Abstract
Clinafloxacin (CI-960) is a potent broad-spectrum, fluoroquinolone antibiotic that has been studied for parenteral and oral administration in patients with serious infections. The objectives of these studies were to examine the pharmacokinetics and safety of clinafloxacin following administration of single and twice-daily intravenous (i.v.) and oral doses to volunteers. Plasma and urine samples were assayed by validated liquid chromatographic methods, and pharmacokinetic parameter values were determined by noncompartmental methods. Safety was evaluated by clinical observation and laboratory tests. Absorption was rapid after oral administration, with maximum concentrations in plasma (Cmax) generally occurring within 2 h. Concentrations in plasma declined biexponentially, with an average terminal half-life of 4 to 6 h after single doses and 5 to 7 h after multiple doses. Increases in Cmax and area under the concentration-time curves (AUC) were generally proportional to the dose. The volume of distribution was much greater than total body water. Approximately 40 to 75% of the clinafloxacin doses were excreted unchanged into urine. Absolute bioavailability of orally administered clinafloxacin was approximately 90% and did not change with increasing dose. Therefore, switching patients from i.v. to oral dosing should achieve similar concentrations in plasma. The tolerability of clinafloxacin was acceptable. No serious adverse events occurred. Cmax values and minimum plasma clinafloxacin concentrations during multiple dosing exceeded MICs for a wide range of organisms.
Clinafloxacin [(±)-7-(3-amino-1-pyrrolidinyl)-8-chloro-1- cyclopropyl-6-fluoroquinolones-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid monohydrochloride] (CI-960) (Fig. 1) is a broad-spectrum fluoroquinolone antibiotic that has been studied for parenteral and oral administration in patients with serious infections. Microbiological studies demonstrated that clinafloxacin is highly active against a broad spectrum of bacterial species (3, 4, 5, 7, 11). These include gram-positive and -negative species, including Enterobacteriaceae, nonfermenters, obligate intracellular pathogens, and obligate anaerobes. Furthermore, clinafloxacin is active against many bacteria that are resistant to a wide variety of other antibiotics (12).
FIG. 1.
Chemical structure of clinafloxacin.
Clinafloxacin has been studied primarily in adults hospitalized for the treatment of serious and potentially life-threatening infections, including nosocomial and community-acquired pneumonia, complicated intra-abdominal infections, complicated skin and soft tissue infections, endocarditis, and acute gynecologic infections. Clinafloxacin may also be of value in the empirical treatment of patients with febrile neutropenia (12). The option of switching to an oral formulation to reduce hospital costs while maintaining therapy is of concern to all patients, especially neutropenic patients who may have damaged gastrointestinal mucosa consequent to chemotherapy.
This report describes pharmacokinetic and safety results following administration of single oral and intravenous (i.v.) doses, multiple oral and i.v. doses, single oral doses in a repeated-measures study, and single oral and i.v. doses in neutropenic patients undergoing chemotherapy. It became apparent from initial clinical studies that twice-daily 100- to 400-mg doses were required to maintain concentrations in plasma within the effective range for most infections. Therefore, multiple-dose studies of both oral and i.v. routes were examined over this dose range.
Preliminary results have been presented elsewhere (E. J. Randinitis, J. I. Brodfuehrer, and A. B. Vassos, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-017, 1998), and a brief report of portions of this work has been published (13). Clinafloxacin pharmacokinetic profiles following single oral doses to healthy volunteers have been previously reported (2). In addition, the pharmacokinetic profiles of the (R)- and (S)-enantiomers of clinafloxacin have been described (10). Pharmacokinetic profiles of the enantiomers are nearly identical following i.v. and oral administration to healthy volunteers as well as patients treated with clinafloxacin in clinical studies. The in vitro antibacterial activities of the enantiomers are equipotent.
MATERIALS AND METHODS
Subjects and study conduct.
Study protocols were approved by institutional review boards at the study sites and all studies were conducted according to the ethical principles stated in the Declaration of Helsinki. All subjects were informed of potential risks and provided written informed consent before entering the study. Subjects were free to withdraw at any time at their own discretion. Eligible subjects were adult men and women who were, except as noted, in good health as determined by medical history, physical examination, electrocardiogram, electroencephalogram, and clinical laboratory measurements. Clinafloxacin demonstrated genotoxic effects in various in vitro and in vivo tests, including clastogenicity in human and animal cells at concentrations of >10 μg/ml of plasma (D. L. Bailey, T. Boyea, M. A. Cohen, S. Priebe, E. J. Randinitis, L. Welling, and M. M. Wolf, unpublished data). As the clinical significance of these results was unclear, multiple-dose studies were conducted with physiologically normal subjects with terminal cancer whose genotoxic risk was minimized. Patients who were neutropenic (absolute neutrophil count of ≤500/mm3) due to cancer chemotherapy or expected to be neutropenic for a minimum of 5 days and exhibited grade 2 or higher mucositis were recruited for the study of the absolute bioavailability of clinafloxacin in this patient group. Healthy volunteers participated in the single-dose studies. Subjects participated in only one study each.
Design of studies.
All five studies were of an open-label, parallel-group design. In the single-dose study, five groups of subjects received i.v. and oral 25-, 50-, 100-, 200-, or 400-mg clinafloxacin doses separated by 1 week. In the multiple oral dose study, three groups of subjects received 100-, 200-, or 400-mg clinafloxacin doses twice daily doses for 14 days, followed by a single dose on the last day. In the multiple i.v. dose study, two groups of subjects received a single 200- or 400-mg clinafloxacin dose on the first day and then every 12 h for 3 days, and this was followed by a single dose on the last day. In the repeated measures study, all subjects received single 400-mg oral clinafloxacin doses on three separate occasions separated by 1 week. In the bioavailability study, all neutropenic patients received single oral and i.v. clinafloxacin doses separated by 48 h. Subjects fasted overnight before and for 4 h following administration of clinafloxacin doses. Identical meals were served after collection of the 4-h sample and 10 h postdose, respectively. Clinafloxacin was administered in 250 ml of distilled water or 5% dextrose in water by 1-h constant-rate i.v. infusion into an antecubital, hand, or wrist vein. Clinafloxacin capsules were administered with 240 ml of water.
Safety.
Safety was evaluated by observation, a physical examination that included the taking of vital signs, an ocular examination, electrocardiograms, electroencephalograms, and clinical laboratory tests.
Sampling.
Venous blood samples for the determination of clinafloxacin concentration in plasma were collected into heparinized tubes. Plasma was immediately separated and stored frozen in plastic containers at −20°C until analysis. After measuring total volume, a 20-ml urine sample was stored frozen at −20°C until analysis. In the single-dose study, blood samples (5 ml) were collected before i.v. dosing and at 0.25, 0.5, 0.75, 1, 1.25, 1.5, 1.75, 2, 2.5, 3, 4, 6, 9, 12, and 24 h postdose. Blood samples were collected before oral dosing and at 0.5, 0.75, 1, 2, 3, 4, 6, 9, 12, and 24 h postdose. Urine was collected before dosing and from 0 to 6, 6 to 12, 12 to 24, and 24 to 48 h postdose. In the multiple oral dose study, blood samples were collected before dosing and at 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 9, and 12 h following the first dose and before as well as at 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 9, 12, and 24 h following the last dose. Urine was collected predose and from 0 to 6 and 6 to 12 h following the first dose as well as for 12 h following the last dose. In the multiple i.v. dose study, blood samples (5 ml) were collected before the morning doses for 5 days as well as at 0.25, 0.5, 0.75, 1, 1.25, 1.5, 1.75, 2, 2.5, 3, 4, 6, 9, and 12 h after beginning the 1-h i.v. infusion dose following the first and last doses. Samples were also collected at 24, 36, and 48 h after the last dose. A urine sample was collected before dosing as well as during the 0- to 6-, 6- to 12-, and 12- to 24-h time intervals after the first and last dose and during the 24- to 36- and 36- to 48-h time intervals after the last dose. In the repeated-measures study, blood samples were collected before and at 0.5, 0.75, 1, 2, 3, 4, 6, 9, 12, 24, 36, and 48 h postdose. A urine sample was collected before the dose as well as during the 0- to 6-, 6- to 12-, 12- to 24-, 24- to 36-, and 36- to 48-h time intervals after each dose. In the bioavailability study in neutropenic subjects, blood samples (3 ml) were collected before oral dosing and at 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, 12, and 24 h postdose and before i.v. dosing and at 1, 1.15, 1.5, 1.75, 2, 2.5, 3, 4, 5, 6, 8, 12, and 24 h postdose.
Assay of clinafloxacin.
Clinafloxacin concentrations in plasma and urine samples were assayed by validated high-performance liquid chromatographic methods. Sample processing was conducted under sodium lighting or in as little light as possible. An internal standard—either 1-cylopropyl-6,8-difluoro-1,4-dihydro-7-[3-(methylamino)methyl-1-pyrrolidinyl]-4-oxo-3-quinolinecarboxylic acid or norfloxacin—was added to 0.2-ml plasma samples before analysis. Clinafloxacin and the internal standard were isolated from human plasma by precipitating plasma proteins with acetonitrile-perchloric acid (4:1, vol/vol). Supernatant was recovered and analyzed by separation on a reverse-phase C18 column using an isocratic eluant consisting of ion-pairing solution–acetonitrile (80:20, vol/vol). The aqueous ion-pairing solution was 0.05 M citric acid, 1.15 mM tetrabutylammonium hydroxide, and 0.1% ammonium perchlorate. The column effluent was monitored at 340 nm. Internal standard was added to 0.5-ml urine samples, which were diluted to 5 ml with water and acidified with acetonitrile-perchloric acid (4:1, vol/vol). The diluent was then analyzed as described for the plasma assay. Sensitivity was determined during assay validation. The minimum quantitation limit was 0.025 μg/ml for a 0.200-ml plasma sample and 2.5 μg/ml for a 0.5-ml urine sample. Concentrations below these limits were reported as zero. The precision of quality control samples assayed with study samples, expressed as percent coefficient of variation (%CV), was <15% for plasma and urine. Detector responses were linear over the calibration ranges of 0.025 to 10 μg/ml and 2.5 to 200 μg/ml for plasma and urine samples, respectively. Sample processing was identical for all five studies.
Pharmacokinetic analysis.
Maximum concentrations in plasma (Cmax) and the time to reach Cmax (Tmax) were recorded as observed. The terminal-phase elimination rate constants (λz), areas under plasma concentration-time curves (AUC), and areas under the first moment of the concentration-time curves (AUMC) were estimated using standard noncompartmental methods (8). Values for λz were estimated as the absolute values of the slope of a least-squares regression line of the natural logarithm (ln) of concentration-time profiles during the terminal elimination phase. Terminal elimination half-life (t1/2) values were calculated as ln(2)/λz. In the multiple i.v. dose study, t1/2 values were determined using concentrations to 12 h postdose during steady state and to 48 h following the last dose. Values were similar for both methods. AUC were estimated using the linear trapezoidal rule. The AUC from 0 h to the time of the last quantifiable concentration (AUCLQC) was calculated. The AUC from 0 h to infinity (AUC∞) was determined by summing the AUC from 0 h to the LQC and LQC/λz. The AUC12 was determined during multiple dose studies. In the multiple i.v. and oral dose studies, the ratio R was calculated as AUC12(last dose)/AUC∞(first dose). Minimum plasma clinafloxacin concentrations (Cmin) were calculated as the average of the predose concentrations after steady state had been achieved and concentrations at 12 h after administration of the last dose. Total body clearance of clinafloxacin (CL) after single i.v. administration (CLi.v.) and after single oral administration (CLoral) was calculated as dose/AUC∞. Following multiple doses, CLoral and CLi.v. were calculated as dose/AUC12 after the last dose. The volume of distribution at steady state (Vss) after i.v. administration was calculated as [(dose · AUMC)/(AUMC)2] − [(dose · T)/(2 · AUC∞)], where T is the duration of the constant-rate i.v. infusion. In the single-dose study, absolute bioavailability (F) was determined as AUClqc(oral)/AUCLQC(i.v.), where AUClqc(oral) is AUClqc after the oral dose, and AUCLQC(i.v.) is AUCLQC after the i.v. dose. Times for LQC following the oral and i.v. doses were identical. In the bioavailability study of neutropenic subjects, F was calculated as AUC∞(oral)/AUC∞(i.v.). The amount of unchanged clinafloxacin excreted into urine (Ae) was calculated as the product of urine volume and urine clinafloxacin concentration. The percentage of dose excreted into urine as unchanged clinafloxacin at time t (Ae%) was calculated as (Ae/dose) · 100%. Renal clearance (CLr) was calculated in the single-dose study as Ae48/AUC∞; in the multiple-dose studies as Ae12/AUC12; and in the repeated-measures study as Ae48/AUC∞, where Ae48 and Ae12 are Ae at 48 and 12 h, respectively. Because the F of clinafloxacin was approximately 90% (see Table 2), nonrenal clearance (CLnr) was calculated as the difference between CLi.v. and CLR. The volume of distribution after i.v. administration (Vi.v.) and after oral administration (Voral) was calculated as CLi.v./λz and CLoral/λz, respectively.
TABLE 2.
Clinafloxacin pharmacokinetic parameter values (n = 4) following administration of single i.v. and oral doses
| Route and clinafloxacin dose (mg) | Mean (%CVa) for parameter
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cmax (μg/ml) | Tmax (h) | AUC∞ (μg · h/ml) | t1/2 (h) | CLR (ml/min) | Ae48% | CLi.v. (ml/min) | Vss (liters) | F (%) | |
| i.v. | |||||||||
| 25 | 0.24 (9) | 1.12 (4) | 4.6 (20) | 206 (13) | 55.4 (11) | 372 (4) | 137 (13) | ||
| 50 | 0.38 (26) | 1.82 (18) | 4.9 (6) | 342 (14) | 73.7 (8) | 468 (17) | 175 (23) | ||
| 100 | 0.90 (23) | 4.36 (25) | 5.6 (32) | 270 (24) | 67.4 (6) | 399 (22) | 150 (32) | ||
| 200 | 1.61 (16) | 8.82 (11) | 6.0 (17) | 262 (12) | 68.8 (6) | 381 (10) | 167 (18) | ||
| 400 | 3.52 (22) | 21.3 (16) | 5.8 (5) | 235 (8) | 74.8 (15) | 319 (14) | 136 (13) | ||
| Oral | |||||||||
| 25 | 0.14b (9) | 3.0b (47) | 1.17b (12) | 5.9b (31) | 221b (18) | 69.2 (15) | 92.2b (4) | ||
| 50 | 0.25 (16) | 1.8 (28) | 1.76 (16) | 5.6 (54) | 281 (17) | 57.9 (2) | 97.9 (14) | ||
| 100 | 0.77c (51) | 1.2c (58) | 4.52c (34) | 6.3c (56) | 256c (34) | 64.8c (26) | 94.4c (17) | ||
| 200 | 0.89 (20) | 1.2 (50) | 7.08 (12) | 5.9 (3) | 251 (14) | 53.4 (20) | 80.6 (9) | ||
| 400 | 2.39c (21) | 1.6c (44) | 18.5c (15) | 6.1c (23) | 232c (14) | 63.6c (4) | 86.9c (6) | ||
%CV, percent coefficient of variation.
n = 2.
n = 3.
Statistical analysis.
Contributions to the total variance due to intrasubject and intersubject variabilities were determined using the Proc Varcomp procedure (version 6.12; SAS Institute, Cary, N.C.).
RESULTS
Subject demographics and safety.
Subject demographics are summarized in Table 1. In general, clinafloxacin was well tolerated. Three subjects withdrew participation in the single-dose studies because of adverse events, including itchy eyes, pharyngitis, and rhinitis; nausea, vomiting, and chills; and increased liver function values. All subjects completed the multiple-dose studies. No deaths or serious adverse events occurred during these studies and clinical laboratory abnormalities were sporadic, transient, and appeared unrelated to drug administration.
TABLE 1.
Subject demographics
| Study description | Subject description | Source | Sex
|
Mean (range) for:
|
||
|---|---|---|---|---|---|---|
| No. of males | No. of females | Age (yr) | Wt (kg) | |||
| Single oral and i.v. doses | Healthy volunteers | Ann Arbor, Mich.a | 13 | 7 | 32 (20–45) | 82 (64–111) |
| Repeated measures | Healthy volunteers | Ann Arbor, Mich.a | 8 | 4 | 38 (21–68) | 77 (51–119) |
| Multiple oral dose | Terminal cancer patients | Little Rock, Ark.b | 6 | 10 | 59 (44–73) | 77 (58–110) |
| Multiple i.v. dose | Terminal cancer patients | Little Rock, Ark.b | 7 | 4 | 60 (45–77) | 89 (73–128) |
| Neutropenic subjects | Patients treated for cancer | Houston, Tex.c | 5 | 4 | 45 (18–76) | 65 (51–93) |
A. Sedman, Community Research Clinic.
W. Tranum, Oncology Clinic.
K. Rolston, M. D. Anderson Cancer Center.
Pharmacokinetics. (i) Single-dose studies.
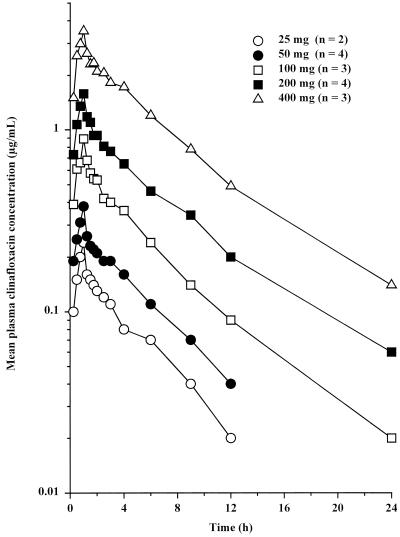
Mean clinafloxacin concentrations in plasma following administration of single i.v. doses are shown in Fig. 2. Pharmacokinetic parameters are summarized in Table 2. Plasma clinafloxacin concentrations declined biphasically following the end of the i.v. infusion. Increases in Cmax and AUC∞ were generally proportional to dose. CLi.v., Vss, t1/2, and Ae48% values did not change with increasing dose, indicating linearity of clinafloxacin pharmacokinetics. Vss was greater than body water, suggesting extensive tissue distribution.
FIG. 2.
Mean plasma clinafloxacin concentrations following administration of single intravenous doses.
Pharmacokinetic parameters following oral administration are also summarized in Table 2. Clinafloxacin absorption was rapid after oral administration, with Cmax occurring generally within 2 h. Concentrations in plasma declined biphasically thereafter. Concentrations in plasma observed in the oral portion of this study were similar to those reported previously (2). The F, approximately 90%, did not change as a function of increasing dose. Approximately 50 to 75% of the administered dose was excreted unchanged into urine after oral and i.v. administration.
Pharmacokinetic parameter values after repeated single oral doses are shown in Table 3. Intrasubject and intersubject variabilities are shown in Table 4. Clinafloxacin was rapidly absorbed, with a mean Tmax value of 1.5 h. Plasma clinafloxacin concentrations declined biphasically, with a mean t1/2 of about 6 h. Contributions to total variance due to intersubject and intrasubject variabilities of t1/2 were similar. Variation in Cmax, AUC, and clearance values after administration of clinafloxacin was primarily due to differences between subjects. Variations in Tmax and Ae48% values were primarily due to differences within a given subject.
TABLE 3.
Pharmacokinetic parameter values following administration of 400-mg oral clinafloxacin doses on three separate occasions to the same subjects (n = 11)
| Dose occasion no. | Mean (%CVa) for parameter
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Cmax (μg/ml) | Tmax (h) | AUC∞ (μg · h/ml) | t1/2 (h) | CLoral (ml/min) | CLR (ml/min) | Voral (liters) | Ae48% | |
| 1 | 3.11 (30) | 1.64 (41) | 23.7 (26) | 6.13 (11) | 300 (26) | 184 (34) | 161 (34) | 60.9 (18) |
| 2 | 3.22 (30) | 1.30 (44) | 23.3 (22) | 5.94 (10) | 299 (23) | 170 (27) | 156 (30) | 56.9 (14) |
| 3 | 3.17 (38) | 1.43 (71) | 24.0 (29) | 5.99 (15) | 298 (27) | 179 (27) | 158 (40) | 60.4 (12) |
%CV, percent coefficient of variation.
TABLE 4.
Contributions to overall variability due to intrasubject and intersubject variabilities after oral administration of clinafloxacin on three separate occasions to the same subjects (n = 11)
| Parameter | Overall
|
Intersubjecta
|
Intrasubjectb
|
|||||
|---|---|---|---|---|---|---|---|---|
| Mean | %CV | Variancec | %CV | % of total | Variancec | %CV | % of total | |
| Cmax (μg/ml) | 3.17 | 31.7 | 0.900 | 29.9 | 83.9 | 0.173 | 13.2 | 16.1 |
| Tmax (h) | 1.45 | 52.5 | 0.073 | 18.6 | 12.1 | 0.528 | 50.1 | 87.9 |
| AUC∞ (μg · h/ml) | 23.7 | 25.2 | 34.5 | 24.8 | 90.8 | 3.49 | 7.9 | 9.2 |
| t1/2 (h) | 6.02 | 12.0 | 0.290 | 8.9 | 52.5 | 0.262 | 8.5 | 47.5 |
| CLoral (ml/min) | 299 | 24.6 | 5023 | 23.7 | 86.9 | 759 | 9.2 | 13.1 |
| CLR (ml/min) | 178 | 28.8 | 2240 | 26.6 | 81.5 | 509 | 12.7 | 18.5 |
| Voral (liters) | 158 | 33.8 | 2606 | 32.3 | 85.6 | 440 | 13.3 | 14.4 |
| Ae48% | 59.4 | 15.0 | 24.3 | 8.3 | 30.0 | 56.7 | 12.7 | 70.0 |
Variance and percent coefficient of variation (%CV) due to intersubject (between subject) variability.
Variance and %CV due to intrasubject (within subject) variability.
Estimate of variance component.
(ii) Multiple oral dose study.
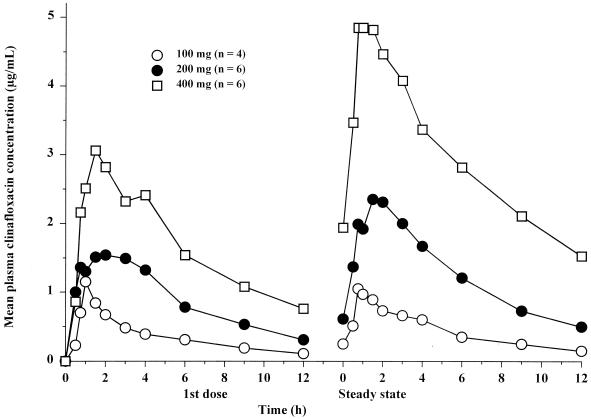
Mean plasma clinafloxacin concentrations during multiple oral dosing are shown in Fig. 3, and pharmacokinetic parameters are summarized in Table 5. Steady state was achieved within 3 days of dosing every 12 h. Increases in Cmax were generally proportional to dose, whereas increases in AUC12 values were slightly greater than dose proportional. Accumulation (R) following all doses was slight and ranged from 1.1 to 1.3. After the first dose and at steady state, the Ae12% values were approximately 40 and 60%, respectively.
FIG. 3.
Mean plasma clinafloxacin concentrations following oral administration of the first dose and at steady state of a regimen of dosing every 12 h.
TABLE 5.
Clinafloxacin pharmacokinetic parameters following oral administration every 12 h following administration of the first dose and at steady state
| Clinafloxacin dosea (mg) | Mean (%CVb) for parameter
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Cmax (μg/ml) | Tmax (h) | AUC12 (μg · h/ml) | AUC∞ (μg · h/ml) | R | t1/2 (h) | CLR (ml/min) | Ae12% | |
| 100 | ||||||||
| First dose | 1.15 (33) | 1.0 (0) | 4.19 (17) | 4.78 (17) | NAd | 3.8 (11) | 158 (42) | 37.8 (28) |
| Steady state | 1.10 (26) | 1.0 (40) | 5.26 (20) | NA | 1.10 | 6.0 (10) | 184 (37) | 55.8 (31) |
| 200 | ||||||||
| First dose | 1.97 (17) | 1.5 (60) | 10.6 (18) | 12.5 (22) | NA | 4.3 (14) | 126 (31) | 39.5 (30) |
| Steady state | 2.76 (25) | 1.5 (40) | 15.3 (24) | NA | 1.22 | 5.7 (11) | 115 (37) | 52.9 (45) |
| 400 | ||||||||
| First dose | 3.43 (11) | 1.4 (29) | 19.6 (8) | 26.5 (11) | NA | 6.1 (21) | 113c (28) | 33.3c (23) |
| Steady state | 5.22 (11) | 1.3 (46) | 35.4 (23) | NA | 1.34 | 7.6 (18) | 113c (17) | 59.8c (10) |
For 100-, 200-, and 400-mg doses there were four, six, and six subjects, respectively.
%CV, percent coefficient of variation.
n = 5 (urine not collected from one subject).
NA, not applicable.
(iii) Multiple i.v. dose study.
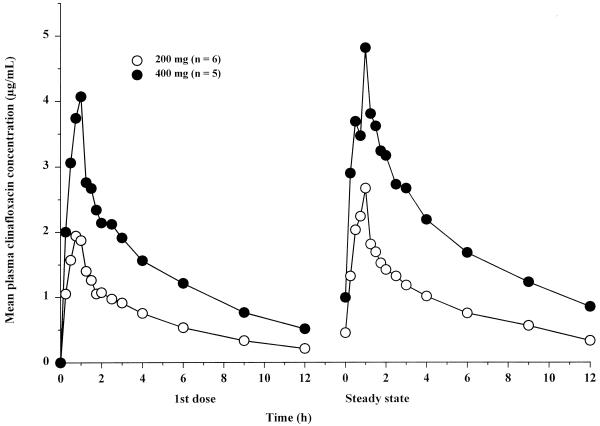
Mean plasma clinafloxacin concentrations during multiple i.v. dosing are shown in Fig. 4, and pharmacokinetic parameters are summarized in Table 6. Plasma clinafloxacin concentrations declined biphasically after the end of the 1-h i.v. infusion. Steady state was achieved within 3 days of dosing every 12 h. Clinafloxacin Cmax occurred at 1 h (the end of infusion) and were directly proportional to dose in the 200- to 400-mg dose range, both after the first dose and at steady state. Cmax at steady state were slightly higher than those after the first dose, reflecting a modest accumulation of clinafloxacin during twice-daily dosing (R = 1.4). AUC were also directly proportional to dose after the first dose and at steady state. During administration of 200- and 400-mg i.v. doses every 12 h, Ae12% values at steady state ranged from approximately 40 to 65%.
FIG. 4.
Mean plasma clinafloxacin concentrations following intravenous administration of the first dose and at steady state of a regimen of dosing every 12 h.
TABLE 6.
Clinafloxacin pharmacokinetic parameter values following i.v. administration of 200- and 400-mg doses every 12 h
| Clinafloxacin dosea (mg) | Mean (%CVb) for parameter
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cmax (μg · ml) | Tmax (h) | AUC12 (μg · h/ml) | AUC∞ (μg · h/ml) | t1/2 (h) | Cmin (μg/ml) | CLi.v. (ml/min) | CLR (ml/min) | CLNR (ml/min) | Vi.v. (liters) | Ae% | R | |
| 200 | ||||||||||||
| First dose | 2.11 (31) | 0.88 (16) | 7.81 (29) | 10.2 (33) | 5.3 (25) | NAc | 365 (35) | 185 (26) | 179 (52) | 146 (31) | 49.7 (16) | NA |
| Steady state | 2.62 (33) | 0.92 (22) | 11.0 (34) | NA | 5.04 (23) | 0.38 (55) | 335 (34) | 196 (30) | 139 (41) | NA | 59.3 (7) | 1.41 |
| 400 | ||||||||||||
| First dose | 4.22 (12) | 0.95 (12) | 16.7 (12) | 22.6 (11) | 6.23 (18) | NA | 298 (11) | 109 (34) | 189 (10) | 167 (18) | 41.6 (15) | NA |
| Steady state | 5.0 (18) | 1.05 (11) | 23.5 (17) | NA | 6.77 (11) | 0.93 (23) | 290 (18) | 135 (17) | 155 (76) | NA | 47.1 (16) | 1.41 |
For 200- and 400-mg doses there were six and five subjects,
%CV, percent-coefficient of variation
NA, not applicable.
(iv) Bioavailability in neutropenic subjects.
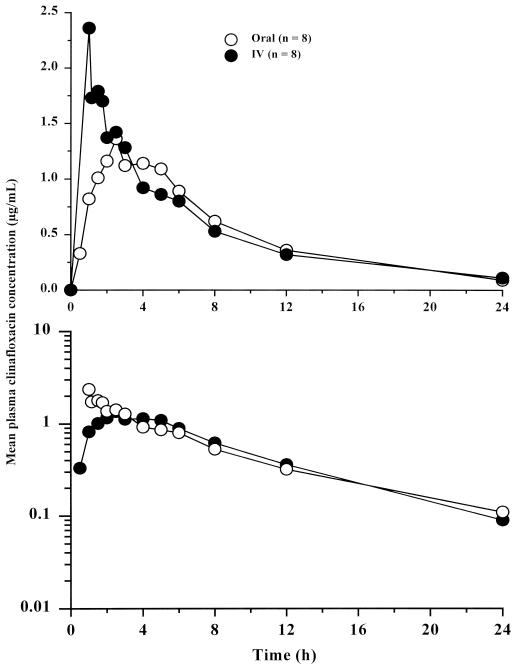
Mean plasma clinafloxacin concentrations following administration of single i.v. and oral doses to neutropenic subjects are shown in Fig. 5, and corresponding pharmacokinetic parameters are summarized in Table 7. Pharmacokinetic profiles in neutropenic subjects were similar to those observed in healthy subjects. Based on dose-normalized AUC, the F of orally administered clinafloxacin in neutropenic subjects with damaged mucosa was approximately 88%, similar to that in healthy subjects (Table 2).
FIG. 5.
Mean plasma clinafloxacin concentrations following administration of single 186-mg i.v. and 200-mg oral doses. Upper and lower panels are linear and semilogarithmic scales, respectively.
TABLE 7.
Clinafloxacin pharmacokinetic parameter values (n = 8) following administration of single 186-mg i.v. and 200-mg oral doses to neutropenic subjects
| Adminstration | Mean (%CVa) for parameter
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Cmax (μg/ml) | Cmax/dose [(μg/ml)/mg] | Tmax (h) | AUC∞ (μg · h/ml) | AUC∞/dose [(μg · h/ml)/mg] | t1/2 (h) | CLi.v., CLoral (ml/min) | Vi.v., Voral (liters) | |
| i.v. | 2.18 (43) | 0.0117 | 1.04 (7) | 13.4 (35) | 0.0720 | 6.82 (49) | 258 (34) | 139 (30) |
| Oral | 1.55 (29) | 0.00775 | 2.75 (55) | 12.6 (28) | 0.0630 | 5.58 (47) | 284 (29) | 130 (34) |
| Ratio | 71.1 | 66.2 | 264 | 94.0 | 87.5 | 81.8 | 110 | 93.5 |
%CV, percent-coefficient of variation.
Ratio = 100% · oral/i.v.
DISCUSSION
About 50 to 70% of an oral or i.v. clinafloxacin dose was excreted unchanged into urine, indicating that urinary excretion is the primary route of drug elimination. CLR exceeded the glomerular filtration rate of 125 ml/min (1), indicating active tubular secretion, similar to that of other fluoroquinolone antibiotics (6). The rate of clinafloxacin absorption, total systemic clearance, and CLR did not differ across the dose range studied. Cmax and AUC values increased generally with increasing dose, within the dose range studied. Therefore, clinafloxacin pharmacokinetics are linear following administration of single i.v. and oral doses ranging from 25 to 400 mg.
Multiple-dose administration resulted in slightly greater than expected accumulation, suggesting that there was a modest degree of nonlinearity in clinafloxacin clearance within an individual. This modest nonlinearity is not clinically important. In the multiple i.v. dose study, Cmax and AUC values were directly proportional to dose. Concentrations in plasma declined biphasically after the 1-h i.v. infusion, with mean t1/2 values of approximately 5.2 and 6.5 h after administration of the 200- and 400-mg doses, respectively. Slightly higher t1/2 values in subjects receiving 400-mg doses might be related to the ability to measure clinafloxacin concentrations in plasma samples collected at 36 and 48 h after dosing and inclusion of these concentrations in the t1/2 value calculation. Slight differences in mean values between dose groups might also be related to differences between subjects in this parallel-group study. CLi.v. did not change with increasing i.v. dose. CLR values were slightly lower after administration of the 400-mg dose relative to those after administration of the 200-mg dose.
Absolute bioavailability of orally administered clinafloxacin, approximately 90%, did not change as a function of increasing dose. Absolute bioavailability was also approximately 90% in subjects with damaged mucosa. Therefore because of excellent bioavailability, patients can be switched from i.v. to oral dosing without change in dose or dosing regimen. Based on intrasubject %CV values of <15%, clinafloxacin Cmax, AUC, and clearance values within a subject are expected to be constant from dose to dose.
The volume of distribution was much greater than body water, suggesting extensive distribution into tissues, which was confirmed by tissue distribution studies. Observed clinafloxacin Cmax and Cmin together with wide tissue distribution resulting in concentrations greater than the MICs for a wide variety of organisms in lung and associated tissues (9), skin and skin-blister fluid (14), bile and gall-bladder wall, heart valve, as well as cerebral spinal fluid (E. J. Randinitis, C. W. Alvey, A. B. Vassos, N. J. Hounslow, N. J. Bron, J. M. Tellado, and P. R. Belcher, unpublished data) suggest that clinafloxacin will be useful in preventing and treating a multitude of serious infections caused by gram-positive and gram-negative organisms, as well as organisms resistant to many current antibiotic therapies.
ACKNOWLEDGMENTS
We thank W. Tranum of the Oncology Clinic, Little Rock, Ark., as well as K. J. Rolston of the M. D. Anderson Cancer Center, University of Texas, Houston, for conducting clinical portions of these studies. Thanks are also extended to Analytical Development Corporation, Colorado Springs, Colo., for clinafloxacin analysis in biological samples.
REFERENCES
- 1.Brenner B M, Mackenzie H S. Disturbances of renal function. In: Fauci A S, Braunwald E, Isselbacher K J, editors. Harrison's principles of internal medicine. 14th ed. New York, N.Y: McGraw-Hill; 1998. pp. 1498–1504. [Google Scholar]
- 2.Bron N J, Dorr M B, Mant T G, Webb C L, Vassos A B. The tolerance and pharmacokinetics of clinafloxacin in healthy subjects. J Antimicrob Chemother. 1996;38:1023–1029. doi: 10.1093/jac/38.6.1023. [DOI] [PubMed] [Google Scholar]
- 3.Cohen M A, Huband M D. Activity of clinafloxacin, trovafloxacin, quinupristin/dalfopristin, and other antimicrobial agents versus Staphylococcus aureus isolates with reduced susceptibility to vancomycin. Diagn Microbiol Infect Dis. 1999;33:43–46. doi: 10.1016/s0732-8893(98)00121-7. [DOI] [PubMed] [Google Scholar]
- 4.Cohen M A, Huband M D, Gage J W, Yoder S L, Roland G E, Gracheck S J. In-vitro activity of clinafloxacin, trovafloxacin, and ciprofloxacin. J Antimicrob Chemother. 1997;40:205–211. doi: 10.1093/jac/40.2.205. [DOI] [PubMed] [Google Scholar]
- 5.Cohen M A, Huband M D, Yoder S L, Gage J W, Roland G E. Bacterial eradication by clinafloxacin, CI-990, and ciprofloxacin employing MBC test, in-vitro time-kill and in-vivo time-kill studies. J Antimicrob Chemother. 1998;41:605–614. doi: 10.1093/jac/41.6.605. [DOI] [PubMed] [Google Scholar]
- 6.Fitton A. The quinolones: an overview of their pharmacology. Clin Pharmacokinet. 1992;22(Suppl. 1):1–11. doi: 10.2165/00003088-199200221-00003. [DOI] [PubMed] [Google Scholar]
- 7.Fuchs P C, Barry A L, Brown S D. In vitro activities of clinafloxacin against contemporary clinical bacterial isolates from 10 North American centers. Antimicrob Agents Chemother. 1998;42:1274–1277. doi: 10.1128/aac.42.5.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibaldi M, Perrier D. Pharmacokinetics. 2nd ed. New York, N.Y: Marcel Dekker; 1982. [Google Scholar]
- 9.Honeybourne D, Andrews J M, Cunningham B, Jevons G, Wise R. The concentrations of clinafloxacin in alveolar macrophages, epithelial lining fluid, bronchial mucosa and serum after administration of single 200 mg oral doses to patients undergoing fibre-optic bronchoscopy. J Antimicrob Chemother. 1999;43:153–155. doi: 10.1093/jac/43.1.153. [DOI] [PubMed] [Google Scholar]
- 10.Humphrey G H, Shapiro M A, Randinitis E J, Guttendorf R J, Brodfuehrer J I. Pharmacokinetics of clinafloxacin enantiomers in humans. J Clin Pharmacol. 1999;39:1143–1150. [PubMed] [Google Scholar]
- 11.Jorgensen J H, Weigel L M, Ferraro M J, Swenson J M, Tenover F C. Activities of newer fluoroquinolones against Streptococcus pneumoniae clinical isolates including those with mutations in the gyrA, parC, and parE loci. Antimicrob Agents Chemother. 1999;43:329–334. doi: 10.1128/aac.43.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moellering R C., Jr The emergence of bacterial resistance to antibiotics: achieving optimum outcomes with clinafloxacin, a seminar-in-print. Clin Drug Investig. 1998;15(Suppl. 1):1–48. [Google Scholar]
- 13.Randinitis E J, Brodfuehrer J I, Vassos A B. Pharmacokinetics of oral and intravenous clinafloxacin. Drugs. 1999;58(Suppl. 2):252–253. [Google Scholar]
- 14.Wise R, Jones S, Das I, Andrews J M. Pharmacokinetics and inflammatory fluid penetration of clinafloxacin. Antimicrob Agents Chemother. 1998;42:428–430. doi: 10.1128/aac.42.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]