd1/rd1, respectively. Similarly, Phosphacan labelling was seen at the outer margin of the OPL between P10 and 3 weeks in wildtype, but not in Aipl1-/- and Pde6brd1/rd1, retinae. Observationally, the separation of photoreceptors in the ONL and the OPL was also less well defined in both Aipl1-/- and Pde6brd1/rd1 mice, compared to wildtype. While the reduced levels may simply reflect a reduction in the number of photoreceptor neurons that may produce these proteins, the different patterns of expression seen in wildtype and diseased retina, together with previous studies examining Neurocan cleavage, suggest that Neurocan and Phosphacan may play a role in the establishment and maintenance of retinal lamination.
In summary, our study provides the first comprehensive characterisation of individual CSPGs and how their expression changes both with age and with progressive retinal degeneration. It also highlights an important heterogeneity in the retinal environments that arise in degeneration due to different genetic causes and the importance for researchers to characterize the specific models they use. Understanding the distinct changes in expression and distribution of individual CSPGs may help us to modulate specific diseased microenvironments, affecting synaptic plasticity, regeneration and repair.
Supplementary Information
Acknowledgements
We thank Professor James Fawcett for the kind gift of Neurocan-N and Neurocan-C antibodies, and members of the Pearson and Ali groups for constructive criticism and discussion of earlier drafts of the manuscript, with particular thanks to Dr C Procyk. This work was supported by grants from Fight for Sight (1566/1567), Moorfields Eye Charity (R180005A), and Medical Research Council UK (MR/T002735/1) and funding from Guy’s and St Thomas Charity and King’s Health Partners. A.K.K. was a Frankenburg Fight for Sight PhD Student (2015-2019).
Abbreviations
- CNS
Central nervous system
- CSPG
Chondroitin sulphate proteoglycan
- GAG
Glycosaminoglycan
- GCL
Ganglion cell layer
- GFAP
Glial fibrillary acidic protein
- INL
Inner nuclear layer
- IPL
Inner plexiform layer
- IS
Inner segment
- ONL
Outer nuclear layer
- OPL
Outer plexiform layer
- OS
Outer segment
Author contributions
A.M., A.A.K. and R.A.P. conceived the project; A.M., A.A.K., A.J.S., and R.A.P. devised the methodology; A.M and A.A.K. carried out the experiments; All authors contributed to determining the methods of analysis and formal analysis was performed by A.M. and A.K.K.. R.R.A. and R.A.P. provided funding and resources; A.M. and R.A.P. wrote the original manuscript and A.M. prepared the figures. All authors contributed to reviewing, editing and approving the final manuscript.
Data availability
According to UK research council's Common Principles on Data Policy, data supporting this study will be openly available at https://github.com/RPearsonLab/CSPG-expression-in-the-retina.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
A. Matsuyama, Email: ayako.matsuyama@riken.jp
R. A. Pearson, Email: rachael.pearson@kcl.ac.uk
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-11204-w.
References
- 1.Barber AC, et al. Repair of the degenerate retina by photoreceptor transplantation. Proc. Natl. Acad. Sci. 2013;110:354–359. doi: 10.1073/pnas.1212677110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nori S, et al. Human oligodendrogenic neural progenitor cells delivered with chondroitinase ABC facilitate functional repair of chronic spinal cord injury. Stem Cell Rep. 2018;11:1433–1448. doi: 10.1016/j.stemcr.2018.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fawcett JW, Oohashi T, Pizzorusso T. The roles of perineuronal nets and the perinodal extracellular matrix in neuronal function. Nat. Rev. Neurosci. 2019;20:451–465. doi: 10.1038/s41583-019-0196-3. [DOI] [PubMed] [Google Scholar]
- 4.Burns ME, Stevens B. Report on the National Eye Institute’s audacious goals initiative: Creating a cellular environment for neuroregeneration. eneuro. 2018 doi: 10.1523/ENEURO.0035-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muir E, De Winter F, Verhaagen J, Fawcett J. Recent advances in the therapeutic uses of chondroitinase ABC. Exp. Neurol. 2019;321:113032. doi: 10.1016/j.expneurol.2019.113032. [DOI] [PubMed] [Google Scholar]
- 6.Sami A, Selzer ME, Li S. Advances in the signaling pathways downstream of glial-scar axon growth inhibitors. Front. Cell. Neurosci. 2020;14:174. doi: 10.3389/fncel.2020.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith PD, Coulson-Thomas VJ, Foscarin S, Kwok JCF, Fawcett JW. “GAG-ing with the neuron”: The role of glycosaminoglycan patterning in the central nervous system. Exp. Neurol. 2015;274:100–114. doi: 10.1016/j.expneurol.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Wu Y, et al. Versican V1 isoform induces neuronal differentiation and promotes neurite outgrowth. Mol. Biol. Cell. 2004;15:2093–2104. doi: 10.1091/mbc.e03-09-0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inatani M, et al. Inhibitory effects of neurocan and phosphacan on neurite outgrowth from retinal ganglion cells in culture. Invest. Ophthalmol. Vis. Sci. 2001;42:1930–1938. [PubMed] [Google Scholar]
- 10.Oakley RA, Tosney KW. Peanut agglutinin and chondroitin-6-sulfate are molecular markers for tissues that act as barriers to axon advance in the avian embryo. Dev. Biol. 1991;147:187–206. doi: 10.1016/S0012-1606(05)80017-X. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz NB, Domowicz M, Krueger RC, Li H, Mangoura D. Brain aggrecan. Perspect. Dev. Neurobiol. 1996;3:291–306. [PubMed] [Google Scholar]
- 12.Afshari FT, Kwok JC, White L, Fawcett JW. Schwann cell migration is integrin-dependent and inhibited by astrocyte-produced aggrecan. Glia. 2010;58:857–869. doi: 10.1002/glia.20970. [DOI] [PubMed] [Google Scholar]
- 13.Lemons ML, Sandy JD, Anderson DK, Howland DR. Intact aggrecan and chondroitin sulfate-depleted aggrecan core glycoprotein inhibit axon growth in the adult rat spinal cord. Exp. Neurol. 2003;184:981–990. doi: 10.1016/S0014-4886(03)00383-2. [DOI] [PubMed] [Google Scholar]
- 14.Asher RA, et al. Neurocan is upregulated in injured brain and in cytokine-treated astrocytes. J. Neurosci. 2000;20:2427–2438. doi: 10.1523/JNEUROSCI.20-07-02427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asher RA, et al. Versican is upregulated in CNS injury and is a product of oligodendrocyte lineage cells. J. Neurosci. 2002;22:2225–2236. doi: 10.1523/JNEUROSCI.22-06-02225.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sobel RA, Ahmed AS. White matter extracellular matrix chondroitin sulfate/dermatan sulfate proteoglycans in multiple sclerosis. J. Neuropathol. Exp. Neurol. 2001;60:1198–1207. doi: 10.1093/jnen/60.12.1198. [DOI] [PubMed] [Google Scholar]
- 17.Jones LL, Margolis RU, Tuszynski MH. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp. Neurol. 2003;182:399–411. doi: 10.1016/S0014-4886(03)00087-6. [DOI] [PubMed] [Google Scholar]
- 18.Sajad M, Zargan J, Chawla R, Umar S, Khan HA. Upregulation of CSPG3 accompanies neuronal progenitor proliferation and migration in EAE. J. Mol. Neurosci. 2011;43:531–540. doi: 10.1007/s12031-010-9476-0. [DOI] [PubMed] [Google Scholar]
- 19.Andrews EM, Richards RJ, Yin FQ, Viapiano MS, Jakeman LB. Alterations in chondroitin sulfate proteoglycan expression occur both at and far from the site of spinal contusion injury. Exp. Neurol. 2012;235:174–187. doi: 10.1016/j.expneurol.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inatani M, et al. Spatiotemporal expression patterns of 6B4 proteoglycan/phosphacan in the developing rat retina. Invest. Ophthalmol. Vis. Sci. 2000;41:1990–1997. [PubMed] [Google Scholar]
- 21.Popp S, Maurel P, Andersen JS, Margolis RU. Developmental changes of aggrecan, versican and neurocan in the retina and optic nerve. Exp. Eye Res. 2004;79:351–356. doi: 10.1016/j.exer.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 22.Keenan TDL, et al. Mapping the differential distribution of proteoglycan core proteins in the adult human retina, choroid, and sclera. Investig. Ophthalmol. Vis. Sci. 2012;53:7528–7538. doi: 10.1167/iovs.12-10797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Felemban M, et al. Extracellular matrix component expression in human pluripotent stem cell-derived retinal organoids recapitulates retinogenesis in vivo and reveals an important role for IMPG1 and CD44 in the development of photoreceptors and interphotoreceptor matrix. Acta Biomater. 2018;74:207–221. doi: 10.1016/j.actbio.2018.05.023. [DOI] [PubMed] [Google Scholar]
- 24.Hippert C, et al. Müller glia activation in response to inherited retinal degeneration is highly varied and disease-specific. PLoS ONE. 2015;10:e0120415. doi: 10.1371/journal.pone.0120415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y, et al. Mechanism of all-trans-retinal toxicity with implications for stargardt disease and age-related macular degeneration. J. Biol. Chem. 2012;287:5059–5069. doi: 10.1074/jbc.M111.315432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reinhard J, et al. Ischemic injury leads to extracellular matrix alterations in retina and optic nerve. Sci. Rep. 2017;7:43470. doi: 10.1038/srep43470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishiguchi KM, et al. Gene therapy restores vision in rd1 mice after removal of a confounding mutation in Gpr179. Nat. Commun. 2015;6:6006. doi: 10.1038/ncomms7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramamurthy V, Niemi GA, Reh TA, Hurley JB. Leber congenital amaurosis linked to AIPL1: A mouse model reveals destabilization of cGMP phosphodiesterase. Proc. Natl. Acad. Sci. U. S. A. 2004;101:13897. doi: 10.1073/pnas.0404197101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kruczek K, et al. Differentiation and transplantation of embryonic stem cell-derived cone photoreceptors into a mouse model of end-stage retinal degeneration. Stem Cell Reports. 2017;8:1659–1674. doi: 10.1016/j.stemcr.2017.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pearson CS, et al. Spatiotemporal distribution of chondroitin sulfate proteoglycans after optic nerve injury in rodents. Exp. Eye Res. 2020;190:107859. doi: 10.1016/j.exer.2019.107859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zuo J, Ferguson TA, Hernandez YJ, Stetler-Stevenson WG, Muir D. Neuronal matrix metalloproteinase-2 degrades and inactivates a neurite- inhibiting chondroitin sulfate proteoglycan. J. Neurosci. 1998;18:5203–5211. doi: 10.1523/JNEUROSCI.18-14-05203.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stanton H, Melrose J, Little CB, Fosang AJ. Proteoglycan degradation by the ADAMTS family of proteinases. Biochim. Biophys. Acta Mol. Basis Dis. 2011;1812:1616–1629. doi: 10.1016/j.bbadis.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Lemarchant S, et al. ADAMTS proteoglycanases in the physiological and pathological central nervous system. J. Neuroinflammation. 2013;10:133. doi: 10.1186/1742-2094-10-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wride MA, Geatrell J, Guggenheim JA. Proteases in eye development and disease. Birth Defects Res. Part C Embryo Today Rev. 2006;78:90–105. doi: 10.1002/bdrc.20063. [DOI] [PubMed] [Google Scholar]
- 35.Aldahmesh MA, et al. The syndrome of microcornea, myopic chorioretinal atrophy, and telecanthus (MMCAT) is caused by mutations in ADAMTS18. Hum. Mutat. 2013;34:1195–1199. doi: 10.1002/humu.22374. [DOI] [PubMed] [Google Scholar]
- 36.Nandadasa S, Foulcer S, Apte SS. The multiple, complex roles of versican and its proteolytic turnover by ADAMTS proteases during embryogenesis. Matrix Biol. 2014;35:34–41. doi: 10.1016/j.matbio.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Demircan K, et al. ADAMTS4 and ADAMTS5 knockout mice are protected from versican but not aggrecan or brevican proteolysis during spinal cord injury. Biomed Res. Int. 2014;2014:1–8. doi: 10.1155/2014/693746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shin JA, et al. Inhibition of matrix metalloproteinase 9 enhances rod survival in the s334ter-Line3 retinitis pigmentosa model. PLoS ONE. 2016;11:e0167102. doi: 10.1371/journal.pone.0167102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campbell WA, et al. Matrix-metalloproteinase expression and gelatinase activity in the avian retina and their influence on Müller glia proliferation. Exp. Neurol. 2019;320:112984. doi: 10.1016/j.expneurol.2019.112984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galtrey CM, Fawcett JW. The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res. Rev. 2007;54:1–18. doi: 10.1016/j.brainresrev.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki T, et al. Chondroitinase ABC treatment enhances synaptogenesis between transplant and host neurons in model of retinal degeneration. Cell Transplant. 2007;16:493–503. doi: 10.3727/000000007783464966. [DOI] [PubMed] [Google Scholar]
- 42.Singhal S, et al. Chondroitin sulfate proteoglycans and microglia prevent migration and integration of grafted müller stem cells into degenerating retina. Stem Cells. 2008;26:1074–1082. doi: 10.1634/stemcells.2007-0898. [DOI] [PubMed] [Google Scholar]
- 43.Ma J, Kabie M, Tucker BA, Ge J, Young MJ. Combining chondroitinase ABC and growth factors promotes the integration of murine retinal progenitor cells transplanted into Rho-/- mice. Mol. Vis. 2011;17:1759–1770. [PMC free article] [PubMed] [Google Scholar]
- 44.Mandai M, et al. Adequate time window and environmental factors supporting retinal graft cell survival in rd mice. Cell Med. 2012;4:45–54. doi: 10.3727/215517912X639315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grüter O, et al. Lentiviral vector-mediated gene transfer in adult mouse photoreceptors is impaired by the presence of a physical barrier. Gene Ther. 2005;12:942–947. doi: 10.1038/sj.gt.3302485. [DOI] [PubMed] [Google Scholar]
- 46.Cehajic-Kapetanovic J, le Goff MM, Allen A, Lucas RJ, Bishop PN. Glycosidic enzymes enhance retinal transduction following intravitreal delivery of AAV2. Mol. Vis. 2011;17:1771–1783. [PMC free article] [PubMed] [Google Scholar]
- 47.Cehajic-Kapetanovic J, Milosavljevic N, Bedford RA, Lucas RJ, Bishop PN. Efficacy and safety of glycosidic enzymes for improved gene delivery to the retina following intravitreal injection in mice. Mol. Ther. Methods Clin. Dev. 2018;9:192–202. doi: 10.1016/j.omtm.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tester NJ, Plaas AH, Howland DR. Effect of body temperature on chondroitinase ABC’s ability to cleave chondroitin sulfate glycosaminoglycans. J. Neurosci. Res. 2007;85:1110–1118. doi: 10.1002/jnr.21199. [DOI] [PubMed] [Google Scholar]
- 49.Shirdel SA, Khalifeh K, Golestani A, Ranjbar B, Khajeh K. Critical role of a loop at C-terminal domain on the conformational stability and catalytic efficiency of chondroitinase ABC I. Mol. Biotechnol. 2015;57:727–734. doi: 10.1007/s12033-015-9864-3. [DOI] [PubMed] [Google Scholar]
- 50.Lemons ML, Howland DR, Anderson DK. Chondroitin sulfate proteoglycan immunoreactivity increases following spinal cord injury and transplantation. Exp. Neurol. 1999;160:51–65. doi: 10.1006/exnr.1999.7184. [DOI] [PubMed] [Google Scholar]
- 51.Ali SAM, Hosaka YZ, Uehara M. Spatiotemporal distribution of chondroitin sulfate proteoglycans in the developing mouse retina and optic nerve. J. Vet. Med. Sci. 2011;73:13–18. doi: 10.1292/jvms.10-0201. [DOI] [PubMed] [Google Scholar]
- 52.Chen L-F, FitzGibbon T, He J-R, Yin ZQ. Localization and developmental expression patterns of CSPG-cs56 (aggrecan) in normal and dystrophic retinas in two rat strains. Exp. Neurol. 2012;234:488–498. doi: 10.1016/j.expneurol.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 53.Sarin S, et al. Role for Wnt signaling in retinal neuropil development: Analysis via RNA-seq and in vivo somatic CRISPR mutagenesis. Neuron. 2018 doi: 10.1016/j.neuron.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Inatani M, Tanihara H, Oohira A, Honjo M, Honda Y. Identification of a nervous tissue-specific chondroitin sulfate proteoglycan, neurocan, in developing rat retina. Invest. Ophthalmol. Vis. Sci. 1999;40:2350–2359. [PubMed] [Google Scholar]
- 55.Leung K, Margolis R, Chan S. Expression of phosphacan and neurocan during early development of mouse retinofugal pathway. Dev. Brain Res. 2004;152:1–10. doi: 10.1016/j.devbrainres.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 56.Bevitt DJ, et al. Expression of ADAMTS metalloproteinases in the retinal pigment epithelium derived cell line ARPE-19: Transcriptional regulation by TNFα. Biochim. Biophys. Acta Gene Struct. Expr. 2003;1626:83–91. doi: 10.1016/S0167-4781(03)00047-2. [DOI] [PubMed] [Google Scholar]
- 57.McRae PA, Rocco MM, Kelly G, Brumberg JC, Matthews RT. Sensory deprivation alters aggrecan and perineuronal net expression in the mouse barrel cortex. J. Neurosci. 2007;27:5405–5413. doi: 10.1523/JNEUROSCI.5425-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rowlands D, et al. Aggrecan directs extracellular matrix-mediated neuronal plasticity. J. Neurosci. 2018;38:10102–10113. doi: 10.1523/JNEUROSCI.1122-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harten IA, et al. The synthesis and secretion of versican isoform V3 by mammalian cells: A role for N-linked glycosylation. Matrix Biol. 2020;89:27–42. doi: 10.1016/j.matbio.2020.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu YJ, Pierre DP, Wu J, Yee AJ, Yang BB. The interaction of versican with its binding partners. Cell Res. 2005;15:483–494. doi: 10.1038/sj.cr.7290318. [DOI] [PubMed] [Google Scholar]
- 61.Wight TN. Provisional matrix: A role for versican and hyaluronan. Matrix Biol. 2017;60–61:38–56. doi: 10.1016/j.matbio.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiang Y-Y, et al. Versican G3 domain regulates neurite growth and synaptic transmission of hippocampal neurons by activation of epidermal growth factor receptor. J. Biol. Chem. 2006;281:19358–19368. doi: 10.1074/jbc.M512980200. [DOI] [PubMed] [Google Scholar]
- 63.Inatani M, et al. Upregulated expression of neurocan, a nervous tissue specific proteoglycan, in transient retinal ischemia. Invest. Ophthalmol. Vis. Sci. 2015;41:2748–2754. [PubMed] [Google Scholar]
- 64.Matsui F, et al. Occurrence of a N-terminal proteolytic fragment of neurocan, not a C- terminal half, in a perineuronal net in the adult rat cerebrum. Brain Res. 1998;790:45–51. doi: 10.1016/S0006-8993(98)00009-2. [DOI] [PubMed] [Google Scholar]
- 65.Deguchi K, et al. Expression of neurocan after transient middle cerebral artery occlusion in adult rat brain. Brain Res. 2005;1037:194–199. doi: 10.1016/j.brainres.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 66.Mohan V, et al. Neurocan inhibits semaphorin 3F induced dendritic spine remodeling through NrCAM in cortical neurons. Front. Cell. Neurosci. 2018;12:346. doi: 10.3389/fncel.2018.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
According to UK research council's Common Principles on Data Policy, data supporting this study will be openly available at https://github.com/RPearsonLab/CSPG-expression-in-the-retina.