Highlights
-
•
Recurrent cervical cancer with peritoneal carcinomatosis is rare.
-
•
Presenting two cases of recurrent cervical cancer with peritoneal carcinomatosis.
-
•
HIPEC used in recurrent cervical cancer cases with peritoneal carcinomatosis.
Keywords: Recurrent cervical cancer, HIPEC, Peritoneal carcinomatosis
Abstract
Cervical cancer is the fourth most common malignancy in women in the world; however, a substantial portion of these malignancies are declining with increasingly sophisticated screening. Unfortunately, recurrent cervical cancer has a dismal prognosis and its management continues to be a growing area of research. While the foundation of treatment remains platinum-based chemotherapies, new techniques such as HIPEC have been evaluated. We present two patients with recurrent cervical adenocarcinoma with peritoneal carcinomatosis who were treated with HIPEC during de-bulking surgery with substantial disease-free survival.
One of our patients had 15 months of disease-free survival before developing biliary metastases and the other remains disease free for over 24 months.
1. Introduction
An estimated 13,170 new cases of cervical cancer are diagnosed each year in the United States, and it is the fourth most common cancer in women worldwide (National Comprehensive Cancer, 2021). Squamous cell carcinomas, which account for 80% of these cancers, are declining with effective screening, though rates of cervical adenocarcinoma have risen in the last three decades. This stark difference is likely in part due to less effective identification of adenocarcinomas than squamous cell carcinomas by cervical cancer screening. While controversy exists as to which histologic type has a worse prognosis, a majority of studies demonstrate that adenocarcinomas have higher rates of metastasis and recurrence (Gien et al., 2010). Recurrent metastatic cervical cancer develops in 11–64% of women with cervical cancer, usually within the first two years of initial therapy completion (Boussios et al., 2016). Of these women, about 1% will have peritoneal metastasis (Burg et al., 2020). Few case reports describe cervical cancer with peritoneal carcinomatosis; and of those, even fewer offer feasible interventions. With few molecular targets or actionable mutations, platinum-based chemotherapies are the mainstay of treatment for recurrent metastatic cervical cancer (Chao et al., 2014, Orgiano et al., 2016). However, new options have emerged to treat peritoneal carcinomatosis from gastrointestinal or other gynecologic malignancies (Huo et al., 2015). Hyperthermic intraperitoneal chemotherapy (HIPEC) during cytoreductive surgery (CRS) is one of such therapies in which high dose intraperitoneal chemotherapy is administered intraoperatively throughout the abdomen to eliminate residual microscopic cancer cells (Sugarbaker, 2006). This technique uses heat to enhance the effect of intraperitoneal chemotherapy by 3 suspected mechanisms: I) heat has more toxicity for cancer tissue compared to non-cancer tissue, II) it increases the penetration of the chemotherapy, and III) it increases the cytotoxicity of the chemotherapy itself. Furthermore, when HIPEC is used in conjunction with CRS, it has demonstrated more direct drug-cancer tissue contact and, in some studies, improved outcomes for pseudomyxoma peritonei, colorectal cancer and appendiceal neoplasm (Bendifallah et al., 2019, Glehen et al., 2010). While HIPEC has been studied in abdominal malignancies, its application to gynecologic malignancies has been studied less until recently. van Driel et al demonstrated that in the setting of neoadjuvant chemotherapy and interval debulking in advanced ovarian cancer, HIPEC improved recurrence free survivorship compared to non-HIPEC regimens, giving rise to the implementation of HIPEC in advanced ovarian cancer (van Driel et al., 2018). In endometrial cancer with peritoneal carcinomatosis, Delotte et al demonstrated clinical safety in the implementation of HIPEC (Delotte et al., 2014). However, not all studies in the literature demonstrate a positive response to HIPEC in gynecologic malignancies. Heijkoop et al evaluated weekly HIPEC in patients with recurrent cervical cancer and demonstrated poor response and survival (Heijkoop et al., 2014). Heijkoop et al’s study of 38 patients put an end to further investigation of HIPEC for patients with recurrent cervical cancer. While some of HIPEC’s results are encouraging in certain advanced gynecologic malignancies, its efficacy in cervical cancer with peritoneal carcinomatosis remains undefined. Here, we present two cases of patients with recurrent cervical cancer with peritoneal carcinomatosis who underwent HIPEC. Unfortunately, neither of these patients qualified for clinical trials at our institution at the time that they underwent HIPEC for recurrent cervical cancer. The choice of which intra-peritoneal chemotherapy was used was based on individual circumstance, chemotherapies that are active in cervical adenocarcinoma and prior patient responses to chemotherapies (Sugarbaker et al., 2014). To our knowledge, these are the only cases in the literature that describe the use of HIPEC in recurrent metastatic cervical adenocarcinoma with peritoneal carcinomatosis.
2. Case presentation
Our first case is a 35-year-old woman who was first diagnosed with stage 1B1 endocervical adenocarcinoma and underwent surgical staging robotic assisted radical hysterectomy and bilateral salpingectomy followed by surveillance (Fig. 3A). Her surveillance was notable for physical exams in a facility closer to her home. She had a PET scan 4 months after resection which was negative for disease recurrence. 10 months later, she presented with abdominal bloating and was found to have an abdominal mass with peritoneal carcinomatosis and malignant ascites without disease in her chest. She was initiated on carboplatin/paclitaxel/bevacizumab for 6 cycles. Notably she received carboplatin instead of cisplatin due to anaphylaxis to fosaprepitant. Her genomic testing demonstrated no actionable alterations though identified a ARID1A mutation, PIK3CA mutation and PTEN mutation, also her tumor was microsatellite stable and the tumor mutational burden was low. She had a partial response to chemotherapy with resolution of symptoms and ascites though a persistent pelvic mass (Fig. 1a). She then underwent cytoreductive surgery with HIPEC with cisplatin and paclitaxel. The patient had a peritoneal cancer index (PCI) of 7 with disease on the pelvic sidewalls, cul-de-sac, and bladder, all of which was stripped or ablated with an argon beam. In addition, the patient underwent omentectomy, bilateral oophorectomy and appendectomy with completeness of cytoreduction 0/1. Afterwards, 3 L of plasmalyte were instilled and circulated at 1400 mL/minute. Once temperatures were between 42 and 43C, cisplatin 100 mg/m2 and paclitaxel 175 mg/m2 were instilled (Hoppenot et al., 2020). These chemotherapies were chosen because of her prior response to platinum based chemotherapy. The abdomen was agitated for 90 min and then all the fluid was removed via outflow catheters. Following HIPEC, she completed 3 more cycles of carboplatin/paclitaxel. She then transitioned to bevacizumab maintenance for 13 cycles. She is currently in surveillance with CT imaging and exams every 3 months. She remains without evidence of disease 24 months since HIPEC therapy.
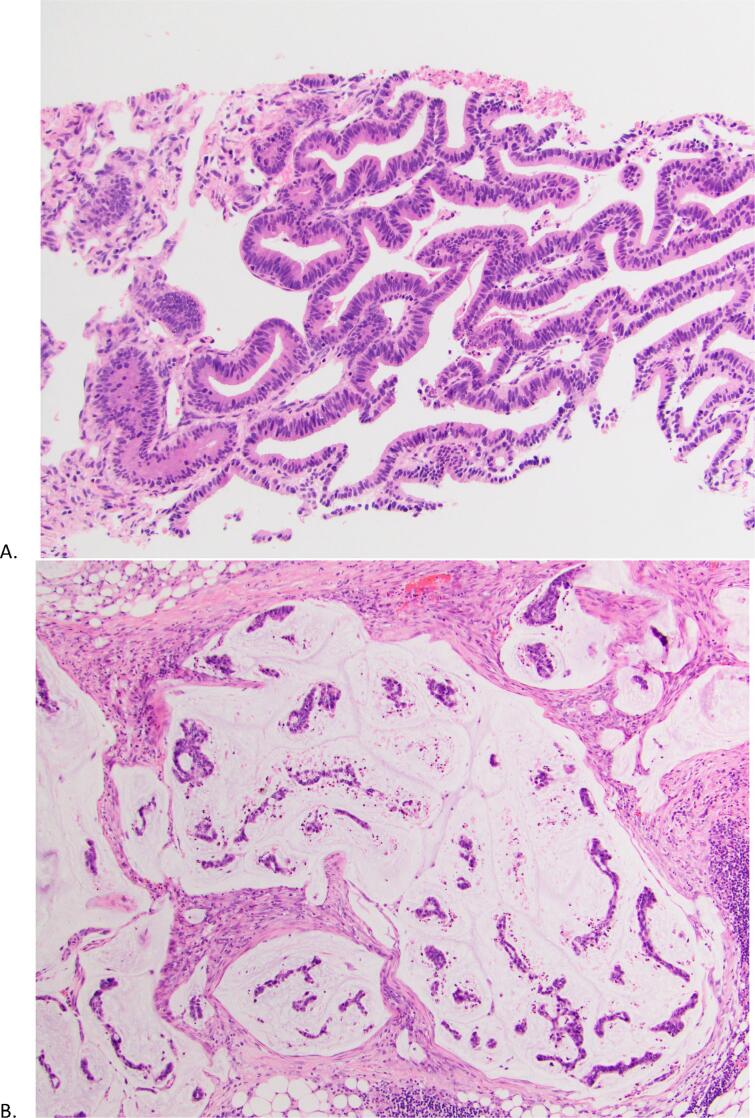
Fig. 3.
Pathology. Figure A is an H&E stain of the pelvic mass from patient 1 taken prior to CRS/HIPEC consistent with cervical adenocarcinoma. Figure B is an H&E stain of an omental biopsy from patient 2 taken prior to CRS/HIPEC demonstrating cervical adenocarcinoma.
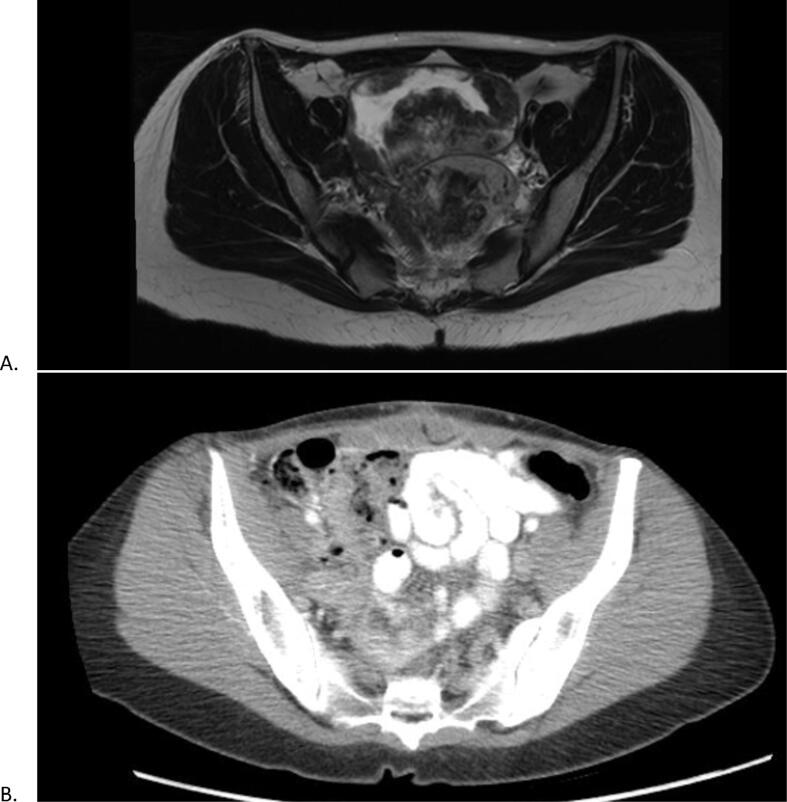
Fig. 1.
1st Patient Pre and Post CRS/HIPEC. Figure A an MRI transverse cross section, prior to CRS/HIPEC, of a bilobed 13.6 cm pelvic mass with small discrete enhancing nodules in the right lower quadrant representing metastasis. Figure B is a CT transverse cross section demonstrating no evidence of disease recurrence.
Our second case is a 33-year-old woman who was first diagnosed with stage IA1 cervical adenocarcinoma. The patient underwent cold knife conization with multiple local recurrences. During her course, she received a total of 4 cold knife conizations in attempts to preserve fertility. Three years after diagnosis with stage 1A1 cervical adenocarcinoma with positive endocervical curettage and deep endocervical margins, the patient underwent total laparoscopic hysterectomy and bilateral salpingectomy followed by adjuvant chemoradiation with cisplatin given high risk feature of close margins (Fig. 3B). She started on a surveillance regimen with exams every three months. Unfortunately, 16 months into surveillance, the patient presented with abdominal distension and pain. CT torso demonstrated large volume ascites with extensive peritoneal nodularity and omental caking without evidence of disease in her chest. She underwent diagnostic laparoscopy and omental biopsy in which pathology confirmed recurrent cervical adenocarcinoma. She received cisplatin/paclitaxel/bevacizumab for 6 cycles with partial response, yet had residual peritoneal carcinomatosis (Fig. 2a). Her genomic testing sequencing from tumor resection demonstrated genomic alterations in FANCC, MLL2 and SMAD4. Microsatellite status and tumor mutation burden could not be determined. After a failed attempt to qualify for an immunotherapy clinical trial due to the adenocarcinoma histology, she received an additional cycle of paclitaxel and bevacizumab and then underwent cytoreductive surgery with HIPEC. The patient had a PCI of 26 with disease on the omentum, spleen, gallbladder, porta hepatis, lesser curve of the stomach, small and large bowel and 2 masses in her pelvis measuring between 12 and 15 cm. In addition, the patient underwent cholecystectomy, appendectomy, splenectomy, omentectomy, bilateral oophorectomy and right sided peritoneal stripping. Once all visible disease was removed, 3 L of plasmalyte was instilled with fluid circulation at 1500 mL/minute until fluid temperature reached 42-43C. The patient’s mucinous tumor component was concerning for being chemo-resistant and 30 mg mitocycin-C was used due to its known responses for pseudomyxoma peritonei from other disease sites as well as a second or third line agent for cervical cancer. A lower dose 75 mg/m2 cisplatin was used due to the patients prior mild renal insufficiency during systemic chemotherapy. The patient received sodium thiosulfate as a bolus and then as a continuous drip for 6 h per weight-based protocol. 60 minutes into the instillation, the patient received another 10 mg of mitomycin-C. During this time, the abdomen was agitated. All the fluid was drained via outflow catheters after 90 minutes. She did not receive any adjuvant therapy following HIPEC. Surveillance consisted of CT/MRI imaging and exam every 3 months. She remained disease free for 15 months from time of HIPEC. Unfortunately, she developed biliary disease as demonstrated on CT torso: new metastatic infiltrative mass/lymph node involving the hepatic hilum causing moderate intrahepatic biliary ductal dilation and a new focus of peritoneal carcinomatosis. She was started on cisplatin/bevacizumab given the platinum free interval; however, she had progression of disease and was then treated with nivolumab/ipilimumab with initial response but then further disease progression. She ultimately passed away over 3 years from her initial diagnosis of recurrent metastatic disease.
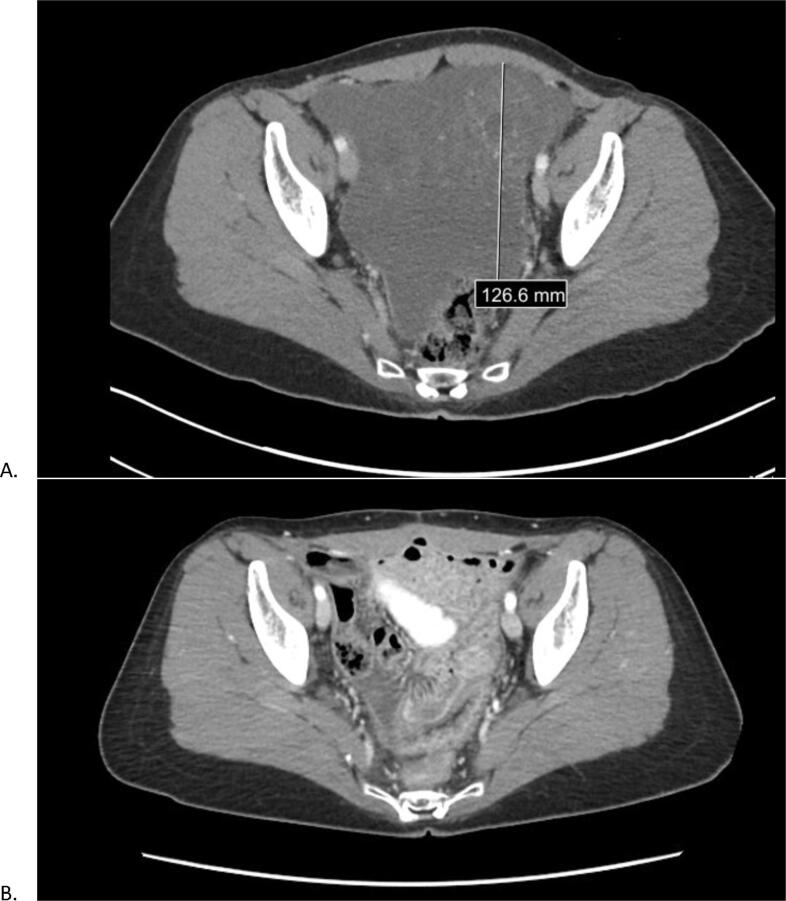
Fig. 2.
2nd Patient Pre and Post CRS/HIPEC. Figure A is a CT transverse cross section, prior to CRS/HIPEC, of a mass occupying the pelvis with peritoneal thickening. Figure B is a CT transverse cross section, post CRS/HIPEC, with no mass and resolved peritoneal thickening and no signs of metastasis.
3. Discussion
In both of these cases, these women presented with cervical adenocarcinoma with abdominal recurrences with peritoneal carcinomatosis after minimally invasive hysterectomy. Based on the conclusions drawn in the LACC trial, our patients were perhaps at higher risk for distant peritoneal recurrence because they underwent minimally invasive surgical hysterectomy (Vergote et al., 2020). Furthermore, our second patient with stage 1A1 cervical cancer with positive margins, positive endocervical curettage and deep endocervical margins would have been a candidate for radical hysterectomy. Nevertheless, given the rarity of such a presentation, few protocols for recurrent cervical cancer with peritoneal carcinomatosis exist. Much of the management of recurrent cervical cancer with abdominal metastases was extrapolated from pathways in advanced ovarian carcinoma. While Heijkoop et al’s study follows 38 patients with locally advanced cervical cancer, their findings may be less applicable to our patients. First, their study does not specifically target patients with peritoneal carcinomatosis, rather they do not comment on the presence of peritoneal carcinomatosis in their patients. Furthermore, our HIPEC protocols greatly differ from those used in Heijkoop et al, the patients discussed in this case report underwent a single HIPEC session, while the patients in Heijkoop’s study underwent weekly sessions. Lastly, only 4 of the 38 patients in Heijkoop et al had adenocarcinoma of the cervix as both of the patients in our case report. The use of HIPEC in peritoneal carcinomatosis continues to be an area of intense research with no single regimen. In particular, the use of bevacizumab around the time of HIPEC and cytoreductive surgery remains controversial. While King et al demonstrate that bevacizumab is not associated with increased morbidity or mortality following CRS/HIPEC, Eveno et al demonstrate that administration of bevacizumab before CRS/HIPEC results in twofold increased morbidity (King et al., 2020, Eveno et al., 2014). Lastly, the significant disease reduction after HIPEC must be considered in the setting of significant cytoreductive surgery. These two cases of recurrent cervical cancer with peritoneal carcinomatosis with remission after CRS/HIPEC incite further questions regarding the appropriate timing and candidacy for HIPEC that could be evaluated in a future prospective study in patients with recurrent/metastatic cervical cancer with peritoneal carcinomatosis.
4. Consultation
These two cases of recurrent cervical cancer with peritoneal carcinomatosis with use of HIPEC during de-bulking surgery with significant disease survival, 15 months and 24 months respectively, demonstrate a possible role for HIPEC in for this disease presentation that does not have many treatment options other than platinum-based therapy. This opens the door to further investigation of HIPEC in patients with cervical cancer with peritoneal carcinomatosis.
Informed consent
Each patient and/or the individual's legal guardian or other person with legal authority to act on the individual's behalf who in this case report is aware of this case report and has given his/her explicit consent.
CRediT authorship contribution statement
Taliya Lantsman: Writing – original draft, Writing – review & editing. Marcos Lepe: Data curation, Formal analysis. Leslie Garrett: Conceptualization, Methodology, Writing – review & editing. Martin Goodman: Conceptualization, Methodology, Writing – review & editing. Meghan Shea: Conceptualization, Methodology, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Bendifallah S., de Foucher T., Bricou A., Ouldamer L., Lavoue V., Varinot J., Canlorbe G., Carcopino X., Raimond E., Huguet F., Graesslin O., Touboul C., Collinet P., Huchon C., Daraï E., Ballester M. Groupe de Recherche FRANCOGYN, FRANCE. Cervical cancer recurrence: Proposal for a classification based on anatomical dissemination pathways and prognosis. Surg. Oncol. 2019 Sep;30:40–46. doi: 10.1016/j.suronc.2019.05.004. Epub 2019 May 23. PMID: 31500783. [DOI] [PubMed] [Google Scholar]
- Boussios S., Seraj E., Zarkavelis G., Petrakis D., Kollas A., Kafantari A., Assi A., Tatsi K., Pavlidis N., Pentheroudakis G. Management of patients with recurrent/advanced cervical cancer beyond first line platinum regimens: Where do we stand? A literature review. Crit. Rev. Oncolo./Hematol. 2016;108:164–174. doi: 10.1016/j.critrevonc.2016.11.006. [DOI] [PubMed] [Google Scholar]
- Burg L., Timmermans M., van der Aa M., Boll D., Rovers K., de Hingh I., van Altena A. Incidence and predictors of peritoneal metastases of gynecological origin: a population-based study in the Netherlands. J. Gynecol. Oncol. 2020 Sep;31(5) doi: 10.3802/jgo.2020.31.e58. PMID: 32808491; PMCID: PMC7440978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao A., Lin C.T., Lai C.H. Updates in systemic treatment for metastatic cervical cancer. Curr. Treat Options Oncol. 2014 Mar;15(1):1–13. doi: 10.1007/s11864-013-0273-1. PMID: 24429797. [DOI] [PubMed] [Google Scholar]
- Delotte J., Desantis M., Frigenza M., Quaranta D., Bongain A., Benchimol D., Bereder J.M. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for the treatment of endometrial cancer with peritoneal carcinomatosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014 Jan;172:111–114. doi: 10.1016/j.ejogrb.2013.10.026. Epub 2013 Nov 5 PMID: 24300558. [DOI] [PubMed] [Google Scholar]
- Eveno C., Passot G., Goéré D., Soyer P., Gayat E., Glehen O., Elias D., Pocard M. Bevacizumab doubles the early postoperative complication rate after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC) for peritoneal carcinomatosis of colorectal origin. Ann. Surg. Oncol. 2014 Jun;21(6):1792–1800. doi: 10.1245/s10434-013-3442-3. Epub 2013 Dec 15 PMID: 24337648. [DOI] [PubMed] [Google Scholar]
- Gien L.T., Beauchemin M.C., Thomas G. Adenocarcinoma: a unique cervical cancer. Gynecol. Oncol. 2010 Jan;116(1):140–146. doi: 10.1016/j.ygyno.2009.09.040. Epub 2009 Oct 31 PMID: 19880165. [DOI] [PubMed] [Google Scholar]
- Glehen O., Gilly F.N., Boutitie F., et al. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: a multi-institutional study of 1,290 patients. Cancer. 2010;116(24):5608–5618. doi: 10.1002/cncr.25356. [DOI] [PubMed] [Google Scholar]
- Heijkoop S.T., van Doorn H.C., Stalpers L.J., Boere I.A., van der Velden J., Franckena M., Westermann A.M. Results of concurrent chemotherapy and hyperthermia in patients with recurrent cervical cancer after previous chemoradiation. Int. J. Hyperthermia. 2014 Feb;30(1):6–10. doi: 10.3109/02656736.2013.844366. Epub 2013 Oct 24 PMID: 24156619. [DOI] [PubMed] [Google Scholar]
- Hoppenot C., Schuitevoerder D., Izquierdo F.J., Plana A., Pothuri B., Diane Yamada S., Kim J., Lee N.K., Abbott D.E., Abdel‐Misih S., Ahrendt S.A., Al‐Kasspooles M., Amersi F., Arrington A.K., Badgwell B., Barone R.M., Baumgartner J.M., Berri R.N., Bijelic L., Blazer D.G., Bowne W.B., Brown C.K., Catenacci D.V., Chan C.H.F., Chapel D.B., Cho C.S., Choudry M.H.A., Clarke C.N., Cusack J.C., Dachman A.H., Deneve J.L., Dineen S.P., Eng O.S., Fernandez L.J., Fleshman J., Clark Gamblin T., Gangi A., Georgakis G.V., Gilbert E.W., Goodman M.D., Grotz T.E., Gushchin V., Hanna N., Harmath C., Hayes‐Jordan A., Husain A.N., Idrees K., Ihemelandu C., Johnston F.M., Jiang D., Kane J.M., Karakousis G., Kelly K.J., Kennedy T.J., Keutgen X.M., Kindler H.L., Kluger M.D., Lastra R.R., Lee B., Mack L.A., Maduekwe U.N., Mak G.Z., Mammen J.M.V., Mathew M.S., Melis M., Melnitchouk N., Merkow R.P., Mogal H., Möller M.G., Moroney J., Oto A., Pameijer C.R., Pappas S.G., Polanco P.M., Polite B.N., Reddy S.S., Royal R., Salti G., Sardi A., Senthil M., Setia N., Sherman S.K., Sideris L., Skitzki J., Tun S., Veerapong J., Votanopoulos K.I., White M.G., Winer J.H., Xiao S.-Y., Yantiss R.K., Ahuja N., Lowy A.M., Richard Alexander H., Esquivel J., Foster J.M., Labow D.M., Lambert L.A., Levine E.A., Staley C., Sugarbaker P.H., Bartlett D.L., Turaga K. The Chicago Consensus on peritoneal surface malignancies: Management of ovarian neoplasms. Cancer. 2020;126(11):2553–2560. doi: 10.1002/cncr.32867. [DOI] [PubMed] [Google Scholar]
- Huo Y.R., Richards A., Liauw W., Morris D.L. Hyperthermic intraperitoneal chemotherapy (HIPEC) and cytoreductive surgery (CRS) in ovarian cancer: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2015 Dec;41(12):1578–1589. doi: 10.1016/j.ejso.2015.08.172. Epub 2015 Sep 25 PMID: 26453145. [DOI] [PubMed] [Google Scholar]
- King B.H., Baumgartner J.M., Kelly K.J., Marmor R.A., Lowy A.M., Veerapong J. Preoperative bevacizumab does not increase complications following cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. PLoS One. 2020 Dec 3;15(12) doi: 10.1371/journal.pone.0243252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network, 2021. Cervical cancer (version 1.2021). Retrieved from https://www.nccn.org/professionals/physician_gls/pdf/cervical_blocks.pdf.
- Orgiano L., Pani F., Astara G., Madeddu C., Marini S., Manca A., Mantovani G. The role of “closed abdomen” hyperthermic intraperitoneal chemotherapy (HIPEC) in the palliative treatment of neoplastic ascites from peritoneal carcinomatosis: report of a single-center experience. Support. Care Cancer. 2016 Oct;24(10):4293–4299. doi: 10.1007/s00520-016-3262-7. Epub 2016 May 12 PMID: 27169699. [DOI] [PubMed] [Google Scholar]
- Sugarbaker P.H. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? LancetOncol. 2006;7:69–76. doi: 10.1016/S1470-2045(05)70539-8. [DOI] [PubMed] [Google Scholar]
- Sugarbaker P.H., Rangole A.K., Carr N.J. Peritoneal metastases from mucinous endocervical adenocarcinoma. Gynecol. Oncol. Rep. 2014 Dec;6(10):5–8. doi: 10.1016/j.gore.2014.07.003. PMID: 26075991; PMCID: PMC4434145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Driel W., et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018;378(14):1362–1364. doi: 10.1056/nejmc1802033. [DOI] [PubMed] [Google Scholar]
- Vergote I., Magrina J.F., Zanagnolo V., Magtibay P.M., Butler K., Gil-Moreno A., Feijoo B.D., Kimmig R., Canis M., Bourdel N., Ind T., Estape R., Persson J., Lim P., Coronado P., Ponce J., Lambaudie E., Van Gorp T., Maggioni A., Narducci F., Van Niewwenhuysen E., Van Trappen P. The LACC Trial and Minimally Invasive Surgery in Cervical Cancer. J. Minim. Invasive Gynecol. 2020 Feb;27(2):462–463. doi: 10.1016/j.jmig.2019.09.767. Epub 2019 Sep 11 PMID: 31520725. [DOI] [PubMed] [Google Scholar]