Abstract
MAPK signaling modules play crucial roles in regulating numerous biological processes in all eukaryotic cells. How MAPK signaling specificity and strength are tightly controlled remains a major challenging question. In Arabidopsis stomatal development, the MAPKK Kinase YODA (YDA) functions at the cell periphery to inhibit stomatal production by activating MAPK 3 and 6 (MPK3/6) that directly phosphorylate stomatal fate-determining transcription factors for degradation in the nucleus. Recently, we demonstrated that BSL1, one of the four BSL protein phosphatases, localizes to the cell cortex to activate YDA, elevating MPK3/6 activity to suppress stomatal formation. Here, we showed that at the plasma membrane, all four members of BSL proteins contribute to the YDA activation. However, in the nucleus, specific BSL members (BSL2, BSL3, and BSU1) directly deactivate MPK6 to counteract the linear MAPK pathway, thereby promoting stomatal formation. Thus, the pivotal MAPK signaling in stomatal fate determination is spatially modulated by a signaling dichotomy of the BSL protein phosphatases in Arabidopsis, providing a prominent example of how MAPK activities are integrated and specified by signaling compartmentalization at the subcellular level.
Subject terms: Leaf development, Plant signalling, Stomata
In Arabidopsis, BSL1 localizes to the cell cortex and activates the MAPKK Kinase YDA to inhibit stomatal production. Here the authors show that three other BSL proteins BSL2, BSL3, and BSU1 act in the nucleus to deactivate MPK6 and promote stomatal formation.
Introduction
Mitogen-Activated Protein Kinases (MAPKs) are universally important signaling molecules that transduce extracellular signals to induce cellular responses. In plants, a MAPK cascade usually is composed of three tiers, including MAPK Kinase Kinase (MAPKKK), MAPK Kinase (MKK), and MAPK (MPK), that relay, amplify and integrate signals from a diverse range of stimuli to elicit appropriate cellular responses and control developmental processes1, such as stomatal development and patterning. Stomata are microscopic pores in the epidermis of aerial organs of a plant. Stomatal movement allows gas exchange between a plant and the environment and restricts excessive water evaporation from the plant. As the number of stomata needs to be optimal for different plant species growing in the changing environment, stomatal development is heavily shaped by environmental cues2,3.
In the model plant Arabidopsis, mutating components in the key canonical MAPK cascade, including the MAPKKK YODA (YDA), MAPKK 4 and 5 (MKK4/5), or MAPK 3 and 6 (MPK3/6), results in comparable phenotypes of overly proliferating and abnormally patterned stomatal guard cells4,5. Previous studies suggest that the MAPKKK YDA is activated by the EPIDERMAL PATTERNING FACTOR (EPF) peptides via their interaction with the ERECTA receptor-like kinase (RLK) family (ERf)6 that partner with the receptor-like protein TOO MANY MOUTHS (TMM) and the RLK SOMATIC EMBRYOGENESIS RECEPTOR KINASES (SERKs)7,8. Activated MPK3/6 directly phosphorylate the master regulators of stomatal development, the basic helix–loop–helix (bHLH) transcription factor SPEECHLESS (SPCH) and its partners ICE1/SCREAM(SCRM) and SCRM2, leading to their degradation9–11. Activities of YDA and MKK4/5 can be negatively regulated by the GSK3-like BIN2 kinase in stomatal development12,13. However, we know little about how different tiers of the MAPK cascade are precisely regulated and integrated for signaling strength and specificity in plant developmental processes and environmental responses.
The BSU1 (bri1 Suppressor 1) phosphatase was initially characterized to promote Brassinosteroid (BR) responses by directly dephosphorylating and deactivating the BIN2 kinase, the key negative regulator of BR signaling in plants14. We recently showed that members of the BSU1-like (BSL) family of Ser/Thr protein phosphatases play key roles in stomatal development15. Three BSL proteins (BSL1, BSL2, and BSL3) directly interact with the polarity protein BREAKING OF ASYMMETRY IN THE STOMATAL LINEAGE (BASL) to enable the progression of stomatal asymmetric cell division15. BSL1 becomes polarized at the cortex of meristemoid mother cells (MMCs) and acts at the plasma membrane (PM) to activate YDA kinase activity and promote stomatal asymmetric cell-fate differentiation15.
In this study, we uncover that the BSL signaling conveys novel dichotomy effects, through which stomatal production in Arabidopsis can be positively and negatively controlled. We demonstrate that at the plasma membrane (PM), all four BSL members contribute to the positive regulation of the MAPKKK YDA, whereas BSL2, BSL3, and BSU1 enforce a negative regulation on MPK6 in the nucleus. Thus, we establish a signaling dichotomy of the BSL proteins occurring at distinct subcellar locations regulates a linear MAPK cascade, providing a prominent example of spatial control of MAPK signaling in decision-making during plant development.
Results
Positive and negative regulation of BSL members in stomatal development
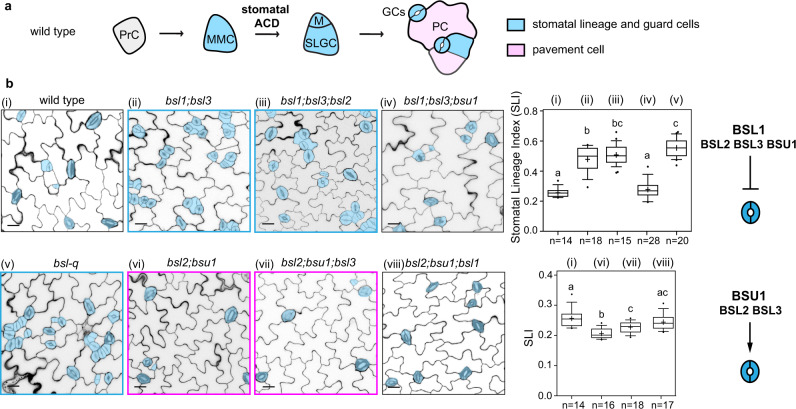
In Arabidopsis, the stomatal lineage is initiated from the conversion of a protodermal cell into a meristemoid mother cell (MMC), which divides asymmetrically to generate a meristemoid (M, small) and a stomatal lineage ground cell (large, SLGC) (Fig. 1a). Both M and SLGC have the potential to further divide to eventually differentiate into stomatal guard cells and non-stomatal pavement cells in the epidermis, respectively. It has been previously shown that mutating all four BSL genes (bsl-q, containing T-DNA insertional bsu1 and bsl1 combined with knocking-down amiRNA-BSL2;3) results in overproliferated stomatal lineage and guard cells12,15. The phenotype of bsl-q suggested the collective role of all four BSL genes in the inhibition of stomatal production. While none of the single bsl mutants (T-DNA insertional lines) showed discernable stomatal and growth defects, our previous work also demonstrated that mutating more than two of the three BASL-interacting members, i.e., BSL1, BSL2, and BSL3, led to slightly increased stomatal production, while mutating all three led to moderately increased stomatal production15 (Fig. 1b). To thoroughly determine the contribution of BSU1 in the BSL1/BSL2/BSL3-mediated negative regulation, we compared bsl-q with the null triple mutant bsl1;bsl2;bsl3 and found that the quadruple mutant indeed showed increased stomatal production (quantified by stomatal lineage index, SLI, measured in the central region of adaxial side cotyledons in 5-day seedlings) (Fig. 1b). Moreover, the quadruple mutant was featured with severely clustered stomata often appearing as patches at the leaf edges (Supplementary Fig. 1a) that were not observed in the triple mutants. As the bsl-q mutant expresses amiR-BSL2;3 that silences the two close homologs (BSL2 and BSL3), the possibility of off-targeting was ruled out by the detection of wild-type level of BSL1 and BSU1 transcripts in plants containing amiR-BSL2;3 and by comparable phenotypes of bsl1;bsl2;bsl3 and bsl1;amiRNA-BSL2;3 (Supplementary Fig. 1b, c). Thus, our data indicated that BSU1 positively contributes to the BSL1/BSL2/BSL3-mediated inhibition of stomatal production in Arabidopsis (Supplementary Fig. 1).
Fig. 1. Opposing regulation of BSL genes in stomatal development.
a Schematic diagrams to describe stomatal development in Arabidopsis. PrC (Protodermal Cell), MMC (Meristemoid Mother Cell), M (Meristemoid), SLGC (Stomatal Lineage Ground Cell), GCs (Guard Cells), and PC (Pavement Cell). Blue shade, stomatal lineage, and guard cells; Pink shade, non-stomatal pavement cells. b Left: (i–viii) Confocal images show stomatal phenotypes in adaxial cotyledon epidermis of a 5-dpg (day post germination) wild-type or a bsl mutant plant. Stomatal lineage and guard cells are manually traced (blue). Scale bars, 20 μm. Blue boxes highlight the genotypes producing more stomatal lineage cells and pink boxes highlight the genotypes producing fewer stomatal lineage cells. Middle: Plot boxes show Stomatal Lineage Index (# stomata lineages relative to # total cells) of the designated genotypes. Box plot shows first and third quartiles, median (line), and mean (cross). Letters were assigned by ANOVA and Tukey’s multiple comparison test (P ≤ 0.01). Exact P values obtained by unpaired t-test are 7.58e−10 for i vs. ii, 7.569e−20 for i vs. iii, 0.2435 for i vs. iv, 5.165e−18 for i vs. v, 1.77718e−05 for i vs. vi, 0.0045948 for i vs. vii, 0.2213566 for i vs. viii, 0.2179877 for ii vs. iii, 9.5308e−09 for ii vs. iv, 0.003676 for ii vs. v, 0.01535 for iii vs. v, 0.0014766 for vi vs. vii, 1.33323e−05 for vi vs. viii, 0.0336666 for vii vs. viii. n, number of cotyledons used for quantification of each genetic background. Right: Graphics describe differential regulation of the BSL genes in stomatal development. A complete panel of bsl mutant analysis can be found in Supplementary Fig. 2.
However, while we characterized all possible combinations of bsl double and triple mutations, surprisingly we found that differential combination of bsl mutations resulted in distinct phenotypes with regards to stomatal production (Fig. 1b, Supplementary Fig. 2). While bsl1;bsl2;bsl3 produced more stomatal lineage cells15 (Fig. 1b), mutating the other three members, i.e., bsl2;bsl3;bsu1 led to an opposite phenotype—lowered stomatal production (Fig. 1b, quantification based on stomatal lineage index). Combination of two mutations among these three genes (BSL2, BSL3, BSU1) also generated similarly lowered stomatal production in the mutants, e.g., bsl2;bsu1 and bsl3;bsu1 (Fig. 1b, Supplementary Fig. 2f, g). Thus, the results suggest that the combined function of BSL2, BSL3, and BSU1 confers an opposite regulation to promote stomatal production.
The strikingly opposite phenotypes of the bsl-q mutant (high production) vs. the triple bsl2;bsl3;bsu1 (low production) suggested that BSL1 may play a prominent role in suppressing stomatal production. Indeed, while bsl2;bsl3 was almost wild-type looking, the addition of bsl1 generated stomatal overproduction in bsl1;bsl2;bsl3 (Supplementary Fig. 2e), whereas the addition of bsl1 reversed the phenotype of low stomatal production in bsl2;bsu1 and bsl3;bsu1 to the wild-type levels in bsl1;bsl2;bsu1 and bsl1;bsl3;bsu1 mutants, respectively (Supplementary Fig. 2f, g). Thus, results of genetic analyses suggested that among the four BSL members, BSL1 appeared to be the major regulator in inhibition of stomatal production.
Also, among BSL2, BSL3, and BSU1 identified as positive regulators, BSU1 was revealed as the major player in promoting stomatal production. Our data showed that, while bsl2;bsl3 is wild-type looking, either bsl2;bsu1 or bsl3;bsu1 exhibited lowered stomatal production (Supplementary Fig. 2e-g). Furthermore, in bsl1;bsl3;bsu1, the addition of bsu1 reversed the stomatal overproduction phenotype of bsl1;bsl3 to the wild-type level (Fig. 1b, Supplementary Fig. 2c). Thus, our genetic data suggested that, among the three positive regulators (BSL2, BSL3, and BSU1), BSU1 appears to play a predominant role in promoting stomatal production.
Differential overexpression phenotypes and subcellular localization of the BSL proteins
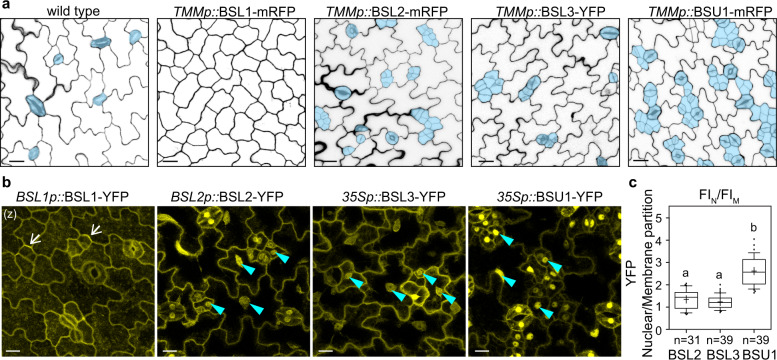
Based on our mutant analyses described above, we proposed that BSL2, BSL3, and BSU1 participate in both negative and positive regulations of stomatal production, while BSL1 only participates in the negative regulation pathway. To further test this hypothesis, we overexpressed each of the individual BSL members driven by the stomatal lineage-specific TMM promoter7 in Arabidopsis wild-type plants (TMMp::BSL1 was first reported in ref. 15). Results showed that overexpression of BSL1 strongly suppressed stomatal formation, whereas overexpression of BSL2 and BSL3 induced moderately increased stomatal production, and overexpression of BSU1 induced severely proliferative stomatal lineage cells (Fig. 2a, Supplementary Fig. 3a, transgene expression levels examined in Supplementary Fig. 3b). Thus, consistent with the loss-of-function mutant analysis, the overexpression data suggested that BSL1 is a strong inhibitor of stomatal production, whereas all the other three BSL members can promote stomatal production, with BSU1 demonstrating the highest activity.
Fig. 2. Differential overexpression phenotypes and subcellular localization of BSL proteins in stomatal lineage cells.
a Confocal images show stomatal phenotypes induced by overexpression of the BSL genes in the stomatal lineage cells (driven by the stomatal lineage TMM promoter) in 5-dpg adaxial cotyledon epidermis. Cell outlines were visualized with propidium iodide (PI)-staining. Stomatal lineage and guard cells are manually traced (blue). Data represent results of three independent experiments. Scale bars, 20 μm. b Subcellular localization of the BSL proteins (yellow) in stomatal lineage cells in 3-dpg Arabidopsis adaxial cotyledon epidermis. Note, BSL1 is mainly cytoplasmic, close to the cell periphery and polarized (arrows); BSL2, BSL3, and BSU1 are cytoplasmic and nuclear (cyan arrowheads). Data represent results of three independent experiments. Scale bars, 10 μm. (z), all images are z-stacked. c Quantification of nuclear/membrane (N/M) partition of BSL–YFP by measuring fluorescence intensity (FI) of z-stacked images. Box plots show the first and third quartiles, split by the median (line) and mean (cross). n, number of stomatal lineage cells used for quantification. Letters were assigned by ANOVA and Tukey’s multiple comparison test (P ≤ 0.01). Exact P values are 0.2208 for BSL3 vs. BSL2 and 1.69335e−12 for BSU1 vs. BSL2, respectively, by unpaired t-test.
Next, we analyzed possible differences among the four BSL proteins that might explain their differentially functional contribution. Based on the protein-sequence alignment, we did not observe striking domain features that may make BSL1 distinct, except that BSL1 contains a unique “GTLDE” motif required for its membrane association (Supplementary Fig. 3c)16. Indeed, we observed that the expression of BSL1-YFP driven by the native promoter showed predominantly cytoplasmic and plasma-membrane localization in the leaf epidermal cell. As reported previously, BSL1-YFP is also polarized in stomatal lineage cells and is absent from the nucleus (Fig. 2b)15. In contrast, all the other three BSL members (BSL2, BSL3, and BSU1) showed both cytoplasmic and nuclear localization15 (Fig. 2b, blue arrowheads show nuclear localization), with BSU1-YFP more abundantly partitioned in the nucleus (Fig. 2c, quantified for nuclear/membrane partition). Correspondingly, overexpression of BSL2/3 and BSU1 generated differential levels of stomatal production (BSL2/3 moderate and BSU1 severe) (Supplementary Fig. 3a). Thus, we hypothesized that the PM/cytoplasmic pool of all four BSL proteins contributes to the negative regulation of stomatal production, whereas the nuclear partition of BSL2, BSL3, and BSU1 contributes to the positive regulation.
BSL phosphatases promote stomatal production in the nucleus
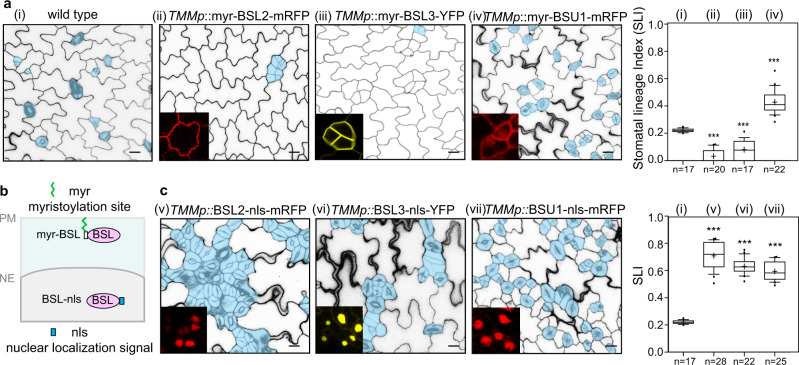
To explicitly assay the functional contribution of the BSL proteins at the subcellular level, we first used BSL2, a family member functioning at both places, as an example to examine its nuclear/membrane partition in developing stomatal lineage cells. By co-expression with the BASL polarity protein15, we found that BSL2-mRFP is more enriched in the nucleus when BASL is not polarized, but BSL2-mRFP became more membrane-associated when BASL is polarized (Supplementary Fig. 4). The results indicated the subcellular distribution thus the function of BSL2 can be dynamically and spatially regulated by developmental regulators. Next, we engineered individual proteins, either by adding an N-terminal myristoylation site to tether the target protein to the PM17, or by adding a nuclear localization sequence (nls) to target protein to the nucleus (Fig. 3b). Because BSL1 was not present in the nucleus, the other three family members, BSL2, BSL3, and BSU1, were included to be overexpressed in the stomatal lineage cells (driven by the TMM promoter, transcript levels examined in Supplementary Fig. 5a). Examination of protein subcellular localization in transgenic plants showed that most of the engineered myr- or nls-BSL2/BSL3/BSU1 proteins exhibited the expected patterns, except that myr-BSU1 showed distribution both at the PM and in the nucleus (Fig. 3a, c, Supplementary Fig. 5b). Examination of stomatal phenotypes revealed an interesting trend that myr-tagged BSL proteins strongly suppressed stomatal production, whereas nls-tagged BSL proteins strongly promoted stomatal production (Fig. 3a, c). This rule is evidently observed for plants expressing myr-BSL2/BSL3 and nls-tagged BSL2/BSL3/BSU1 but not quite applicable to the PM/cytoplasmic/nuclear myr-BSU1-RFP. BSU1 was previously reported to mainly enriched in the nucleus16. Overexpression of myr-BSU1 (both PM and nuclear), when compared with the PM-only myr-BSL2/BSL3, was less capable of suppressing stomatal overproduction. On the other hand, when compared with the native, nuclear BSU1, myr-BSU1 was less capable of promoting stomatal production (Fig. 3a, Supplementary Fig. 3a). The mild effect of myr-BSU1 was likely due to its original nuclear retention greatly alleviated by the myr-mediated membrane association. Taken together, the results indicated that the BSL2, BSL3, and BSU1 proteins likely suppress stomatal formation at the PM/cytoplasm but promote stomatal formation in the nucleus.
Fig. 3. Opposite regulation of BSL proteins at the PM vs. in the nucleus.
a Stomatal phenotypes in 5-dpg adaxial cotyledon epidermis overexpressing myristoylated (myr) BSL proteins (driven by the TMM promoter). Stomatal lineage cells are manually traced (blue). Insets (red and yellow) show protein localization in stomatal lineage cells. Note, myr-BSL2/BSL3 were solely found at the plasma membrane and myr-BSU1 was localized to the PM, cytoplasm, and nucleus. Each representative confocal images were selected from at least 10 individual cotyledons. Scale bar, 20 μm. b Schematic diagram depicts engineered BSL proteins for functional testing at the PM (myr-BSL) or in the nucleus (BSL-nls). PM, plasma membrane; NE, nuclear envelope. c Stomatal phenotypes in 5-dpg adaxial cotyledon epidermis overexpressing nuclear BSL-nls proteins (driven by the TMM promoter). Insets (red and yellow) show protein localization in the nucleus. Box plots in a and c show the first and third quartiles, split by the median (line) and mean (cross). n, number of biologically independent cotyledons from two homozygous transgenic lines (T3). Statistical analysis was performed to compare with the wild-type values. Unpaired t-test, ***P < 0.0001. Exact P values are 6.65262e−15 for i vs. ii, 1.19704e−7 for i vs. iii, 9.91101e−11 for i vs. iv, 6.4315e−21 for i vs. v, 1.70435e−21 for i vs. vi, 5.90558e−21 for i vs. vii.
Genetic positioning of BSL function at the plasma membrane (PM)
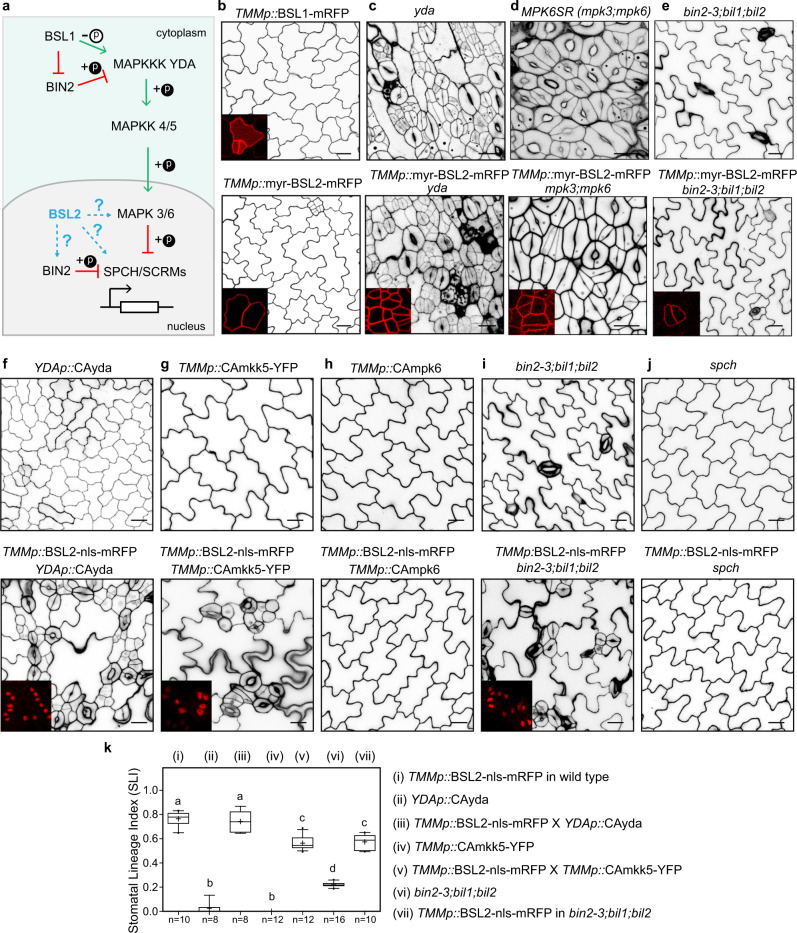
Our previous work had demonstrated that at the PM, the founding member BSL1 relied on its phosphatase activity to directly activate the kinase activity of YDA and jointly release the BIN2-mediated inhibition on YDA, thereby MAPK signaling is elevated in the stomatal lineage cells (Fig. 4a)15. Here, in our assays, overexpression of myr-BSL2/BSL3 in the stomatal lineage cells recapitulated the stomata-less phenotype of BSL1 overexpression plants15 (Figs. 3a, 4b), presumably through the same regulations on YDA and BIN2. Indeed, in the absence of YDA or MPK3/6, myr-BSL2 failed to suppress stomatal formation, and the yda or mpk3;mpk6 mutant phenotypes were epistatic (Fig. 4c, d, Supplementary Fig. 6a), indicating that the PM function of BSL2 just as BSL1 requires the YDA MAPK signaling pathway. Also, in the absence of three GSK3-like BIN2 and BIN2-like kinases (BIL1/BIL2), the effect of myr-BSL2-mRFP was greatly alleviated in bin2-3;bil1;bil2 mutants (Fig. 4e, quantification in Supplementary Fig. 6a), suggesting that the BIN2 kinases are also downstream of myr-BSL2. Thus, by using myr-BSL2 as a tool, our results suggested that the PM function of the other BSL members (BSL2/BSL3/BSU1), just as BSL1, requires the YDA MAPK and BIN2 pathways to suppress stomatal differentiation.
Fig. 4. Nuclear BSL2 functions upstream of MPK6 in stomatal development.
a Graphic diagram describes known (solid lines) and unknown (dashed lines) functional regulations surrounding the linear YDA MAPK signaling module in Arabidopsis stomatal development. Arrows and T-bars indicate positive and negative effects, respectively. Phosphorylation modifications are indicated by +p or −p. b–j Stomatal phenotypes in 5-dpg adaxial cotyledon epidermis. b–e Overexpression of membrane-localized BSL2 (myr-BSL2) was introduced in the wild type (b), yda (c), mpk3;mpk6 (d), or loss-of-function bin2-3;bil1;bil2 (e) mutants (bottom panels) to compare with their respective genetic background (top panels). f–j Overexpression of nuclear BSL2 (BSL2-nls) was introduced in the constitutively active variant of YDA (YDAp::CAyda, f), constitutively active variant of MKK5 (TMMp::CAmkk5, g), constitutively active variant of MPK6 (TMMp::CAmpk6, h), bin2-3;bil1;bil2 triple mutant (i), or spch loss-of-function mutant (j) (bottom panels), to compare with their respective genetic backgrounds (top panels). Cell outlines were stained with PI, images were captured by the confocal microscope and converted to black/white. Insets show protein localization (red) in stomatal lineage cells. Data represent results of three independent experiments. Scale bar, 20 μm. k Quantification of stomatal lineage index (SLI) for the designated genotypes. Box plot shows first and third quartiles, median (line) and mean (cross). n, number of individual cotyledons. Letters were assigned by ANOVA and Tukey’s multiple comparison test (P ≤ 0.01). Exact P values are 4.7192e−15 for i vs. ii, 0.557 for i vs. iii, 3.201e−11 for i vs. iv, 3.90248e−7 for i vs. v, 1.39073e−10 for i vs. vi, 2.32396e−6 for i vs. vii, 6.31636e−10 for ii vs. iii, 2.12926e−12 for iv vs. v, 5.41428e−9 for vi vs. vii, by unpaired t-test.
Genetic positioning of BSL function in the nucleus
We were intrigued by the phenotype of BSL2/BSL3/BSU1-nls and their positive regulation in stomatal development, which strikingly contrasts to their negative regulation at the PM. Because BSL2 showed strong functions at both locations, we used BSL2 as a representative member to genetically characterize the nuclear function of BSL2/BSL3/BSU1. To determine the molecular basis for these BSL proteins to promote stomatal production in the nucleus, we considered the key stomatal regulators, including the MAPK components (YDA, MKK4/5 and MPK3/6), the GSK3-like BIN2 kinases, and the transcription factors SPCH and ICE1/SCRMs5,18–20. Particularly in the nucleus, both MAPKs and BIN2 kinases may phosphorylate SPCH for degradation9,20, and MAPKs also regulate the degradation of ICE1/SCRMs10,21. To test the genetic interaction with the MAPK pathway, first, we introduced stomata-inducing BSL2-nls into transgenic plants overexpressing one of the constitutively active (CA) MAPK components4,5,22, such as CAyda (driven by the YDA promoter), CAmkk5, or CAmpk6 (both driven by the TMM promoter), all of which generated a stomata-less phenotype (Fig. 4f–h, top). We found that, strikingly, the overexpression of BSL2-nls was able to recover stomatal production in CAyda and CAmkk5, but not at all in CAmpk6 plants (Fig. 4f–h, bottom, quantification in Fig. 4k, transcript levels or genotype examined in Supplementary Fig. 6b, c). The results suggested that BSL2-nls acts downstream or in parallel with MKK4/5 but likely upstream of MPK6 and that the positive regulation of BSL in the nucleus requires MAPK signaling in stomatal development.
Next, we analyzed the relationship between nuclear BSLs and BIN2/BILs. The loss-of-function bin2-3;bil1;bil2 mutant, due to the defective regulation on YDA and SPCH12,20, produces fewer stomatal lineage cells in the cotyledons. Interestingly, overexpression of BSL2-nls induced stomatal overproduction in bin2-3;bil1;bil2 but to a much lesser extent compared to in the wild-type background (Fig. 4i and quantification in Fig. 4k). The results suggested that the effect of BSL-nls at least partially requires the BIN2 kinases or that BLS-nls and BIN2 may act in parallel. Similar results were obtained that the BIN2 kinase inhibitor, bikini, greatly alleviated the effect of BSL2-nls overexpression in plants (Supplementary Fig. 6d). Thus, these results consistently suggested that the GSK3-like BIN2 kinases are partially required for the BSL2-nls. Finally, as anticipated, BSL2-nls overexpression failed to induce stomatal formation in the stomata-less mutants spch and ice1-2;ice2-118,19 (Fig. 4j, Supplementary Fig. 6e), suggesting these transcription factors are absolutely required for the stomata-promoting function of BSL2-nls. Taken together, our genetic analyses suggested the nuclear function of BSL2 can be connected to BIN2, MPK3/6, SPCH, and/or ICE1/SCRM.
BSL2 directly interacts with MPK6
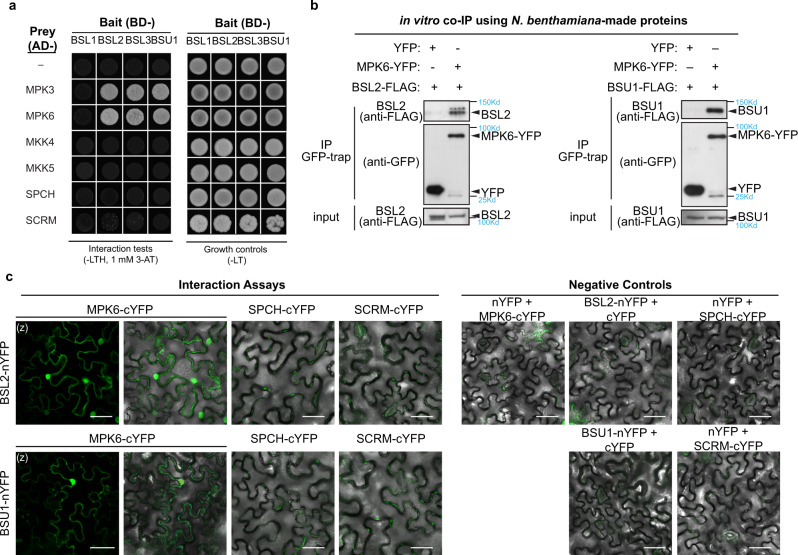
Next, we asked whether the nuclear function of BSL2/BSL3/BSU1 can be achieved by direct physical interaction with the candidates we identified above, i.e., BIN2, MPK3/6, or SPCH/SCRMs. In Arabidopsis, it was previously reported that BSU1 directly dephosphorylates BIN2 to suppress its kinase activity in BR signaling14, so that BIN2 was not included in our interaction assays. However, whether the BSL proteins can interact with MPK3/6, SPCH, and ICE1/SCRMs have not yet been investigated. The yeast two-hybrid results showed that none of the four BSL proteins interacted with MKK4, MKK5, SPCH, or ICE1/SCRM (Fig. 5a). However, BSL2/BSL3/BSU1, but not BSL1, interacted with MPK3/6 (Fig. 5a). Further analysis on the subdomains showed that the interaction of BSL2-MPK6 appeared to require the N-terminal half of BSL2 that contains the Kelch domain (Supplementary Fig. 7a, b).
Fig. 5. BSL2/BSL3/BSU1 interact with MPK6.
a Results of yeast two-hybrid assays show BSL2/BSL3/BSU1 proteins directly interact with MPK3/6. Bait, “BD-” indicates Gal4 DNA-binding domain; Prey, “AD-” indicates Gal4 activation domain. “Growth controls”, assays performed using rich media (-Leu-Trp); “Interaction tests”, assays performed using synthetic dropout medium (-Leu-Trp-His; 1 mM 3-AT added to suppress bait auto-activation). b Co-IP assays using purified fusion proteins produced by N. benthamiana leaves show physical association of BSL2-FLAG (left) or BSU1-FLAG (right) with MPK6-YFP. YFP (left lane, - control) or MPK6-YFP (right lane) was used as bait to bind to the GFP-Trap agarose. Immunoprecipitated proteins were detected by anti-FLAG. Data represent results of three biological repeats. c Results of bimolecular fluorescence complementation (BiFC) assays in N. benthamiana leaf epidermal cells show BSL2 and BSU1 interact with MPK6 in plant cells. YFP signals (green) indicate protein-protein interactions. No interactions were detected between BSL2/BSU1 and the transcription factors SPCH/SCRM. nYFP, N-terminal YFP; cYFP, C-terminal YFP. Data represent results of three independent experiments. Scale, 50 μm. (z), z-staked confocal images.
Because the plasma membrane BSL1 activates YDA15, the potential interaction of BSL2/BSL3/BSU1 with downstream MPK3/6 is intriguing. The physical interactions between BSL2/BSU1 (BSL3 88% identical BSL2) with MPK6 were further verified by in vitro pull-down assays with proteins produced by Nicotiana benthamiana leaf cells. Results showed that BSL2-Flag or BSU1-Flag was co-immunoprecipitated with MPK6-YFP, but not with the YFP-only negative controls (Fig. 5b). Thus, these in vitro assays demonstrated that BSL2/BSL3/BSU1 can directly interact with MPK3/MPK6.
Next, we tested protein-protein interaction via the bimolecular fluorescence complementation (BiFC) assays in leaf epidermal cells of N. benthamiana. While the negative controls did not produce detectable signals, complemented YFP signals were detected between nYFP-tagged BSL2/BSU1 and cYFP-tagged MPK6, but not with SPCH-cYFP or SCRM-cYFP (Fig. 5c). In addition, the interactions were found to occur in the cytoplasm and nucleus, like those of the BSL2/BSU1 single-protein expressions (Supplementary Fig. 7c). Consistent with the yeast two-hybrid results, BSL2 appeared to interact with MPK6 with its N-terminal Kelch-containing domain (Supplementary Fig. 7d). Taken together, our results demonstrated physical interactions between BSL2/BSL3/BSU1 and MPK6 both in vitro and in a heterologous plant cell system.
BSL2 deactivates MPK6
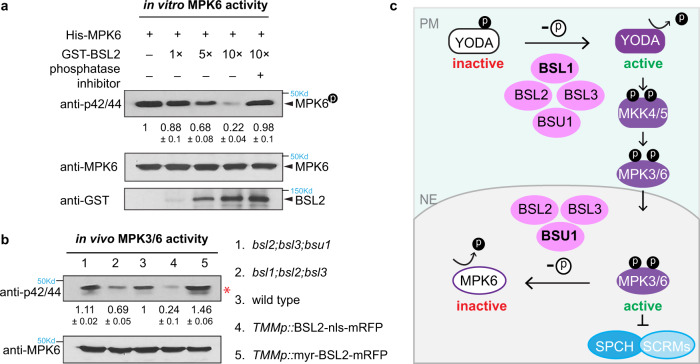
Given the established phosphatase activity of BSU1 on BIN2 in BR signaling and BSL1 on YDA in stomatal development14,15, we tested whether BSL2, as a representative nuclear member, may regulate the activity of MPK6. In in vitro kinase assays, when BSL2 was increasingly added into the system, the MPK6 phosphorylation levels were gradually reduced (Fig. 6a). Importantly, the addition of a blend of phosphatase inhibitors (PhosSTOP) abolished the effect of BSL2 (Fig. 6a). Consistent results were obtained when we performed the in vitro kinase assays using myelin basic protein (MBP) as a substrate of MPK6 (Supplementary Fig. 8a). Thus, the results suggested that the kinase activity of MPK6 is inhibited by BSL2 in a dosage-dependent manner.
Fig. 6. Negative regulation of BSL phosphatases on MAPKs in vitro and in vivo.
a Kinase activity of the recombinant MPK6 protein in the presence or absence of BSL2 was examined in vitro. MPK6 and BSL2 were fused histidine-tag (His) and glutathione S-transferase (GST), respectively. phosphorylated MPK6 was detected by immunoblot using anti-phospho-p42/p44 antibody. Protein levels of His-MPK6 or GST-BSL2 were examined using anti-MPK6 or anti-GST antibody, respectively. Numbers indicate relative amount of phosphorylated MPK6 protein of three biological replicates. Data are presented as mean ± SD. b Phosphorylated MPK3/6 (activity) levels in 5-dpg Arabidopsis seedlings were detected by anti-phospho-p42/p44 (top). Total proteins were extracted, and immunoblotting assays were performed using anti-phospho-p42/p44 (top) and anti-MPK6 (bottom). Numbers are mean ± SD, intensity quantification of phosphorylated MPK6 bands (red asterisk). n = three biological replicates. a, b Representative results of assays performed three times. c A working model: A BSL phosphatases-based signaling dichotomy, through spatial compartmentalization of the regulation on distinct components of the linear YODA MAPK pathway, controls stomatal development in Arabidopsis. At the cell cortex close to the PM, BSL1 is a predominant regulator, together with the other three BSL phosphatases, activating the MAPKKK YODA to promote MAPK signaling. Activated MPK3/6 molecules phosphorylate the key stomatal fate transcription factors, SPCH and ICE1/SCRMs, for degradation, thereby suppressing stomatal production. In the nucleus, BSU1 plays a primary role, together with BSL2 and BSL3, deactivating MPK3/6, resulting in stabilized SPCH and ICE1/SCRMs, thereby promoting stomatal production.
We further tested whether and how in vivo MPK3/6 activity levels might be eventually altered by the spatially directed overexpression of BSL2 in Arabidopsis plants. By using the p42/p44 antibodies to evaluate phosphorylated levels of MPK3/6 in vivo, the immunoblotting results showed that indeed, overexpression of the nuclear enriched BSL2-nls lowered the levels of activated MPK3/6, whereas the PM-enriched myr-BSL2 induced elevated levels of activated MPK3/6 in plants (Fig. 6b). In addition, we found that mutating the three major nuclear BSL members, i.e., BSL2, BSL3, and BSU1, resulted in elevated levels of activated MPK3/6 in bsl2;bsl3;bsu1 plants, whereas mutating BSL1, the predominant regulator at the PM, in combination with bsl2;bsl3 mutations converted the levels of activated MPK3/6 to the lower side in bsl1;bsl2;bsl3 plants (Fig. 6b). The negative regulation of nuclear-targeted BSL2/BSL3/BSU1 on MPK3/6 was further supported by examining the protein levels of SPCH (SPCHp::SPCH-CFP) relative to its transcription levels (SPCHp::nucYFP) in vivo. As a MPK3/6 substrate, the protein abundance of SPCH was downregulated by MPK3/6 in wild-type plants (fewer CFP-positive cells than YFP-positive cells at the same developmental stage). But this downregulation was greatly alleviated by BSL2-nls or BSU1 overexpression (Supplementary Fig. 8b–e). Taken together, our results suggested that the nuclear function of BSL proteins may directly deactivate MPK3/6 to promote SPCH protein abundance, thereby stomatal production in Arabidopsis (Fig. 6c).
Discussion
In this study, our genetic and cell biological analyses reveal a functional dichotomy of the four Kelch-domain containing BSL phosphatases in the regulation of Arabidopsis stomatal development (Fig. 6c and Supplementary Fig. 8f). Through phenotypic analyses of the loss-of-function mutants, we identified an opposing contribution from differentially combined family members, i.e., BSL2 and BSL3 can be either negative or positive regulators of stomatal production depending on whether they are combined with BSL1 or BSU1, respectively. When combined with BSL1, BSL2/BSL3 confer a negative regulation, whereas when combined with BSU1, BSL2/BSL3 confer a positive regulation (Fig. 1, Supplementary Fig. 2). This functional paradox can be explained by the spatial compartmentation of the BSL-mediated signaling events at the subcellular level (Fig. 6c). At the cell cortex, predominantly driven by BSL1, all four BSL proteins contribute to the activation of the YDA MAPK signaling pathway, which triggers the phosphorylation and degradation of SPCH and ICE1/SCRMs, resulting in the suppression of stomatal production. Therefore, in bsl-q mutants, the loss of activation on YDA kinase results in the loss of MAPK signaling, leading to elevated SPCH abundance and stomatal overproduction. In the nucleus, the predominant contributor BSU1, together with BSL2/BSL3, deactivates MPK3/6, thereby stabilizing SPCH and ICE1/SCRMs to promote stomatal production. Therefore, in bsl2;bsl3;bsu1 triple mutants, the presence of BSL1 is sufficient to activate YDA kinase but the absence of the nuclear inhibitors (BSL2/BSL3/BSU1) results in elevated MAPK signaling, leading to reduced SPCH abundance and stomatal inhibition. Thus, our study identified a signaling dichotomy of the BSL phosphatases, which can positively or negatively regulate a specific tier of the YDA MAPK cascade to control stomatal development (Fig. 6c). Hence, by shifting the spatial distribution of BSL2 and BSL3 that are dually localized to the nucleus and the PM/cytoplasm, the meristemoid and SLGC can decide its stomatal or non-stomatal cell fate, thereby regulating the density of guard cells in the leaf epidermis.
The BSL proteins belong to the family of Protein Phosphatase with Kelch-like domains (PPKL). While BSL1, BSL2, and BSL3 are highly conserved and universally present in all green plants, BSU1-type genes are exclusively found in Brassicaceae23. The four BSL members were initially thought to function redundantly in BR signaling14,24, but more recent genetic data suggest that BSL1/BSL2/BSL3 and BSU1 have contrasting effects on BR signaling23, The mechanism for this functional divergence remains unknown. In our study, phenotypic comparison of the various bsl mutants also supported contrasting roles of the BSL proteins in stomatal production, particularly for BSL1 vs. BSU1 (Supplementary Fig. 2b). Apart from many other reasons, such as cell-type-specific expression, conferring the differential functions of individual BSL members, our studies here showed that spatial compartmentation of signaling events, centering on the components of a MAPK cascade, provides a powerful mechanism for the BSL proteins to both positively and negatively modulate stomatal formation in Arabidopsis.
In our working model, all four BSL members at the PM contribute to the negative regulation of stomatal differentiation (Fig. 6c). This was based on the facts that (1) gradually more severe stomatal overproduction phenotypes were observed in the double, triple, and quadruple mutants, and (2) overexpression of membrane-associated/enriched BSL1 and myr-BSL2/BSL3/BSU1 were all capable of suppressing stomatal production (Fig. 3a). The previous studies by Kim et al.16 and Guo et al.15 demonstrated that BSL2, BSL3, and BSU1 are cytoplasmic and nuclear, whereas BSL1 is exclusively cytoplasmic and close to the PM. A specific amino acid sequence (GTLDE) was later identified to direct the BSL1 PM/cytoplasmic retention by an unknown mechanism16. In the stomatal lineage cells, direct physical interactions between the polarity protein BASL and BSL1/BSL2/BSL3 may recruit these BSLs to the PM polarity site. BSL1 was demonstrated to directly interact with and dephosphorylate YDA, leading to YDA activation15. Because myr-BSL2 promoted the YDA pathway, and because yda and mpk3;mpk6 were epistatic to myr-BSL2 (Fig. 4c, d), we suggest that, like BSL1, the other BSL phosphatases also participate in the direct dephosphorylation of YDA, resulting in elevated YDA activity and MAPK signaling in plants.
Then, what activates the BSL phosphatases before they can activate YDA? In embryo development, the membrane-associated pseudokinase SHORT SUSPENSOR (SSP) was found to constitutively activate YDA by physical binding25,26, a process likely facilitated by the Gβ protein AGB127. BRASSINOSTEROID SIGNALING KINASE 1 (BSK1) and BSK2, two close homologs of SSP, were recently identified to participate in YDA-dependent signaling in stomatal development26. In addition, in BR signaling, the activation of BSU1 is triggered by BSK1 that is phosphorylated by the cell-surface brassinosteroid receptor BRASSINOSTEROID INSENSITIVE 1 (BRI1)12,14. Thus, the outstanding question is whether the BSL phosphatases are activated by BSKs prior to they can activate the YDA MAPK signaling. it is possible that the BSL family members function between BSKs and YDA to relay the Brassinosteriods or ligand-receptor triggered signaling to the MAPK cascade.
While YDA is predominantly localized in the cytoplasm and tightly associated with the PM28, both MKK4/5 and MPK3/6 are broadly distributed in the cytoplasm and the nucleus29. It is not uncommon that more than one tier of the MAPK cascade is targeted by one regulator. For example, the conserved GSK3-like BIN2 kinase was first determined to phosphorylate and inhibit YDA in BR-regulated stomatal development12, and later found to catalyze the phosphorylation of the MAPK Kinase 4, reducing MKK4 activity against its substrate MPK613. However, our demonstration of opposing functions for the BSL proteins to regulate multiple tiers of a MAPK cascade is striking. In this study, we demonstrated that, besides the previously reported BSL1 interaction and inhibition of the upstream MAPKKK YDA15, BSL2/BSL3/BSU1 interact and deactivate downstream MPK6 signaling in vitro and in vivo (Figs. 5, 6). No direct physical interactions between the BSL proteins with MAPKKs were identified yet.
Conventional MAPKs are known to be activated by MAPKKs on the conserved catalytic loop Thr-X-Tyr (TxY)30 and deactivated by protein phosphatase-mediated dephosphorylation31. Here, we show that BSL2/BSL3/BSU1 bind to MPK3/6 in vitro, deactivate MPK3/6 signaling, and promote the protein stability of key transcription factor SPCH in vivo (Figs. 5, 6, Supplementary Fig. 8). The BSL proteins are members of Ser/Thr phosphatases characterized by a C-terminal catalytic domain. The phosphatase activity is not only crucial for BSL1 to activate YDA and regulate BIN214,15, we found it is also essential for BSL2 to deactivate MPK6 (Fig. 6). It is likely that BSL2/BSL3/BSU1 dephosphorylate the Thr site of the TxY motif to counteract the MAPKK-mediated activation of MPK3/6. Indeed, we observed moderately reduced phenotype of BSL2-nls when co-expressed with the constitutively active MKK5 in Arabidopsis (Fig. 4g), suggesting an antagonistic regulation of BSL phosphatases and MAPKKs against MPK3/6 in stomatal development. Furthermore, the BSL phosphatases contain an N-terminal Kelch repeats that form β-propeller structures, capable of mediating protein-protein interactions32. Our interaction assays did detect direct physical interaction between the N-terminal half of BSL2 and MPK6 (Supplementary Fig. 7). What other regulators bind to the Kelch domain and how they regulate the BSL activities are important questions to pursue in the future.
The striking positive regulation of BSL2/BSL3/BSU1 in stomatal development appeared to occur predominantly in the nucleus (Fig. 3), though BSU1 was previously shown to control BR signaling mainly in the cytoplasm14,33. Clarification of the mechanisms controlling the nuclear/cytoplasmic shutting of BSU1 and BSL2/BSL3 remains challenging because the BSL proteins do not contain a nuclear localization signal, thus their nuclear retention is probably regulated by interacting with substrates or scaffold proteins. Interestingly, BIN2 as one of the substrates of BSU1 was shown to reside both in the nucleus and the cytosol15. In stomatal development, BIN2 is temporally recruited to the cortical polarity complex, while the nuclear retention of BIN2 is promoted by the PM-enriched BSL1 phosphatase15, as well as a negative regulatory loop with the scaffold protein POLAR at the cell cortex34. In the nucleus, BIN2 and MPK3/6 promote SPCH degradation via direct phosphorylation9,20. Therefore, the nuclear enrichment of substrates, such as BIN2 and MPK3/6, can be one of the reasons that promotes the nuclear function of BSL2/BSL3/BSU1 (Supplementary Fig. 8f).
One key question related to the signaling dichotomy of the BSL phosphatase is how opposite signaling events in the regulation of the linear YDA MPK3/6 pathway are differentially triggered and modulated in plant development. The manifested stomatal defects in the loss-of-function bsl-quad mutants (severely over proliferative guard cells) suggest the negative regulation is default for the four BSL genes in this developmental context. Consistently, the primary drivers of the negative regulation, BSL1/BSL2/BSL3, are deeply conserved and essential for many developmental programs, including early endosperm development23. As discussed above, the activation of BSL phosphatases at the cell periphery might rely on the partnership with BSKs and/or other regulators. The predominant nuclear function of BSU1 was suggested to evolutionally split from the other family members and might have undergone a process of neofunctionalization35. What signals trigger the nuclear function of BSL2/BSL3/BSU1 are unknown but BSU1 in BR signaling was found to be activated in the cytoplasm prior to its activity of deactivating BIN2 in the nucleus36. As stomatal development can be heavily influenced by environmental changes2,3, it is reasonable to suspect that hormonal, such as BR, and/or other environmental cues may trigger the shift of the BSL signaling function between the PM/cytoplasm and the nucleus to impact on stomatal production. The next step towards understanding the opposing functions of BSL members in the regulation of stomatal development, probably applicable to other developmental processes as well, is to dissect out the spatially segregated protein networks that associate with BSL at the cell periphery and in the nucleus, respectively. Regulators responding to environmental changes would be great candidates for their possible participation in the regulation of BSL nuclear/cytoplasmic shuttling.
Methods
Plant materials
The Arabidopsis thaliana ecotype Columbia (Col-0) was used as the wild type. All mutants are in the wild type Col-0 background except for bin2-3;bil1;bil2 in the Wassilewskija (Ws-0) background. The bsl T-DNA insertional mutants were obtained from the Arabidopsis Biological Resource Center (ABRC), including bsl1 (SALK_051383), bsl2 (SALK_055335), bsl3 (SALK_072437), and bsu1 (SALK_030721). These null alleles were used for generating higher-order mutants and phenotypic analysis. Mutants and transgenic Arabidopsis lines reported previously were: spch-3, SPCHp::SPCH-CFP;spch-337, SPCHp::nucYFP38, ice1-2;ice2-139, yda-3 (Salk_105078)28, YDAp::CAyda40, bsl-quad (null alleles of bsu1, bsl1 combined with RNAi-mediated silencing of BSL2 and BSL3), bsl1amiRNA-BSL2;3, amiRNA-BSL2;3, and bin2-3;bil1;bil212, mpk3;mpk6-6SR41, BSL1p::BSL1-YFP, BSL2p::BSL2-YFP15, 35Sp::BSL3-YFP, 35Sp::BSU1-YFP12. Primers for genotyping and quantitative real-time PCR are listed in Supplementary Table 1.
Plant growth conditions
In general, Arabidopsis seeds were surface sterilized with 10% bleach and grown on half-strength Murashige and Skoog (MS) basal medium plates or in soil with 16-h light/8-h dark cycles at 22 °C. Wild-type N. benthamiana plants were grown under 14-h light and 10-h darkness at 25 °C.
Plasmid construction
The Gateway cloning technology (Invitrogen) was used for most DNA manipulations unless otherwise specified. For molecular cloning, the coding DNA sequences (CDS) of BSL1, BSL2, BSL3, BSU1, myr-BSL2/3/U1, BSL2/3/U1-nls, MPK6, and CAmkk5 were cloned into pENTR/D/TOPO vectors (Invitrogen). The MPK6D218GE222A(CAmpk6) were obtained by site-directed mutagenesis of MPK6 CDS in pENTR/D/TOPO by QuikChange II Site-Directed Mutagenesis Kit (Agilent). The TMM promoter sequences can be found in ref. 7 and were subcloned into pDONR-P4-P1R (courtesy of Dr. Diego Wengier). Double LR recombination reactions using LR Clonase II (Invitrogen) were performed to integrate pENTR/D containing CDS of the gene-of-interest and pDONR-promoter into the R4pGWB vectors42. For transient protein expression in N. benthamiana, the pENTR/D vectors containing the coding sequences of MPK6, SPCH, SCRM, BSL1, BSL2, BSL3, BSU1, N-terminal, or C-terminal of BSL2 were recombined into the pH35GC/Y43 or pGWB to generate 35Sp::BSLf-CFP, 35Sp::MPK6/SPCH/SCRM-CFP, or 35Sp::BSL2/BSU1-FLAG. The pXNGW and/or pXCGW vectors were used for recombination reactions to generate the BiFC constructs. The resulted binary vectors mentioned above were confirmed by restriction enzyme digestion and DNA sequencing, then transferred into Agrobacterium tumefaciens strains GV3101 for Arabidopsis transformation and/or N. benthamiana leaf infiltration.
Confocal imaging and image processing
Confocal images were acquired by a Leica TCS SP5 II microscope. The excitation/emission spectra for various fluorescent proteins are: CFP, 458 nm/480–500 nm; GFP, 488 nm/501–528 nm; YFP, 514 nm/520–540 nm; mRFP, 594 nm/600–620 nm, and propidium iodide (PI), 594 nm/591–636 nm. Images were taken from similar central areas in the adaxial side of developing cotyledons of Arabidopsis seedlings or N. benthamiana leaves. Cells outlines in Arabidopsis were visualized by propidium iodide (PI, Invitrogen) staining. All imaging processing was performed with Fiji (Image J) software (http://fiji.sc/Fiji). Whenever possible, z-stacked images were obtained. Quantifications and statistical analyses were performed using Fiji and GraphPad Prism 5.1, respectively.
Recombinant protein expression, purification, and in vitro kinase assay
To express recombinant proteins in E. coli, the coding region of MPK6 was cloned into pET28a vector to generate His-tagged MPK6. The coding region of BSL2 or MKK5DD were cloned into pGEX-4T-1 vector to generate GST-tagged BSL2 and GST-tagged MKK5DD. The primer sequences are listed in Supplementary Table 1.
All constructs were transformed into E. coli (BL21 strain) and recombinant protein expression was induced by IPTG (0.5 mM) at 16 °C for 16 h. Bacterial cells were harvested and lysed by sonication in lysis buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 0.5 mM EDTA, and 1 mM PMSF). GST-tagged proteins were purified with Pierce™ Glutathione Superflow Agarose (Thermo Scientific™), and His-tagged proteins were purified with Ni-NTA Agarose (Qiagen) according to the manufacturer’s instructions.
Purified proteins were used for in vitro kinase assay. His-MPK6 proteins were immobilized on Ni-NTA Agarose, which were then incubated with constitutively active MKK5(MKK5DD), in the presence of ATP. The column-bound activated His-MPK6 was washed with buffer to remove any residual MKK5DD. Pre-phosphorylated MPK6 was incubated without or with gradually increased amount of GST-BSL2, in the presence or absence of a mixer of phosphatase inhibitors, PhosSTOP (Sigma-Aldrich). The reaction mixture was subjected to standard western blotting analysis and immunodetection with anti-phospho-p42/p44 antibody (1:1000, Cell Signaling Technology).
When MBP were used as a substrate for in vitro kinase assay. Pre-phosphorylated MPK6 was incubated with MBP, without or with gradually increased amount of GST-BSL2, in the presence or absence of a mixer of phosphatase inhibitors, PhosSTOP (Sigma-Aldrich). After adding p-nitrobenzyl mesylate (PNBM) for alkylating the potential thiophosphoryl group on MBP, the reaction mixture was subjected to standard western blotting analysis and immunodetection with anti-Thiophosphate ester antibody (Abcam, #ab92570).
Protein transient expression in Nicotiana benthamiana leaf epidermis and image analysis
Agrobacterium strains GV3101 harboring the constructs-of-interest in 10 ml of LB medium with appropriate antibiotics were cultured overnight. Bacterial cells were collected at 5000 × g for 10 min and resuspended in 10 mM MgCl2. Cell culture and p19 were mixed to reach an OD600 = 0.5 for each line and co-infiltrated into abaxial side leaves of 4-week-old N. benthamiana plants, as described previously28. Co-infiltrated leaves were checked by confocal microscopy 3–4 days post infiltration.
Co-immunoprecipitation (co-IP) assay using proteins produced by N. benthamiana
To test physical association between BSL2 or BSU1 with MPK6 in plant cells, total cell proteins were extracted from N. benthamiana leaves that transiently expressed the combinations of 35Sp::YFP or 35Sp::MPK6-YFP with 35Sp::BSL2-FLAG or 35Sp::BSU1-FLAG. N. benthamiana leaves were collected and ground in liquid nitrogen with the protein extraction buffer (100 mM Tris-HCl pH 7.5, 5 mM EDTA, 5 mM EGTA, 1 mM Na3VO4, 10 mM NaF, 50 mM b-glycerophosphate, 10 mM DTT, 1 mM phenylmethylsulfonyl fluoride, 5% (v/v) glycerol, 0.5% (v/v) Triton X-100, and 1% (v/v) protease inhibitor cocktail (Sigma-Aldrich, P 9599)). Protein extracts were centrifuged at 18,000 × g at 4 °C for 30 min and the supernatants were incubated with GFP-Trap Agarose beads (Chromotek) at 4 °C for 3 h. Then, the beads were washed three times with extraction buffer, followed by mixing with 2 × SDS sample buffer and boiling for 5 min. Samples were separated by 10% SDS–PAGE and analyzed by corresponding primary antibodies (Monoclonal ANTI-FLAG M2 antibody produced in mouse, F3165, Sigma-Aldrich; anti-GFP Antibody, Roche #11814460001).
In vivo MAPK activity assay
Total cell proteins were extracted with extraction buffer (50 mM HEPES pH 7.5, 150 mM NaCl, 5 mM EDTA, 5 mM EGTA, 10 mM DTT, 10 mM Na3VO4, 20 mM NaF, 50 mM β-glycerophosphate, 10% glycerol, 1 mM PMSF, protease inhibitor cocktail for plant cell extracts (Sigma-Aldrich, P 9599), 1% (v/v) NP-40) from 5-dpg Arabidopsis seedlings of Col-0, bsl2;bsl3;bsu1, bsl1;bsl2;bsl3, TMMp::myr-BSL2-mRFP, and TMMp::BSL2-nls-mRFP. The extracted total cell proteins were resolved on 10% SDS–PAGE, followed by Immunoblot with the primary antibody against phosphor-p42/44 MAP kinase (1:1000, Cell Signaling Technology). Equal loading was indicated by immunoblot analysis with anti-MPK6 antibody.
Yeast two-hybrid assay
Full-length coding sequence of BSL1, BSL2, BSL3, BSU1, N-terminal, or C-terminal of BSL2 was cloned and inserted into the pGBKT7 vector as bait. The full-length coding sequence of MPK3/6, MKK4/5 or SPCH/SCRM was cloned into the pGADT7 vector. Constructs used for testing the interactions were co-transformed into Saccharomyces cerevisiae strain AH109 using EZ-YEAST™ transformation kit (MP Bio-medicals) following the manufacturer’s instructions. The positive transformants were selected on the SD/-Leu/-Trp medium. The interactions were tested on the SD/-Leu/-Trp/-His medium with appropriate concentration of 3-amino-1,2,4-triazole (3-AT). Interactions were observed after 3 days of yeast growth at 30 °C.
Quantitative real-time PCR
Total RNAs were extracted from 50 mg of seedlings at 3 to 5-dpg with RNeasy Plant Mini Kit (Qiagen). cDNAs were generated by the SuperScript™ III First-Strand Synthesis System (Invitrogen). Transcript levels of BSL1, BSL2, BSL3, and BSU1 were amplified with the primers listed in Supplementary Table 1 and the reactions were set up by SYBR™ Select Master Mix (Thermo Fisher Scientific). ACTIN 2 was used as an internal control to normalize expression levels. Data are presented as mean ± SD. Quantitative real-time PCRs were performed by the Stepone real-time PCR system (Applied Biosystems).
Stomatal quantification
In general, to examine stomatal phenotypes in development, 5-dpg (days post germination) cotyledons were stained with PI (Invitrogen) to capture images from similar central regions of adaxial cotyledons. Images were captured by the EC Plan-Neofluar (20×/0.5) lenses on a Carl Zeiss Axio Scope A1 fluorescence microscope equipped with a Progress MF CCD camera (Jenoptik). Typically, 12–20 individual seedlings were picked from each mutant or two representative T2 transgenic lines out of >12 independent transgenic events. Confocal images shown in the figures were false colored with brightness/contrast adjusted by Fiji.
To quantify stomatal phenotypes, the epidermal cells were categorized by size and shape into three groups: guard cells (pairs of kidney-shaped), stomatal lineage cells (small dividing cells, including MMCs, Ms, and SLGCs), and pavement cells (puzzle-shaped epidermal cell and enlarged SLGCs with at least one obvious lobe). Quantification for stomatal lineage index (SLI: number of guard cell pairs + stomatal lineage cells over total number of epidermal cells) were calculated by counting cells in an area of 0.385 mm2 with the cell-counter plug-in in Fiji.
To quantify SPCH expression patterns, seedlings expressing SPCHp::nucYFP or SPCHp::SPCH-CFP in the wild-type, TMMp::BSL2-nls-mRFP, or TMMp::BSU1-mRFP were stained with PI and images of the adaxial side cotyledon epidermis were captured by Confocal. Numbers of Y/CFP-positive cells and total stomatal lineage cells were counted with Fiji to obtain the Y/CFP-positive ratios.
Statistics and reproducibility
All statistical analyses were conducted with GraphPad Prism 5.1 Software. To compare two normally distributed groups, unpaired two-tailed t-tests were used. For multiple comparisons between normally distributed groups, one-way ANOVA followed by Tukey’s post hoc test were used. For all figures, *P < 0.05, **P < 0.005, ***P < 0.0001. To quantify immunoblots, the grid and image counter plug-ins in Image J were used. Numbers of repetitions and replicates for each experiment were indicated in the legends.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
We thank Dr. Dongtao Ren (China Agricultural University) and Dr. Zhiyong Wang (Stanford University) for sharing plant reagents and DNA resources. We thank Dr. Tsuyoshi Nakagawa (Shimane University) for sharing the R4 Gateway Binary Vectors (R4pGWB). We thank the ABRC stock center for providing T-DNA insertional mutants. This research was supported by grants from the National Institute of Health GM109080 and GM131827 to J.D. J.D. is also supported by grants from the National Science Foundation 2049642, 1952823, and 1851907.
Source data
Author contributions
X.G. and J.D. designed the research. X.G. and X.D. performed the experiments. X.G. and J.D. wrote the manuscript.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
All data generated or analyzed during this study are included (figures, figure legends, and Supplementary Information files). Supplementary data, including figures and tables, are available for this paper. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-30254-2.
References
- 1.Rodriguez MC, Petersen M, Mundy J. Mitogen-activated protein kinase signaling in plants. Annu. Rev. Plant Biol. 2010;61:621–649. doi: 10.1146/annurev-arplant-042809-112252. [DOI] [PubMed] [Google Scholar]
- 2.Casson SA, Hetherington AM. Environmental regulation of stomatal development. Curr. Opin. Plant Biol. 2010;13:90–95. doi: 10.1016/j.pbi.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Qi X, Torii KU. Hormonal and environmental signals guiding stomatal development. BMC Biol. 2018;16:21. doi: 10.1186/s12915-018-0488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergmann DC, Lukowitz W, Somerville CR. Stomatal development and pattern controlled by a MAPKK kinase. Science. 2004;304:1494–1497. doi: 10.1126/science.1096014. [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Ngwenyama N, Liu Y, Walker JC, Zhang S. Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis. Plant Cell. 2007;19:63–73. doi: 10.1105/tpc.106.048298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee JS, et al. Competitive binding of antagonistic peptides fine-tunes stomatal patterning. Nature. 2015;522:439–443. doi: 10.1038/nature14561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nadeau JA, Sack FD. Control of stomatal distribution on the Arabidopsis leaf surface. Science. 2002;296:1697–1700. doi: 10.1126/science.1069596. [DOI] [PubMed] [Google Scholar]
- 8.Meng X, et al. Differential function of Arabidopsis SERK family receptor-like kinases in stomatal patterning. Curr. Biol. 2015;25:2361–2372. doi: 10.1016/j.cub.2015.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lampard GR, Macalister CA, Bergmann DC. Arabidopsis stomatal initiation is controlled by MAPK-mediated regulation of the bHLH SPEECHLESS. Science. 2008;322:1113–1116. doi: 10.1126/science.1162263. [DOI] [PubMed] [Google Scholar]
- 10.Zhao C, et al. MAP kinase cascades regulate the cold response by modulating ICE1 protein stability. Dev. Cell. 2017;43:618–629.e615. doi: 10.1016/j.devcel.2017.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Putarjunan A, et al. Bipartite anchoring of SCREAM enforces stomatal initiation by coupling MAP kinases to SPEECHLESS. Nat. Plants. 2019;5:742–754. doi: 10.1038/s41477-019-0440-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim TW, Michniewicz M, Bergmann DC, Wang ZY. Brassinosteroid regulates stomatal development by GSK3-mediated inhibition of a MAPK pathway. Nature. 2012;482:419–422. doi: 10.1038/nature10794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan M, et al. Brassinosteroid-regulated GSK3/Shaggy-like kinases phosphorylate mitogen-activated protein (MAP) kinase kinases, which control stomata development in Arabidopsis thaliana. J. Biol. Chem. 2013;288:7519–7527. doi: 10.1074/jbc.M112.384453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim TW, et al. Brassinosteroid signal transduction from cell-surface receptor kinases to nuclear transcription factors. Nat. Cell Biol. 2009;11:1254–1260. doi: 10.1038/ncb1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo, X., Park, C. H., Wang, Z. Y., Nickels, B. E. & Dong, J. A spatiotemporal molecular switch governs plant asymmetric cell division. Nat. Plants7, 667–680 (2021). [DOI] [PMC free article] [PubMed]
- 16.Kim E-J, Lee S-H, Park C-H, Kim T-W. Functional role of BSL1 subcellular localization in Brassinosteroid signaling. J. Plant Biol. 2018;61:40–49. doi: 10.1007/s12374-017-0363-x. [DOI] [Google Scholar]
- 17.Greenwood S, Struhl G. Progression of the morphogenetic furrow in the Drosophila eye: the roles of Hedgehog, Decapentaplegic and the Raf pathway. Dev. (Camb., Engl.) 1999;126:5795–5808. doi: 10.1242/dev.126.24.5795. [DOI] [PubMed] [Google Scholar]
- 18.MacAlister CA, Ohashi-Ito K, Bergmann DC. Transcription factor control of asymmetric cell divisions that establish the stomatal lineage. Nature. 2007;445:537–540. doi: 10.1038/nature05491. [DOI] [PubMed] [Google Scholar]
- 19.Kanaoka MM, et al. SCREAM/ICE1 and SCREAM2 specify three cell-state transitional steps leading to Arabidopsis stomatal differentiation. Plant Cell. 2008;20:1775–1785. doi: 10.1105/tpc.108.060848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gudesblat GE, et al. SPEECHLESS integrates brassinosteroid and stomata signalling pathways. Nat. Cell Biol. 2012;14:548–554. doi: 10.1038/ncb2471. [DOI] [PubMed] [Google Scholar]
- 21.Li H, et al. MPK3- and MPK6-mediated ICE1 phosphorylation negatively regulates ICE1 stability and freezing tolerance in Arabidopsis. Dev. Cell. 2017;43:630–642.e634. doi: 10.1016/j.devcel.2017.09.025. [DOI] [PubMed] [Google Scholar]
- 22.Wengier DL, Lampard GR, Bergmann DC. Dissection of MAPK signaling specificity through protein engineering in a developmental context. BMC Plant Biol. 2018;18:60. doi: 10.1186/s12870-018-1274-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maselli GA, et al. Revisiting the evolutionary history and roles of protein phosphatases with Kelch-like domains in plants. Plant Physiol. 2014;164:1527–1541. doi: 10.1104/pp.113.233627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mora-Garcia S, et al. Nuclear protein phosphatases with Kelch-repeat domains modulate the response to brassinosteroids in Arabidopsis. Genes Dev. 2004;18:448–460. doi: 10.1101/gad.1174204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bayer M, et al. Paternal control of embryonic patterning in Arabidopsis thaliana. Science. 2009;323:1485–1488. doi: 10.1126/science.1167784. [DOI] [PubMed] [Google Scholar]
- 26.Neu A, et al. Constitutive signaling activity of a receptor-associated protein links fertilization with embryonic patterning in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA. 2019;116:5795–5804. doi: 10.1073/pnas.1815866116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan GL, Li HJ, Yang WC. The integration of Gβ and MAPK signaling cascade in zygote development. Sci. Rep. 2017;7:8732. doi: 10.1038/s41598-017-08230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Wang P, Shao W, Zhu JK, Dong J. The BASL polarity protein controls a MAPK signaling feedback loop in asymmetric cell division. Dev. Cell. 2015;33:136–149. doi: 10.1016/j.devcel.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao C, et al. EDR1 physically interacts with MKK4/MKK5 and negatively regulates a MAP kinase cascade to modulate plant innate immunity. PLoS Genet. 2014;10:e1004389. doi: 10.1371/journal.pgen.1004389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morrison, D. K. MAP kinase pathways. Cold Spring Harb. Perspect. Biol.4, a011254 (2012). [DOI] [PMC free article] [PubMed]
- 31.Bartels S, González Besteiro MA, Lang D, Ulm R. Emerging functions for plant MAP kinase phosphatases. Trends Plant Sci. 2010;15:322–329. doi: 10.1016/j.tplants.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Kutuzov MA, Andreeva AV. Protein Ser/Thr phosphatases with kelch-like repeat domains. Cell Signal. 2002;14:745–750. doi: 10.1016/S0898-6568(02)00018-9. [DOI] [PubMed] [Google Scholar]
- 33.Ryu H, Kim K, Cho H, Hwang I. Predominant actions of cytosolic BSU1 and nuclear BIN2 regulate subcellular localization of BES1 in brassinosteroid signaling. Mol. Cells. 2010;29:291–296. doi: 10.1007/s10059-010-0034-y. [DOI] [PubMed] [Google Scholar]
- 34.Houbaert A, et al. POLAR-guided signalling complex assembly and localization drive asymmetric cell division. Nature. 2018;563:574–578. doi: 10.1038/s41586-018-0714-x. [DOI] [PubMed] [Google Scholar]
- 35.Liu SL, Baute GJ, Adams KL. Organ and cell type-specific complementary expression patterns and regulatory neofunctionalization between duplicated genes in Arabidopsis thaliana. Genome Biol. Evol. 2011;3:1419–1436. doi: 10.1093/gbe/evr114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryu H, et al. Nucleocytoplasmic shuttling of BZR1 mediated by phosphorylation is essential in Arabidopsis brassinosteroid signaling. Plant Cell. 2007;19:2749–2762. doi: 10.1105/tpc.107.053728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lau OS, et al. Direct roles of SPEECHLESS in the specification of stomatal self-renewing cells. Science. 2014;345:1605–1609. doi: 10.1126/science.1256888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bian C, et al. Protein phosphatase 2A promotes stomatal development by stabilizing SPEECHLESS in Arabidopsis. Proc. Natl Acad. Sci. USA. 2020;117:13127–13137. doi: 10.1073/pnas.1912075117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kidokoro S, et al. DREB1A/CBF3 is repressed by transgene-induced DNA methylation in the Arabidopsis ice1-1 mutant. Plant Cell. 2020;32:1035–1048. doi: 10.1105/tpc.19.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lukowitz W, Roeder A, Parmenter D, Somerville C. A MAPKK kinase gene regulates extra-embryonic cell fate in Arabidopsis. Cell. 2004;116:109–119. doi: 10.1016/S0092-8674(03)01067-5. [DOI] [PubMed] [Google Scholar]
- 41.Xu J, et al. A chemical genetic approach demonstrates that MPK3/MPK6 activation and NADPH oxidase-mediated oxidative burst are two independent signaling events in plant immunity. Plant J. 2014;77:222–234. doi: 10.1111/tpj.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakagawa T, et al. Development of R4 gateway binary vectors (R4pGWB) enabling high-throughput promoter swapping for plant research. Biosci., Biotechnol., Biochem. 2008;72:624–629. doi: 10.1271/bbb.70678. [DOI] [PubMed] [Google Scholar]
- 43.Kubo M, et al. Transcription switches for protoxylem and metaxylem vessel formation. Genes Dev. 2005;19:1855–1860. doi: 10.1101/gad.1331305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included (figures, figure legends, and Supplementary Information files). Supplementary data, including figures and tables, are available for this paper. Source data are provided with this paper.