Highlights
-
•
Endometrial cancer is associated with modifiable risk factors including obesity.
-
•
Weight loss and pharmaceutical interventions may prevent endometrial cancer.
-
•
Lifestyle interventions were associated with reduced endometrial proliferation.
-
•
A reduction in circulating insulin levels was seen following lifestyle intervention.
-
•
Endometrial cancer prevention research demonstrates promising preliminary evidence.
Keywords: Endometrial cancer, Lifestyle, Lifestyle intervention, Prevention
Abstract
Endometrial cancer is the most common gynaecological malignancy in Australia. Epidemiological studies have widely documented the association of endometrial cancer with modifiable lifestyle risk factors, most notably obesity. However, preventative strategies for endometrial cancer have not been well reported. The objective of this systematic review was to identify interventions targeted towards modifiable lifestyle risk factors that may reduce the risk of endometrial cancer. Literature published in the past ten years (January 2010 – January 2021) was retrieved from PubMed, Embase and Web of Science literature databases. Of 593 studies potentially eligible, 41 were assessed in full-text, and nine studies were included in the systematic review and synthesis without meta-analysis following the SWiM guidelines. The included studies were highly heterogenous with respect to the type of interventions implemented and the outcomes measured. We identified that diet and physical activity interventions, surgical weight loss interventions, and hormonal interventions were associated with changes in endometrial cancer biomarkers including circulating hormones and tissue markers. We identified a reduction in endometrial proliferation following lifestyle intervention as measured by the ki-67 proliferation index. Furthermore we identified an increase in adiponectin (a circulating biomarker of adiposity) following lifestyle intervention and a reduction in circulating insulin levels following lifestyle intervention. This review highlighted that the area of endometrial cancer prevention research is in its infancy and that further investigation of diet and physical activity interventions, surgical weight loss interventions, and hormonal interventions should be undertaken due to promising preliminary evidence.
1. Introduction
Endometrial cancer is the most common gynaecological malignancy and the fifth most common cancer among Australian women (Australia 2012). Globally the incidence of endometrial cancer is increasing, with the highest rates of change occurring in developed countries (Lortet-Tieulent et al. 2018). Overall, women with endometrial cancer have a good chance of survival, with an 85% five-year survival rate for stage 1 disease. However, survival is poor for women with recurrent or late-stage disease. Women with stage-IV endometrial cancer have only a 15–25% chance of survival (Amant et al. 2005). Furthermore, endometrial cancer survivors are at higher risk of death due to all-cause mortality, and particularly death due to cardiovascular disease when compared to the general population. This is likely due to the development of endometrial cancer being caused by obesity and metabolic syndrome (Lees et al. 2021).
The link between obesity and the development of endometrial cancer is striking. Both type 1 (endometrioid) and type 2 (non-endometrioid) endometrial cancers have been associated with obesity (McCullough et al. 2008); however, obesity is most strongly associated with type 1 (endometrioid) endometrial cancers. Type 1 endometrial cancer accounts for up to 80–90% of cases (Yasin, Taylor, and Ayakannu 2021). For every 5 kg/m2 increase in BMI there is a 1.6 fold increased risk of endometrial cancer and in comparison to women with a normal BMI, women with class III obesity (BMI ≥ 40 kg/m2) have a five-fold increase in lifetime risk of EC (Crosbie et al. 2010). The prevalence of overweight and obesity among Australians aged 18 and over has increased from 57% in 1995 to 67% in 2017–18, with one in three Australians currently classified as overweight or obese (Welfare, 2021). It has been estimated that overweight and obesity will account for over 40% of the endometrial cancer burden and over 12,000 of the endometrial cancers diagnosed in the next decade in Australia (Laaksonen et al. 2019).
Besides maintaining a healthy weight or losing weight, a number of other modifiable lifestyle factors including the ever use of hormonal contraceptives and increased levels of physical activity have been associated with lower risk of endometrial cancer in large epidemiological studies (Collaborative Group on Epidemiological Studies on Endometrial and Cancer, 2015, Maliniak et al., 2021). In addition, the use of menopausal hormonal therapy (MHT) has been associated with both an increase and a decrease in endometrial cancer risk depending on the hormonal preparation prescribed (Brinton and Felix 2014). Given the strong epidemiological evidence in the existing literature that endometrial cancer risk is associated with several modifiable lifestyle risk factors, the current systematic review aimed to identify if health and lifestyle interventions can be effective in the prevention of endometrial cancer.
2. Methods
We conducted a systematic review and synthesis without meta-analysis (SWiM) on the utility of lifestyle interventions in endometrial cancer prevention. Our review was conducted according to PRISMA guidelines and SWiM guidelines (Moher et al., 2009, Campbell et al., 2020). The review was registered with The International Prospective Register of Systematic Reviews; PROSPERO (CRD42021240103). The PROSPERO database is an international register of systematic review protocols in the area of health and social sciences that aims to increase the transparency and to minimise reporting bias of systematic reviews (Page, Shamseer, and Tricco 2018).
2.1. Search strategy and inclusion criteria
Studies were identified through a systematic review of recent published literature available on PubMed, Embase and Web of Science literature databases. The search was restricted to articles published in the English language between 1st January 2010 and 31st January 2021. The following search terms were used to identify relevant literature within each database: (“endometrial cancer” or “endometrial hyperplasia” or “endometrial neoplasm” or “uterine cancer”) and (“Reduce risk” or “prevent” or “risk reduction”) and (intervention or program* or “lifestyle” or “treat” or “therapy”). The search terms were adjusted and combined within each database’s search builder. Full search terms for each database are available in Supplementary table 1. In addition to this search, forwards and backwards citation tracking was performed, and the reference list of articles were scanned for any additional articles potentially eligible.
Original articles (randomised controlled trial and observational studies) investigating the association of endometrial cancer risk with lifestyle interventions were included. Lifestyle is a generic term that for this review was defined to include any interventions encompassing weight management, physical activity levels, diet and nutritional intake, the use and never use of hormonal contraceptives or menopausal hormonal therapy, and alcohol and smoking status. We included only human studies that reported on endometrial cancer risk reduction or surrogate markers of endometrial cancer risk reduction. Surrogate markers of endometrial cancer included tissue markers such as estrogen and progesterone hormone receptor expression and the Ki-67 index as well as circulating blood markers including insulin, estradiol and progesterone. Studies reporting on women with a pre-existing diagnosis of endometrial cancer or endometrial hyperplasia with atypia or women currently or previously treated with tamoxifen were excluded.
2.2. Data extraction and quality assessment
Two reviewers (DR, SS) independently assessed title and abstracts of individual studies for inclusion and extracted data for further analysis from those selected for full text review. Any conflicting opinions were resolved through discussion by consensus agreement with a third author (MJ). Data were extracted with use of Covidence software (version 2, Veritas Health Innovation, Melbourne, VIC, Australia). The following information was extracted from each article: author, publication year, study design, setting, number of participants, age, menopausal status, BMI, nulliparity, type of intervention, duration of intervention and follow up, incidence of endometrial cancer, changes in tissue markers associated with endometrial cancer risk, change in circulating blood markers associated with endometrial cancer risk, change in weight and BMI. The same reviewers independently evaluated the risk of bias for each study using the Newcastle-Ottawa Scale. Using this scale studies were classified as low, medium-, and high-quality based on selection, comparability and outcome assessments (The Newcastle Ottawa Scale for assessing the quality of nonrandomised studies in meta-analyses, 2019). Any conflicts were resolved with consensus through discussion with two authors (DR, SS).
2.3. Synthesis of extracted data
Endometrial cancer prevention research is in its infancy and only a limited number of studies were identified as eligible for inclusion in the review. There was considerable diversity among the included studies with respect to the populations studied, the type of intervention studied, and the outcomes that were reported. This diversity in research methods precluded meta-analysis and statistical pooling of effect estimates. As such, a SWiM analysis was performed. Following data extraction, studies were grouped according to intervention type and outcomes measured for synthesis. For each outcome included in the synthesis the direction of effect was used as a standardised metric for synthesis of results. Where available, the reported P value was included for interest and visualisation of results but this was not included in the synthesis to determine an overall trend (see Table 2 and Table 3). To determine an overall trend, vote counting was performed according to the Cochrane Handbook for Systematic Reviews of Interventions (Cochrane Handbook for Systematic Reviews of Interventions, 2021). Vote counting was performed based on the direction of effect observed in each study with the number of interventions demonstrating a positive effect on endometrial cancer markers being compared to the number of interventions showing a negative or no effect. As described in the Cochrane Handbook for Systematic Reviews of Interventions, statistical significance was not included in the synthesis of results.
Table 2.
Effect and direction of effect of interventions on tissue biomarkers of endometrial cancer risk.
| Risk of bias | Intervention | Mean change in BMI (kg/m2) post intervention |
Tissue markers |
|||
|---|---|---|---|---|---|---|
| Ki-67% | ER H-Score |
PR H-Score | ||||
| Behavioural interventions | ||||||
| Yates, 2018 | Low | Physical activity | −3.15 p = 0.140 |
↓ p = 0.216 |
||
| Drug/therapeutic interventions | ||||||
| Lu, 2013 | Low | Oral contraceptive | Not assessed |
↓ p < 0.001 |
||
| Low | Depo-MPA | Not assessed |
↓ p < 0.001 |
|||
| Derbyshire, 2021 | Moderate | Mirena IUD | −0.5 |
↓ |
↔ |
↓ |
| Surgical interventions | ||||||
| Argenta, 2014 | Moderate | Bariatric surgery | Not recorded Median weight loss post surgery 41 kg |
↓ p = 0.59 |
↓ | ↓ |
| Mackintosh 2018 | Moderate | Bariatric surgery | −18.76 p < 0.0001 |
↓ p = 0.034 |
↓ p = 0.04 |
↓ p = 0.004 |
| Vote counting* | 5/5 decrease |
2/3 decrease |
3/3 decrease | |||
Table 3.
Effect and direction of effect of interventions on circulating biomarkers of endometrial cancer risk.
|
Risk of bias |
Intervention |
Mean change in BMI (kg/m2) post intervention |
Markers of adiposity |
Markers of insulin resistance |
Markers of reproductive function |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adiponectin | Leptin | Insulin | Glucose | HbA1C | FSH | LH | SHBG | Estradiol | ||||
| Behavioural interventions | ||||||||||||
| Linkov 2012 | Low | Physical activity + diet | −4.95 p < 0.01 |
↑ p < 0.01 |
||||||||
| Yates, 2018 | Low | Physical activity | −3.15 p = 0.140 |
↑ p = 0.324 |
↓ p = 0.379 |
↓ p = 0.018 |
↓ p = 0.541 |
↑ p = 0.115 |
↔ p = 0.912 |
↔ p = 0.925 |
||
| Drug/therapeutic interventions | ||||||||||||
| Derbyshire, 2021 | Moderate | Mirena IUD | −0.5 | ↓ |
↑ |
↓ |
↔ |
↑ |
↑ |
↑ |
↓ |
↔ |
| Surgical interventions | ||||||||||||
| Linkov, 2017 | Low | Bariatric surgery | −11.03 p < 0.01 |
↑ p < 0.01 |
↓ p < 0.01 |
|||||||
| Mackintosh, 2018 | Moderate | Bariatric surgery |
−18.76 p < 0.0001 |
↑ p < 0.0001 |
↓ p < 0.0001 |
↓ p < 0.0001 |
↓ p < 0.0001 |
↓ p < 0.0001 |
↑ p = 0.0005 |
↑ p = 0.0051 |
↓ p < 0.001 |
↑ p = 0.55 |
| Vote counting* | 4/5 increase | 2/3 decrease | 4/4 decrease | 2/3 decrease | 2/3 increase | 2/3 increase | 2/2 increase | 2/2 decrease | 2/3 increase | |||
3. Results
3.1. Included studies
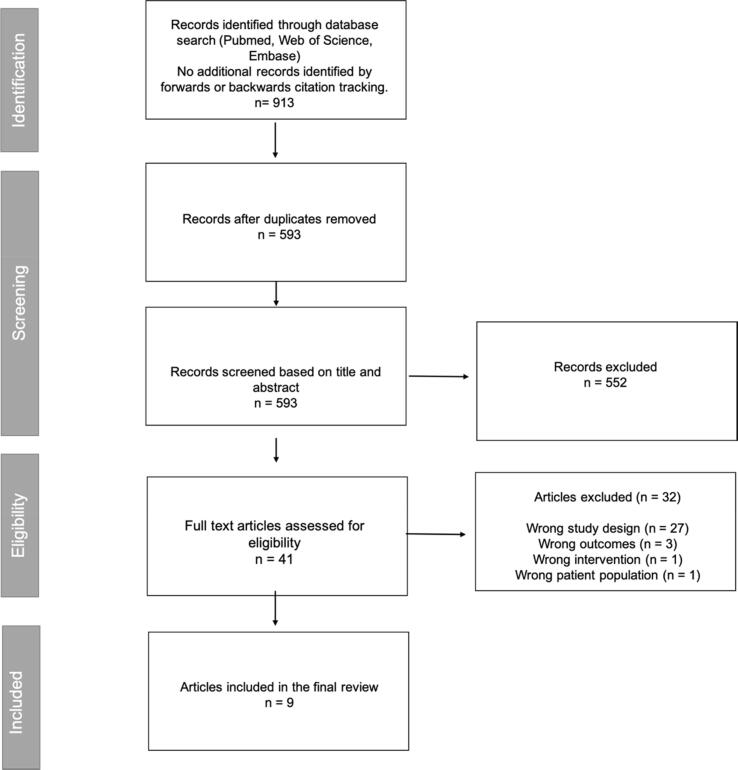
A total of 593 abstracts were identified for primary screening and following title and abstract screening, 41 potentially relevant articles were identified for full-text review and comparison against inclusion and exclusion criteria. Of these studies, 32 were excluded (27 due to unrelated study design, 3 due to reporting of unrelated outcomes, 1 due to unrelated intervention, and 1 due to unrelated patient population). A total of 9 studies met the criteria for inclusion in our systematic review. This is detailed further in the PRISMA flow diagram (Fig. 1). Overall, five studies were judged to be at low risk of bias and four studies were judged to be at a moderate risk of bias. Using the Newcastle-Ottawa Scale the median quality score for included studies was 7 out of 9 (range 4–8). This can be visualised in Supplementary Table 2.
Fig. 1.
PRISMA flowchart of included studies.
3.2. Participants
The majority of included studies (7 of the 9 studies) specifically recruited women with obesity due to the strong association between endometrial cancer and obesity (Linkov et al., 2014, Linkov et al., 2012, Linkov et al., 2017, MacKintosh et al., 2019, Argenta et al., 2014, Yates et al., 2018). Lu et al recruited women between the ages of 25 and 50 with Lynch syndrome (defined by having a known mutation in MLH1, MSH2, or MSH6) a known high-risk population for endometrial cancer. Manson et al., (2013) recruited post-menopausal women without prior hysterectomy for inclusion in their study. Other characteristics of the 9 included studies are outlined in Table 1. Four were randomised clinical trials (Linkov et al., 2012, Yates et al., 2018, Manson et al., 2013, Lu et al., 2013), one was a feasibility study (Derbyshire et al. 2021), and four were prospective cohort studies (Argenta et al., 2014, Linkov et al., 2014, Linkov et al., 2017, MacKintosh et al., 2019). Seven of the nine studies were conducted in the United States of America (Linkov et al., 2014, Linkov et al., 2017, Argenta et al., 2014, Linkov et al., 2012, Manson et al., 2013, Lu et al., 2013, Yates et al., 2018) and two were conducted in the United Kingdom (MacKintosh et al., 2019, Derbyshire et al., 2021).
Table 1.
Study characteristics.
| Author, year | Study type | Population |
Participant characteristics |
Total N | Intervention | Duration of intervention | Outcomes reported | ||
|---|---|---|---|---|---|---|---|---|---|
| Age | Baseline BMI | Menopausal status | |||||||
| Behavioural intervention | |||||||||
| Linkov, 2012 United States of America |
Randomised intervention trial | Hospital outpatient setting Pittsburgh, USA |
Mean (SD) 45.76 (6.58) years | Mean (SD) 38.06 (9.76) | 89 | Weight loss through an intensive diet and physical activity program | 12 months | Circulating biomarkers | |
| Yates, 2018 United States of America |
Randomised intervention trial | Community and hospital outpatient setting Texas, USA |
Mean (SD) 57.5 (4.4) years | Mean (SD) 39.1 (5.7) | All women (100%) post-menopausal | 37 | Physical activity intervention Comparative group with physical activity + therapeutic intervention (metformin)* |
16 weeks Follow up at 12 months |
Tissue biomarkers Circulating biomarkers |
| Drug/therapeutic intervention | |||||||||
| Manson, 2013 United States of America |
Randomised control trial | Multi-national setting | Treatment group mean (SD) 63.2 (7.1) Placebo mean (SD) 63.3 (7) |
Treatment group median 27.5 Placebo median 27.5 |
All women (100%) post-menopausal | 16 608 | Menopausal hormone therapy (conjugated equine estrogen + medroxyprogesterone acetate) vs placebo | Median duration of treatment 5.6 years Follow up 13 years |
Endometrial cancer incidence |
| Lu, 2013 United States of America |
Randomised intervention trial | Hospital outpatient setting Women with Lynch syndrome |
OCP group mean 38.0 Depo group mean 36.8 |
OCP group mean 26.2 Depo group mean 28.4 |
51 | Hormonal contraception:oral contraceptive vs Depo-provera | 3–4 months | Tissue biomarkers | |
| Derbyshire, 2021 United Kingdom |
Feasibility study | Hospital outpatient setting. Manchester, UK |
Median 54 | Median 47 | 64% of women post menopausal | 25 | Hormonal intrauterine device (Mirena) | 8–11 months | Tissue biomarkers Circulating biomarkers |
| Surgical intervention | |||||||||
| Argenta, 2014 United States of America |
Prospective cohort study | Hospital outpatient setting Pittsburgh, USA |
Median 39 | Median 46 | 17% of women post menopausal | 59 | Bariatric surgery (Roux-en-Y gastric bypass) | 12 month follow up | Tissue biomarkers |
| Linkov, 2014 United States of America |
Prospective cohort study | Hospital outpatient setting Pittsburgh, USA |
Mean (range) 42 (22–62) | Mean (range) 46.8 (36–64.3) | 17% of women post menopausal | 59 | Bariatric surgery (Roux-en-Y gastric bypass) | 12 month follow up | Tissue biomarkers |
| Linkov, 2017 United States of America |
Prospective cohort study | Hospital outpatient setting Pittsburgh, USA |
Mean (SD) 43.88 (11.66) | Mean (SD) 45.52 (6.19) | 107 | Bariatric surgery (Roux-en-Y gastric bypass, laparoscopic adjustable gastric banding, or sleeve gastrectomy) | 6 month follow up | Circulating biomarkers | |
| Mackintosh, 2019 United Kingdom |
Prospective cohort study | Hospital outpatient setting Manchester, UK |
Mean (range) 42 (24–65) | Median 52.1 | 24% of women were post menopausal | 72 | Bariatric surgery (laparoscopic gastric bypass or sleeve gastrectomy) | 12 month follow up | Tissue biomarkers Circulating biomarkers |
3.3. Interventions
There was considerable diversity among the included studies with respect to the type of intervention performed.
Behavioural interventions: Two studies reported on behavioural interventions. Yates et al., (2018) performed a 16-week study to examine the effect of a physical activity and diet intervention on circulating and tissue biomarkers. Participants recorded daily calorie intake and step counts for the duration of the intervention. Linkov et al., (2012) reported on a 12-month behavioural intervention including diet and physical activity changes. The primary outcome of this study was to investigate the effect of weight loss in obese women on circulating biomarkers.
Pharmaceutical interventions: Three studies reported on pharmaceutical interventions. Manson et al., (2013) performed a randomized control trial on post-menopausal women to examine the effects of menopausal hormone therapy. Participants received either menopausal hormone therapy (conjugated equine estrogen + medroxyprogesterone acetate) or placebo. Lu et al., (2013) investigated hormonal contraception in women with Lynch syndrome and its effect on endometrial proliferation. Women were randomized to receive a progestin-containing oral contraceptive or depo-medroxyprogesterone acetate. Derbyshire et al., (2021) investigated the effects of progesterone releasing intrauterine device in obese women on biomarkers of endometrial cancer.
Surgical interventions: Four studies reported on surgical interventions, each looking at the influence of bariatric surgery on biomarkers of endometrial cancer (MacKintosh et al., 2019, Linkov et al., 2017, Linkov et al., 2014, Argenta et al., 2014). Linkov et al., 2014, Argenta et al., 2014 investigated the effects of Rou-en-Y bariatric surgery induced weight loss on endometrial proliferation. Linkov et al., (2017) explored all types of bariatric surgery-induced weight loss in relation to change in circulating biomarkers. Mackintosh et al., (2019) investigated changes in circulating and tissue biomarkers from the weight loss achieved by laparoscopic gastric bypass and sleeve gastrectomy.
3.4. Recruitment and attrition
Several studies reported on the recruitment process and in particular difficulties with recruitment and attrition during the intervention period; this occurred in both behavioural and pharmaceutical interventions. Lu et al., (2013) reported screening 700 women over a six-year period in order to identify 51 women for inclusion in their study. Reasons for exclusion included no consent for endometrial biopsies and no consent to hormonal therapy. Yates et al., (2018) reported approaching 576 women in order to identify 52 women eligible to attend for baseline assessment and inclusion in the study. Reasons for slow recruitment were not reported. Mackintosh et al., (2019) screened 248 women, 72 of which presented for baseline assessment, and only 28 presented for follow up assessment on completion of the study. Common reasons reported for withdrawal from the study included women declining ‘hassle’ of follow up and declining painful procedures such as endometrial biopsy.
4. Outcomes measured
4.1. Reduction in the incidence of endometrial cancer
Only one study reported on the overall incidence of endometrial cancer following a preventive intervention. Manson et al., (2013) demonstrated that in post-menopausal women the use of menopausal hormonal therapy (conjugated equine estrogen and medroxyprogesterone acetate) for a median duration of 5.6 years resulted in a reduced risk of endometrial cancer when compared to placebo (Hazard ratio (HR) = 0.58; 95% Confidence Interval (CI) = 0.40–0.80; p = 0.007). However, there were also health risks associated with menopausal hormonal therapy in the trial, including increased risk of cardiovascular disease (HR 1.13, 95% CI = 1.02–1.25), stroke (HR = 1.37, 95% CI = 1.07–1.76), and venous thromboembolism (HR = 1.98, 95% CI = 1.36–2.87), and the authors concluded that these outweighed the benefits.
4.2. Tissue and blood biomarkers
Five studies reported on hormonal and proliferative tissue biomarkers including Ki-67 proliferation index, estrogen receptor H-score, and progesterone receptor H-score (Yates et al., 2018, Lu et al., 2013, Derbyshire et al., 2021, Argenta et al., 2014, MacKintosh et al., 2019, Linkov et al., 2017). One additional study reported on tissue markers of immune function (Linkov et al. 2014) .Five studies reported on circulating biomarkers of endometrial cancer risk (Linkov et al., 2012, Yates et al., 2018, Derbyshire et al., 2021, Linkov et al., 2017, MacKintosh and Crosbie, 2018). Circulating biomarkers commonly reported in the included studies could be divided into groups of markers of adiposity (adiponectin and leptin), markers of insulin resistance (insulin, glucose, and glycated haemoglobin), and markers of reproductive function (follicular stimulating hormone, luteinising hormone, sex hormone binding globulin, and estradiol).
4.3. Tissue biomarkers
There was evidence that lifestyle interventions had an effect on tissue markers of endometrial cancer, in particular the ki-67 proliferation index, with five of five studies reporting a decrease in endometrial proliferation following the intervention. This direction of effect was consistent across the behavioural, drug/therapeutic, and surgical interventions. Two of these five studies were judged to be at a low risk of bias. Lu et al., (2013) demonstrated that the use of either the COCP or Depo-MPA for up to four months in women with Lynch syndrome was associated with a significant post intervention decrease in the Ki-67 index compared to baseline. Furthermore, Yates et al., (2018) demonstrated that a 16-week physical activity and diet intervention was associated with a trend towards reduced endometrial proliferation compared to baseline at completion of the intervention.
Only three studies reported on estrogen and progesterone hormone receptor expression pre and post lifestyle interventions. Each of these studies were judged to be at a moderate risk of bias and no definitive direction of effect for endometrial hormone receptor expression pattern was observed in response to lifestyle interventions. Linkov et al., (2014) was the only study in the current review to report on tissue markers of immune function, including expression of CD20, CD43 and PTEN receptor biomarkers following bariatric surgery. Reduced expression of CD20 was observed following bariatric surgery; however, no significant changes in other immune marker expression was observed.
4.4. Circulating biomarkers
There was evidence that lifestyle interventions had an effect on circulating biomarkers of potential endometrial cancer risk. The most consistent effects were seen for adiponectin and insulin; markers of adiposity and insulin resistance, respectively. Four of five studies demonstrated higher adiponectin levels following an intervention when compared to baseline. All studies reporting on behavioural and surgical interventions demonstrated a trend towards increased adiponectin levels post intervention. Three of the five studies were judged to be at a low risk of bias and each of these demonstrated an increase in adiponectin with intervention. There was only one study judged to be low risk that reported on the effect of an intervention on leptin levels, another biomarker for adiposity. This study demonstrated a significant reduction in leptin levels post bariatric surgery (Table 3).
Four out of four studies reported lower insulin levels following the intervention when compared to baseline, the direction of effect was consistent among behavioural, drug/therapeutic, and surgical interventions. Only three studies included markers of reproductive function as biomarkers for endometrial cancer development, with results conflicting with respect to the direction of effect (Table 3). The present synthesis did not demonstrate a direction of effect of lifestyle interventions on biomarkers of reproductive function and endometrial cancer risk including follicular stimulating hormone, luteinising hormone, sex hormone binding globulin, and estradiol.
5. Discussion
This review summarises clinically relevant lifestyle interventions to prevent the development of endometrial cancer. We identified only few studies devoted to the prevention of endometrial cancer, and these studies used a variety of intervention types including diet/physical activity interventions, surgical interventions (bariatric surgery), and hormonal interventions. Using a SWIM approach summarise the existing evidence, notably this review demonstrated positive effects of lifestyle interventions on endometrial proliferation as measured by the Ki-67 index and on circulating biomarkers of adiposity and insulin resistance (adiponectin and insulin, respectively) that have been shown to play a role in endometrial cancer risk (Soliman et al., 2006, Shevra et al., 2015). The reviewed studies did not report any negative effects of lifestyle interventions on tissue or circulating biomarkers of endometrial cancer, but many reported difficulties in recruiting and retaining participants.
The Ki-67 proliferation index is a commonly used tissue marker of cell proliferation. Ki-67 is readily detected by immunohistochemistry and Ki-67 expression is considered to be on a continuum where 10% increase in Ki-67 expression is equal to cancer-specific survival hazard ratio of 1.31 (Kitson et al. 2017). In addition, studies have demonstrated that increased Ki-67 expression is seen in patients presenting with atypical endometrial hyperplasia and endometrial carcinoma when compared to normal endometrium (Shevra, Ghosh, and Kumar 2015). We observed a consistent decrease in five out of five studies that measured endometrial proliferation across all types of lifestyle interventions in this review. Larger and longer studies would be required to assess whether such declines in Ki-67 would translate in reduced incidence of endometrial cancer.
We also demonstrated a consistent decrease in circulating insulin levels following four out of four lifestyle interventions. Insulin is known to promote cell proliferation by activating the Ras/MAPK and PI3K/AKT pathways and also lead to an increase in circulating estrogen and endometrial proliferation (Mu et al. 2012). Furthermore, women with diabetes have a significantly increased risk of developing endometrial cancer when compared to the general population (Saed et al. 2019). This increased risk may be due to increased rates of overweight and obesity among the diabetic population, or in part be due to the effects of insulin therapy or insulin resistance influencing levels of circulating insulin (Soliman et al. 2006). We observed an increase in four of five studies in circulating adiponectin levels following lifestyle intervention. More significant increases were seen with increased weight loss, particularly following surgical interventions. Adiponectin levels have been shown to be decreased in people with obesity and with type 2 diabetes, each of which are independent risk factors for endometrial cancer (Linkov et al. 2012). Adiponectin has anti-inflammatory properties and higher levels of circulating adiponectin has been shown to be correlated with a reduced risk of endometrial cancer (Barb et al., 2006, Zeng et al., 2015, Cust et al., 2007). Adiponectin levels have been shown to be higher in individuals with a lower body mass (Turer et al. 2011).
Strengths of this review include its focus on healthcare interventions for the prevention of endometrial cancer, and use of a pragmatic SWIM approach to summarise the emerging evidence for a number of potentially useful strategies to reduce endometrial cancer incidence over the past decade. Our review was limited by the low number of studies available in the existing literature regarding lifestyle interventions for endometrial cancer prevention. This does appear to be an evolving area of research and we identified several ongoing clinical trials of lifestyle interventions for the prevention of endometrial cancer through searches of international clinical trials registries. For example, researchers at the University of Utah have registered the TIMESPAN trial (time restricted eating among native Hawaiian / Pacific Islander women at risk of endometrial cancer (ClinicalTrials.gov identifier NCT number NCT04763902). Furthermore, researchers at the University of Manchester have registered a trial investigating the prevention of breast and endometrial cancer using total dietary replacement (ISRCTN 15358157, https://doi.org/10.1186/ISRCTN15358157). Further descriptions of these ongoing trials are available in supplementary table 3.
We identified several possible challenges in the implementation of preventative interventions for endometrial cancer. A large sample size and prolonged duration of follow up is required in order to include endometrial cancer incidence as a primary outcome. As such, surrogate tissue and circulating markers of endometrial cancer pathogenesis have been used, but the relevance of impact on these intermediary outcomes still needs to be conclusively proven. Several studies reported difficulty with patient recruitment and attrition; in these studies, large numbers of women were approached in order to often only achieve a small sample of participants to undergo the intervention. Common reasons reported for participant attrition included reluctance to attend for painful procedures, in particular endometrial sampling, and reluctance to attend follow up due to time commitment. In order to maximise the uptake and benefits of future intervention for the prevention of endometrial cancer, more convenient markers as surrogate measures for endometrial cancer risk should be considered. In particular, the use of blood markers that negate the need for painful endometrial biopsies may improve participation rates in intervention studies. Recently Njoku et al., (2021) have reported accurate endometrial cancer detection through plasma biomarkers glycerophospholipids and hydroxybutyrates. To allow for shorter follow up and continued progression of research in endometrial cancer prevention the identification of biomarkers and risk prediction tools for endometrial cancer would be beneficial. This is an important area of ongoing research.
6. Conclusions
Here we have described potentially beneficial effects on tissue and circulating blood biomarkers that may be associated with the development of endometrial cancer following lifestyle interventions. These interventions were diverse and included diet and physical activity programs, the use of hormonal contraceptives or menopausal hormone therapy, and surgical weight loss. Given the increasing incidence of endometrial cancer and the strong association of endometrial cancer with modifiable risk factors including obesity it is critical to continue research into endometrial cancer prevention. Further research into possible biomarkers of endometrial cancer would aid in greater acceptance and participation of women in prevention trials.
CRediT authorship contribution statement
Dayle Rundle-Thiele: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. Sujal Shrestha: Investigation, Writing – original draft. Monika Janda: Conceptualization, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gore.2021.100900.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Amant F., Moerman P., Neven P., Timmerman D., Van Limbergen E., Vergote I. Endometrial cancer. Lancet. 2005;366:491–505. doi: 10.1016/S0140-6736(05)67063-8. [DOI] [PubMed] [Google Scholar]
- Argenta P., Svendsen C., Elishaev E., Gloyeske N., Geller M.A., Edwards R.P., Linkov F. Hormone receptor expression patterns in the endometrium of asymptomatic morbidly obese women before and after bariatric surgery. Gynecol Oncol. 2014;133:78–82. doi: 10.1016/j.ygyno.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Australia, Australian Institute of Health and Welfare & Cancer. 2012. “Gynaecological cancers in Australia: an overview.” In. Canberra: AIHW.
- Barb D., Pazaitou-Panayiotou K., Mantzoros C.S. Adiponectin: a link between obesity and cancer. Expert Opin Investig Drugs. 2006;15:917–931. doi: 10.1517/13543784.15.8.917. [DOI] [PubMed] [Google Scholar]
- Brinton L.A., Felix A.S. Menopausal hormone therapy and risk of endometrial cancer. J Steroid Biochem Mol Biol. 2014;142:83–89. doi: 10.1016/j.jsbmb.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M., McKenzie J.E., Sowden A., Katikireddi S.V., Brennan S.E., Ellis S., Hartmann-Boyce J., Ryan R., Shepperd S., Thomas J., Welch V., Thomson H. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. 2020;368 doi: 10.1136/bmj.l6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- “Cochrane Handbook for Systematic Reviews of Interventions.” In. 2021. edited by J. P. T. Higgins, J. Thomas, J. Chandler, M. Cumpston, T. Li, M. J. Page and V. Welch. Cochrane.
- Collaborative Group on Epidemiological Studies on Endometrial, Cancer Endometrial cancer and oral contraceptives: an individual participant meta-analysis of 27 276 women with endometrial cancer from 36 epidemiological studies. Lancet Oncol. 2015;16:1061–1070. doi: 10.1016/S1470-2045(15)00212-0. [DOI] [PubMed] [Google Scholar]
- Crosbie E.J., Zwahlen M., Kitchener H.C., Egger M., Renehan A.G. Body mass index, hormone replacement therapy, and endometrial cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2010;19:3119–3130. doi: 10.1158/1055-9965.EPI-10-0832. [DOI] [PubMed] [Google Scholar]
- Cust A.E., Kaaks R., Friedenreich C., Bonnet F., Laville M., Lukanova A., Rinaldi S., Dossus L., Slimani N., Lundin E., Tjonneland A., Olsen A., Overvad K., Clavel-Chapelon F., Mesrine S., Joulin V., Linseisen J., Rohrmann S., Pischon T., Boeing H., Trichopoulos D., Trichopoulou A., Benetou V., Palli D., Berrino F., Tumino R., Sacerdote C., Mattiello A., Quiros J.R., Mendez M.A., Sanchez M.J., Larranaga N., Tormo M.J., Ardanaz E., Bueno-de-Mesquita H.B., Peeters P.H., van Gils C.H., Khaw K.T., Bingham S., Allen N., Key T., Jenab M., Riboli E. Plasma adiponectin levels and endometrial cancer risk in pre- and postmenopausal women. J Clin Endocrinol Metab. 2007;92:255–263. doi: 10.1210/jc.2006-1371. [DOI] [PubMed] [Google Scholar]
- Derbyshire A.E., Allen J.L., Gittins M., Lakhiani B., Bolton J., Shaw J., Pemberton P.W., Needham M., MacKintosh M.L., Edmondson R.J., Kitchener H.C., Crosbie E.J. PROgesterone Therapy for Endometrial Cancer Prevention in Obese Women (PROTEC) Trial: A Feasibility Study. Cancer Prev Res (Phila) 2021;14:263–274. doi: 10.1158/1940-6207.CAPR-20-0248. [DOI] [PubMed] [Google Scholar]
- Kitson S., Sivalingam V.N., Bolton J., McVey R., Nickkho-Amiry M., Powell M.E., Leary A., Nijman H.W., Nout R.A., Bosse T., Renehan A.G., Kitchener H.C., Edmondson R.J., Crosbie E.J. Ki-67 in endometrial cancer: scoring optimization and prognostic relevance for window studies. Mod Pathol. 2017;30(3):459–468. doi: 10.1038/modpathol.2016.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laaksonen M.A., Arriaga M.E., Canfell K., MacInnis R.J., Byles J.E., Banks E., Shaw J.E., Mitchell P., Giles G.G., Magliano D.J., Gill T.K., Klaes E., Velentzis L.S., Hirani V., Cumming R.G., Vajdic C.M. The preventable burden of endometrial and ovarian cancers in Australia: A pooled cohort study. Gynecol Oncol. 2019;153(3):580–588. doi: 10.1016/j.ygyno.2019.03.102. [DOI] [PubMed] [Google Scholar]
- Lees B., Hampton J.M., Trentham-Dietz A., Newcomb P., Spencer R. A population-based study of causes of death after endometrial cancer according to major risk factors. Gynecol Oncol. 2021;160:655–659. doi: 10.1016/j.ygyno.2020.12.020. [DOI] [PubMed] [Google Scholar]
- Linkov F., Elishaev E., Gloyeske N., Edwards R., Althouse A.D., Geller M.A., Svendsen C., Argenta P.A. Bariatric surgery-induced weight loss changes immune markers in the endometrium of morbidly obese women. Surg Obes Relat Dis. 2014;10:921–926. doi: 10.1016/j.soard.2014.03.023. [DOI] [PubMed] [Google Scholar]
- Linkov F., Goughnour S.L., Ma T., Xu Z., Edwards R.P., Lokshin A.E., Ramanathan R.C., Hamad G.G., McCloskey C., Bovbjerg D.H. Changes in inflammatory endometrial cancer risk biomarkers in individuals undergoing surgical weight loss. Gynecol Oncol. 2017;147:133–138. doi: 10.1016/j.ygyno.2017.07.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkov F., Maxwell G.L., Felix A.S., Lin Y., Lenzner D., Bovbjerg D.H., Lokshin A., Hennon M., Jakicic J.M., Goodpaster B.H., DeLany J.P. Longitudinal evaluation of cancer-associated biomarkers before and after weight loss in RENEW study participants: implications for cancer risk reduction. Gynecol Oncol. 2012;125(1):114–119. doi: 10.1016/j.ygyno.2011.12.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lortet-Tieulent J., Ferlay J., Bray F., Jemal A. International Patterns and Trends in Endometrial Cancer Incidence, 1978–2013. J Natl Cancer Inst. 2018;110:354–361. doi: 10.1093/jnci/djx214. [DOI] [PubMed] [Google Scholar]
- Lu K.H., Loose D.S., Yates M.S., Nogueras-Gonzalez G.M., Munsell M.F., Chen L.-M., Lynch H., Cornelison T., Boyd-Rogers S., Rubin M., Daniels M.S., Conrad P., Milbourne A., Gershenson D.M., Broaddus R.R. Prospective multicenter randomized intermediate biomarker study of oral contraceptive versus depo-provera for prevention of endometrial cancer in women with Lynch syndrome. Cancer Prev Res (Phila) 2013;6(8):774–781. doi: 10.1158/1940-6207.CAPR-13-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKintosh M.L., Crosbie E.J. Prevention Strategies in Endometrial Carcinoma. Curr Oncol Rep. 2018;20:101. doi: 10.1007/s11912-018-0747-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKintosh M.L., Derbyshire A.E., McVey R.J., Bolton J., Nickkho-Amiry M., Higgins C.L., Kamieniorz M., Pemberton P.W., Kirmani B.H., Ahmed B., Syed A.A., Ammori B.J., Renehan A.G., Kitchener H.C., Crosbie E.J. The impact of obesity and bariatric surgery on circulating and tissue biomarkers of endometrial cancer risk. Int J Cancer. 2019;144:641–650. doi: 10.1002/ijc.31913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maliniak M.L., Gapstur S.M., McCullough L.E., Rees-Punia E., Gaudet M.M., Um C.Y., Guinter M.A., Flanders W.D., Patel A.V. Joint associations of physical activity and body mass index with the risk of established excess body fatness-related cancers among postmenopausal women. Cancer Causes Control. 2021;32(2):127–138. doi: 10.1007/s10552-020-01365-2. [DOI] [PubMed] [Google Scholar]
- Manson J.E., Chlebowski R.T., Stefanick M.L., Aragaki A.K., Rossouw J.E., Prentice R.L., Anderson G., Howard B.V., Thomson C.A., LaCroix A.Z., Wactawski-Wende J., Jackson R.D., Limacher M., Margolis K.L., Wassertheil-Smoller S., Beresford S.A., Cauley J.A., Eaton C.B., Gass M., Hsia J., Johnson K.C., Kooperberg C., Kuller L.H., Lewis C.E., Liu S., Martin L.W., Ockene J.K., O’Sullivan M.J., Powell L.H., Simon M.S., Van Horn L., Vitolins M.Z., Wallace R.B. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA. 2013;310(13):1353. doi: 10.1001/jama.2013.278040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough M.L., Patel A.V., Patel R., Rodriguez C., Feigelson H.S., Bandera E.V., Gansler T., Thun M.J., Calle E.E. Body mass and endometrial cancer risk by hormone replacement therapy and cancer subtype. Cancer Epidemiol Biomarkers Prev. 2008;17:73–79. doi: 10.1158/1055-9965.EPI-07-2567. [DOI] [PubMed] [Google Scholar]
- Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G. and Prisma Group. 2009. 'Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement', Open Med, 3: e123-30. [PMC free article] [PubMed]
- Mu N., Zhu Y., Wang Y., Zhang H., Xue F. Insulin resistance: a significant risk factor of endometrial cancer. Gynecol Oncol. 2012;125:751–757. doi: 10.1016/j.ygyno.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 'The Newcastle Ottawa Scale for assessing the quality of nonrandomised studies in meta-analyses'. 2019. The Ottawa Hospital, Accessed 15th May. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- Njoku, K., A. E. Campbell, B. Geary, M. L. MacKintosh, A. E. Derbyshire, S. J. Kitson, V. N. Sivalingam, A. Pierce, A. D. Whetton, and E. J. Crosbie. 2021. 'Metabolomic Biomarkers for the Detection of Obesity-Driven Endometrial Cancer', Cancers (Basel), 13. [DOI] [PMC free article] [PubMed]
- Page M.J., Shamseer L., Tricco A.C. Registration of systematic reviews in PROSPERO: 30,000 records and counting. Syst Rev. 2018;7:32. doi: 10.1186/s13643-018-0699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saed L., Varse F., Baradaran H.R., Moradi Y., Khateri S., Friberg E., Khazaei Z., Gharahjeh S., Tehrani S., Sioofy-Khojine A.B., Najmi Z. The effect of diabetes on the risk of endometrial Cancer: an updated a systematic review and meta-analysis. BMC Cancer. 2019;19:527. doi: 10.1186/s12885-019-5748-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevra C.R., Ghosh A., Kumar M. Cyclin D1 and Ki-67 expression in normal, hyperplastic and neoplastic endometrium. J Postgrad Med. 2015;61:15–20. doi: 10.4103/0022-3859.147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman P.T., Wu D., Tortolero-Luna G., Schmeler K.M., Slomovitz B.M., Bray M.S., Gershenson D.M., Lu K.H. Association between adiponectin, insulin resistance, and endometrial cancer. Cancer. 2006;106:2376–2381. doi: 10.1002/cncr.21866. [DOI] [PubMed] [Google Scholar]
- Turer A.T., Khera A., Ayers C.R., Turer C.B., Grundy S.M., Vega G.L., Scherer P.E. Adipose tissue mass and location affect circulating adiponectin levels. Diabetologia. 2011;54:2515–2524. doi: 10.1007/s00125-011-2252-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welfare, Australian Institute of Health and. 2021. 'Overweight and obesity', Accessed 10th March. aihw.gov.au/reports/australias-health/overweight-and-obesity.
- Yasin H.K., Taylor A.H., Ayakannu T. A Narrative Review of the Role of Diet and Lifestyle Factors in the Development and Prevention of Endometrial Cancer. Cancers (Basel) 2021;13(9):2149. doi: 10.3390/cancers13092149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates M.S., Coletta A.M., Zhang Q., Schmandt R.E., Medepalli M., Nebgen D., Soletsky B., Milbourne A., Levy E., Fellman B., Urbauer D., Yuan Y., Broaddus R.R., Basen-Engquist K., Lu K. Prospective Randomized Biomarker Study of Metformin and Lifestyle Intervention for Prevention in Obese Women at Increased Risk for Endometrial Cancer. Cancer Prev Res (Phila) 2018;11:477–490. doi: 10.1158/1940-6207.CAPR-17-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng F., Shi J., Long Y., Tian H., Li X., Zhao A.Z., Li R.F., Chen T. Adiponectin and Endometrial Cancer: A Systematic Review and Meta-Analysis. Cell Physiol Biochem. 2015;36:1670–1678. doi: 10.1159/000430327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.