Abstract
Background
To gain a global perspective on short-acting β2-agonist (SABA) prescriptions and associated asthma-related clinical outcomes in patients with asthma, we assessed primary health data across 24 countries in five continents.
Methods
SABINA III was a cross-sectional study that employed electronic case report forms at a study visit (in primary or specialist care) to record prescribed medication(s), over-the-counter (OTC) SABA purchases and clinical outcomes in asthma patients (≥12 years old) during the past 12 months. In patients with ≥1 SABA prescriptions, associations of SABA with asthma symptom control and severe exacerbations were analysed using multivariable regression models.
Results
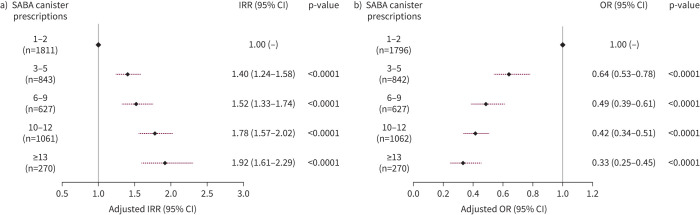
Of 8351 patients recruited (n=6872, specialists; n=1440, primary care), 76.5% had moderate-to-severe asthma and 45.4% experienced ≥1 severe exacerbations in the past 12 months. 38% of patients were prescribed ≥3 SABA canisters; 18.0% purchased OTC SABA, of whom 76.8% also received SABA prescriptions. Prescriptions of 3–5, 6–9, 10–12 and ≥13 SABA canisters (versus 1–2) were associated with increasingly lower odds of controlled or partly controlled asthma (adjusted OR 0.64 (95% CI 0.53–0.78), 0.49 (95% CI 0.39–0.61), 0.42 (95% CI 0.34–0.51) and 0.33 (95% CI 0.25–0.45), respectively; n=4597) and higher severe exacerbation rates (adjusted incidence rate ratio 1.40 (95% CI 1.24–1.58), 1.52 (95% CI 1.33–1.74), 1.78 (95% CI 1.57–2.02) and 1.92 (95% CI 1.61–2.29), respectively; n=4612).
Conclusions
This study indicates an association between high SABA prescriptions and poor clinical outcomes across a broad range of countries, healthcare settings and asthma severities, providing support for initiatives to improve asthma morbidity by reducing SABA overreliance.
Short abstract
Findings from SABINA III, which included 8351 patients from 24 countries, indicate that across treatment steps and clinical care settings, high SABA prescriptions were associated with higher rates of severe exacerbations and poorer asthma control https://bit.ly/2VHBISg
Introduction
Asthma is a common disease worldwide and the most common chronic disease of childhood [1]. In the face of a rising prevalence in a majority of the countries globally [2], the substantial decreases in asthma-related hospitalisations and reduction in asthma deaths by more than one-half, even in countries with relatively poor resources for asthma care [2, 3], are considered to be due largely to the introduction of inhaled corticosteroids (ICSs) and other effective controller therapies. However, this decrease in asthma morbidity has stalled in many countries, including in those with provision and access to the most effective controller therapies [3], suggesting the need for additional measures to avoid morbidity and preventable deaths from asthma. A case-based enquiry into factors associated with asthma deaths in the UK identified several potentially modifiable issues, chief among which were the underuse of ICSs and an excessive use of short-acting β2-agonists (SABAs) [4]. Of concern is that almost one-half of asthma deaths in the UK were among patients considered by their physicians to have asthma of mild-to-moderate severity [4]. Studies performed with inhaler dose counters confirm that much of the SABA overuse occurs during asthma worsening as patients seek relief [5]; occasionally, this may delay initiation of more effective treatment to prevent the attack or delay presentation for medical care [6]. It is salient to recognise that two-thirds of asthma deaths occur outside of medical facilities [4].
Research on alternative approaches to symptom-based titration of as-needed SABA has established the value of replacing a SABA with a combination of a low-dose ICS and a rapid-onset long-acting β2-agonist (LABA) [7] or a SABA as reliever [8, 9]. A single inhaler for maintenance and reliever therapy [7, 10, 11] has been endorsed as the preferred treatment for moderate-to-severe asthma in local guidelines [12, 13] and in the Global Initiative for Asthma (GINA) report [14]. More recently, on the basis of evidence of efficacy and safety observed in randomised controlled trials [15, 16] and in real-life studies [17, 18], GINA now recommends ICS/formoterol combination inhalers taken only as-needed as sole therapy for patients with mild asthma [14, 19]. If applied widely, this approach has the potential to reduce SABA overuse and ensure that more patients receive doses of anti-inflammatory treatment when symptoms develop, targeting the underlying airway inflammation [20]. However, inclusion of these recommendations into national guidelines and formularies can be challenging as they represent a major shift in treatment approach, and there are important considerations of cost and benefit in every country based on local factors. Information on the current status of SABA use and the potential burden associated is not readily available for many countries outside of Europe. A more current and detailed knowledge of local SABA use and its association with continuing asthma morbidity may assist policymakers and clinicians in assessing the potential benefits of switching to ICS-containing relievers as the standard of care for asthma in these countries [14].
SABINA (SABA use IN Asthma) III forms part of the SABINA group of observational studies [21–25] that seek to assess SABA prescriptions for asthma around the world. In SABINA III, we investigated prescriptions and over-the-counter (OTC) purchases of SABAs, other asthma medication prescriptions and associated clinical outcomes among patients with asthma attending primary and specialist care in 24 countries, including several with limited healthcare resources. We employed a standardised methodology that circumvented the need for electronic records and databases.
Methods
Study design
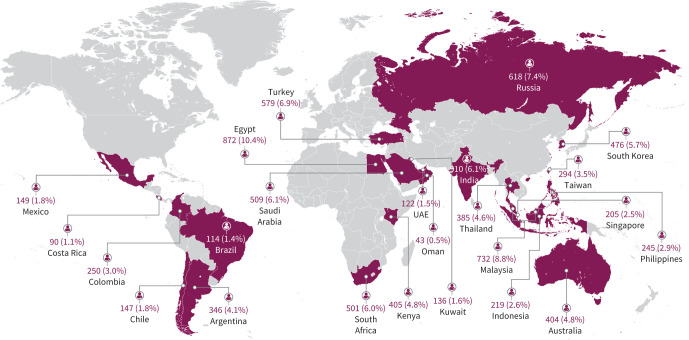
SABINA III was a multi-country, observational, cross-sectional study conducted in 24 countries (figure 1). Retrospective data were obtained from existing medical records, and patient data, including an assessment of current asthma symptom control, were collected during a study visit and entered real-time on an electronic case report form (eCRF). Physicians entered data on exacerbation history, comorbidities and information of medication prescriptions for asthma in the eCRF based on patient medical records. Additionally, physicians were required to enquire and record, at the study visit, whether patients had experienced exacerbations that were not recorded in the medical record. SABA OTC purchase data based on patient recall was obtained directly from the patient at the study visit and entered in the eCRF by the investigator. All site investigators were trained in using the eCRF system. The data collected were checked by monitors and data management teams, who ensured that queries raised (either by the eCRF system or by the monitors) were resolved. The final database was locked and signed off before statistical analyses on the final data were performed. Recruitment occurred from March 2019 to January 2020. We report multi-country aggregated data; regional and country-specific data will be published separately.
FIGURE 1.
Patient enrolment across countries in SABINA III. UAE: United Arab Emirates.
Study population
Purposive sampling of primary and specialist care potential study sites was performed by a national coordinator in each country with the intention of obtaining a sample representative of how patients with asthma were being treated in their country (supplementary table E1). At each site, patients (aged ≥12 years) with a diagnosis of asthma in their medical records, ≥3 prior consultations with their healthcare provider and having medical records containing data for ≥12 months before the study visit were enrolled. Patients with a diagnosis of other chronic respiratory diseases (such as chronic obstructive pulmonary disease) or with an acute respiratory condition were excluded.
Ethics approval
The study was conducted in compliance with the study protocol, the Declaration of Helsinki and local ethics committee approvals. Informed consent was obtained from all patients or their legal guardians.
Statistical analysis
SABA prescriptions in the 12 months before the study visit were categorised as none, 1–2, 3–5, 6–9, 10–12 and ≥13 canisters, and ≥3 SABA canister prescriptions was considered as overuse [18, 22].
The level of asthma symptom control was evaluated using the GINA assessment for asthma symptom control [26]. At least partly controlled asthma (partly controlled plus well-controlled asthma) was used as the outcome of clinical relevance. Severe exacerbations in the 12 months before the study visit were defined based on the American Thoracic Society/European Respiratory Society recommendations [27]. For secondary analyses, logistic regression and negative binomial models were used to analyse the associations of SABA prescriptions with at least partly controlled asthma (reference: uncontrolled asthma) and rate of severe exacerbations, respectively. Patients with missing data on covariates and those for whom there was no record of SABA prescriptions during the past year were excluded from secondary analyses. The latter prevented confounding of the results due to use of other relievers (such as low-dose ICS/formoterol, or oral or nebulised SABA) in these patients with zero SABA prescriptions. All regression models used complete-case analyses and were adjusted for pre-specified covariates and potential confounders (based on the literature and modelling data from SABINA I [22]). Covariates included age (continuous), sex, body mass index (continuous), education (primary/secondary school, high school or university and/or post-university), healthcare insurance (not reimbursed, partially reimbursed or fully reimbursed), practice type (primary or specialist care), investigator-classified asthma severity (guided by GINA 2017 treatment steps [26]: Steps 1–2, mild asthma; Steps 3–5, moderate-to-severe asthma), asthma duration (continuous), number of comorbidities (none, 1–2, 3–4 or ≥5) and smoking status (active, former or never-smoker).
All statistical tests were two-sided and at a 5% level of significance, and were performed using R version 3.6.0 (www.r-project.org).
Results
Study population
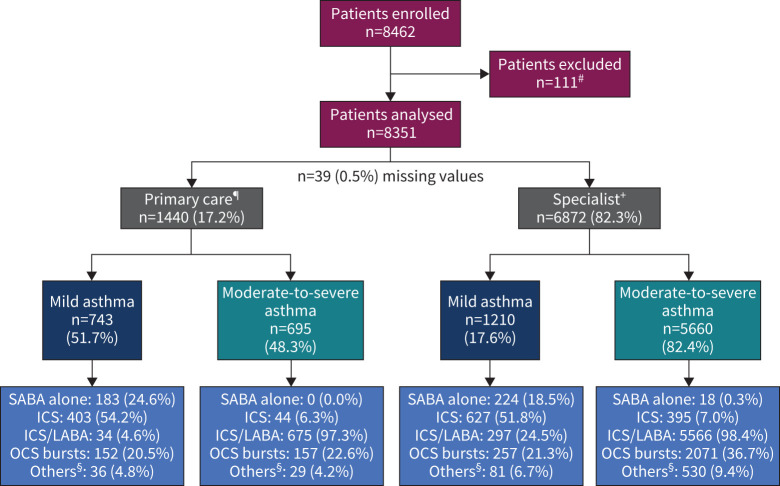
Overall, 8462 patients were recruited; 8351 patients were included in the primary analysis (figure 2): 36.7% from Asia, 21.3% from Africa, 16.6% from the Middle East, 13.1% from Latin America, 7.4% from Russia and 4.8% from Australia (figure 1).
FIGURE 2.
Patient population by practice type and asthma severity. #: excluded because the duration of asthma was <12 months; ¶: missing severity for primary care, n=2; +: missing severity for specialist, n=2; §: “others” includes oral corticosteroid (OCS) maintenance dosing and OCS prescribed for any reason other than asthma. Patients could have been prescribed multiple treatments in the past 12 months. ICS: inhaled corticosteroid; LABA: long-acting β2-agonist; SABA: short-acting β2-agonist.
Most patients (n=6872 (82.3%)) were enrolled by specialists (figure 2) and 76.5% were classified by investigators as having moderate-to-severe asthma. The mean±sd age of enrolled patients was 49.4±16.7 years; a majority were female (n=5691 (68.1%)) and had never smoked (n=6747 (80.8%)) (table 1). Over a quarter of the patients (n=2281 (27.3%)) had no healthcare reimbursement. Overall, 45.4% of patients reported ≥1 severe exacerbations within the past 12 months and 13.1% reported ≥3 severe exacerbations (table 2). Asthma symptom control was assessed as well controlled in 43.3% of patients, partly controlled in 32.2% and uncontrolled in 24.5%.
TABLE 1.
Sociodemographic and disease characteristics presented by asthma severity and practice type
| All (n=8351) | Primary care (n=1440) | Specialists (n=6872) | |||||
| Investigator-classified mild asthma (n=743) | Investigator-classified moderate-to-severe asthma (n=695) | All (n=1440) | Investigator-classified mild asthma (n=1210) | Investigator-classified moderate-to-severe asthma (n=5660) | All (n=6872) | ||
| Age (years) | |||||||
| Mean±sd | 49.4±16.7 | 45.8±16.8 | 50.2±16.4 | 47.9±16.7 | 44.7±18.0 | 50.8±16.2 | 49.7±16.7 |
| Median (IQR) | 51.0 (37.0–62.0) | 47.0 (34.0–58.0) | 51.0 (38.0–62.0) | 49.0 (36.0–60.0) | 44.0 (31.0–59.0) | 52.0 (39.0–63.0) | 51.0 (38.0–62.0) |
| Sex | |||||||
| Female | 5691 (68.1) | 535 (72.0) | 452 (65.0) | 988 (68.6) | 779 (64.4) | 3895 (68.8) | 4676 (68.0) |
| BMI (kg·m−2) | |||||||
| Mean±sd | 27.8±6.19 | 27.7±6.44 | 28.1±6.55 | 27.9±6.49 | 27.2±6.46 | 27.9±6.05 | 27.8±6.13 |
| BMI group (kg·m−2) | |||||||
| <18.5 | 256 (3.1) | 32 (4.3) | 15 (2.2) | 47 (3.3) | 61 (5.0) | 148 (2.6) | 209 (3.0) |
| ≥18.5–24.9 | 2619 (31.4) | 241 (32.4) | 232 (33.4) | 474 (32.9) | 402 (33.2) | 1734 (30.6) | 2136 (31.1) |
| ≥25.0–29.9 | 2954 (35.4) | 247 (33.2) | 230 (33.1) | 477 (33.1) | 430 (35.5) | 2031 (35.9) | 2463 (35.8) |
| ≥30.0 | 2522 (30.2) | 223 (30.0) | 218 (31.4) | 442 (30.7) | 317 (26.2) | 1747 (30.9) | 2064 (30.0) |
| Education level | |||||||
| Primary or secondary school | 2877 (34.5) | 346 (46.6) | 187 (26.9) | 533 (37.0) | 393 (32.5) | 1937 (34.2) | 2330 (33.9) |
| High school | 2013 (24.1) | 166 (22.3) | 151 (21.7) | 318 (22.1) | 373 (30.8) | 1312 (23.2) | 1685 (24.5) |
| University and/or post-university | 2792 (33.4) | 203 (27.3) | 297 (42.7) | 501 (34.8) | 392 (32.4) | 1887 (33.3) | 2281 (33.2) |
| Not established | 668 (8.0) | 28 (3.8) | 60 (8.6) | 88 (6.1) | 52 (4.3) | 523 (9.2) | 575 (8.4) |
| Missing data | 1 | 0 | 0 | 0 | 0 | 1 | 1 |
| Healthcare/medication funding | |||||||
| Not reimbursed | 2281 (27.3) | 320 (43.1) | 191 (27.5) | 511 (35.5) | 444 (36.7) | 1317 (23.3) | 1762 (25.6) |
| Partially reimbursed | 1851 (22.2) | 152 (20.5) | 196 (28.2) | 348 (24.2) | 241 (19.9) | 1253 (22.1) | 1494 (21.7) |
| Fully reimbursed | 3940 (47.2) | 258 (34.7) | 281 (40.4) | 539 (37.4) | 507 (41.9) | 2871 (50.7) | 3379 (49.2) |
| Not specified | 276 (3.3) | 13 (1.7) | 27 (3.9) | 42 (2.9) | 18 (1.5) | 216 (3.8) | 234 (3.4) |
| Missing data | 3 | 0 | 0 | 0 | 0 | 3 | 3 |
| Smoking status history | |||||||
| Active smoker | 497 (6.0) | 27 (3.6) | 63 (9.1) | 91 (6.3) | 81 (6.7) | 322 (5.7) | 403 (5.9) |
| Former smoker | 1105 (13.2) | 97 (13.1) | 119 (17.1) | 216 (15.0) | 146 (12.1) | 741 (13.1) | 887 (12.9) |
| Never-smoker | 6747 (80.8) | 619 (83.3) | 513 (73.8) | 1133 (78.7) | 983 (81.2) | 4595 (81.2) | 5580 (81.2) |
| Missing values | 2 | 0 | 0 | 0 | 0 | 2 | 2 |
| Asthma duration (years) | |||||||
| Mean±sd | 14.9±14.31 | 17.9±14.78 | 16.5±13.91 | 17.2±14.37 | 13.9±13.50 | 14.6±14.43 | 14.4±14.27 |
| Median (IQR) | 10.0 (4.0–21.0) | 13.0 (7.0–25.5) | 13.0 (6.0–22.0) | 13.0 (6.0–24.0) | 9.5 (4.0–20.0) | 10.0 (4.0–20.0) | 10.0 (4.0–20.0) |
| GINA treatment step | |||||||
| Step 1 | 714 (8.5) | 316 (42.5) | 0 (0) | 316 (21.9) | 396 (32.7) | 0 (0) | 396 (5.8) |
| Step 2 | 1244 (14.9) | 427 (57.5) | 0 (0) | 427 (29.7) | 814 (67.3) | 0 (0) | 814 (11.8) |
| Step 3 | 2279 (27.3) | 0 (0) | 371 (53.4) | 371 (25.8) | 0 (0) | 1900 (33.6) | 1900 (27.6) |
| Step 4 | 2872 (34.4) | 0 (0) | 261 (37.6) | 261 (18.2) | 0 (0) | 2595 (45.8) | 2595 (37.8) |
| Step 5 | 1237 (14.8) | 0 (0) | 63 (9.1) | 63 (4.4) | 0 (0) | 1165 (20.6) | 1165 (17.0) |
| Missing data | 5 | 0 | 0 | 2 | 0 | 0 | 2 |
| Comorbidities | |||||||
| None | 2962 (35.5) | 328 (44.1) | 264 (38.0) | 593 (41.2) | 535 (44.2) | 1822 (32.2) | 2358 (34.3) |
| 1–2 | 3900 (46.7) | 319 (42.9) | 276 (39.7) | 596 (41.4) | 512 (42.3) | 2773 (49.0) | 3286 (47.8) |
| 3–4 | 1228 (14.7) | 89 (12.0) | 126 (18.1) | 215 (14.9) | 136 (11.2) | 870 (15.4) | 1006 (14.6) |
| ≥5 | 261 (3.1) | 7 (0.9) | 29 (4.2) | 36 (2.5) | 27 (2.2) | 195 (3.4) | 222 (3.2) |
Data are presented as n (%) or n, unless otherwise stated. IQR: interquartile range; BMI: body mass index; GINA: Global Initiative for Asthma.
TABLE 2.
Asthma-related severe exacerbations in the past year and asthma symptom control presented by asthma severity and practice type
| All (n=8351) | Primary care (n=1440) | Specialists (n=6872) | |||||
| Investigator-classified mild asthma (n=743) | Investigator-classified moderate-to-severe asthma (n=695) | All (n=1440) | Investigator-classified mild asthma (n=1210) | Investigator-classified moderate-to-severe asthma (n=5660) | All (n=6872) | ||
| Severe asthma exacerbations (n) | |||||||
| Mean±sd | 1.1±2.09 | 1.1±2.99 | 0.9±1.60 | 1.0±2.42 | 0.8±1.81 | 1.1±2.03 | 1.1±2.00 |
| Severe asthma exacerbations by group (n) | |||||||
| 0 | 4555 (54.5) | 453 (61.0) | 428 (61.6) | 882 (61.3) | 772 (63.8) | 2880 (50.9) | 3653 (53.2) |
| 1 | 1810 (21.7) | 129 (17.4) | 130 (18.7) | 259 (18.0) | 206 (17.0) | 1338 (23.6) | 1544 (22.5) |
| 2 | 892 (10.7) | 59 (7.9) | 62 (8.9) | 122 (8.5) | 109 (9.0) | 655 (11.6) | 764 (11.1) |
| 3 | 493 (5.9) | 47 (6.3) | 32 (4.6) | 79 (5.5) | 51 (4.2) | 362 (6.4) | 413 (6.0) |
| >3 | 600 (7.2) | 55 (7.4) | 43 (6.2) | 98 (6.8) | 72 (6.0) | 424 (7.5) | 497 (7.2) |
| Missing data | 1 | 0 | 0 | 0 | 0 | 1 | 1 |
| Level of asthma symptom control | |||||||
| Well controlled | 3610 (43.3) | 318 (42.8) | 282 (40.6) | 601 (41.7) | 608 (50.4) | 2388 (42.3) | 2996 (43.7) |
| Partly controlled | 2686 (32.2) | 244 (32.8) | 258 (37.1) | 503 (34.9) | 361 (29.9) | 1805 (32.0) | 2167 (31.6) |
| Uncontrolled | 2034 (24.5) | 181 (24.4) | 155 (22.3) | 336 (23.3) | 237 (19.7) | 1450 (25.7) | 1688 (24.6) |
| Missing data | 21 | 0 | 0 | 0 | 4 | 17 | 21 |
Data are presented as n (%) or n, unless otherwise stated.
Asthma treatment
SABA prescriptions
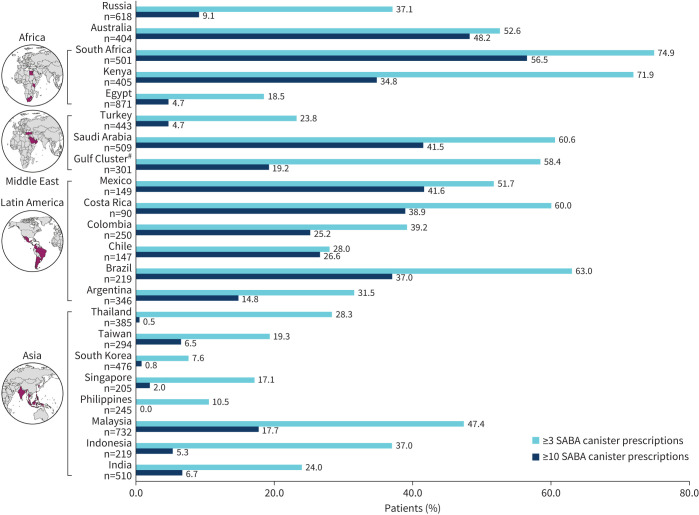
Among all patients, 24.3% were prescribed 1–2 SABA canisters in the past 12 months and 38.0% were prescribe ≥3 SABA canisters. Prescriptions of ≥3 SABA canisters were reported in 45.8% of patients with mild asthma and 35.6% with moderate-to-severe asthma (figure 3). The prevalence of ≥3 SABA prescriptions in the past 12 months varied in the 24 countries, ranging from 7.6% in South Korea to 74.9% in South Africa (figure 4).
FIGURE 3.
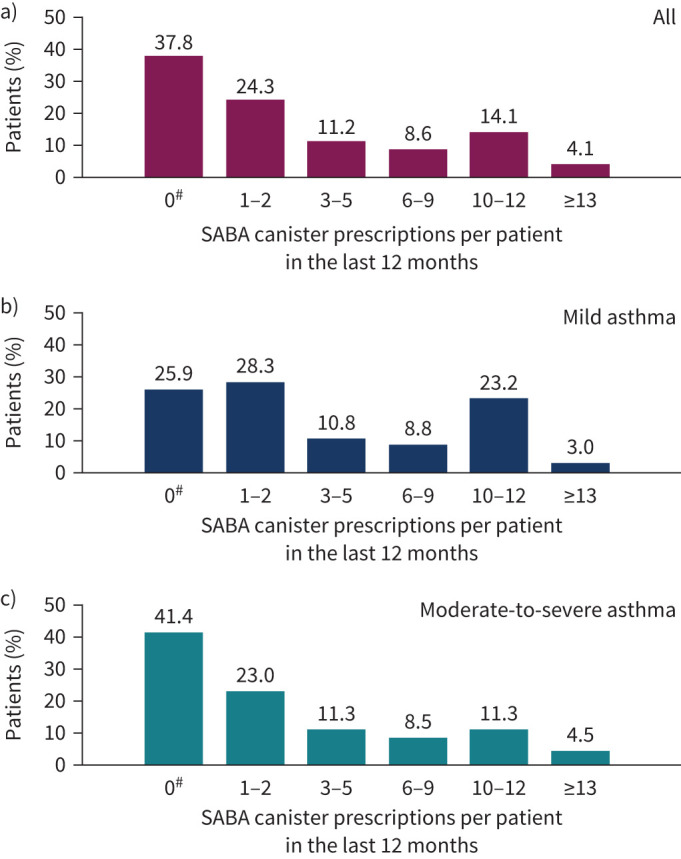
Short-acting β2-agonist (SABA) prescriptions according to asthma severity: a) all patients (n=8147), b) patients with mild asthma (n=1939) and c) patients with moderate-to-severe asthma (n=6203). #: the category of patients classified as having zero SABA canister prescriptions included patients using non-SABA relievers, non-inhaler forms of SABA and/or SABA purchased over the counter. Missing data for the overall population, n=204; mild asthma, n=19; moderate-to-severe asthma, n=18.
FIGURE 4.
Short-acting β2-agonist (SABA) prescriptions across the SABINA III countries. #: United Arab Emirates, Oman and Kuwait.
Prescriptions of SABA as monotherapy were reported in 5.1% of patients, almost exclusively for mild asthma (supplementary table E2). Of these, more than half (53.6%) were prescribed ≥3 SABA canisters in the past year and 29.9% were prescribed ≥10 SABA canisters. Overall, 58.0% of patients on any maintenance therapy were also prescribed SABA (supplementary table E2), of whom 61.7% were prescribed ≥3 SABA canisters and 29.3% were prescribed ≥10 SABA canisters.
No SABA prescriptions were reported in 3076 (37.8%) patients and more commonly in those with moderate-to-severe asthma versus mild asthma (41.4% versus 25.9%) (figure 3). See the supplementary material and supplementary tables E3–E5 for additional details.
SABA obtained OTC without a prescription
Overall, 18.0% of patients reported purchasing SABA OTC (table 3), of whom 48.8% purchased ≥3 canisters. Among patients who purchased SABA OTC (n=1503), 76.8% had also received SABA prescriptions (supplementary figure E1): 69.9% for ≥3 canisters and 35.8% for ≥10 canisters in the past 12 months.
TABLE 3.
Patients who purchased short-acting β2-agonists (SABAs) without a prescription (over the counter) in the past 12 months (all n=8351)
| Patients who purchased SABAs without a prescription | |
| Yes | 1503 (18.0) |
| No | 6512 (78.0) |
| Unknown | 333 (4.0) |
| Missing data | 3 |
| Total | 8348 (100.0) |
| Canisters or inhalers per patient obtained without a prescription | |
| 1–2 | 770 (51.2) |
| 3–5 | 450 (29.9) |
| 6–9 | 114 (7.6) |
| 10–12 | 64 (4.3) |
| ≥13 | 34 (2.3) |
| Not applicable# | 71 (4.7) |
Data are presented as n (%) or n. #: “Not applicable” could be selected in the electronic case report form when patients purchased SABAs in a different form (e.g. oral or nebulised) without a prescription.
Prescriptions for asthma medications other than SABA
ICS as sole maintenance therapy was prescribed for 17.6% of patients overall, of whom >50% had mild asthma (supplementary table E2). The mean±sd number of ICS canisters prescribed was 8.1±8.7, with 51.8% of patients being prescribed ≤6 canisters in the past year (supplementary figure E2).
Most (79.2%) patients were prescribed ICS/LABA, while a total of 264 patients (3.2%) received prescriptions for a biological agent. The majority of the latter were prescribed omalizumab, although mepolizumab, dupilumab and benralizumab were also prescribed. See supplementary table E6 for additional data on other asthma medication prescriptions.
Context of care
For mild asthma, primary care physicians (PCPs) tended to prescribe ≥3 SABA canisters as monotherapy more commonly than specialists (60.6% versus 47.3%, respectively) (supplementary table E2). The number of patients prescribed ≥3 SABA canisters on a background of maintenance therapy by PCPs for patients with mild and moderate-to-severe asthma was also higher (71.8% and 65.9% versus 61.0% and 60.1%, respectively, for PCPs versus specialists) (supplementary table E2).
Association between SABA prescriptions and asthma-related health outcomes
Among patients with ≥1 SABA prescriptions (supplementary figure E3), higher SABA prescriptions were associated with increasing rates of severe exacerbations (figure 5a and supplementary table E7). Patients prescribed 3–5 SABA canisters (versus 1–2 SABA canisters) had 40% more severe exacerbations (adjusted incidence rate ratio (IRR) 1.40 (95% CI 1.24–1.58)) and this increased further with increasing SABA prescriptions (range of adjusted IRRs 1.40–1.92). Prescription of 3–5 SABA canisters (versus 1–2 SABA canisters) was also associated with significantly lower odds of having at least partly controlled asthma (adjusted OR 0.64 (95% CI 0.53–0.78)) and this decreased further with increasing SABA prescriptions (range of adjusted ORs 0.64–0.33) (figure 5b and supplementary table E7). See supplementary table E8 for unadjusted analyses.
FIGURE 5.
Association of short-acting β2-agonist (SABA) prescriptions with severe exacerbations in the past 12 months and the level of asthma symptom control: a) adjusted incidence rate ratio (IRR) of experiencing a severe asthma exacerbation by SABA canister prescriptions in the past year (n=4612) and b) adjusted odds ratio (OR) of achieving at least partly controlled asthma according to SABA canister prescriptions in the past year (reference: uncontrolled asthma) (n=4597). Based on the covariable significance in the models, IRRs are corrected by country, age, sex, body mass index (BMI), smoking history, Global Initiative for Asthma (GINA) step and education level. ORs are corrected by country, age, sex, BMI, asthma duration, smoking history, comorbidity, GINA step and education level.
Discussion
Our study describing asthma medication prescriptions by PCPs and specialists for patients with mild or moderate-to-severe asthma in 24 countries with a wide global representation confirms high levels of SABA prescriptions, with 38% of patients being prescribed ≥3 SABA canisters in the past 12 months. Use of ≥3 SABA canisters per year is considered undesirable since it indicates overreliance on SABA for the management of persistent symptoms [28], usually related to the underuse of ICS and other controllers. GINA-defined controlled or partly controlled asthma specifies that SABA reliever use should not be >2 doses per week, which equates to <2 standard SABA canisters per year. In support of this threshold, in our study, even after adjusting for known confounding factors, SABA prescriptions for >2 canisters per year were associated with an increasing rate of severe exacerbations and a lower likelihood of satisfactory symptom control. More than half of the patients with mild asthma receiving SABA alone for as-needed use were prescribed ≥3 canisters and almost one-third were prescribed ≥10 canisters, suggesting that a majority should have been considered for additional maintenance treatment with controllers. Among patients prescribed controller treatment, >60% received ≥3 SABA canister prescriptions in the past year and almost one-third received ≥10 SABA canister prescriptions, suggesting overuse of SABA instead of optimisation of controller treatments. Although there were differences in prescribing between PCPs and specialists, the pattern of SABA overreliance was common to both. SABA monotherapy for mild asthma was more commonly prescribed by PCPs and in higher numbers, and SABA prescriptions for moderate-to-severe asthma by both categories of prescriber were similarly high.
In some of the countries surveyed, SABA may be obtained without a prescription, increasing the potential for SABA overuse [29, 30]. Overall, one-fifth of patients in our study reported obtaining SABA in this way, of whom one-half purchased ≥3 canisters in the past year. In most cases (77%), these canisters were in addition to those prescribed by their physician. The potential for overuse by patients receiving SABA from two sources is suggested by the fact that among such patients, 70% also received prescriptions for ≥3 SABA canisters in the past year and 35% also received prescriptions for ≥10 SABA canisters.
Overall, these findings are similar to what has been observed in the SABINA I and II studies in Europe. Across the UK, Germany, Spain, Sweden and Italy [22], SABA prescription/possession of ≥3 SABA canisters per year (33%) was slightly lower than that in SABINA III, although differences were observed between countries. SABA overuse ranged from 38% in the UK to 9% in Italy. Subsequently, it was confirmed that in Italy, SABA overuse was higher (>50%) when SABAs dispensed by pharmacists, including those purchased without a prescription, were included [25].
The findings in our study confirming the association between SABA prescriptions and poor asthma outcomes contribute to the growing evidence that SABA overuse in asthma needs to be addressed if further reductions in asthma morbidity and mortality are to be achieved. An association between SABA prescription/possession and severe exacerbations [22, 24, 25], and even asthma deaths [24], has been reported in SABINA I (UK) and II (Sweden and Italy). Similar findings have been observed in other studies of SABA use in asthma [28], with high SABA overuse, which may occur even on symptom-free days [31], being associated with worse asthma control.
The growing concern about the negative effects of SABA use on global efforts to improve asthma outcomes has led to research into alternative treatment options for providing quick relief from asthma symptoms either for occasional symptom relief or, more importantly, when breakthrough symptoms herald an approaching severe exacerbation. Foremost has been examining the potential of ensuring that use of a rapid-onset bronchodilator is always accompanied by use of an ICS to ensure that the underlying airway inflammation is also addressed at these critical times. The single-inhaler maintenance and reliever approach was initially trialled with formoterol, a long-acting bronchodilator with a rapid onset of action like that of a SABA, combined with budesonide. Most research has been focused on this combination, but efficacy has also been shown for the combination of formoterol with beclomethasone [7] and is currently being evaluated for combinations of a SABA with an ICS [8, 9]. The anti-inflammatory reliever approach has been shown to be highly effective in mild asthma [15–18], where it may be used without maintenance dosing, and in moderate-to-severe asthma (with fixed daily dosing of the same combination as maintenance treatment) [7]. Consequently, an anti-inflammatory reliever approach has become the preferred option in both the GINA report and the recently published updated report of the National Asthma Education and Prevention Program in the USA, and in other national guidelines and formularies [13, 19, 32]. The 2019 World Health Organization Model List of Essential Medicines, which represent “minimum medicine needs for a basic healthcare system”, includes budesonide/formoterol for use in asthma [33].
Given the entrenched and time-honoured position of SABAs in asthma care spanning >50 years, SABINA and similar studies provide potentially useful information about the magnitude of the problem relating to SABA use that may be used to assess the gains that are possible if this alternative reliever strategy were to be introduced globally. The current study is focused on data from several countries, most of which do not have national databases from which to gauge SABA use. Although not fully representative of asthma care within each country and biased towards specialist services for asthma patients, it provides a snapshot of the situation in a range of countries, including some with limited healthcare insurance or national provision of care. Our findings reveal overuse of SABAs by both PCPs and specialists, and although the assessment of asthma control was not as poor as that reported in many cross-sectional surveys [34, 35], most patients were not optimally controlled, asthma attacks remained common and both were associated with SABA use. On the other hand, 37.8% were recorded as having no SABA prescriptions, a proportion similar to that seen in SABINA I in the UK [22]. Although some had obtained SABA OTC, it is likely that many such patients, 89.2% of whom had been prescribed an ICS/LABA combination, may have already been switched to ICS/formoterol as reliever. Unfortunately, the size of this group could not be accurately assessed in our study.
In strategising how to encourage the use of the preferable reliever option, several approaches are needed. First, OTC SABA purchases may need to be better regulated in some countries as part of the education process on reliever use and limits should be put in place. Entrenched prescribing habits in well-resourced health services, such as automatic repeat prescriptions, may result in high and unnecessary SABA prescriptions [36]. Although easier to enact in developed countries, such a limitation will be difficult in poorer nations where the relatively low cost and accessibility of SABAs are relied on for short-term benefit despite the fact that they may help to entrench poor asthma care. In such settings, the bias towards using relievers rather than more costly controller medications is likely to be greater. Access to affordable combination medications should be a key priority as it is likely that in these countries, the single-inhaler maintenance and reliever approach will be of greatest benefit in view of its strong effects on reducing asthma worsening and attacks, which pose an avoidable high burden on health services [1, 37]. These approaches will need to be accompanied by national initiatives targeting patients, physicians and other stakeholders such as pharmacists to increase awareness of updated treatment guidelines. Creation of national asthma programmes based on current evidence-based asthma guidelines and tailored to the context of clinical practice and local resources can play a critical role in this endeavour. National or regional asthma programmes have been shown to be more effective than conventional treatment guidelines in improving asthma care [38]. Patient involvement is also crucial and patient advocacy groups can play a significant role in disseminating appropriate treatment information [39]. Besides these measures, the current move away from a SABA as reliever to be replaced by an ICS-containing rapid-onset reliever for all treatment steps, as now proposed by GINA, may, in some countries, represent the most significant step towards addressing overreliance and overuse of SABAs. This trend was already evident in our study in the high proportion of patients who received prescriptions for ICS/LABA and no provision for a SABA.
As a limitation, first, it is recognised that SABA prescriptions may not necessarily reflect actual usage, which is likely to be lower. However, overprescribing, particularly in poorly resourced settings, may result in medications being passed on to family and friends, increasing the potential for mis-assessments and haphazard treatment. Second, the assignment of asthma severity based on GINA treatment steps appeared to be poorly adhered to by investigators, as evidenced by the large proportion of patients with mild asthma who received an ICS/LABA prescription. It is possible that instead of assigning severity according to the 2017 GINA classification [26], a later version that proposed as-needed ICS/formoterol for mild asthma may have been followed. In our study, the nonrandom selection of sites with a majority representing specialist care resulted in the enrolment of more patients with moderate-to-severe asthma. In view of this bias, we have avoided comparisons of data obtained from participants enrolled by specialists with those recruited in primary care. However, these data from different contexts and of differing severities of asthma permit broad generalisations. Furthermore, this cross-sectional study does not permit an assessment of a causal link between SABA prescriptions and asthma outcomes, and does not discount reverse causality; the results simply represent an association. Our aim to include data from a large number of countries and practices with different methods of recording clinical data necessitated acceptance of limitations in methods of collecting source data, such as reliance on patient recall for some questions and limiting the scope of the questionnaire. For example, data on comorbidities and the number and type of all maintenance medications was not obtained. Lastly, the basis for an asthma diagnosis in each participant was not requested. However, misdiagnosis is not likely to have had an impact on the main findings of this study.
Our SABINA III findings demonstrating that 38% of patients in 24 countries in five continents are overprescribed SABAs (≥3 canisters per year) extend the data from the SABINA studies in Europe [22–25]. Although drivers for SABA prescribing may differ by country, SABA overprescription results in an unnecessary burden of poor asthma symptom control and severe asthma exacerbations with their attendant risks. These findings support the need for continued efforts to improve asthma care in these countries, particularly relating to the prescribing of SABAs and the need to switch to combination medications that provide both quick symptom relief and an anti-inflammatory effect.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-01402-2021.Supplement (698.7KB, pdf)
Shareable PDF
Acknowledgements
Editorial support was provided by Michelle Rebello of Cactus Life Sciences (part of Cactus Communications, Mumbai, India) in accordance with Good Publication Practice (GPP3) guidelines (www.ismpp.org/gpp3). This support was fully funded by AstraZeneca.
Footnotes
This article has supplementary material available from erj.ersjournals.com
This study is registered at ClinicalTrials.gov with identifier number NCT03857178. Data underlying the findings described in this article may be obtained in accordance with AstraZeneca's data sharing policy described at: https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Author contributions: E.D. Bateman, D.B. Price, A. Catanzariti, R.J.P. van der Valk and M.J.H.I. Beekman designed the study. E.D. Bateman, D.B. Price, H-C. Wang, A. Khattab, P. Schonffeldt, A. Catanzariti, R.J.P. van der Valk and M.J.H.I. Beekman contributed to data collection, data analysis, data interpretation and writing. E.D. Bateman, D.B. Price, A. Catanzariti and M.J.H.I. Beekman act as guarantors.
Conflict of interest: E.D. Bateman is a member of the Science Committee and Board of GINA, and reports personal fees from ALK, AstraZeneca, Boehringer Ingelheim, Chiesi, Menarini, Novartis, Orion, Regeneron Pharmaceuticals and Sanofi Genzyme.
Conflict of interest: D.B. Price has board membership with Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, Mylan, Mundipharma, Novartis, Regeneron, Sanofi Genzyme, Teva Pharmaceuticals and Thermo Fisher; consultancy agreements with Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Mylan, Mundipharma, Novartis, Pfizer, Teva and Theravance; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, Mylan, Mundipharma, Novartis, Pfizer, Regeneron, Respiratory Effectiveness Group, Sanofi Genzyme, Teva, Theravance and the UK National Health Service; payment for lectures/speaking engagements from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Mylan, Mundipharma, Novartis, Regeneron, Sanofi Genzyme and Teva; payment for the development of educational materials from Mundipharma and Novartis; payment for travel/accommodation/meeting expenses from AstraZeneca, Boehringer Ingelheim, Mundipharma, Mylan, Novartis and Thermo Fisher; funding for patient enrolment or completion of research from Novartis; stock/stock options from AKL Research and Development Ltd, which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd (Australia and UK) and 74% of Observational and Pragmatic Research Institute Pte Ltd (Singapore); is a peer reviewer for grant committees of the Efficacy and Mechanism Evaluation programme and Health Technology Assessment; and was an expert witness for GlaxoSmithKline.
Conflict of interest: H-C. Wang has nothing to disclose.
Conflict of interest: A. Khattab has nothing to disclose.
Conflict of interest: P. Schonffeldt reports lectures on medical education and inclusion as a researcher on clinical study protocols funded by AstraZeneca, GlaxoSmithKline, Teva, ITF Labomed, Boehringer Ingelheim and Sanofi Genzyme.
Conflict of interest: A. Catanzariti is an employee of AstraZeneca.
Conflict of interest: R.J.P. van der Valk is an employee of AstraZeneca and has shares in GlaxoSmithKline and shares and options in AstraZeneca.
Conflict of interest: M.J.H.I. Beekman was an employee of AstraZeneca at the time the study was conducted and has shares in AstraZeneca.
Support statement: AstraZeneca funded the study; was involved in the study design, protocol development, study conduct and statistical analysis; and was given the opportunity to review the manuscript before submission. AstraZeneca also funded medical writing support. All authors had full access to all the data, wrote the report and accept responsibility for its publication. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Global Asthma Network . The Global Asthma Report 2018. 2018. Date last accessed: 12 May 2021. http://globalasthmareport.org/resources/Global_Asthma_Report_2018.pdf
- 2.Asher MI, García-Marcos L, Pearce NE, et al. . Trends in worldwide asthma prevalence. Eur Respir J 2020; 56: 2002094. doi: 10.1183/13993003.02094-2020 [DOI] [PubMed] [Google Scholar]
- 3.Ebmeier S, Thayabaran D, Braithwaite I, et al. . Trends in international asthma mortality: analysis of data from the WHO Mortality Database from 46 countries (1993–2012). Lancet 2017; 390: 935–945. doi: 10.1016/S0140-6736(17)31448-4 [DOI] [PubMed] [Google Scholar]
- 4.Royal College of Physicians . Why Asthma Still Kills: the National Review of Asthma Deaths (NRAD) Confidential Enquiry Report 2014. 2015. Date last accessed: 12 May 2021. www.rcplondon.ac.uk/projects/outputs/why-asthma-still-kills
- 5.Patel M, Pilcher J, Hancox RJ, et al. . The use of β2-agonist therapy before hospital attendance for severe asthma exacerbations: a post-hoc analysis. NPJ Prim Care Respir Med 2015; 25: 14099. doi: 10.1038/npjpcrm.2014.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pilcher J, Patel M, Pritchard A, et al. . Beta-agonist overuse and delay in obtaining medical review in high risk asthma: a secondary analysis of data from a randomised controlled trial. NPJ Prim Care Respir Med 2017; 27: 33. doi: 10.1038/s41533-017-0032-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sobieraj DM, Weeda ER, Nguyen E, et al. . Association of inhaled corticosteroids and long-acting β-agonists as controller and quick relief therapy with exacerbations and symptom control in persistent asthma: a systematic review and meta-analysis. JAMA 2018; 319: 1485–1496. doi: 10.1001/jama.2018.2769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papi A, Canonica GW, Maestrelli P, et al. . Rescue use of beclomethasone and albuterol in a single inhaler for mild asthma. N Engl J Med 2007; 356: 2040–2052. doi: 10.1056/NEJMoa063861 [DOI] [PubMed] [Google Scholar]
- 9.Chipps BE, Albers FC, Reilly L, et al. . Evaluation of the efficacy and safety of as-needed PT027 (budesonide/albuterol MDI) compared to as-needed albuterol MDI in adults and children 4 years of age or older with uncontrolled moderate to severe asthma: design of the Mandala Study. Am J Respir Crit Care Med 2020; 201: A3015. doi: 10.1164/ajrccm-conference.2020.201.1_MeetingAbstracts.A3015 [DOI] [Google Scholar]
- 10.Cates CJ, Karner C. Combination formoterol and budesonide as maintenance and reliever therapy versus current best practice (including inhaled steroid maintenance), for chronic asthma in adults and children. Cochrane Database Syst Rev 2013; 4: CD007313. doi: 10.1002/14651858.CD007313.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kew KM, Karner C, Mindus SM, et al. . Combination formoterol and budesonide as maintenance and reliever therapy versus combination inhaler maintenance for chronic asthma in adults and children. Cochrane Database Syst Rev 2013; 12: CD009019. doi: 10.1002/14651858.CD009019.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.British Thoracic Society/Scottish Intercollegiate Guidelines Network . BTS/SIGN British Guideline on the Management of Asthma. 2019. Date last accessed: 12 May 2021. www.brit-thoracic.org.uk/quality-improvement/guidelines/asthma
- 13.Cloutier MM, Dixon AE, Krishnan JA, et al. . Managing asthma in adolescents and adults: 2020 asthma guideline update from the National Asthma Education and Prevention Program. JAMA 2020; 324: 2301–2317. doi: 10.1001/jama.2020.21974 [DOI] [PubMed] [Google Scholar]
- 14.Global Initiative for Asthma (GINA) . Global Strategy for Asthma Management and Prevention. 2021. Available from: http://ginasthma.org/
- 15.O'Byrne PM, FitzGerald JM, Bateman ED, et al. . Inhaled combined budesonide–formoterol as needed in mild asthma. N Engl J Med 2018; 378: 1865–1876. doi: 10.1056/NEJMoa1715274 [DOI] [PubMed] [Google Scholar]
- 16.Bateman ED, Reddel HK, O'Byrne PM, et al. . As-needed budesonide–formoterol versus maintenance budesonide in mild asthma. N Engl J Med 2018; 378: 1877–1887. doi: 10.1056/NEJMoa1715275 [DOI] [PubMed] [Google Scholar]
- 17.Beasley R, Holliday M, Reddel HK, et al. . Controlled trial of budesonide–formoterol as needed for mild asthma. N Engl J Med 2019; 380: 2020–2030. doi: 10.1056/NEJMoa1901963 [DOI] [PubMed] [Google Scholar]
- 18.Hardy J, Baggott C, Fingleton J, et al. . Budesonide-formoterol reliever therapy versus maintenance budesonide plus terbutaline reliever therapy in adults with mild to moderate asthma (PRACTICAL): a 52-week, open-label, multicentre, superiority, randomised controlled trial. Lancet 2019; 394: 919–928. doi: 10.1016/S0140-6736(19)31948-8 [DOI] [PubMed] [Google Scholar]
- 19.Reddel HK, FitzGerald JM, Bateman ED, et al. . GINA 2019: a fundamental change in asthma management. Eur Respir J 2019; 53: 1901046. doi: 10.1183/13993003.01046-2019 [DOI] [PubMed] [Google Scholar]
- 20.van der Valk RJ, Baraldi E, Stern G, et al. . Daily exhaled nitric oxide measurements and asthma exacerbations in children. Allergy 2012; 67: 265–271. doi: 10.1111/j.1398-9995.2011.02734.x [DOI] [PubMed] [Google Scholar]
- 21.Cabrera CS, Nan C, Lindarck N, et al. . SABINA: global programme to evaluate prescriptions and clinical outcomes related to short-acting β2-agonist use in asthma. Eur Respir J 2020; 55: 1901858. doi: 10.1183/13993003.01858-2019 [DOI] [PubMed] [Google Scholar]
- 22.Bloom CI, Cabrera C, Arnetorp S, et al. . Asthma-related health outcomes associated with short-acting β2-agonist inhaler use: an observational UK study as part of the SABINA global program. Adv Ther 2020; 37: 4190–4208. doi: 10.1007/s12325-020-01444-5 [DOI] [PubMed] [Google Scholar]
- 23.Janson C, Menzies-Gow A, Nan C, et al. . SABINA: an overview of short-acting β2-agonist use in asthma in European countries. Adv Ther 2020; 37: 1124–1135. doi: 10.1007/s12325-020-01233-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nwaru BI, Ekström M, Hasvold P, et al. . Overuse of short-acting β2-agonists in asthma is associated with increased risk of exacerbation and mortality: a nationwide cohort study of the global SABINA programme. Eur Respir J 2020; 55: 1901872. doi: 10.1183/13993003.01872-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Marco F, D'Amato M, Lombardo FP, et al. . The burden of short-acting β2-agonist use in asthma: is there an Italian case? An update from SABINA program. Adv Ther 2021; 38: 3816–3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Global Initiative for Asthma (GINA) . Global Strategy for Asthma Management and Prevention. 2017. Available from: http://ginasthma.org/
- 27.Reddel HK, Taylor DR, Bateman ED, et al. . An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med 2009; 180: 59–99. doi: 10.1164/rccm.200801-060ST [DOI] [PubMed] [Google Scholar]
- 28.Amin S, Soliman M, McIvor A, et al. . Usage patterns of short-acting β2-agonists and inhaled corticosteroids in asthma: a targeted literature review. J Allergy Clin Immunol Pract 2020; 8: 2556–2564. doi: 10.1016/j.jaip.2020.03.013 [DOI] [PubMed] [Google Scholar]
- 29.Van Sickle D. Management of asthma at private pharmacies in India. Int J Tuberc Lung Dis 2006; 10: 1386–1392. [PubMed] [Google Scholar]
- 30.Azzi EA, Kritikos V, Peters MJ, et al. . Understanding reliever overuse in patients purchasing over-the-counter short-acting beta2 agonists: an Australian community pharmacy-based survey. BMJ Open 2019; 9: e028995. doi: 10.1136/bmjopen-2019-028995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerald JK, Carr TF, Wei CY, et al. . Albuterol overuse: a marker of psychological distress? J Allergy Clin Immunol Pract 2015; 3: 957–962. doi: 10.1016/j.jaip.2015.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang CL, Hicks EA, Mitchell P, et al. . 2021 Canadian Thoracic Society Guideline – a focused update on the management of very mild and mild asthma. Can J Respir Crit Care Sleep Med 2021; 5: 205–245. doi: 10.1080/24745332.2021.1877043. [DOI] [Google Scholar]
- 33.World Health Organization . WHO Model List of Essential Medicines – 21st List, 2019. Geneva, WHO, 2019. [Google Scholar]
- 34.Nathan RA, Thompson PJ, Price D, et al. . Taking aim at asthma around the world: global results of the asthma insight and management survey in the Asia-Pacific region, Latin America, Europe, Canada, and the United States. J Allergy Clin Immunol Pract 2015; 3: 734–742. doi: 10.1016/j.jaip.2015.04.013 [DOI] [PubMed] [Google Scholar]
- 35.Price D, David-Wang A, Cho S-H, et al. . Time for a new language for asthma control: results from REALISE Asia. J Asthma Allergy 2015; 8: 93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saini B, Krass I, Smith L, et al. . Role of community pharmacists in asthma – Australian research highlighting pathways for future primary care models. Australas Med J 2011; 4: 190–200. doi: 10.4066/AMJ.2011.790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lalloo U, Walters R, Adachi M, et al. . Asthma programmes in diverse regions of the world: challenges, successes and lessons learnt. Int J Tuberc Lung Dis 2011; 15: 1574–1587. doi: 10.5588/ijtld.11.0289 [DOI] [PubMed] [Google Scholar]
- 38.Selroos O, Kupczyk M, Kuna P, et al. . National and regional asthma programmes in Europe. Eur Respir Rev 2015; 24: 474–483. doi: 10.1183/16000617.00008114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Localio AM, Black HL, Park H, et al. . Filling the patient–provider knowledge gap: a patient advocate to address asthma care and self-management barriers. J Asthma 2019; 56: 1027–1036. doi: 10.1080/02770903.2018.1520864 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-01402-2021.Supplement (698.7KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-01402-2021.Shareable (339KB, pdf)