Abstract
Psychedelic research across different disciplines and biological levels is growing at a remarkably fast pace. In the prospect of a psychedelic drug becoming again an approved treatment, much of these efforts have been oriented toward exploring the relationship between the actual psychedelic effects and those manifestations of therapeutic interest. Considering the central role of the serotonin 5-HT2A receptor in the distinct effects of psychedelics in human psyche, neuropharmacology sits at the center of this debate and exploratory continuum. Here we discuss some of the most recent findings in human studies and contextualize them considering previous preclinical models studying phenomena related to synaptic plasticity. A special emphasis is placed on knowledge gaps, challenges, and limitations to evaluate the underpinnings of psychedelics’ potential antidepressant action.
Keywords: 5-HT2A receptor, GPCR, hallucinogens, LSD, psychedelics, serotonin
Psychedelics have been part of our cultures since ancient times (Fantegrossi et al., 2008; Glennon, 1994; Hanks & González-Maeso, 2016a, 2016b; Nichols, 2016; Rucker et al., 2018). This family of psychoactive drugs includes naturally occurring alkaloids such as psilocybin which is the main active component found in more than 100 species of “magic” mushrooms including Psilocybe cyanescens in Europe and the west coast of the U.S., and Psilocybe cubensis in South America and parts of Mexico, mescaline which is the principal active compound in the peyote cactus (Lophophora williamsii) native to Mexico and southwestern Texas, bufotenin which is found in the gland secretion of certain toads’ skin (Bufonidae), and dimethyltryptamine (DMT)—found in different vegetable sources such as Mimosa sp. root bark and Psychotria viridis leaves, one the main ingredients of ayahuasca, a brew traditionally used for ceremonial purposes in the Amazon region made in combination with roots of Banisteriopsis caapi that render this psychedelic orally active by virtue of the monoamine oxidase A inhibitors contained in this vine (Abraham et al., 1996; Halpern, 2004). The paradigmatic psychedelic of the 20th century, lysergic acid diethylamide (LSD), however, is hemisynthetic in nature. It was first synthesized in 1938 by Dr Albert Hofmann, who also serendipitously discovered its psychopharmacological effects in 1943 while working on ergot derivatives from Claviceps purpurea in search for respiratory stimulants at Sandoz Pharmaceuticals in Basel, Switzerland (Hofmann, 1979). All these drugs have in common a battery of acute (within minutes to hours) effects in humans that include profound changes in processes related to perception, cognition, sensory processing, and mood. As substances listed in DEA Schedule I, elucidating the mechanisms by which psychedelics induce their unique neuropsychological effects has been an important objective of drug abuse research for decades (Halpern & Pope, 1999; Heal et al., 2018; Krebs & Johansen, 2013). Currently, however, psychedelics are not considered addictive—although some can produce tolerance in rodents (Smith et al., 2014), they lack reinforcement effects (Johnson et al., 2018). Notably, more recent clinical work as well as preclinical findings in rodents suggest that psychedelics, and particularly psilocybin, behave as fast-acting and long-lasting therapeutic agents against a number of psychiatric conditions that include depression (Carhart-Harris et al., 2016, 2021; Davis et al., 2020; Griffiths et al., 2016), and substance use disorders (Bogenschutz et al., 2015; Krebs & Johansen, 2012). Although the molecular pharmacology of this family of drugs is notoriously complex (Inserra et al., 2021), one receptor emerges from the plethora of neurotransmitter receptors that psychedelics interact with as the main mediator of psychedelics’ most distinct effects. Thus, previous gene deletion models in mice (Gonzalez-Maeso et al., 2003, 2007) and pharmacological antagonism in both rodents (de la Fuente Revenga et al., 2019, 2020; Halberstadt & Geyer, 2013) and healthy volunteers (Schmid et al., 2015; Vollenweider et al., 1998) have clearly established the prominent role of the serotonin (or 5-hydroxytryptamine) 5-HT2A receptor (5-HT2AR) in the mechanism of action of psychedelics. For a general review about classification and pharmacology of serotonin 5-HT receptors, see here (Barnes et al., 2021).
Serotonergic psychedelics are classified into two main groups that include phenethylamines such as mescaline and its synthetic analog 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI), and tryptamines. The latter group can be subdivided into two subgroups: simple tryptamines such as psilocybin and its active metabolite psilocin, and DMT, which contains an indole ring and shows substantial structural flexibility, and ergoline derivatives such as LSD (Hanks & González-Maeso, 2016a, 2016b; Nichols, 2004). Most of the medicinal chemistry, molecular pharmacology, and behavioral work in rodent models has been conducted with psychedelics that belong to these three families and hence most of the chemical structure–psychedelic activity relationships reported to date belong into a relatively narrow chemical space. This, however, does not exclude the possibility that compounds that possess different structural cores may also behave as psychedelics so long they exhibit pharmacological mimicry. Recent examples that support this concept include the HIV antiretroviral medication efavirenz (Dalwadi et al., 2016, 2018; Gatch et al., 2013) and quipazine (de la Fuente Revenga et al., 2021a), an N-substituted piperazine in which development as a potential antidepressant was halted. It has been demonstrated that both efavirenz and quipazine activate the serotonin 5-HT2AR and induce behavioral signatures characteristic of psychedelic action in rodent models. While largely anecdotal, their departure from the structural orthodoxy of phenethylamines and tryptamines sets a new frontier in the exploration of chemical spaces populated by novel 5-HT2AR agonists as well as the role of additional monoaminergic G protein-coupled receptors (GPCRs).
Preclinical research with psychedelics largely relies on rodent models that bear high predictive values but are not exempt from substantial limitations (Hanks & Gonzalez-Maeso, 2013). One of such limitations is their translational validity. It is tempting to speculate that the alterations induced upon acute administration of psychedelics in processes related to perception and sensory processing are uniquely human and therefore difficult, if not impossible, to model in rats and mice. In 1990, it was reported that the phenethylamine psychedelic DOI induced a dose-dependent increase in ear-scratch response in mice (Darmani et al., 1990). While this behavior is not preserved in humans exposed to a psychedelic drug, the effect in rodents was prevented by pre-administration of serotonin 5-HT2A/2CR antagonists such as ketanserin (Darmani et al., 1990)—a drug that was also shown to block the hallucinogenic effects of psilocybin and LSD in humans (Schmid et al., 2015; Vollenweider et al., 1998). Head-twitch behavior (a rapid side-to-side movement of the head) represents a similar case—while naturally present in rodents, the frequency of manifestation increases abruptly with all the psychedelic serotonin 5-HT2AR agonists studied so far (Canal, 2012; Hanks & Gonzalez-Maeso, 2013). This behavior, which was originally reported in 1955 (Woolley, 1955), is not induced by closely related 5-HT2AR agonists that lack psychedelic potential in humans, such as lisuride and ergotamine (Gonzalez-Maeso et al., 2003, 2007). It has also been demonstrated that there is a high correlation between the potency of psychedelic-induced head-twitch behavior in rodents and their behavioral subjective effects in humans (Halberstadt et al., 2020). Based on these and other preclinical findings, this behavior is widely used as a mouse behavioral proxy of human hallucinogenic potential. However, it is important to remark that there are still a few examples of false positives that are able to induce head-twitch behavior in rodents—these include cannabinoid CB1 receptor agonists (Darmani and Pandya, 2000; Darmani et al., 2003) and α2-adrenergic receptor antagonists (Matsumoto et al., 1997). Another potential false positive is the serotonin precursor 5-hydroxytryptophan (5-HTP). At relatively high doses, this intermediate metabolite of the essential amino acid L-tryptophan has been shown to induce a robust increase in mouse head-twitch behavior that is prevented by 5-HT2AR antagonists (Schmid & Bohn, 2010; Schmid et al., 2008). 5-HTP has been used for the treatment of a variety of conditions related to potential alterations in serotonin levels that include depression, insomnia, and migraines. While psychedelic-like events do not appear to concern in such studies, the allometric scales of the doses of 5-HTP employed in the rodent head-twitch studies can exceed by orders of magnitude the doses of 5-HTP used safely in humans.
Related to the molecular target responsible for the distinct interoceptive cue elicited by psychedelics, Richard Glennon and his team reported a significant correlation between the affinity of multiple psychedelic agents and their potencies (EC50 values) using both drug discrimination with 2,5-dimethoxy-4-methylamphetamine (DOM) as the training drug in rats and human hallucinogenic potencies of these agents (Glennon et al., 1984). About 15 years later, studies in healthy volunteers suggested the psychedelic psilocybin-induced schizophrenia-like related effects, including ego-disorders, affective changes, loosened associations, and perceptional alterations–these behavioral disruptions were blocked dose-dependently by the 5-HT2A/2CR antagonist ketanserin (Vollenweider et al., 1998). A similar conclusion related to the role of the 5-HT2AR in the acute effects of psychedelics was recently reached by testing the effects of LSD in healthy subjects (Schmid et al., 2015). Mouse and rat head-twitch behavior induced by psychedelics is also blocked by 5-HT2A/2CR and 5-HT2AR antagonists, including ketanserin and M100907, respectively, as well as by genetic deletion of the 5-HT2AR (Htr2a) gene in mice (Hanks & Gonzalez-Maeso, 2013). It is therefore accepted that the 5-HT2AR is the principal target responsible for the hallucinogenic-like effects in humans and head-twitch behavior in mice upon acute psychedelic administration. However, the potential role of this serotonin receptor in the post-acute antidepressant-like behavioral and synaptic plasticity effects induced by psychedelics still remains to be fully elucidated.
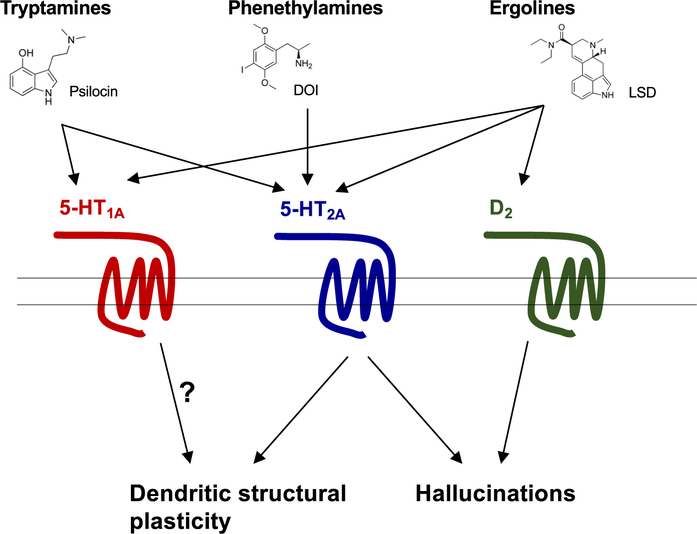
Depression and other mood disorders represent a psychiatric condition that affects approximately 5% of the adult population (Kessler et al., 2003; Krishnan & Nestler, 2008). Traditional monoaminergic antidepressants, such as fluoxetine, have the ability to reduce depression in some depressive patients, but their clinical effects require a long-lasting (weeks to months) administration and there is a high percentage of patients with depression that does not respond to the currently available antidepressant medications (Duman & Aghajanian, 2012; Insel & Wang, 2009). Based on the relatively recent clinical effects of the dissociative drug ketamine, a non-competitive antagonist of the N-methyl-d-aspartate (NMDA) receptor, showing robust, rapid (a few hours following administration) and sustained (several days on average) antidepressant effects following a single administration (typically intravenous) at a sub-anesthetic dose (Abdallah et al., 2015; Berman et al., 2000), more recent studies have provided convincing evidence that classical psychedelics, particularly naturally occurring compounds such as psilocybin and DMT in the ayahuasca brew, also behave as rapid-acting antidepressant medications (Carhart-Harris et al., 2016, 2021; Davis et al., 2020; Griffiths et al., 2016; Kyzar et al., 2017; Vollenweider & Kometer, 2010). Dendritic spines are small protrusions studding neuronal dendrites located at the postsynaptic sites of most excitatory synapses in brain regions including frontal cortex and the hippocampus (Koleske, 2013; Spruston, 2008). Santiago Ramón y Cajal was the first to observe these small protrusions using his famous Golgi staining. A number of studies in animal models as well as postmortem human brain samples from subjects with depression and controls has provided evidence that mood disorders occur in conjunction with a reduction in the density of dendritic spines, particularly in the frontal cortex (Duman & Aghajanian, 2012; Liu & Aghajanian, 2008; Nestler et al., 2002; Russo & Nestler, 2013). In 2009, studies in rat cortical neuronal primary cultures suggested that the psychedelic DOI induced a rapid (observed 30 min upon drug administration) and transient (returned to control levels by 60 min) effect on dendritic spine remodeling (Jones et al., 2009). This was corroborated with additional in vitro studies in cortical neuronal primary cultures with the psychedelics DOI, DMT, and LSD (Ly et al., 2018). Additionally, these studies showed that the effects of psychedelics on in vitro structural plasticity were blocked by the 5-HT2A/2CR antagonist ketanserin (Ly et al., 2018). Using an in vivo 3D automated method for quantitative structural analysis, it has also been suggested that a single administration of the psychedelic DOI produced fast-acting effects on mouse frontal cortex dendritic spine structure with a selective augmentation of the density of transitional stubby and dynamic thin spines in cortical pyramidal neurons, but not mature mushroom spine density (de la Fuente Revenga et al., 2021b). This structural synaptic remodeling event induced by DOI was not observed in 5-HT2AR knockout mice (de la Fuente Revenga et al., 2021b). Together, these in vitro (Ly et al., 2018) and in vivo (de la Fuente Revenga et al., 2021b) findings suggest that expression of the 5-HT2AR is necessary for the effects of a single administration of the psychedelics DOI, DMT, and LSD on changes in frontal cortex dendritic spine structural plasticity (Figure 1).
FIGURE 1.
Schematic summarizing receptor targets and their potential involvement in therapeutic effects of different structural classes of psychedelics. Tryptamine psilocin activates both serotonin 5-HT1A and 5-HT2A receptors, contributing to hallucinogenic-like behavior, but the role of the 5-HT1A in structural plasticity remains unknown. Phenethylamine DOI is more selective for 5-HT2 receptors, with a higher affinity to 5-HT2AR, which is thought to contribute to both structural plasticity and hallucinogenic-like behavior. Ergoline LSD activates dopamine D2 receptors as well as 5-HT1A and 5-HT2A receptors, which leads to hallucinogenic-like behavior, but it is unknown how LSD’s polypharmacology influences synaptic structural plasticity
Intriguingly, this does not seem to be the case for the tryptamine psychedelic psilocybin. Thus, it has been reported that ketanserin pretreatment did not prevent phenotypes related to psilocybin-induced synaptic strengthening of hippocampal synapses, although under these experimental conditions ketanserin was only able to partially, and not fully, reduce psilocybin-induced head-twitch behavior (Hesselgrave et al., 2021). Additional investigation is necessary to fully understand the pharmacological reason behind this lack of effect of the 5-HT2A/2CR antagonist ketanserin on synaptic plasticity events induced by psilocybin, but a potential explanation is the time (1 h) between antagonist and agonist administration when ketanserin might be metabolized. More recently, it has also been reported that ketanserin given 10 min prior to psilocybin was able to fully block head-twitch behavior, and under these experimental conditions, the effect of coadministration of this 5-HT2A/2CR antagonist with psilocybin on frontal cortex dendritic spine density was not statistically different from that of the ketanserin alone group, suggesting that pharmacological blockade of 5-HT2A/2CRs prevents the post-acute effects of psilocybin on frontal cortex structural plasticity. However, under these experimental conditions, the ketanserin +psilocybin group showed an increase in frontal cortex dendritic spine head width as compared to the ketanserin alone group (Shao et al., 2021). Ketanserin targets the human 5-HT2AR with higher affinity as compared to human 5-HT2CR, whereas this ligand is considered a non-selective mouse 5-HT2R antagonist (Barnes et al., 2021). Since these inter-species differences in its pharmacological profile also imply that ketanserin may not fully bock the 5-HT2AR population in psychedelic-target brain regions, such as the frontal cortex in mouse models, it is clear that additional work will be necessary to fully understand the main molecular target/s on the cell surface responsible for the long-lasting effects of the different classes of psychedelics on synaptic plasticity and associated behaviors via engagement of downstream mechanisms known to involve processes such as tropomyosin receptor kinase B (TrkB), mammalian target of rapamycin (mTOR), brain-derived neurotrophic factor (BDNF), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type glutamate receptors, and/or metabotropic glutamate receptor 2 (mGluR2) (Benvenga et al., 2018; De Gregorio et al., 2021; Ly et al., 2018; Moreno et al., 2011). This exploration of cell membrane transducers becomes particularly relevant considering the pronounced pharmacological promiscuity of psilocybin and other psychedelics. It is worth noting that both 5-HT1AR and 5-HT2AR constitute targets within range for drugs currently approved for the treatment of mood disorders, anxiety, and depression, such as buspirone and trazodone (Kessler et al., 2003; Krishnan & Nestler, 2008). Potential alternative molecular targets include a number of class A GPCRs, such as 5-HT2CR (Canal et al., 2010), and 5-HT5AR (Grailhe et al., 1999) as well as dopaminergic (De Gregorio et al., 2016; Inserra et al., 2021; Marona-Lewicka & Nichols, 2007; Marona-Lewicka et al., 2005; Rickli et al., 2016) and adrenergic (Inserra et al., 2021; Rickli et al., 2016) receptors, which have already been involved in some of the acute behavioral effects of psychedelics in rodents. Particularly, it has been suggested that the later (90–120 min after psychedelic exposure) temporal phase of LSD administration on discriminative stimulus is mediated via D2 receptors (Marona-Lewicka & Nichols, 2007; Marona-Lewicka et al., 2005) (Figure 1). Additionally, some fundamental questions still remain open as the pro-synaptic effects of psychedelics cannot simply be generalized as therapeutically positive. Synaptic plasticity events in regions related to reward can reinforce drug-seeking behaviors in addiction. For example, psychoactive drugs of abuse such as cocaine increased the density of dendritic spines in medium spiny neurons of the nucleus accumbens (Maze et al., 2010). Similarly, plasticity events underlie the development of chronic pain (Cunha et al., 2020). Causal research is also necessary to determine if psychedelic-induced changes in frontal cortex dendritic spine density are responsible for the post-acute effects of psychedelics. Related to this concept, an increase in the density of immature thin dendritic spines has also been shown in the frontal cortex of a genetic rat model related to schizophrenia (Sanchez-Gonzalez et al., 2021).
Dosing is also deserving of consideration. The occurrence of psychedelics’ subjective effects on human psyche occurs once a certain threshold is exceeded. However, it has been posed that below such threshold, therapeutic and/or adaptive benefits can readily manifest. Earlier anecdotal reports suggested that psychedelic microdosing, which involves regularly consuming a small, sub-perceptual amount of psychedelics substances such as psilocybin or LSD, may have the potential to enhance creativity and efficiency, increase performance on problem-solving tasks, and treat neuropsychiatric conditions including depression and Alzheimer's disease (Cameron et al., 2020; Kuypers et al., 2019). These overarching claims were recently challenged by studies suggesting that subjective effects of microdosing in volunteers were not different than placebo and hence largely explained by positive expectation (Kaertner et al., 2021; Szigeti et al., 2021). The study of microdosing in human subjects bears significant challenges considering that the drugs of study are subject to heavy legal burdens and the so-called positive effects appear to be of interest in the community rather than in a clinical-controlled setting. In this regard, preclinical research offers a more accessible platform to characterize the molecular and behavioral fingerprints of psychedelics across the dose range. Although the additional investigation is necessary to further define better preclinical models of microdosing in animal models, it is worth mentioning the recent studies in male and female Sprague Dawley rats showing either reduced effects of microdosing with DMT on models of depression such as reduced immobility time in the forced swim test or absence of effect in more elaborated behaviors such as fear conditioning and extinction (Cameron et al., 2019). Additionally, and opposite to the effects observed upon post-acute DMT administration, these findings also suggested that chronic, intermittent low doses of DMT led to a decrease in the dendritic spine density in the frontal cortex of female, but not, male rats (Cameron et al., 2019).
One of the still open questions is whether the fast-acting and long-lasting beneficial effects of psychedelics, particularly psilocybin, on a number of neuropsychiatric conditions that range from depression and alcoholism to post-traumatic stress disorder and cluster headache are observed as a repercussion of the action of these agents in complex neuropsychological processes such as ego-dissolution, depersonalization, oceanic boundlessness or visionary re-structuralization (Schmid et al., 2015; Vollenweider et al., 1998). It has also been reported that classical psychedelics occasion reliable and measurable mystical-type experiences in healthy volunteers (Griffiths et al., 2008). In this case, preclinical research and animal models offer little value as surrogates to study the phenomenology of such events. From a translational perspective, it would be challenging, perhaps unfeasible, to ascertain whether these intricate behavioral disruptions are necessary for their therapeutic-related phenotypes in rodents. Despite such limitations, it has been shown that rodent models replicate some of the clinically relevant post-acute effects of psychedelics in human subjects. Thus, a number of recent publications supports the notion that a single administration of different families of psychedelics such as the phenethylamine DOI and the tryptamines psilocybin and LSD produce long-lasting (5 weeks after administration) antidepressant-related behavior in mice and rats when evaluating behavioral despair or passivity such as the forced swim test (Cameron et al., 2018; de la Fuente Revenga et al., 2021b; Hibicke et al., 2020). Additionally, it has also been reported that a single dose with the DMT facilitates the extinction of cued fear memory in rats (Cameron et al., 2018), whereas a single administration of the psychedelic DOI accelerated contextual fear extinction via the 5-HT2AR in mice (de la Fuente Revenga et al., 2021b). Using sucrose and female urine preference as a model of anhedonic behavior, it was validated the chronic stress led to anhedonic-related behavior in male mice. A single injection with psilocybin reduced stress-induced anhedonic behavior (Hesselgrave et al., 2021). However, this behavioral phenotype was not prevented by pretreatment with the serotonin 5-HT2A/2CR antagonist ketanserin (Hesselgrave et al., 2021). Although as mentioned above under these experimental conditions ketanserin only partially blocked psilocybin-induced head-twitch behavior (Hesselgrave et al., 2021), these findings suggest that opposite to what had been observed with DOI, DMT, and LSD (de la Fuente Revenga et al., 2021b; Ly et al., 2018), the effects of psilocybin on post-acute antidepressant-related behavior do not require activation of the 5-HT2AR. Considering that both newly synthesized putative non-psychedelic yet highly selective 5-HT2AR agonists such as tabernanthalog (Cameron et al., 2021), as well as already classical non-psychedelic 5-HT2AR agonists such as lisuride (Nakamura et al., 1989), reduce alcohol-and heroin-seeking behavior, and produce antidepressant-like effects antidepressant-like effects in rodents, it is tempting to speculate that the psychosis-related behavior induced by psychedelic 5-HT2AR agonists is not necessary for the post-acute beneficial effects induced by other psychedelics such as psilocybin or LSD. Alternatively, although their antidepressant and anxiolytic clinical effects are mild, it is rather surprising that the involvement of the 5-HT1AR on the abovementioned traits has not been established considering that this target has been validated in the treatment of anxiety by azapirones such as buspirone and the high affinity for the 5-HT1AR of LSD and psilocybin's active form: psilocin. Additional investigation will be necessary to unravel this still open question about the molecular target and signaling mechanisms responsible for the therapeutic potential of these ligands.
Most cell signaling pathways regulate gene expression in response to extracellular cues, and receptor activation can cause concentration-dependent changes in the level of induction of specific genes in vitro in cell cultures (Wurmbach et al., 2002; Yuen et al., 2002). Using traditional gene expression assays including oligonucleotide and cDNA microarrays, it was reported that intraperitoneal administration of psychedelics such as DOI and LSD leads to changes in expression of a number of genes including transcription factors such as c-Fos, Egr-1, and Egr-2 that showed maximal change at approximately 60 min following drug exposure (Gonzalez-Maeso et al., 2003, 2007; Nichols et al., 2003; Nichols & Sanders-Bush, 2002). However, these changes in gene expression were not observed two hours after psychedelic administration. More recent findings have suggested that gene expression depends on the ability of the transcriptional machinery to access DNA, which is tightly packed into chromatin. The status of chromatin organization depends on epigenetic factors, such as DNA methylation and histone modifications that primarily occur on their amino-terminal tails. Hence these epigenetic mechanisms lead to stable changes in gene expression that are mediated via altered chromatin structure without modification of DNA sequence and remain largely plastic throughout all periods of brain development and aging (Bastle & Maze, 2019; Graff & Tsai, 2013). Unlike psilocybin, DOI exhibits a pronounced preference for 5-HT2Rs thus offering some built-in discriminative power to segregate the effects of psychedelics via 5-HT2AR from other interactions that can operate as potential confounding factors. Using MOWChIP-seq with H3K27ac (Cao et al., 2015; Zhu et al., 2019) and Smart-seq2 (Picelli et al., 2013, 2014) assays in neuronal nuclei from the frontal cortex of mice, recent findings suggest that a single dose of DOI leads to changes in chromatin organization particularly at enhancer regions of genes involved in the synaptic assembly that stretched for at least one week after the psychedelic exposure (de la Fuente Revenga et al., 2021b). Further, DOI-induced alterations in the neuronal epigenomic landscape overlapped with genetic loci associated with psychiatric conditions that included schizophrenia, depression, and attention deficit hyperactivity disorder (de la Fuente Revenga et al., 2021b). These data suggest that epigenetic mechanisms may underlie at least some of the long-lasting antidepressant action induced upon psychedelic administration, but also raise concerns about the limitations in patients with underlying genetic risk for psychosis.
Psychedelics produce a myriad of acute and post-acute behavioral alterations in both humans and animal models, including the promotion of spinogenesis in brain regions such as the frontal cortex. The robust and sustained antidepressant and plastic effects reported are suggestive of therapeutic potential for treatment-resistant depression and other neuropsychiatric disorders. Although recent findings provided evidence about the pathways affected by psychedelics and potentially involved in their post-acute beneficial effects, questions about the initiator molecular target(s) and causality of downstream signaling pathways still remain open. Utilizing behavioral, molecular, and epigenetic tools, ongoing and future studies are working to determine how psychedelics are exerting their beneficial effects and what factors may be important to consider when using these compounds to treat depression and other neuropsychiatric conditions.
ACKNOWLEDGMENTS
This work was supported in part by NIH-R01MH084894 (J.G.-M.), NIH-R01MH111940 (J.G.-M.), and NIH-T32MH020030 (M.d.l.F.R.).
Funding information
National Institute of Mental Health, Grant/Award Number: R01MH084894, R01MH111940 and T32MH020030
Abbreviations
- 5-HTP
5-hydroxytryptophan
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type glutamate receptors
- BDNF
brain-derived neurotrophic factor
- DMT
dimethyltryptamine
- DOI
1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane
- DOM
2,5-dimethoxy-4-methylamphetamine
- GPCR
G protein-coupled receptor
- LSD
lysergic acid diethylamide
- mGluR2
metabotropic glutamate receptor 2
- MOWChIP-seq
microfluidic oscillatory washing-based chromatin immunoprecipitation followed by sequencing
- mTOR
mammalian target of rapamycin
- TrkB
tropomyosin receptor kinase B
- NMDA
N-methyl-d-aspartate
- 5-HT
serotonin or 5-hydroxytryptamine
Footnotes
CONFLICT OF INTEREST
J.G.-M. has a sponsored research contract with NeuRistic, and M.d.l.F.R. has a consulting agreement with Noetic. The remaining authors declare that they have no conflict of interest.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Abdallah CG, Sanacora G, Duman RS, & Krystal JH (2015). Ketamine and rapid-acting antidepressants: A window into a new neurobiology for mood disorder therapeutics. Annual Review of Medicine, 66, 509–523. 10.1146/annurev-med-053013-062946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham HD, Aldridge AM, & Gogia P (1996). The psychopharmacology of hallucinogens. Neuropsychopharmacology, 14, 285–298. 10.1016/0893-133X(95)00136-2. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Ahern GP, Becamel C, Bockaert J, Camilleri M, Chaumont-Dubel S, Claeysen S, Cunningham KA, Fone KC, Gershon M, Di Giovanni G, Goodfellow NM, Halberstadt AL, Hartley RM, Hassaine G, Herrick-Davis K, Hovius R, Lacivita E, Lambe EK, … Hoyer D (2021). International union of basic and clinical pharmacology. CX. classification of receptors for 5-hydroxytryptamine; pharmacology and function. Pharmacological Reviews, 73, 310–520. 10.1124/pr.118.015552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastle RM, & Maze I (2019). Chromatin regulation in complex brain disorders. Current Opinion in Behavioral Sciences, 25, 57–65. 10.1016/j.cobeha.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benvenga MJ, Chaney SF, Baez M, Britton TC, Hornback WJ, Monn JA, & Marek GJ (2018). Metabotropic Glutamate2 receptors play a key role in modulating head twitches induced by a serotonergic hallucinogen in mice. Frontiers in Pharmacology, 9, 208. 10.3389/fphar.2018.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, & Krystal JH (2000). Antidepressant effects of ketamine in depressed patients. Biological Psychiatry, 47, 351–354. 10.1016/S0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Bogenschutz MP, Forcehimes AA, Pommy JA, Wilcox CE, Barbosa PC, & Strassman RJ (2015). Psilocybin-assisted treatment for alcohol dependence: A proof-of-concept study. Journal of Psychopharmacology, 29, 289–299. 10.1177/0269881114565144. [DOI] [PubMed] [Google Scholar]
- Cameron LP, Benson CJ, DeFelice BC, Fiehn O, & Olson DE (2019). Chronic, intermittent Microdoses of the psychedelic N, N-dimethyltryptamine (DMT) produce positive effects on mood and anxiety in rodents. ACS Chemical Neuroscience, 10, 3261–3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron LP, Benson CJ, Dunlap LE, & Olson DE (2018). Effects of N, N-dimethyltryptamine on rat behaviors relevant to anxiety and depression. ACS Chemical Neuroscience, 9, 1582–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron LP, Nazarian A, & Olson DE (2020). Psychedelic microdosing: Prevalence and subjective effects. Journal of Psychoactive Drugs, 52, 113–122. 10.1080/02791072.2020.1718250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron LP, Tombari RJ, Lu JU, Pell AJ, Hurley ZQ, Ehinger Y, Vargas MV, McCarroll MN, Taylor JC, Myers-Turnbull D, Liu T, Yaghoobi B, Laskowski LJ, Anderson EI, Zhang G, Viswanathan J, Brown BM, Tjia M, Dunlap LE, … Olson DE (2021). A non-hallucinogenic psychedelic analogue with therapeutic potential. Nature, 589, 474–479. 10.1038/s41586-020-3008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal CE (2012). Head-twitch response in rodents induced by the hallucinogen 2, 5-dimethoxy-4-iodoamphetamine: A comprehensive history, a re-evaluation of mechanisms, and its utility as a model. Drug Testing and Analysis, 4, 556–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal CE, Olaghere da Silva UB, Gresch PJ, Watt EE, Sanders-Bush E, & Airey DC (2010). The serotonin 2C receptor potently modulates the head-twitch response in mice induced by a phenethylamine hallucinogen. Psychopharmacology (Berl), 209, 163–174. 10.1007/s00213-010-1784-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Chen C, He B, Tan K, & Lu C (2015). A microfluidic device for epigenomic profiling using 100 cells. Nature Methods, 12, 959–962. 10.1038/nmeth.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Bolstridge M, Rucker J, Day CMJ, Erritzoe D, Kaelen M, Bloomfield M, Rickard JA, Forbes B, Feilding A, Taylor D, Pilling S, Curran VH, & Nutt DJ (2016). Psilocybin with psychological support for treatment-resistant depression: An open-label feasibility study. Lancet Psychiatry, 3, 619–627. 10.1016/S2215-0366(16)30065-7. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris R, Giribaldi B, Watts R, Baker-Jones M, Murphy-Beiner A, Murphy R, Martell J, Blemings A, Erritzoe D, & Nutt DJ (2021). Trial of psilocybin versus escitalopram for depression. New England Journal of Medicine, 384, 1402–1411. 10.1056/NEJMoa2032994. [DOI] [PubMed] [Google Scholar]
- Cunha AM, Pereira-Mendes J, Almeida A, Guimaraes MR, & Leite-Almeida H (2020). Chronic pain impact on rodents’ behavioral repertoire. Neuroscience and Biobehavioral Reviews, 119, 101–127. 10.1016/j.neubiorev.2020.09.022. [DOI] [PubMed] [Google Scholar]
- Dalwadi DA, Kim S, Amdani SM, Chen Z, Huang RQ, & Schetz JA (2016). Molecular mechanisms of serotonergic action of the HIV-1 antiretroviral efavirenz. Pharmacological Research, 110, 10–24. 10.1016/j.phrs.2016.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalwadi DA, Ozuna L, Harvey BH, Viljoen M, & Schetz JA (2018). Adverse neuropsychiatric events and recreational use of efavirenz and other HIV-1 antiretroviral drugs. Pharmacological Reviews, 70, 684–711. 10.1124/pr.117.013706. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Janoyan JJ, Kumar N, & Crim JL (2003). Behaviorally active doses of the CB1 receptor antagonist SR 141716A increase brain serotonin and dopamine levels and turnover. Pharmacology, Biochemistry and Behavior, 75, 777–787. 10.1016/S0091-3057(03)00150-3. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Martin BR, Pandey U, & Glennon RA (1990). Pharmacological characterization of ear-scratch response in mice as a behavioral model for selective 5-HT2-receptor agonists and evidence for 5-HT1B-and 5-HT2-receptor interactions. Pharmacology, Biochemistry and Behavior, 37, 95–99. 10.1016/0091-3057(90)90047-L. [DOI] [PubMed] [Google Scholar]
- Darmani N. a, & Pandya D. k (2000). Involvement of other neurotransmitters in behaviors induced by the cannabinoid CB 1 receptor antagonist SR 141716A in naive mice. Journal of Neural Transmission, 107(8–9), 931–945. 10.1007/s007020070043. [DOI] [PubMed] [Google Scholar]
- Davis AK, Barrett FS, May DG, Cosimano MP, Sepeda ND, Johnson MW, Finan PH, & Griffiths RR (2020). Effects of psilocybin-assisted therapy on major depressive disorder: A randomized clinical trial. JAMA Psychiatry, 78(5), 481– 10.1001/jamapsychiatry.2020.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gregorio D, Popic J, Enns JP, Inserra A, Skalecka A, Markopoulos A, Posa L, Lopez-Canul M, Qianzi H, Lafferty CK, Britt JP, Comai S, Aguilar-Valles A, Sonenberg N, & Gobbi G (2021). Lysergic acid diethylamide (LSD) promotes social behavior through mTORC1 in the excitatory neurotransmission. Proceedings of the National Academy of Sciences of the United States of America, 118, e2020705118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gregorio D, Posa L, Ochoa-Sanchez R, McLaughlin R, Maione S, Comai S, & Gobbi G (2016). The hallucinogen d-lysergic diethylamide (LSD) decreases dopamine firing activity through 5-HT1A, D2 and TAAR1 receptors. Pharmacological Research, 113, 81–91. 10.1016/j.phrs.2016.08.022. [DOI] [PubMed] [Google Scholar]
- de la Fuente Revenga M, Shah UH, Nassehi N, Jaster AM, Hemanth P, Sierra S, Dukat M, & Gonzalez-Maeso J (2021a). Psychedelic-like properties of quipazine and its structural analogues in mice. ACS Chemical Neuroscience, 12(5), 831–844. 10.1021/acschemneuro.0c00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente Revenga M, Shin JM, Vohra HZ, Hideshima KS, Schneck M, Poklis JL, & Gonzalez-Maeso J (2019). Fully automated head-twitch detection system for the study of 5-HT2A receptor pharmacology in vivo. Scientific Reports, 9, 14247. 10.1038/s41598-019-49913-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente Revenga M, Vohra HZ, & Gonzalez-Maeso J (2020). Automated quantification of head-twitch response in mice via ear tag reporter coupled with biphasic detection. Journal of Neuroscience Methods, 334, 108595. 10.1016/j.jneumeth.2020.108595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente Revenga M, Zhu B, Guevara CA, Naler LB, Saunders JM, Zhou Z, Toneatti R, Sierra S, Wolstenholme JT, Beardsley PM, Huntley GW, Lu C, & González-Maeso J (2021b). Prolonged epigenomic and synaptic plasticity alterations following single exposure to a psychedelic in mice. Cell Reports, 37, 109836. 10.1016/j.celrep.2021.109836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, & Aghajanian GK (2012). Synaptic dysfunction in depression: Potential therapeutic targets. Science, 338, 68–72. 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Murnane KS, & Reissig CJ (2008). The behavioral pharmacology of hallucinogens. Biochemical Pharmacology, 75, 17–33. 10.1016/j.bcp.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Kozlenkov A, Huang R-Q, Yang W, Nguyen JD, González-Maeso J, Rice KC, France CP, Dillon GH, Forster MJ, & Schetz JA (2013). The HIV antiretroviral drug efavirenz has LSD-like properties. Neuropsychopharmacology, 38(12), 2373–2384. 10.1038/npp.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon RA (1994). Classical hallucinogens: An introductory overview. NIDA Research Monograph, 146, 4–32. [PubMed] [Google Scholar]
- Glennon RA, Titeler M, & McKenney JD (1984). Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sciences, 35, 2505–2511. 10.1016/0024-3205(84)90436-3. [DOI] [PubMed] [Google Scholar]
- González-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, & Gingrich JA (2007). Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron, 53, 439–452. 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Yuen T, Ebersole BJ, Wurmbach E, Lira A, Zhou M, Weisstaub N, Hen R, Gingrich JA, & Sealfon SC (2003). Transcriptome fingerprints distinguish hallucinogenic and nonhallucinogenic 5-hydroxytryptamine 2A receptor agonist effects in mouse somatosensory cortex. Journal of Neuroscience, 23, 8836–8843. 10.1523/JNEUROSCI.23-26-08836.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff J, & Tsai LH (2013). Histone acetylation: Molecular mnemonics on the chromatin. Nature Reviews Neuroscience, 14, 97–111. 10.1038/nrn3427. [DOI] [PubMed] [Google Scholar]
- Grailhe R, Waeber C, Dulawa SC, Hornung JP, Zhuang X, Brunner D, Geyer MA, & Hen R (1999). Increased exploratory activity and altered response to LSD in mice lacking the 5-HT(5A) receptor. Neuron, 22, 581–591. 10.1016/S0896-6273(00)80712-6. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Carducci MA, Umbricht A, Richards WA, Richards BD, Cosimano MP, & Klinedinst MA (2016). Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. Journal of Psychopharmacology, 30, 1181–1197. 10.1177/0269881116675513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths R, Richards W, Johnson M, McCann U, & Jesse R (2008). Mystical-type experiences occasioned by psilocybin mediate the attribution of personal meaning and spiritual significance 14 months later. Journal of Psychopharmacology, 22, 621–632. 10.1177/0269881108094300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Chatha M, Klein AK, Wallach J, & Brandt SD (2020). Correlation between the potency of hallucinogens in the mouse head-twitch response assay and their behavioral and subjective effects in other species. Neuropharmacology, 167, 107933. 10.1016/j.neuropharm.2019.107933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, & Geyer MA (2013). Characterization of the head-twitch response induced by hallucinogens in mice: Detection of the behavior based on the dynamics of head movement. Psychopharmacology (Berl), 227, 727–739. 10.1007/s00213-013-3006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern JH (2004). Hallucinogens and dissociative agents naturally growing in the United States. Pharmacology & Therapeutics, 102, 131–138. 10.1016/j.pharmthera.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Halpern JH, & Pope HG Jr (1999). Do hallucinogens cause residual neuropsychological toxicity? Drug and Alcohol Dependence, 53, 247–256. 10.1016/S0376-8716(98)00129-X. [DOI] [PubMed] [Google Scholar]
- Hanks JB, & Gonzalez-Maeso J (2013). Animal models of serotonergic psychedelics. ACS Chemical Neuroscience, 4, 33–42. 10.1021/cn300138m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks JB, & González-Maeso J (2016a). Hallucinogens: Circuits, behavior and translational models. In Preedy VR (Ed.), The Neuropathology of drug addiction and substance misuse (Vol. 75, pp. 812–820). Elsevier. [Google Scholar]
- Hanks JB, & González-Maeso J (2016b). Molecular and cellular basis of hallucinogenic drug action. In Preedy VR (Ed.), The Neuropathology of drug addiction and substance misuse (Vol. 73, pp. 803–812). Elsevier. [Google Scholar]
- Heal DJ, Gosden J, & Smith SL (2018). Evaluating the abuse potential of psychedelic drugs as part of the safety pharmacology assessment for medical use in humans. Neuropharmacology, 142, 89–115. 10.1016/j.neuropharm.2018.01.049. [DOI] [PubMed] [Google Scholar]
- Hesselgrave N, Troppoli TA, Wulff AB, Cole AB, & Thompson SM (2021). Harnessing psilocybin: Antidepressant-like behavioral and synaptic actions of psilocybin are independent of 5-HT2R activation in mice. Proceedings of the National Academy of Sciences, 118(17), e2022489118. 10.1073/pnas.2022489118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibicke M, Landry AN, Kramer HM, Talman ZK, & Nichols CD (2020). Psychedelics, but not ketamine, produce persistent antidepressant-like effects in a rodent experimental system for the study of depression. ACS Chemical Neuroscience, 11, 864–871. 10.1021/acschemneuro.9b00493. [DOI] [PubMed] [Google Scholar]
- Hofmann A (1979). How LSD originated. Journal of Psychedelic Drugs, 11(1–2), 53–60. 10.1080/02791072.1979.10472092. [DOI] [PubMed] [Google Scholar]
- Insel TR, & Wang PS (2009). The STAR*D trial: revealing the need for better treatments. Psychiatric Services (Washington, D. C.), 60, 1466–1467. 10.1176/ps.2009.60.11.1466. [DOI] [PubMed] [Google Scholar]
- Inserra A, De Gregorio D, & Gobbi G (2021). Psychedelics in psychiatry: Neuroplastic, immunomodulatory, and neurotransmitter mechanisms. Pharmacological Reviews, 73, 202–277. 10.1124/pharmrev.120.000056. [DOI] [PubMed] [Google Scholar]
- Johnson MW, Griffiths RR, Hendricks PS, & Henningfield JE (2018). The abuse potential of medical psilocybin according to the 8 factors of the Controlled Substances Act. Neuropharmacology, 142, 143–166. 10.1016/j.neuropharm.2018.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KA, Srivastava DP, Allen JA, Strachan RT, Roth BL, & Penzes P (2009). Rapid modulation of spine morphology by the 5-HT2A serotonin receptor through kalirin-7 signaling. Proceedings of the National Academy of Sciences, 106(46), 19575–19580. 10.1073/pnas.0905884106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaertner LS, Steinborn MB, Kettner H, Spriggs MJ, Roseman L, Buchborn T, Balaet M, Timmermann C, Erritzoe D, & Carhart-Harris RL (2021). Positive expectations predict improved mental-health outcomes linked to psychedelic microdosing. Scientific Reports, 11, 1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS, Survey NCR (2003). The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R). JAMA, 289, 3095–3105. 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Koleske AJ (2013). Molecular mechanisms of dendrite stability. Nature Reviews Neuroscience, 14, 536–550. 10.1038/nrn3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs TS, & Johansen PO (2012). Lysergic acid diethylamide (LSD) for alcoholism: Meta-analysis of randomized controlled trials. Journal of Psychopharmacology, 26, 994–1002. 10.1177/0269881112439253. [DOI] [PubMed] [Google Scholar]
- Krebs TS, & Johansen PO (2013). Over 30 million psychedelic users in the United States. F1000Research, 2, 98- 10.12688/f1000research.2-98.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, & Nestler EJ (2008). The molecular neurobiology of depression. Nature, 455, 894–902. 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuypers KP, Ng L, Erritzoe D, Knudsen GM, Nichols CD, Nichols DE, Pani L, Soula A, & Nutt D (2019). Microdosing psychedelics: More questions than answers? An overview and suggestions for future research. J Psychopharmacol, 33, 1039–1057. 10.1177/0269881119857204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyzar EJ, Nichols CD, Gainetdinov RR, Nichols DE, & Kalueff AV (2017). Psychedelic drugs in biomedicine. Trends in Pharmacological Sciences, 38, 992–1005. 10.1016/j.tips.2017.08.003. [DOI] [PubMed] [Google Scholar]
- Liu RJ, & Aghajanian GK (2008). Stress blunts serotonin-and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proceedings of the National Academy of Sciences, 105, 359–364. 10.1073/pnas.0706679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly C, Greb AC, Cameron LP, Wong JM, Barragan EV, Wilson PC, Burbach KF, Soltanzadeh Zarandi S, Sood A, Paddy MR, Duim WC, Dennis MY, McAllister AK, Ori-McKenney KM, Gray JA, & Olson DE (2018). Psychedelics promote structural and functional neural plasticity. Cell Reports, 23, 3170–3182. 10.1016/j.celrep.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marona-Lewicka D, & Nichols DE (2007). Further evidence that the delayed temporal dopaminergic effects of LSD are mediated by a mechanism different than the first temporal phase of action. Pharmacology, Biochemistry and Behavior, 87, 453–461. 10.1016/j.pbb.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Marona-Lewicka D, Thisted RA, & Nichols DE (2005). Distinct temporal phases in the behavioral pharmacology of LSD: dopamine D2 receptor-mediated effects in the rat and implications for psychosis. Psychopharmacology (Berl), 180, 427–435. 10.1007/s00213-005-2183-9. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Mizowaki M, Thongpraditchote S, Murakami Y, & Watanabe H (1997). Alpha2-Adrenoceptor antagonists reverse the 5-HT2 receptor antagonist suppression of head-twitch behavior in mice. Pharmacology, Biochemistry and Behavior, 56, 417–422. [DOI] [PubMed] [Google Scholar]
- Maze I, Covington HE, Dietz DM, LaPlant Q, Renthal W, Russo SJ, Mechanic M, Mouzon E, Neve RL, Haggarty SJ, Ren Y, Sampath SC, Hurd YL, Greengard P, Tarakhovsky A, Schaefer A, & Nestler EJ (2010). Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science, 327, 213–216. 10.1126/science.1179438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JL, Holloway T, Albizu L, Sealfon SC, & Gonzalez-Maeso J (2011). Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neuroscience Letters, 493, 76–79. 10.1016/j.neulet.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Ikoma Y, Kimura K, Nakada Y, Kobayashi S, Yamaguchi M, & Nakagawa H (1989). Effects in animal models of depression of lisuride alone and upon coadministration with antidepressants. Nihon Yakurigaku Zasshi, 94, 81–89. 10.1254/fpj.94.81. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, & Monteggia LM (2002). Neurobiology of depression. Neuron, 34, 13–25. 10.1016/S0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Nichols CD, Garcia EE, & Sanders-Bush E (2003). Dynamic changes in prefrontal cortex gene expression following lysergic acid diethylamide administration. Molecular Brain Research, 111, 182–188. 10.1016/S0169-328X(03)00029-9. [DOI] [PubMed] [Google Scholar]
- Nichols CD, & Sanders-Bush E (2002). A single dose of lysergic acid diethylamide influences gene expression patterns within the mammalian brain. Neuropsychopharmacology, 26, 634–642. 10.1016/S0893-133X(01)00405-5. [DOI] [PubMed] [Google Scholar]
- Nichols DE (2004). Hallucinogens. Pharmacology & Therapeutics, 101, 131–181. 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Nichols DE (2016). Psychedelics. Pharmacological Reviews, 68, 264–355. 10.1124/pr.115.011478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picelli S, Bjorklund AK, Faridani OR, Sagasser S, Winberg G, & Sandberg R (2013). Smart-seq2 for sensitive full-length transcriptome profiling in single cells. Nature Methods, 10, 1096–1098. 10.1038/nmeth.2639. [DOI] [PubMed] [Google Scholar]
- Picelli S, Faridani OR, Bjorklund AK, Winberg G, Sagasser S, & Sandberg R (2014). Full-length RNA-seq from single cells using Smart-seq2. Nature Protocols, 9, 171–181. 10.1038/nprot.2014.006. [DOI] [PubMed] [Google Scholar]
- Rickli A, Moning OD, Hoener MC, & Liechti ME (2016). Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens. European Neuropsychopharmacology, 26, 1327–1337. 10.1016/j.euroneuro.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Rucker JJH, Iliff J, & Nutt DJ (2018). Psychiatry & the psychedelic drugs. Past, present & future. Neuropharmacology, 142, 200–218. [DOI] [PubMed] [Google Scholar]
- Russo SJ, & Nestler EJ (2013). The brain reward circuitry in mood disorders. Nature Reviews Neuroscience, 14, 609–625. 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-González A, Thougaard E, Tapias-Espinosa C, Cañete T, Sampedro-Viana D, Saunders JM, Toneatti R, Tobeña A, Gónzalez-Maeso J, Aznar S, & Fernández-Teruel A (2021). Increased thin-spine density in frontal cortex pyramidal neurons in a genetic rat model of schizophrenia-relevant features. European Neuropsychopharmacology, 44, 79–91. 10.1016/j.euroneuro.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, & Bohn LM (2010). Serotonin, but not N-methyltryptamines, activates the serotonin 2A receptor via a β-arrestin2/SRC/AKT signaling complex in vivo. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience, 30, 13513–13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Raehal KM, & Bohn LM (2008). Agonist-directed signaling of the serotonin 2A receptor depends on beta-arrestin-2 interactions in vivo. Proceedings of the National Academy of Sciences of the United States of America, 105, 1079–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid Y, Enzler F, Gasser P, Grouzmann E, Preller KH, Vollenweider FX, Brenneisen R, Muller F, Borgwardt S, & Liechti ME (2015). Acute effects of lysergic acid diethylamide in healthy subjects. Biological Psychiatry, 78(8), 544–553. 10.1016/j.biopsych.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Shao LX, Liao C, Gregg I, Davoudian PA, Savalia NK, Delagarza K, & Kwan AC (2021). Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo. Neuron, 109, 2525–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DA, Bailey JM, Williams D, & Fantegrossi WE (2014). Tolerance and cross-tolerance to head twitch behavior elicited by phenethylamine-and tryptamine-derived hallucinogens in mice. Journal of Pharmacology and Experimental Therapeutics, 351, 485–491. 10.1124/jpet.114.219337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruston N (2008). Pyramidal neurons: Dendritic structure and synaptic integration. Nature Reviews Neuroscience, 9, 206–221. 10.1038/nrn2286. [DOI] [PubMed] [Google Scholar]
- Szigeti B, Kartner L, Blemings A, Rosas F, Feilding A, Nutt DJ, Carhart-Harris RL, & Erritzoe D (2021). Self-blinding citizen science to explore psychedelic microdosing. Elife, 10, e62878. 10.7554/eLife.62878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider FX, & Kometer M (2010). The neurobiology of psychedelic drugs: Implications for the treatment of mood disorders. Nature Reviews Neuroscience, 11, 642–651. 10.1038/nrn2884. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Babler A, Vogel H, & Hell D (1998). Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. NeuroReport, 9, 3897–3902. 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- Woolley DW (1955). Production of Abnormal (Psychotic?) behavior in mice with lysergic acid diethylamide, and its partial prevention with cholinergic drugs and serotonin. Proceedings of the National Academy of Sciences, 41, 338–344. 10.1073/pnas.41.6.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurmbach E, Gonzalez-Maeso J, Yuen T, Ebersole BJ, Mastaitis JW, Mobbs CV, & Sealfon SC (2002). Validated genomic approach to study differentially expressed genes in complex tissues. Neurochemical Research, 27, 1027–1033. [DOI] [PubMed] [Google Scholar]
- Yuen T, Zhang W, Ebersole BJ, & Sealfon SC (2002). Monitoring G-protein-coupled receptor signaling with DNA microarrays and real-time polymerase chain reaction. Methods in Enzymology, 345, 556–569. [DOI] [PubMed] [Google Scholar]
- Zhu B, Hsieh YP, Murphy TW, Zhang Q, Naler LB, & Lu C (2019). MOWChIP-seq for low-input and multiplexed profiling of genome-wide histone modifications. Nature Protocols, 14, 3366–3394. 10.1038/s41596-019-0223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.